Abstract
The extracellular framework and two-thirds of the dry mass of adult articular cartilage are polymeric collagen. Type II collagen is the principal molecular component in mammals, but collagens III, VI, IX, X, XI, XII and XIV all contribute to the mature matrix. In developing cartilage, the core fibrillar network is a cross-linked copolymer of collagens II, IX and XI. The functions of collagens IX and XI in this heteropolymer are not yet fully defined but, evidently, they are critically important since mutations in COLIX and COLXI genes result in chondrodysplasia phenotypes that feature precocious osteoarthritis. Collagens XII and XIV are thought also to be bound to fibril surfaces but not covalently attached. Collagen VI polymerizes into its own type of filamentous network that has multiple adhesion domains for cells and other matrix components. Collagen X is normally restricted to the thin layer of calcified cartilage that interfaces articular cartilage with bone.
Keywords: articular cartilage, collagens, extracellular matrix
Introduction
Collagen accounts for about two-thirds of the dry weight of adult articular cartilage. The tissue's material strength depends on the extensive cross-linking of the collagen and the apparent zonal changes in fibrillar architecture with tissue depth. Once laid down during development, there appears to be little capacity for articular chondrocytes to recapitulate the overall collagen architecture if the mature tissue is injured or undergoes advanced degenerative changes. The ability of chondrocytes to remodel the collagen at ultrastructural and molecular levels is poorly understood, but it may be more significant than previously thought and possible molecular mechanisms are a topic of growing interest.
The four zones of articular cartilage visible by light microscopy (superficial or tangential, intermediate or transitional, deep or radial, and calcified) differ in their collagen fibril orientation [1]. In general, collagen fibrils seen by transmission electron microscopy (TEM) (Fig. 1) form a random network compared with those of other connective tissues but, both macroscopically and ultrastructurally, preferred fibril patterns are evident [2]. In the superficial zone (~200 μm), the fibrils are thin and tend to run primarily parallel to the plane of the articular surface with some degree of parallel orientation in that plane. A greater range of fibril diameters is seen in the deeper zones, and the organization appears more random when viewed by TEM. In the radial zone of some joint regions, a preferred orientation of fibril bundles orthogonal to the surface can be seen by scanning electron microscopy, also visible by TEM in regions of pathologically softened cartilage [2]. The arcade-like macro-architecture of collagen responsible for this zonal appearance described by Benninghoff [3] appears, on scanning electron microscopy, to reflect a folding over of radial fiber bundles to lie in the plane of the surface in a series of layers or leaflets that makes up the tangential zone [4]. In mammalian articular cartilage, the primary collagen components (collagens II, IX and XI) do not appear to alter dramatically in proportion between zones. (In birds, type I collagen predominates at the articular surface and decreases with depth in an interchanging gradient with type II collagen [5].) The greatest quantitative difference occurs with maturation from the exclusively fine fibrils of young growth cartilages (≥ 10% collagen IX, ≥ 10% collagen XI, ≤ 80% collagen II) to the thicker and more varied fibril diameters of mature articular cartilage (~1% collagen IX, ~3% collagen XI, ≥ 90% collagen II) [6].
Figure 1.
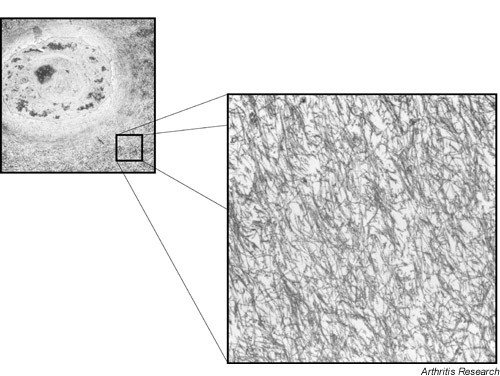
The chondrocyte and extracellular matrix of articular cartilage showing the underlying collagen fibril meshwork (transmission electron microscopy).
The collagen phenotype of the calcified zone of cartilage that interfaces with bone also includes type X collagen surrounding the cells, as in the hypertrophic zone of the growth plate [7].
Ultrastructural fabric
In finer detail, the fibrillar appearance of the mature tissue differs for the pericellular and the intercellular (interterritorial) matrix. Fibrils become coarser and more obviously banded, as seen by TEM, going further from the chondrocyte [1]. The proportion of type IX [8] and type XI [9] collagens is highest in the thinnest fibrils that form the pericellular basket, or the chondron described by Poole et al. [8]. Remodeling and maturation of thin, newly made fibrils presumably involves removal of collagens IX and XI, and/or their dilution by addition of new type II collagen. To what degree thin fibrils fuse laterally in the matrix versus growing by accretion of new monomers is unclear, although both processes are thought to occur [10,11].
The collagen II:IX:XI heteropolymer
Collagens II, IX and XI resist extraction with denaturants or serial digestion with streptomyces hyaluronidase, chondroitinase ABC, and trypsin at 37°C. Such serial digestion leaves little else in the cartilage but these three collagens as cross-linked polymers [12]. The exact spatial relationships, manner and temporal order of assembly of these different collagen types into heteromeric fibrils are not well understood. Their interaction and existence as subunits of the same fibril network have been shown by immunoelectron microscopy [13] and the isolation and structural identification of cross-linked heterotypic peptides [14,15]. The basic structure of the fibrils seen by TEM is a four-dimensional (4D)-staggered polymer of collagen type II molecules heavily cross-linked head-to-tail by hydroxylysyl pyridinoline residues at the two telopeptide-to-helix sites.
Collagen IX molecules can decorate fibril surfaces, particularly those of thin fibrils in the pericellular basket [16]. Cross-linking studies have identified at least six sites of cross-linking within the collagen IX molecule where covalent bonds form with either collagen II molecules or with other collagen IX molecules [14,17,18] (Eyre D, Wu J, Weis M, unpublished observations, 2001; Fig. 2). The cross-linking residues are either trivalent pyridinolines or divalent borohydride-reducible intermediates formed by the same lysyl oxidase-mediated mechanism as occurs in the major fibril-forming collagens.
Figure 2.
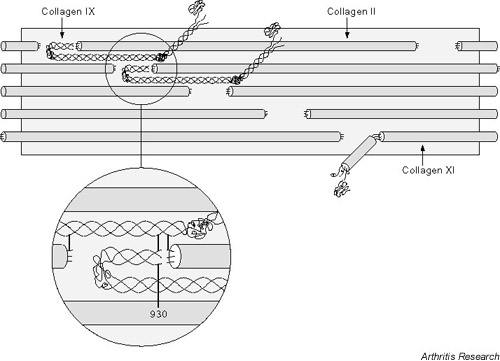
The collagen II:IX:XI heterofibril. A molecular model of the collagen type IX fold and interaction site with a collagen II microfibril that can account for all known cross-linking sites between collagen II and IX molecules.
Each of the three collagen IX chains, α1(IX), α2(IX), and α3(IX), has one to three cross-linking sites, all of which are occupied in the matrix pool of type IX collagen, as judged from peptide mapping studies [17]. The role of collagen IX in the matrix apparently requires the molecules to be covalently linked to the surface of type II collagen fibrils, which suggests a mechanical restraint of some kind. It is tempting to speculate from the biochemical evidence that collagen IX can also form a covalent bridge between fibrils, increasing network mechanical integrity and providing a restraint for entrapped proteoglycan osmotic swelling pressure. Interfibrillar cross-linking has not been proven, however, and it could be that covalently anchored molecular projections from fibril surfaces (the COL3 domain and terminal NC4 globular domain of α1(IX) project from the fibril surfaces) could restrict shear strains between fibrils in a mesh of thin fibrils embedded in a proteoglycan gel, without a need for direct covalent bonds between fibrils. Figure 2 shows how collagen IX molecules can be accommodated on a fibril surface and can satisfy all the covalent interactions so far identified. In this model proposed by Miles et al. [19], the COL1/NC1 domain docks in the hole region, oriented as shown in Figure 2, and the molecule hinges back on itself at the NC2 domain.
Collagen XI is found in developing cartilage as a heterotrimeric molecule of two novel collagen gene products (α1(XI) and α2(XI)) and a third chain (α3(XI)) identical in primary sequence to α1(II)B, the common form of splicing variant of the type II collagen gene [6]. From mature articular cartilage, the isolated collagen XI fraction contains α1(V) and α1(XI) in roughly equal amounts [6]. The α1(V) chain appears to occur in hybrid molecules together with α1(XI) and/or α2(XI) rather than in typical type V collagen molecules found in non-cartilaginous tissues. The biological significance of this is unknown.
The N-propeptide domains of all these chains are retained in the matrix and alternatively spliced variants can be expressed [20]. Selective binding interactions with other matrix macromolecules can be expected as part of the distinctive function of these molecules. Immunolocalization studies [13] and analyses of cross-linked peptides [15] have shown that the collagen XI pool is intimately copolymerized with type II collagen. The type XI N-propeptide domains are thought to poke out of the hole domains of the collagen 4D-staggered lattice, perhaps acting to limit growth in fibril diameters [20]. Collagen XI is most concentrated in the pericellular network of thin fibrils, and recent work has shown high-affinity binding sites for heparan and heparin sulfate in the triple helical domains [9].
Cross-linked peptide analyses have shown that collagen XI molecules are cross-linked to each other through their N-telopeptide-to-helix interaction sites [15]. They lack a cross-linking lysine in the C-telopeptide except in the α3(XI) (αI(II)) chain. Interestingly, the N-telopeptide cross-linking lysines are located external to candidate metalloproteinase cleavage sites, in α1(XI), α1(V) and α2(XI), implying that any such cleavages could selectively depolymerize collagen XI [15]. The N-terminal helical cross-linking site of collagen XI molecules was occupied (in α1(XI)) by the α1(II)C-telopeptide. By analogy to findings with the type I/V collagen heteromer of bone [21], this is consistent with the formation of lateral cross-links between collagen II and XI molecules at this locus. Together, these findings can be interpreted as collagen XI initially forming a head-to-tail self-cross-linked filament that becomes integrated and cross-linked laterally onto or within the body of collagen II fibrils. Collagen XI could conceivably form an interconnecting, secondary filamentous network that provides links between fibrils as well as running within fibrils, not inconsistent with the current concept that collagen XI restricts the lateral growth of collagen II fibrils [22]. Clearly, the majority of the covalent links of collagen XI are type XI to type XI [15] and this fact needs to be accommodated in any workable model of fibril assembly.
Proteolytic and mechanical damage to the fibrillar network is believed to be a key, perhaps irreversible, stage in the destruction of joint cartilages in arthritis. Defining and being able to monitor the structure, assembly and biological mechanisms of degradation of the cartilage collagen heterotypic polymer are therefore important for the development and validation of rational therapeutic targets for treating and preventing joint disease.
Collagen type III
Collagen type III is consistently detected by immunofluorescence in samples of normal and osteoarthritic human articular cartilage [23,24]. By electron microscopy study, it has been found to colocalize with type II collagen in the same banded fibrils and to retain its N-propeptide domain [25]. Cross-linking studies confirm that type III collagen is copolymerized and linked to collagen II in human articular cartilage as a minor but regular component [26]. In osteoarthritic cartilage, collagen III tended to be concentrated in the superficial and upper middle zones and to be synthesized by the chondrocytes in the absence of collagen I expression [23]. It is tempting to speculate that collagen III is made by chondrocytes in addition to collagen II in response to matrix damage akin to the wound-healing role of collagen III in type I collagen-based tissues.
Collagens VI, XII and XIV
Type VI collagen is a ubiquitous matrix constituent of most tissues, including articular cartilage (≤ 1% of the collagen) [27]. This protein self-assembles into disulfide-bonded dimers, tetramers and a distinctive filamentous network that is most concentrated around cells [28] but also interspersed loosely in spaces throughout the fibrillar matrix. Among different cartilage types, collagen VI is most concentrated in fibrocartilages such as the meniscus and the intervertebral disc [27]. Type XII and XIV collagens are two members of the FACIT collagen subfamily, in addition to collagen IX, that can be extracted from the cartilage matrix [29]. The FACIT molecules share sequence similarities, most conserved in their COL1 domains [30]. The proteins can be extracted without proteolysis, so they appear not to be covalently polymerized in the matrix [29], but are thought to bind physically to collagen fibril surfaces via their COL1/NC1 domains. Their function is unknown, but cooperation and/or competition between them and various other fibril-binding proteins in cartilage, notably the leucine-repeat small proteoglycans, decorin, biglycan and fibromodulin, seems probable.
Assembly and turnover
After skeletal growth has ceased, the synthetic rate of type II collagen by articular chondrocytes drops dramatically as assessed by proline labeling in vivo. In the adult tissue, however, some synthesis continues, and this can be accelerated up to 10-fold within 2 weeks after joint injury, for example after anterior cruciate ligament section in the mature dog [31]. Little is known of synthetic rates of the other collagen types in adult articular cartilage. Observations based on the synthetic rate of hydroxyproline indicate very little turnover of the collagenous component of the matrix as a whole, with an estimated turnover time of 400 years for human femoral head cartilage [32]. This still leaves the possibility that a subfraction of the collagenous matrix (e.g. fibril surface molecules and the pericellular domain) is remodeled more rapidly by chondrocytes in response to mechanical and molecular signals. If the bulk of the collagen mass, which is embodied in the thicker, mature fibrils of the interterritorial matrix, persists in maturity without turnover, then the average turnover rate of the collagen as a whole would still be very slow. Indeed, the mean diameter of banded collagen fibrils in mature human articular cartilage increases with age [1], consistent with this remodeling concept.
It will be important to define how chondrocytes control the assembly of the heterotypic fibril polymer. Studies on a rat chondrosarcoma cell line that fails to remove the N-propeptides from collagen II show that, although fibril growth is arrested at the stage of fine filaments, collagens II, IX and XI are already cross-linked in fetal proportions [33]. Is an initial protofibril containing collagens II, IX and XI preassembled from monomers as early as a secretory organelle or are, for example, collagen IX monomers secreted that can interact with either nascent or mature fibrils outside the cell? Are collagen XI 4D-staggered filaments assembled independently and do they provide the template intracellularly or extracellularly for collagen II fibril growth? By building on such studies and knowledge derived from studying procollagen I assembly into fibrils in vitro, and using antibodies and other imaging techniques to study fibrils in tissues by electron microscopy [34], these questions should be answerable.
Mechanisms of degradation
Tissue sites of proteolysis and denaturation of matrix type II collagen can be observed in normal and osteoarthritic joint surfaces [35] using specific antibodies. The classical concept of collagen fibril degradation is through an initial cleavage of the collagen molecule (type I, II or III) by collagenase into three-quarter and one-quarter length fragments. Articular chondrocytes can express collagenases, including collagenase-3 (MMP13) (which is the most active in cleaving type II collagen), as demonstrated in culture under interleukin-1 stimulation or directly in tissue removed from arthritic joints [36]. This enzyme, therefore, is implicated in the breakdown of cartilage collagen in osteoarthritis. Of the growing number of matrix metalloproteinases that may contribute to matrix protein metabolism [37], the collagenases are perhaps the best understood in terms of their natural substrate. However, an essential role for collagenases in all forms of collagen breakdown and turnover is becoming less certain. For instance, in mice genetically engineered to express type I collagen lacking a functional cleavage sequence at the three-quarter site, no phenotype was evident at birth. Only later did mild skin thickening and uterine fibroses develop, implying that alternative degradation mechanisms not requiring the three-quarter cleavage can provide for essentially normal development, growth and remodeling of most collagen type I-based tissues [38].
This may also be true in articular cartilage. Collagenases, although active at cleaving soluble monomers of collagen (types I, II and III) in vitro, have limited activity against cross-linked native fibrils. There is evidence to suspect, based on findings with stromelysin-1 (MMP3) against bovine cartilage collagen in vitro [39] and in considering the placement of cross-links, that telopeptide cleavages have to be critical early events in fibril depolymerization, and that they may be the initiating event. In theory, telopeptide cleavages alone could depolymerize a fibril if they are internal to the cross-linking residue. Given the complexity of the collagen II, IX and XI heteromer, and the need for selective removal of collagen IX and perhaps XI to allow for lateral growth of young fibrils, cleavages adjacent to the telopeptide cross-links are an attractive mechanism for cellular control of turnover. The large array of matrix and membrane-associated proteases that chondrocytes can potentially express offers the basis for substrate specificity and fine control of the temporal sequence of collagen depolymerizing action. Perhaps collagenases only come into play in tissues when a particularly rapid degradation and a more efficient disposal of released fibral monomers is required.
Collagen gene defects
The effects of mutations in cartilage collagen genes on the matrix structure offer unique insights into the function of the individual gene products. Collagen II mutations cause a spectrum of diseases from lethal in utero (e.g. achondrogenesis) to early-onset osteoarthritis and minimal skeletal dysplasia [40]. Mutations in collagen IX genes have been found to cause multiple epiphyseal dysplasia, as have mutations in the cartilage oligomeric matrix protein gene [41]. In COL9A2 and COL9A3, splice junction mutations predicted deletion of the equivalent 12 amino acids (exon3) of the COL3 domain of α2(IX) or α3(IX) [42]. Protein analysis of iliac crest cartilage from an affected 10-year-old boy heterozygous for the COL9A3 defect showed collagen IX in the matrix, but showed evidence of its inefficient cross-linking [43]. Two relatively common polymorphisms that introduce a tryptophan residue into the α2(IX) or α3(IX) chain have been shown to be linked to an increased risk of lumbar disc disease [44]. Understanding their presumed negative effects on disc biology is likely to be informative about collagen IX function.
Mutations in COL11A1 and COL2A1 have been found to cause forms of Stickler and Marshall syndrome, in which eye and/or cartilage problems are manifested [45]. Homozygous or compound heterozygous mutations in COL11A2, which are predicted to cause a lack of α2(XI) chains, underlie the recessively inherited otospondylomegaepiphyseal dysplasia syndrome [46]. The mechanisms by which these and other collagen gene defects disturb cartilage matrix assembly and function will be important to define.
Conclusions
The collagenous matrix of articular cartilage is a highly complex assemblage of multiple gene products. Neither the functions of the individual components nor the molecular mechanisms controlling the assembly, turnover or degradation in disease of the collagen heteropolymer are yet well understood. Collagen breakdown is considered to be a critical and perhaps irreversible step in the progression of osteoarthritis. Better insight into molecular mechanisms by which chondrocytes control the functional integrity of the collagenous component of adult articular cartilage is needed.
Abbreviations
4D = four-dimensional; TEM = transmission electron microscopy.
Acknowledgments
Acknowledgements
Work described in the author's laboratory was supported in part by grants from the National Institutes of Health and the Burgess Chair Endowment of the University of Washington. The author thanks Tom Eykemans for the figures and Kae Ellingsen for preparing the manuscript.
References
- Lane JM, Weiss C. Review of articular cartilage collagen research. Arth Rheum. 1975;18:553–562. doi: 10.1002/art.1780180605. [DOI] [PubMed] [Google Scholar]
- Chen M-H, Broom N. On the ultrastructure of softened cartilage: A possible model for structural transformation. J Anat. 1998;192:329–341. doi: 10.1017/S0021878298003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff A. Form und bau der Gelenknorpel in ihren Bezeihungen zur Funktion. Z Zellforsch Mikrosk Anat. 1925;2:783–825. [Google Scholar]
- Notzli H, Clark J. Deformation of loaded articular cartilage prepared for scanning electronmicroscopy with rapid freezing and freeze-substituion fixation. J Orthop Res. 1997;15:76–86. doi: 10.1002/jor.1100150112. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Brickley-Parsons DM, Glimcher MJ. Predominance of type I collagen at the surface of avian articular cartilage. FEBS Lett. 1978;85:259–263. doi: 10.1016/0014-5793(78)80468-2. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Wu JJ. Type XI or 1α2α3α collagen. In Structure and Function of Collagen Types Edited by Mayne R, Burgeson RE New York: Academic Press; 1987. pp. 261–281.
- Gannon JM, Walker G, Fischer M, Carpenter R, Thompson RC, Jr, Oegema TR., Jr Localization of type X collagen in canine growth plate and adult canine articular cartilage. J Orthop Res. 1991;9:485–494. doi: 10.1002/jor.1100090404. [DOI] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: Ultrastructural analysis of the pericellular microenvironment in adult human articular cartilage. J Orthop Res. 1987;5:509–522. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- Vaughan-Thomas A, Young RD, Phillips AC, Duance VC. Characterization of type XI collagen-glycosaminoglycan interactions. J Biol Chem. 2001;276:5303–5309. doi: 10.1074/jbc.M008764200. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Michel M, Studer C. Ultrasturcture of adult human articular cartilage matrix after cryotechnical processing. Micro Res Tech. 1997;37:271–284. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Holmes DF, Graham HK, Trotter JA, Kadler KE. STEM/TEM studies of collagen fibril assembly. Micron. 2001;32:273–285. doi: 10.1016/S0968-4328(00)00040-8. [DOI] [PubMed] [Google Scholar]
- Chun LE, Koob TJ, Eyre DR. Sequential enzymic dissection of the proteoglycan complex from articular cartilage [abstract]. Trans Orthop Res Soc. 1986;11:96. [Google Scholar]
- Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Woods PE, Eyre DR. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J Biol Chem. 1992;267:23007–23014. [PubMed] [Google Scholar]
- Wu JJ, Eyre DR. Structural analysis of cross-linking domains in cartilage type XI collagen: Insights on polymeric assembly. J Biol Chem. 1995;270:18865–18870. doi: 10.1074/jbc.270.32.18865. [DOI] [PubMed] [Google Scholar]
- Hagg R, Bruckner P, Hedbom E. Cartilage fibrils of mammals are biochemically heterogeneous: Differential distribution of decorin and collagen IX. J Cell Biol. 1998;142:285–294. doi: 10.1083/jcb.142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura S, Wu JJ, Eyre DR. Two-dimensional peptide-mapping of cross-linked type IX collagen in human cartilage. Arch Biochem Biophys. 2000;378:33–39. doi: 10.1006/abbi.2000.1805. [DOI] [PubMed] [Google Scholar]
- Diab M, Wu J-J, Eyre DR. Collagen type IX from human cartilage. A structural profile of intermolecular cross-linking sites. Biochem J. 1996;314:327–332. doi: 10.1042/bj3140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles CA, Knott L, Sumner IG, Bailey AJ. Differences between the thermal stabilities of the three triple-helical domains of type IX collagen. J Mol Biol. 1988;277:135–144. doi: 10.1006/jmbi.1997.1603. [DOI] [PubMed] [Google Scholar]
- Gregory KE, Oxford JT, Chen Y, Gambee JE, Gygi SP, Aebersold R, Neame PJ, Mechling DE, Bachinger HP, Morris NP. Structural organization of distinct domains within the non-collagenous N-terminal region of collagen type XI. J Biol Chem. 2000;275:11498–11506. doi: 10.1074/jbc.275.15.11498. [DOI] [PubMed] [Google Scholar]
- Niyibizi C, Eyre DR. Structural characteristics of cross-linking sites in type V collagen of bone: Chain specificities and heterotypic links to type I collagen. Eur J Biochem. 1994;224:943–950. doi: 10.1111/j.1432-1033.1994.00943.x. [DOI] [PubMed] [Google Scholar]
- Blaschke UK, Eikenberry EF, Hulmes DJS, Galla H-J, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–10378. doi: 10.1074/jbc.275.14.10370. [DOI] [PubMed] [Google Scholar]
- Aigner T, Bertling W, Stöss H, Weseloh G, von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993;91:829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton SF, Duance VC. Type III collagen in normal human articular cartilage. Histochem J. 1994;26:412–416. doi: 10.1007/BF00160053. [DOI] [PubMed] [Google Scholar]
- Young RD, Lawrence PA, Duance VC, Aigner T, Monaghan P. Immunolocalization of collagen types II and III in single fibrils of human articular cartilage. J Histochem Cytochem. 2000;48:423–432. doi: 10.1177/002215540004800312. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Murray J, Eyre DR. Evidence for copolymeric cross-linking between types II and III collagens in human articular cartilage [abstract]. Trans Orthop Res Soc. 1996;21:42. [Google Scholar]
- Wu JJ, Eyre DR, Slayter HS. Type VI collagen of the intervertebral disc. Biochemical and electronmicroscopic characterization of the native protein. Biochem J. 1987;248:373–381. doi: 10.1042/bj2480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Poole CA. Sequestration of type VI collagen in the pericellular microenvironment of adult chondrocytes cultured in agarose. Osteoarth Cart. 1996;4:275–285. doi: 10.1016/s1063-4584(05)80105-0. [DOI] [PubMed] [Google Scholar]
- Watt SL, Lundstrum GP, McDonough AM, Keene DR, Burgeson RE, Morris NP. Characterization of collagen types XII and XIV from fetal bovine cartilage. J Biol Chem. 1992;267:20093–20099. [PubMed] [Google Scholar]
- Gordon MK, Castagnola P, Dublet B, Linsenmayer TF, Van Der Rest M, Mayne R, Olsen BR. Cloning of a cDNA for a new member of the class of fibril-associated collagens with interrupted triple helices. Eur J Biochem. 1991;201:333–338. doi: 10.1111/j.1432-1033.1991.tb16290.x. [DOI] [PubMed] [Google Scholar]
- Eyre DR, McDevitt CA, Billingham MEJ, Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980;188:823–827. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A. Physicochemical properties of articular cartilage. In Adult Articular Cartilage, 2nd edition Edited by Freeman MAR London: Pitman Medical; 1979. pp. 215–290.
- Fernandes RJ, Schmid TM, Harkey MA, Eyre DR. Incomplete processing of type II procollagen by a rat chondrosarcoma cell line. Eur J Biochem. 1997;247:620–624. doi: 10.1111/j.1432-1033.1997.00620.x. [DOI] [PubMed] [Google Scholar]
- Bos KJ, Holmes DF, Kadler KE, McLeod D, Morris NP, Bishop PN. Axial structure of the heterotypic collagen fibrils of vitreous humour and cartilage. J Mol Biol. 2001;306:1011–1022. doi: 10.1006/jmbi.2000.4429. [DOI] [PubMed] [Google Scholar]
- Poole AR, Nelson F, Hollander A, Reiner A, Pidoux I, Ionescu M. Collagen II turnover in joint disease. Acta Orthop Scand. 1995;266(suppl):88–91. [PubMed] [Google Scholar]
- Dahlberg L, Billinghurst RC, Manner P, Nelson F, Webb G, Ionescu M, Reiner A, Tanzer M, Zukor D, Chen J, van Wart HE, Poole AR. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (Matrix Metalloproteinase 1). Arth Rheum. 2000;43:673–682. doi: 10.1002/1529-0131(200003)43:3<673::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Murphy G, Knauper V, Cowell S, Hembry R, Stanton H, Butler G, Freije J, Pendas AM, Lopez-Otin C. Evaluation of some newer matrix metalloproteinases. Ann NY Acad Sci. 1999;878:25–39. doi: 10.1111/j.1749-6632.1999.tb07672.x. [DOI] [PubMed] [Google Scholar]
- Krane SM, Byrne MH, Lemaitre V, Henriet P, Jeffrey JJ, Witter JP, Liu X, Wu H, Jaenisch R, Eeckhout Y. Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem. 1996;271:28509–28515. doi: 10.1074/jbc.271.45.28509. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Lark MW, Chun LE, Eyre DR. Sites of stromelysin cleavage in collagen types II, IX, X and XI of cartilage. J Biol Chem. 1991;266:5625–5628. [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- Unger SL, Briggs MD, Holden P, Zabel B, Ala-Kokko L, Paassilta P, Lohiniva J, Rimoin DL, Lachman RS, Cohn DH. Multiple epiphyseal dysplasia: Radiographic abnormalities correlated with genotype. Pediatr Radiol. 2001;31:10–18. doi: 10.1007/s002470000362. [DOI] [PubMed] [Google Scholar]
- Lohiniva J, Paassilta P, Seppanen U, Vierimaa O, Kivirikko S, Ala-Kokko L. Splicing mutations in the COL3 domain of collagen IX cause multiple epiphyseal dysplasia. Am J Med Genet. 2000;90:216–222. doi: 10.1002/(SICI)1096-8628(20000131)90:3<216::AID-AJMG6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bönnemann CG, Cox GF, Shapiro F, Wu JJ, Feener CA, Thompson T, Anthony CD, Eyre DR, Darras B, Kunkel LM. A mutation in the α3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy. Proc Natl Acad Sci USA. 2000;97:1212–1217. doi: 10.1073/pnas.97.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paassilta P, Lohiniva J, Goring HH, Perala M, Raina SS, Karppinen J, Hakala M, Palm T, Kroger H, Kaitila I, Vanharanta H, Ott J, Ala-Kokko L. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA. 2001;285:1843–1849. doi: 10.1001/jama.285.14.1843. [DOI] [PubMed] [Google Scholar]
- Griffith AJ, Sprunger LK, Sirko-Osadsa DA, Tiller GE, Meisler MH, Warman ML. Marshall syndrome associated with a splicing defect at the COL11A1 locus. Am J Hum Genet. 1998;62:816–823. doi: 10.1086/301789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkoniemi M, Brunner HG, Manouvrier S, Hennekam R, Superti-Furga A, Kaariainen H, Pauli RM, van Essen T, Warman ML, Bonaventure J, Miny P, Ala-Kokko L. Autosomal recessive disorder otospondylomegaepiphyseal dysplasia is associated with loss-of-function mutations in the COL11A2 gene. Am J Hum Genet. 2000;66:368–377. doi: 10.1086/302750. [DOI] [PMC free article] [PubMed] [Google Scholar]


