Abstract
The present study tests the clinical postulate that elevated testosterone (Te) and estradiol (E2) concentrations modulate the effects of constant iv infusion of saline vs recombinant human IGF-I on free IGF-I, IGFBP-1 and dimeric IGF-I/IGFBP-1 concentrations in healthy aging adults. To this end, comparisons were made following administration of placebo (Pl) vs Te in 8 older men (age 61 ± 4 y) and after Pl vs E2 in 8 postmenopausal women (62 ± 3 y). In the saline session: (i) E2 lowered and Te increased total IGF-I; (ii) E2 specifically elevated IGFBP-1 by 1.5-fold and suppressed free IGF-I by 34%; and (iii) E2 increased binary IGF-I/IGFBP-1 by 5-fold more than Te. During IGF-I infusion: (i) total and free IGF-I rose 1.4–2.0 fold [Pl] and 2.1–2.5 fold [Te] more rapidly in men than women; (ii) binary IGF-I/IGFBP-1 increased 3.4-fold more rapidly in men [Te] than women [E2]; and (iii) end-infusion free IGF-I was 1.6-fold higher in men than women.
In summary, E2 compared with Te supplementation lowers concentrations of total and ultrafiltratably free IGF-I and elevates those of IGFBP-1 and binary IGF-I/IGFBP-1, thus putatively limiting IGF-I bioavailability. If free IGF-I mediates certain biological actions, then exogenous Te and E2 may modulate the tissue effects of total IGF-I concentrations unequally.
Keywords: insulin-like growth factor, IGFBP-1, binary complex, estrogen, androgen
Introduction
Concentrations of total IGF-I and sex-steroid hormones fall pari passu in aging adults, thereby putatively contributing to relative frailty (1–6). Both testosterone (Te) and estradiol (E2) stimulate GH secretion, but only Te consistently elevates total IGF-I concentrations (7–16). This mechanistic distinction reflects in part the capability of estrogenic steroids to inhibit the hepatic actions of GH (17). On the other hand, relative IGF-I depletion in aging individuals is due primarily to reduced GH secretion, inasmuch as small doses of rh GH elevate IGF-I concentrations by comparable increments in older and young adults (18,19).
Although free IGF-I concentrations also decrease dramatically in healthy older individuals (20,21), how concomitant deficiency of sex steroids modifies free IGF-I availability is not known (22–24). Physiological regulation of free IGF-I concentrations is important, in that the unbound peptide appears to mediate certain biological effects of this trophic hormone (25). For example, in experimental animals, free IGF-I concentrations correlate positively with somatic growth, cellular replication, glucose disposal and neuronal viability (26–29).
IGF-I associates with six major binding proteins in plasma (30). Among these, IGFBP-3 and IGFBP-1 control free IGF-I concentrations in a sustained (h) and rapid (min) fashion, respectively. Administration of E2 or Te does not alter IGFBP-3 concentrations in short-term (several wk-long) clinical experiments, whereas E2 (but not Te) elevates IGFBP-1 concentrations (16,31,32). In view of such data, we reasoned that E2 and/or aromatized Te may enhance formation of the stable dimeric IGF-I/IGFBP-1 complex in plasma (33), and thereby lower fasting free IGF-I concentrations (20,34). In addition, we postulated that constant iv infusion of rh IGF-I will unmask E2 and Te-dependent control of dynamic adaptations of free IGF-I, IGFBP-1 and binary IGF-I/IGFBP-1 concentrations. To test these hypotheses, we studied healthy aging men and women, in whom IGF-I concentrations are relatively reduced (15,16,35); administered placebo (Pl) vs E2 or Te in randomized order before each infusion; and utilized specific high-sensitivity immunofluorometric assays to quantitate total and ultrafiltrably free IGF-I, IGFBP-1 and the dimeric IGF-I/IGFBP-1 complex [Methods].
Methods
Overview
The effect of GHRH on GH secretion in these two studies was reported earlier (36,37). The present analyses and measurements of IGF-I and IGFBP-1 do not overlap.
Subjects
The project was reviewed by the National Institutes of Health and U.S. Food and Drug Administration under an investigator-initiated new drug file for the use of rh IGF-I by iv infusion. Inclusion criteria required an unremarkable medical history and physical examination, and normal outpatient screening laboratory tests of hepatic, renal, endocrine, metabolic and hematologic function. Exclusion criteria included known or suspected cardiac, cerebrovascular, peripheral arterial or venous thromboembolic disease; a history of chronic smoking; personal history of breast or endometrial cancer; elevated PSA and neoplastic or obstructive prostatic disease; concomitant or recent use of neuroactive medications; anemia; allergy to peanuts (in men due to the testosterone excipient); and, failure to provide voluntary written informed consent. There was no recent transmeridian travel (within 10 days), night-shift work, significant weight change (≥ 2 kg in 6 weeks), acute or chronic disease, psychiatric illness requiring treatment or substance abuse. Some enrollees continued to take multivitamins and ferrous sulfate, one volunteer was using triamcinolone nasal spray and another was taking stable thyroxine replacement.
Eight postmenopausal and 8 older male volunteers enrolled in and completed all four infusion sessions. Participants provided written informed consent approved by the Institutional Review Board. In women, the mean (± SEM) age was 62 ± 3 y and body mass index [BMI] 25 ± 0.8 kg/m2. Individuals were clinically postmenopausal for at least 1 year, and ovariprival status was confirmed by appropriate screening concentrations of FSH (> 50 IU/L) and LH (> 20 IU/L) and estradiol (< 20 pg/mL, < 7.4 pmol/L). Subjects discontinued any sex-hormone replacement at least 4 weeks prior to participation. In men, the mean age was 61 ± 4 y and BMI 27 ± 1.3 kg/m2. Normal gonadal status was corrobrated by unremarkable pubertal, fertility and sexual histories, physical examination and concentrations of LH (4.5 ± 0.8 IU/L), FSH (7.8 ± 2.1 IU/L) and total testosterone (439 ± 47 ng/dL or 15 ±1.6 nmol/L).
Protocol design
The design was a prospectively randomized, placebo-controlled, patient-blinded, within-subject crossover intervention. Each participant underwent a total of 4 admissions (2 during placebo and 2 during estrogen or testosterone supplementation). Estrogen was administered orally twice daily for 10 days as 1 mg micronized 17 β-estradiol [Estrace, Bristol-Myers Squibb, Princeton, NJ] (15,36). Testosterone was given as a single im injection of enanthate ester [300 mg in oil] (16). Each of 4 infusion sessions was performed on the morning of day 10 of Pl and sex-steroid administration. Interventions were separated by a minimum of 6 wk. Thus, individual study duration was 4–6 mo.
Volunteers were admitted to the General Clinical Research Center (GCRC) on the evening of day 9 of Pl or sex-hormone administration to allow overnight adaptation to the Unit. To obviate food-related confounds, subjects received a constant evening meal (turkey sandwich or vegetarian alternative) of 500 kcal containing 55% carbohydrate, 15% protein, and 30% fat at 1800 h. Participants remained fasting overnight and until 1400 h the next day. Caffeinated beverages, sleep and vigorous exercise were disallowed during the sampling session.
Infusions
At 0600 h on the morning of study, iv catheters were inserted in (contralateral) forearm veins. Blood was withdrawn at 0600 h for later assay of estradiol and testosterone concentrations, and then sampled (2 mL) every 10 min for a total of 8 h (0600–1400 h). The 10-min samples were used to create hourly serum pools for measurements of free and total IGF-I, IGFBP-1 and dimeric IGF-I/IGFBP-1. After 2 h of baseline sampling (0600–0800 h), saline (50 mL/h) or rh IGF-I (10 μg/kg/h) [Genentech, Inc., South San Francisco, CA] was infused continuously iv for 6 h during the interval 0800–1400 h. A single iv bolus of GHRH (1.0 μg/kg) [Geref, Serono, Rockland, MA) was injected at 1200 h. The latter did not affect serial IGF-I concentrations in the presence or absence of IGF-I infusion. The time points after GHRH injection were included to establish a full time course of 6 h of rh IGF-I infusion. For safety monitoring, plasma potassium and phosphorus were measured at baseline and at the end of each infusion; the electrocardiogram was recorded continuously and plasma glucose concentrations hourly.
Hormone assays
Estradiol concentrations were quantitated in a single batch (32 samples) by double-antibody RIA with a sensitivity of 2.5 pg/mL and within-assay CV of 4.8% [Diagnostic Systems Laboratories, Baxter, TX]. Total (acid-ethanol extractable) IGF-I concentrations were measured by time-resolved double-monoclonal immunofluorometric assay (20,21). Sensitivity is 0.0025 μg/L; IGF-II cross-reactivity is < 0.0002%; and, intraassay and interassay CVs are 3.2% and 8.6%, respectively. Free IGF-I concentrations were determined by the same means after centrifugal ultrafiltration of undiluted serum at 37C pH 7.4. The sensitivity is that of total IGF-I, and the precision is 5.3% (intraassay) and 9.8% (interassay) (38).
IGFBP-1 was determined by an in-house radioimmunoassay (RIA) performed according to Westwood et al. (39) with modifications as previously described (40). The RIA is based on a monoclonal IGFBP-1 antibody, which recognizes all human phosphoforms of IGFBP-1 in serum (MAB 6303 from Medix Biochemica, Kauniainen, Finland). Iodinated rhIGFBP-1 served as tracer, and unlabelled rhIGFBP-1 as standard (HyTest, Turku, Finland). The lower detection limit was estimated as ~2.5 μg/L; half-maximal displacement occurred at 25 μg/L; and the highest IGFBP-1 standard was 200 μg/L. Within and between-assay CV’s averaged 4.8 and 12%, respectively. Addition of rhIGF-I and -II (from Austral Biologicals; San Ramon, CA, USA), and rh IGFBP-2, -3, -4 and -5 (from R&D Systems, Abingdon, UK) up to 10,000 μg/L did not affect the measured concentration of IGFBP-1 to any significant degree.
The dimeric complex of IGF-I and IGFBP-1 was determined by a specific time-resolved immunofluorometric assay as previously described (33). The dimeric complex was captured by an IGFBP-1 antibody (MAB 6303) and detected by an Europium-labeled monoclonal IGF-I antibody (from DSL Inc., Webster, TX, USA), prepared as previously described (21). The assay is highly specific for the dimeric complex of IGFBP-1 and IGF-I. No signal is obtained unless both IGF-I and IGFBP-1 are present, and none of IGFBP-2, -3, -4 or IGF-II caused any cross-reaction. Within and between-assay CV’s were 4.7 and 13%, respectively.
Rate estimates
The initial rate of progress of free or total IGF-I, IGFBP-1 or dimeric IGF-I/IGFBP-1 concentrations toward equilibrium during rh IGF-I infusion was estimated as the slope of the corresponding linear regression on time (41,42).
Statistical comparisons
One-way repeated-measures ANOVA was used to compare peptide concentrations. Subsequent post hoc contrasts were made using Tukey’s honestly significantly different (HSD) criterion for multiple comparisons at an experiment-wise protected P value of 0.05 (43). Unless otherwise indicated, P values reflect the overall interventional effect, unless further specified. Slope values were compared by cohort- and intervention-specific 95% statistical confidence intervals (44). Other data are given as the mean ± SEM.
Results
Doses of E2 and Te were selected that are known to decrease and increase total IGF-I concentrations in postmenopausal women and older men, respectively (15,16). Compared with placebo (Pl), estrogen administration in women elevated concentrations of E2 from 4.4 ± 0.77 to 367 ± 28 pg/mL (to convert to pmol/L, multiply by 3.67), and testosterone supplementation in men increased Te concentrations from 439 ± 42 to 1043 ± 51 ng/dL (to convert to nmol/L, multiply by 0.0347) [P < 0.01 for both].
Figure 1 summarizes preinfusion (2-h baseline) concentrations of total (top) and free (bottom) IGF-I in the 8 interventional settings. In the placebo context, fasting total IGF-I concentrations were lower in women than men (P = 0.013). Exposure to E2 decreased total and free IGF-I (both P < 0.025), whereas Te did not alter either measurement. Comparison of free IGF-I concentrations by gender showed that women receiving either Pl or E2 supplementation maintained higher values than men given Pl, but not Te (P < 0.01).
Figure 1.
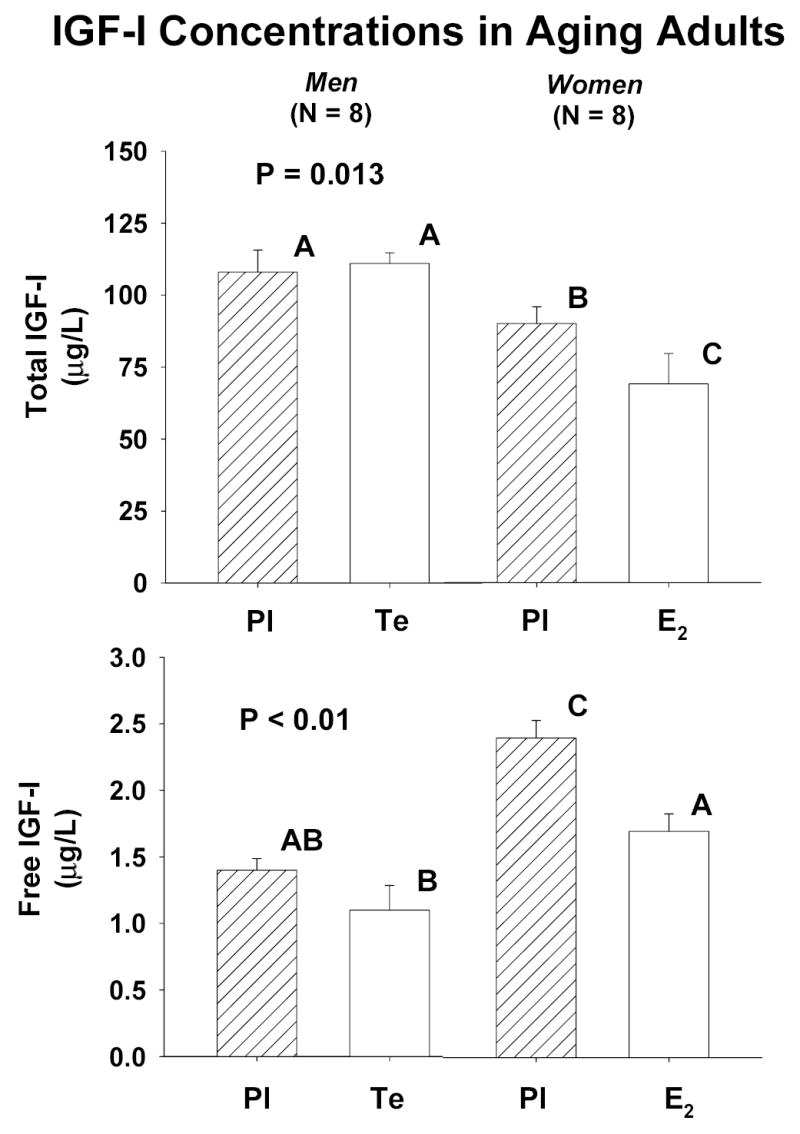
Fasting preinfusion concentrations of total (top) and free (bottom) IGF-I in older men (N = 8) and postmenopausal women (N = 8) pretreated for 10 days with placebo (Pl), estradiol (E2) or testosterone (Te). Data are the mean ± SEM. The P value reflects the overall interventional effect. Means with unshared (unique) alphabetic superscripts differ significantly (see Results).
Figure 2 presents the time course of total [Panel A] and free [Panel B] IGF-I concentrations monitored once each h for 8 h in men (top) and women (bottom). Measurements were made twice before and six times during constant iv infusion of saline vs rh IGF-I. Infusion of IGF-I elevated total and free IGF-I concentrations to consistently higher values in men than women after both Pl and sex-steroid supplementation (P = 0.014). End-infusion values are compared statistically in Table 1. Note that the final total IGF-I concentration was higher after Te than Pl administration in men.
Figure 2.
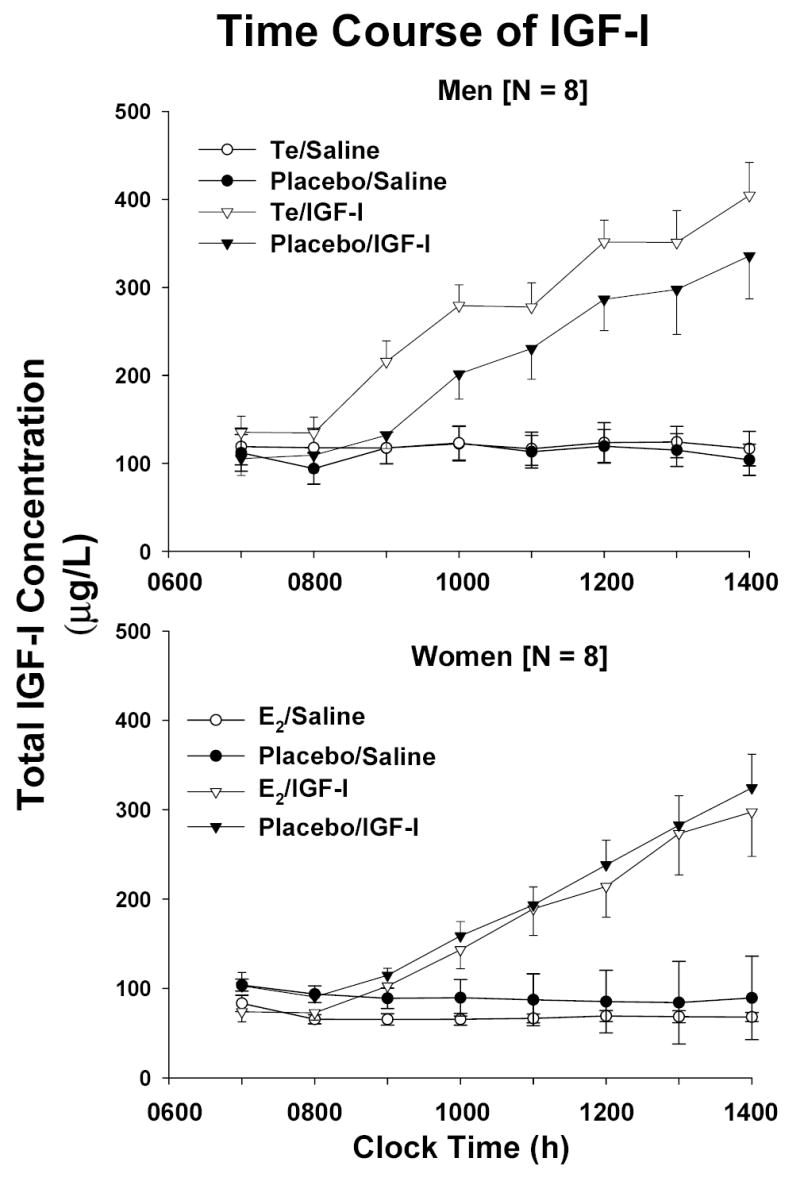
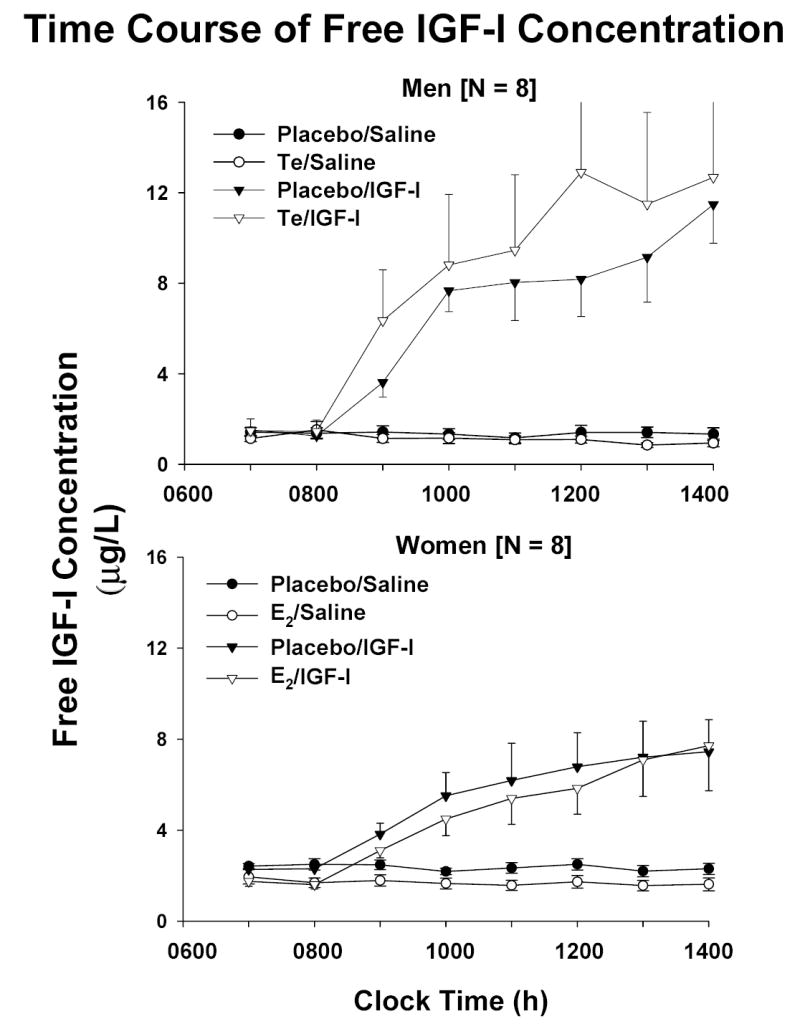
Time course of total [Panel A] and free [Panel B] IGF-I concentrations sampled every hour for 2 h before and 6 h during constant iv infusion of saline (50 mL/h) or rh IGF-I (10 μg/kg/h). Measurements were made following randomly ordered supplementation with placebo, estradiol (E2) or testosterone (Te). Data are the mean ± SEM (N = 8 men and N = 8 women).
Table 1.
Maximal Observed Concentrations of Total IGF-I, Free IGF-I, IGFBP-1 and Binary IGF-1/IGFBP-1 Driven by Constant Infusion of Rh IGF-I
| Men
|
Women
|
|||
|---|---|---|---|---|
| Measure | Pl | Te | Pl | E2 |
| Total IGF-I (μg/L) | 336 ± 50A* | 405 ± 38B | 297 ± 50A | 324 ± 38 A |
| Free IGF-I (μg/L) | 11.5 ± 1.7A** | 12.7 ± 1.8A | 7.4 ± 1.4B | 7.7 ± 2.0B |
| IGFBP-1 (μg/L) | 169 ± 26NS | 154 ± 51 | 173 ± 27 | 159 ± 36 |
| IGF-I/IGFBP-1 Complex (μg/L) | 32 ± 17A* | 58 ± 19AB | 89 ± 22B | 72 ± 24B |
Data are the mean ± SEM (N = 8 men, N = 8 women).
Superscripts that are unshared (unique) differ significantly at
P < 0.05 and
P < 0.01.
NS denotes P > 0.05. Thus, A differs from B but not from AB.
Figure 3 summarizes the initial rates (slopes) of increase of total and free IGF-I concentrations during saline and rh IGF-I infusion. Te compared with Pl administration increased the rate of rise of total IGF-I concentrations. Statistical analysis by gender revealed more rapid elevation of: (a) total IGF-I concentrations in Te-treated men than either E2 or Pl-exposed women [P < 0.05]; and (b) free IGF-I concentrations in men than women independently of steroid supplementation [P < 0.025].
Figure 3.
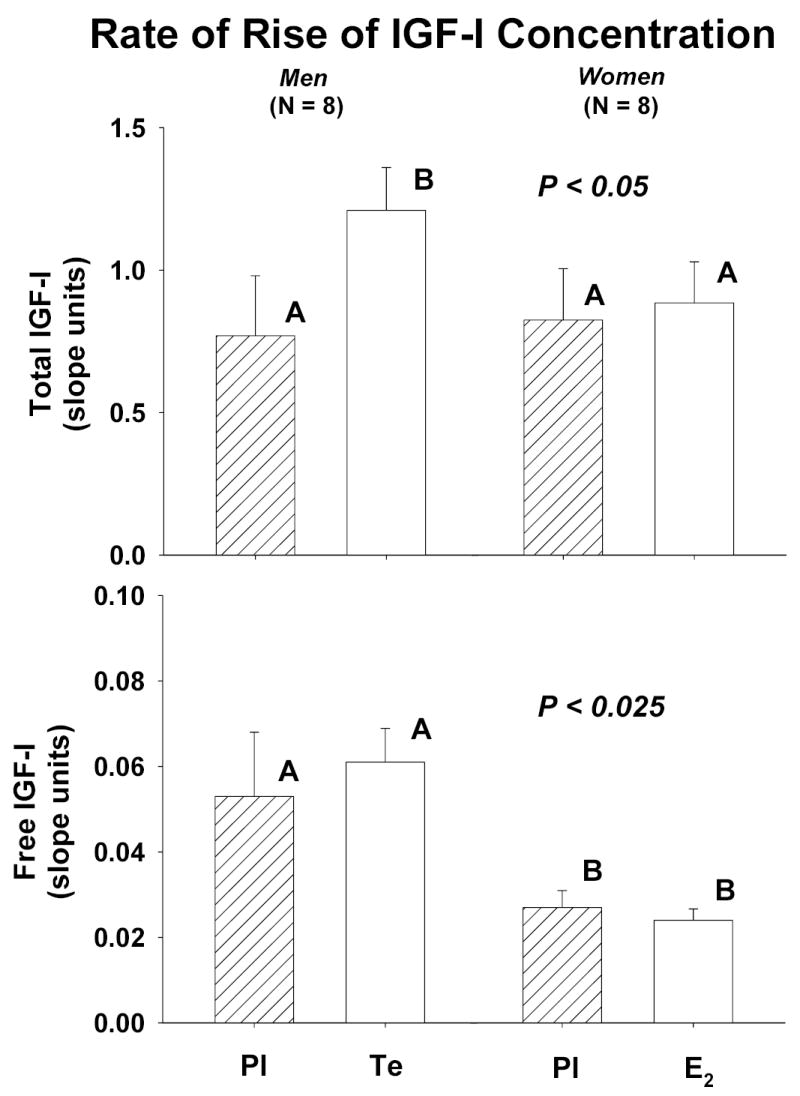
Initial rates of rise (slopes) of concentrations of total [top] and free [bottom] IGF-I concentrations over time during saline and rh IGF-I infusion in men and women pretreated with placebo (Pl), testosterone (Te) and estradiol (E2). Statistical representations are described in Figure 1.
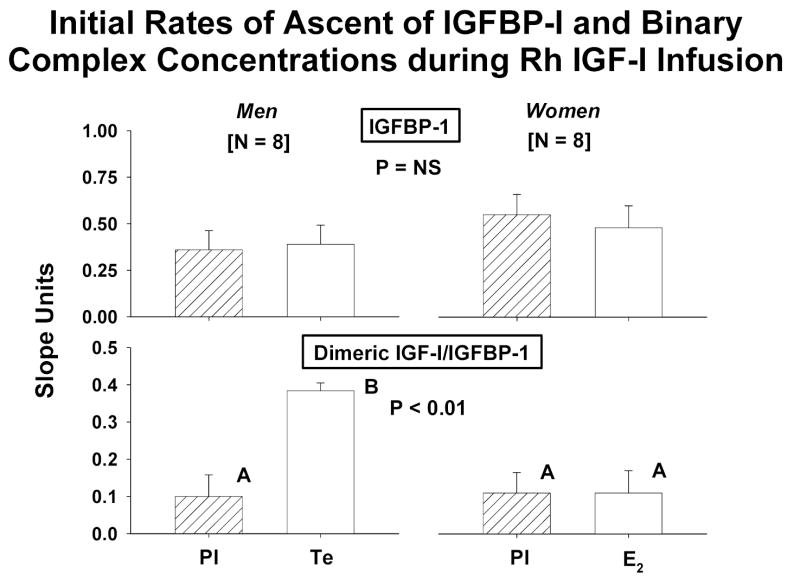
Baseline concentrations and time courses of rising IGFBP-1 and binary IGF-I/IGFBP-1 complexes were compared by gender and sex-steroid intervention. Principal similarities and distinctions included: (a) fasting concentrations of IGFBP-1 and IGF-I/IGFBP-1 were 1.4- and 1.7-fold higher in women than men given Pl [both P < 0.01]: Figure 4; (b) E2 but not Te increased fasting IGFBP-1 concentrations significantly (P = 0.0062 within-gender); (c) E2 and Te increased dimeric IGF-I/IGFBP-1 concentrations significantly [both P < 0.05]; (d) IGF-I infusion elevated IGFBP-1(Figure 5A) and dimeric IGF-I/IGFBP-1 (Figure 5B) concentrations in both sexes; (e) the rate of increase in IGFBP-1 concentrations was independent of gender and sex-steroid intervention: Figure 6; (f) Te stimulated a 3.4-fold more rapid rise of binary IGF-I/IGFBP-1 concentrations than E2 (P < 0.01); and (g) men manifested an (unexplained) delay in the rh IGF-I-stimulated increase in dimeric IGF-I/IGFBP-1, and women did the same for monomeric IGFBP-1 concentrations.
Figure 4.
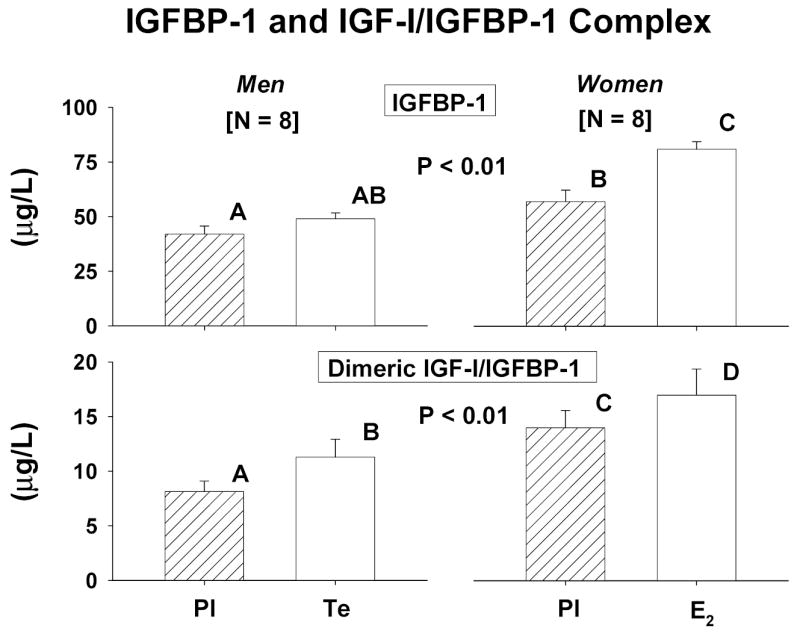
Fasting concentrations of IGFBP-1 [top] and the binary IGF-I/IGFBP-1 complex [bottom] in men and women. See Figure 1 for data format.
Figure 5.
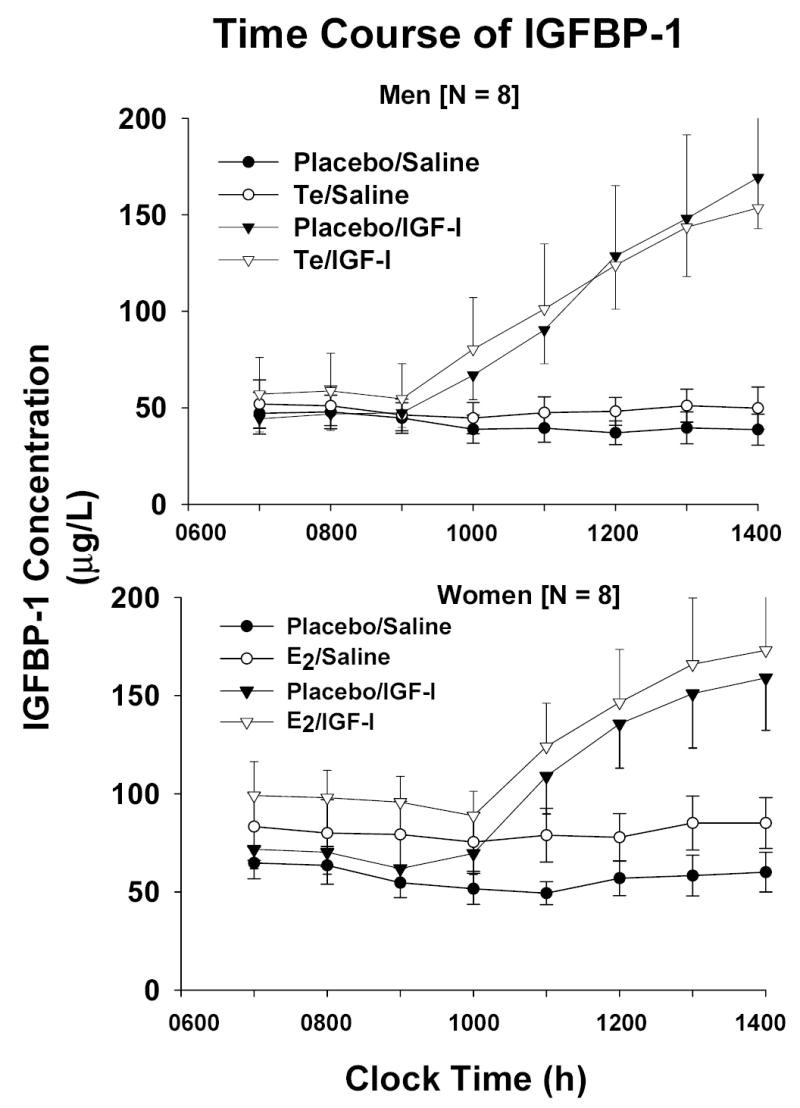
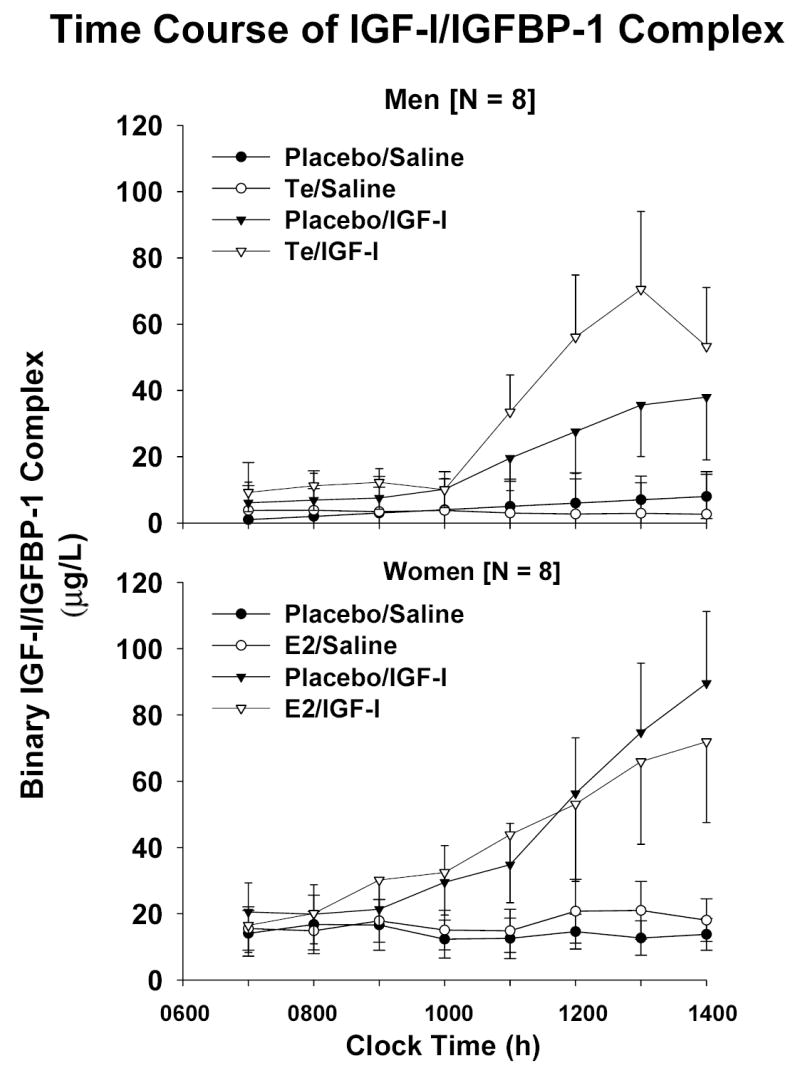
Time profiles of IGFBP-1 [Panel A] and binary IGF-I/IGFBP-1 [Panel B] concentrations during continuous iv infusion of saline vs rh IGF-I. Data are presented as described in the legend of Figure 2
Figure 6.
Initial rates of rise (slope) of concentrations of IGFBP-1 [top] and binary IGF-I/IGFBP-1 [bottom] in men and women: see legend of Figure 1.
Discussion
The present investigation disclosed that administration of E2, but not Te, lowers circulating free IGF-I availability, and that this decrease is attributable to a significant rise in both IGFBP-1 and dimeric IGF-I/IGFBP-2 in fasting healthy middle-aged and older adults. In the setting of iv infusion of rh IGF-I, supplementation with Te compared with E2 conferred higher final values of and more rapid elevations in the concentrations of free IGF-I and binary IGF-I/IGFBP-1. Based upon these collective outcomes, we postulate that gonadal steroids can modulate the synthesis and/or metabolic clearance of IGF-I and IGFBP-1 differentially. In contrast, earlier studies indicate that IGFBP-3 concentrations are not influenced by E2 or Te supplementation (16,45).
An inverse relationship between IGFBP-1 and free IGF-I concentrations emerges in fasting and renal failure (increased IGFBP-1) as well as in hyperinsulinemia, visceral obesity and after a high dose of GH (decreased IGFBP-1) (38,46,47). Such differences are important, inasmuch as low free IGF-I concentrations are associated with reduced somatic growth in puberty and, conversely, for high free IGF-I concentrations (48,49). Accordingly, we postulate that higher free IGF-I concentrations in men than women receiving exogenous gonadal steroids may contribute to greater anabolism in men. The experimental finding that transgenic overexpression of IGFBP-1 impairs GH-stimulated growth in the immature hypohysectomized animal would support this notion (27). In murine models, the trimeric complex of IGF-I, acid-labile subunit (ALS) and IGFBP-3 is also required for optimal growth (30,34,50,51).
Constant iv infusion of rh IGF-I unveiled three salient contrasts between the effects of Te and E2. In particular, men with high physiological (mid-to-late puberty) Te concentrations compared with women with high physiological (late follicular-phase) E2 concentrations given rh IGF-I evinced: (i) higher peak free IGF-I concentrations; and (ii)/(iii) more rapid increases in free IGF-I and binary IGF-I/IGFBP-1 concentrations. Plausible bases for all three observations would be a smaller distribution volume for and/or shorter half-life of free IGF-I in men (52).
Infusion of rh IGF-I for 6 h stimulated > 3-fold increases in IGFBP-1 concentrations in men and women. Earlier clinical investigations indicate that infusion of rh IGF-I inhibits beta-cell insulin secretion rapidly (53). This effect may be pertinent, in that insulin represses hepatic IGFBP-1 gene transcription and lowers serum IGFBP-1 concentrations (46,54).
Several caveats are relevant in interpreting the current outcomes. First, constant iv infusion of rh IGF-I over 6 h is a nonequilibrium intervention, described by initial rates of peptide distribution and elimination rather than by equilibrium half-lives (55). In the latter regard, earlier steady-state estimates of the half-lives of total IGF-I, free IGF-I and binary IGF-I/IGFBP-I are 8-15 h, 12–18 min, and 25 min, respectively (50,56). Whether equilibrium kinetics are altered by gender or sex hormones has not been ascertained. Second, the doses of E2 and Te used experimentally exceed physiological replacement. Actual threshold doses are not known in this type of model (48,49). Third, in one study oral but not transdermal E2 replacement in postmenopausal women increased IGFBP-1 concentrations, and norethisterone inhibited this effect (32). Thus, extrapolation of our data to other hormone-replacement regimens would not be practicable. And, fourth, the present assay configuration quantitates primarily, but not exclusively, high-affinity phosphorylated IGFBP-1 (33,40). Whether comparable outcomes apply to the less abundant nonphosphorylated form is not known.
In summary, short-term administration of E2 in postmenopausal women diminishes the fasting concentration of free IGF-I and elevates that of IGFBP-1 and binary IGF-I/IGFBP-1. In contradistinction, Te supplementation in comparably aged men does not alter the fasting concentration of free IGF-I or IGFBP-1, but increases that of total IGF-I and binary IGF-I/IGFBP-1. Constant infusion of rh IGF-I induces higher final free IGF-I concentrations and more rapid increases in free IGF-I and binary IGF-I/IGFBP-1 concentrations in a Te-predominant than E2-enriched milieu. From these data, we postulate that sex steroids modulate the bioavailability of IGF-I in healthy adults.
Acknowledgments
We thank Kris Nunez, Kimberly Coulter, Gail Bierbaum and Kandace Bradford for excellent support of manuscript preparation; Dr. Peter O’Brien for statistical consultation, the General Clinical Research Center Core Assay Laboratory for performing the immunoassays; and the nursing staff for conducting the research protocol; and Dr. Stacey Anderson, who screened, visited and infused patients in the GCRC on a fee-for-service basis. Studies were funded in part by grants MO1 RR00847 and RR00585 to the General Clinical Research Centers of the University of Virginia and Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD); R01 NIA AG14799 and AG19695 from the National Institutes of Health (Bethesda,MD); and the Hørslev Foundation, Danish Health Research Council (grant # 22020141) and Aarhus University-Novo Nordisk Center for Research in Growth and Regeneration.
References
- Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman L. Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J Clin Endocrinol Metab. 1972;35:665–670. doi: 10.1210/jcem-35-5-665. Ref ID: 119100. [DOI] [PubMed] [Google Scholar]
- Zadik Z, Chalew SA, McCarter RJ, Jr, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60:513–516. doi: 10.1210/jcem-60-3-513. Ref ID: 2610. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. Ref ID: 5617. [DOI] [PubMed] [Google Scholar]
- De Boer H, Blok G-J, van der Veen E. Clinical aspects of growth hormone deficiency in adults. Endo Rev. 1995;16:63–86. doi: 10.1210/edrv-16-1-63. Ref ID: 118672. [DOI] [PubMed] [Google Scholar]
- Blum WF, Shavrikova EP, Edwards DJ, Rosilio M, Hartman ML, Marin F, Valle D, van der Lely AJ, Attanasio AF, Strassburger CJ, Henrich G, Herschbach P. Decreased quality of life in adult patients with growth hormone deficiency compared with general populations using the new, validated, self-weighted questionnaire, questions on life satisfaction hypopituitarism module. J Clin Endocrinol Metab. 2003;88:4158–4167. doi: 10.1210/jc.2002-021792. Ref ID: 128270. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73:1081–1088. doi: 10.1210/jcem-73-5-1081. Ref ID: 168. [DOI] [PubMed] [Google Scholar]
- Frantz AG, Rabkin MT. Effects of estrogen and sex difference on secretion of human growth hormone. J Clin Endocrinol Metab. 1965;25:1470–1480. doi: 10.1210/jcem-25-11-1470. Ref ID: 3787. [DOI] [PubMed] [Google Scholar]
- Mauras N, Rogol AD, Veldhuis JD. Increased hGH production rate after low-dose estrogen therapy in prepubertal girls with Turner’s syndrome. Pediatr Res. 1990;28:626–630. doi: 10.1203/00006450-199012000-00018. Ref ID: 141. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Metzger DL, Martha PM, Jr, Mauras N, Kerrigan JR, Keenan B, Rogol AD, Pincus SM. Estrogen and testosterone, but not a non-aromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab. 1997;82:3414–3420. doi: 10.1210/jcem.82.10.4317. Ref ID: 349. [DOI] [PubMed] [Google Scholar]
- van Kesteren P, Lips P, Deville W, Popp-Snijders C, Asscheman H, Megens J, Gooren L. The effect of one-year cross-sex hormonal treatment on bone metabolism and serum insulin-like growth factor-1 in transsexuals. J Clin Endocrinol Metab. 1996;81:2227–2232. doi: 10.1210/jcem.81.6.8964856. Ref ID: 115930. [DOI] [PubMed] [Google Scholar]
- Friend KE, Hartman ML, Pezzoli SS, Clasey JL, Thorner MO. Both oral and transdermal estrogen increase growth hormone release in postmenopausal women -- a clinical research center study. J Clin Endocrinol Metab. 1996;81:2250–2256. doi: 10.1210/jcem.81.6.8964860. Ref ID: 115895. [DOI] [PubMed] [Google Scholar]
- Burman P, Johansson AG, Siegbahn A, Vessby B, Karlsson FA. Growth hormone (GH)-deficient men are more responsive to GH replacement therapy than women. J Clin Endocrinol Metab. 1997;82:550–555. doi: 10.1210/jcem.82.2.3776. Ref ID: 116290. [DOI] [PubMed] [Google Scholar]
- Janssen YJH, Helmerhorst F, Frolich M, Roelfsema F. A switch from oral (2 mg/day) to transdermal (50 μg/day) 17β-estradiol therapy increases serum insulin-like growth factor-I levels in recombinant human growth hromone (GH)-substituted women with GH deficiency. J Clin Endocrinol Metab. 2000;85:464–467. doi: 10.1210/jcem.85.1.6311. Ref ID: 119216. [DOI] [PubMed] [Google Scholar]
- Span JP, Pieters GF, Sweep CG, Hermus AR, Smals AG. Gender difference in insulin-like growth factor I response to growth hormone (GH) treatment in GH-deficient adults: role of sex hormone replacement. J Clin Endocrinol Metab. 2000;85:1121–1125. doi: 10.1210/jcem.85.3.6463. Ref ID: 123084. [DOI] [PubMed] [Google Scholar]
- Shah N, Evans WS, Veldhuis JD. Actions of estrogen on the pulsatile, nyctohemeral, and entropic modes of growth hormone secretion. Am J Physiol. 1999;276:R1351–R1358. doi: 10.1152/ajpregu.1999.276.5.R1351. Ref ID: 395. [DOI] [PubMed] [Google Scholar]
- Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. Ref ID: 486. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Shah N, Story S, Bray MJ, Andersonm SM 1999 Proposed mechanisms of sex-steroid hormone neuromodulation of the human GH-IGF-I axis. Veldhuis, J. D. and Giustina, A. Sex-steroid interactions with growth hormone. 93-121 New York, Springer-Verlag.Ref ID: 1144
- Lissett CA, Shalet SM. The insulin-like growth factor-I generation test: peripheral responsiveness to growth hormone is not decreased with ageing. Clin Endocrinol (Oxf) 2003;58:238–245. doi: 10.1046/j.1365-2265.2003.01703.x. Ref ID: 128990. [DOI] [PubMed] [Google Scholar]
- Arvat E, Ceda G, Ramunni J, Lanfranco F, Aimaretti G, Gianotti L, Broglio F, Ghigo E. The IGF-I response to very low rhGH doses is preserved in human ageing. Clin Endocrinol (Oxf) 1998;49:757–763. doi: 10.1046/j.1365-2265.1998.00613.x. Ref ID: 128989. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Skjaerbaek C, Dinesen B, Orskov H. Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS Lett. 1994;348:185–191. doi: 10.1016/0014-5793(94)00602-4. Ref ID: 119262. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Dinesen B, Orskov H. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul. 1995;5:169–176. Ref ID: 122804. [PubMed] [Google Scholar]
- Johannsson G, Bengtsson BA. Influence of gender and gonadal steroids on responsiveness to growth hormone replacement therapy in adults with growth hormone deficiency. Growth Horm IGF Res. 1998;8:69–75. doi: 10.1016/s1096-6374(98)80026-9. Ref ID: 118669. [DOI] [PubMed] [Google Scholar]
- Fisker S, Jorgensen JO, Vahl N, Orskov H, Christiansen JS. Impact of gender and androgen status on IGF-I levels in normal and GH-deficient adults. Eur J Endocrinol. 1999;141:601–608. doi: 10.1530/eje.0.1410601. Ref ID: 122203. [DOI] [PubMed] [Google Scholar]
- Ghigo E, Aimaretti G, Maccario M, Fanciulli G, Arvat E, Minuto F, Giordano G, Delitala G, Camanni F. Does-response study of GH effects on circulating IGF-I and IGFBP-3 levels in healthy young men and women. Am J Physiol. 1999;276:E1009–E1013. doi: 10.1152/ajpendo.1999.276.6.E1009. Ref ID: 119107. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. Ref ID: 120344. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Liu XJ, Lu ZX, Liu C, Behan DP, Chalmers DC, Foster AC, Vale WW, Ling N, De S. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc Natl Acad Sci USA. 1998;95:1894–1898. doi: 10.1073/pnas.95.4.1894. Ref ID: 123285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GN, McDermott MJ, Merkel E, Stroh CA, Ko SC, Squires CH, Gleason TM, Russell D. Recombinant human insulin-like growth factor (IGF)-binding protein-1 inhibits somatic growth stimulated by IGF-I and growth hormone in hypophysectomized rats. Endocrinol. 1994;135:1913–1920. doi: 10.1210/endo.135.5.7525258. Ref ID: 126805. [DOI] [PubMed] [Google Scholar]
- van Buul-Offers SC, Van Kleffens M, Koster JG, Lindenbergh-Kortleve DJ, Gresnigt MG, Drop SL, Hoogerbrugge CM, Bloemen RJ, Koedam JA, van Neck JW. Human insulin-like growth factor (IGF) binding protein-1 inhibits IGF-I-stimulated body growth but stimulates growth of the kidney in snell dwarf mice. Endocrinol. 2000;141:1493–1499. doi: 10.1210/endo.141.4.7418. Ref ID: 130514. [DOI] [PubMed] [Google Scholar]
- Lewitt MS, Denyer GS, Cooney GJ, Baxter RC. Insulin-like growth factor-binding protein-1 modulates blood glucose levels. Endocrinol. 1991;129:2254–2256. doi: 10.1210/endo-129-4-2254. Ref ID: 126827. [DOI] [PubMed] [Google Scholar]
- Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol. 2000;278:E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. Ref ID: 122802. [DOI] [PubMed] [Google Scholar]
- Evans WS, Anderson SM, Hull LT, Azimi PP, Bowers CY, Veldhuis JD. Continuous 24-hour intravenous infusion of recombinant human growth hormone (GH)-releasing hormone-(1,44)-amide augments pulsatile, entropic, and daily rhythmic GH secretion in postmenopausal women equally in the estrogen-withdrawn and estrogen-supplemented states. J Clin Endocrinol Metab. 2001;86:700–712. doi: 10.1210/jcem.86.2.7195. Ref ID: 474. [DOI] [PubMed] [Google Scholar]
- Helle SI, Omsjo IH, Hughes SC, Botta L, Huls G, Holly JM, Lonning PE. Effects of oral and transdermal oestrogen replacement therapy on plasma levels of insulin-like growth factors and IGF binding proteins 1 and 3: a cross-over study. Clin Endocrinol (Oxf) 1996;45:727–732. doi: 10.1046/j.1365-2265.1996.8610870.x. Ref ID: 126806. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Hojlund K, Rasmussen KN, Jorgensen SP, Wildner-Christensen M, Orskov H. Development and clinical evaluation of a novel immunoassay for the binary complex of IGF-I and IGF-binding protein-1 in human serum. J Clin Endocrinol Metab. 2002;87:260–266. doi: 10.1210/jcem.87.1.8147. Ref ID: 132262. [DOI] [PubMed] [Google Scholar]
- Jones JI, D’Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci USA. 1991;88:7481–7485. doi: 10.1073/pnas.88.17.7481. Ref ID: 126823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol. 2003;285:R664–R673. doi: 10.1152/ajpregu.00195.2003. Ref ID: 561. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Anderson SM, Kok P, Iranmanesh A, Frystyk J, Orskov H, Keenan DM. Estradiol supplementation modulates growth hormone (GH) secretory-burst waveform and recombinant human insulin-like growth factor-I-enforced suppression of endogenously driven GH release in postmenopausal women. J Clin Endocrinol Metab. 2004;89:1312–1318. doi: 10.1210/jc.2003-031482. Ref ID: 573. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Anderson SM, Iranmanesh A, Bowers CY 2004 Testosterone blunts feedback inhibition of GH secretion by experimentally elevated IGF-I concentrations. J Clin Endocrinol Metab (in press)Ref ID: 511 [DOI] [PubMed]
- Frystyk J, Ivarsen P, Stoving RK, Dall R, Bek T, Hagen C, Orskov H. Determination of free insulin-like growth factor-I in human serum: comparison of ultrafiltration and direct immunoradiometric assay. Growth Horm IGF Res. 2001;11:117–127. doi: 10.1054/ghir.2001.0197. Ref ID: 120919. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, Davies AJ, Young RJ, White A. The phosphorylation pattern of insulin-like growth factor-binding protein-1 in normal plasma is different from that in amniotic fluid and changes during pregnancy. J Clin Endocrinol Metab. 1994;79:1735–1741. doi: 10.1210/jcem.79.6.7527409. Ref ID: 122712. [DOI] [PubMed] [Google Scholar]
- Krassas GE, Pontikides N, Kaltsas T, Dumas A, Frystyk J, Chen JW, Flyvbjerg A. Free and total insulin-like growth factor (IGF)-I, -II, and IGF binding protein-1, -2, and -3 serum levels in patients with active thyroid eye disease. J Clin Endocrinol Metab. 2003;88:132–135. doi: 10.1210/jc.2002-021349. Ref ID: 132261. [DOI] [PubMed] [Google Scholar]
- Schaefer F, Baumann G, Faunt LM, Haffner D, Johnson ML, Mercado M, Ritz E, Mehls O, Veldhuis JD. Multifactorial control of the elimination kinetics of unbound (free) GH in the human: regulation by age, adiposity, renal function, and steady-state concentrations of GH in plasma. J Clin Endocrinol Metab. 1996;81:22–31. doi: 10.1210/jcem.81.1.8550755. Ref ID: 286. [DOI] [PubMed] [Google Scholar]
- Shah N, Aloi J, Evans WS, Veldhuis JD. Time-mode of growth hormone (GH) entry into the bloodstream and steady-state plasma GH concentrations rather than sex, estradiol, or menstrual-cycle stage primarily determine the GH elimination rate in healthy young women and men. J Clin Endocrinol Metab. 1999;84:2862–2869. doi: 10.1210/jcem.84.8.5881. Ref ID: 422. [DOI] [PubMed] [Google Scholar]
- O’Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39:787–794. Ref ID: 126643. [PubMed] [Google Scholar]
- Johnson ML, Frasier SG. Nonlinear least squares analysis. Methods Enzymol. 1985;117:301–342. Ref ID: 6030. [Google Scholar]
- Veldhuis JD, Evans WS, Bowers CY. Impact of estradiol supplementation on dual peptidyl drive of growth-hormone secretion in postmenopausal women. J Clin Endocrinol Metab. 2002;87:859–866. doi: 10.1210/jcem.87.2.8251. Ref ID: 496. [DOI] [PubMed] [Google Scholar]
- Suikkari AM, Koivisto VA, Rutanen EM, Yki-Jarvinen H, Karonen SL, Seppala M. Insulin regulates the serum levels of low molecular weight insulin-like growth factor-binding protein. J Clin Endocrinol Metab. 1988;66:266–272. doi: 10.1210/jcem-66-2-266. Ref ID: 126829. [DOI] [PubMed] [Google Scholar]
- Skjaerbaek C, Frystyk J, Moller J, Christiansen JS, Orskov H. Free and total insulin-like growth factors and insulin-like growth factor binding proteins during 14 days of growth hormone administration in healthy adults. Eur J Endocrinol. 1996;135:672–677. doi: 10.1530/eje.0.1350672. Ref ID: 124644. [DOI] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. Ref ID: 384. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY 2005 Endocrine control of body composition in infancy, childhood and puberty. Endocr Rev (in press)Ref ID: 516 [DOI] [PubMed]
- Frystyk J, Hussain M, Skjaerbaek C, Porksen N, Froesch ER, Orskov H. The pharmacokinetics of free insulin-like growth factor-I in healthy subjects. Growth Horm IGF Res. 1999;9:150–156. doi: 10.1054/ghir.1999.0100. Ref ID: 120285. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. Ref ID: 128047. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Faunt LM, Johnson ML. Analysis of nonequilibrium dynamics of bound, free, and total plasma ligand concentrations over time following nonlinear secretory inputs: evaluation of the kinetics of two or more hormones pulsed into compartments containing multiple variable-affinity binding proteins. Meth Enzymol. 1994;240:349–377. doi: 10.1016/s0076-6879(94)40055-5. Ref ID: 248. [DOI] [PubMed] [Google Scholar]
- Porksen N, Hussain MA, Bianda TL, Nyholm B, Christiansen JS, Butler PC, Veldhuis JD, Foresch ER, Schmitz O. IGF-I inhibits burst mass of pulsatile insulin secretion at supraphysiological and low IGF-I infusion rates. Am J Physiol. 1997;272:E352–E358. doi: 10.1152/ajpendo.1997.272.3.E352. Ref ID: 297. [DOI] [PubMed] [Google Scholar]
- Villafuerte BC, Goldstein S, Robertson DG, Pao CI, Murphy LJ, Phillips LS. Nutrition and somatomedin XXIX. Molecular regulation of IGFBP-1 in hepatocyte primary culture. Diabetes. 1992;41:835–842. doi: 10.2337/diab.41.7.835. Ref ID: 126822. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements in interglandular signaling. Proc Natl Acad Sci USA. 2004;101:6740–6745. doi: 10.1073/pnas.0300619101. Ref ID: 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–322. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. Ref ID: 120292. [DOI] [PubMed] [Google Scholar]