Abstract
The basic mechanisms that drive the renewal of GH pulses in the human are not understood. Recent ensemble models predict that pulse regeneration requires quenching of an ongoing GH pulse by somatostatin outflow and evocation of a new burst by rebound GHRH release. We reasoned that related principles might explain why women consistently maintain higher-amplitude GH secretory bursts than men. Accordingly, the present study tests the hypothesis that gender modulates the successive dynamics of GH feedback and escape in the morning fasting, when GH pulses are larger in women. To this end, we infused single iv pulses of recombinant human (rh) GH (0, 1, and 3 μg/kg) in eight young men and six women on separate randomly ordered mornings fasting and quantitated serial inhibition and recovery of GH secretion by frequent sampling, immunochemiluminometry, a deconvolution procedure, and regularity analysis. Statistical contrasts revealed gender-comparable peak concentrations and kinetics of rhGH. However, women differed from men by way of: (1) 3.5-and 4.0-fold less feedback suppression of GH secretory-burst mass; (2) more irregular patterns of GH release during negative feedback; and (3) 12-and 14-fold greater postnadir rebound-like GH secretion after rhGH pulses. Mechanistic analyses based on a minimal feedback construct predicted that women generate higher endogenous secretagogue stimulation per unit somatostatin outflow than men.
In summary, negative feedback induced by near-physiological GH pulses unmasks prominent gender-related contrasts in hypothalamo-pituitary autoregulation in young adults. A frugal but sufficient explanation of the ensemble outcomes is that women sustain greater hypothalamo-pituitary agonist input than men.
Abbreviations: ANCOVA, Analysis of covariance; ApEn, approximate entropy; GHRP, GH-releasing peptide; rh, recombinant human; SS, somatostatin
Gh is secreted (>85%) in prominent discrete bursts, which stimulate somatic growth and mediate certain metabolic adaptations (1–4). Laboratory investigations indicate that the generation of successive high-amplitude GH pulses requires rapid reversible negative feedback followed by rebound-like recovery of GH release (5–8). Accordingly, inactivating mutations of the GH receptor gene and administration of peptidyl antagonists of the human GH receptor disinhibit feedback and elevate pulsatile GH secretion by several-fold (3, 9). In experimental animals, autoinhibition proceeds via hypothalamic GH receptors, which stimulate somatostatin (SS) release and repress GHRH outflow to the pituitary gland (10–12). During the postinhibitory phase, intrahypothalamic SS withdrawal evokes a burst of GHRH release, which triggers GH secretion (13–17). In simplified biomathematical constructs, such cycles of autoinhibition and recovery are sufficient to confer self-renewable GH pulsatility (18–21).
Negative feedback is more prominent in the male than female rodent (3). This basic sex contrast putatively contributes to the higher amplitude, lower frequency, and lesser irregularity of GH secretory patterns as well as sex-specific gene expression in the male animal (22–24). Sexual dimorphism of the human somatotropic axis differs in certain fundamental ways (25). In particular, women secrete 2-fold more GH per burst than men (and, thus, have double the peak amplitude); maintain the same mean GH pulse frequency; and generate quantitatively more irregular GH secretory patterns (26–33). The mechanisms that mediate such gender-defined regulatory features are not known. Among other considerations (25), we postulated that men and women sustain distinct dynamics of GH pulse renewal, as transduced by sequential autofeedback and recovery. In this context, the only direct gender comparison of feedback properties used a single pharmacological dose of recombinant human (rh) GH (10 μg/kg). This paradigm monitored maximal suppression but abolished the rebound recovery phase (34). In that study, women manifested larger spontaneous GH pulses than men but comparable absolute (maximal) inhibition. In mechanistic terms, the outcome would signify that inhibitory efficacy does not differ significantly by gender. Thus, how gender impacts physiological mechanisms that mediate dynamic feedback on and recovery of self-renewing GH pulses remains unknown.
The present study adopts a nonpharmacological strategy to dissect the basis of gender-specific control of GH-pulse regeneration in young adults. Studies were performed in the morning fasting to assess the hypothesis that larger GH pulses in women at the time (25) reflect gender-related muting of negative feedback by a GH pulse. To this end, the design comprised iv infusion of saline or mid- and high-physiological pulses of rhGH to impose submaximal inhibition and evoke rebound recovery of GH secretion; intensive blood sampling to capture both suppression and rebound phases of GH secretion; ultrasensitive GH immunochemiluminometry to measure low GH concentrations accurately; and complementary analytical tools to quantitate sequential repression and escape of GH secretion. We postulated that gender would specifically determine feedback-driven inhibition of GH secretory-burst mass, rebound GH release, and regularity of GH secretion patterns. The choice of these end points reflects evidence of mechanistically distinguishable control of each (see Discussion).
Subjects and Methods
Clinical protocol
The same subjects participated in this and an earlier pharmacological feedback study (34). Volunteers provided a detailed medical history and underwent a complete physical examination, after giving written informed consent for the protocol as approved by the institutional review board. The U.S. Food and Drug Administration authorized conduct of the protocol under an investigator-initiated new drug file. Inclusion criteria were healthy young adults who undertook recreational (but not competitive) aerobic exercise three or four times per week. Eight men and six women participated. Characteristics were (men) age 26 ± 0.5 yr, height 181 ± 1.0 cm, and weight 82 ± 1.6 kg; and (women) age 22 ± 0.5 yr, height 164 ± 1.0 cm, and weight 60 ± 1.2 kg. Exclusion criteria included pregnancy or breast-feeding; age 30 yr or older; glucocorticoid, sex steroid, or other hormone use; alcohol or drug abuse; clinical depression; acute or chronic systemic illness; endocrinopathy; hematologic, pulmonary, or hepatorenal disease; diabetes mellitus; anemia (hematocrit <38%); exposure to neuro- or psychoactive medications within 10 biological half-lives; recent transmeridian travel (more than three time zones traversed within 1 wk) or shift work; weight gain or loss (exceeding 2 kg in the preceding 6 wk); and failure to provide written, witnessed informed consent.
Women were studied during the early follicular phase (d 2–8) of the menstrual cycle. Volunteers were admitted to the General Clinical Research Center on three separate occasions to receive saline and 1 or 3 μg/kg rhGH in prospectively randomized order at least 3 d apart. To obviate nutritional confounds, participants ingested a constant meal at 1800 h the evening before, which contained 500 kcal (60% carbohydrate, 20% protein, and 20% fat). Subjects then remained fasting overnight and until the end of sampling on the next day. Use of coffee, alcohol, and tobacco and vigorous exercise were disallowed during the study protocol.
Negative-feedback paradigm
To allow simultaneous sampling and infusion, forearm venous catheters were inserted contralaterally at 0600 h. Blood samples (1.5 ml) were withdrawn every 10 min for a total of 7.5 h from 0630 to 1400 h. After a 60-min baseline, rhGH (1 or 3 μg/kg) or saline was infused iv as a 6-min square-wave pulse (0730 h) by programmable infusion pump. Thereafter, blood was sampled every 2.5 min for 10 min (0730–0740 h) and every 5 min for 50 min (0740–0830 h) for kinetic analyses, followed by every 10 min for 5 h 30 min (0830–1400 h).
Assays
GH concentrations were measured in duplicate in each sample by ultrasensitive immunochemiluminescence assay (Nichols, San Juan Capistrano, CA) (35, 36). Sensitivity is 0.005 μg/liter, when defined as 3 sd above the zero-dose tube. Median intra- and interassay coefficients of variation were 5.8 and 6.7%, respectively. GH concentration-dependent intraassay variance (sd2) was modeled as a power function of sample means using all replicates from each time series (37). Concentrations of total testosterone and estradiol were quantitated by solid-phase RIA (Diagnostic Products Corp., Los Angeles, CA) (38). Comparisons were made on the mean of all four fasting 0630 h samples collected in each subject. Mean intra- and interassay coefficients of variation were, respectively, 6.9 and 8.3% (total testosterone) and 5.9 and 9.1% (estradiol) with sensitivities of 0.35 nmol/liter and 37 pmol/liter.
Deconvolution analysis
GH secretion was quantitated by deconvolution analysis, using the previously determined rapid-phase GH half-life of 3.5 min, an analytically estimated slow-phase half-life, and a fixed fractional (slow/total) decay amplitude of 0.63 (39, 40). For statistical validity, the analysis was conditioned on pulse times estimated independently by Cluster analysis (37, 41). The combined approach accounts mathematically for basal (nonpulsatile) secretion, partially overlapping GH pulses, and decay of hormone concentrations during the observation interval. The entire 7.5-h GH time series was analyzed, followed by computation of the summed mass of GH secreted in bursts (micrograms per liter): (1) beginning 1.5 h after saline vs. rhGH injection and continuing for 3 h until the nadir (thus defining the interval when GH-negative feedback is evident); and (2) beginning at the nadir and continuing for a mean of 2 h until the end of sampling (interval when initial rebound/recovery emerges) (34, 42, 43). The nadir was defined as the single lowest value of a three-point moving average of GH concentrations (hence the mean of three consecutive measurements).
The half-life of infused GH was evaluated by deconvolving the injected peaks. The distribution volume of rhGH (milliliters per kilogram) was computed as 1000-fold the quotient of the dose (micrograms per kilogram) and the deconvolution-calculated mass (micrograms per liter) of infused rhGH.
Approximate entropy
Approximate entropy (ApEn) analysis was applied to first-differenced (stationarized or epoch-detrended) postinfusion GH concentration time series (44, 45). ApEn pattern length and threshold, as validated for data series of this size, were, respectively, m = 1 and r = 0.85 (46). ApEn is a model-free statistic, which quantitates feedback-sensitive subpattern regularity. ApEn calculations are independent of absolute concentrations or deconvolution analysis (47, 48). Higher ApEn values denote more irregular (less orderly) secretory patterns, as observed in GH-secretory tumors, aging adults, puberty, and women, compared with men (26–28, 38, 45). Mathematical simulations and clinical experiments have demonstrated that deterioration of expected pattern regularity in an interlinked system denotes erosion of balanced signal coordination (44, 46, 48). In the GH axis, irregularity provides a measure of unopposed feedforward drive by GHRH or GH-releasing peptide (GHRP) (49–51) and attenuated feedback restraint by SS or GH/IGF-I (20, 48, 52).
Statistical procedures
Statistical comparisons of derived measures, GH secretory-burst mass, and ApEn were made on logarithmically transformed data to limit heterogeneity of variance. The model was two-way analysis of covariance (ANCOVA) to test the effects of gender and two doses of rhGH, compared with the response to saline, considered as a statistical covariate (53). This structure accommodates the repeated-measures design, includes expected serial correlation within a subject, and examines the individual effects and the interaction between genders (two factors) and rhGH dose (two factors). Post hoc contrasts were based on Tukey’s honestly significantly different criterion at an overall (experiment-wise) protected type I error rate of 0.05 (54). Data are presented as the mean ± sem.
Simulation of GH network
To simulate inferences made in Results and Discussion, we assumed greater GHRH potency, GHRP/ghrelin efficacy, and GH feedback-induced SS release in women than men (at nominal respective female to male ratios of 1.2, 2.2, and 2.5).
Results
Screening concentrations of testosterone were 20 ± 1.5 and 1.6 ± 0.28 nmol/liter (P < 0.001) and of estradiol 92 ± 11 and 140 ± 15 pmol/liter (P > 0.10) in men and women, respectively.
Figure 1 depicts cohort mean (± sem) GH concentration profiles in the eight men and six women sampled every 10 min for 1.0 h before and 6.5 h after iv injection of a 6-min pulse of saline or rhGH. The data illustrate higher mean GH concentrations in women (3.2 ± 0.61 μg/liter) than men (1.3 ± 0.25 μg/liter) after saline infusion (P < 0.05). Visual inspection revealed dose-varying and gender-comparable peak concentrations and kinetics of infused GH; relative failure of the 1 μg/kg dose of rhGH to suppress ongoing GH release in women; and accentuated initial rebound recovery of GH release after the 3 μg/kg rhGH dose in women, compared with men (see below).
Fig. 1.
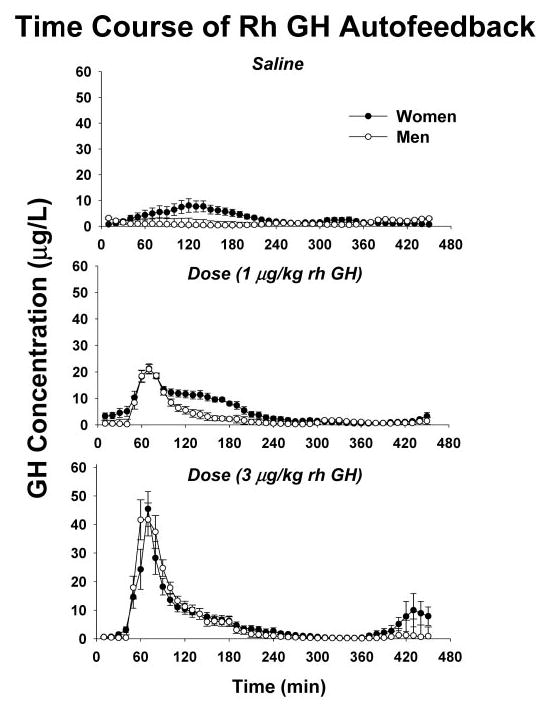
Serum GH concentrations (y-axis) sampled every 10 min for a total of 7.5 h beginning 60 min before a 6-min iv bolus injection of saline or rhGH (time, x-axis). The experimental negative-feedback signal (top to bottom) was 0 (saline), 1, or 3 μg/kg rhGH administered fasting on separate mornings in randomly assigned order. Peak GH concentrations occurred uniformly at 70 min. GH was measured by immunochemiluminometry (see Subjects and Methods). Data are the mean ± sem (n = 8 men, n = 6 women).
Table 1 summarizes peak concentrations, half-lives, and distribution volumes of rhGH in men and women at the two doses of rhGH studied. No kinetic measures differed by gender. Peak-infused GH concentrations were mid- and high physiological; viz. (pooled median) values were 20 and 46 μg/liter after injection of 1 and 3 μg/kg, respectively. These data verify that gender-related autofeedback differences (see below) are not attributable to sex-specific GH kinetics.
TABLE 1.
Estimated kinetics of rhGH in men and women
| Dose of Rh GH infused
|
||
|---|---|---|
| Kinetic parameter | 1 μg/kg | 3 μg/kg |
| Half life (min) | ||
| Men | 13.6 ± 0.8 | 14.5 ± 1.2 |
| Women | 12.4 ± 1.2 | 14.6 ± 2.9 |
| Distribution volume (ml/kg) | ||
| Men | 38 ± 5.6 | 41 ± 3.7 |
| Women | 34 ± 2.5 | 43 ± 5.4 |
| Peak GH (μg/liter) | ||
| Men | 23 ± 3.2 | 51 ± 7.6 |
| Women | 22 ± 1.4 | 47 ± 6.8 |
Gender and dose did not affect any values shown. n = 8 men; n = 6 women.
Absolute nadir (mean lowest three consecutive) GH concentrations induced by each pulse of saline or rhGH were used as a model-free estimate of negative feedback (Fig. 2). Nadir GH concentrations were higher in women than men after infusion of saline and each dose of rhGH (P = 0.014). Nadir values decreased significantly only in response to the higher feedback signal in women (3 μg/kg rhGH, P < 0.025). Time latencies to reach nadir GH concentrations after the injected GH peak at 70 min were influenced by gender and feedback dose, viz. in men and women, nadir values occurred, respectively, 274 ± 23 and 307 ± 19 min (P < 0.01) (1 μg/kg) and 319 ± 13 and 318 ± 12 min (3 μg/kg) after the peak GH concentration.
Fig. 2.
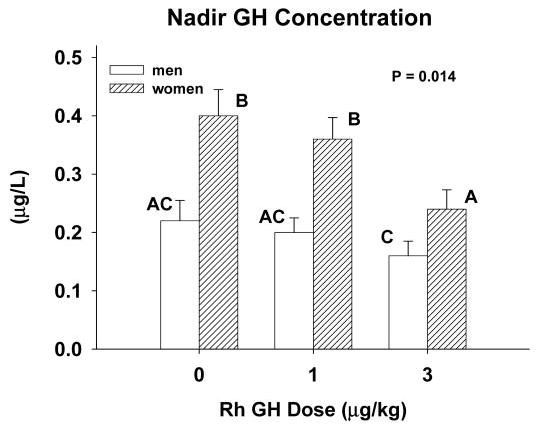
Nadir GH concentrations induced by iv injection of saline vs. 1 or 3 μg/kg rhGH in young men and women. Data are the mean ± sem (n = 8 men, n = 6 women). Means with different (unshared) alphabetic superscripts differ significantly by the post hoc Tukey test. ANCOVA was used to estimate the overall P value indicated for the gender-by-intervention interaction (see Subjects and Methods).
Figure 3 depicts the dose dependency of rhGH-induced inhibition of summed GH secretory-burst mass determined during the 3-h interval beginning 1.5 h after saline/rhGH injection in men and women. A 1.5-h delay was chosen because stimulation of GH secretion by a maximally effective dose of GHRH and a high dose of GHRP-2 is blocked within 2 h after injection of rhGH (42, 43, 55). ANCOVA predicted P < 0.001 for the dose effect of rhGH dose, P < 0.001 for the gender effect, and P = 0.0031 for the dose × gender interaction. In men, administration of rhGH suppressed GH secretory-burst mass progressively across the dose range 0, 1, and 3 μg/kg (Fig. 3). On the other hand, in women, the low dose of 1 μg/kg was not inhibitory (P = NS vs. saline injection). Post hoc gender comparisons by Tukey’s honestly significantly different test revealed 3.5- and 4-fold higher noninhibitable pulsatile GH secretion (micrograms per liter per 3 h) after the 1 and 3 μg/kg doses of rhGH in women than men (both P < 0.005).
Fig. 3.
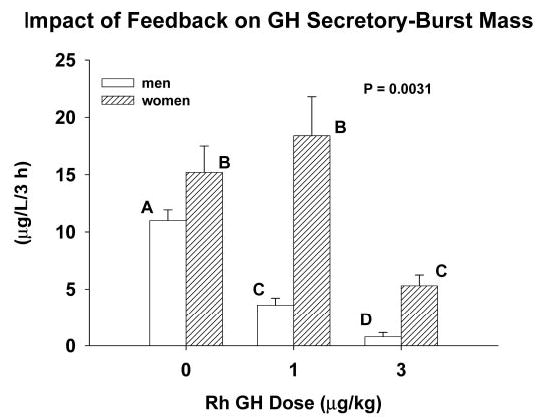
Dose-dependent inhibition of the amount (mass) of GH secreted in bursts in healthy young adults. Observations reflect the 3-h time interval beginning 1.5 h after iv injection of a pulse of saline vs. the indicated dose of rhGH. Data are presented as described in the legend of Fig. 2. The overall P value reflects the effect of rhGH dose.
Figure 4 summarizes gender differences in the delayed recovery (initial rebound) of GH release, viz. during the mean 2-h (± 0.23 h) time window beginning at the absolute nadir. ANCOVA disclosed 12- and 14-fold greater summed GH secretory-burst mass normalized per 2 h during initial postnadir recovery in women than men after infusion of 1 and 3 μg/kg rhGH, respectively (P < 0.001).
Fig. 4.

Recovery of GH secretory-burst mass over a mean 2-h time interval after the nadir GH concentration induced by bolus iv infusion of saline vs. the indicated doses of rhGH in young men and women. Data are presented as noted in the legend of Fig. 3.
ApEn was used as a validated scale-independent measure of feedback-signal strength during the 3-h interval beginning 1.5 h after the iv pulse of saline or rhGH and continuing until the nadir (see above) (44, 48). As shown in Figure 5 (top), the overall feedback effect to enhance GH regularity was significant (P < 0.001). After infusion of saline and the lower dose of rh GH, women maintained significantly higher ApEn values, signifying less feedback defined by more irregular (disorderly) patterns of GH release (P < 0.01). Infusion of 3 μg/kg rh GH enforced equivalent orderliness, consistent with gender-comparable feedback efficacy (maximal inhibition).
Fig. 5.
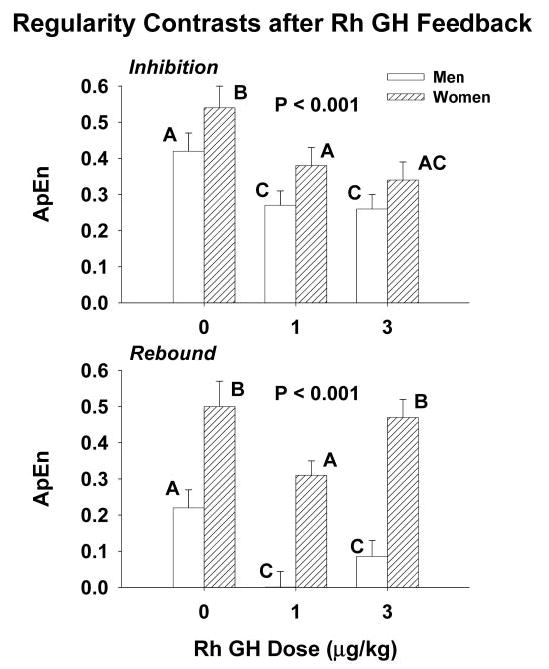
Feedback imposed by a midphysiological pulse of rhGH enhances the regularity (orderliness) of GH release to a lesser degree in women than men (top panel). Initial rebound-like recovery of GH release also is less regular in women than men (bottom panel). Higher values of ApEn (regularity statistic) denote decreased pattern reproducibility (greater relative randomness) due to greater feedforward and/or less feedback within an interlinked system, e.g. greater GHRH and/or less SS release. See legend of Fig. 3 for format of data presentation.
The orderliness of GH secretion during the initial rebound phase was assessed by applying ApEn to the 2-h GH time series after the nadir (Figure 5bottom). Initial rebound recovery of GH release after the lower dose of GH yielded more orderly patterns (lower ApEn) than after saline in both men and women, indicating persistence of SS release (P < 0.001). Women manifested significantly more irregular GH release (higher ApEn values) than men during the initial recovery phase of GH secretion after injection of saline and both doses of rhGH (P < 0.001).
Figure 6 presents model-based predictions that greater endogenous GHRH drive in women could: (1) potentiate initial rebound GH secretion after the low dose of rhGH (as observed); (2) prevent nadir suppression by the low dose of rhGH by opposing the effect of low SS outflow; and (3) overcome low GH-induced SSergic inhibition of GH secretory-burst mass.
Fig. 6.
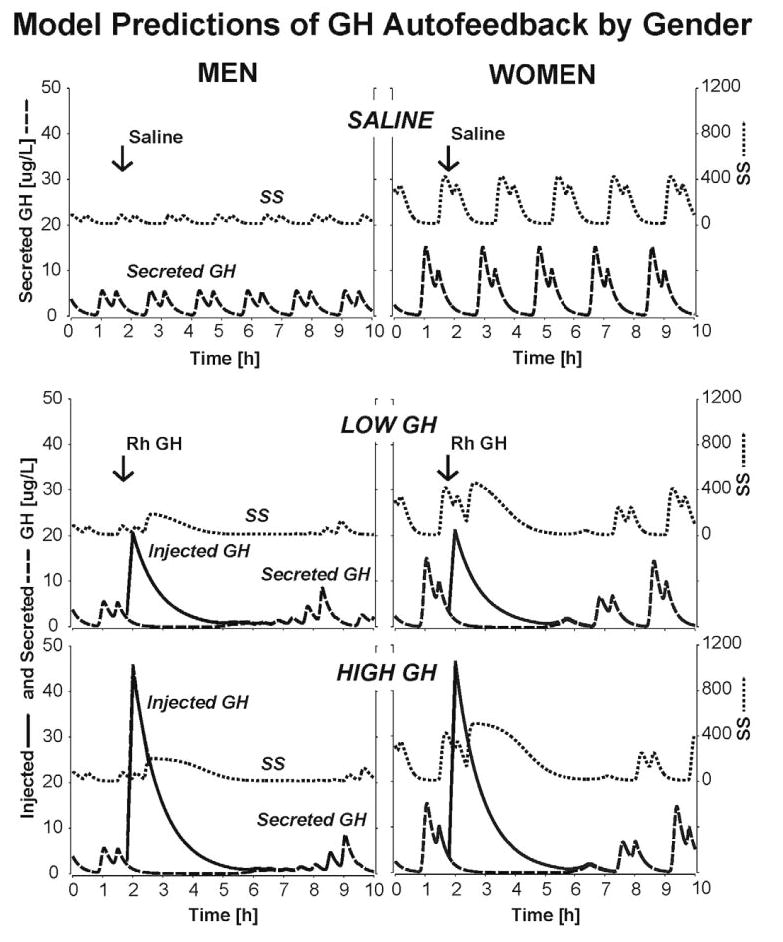
Output of a simplified three-peptide model linking GHRH feedforward and GH feedback via SS to pulsatile GH secretion via objective mathematical connections. Each curve is a computer-driven plot of SS or GH release and injected GH pulses over time. The model parameters (see Subjects and Methods) reflect the present clinical inference that women maintain greater GHRH feedforward potency, maximal GH-induced SS outflow, and GHRP/ghrelin efficacy than men. The three paired panels (top to bottom) depict predicted responses to infusion of saline vs. 1 and 3 μg/kg rhGH in men (left) and women (right). The separate curves in each panel represent injected (solid line) and secreted (broken line) GH and SS (dotted line) outflow. GH pulses (saline) in the absence of exogenous feedback occur at the same frequency but attain a higher mean amplitude in women than men. The delayed emergence of GH peaks about 5 h after each rhGH bolus reflects feedback-induced rebound-like secretion of GHRH and thereby GH as shown in Fig. 1.
Discussion
The present study reveals that gender determines both the inhibition and initial recovery phase of GH autofeedback. In particular, absolute nadir GH concentrations after a pulse of rhGH are higher, whereas the extent of suppression of GH secretory-burst mass and the induced regularity of GH release are less in young women than men. Moreover, initial rebound-like recovery of GH secretion after autoinhibition is markedly greater in women. Assuming that GHRH and SS act antagonistically, these data indicate that a physiological GH feedback signal evokes greater rebound-like release (agonist input) and/or stimulates less SS outflow (antagonistic input) in women than men. Detecting this gender distinction required the use of near-physiological rather than pharmacological feedback by exogenous GH.
Two gradations of GH autofeedback were compared with endogenous GH pulses in men and women. To this end, the low dose of rhGH (1 μg/kg) approximated a nocturnal GH pulse (peak concentration 20 μg/liter), whereas the higher dose (3 μg/kg) mimicked a high-physiological GH peak (maximum 46 μg/liter) (27, 28, 45, 56, 57). The resultant responses establish the dose dependence of GH feedback on nadir GH concentrations, GH secretory-burst mass, regularity of GH release, and initial GH recovery in both genders. To our knowledge, these are the first dose-response comparisons of feedback in men and women. Statistical comparisons disclosed that sex differences operate prominently in the physiological GH feedback range. Therefore, the present paradigm supports the relevance of endogenous GH pulses in enforcing interburst nadirs and generating rebound-like secretory bursts.
Available studies indicate that iv infusions of GH do not significantly elevate IGF-I concentrations within the brief interval studied here (5, 6). Thus, the main feedback signal tested is the rapid increase in blood GH concentrations. In experimental animals, a pulse of GH stimulates hypothalamic SS secretion in vitro and in vivo within 45 min (58). More sustained increases in GH and IGF-I concentrations induce periventricular SS and repress arcuate-nucleus GHRH gene expression (24, 59, 60). Assuming an acute role of SS release in GH autofeedback (61–64), the responses to midphysiological rhGH pulses permit indirect inferences about hypothalamic SS outflow, as assisted by an objective three-peptide model of GHRH-SS-GH interactions (20, 21, 65). The hypothesis was that sex differences observed could be accounted for by reported effects of estradiol to: (1) attenuate the inhibitory potency of available SS (52); (2) augment post-SS rebound-like release of hypothalamic GHRH and thereby pituitary GH (10, 14–16, 64, 66, 67); (3) amplify the potency of individual GHRH pulses (68); and (4) potentiate stimulation by GHRP (69). Objective modeling verified that these ensemble observations are sufficient to predict the accompanying gender differences of higher interpulse (nadir) GH concentrations, less negative feedback by a submaximal but not maximal GH pulse, and greater initial rebound-like recovery of GH release in young women than men (18–21).
Passive immunoneutralization of GHRH inhibits rebound-like GH release after SS withdrawal in the rat (14, 16, 17). In addition, bolus octreotide administration initially suppresses and then stimulates GHRH secretion into hypothalamo-pituitary portal-venous blood in the sheep (15). Assuming that an analogous mechanism operates in the human, then heightened initial rebound-like GH secretion in women would predict accentuated GHRH stimulation. This inference agrees with the capabilities of estradiol to augment rebound-like GH release after iv infusion of SS (66) and double the potency of GHRH pulses (68). Accordingly, we hypothesize that both the release and action of GHRH are greater in young women than men.
ApEn, a regularity statistic, is a scale- and model-free measure of relative feedback/feedforward strength in interlinked mathematical and biological systems (22, 26, 44–46, 48, 70). Thus, infusion of somatostatin vs. GHRH enhances vs. degrades the regularity of GH patterns by imposing feedback vs. feedforward, respectively (20, 21, 48, 49, 52, 71). ApEn analyses disclosed that GH-induced feedback increases pattern regularity in both women and men, consistent with SS release and GHRH withdrawal. During the initial rebound phase, GH release remains more irregular in women than men, which would denote higher GHRH (and possibly ghrelin) drive than SS inhibition. This gender difference is consistent with greater hypothalamic GHRH drive and/or less SS outflow during initial rebound in women than men. The rise in ApEn between the low and higher dose of GH in women also forecasts greater GHRH outflow in women. The more than 12-fold greater mass of GH secreted during initial rebound in women than men further points to heightened secretagogue action for the given degree of SSergic restraint.
In conclusion, gender is a prominent determinant of GH autofeedback in healthy young adults. The present mechanistic analyses suggest that women maintain greater feedforward by GHRH for any given degree of SS inhibition than men, thus accounting for higher amplitude GH pulses.
Acknowledgments
We thank Kris Nunez for excellent support of manuscript preparation; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo Research Unit nursing staff for conducting the protocol.
Footnotes
This work was supported in part by the General Clinical Research Center Grant MO1 RR00585 (to the Mayo Clinic and Foundation) from the National Center for Research Resources (Rockville, MD) and Grants R01 NIA AG 14799, AG 19695, and K25 HD01474 from the National Institutes of Health (Bethesda, MD).
Current address for L.W.: University of North Carolina-Greensboro, School of Health and Human Performance, Department of Exercise and Sport Science, P.O. Box 26169, Greensboro, North Carolina 27402-6169.
References
- 1.Achermann JC, Brook CG, Robinson IC, Matthews DR, Hindmarsh PC. Peak and trough growth hormone (GH) concentrations influence growth and serum insulin like growth factor-1 (IGF-1) concentrations in short children. Clin Endocrinol (Oxf) 1999;50:301–308. doi: 10.1111/j.1365-2265.1999.tb03945.x. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen JO, Moller J, George K. Marked effects of sustained low growth hormone (GH) levels on day-to-day fuel metabolism. Studies in GH-deficient patients and healthy untreated subjects. J Clin Endocrinol Metab. 1993;77:1589–1596. doi: 10.1210/jcem.77.6.8263146. [DOI] [PubMed] [Google Scholar]
- 3.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 4.Mueller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- 5.Pontiroli AE, Lanzi R, Pozza G. Inhibition of the growth hormone (GH) response to GH-releasing hormone by constant met-GH infusions. J Clin Endocrinol Metab. 1989;68:956–959. doi: 10.1210/jcem-68-5-956. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal SM, Kaplan SL, Grumbach MM. Short term continuous intravenous infusion of growth hormone (GH) inhibits GH-releasing hormone-induced GH secretion: a time-dependent effect. J Clin Endocrinol Metab. 1989;68:1101–1105. doi: 10.1210/jcem-68-6-1101. [DOI] [PubMed] [Google Scholar]
- 7.Robinson ICAF. The growth hormone secretory pattern: a response to neuroendocrine signals. Acta Paediatr Scand Suppl. 1991;372:70–78. doi: 10.1111/j.1651-2227.1991.tb17975.x. [DOI] [PubMed] [Google Scholar]
- 8.Sato M, Chihara K, Kita T, Kashio Y, Okimura Y, Kitajima N, Fujita T. Physiological role of somatostatin-mediated autofeedback regulation for growth hormone: importance of growth hormone in triggering somatostatin release during a trough period of pulsatile growth hormone release in conscious male rats. Neuroendocrinology. 1989;50:139–151. doi: 10.1159/000125213. [DOI] [PubMed] [Google Scholar]
- 9.Veldhuis JD, Bidlingmaier M, Anderson SM, Wu Z, Strassburger CJ. Lowering total plasma insulin-like growth factor I concentrations by way of a novel, potent, and selective growth hormone (GH) receptor antagonist, pegvisomant (B2036-peg), augments the amplitude of GH secretory bursts and elevates basal/nonpulsatile GH release in healthy women and men. J Clin Endocrinol Metab. 2001;86:3304–3310. doi: 10.1210/jcem.86.7.7656. [DOI] [PubMed] [Google Scholar]
- 10.Clark RG, Robinson ICAF. Growth hormone responses to multiple injections of a fragment of human growth hormone-releasing factor in conscious male and female rats. J Endocrinol. 1985;106:281–289. doi: 10.1677/joe.0.1060281. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi N, Shibasaki N, Ling N, Demura H. In vitro release of growth hormone-releasing factor (GRF) from the hypothalamus: somatostatin inhibits GRF release. Regul Pept. 1991;33:71–78. doi: 10.1016/0167-0115(91)90016-a. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrini E, Bluet-Pajot MT, Mounier F, Bennett P, Kordon C, Epelbaum J. Central administration of a growth hormone (GH) receptor mRNA antisense increases GH pulsatility and decreases hypothalamic somatostatin expression in rats. J Neurosci. 1996;16:8140–8148. doi: 10.1523/JNEUROSCI.16-24-08140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stachura ME, Tyler JM, Farmer PK. Combined effects of human growth hormone (GH)-releasing factor-44 (GRF) and somatostatin (SRIF) on post-SRIF rebound release of GH and prolactin: a model for GRF-SRIF modulation of secretion. Endocrinology. 1988;123:1476–1482. doi: 10.1210/endo-123-3-1476. [DOI] [PubMed] [Google Scholar]
- 14.Clark RG, Carlsson LMS, Rafferty B, Robinson ICAF. The rebound release of growth hormone (GH) following somatostatin infusion in rats involves hypothalamic GH-releasing factor release. J Endocrinol. 1988;119:397–404. doi: 10.1677/joe.0.1190397. [DOI] [PubMed] [Google Scholar]
- 15.Magnan E, Cataldi M, Guillaume V, Conte-Devolx B, Graziani N, Figaroli JC, Thomas F, Chihara K, Oliver C. Acute changes in growth hormone-releasing hormone secretion after injection of BIM 23014, a long acting somatostatin analog, in rams. Life Sci. 1992;51:831–838. doi: 10.1016/0024-3205(92)90610-2. [DOI] [PubMed] [Google Scholar]
- 16.Miki N, Ono M, Shizume K. Withdrawal of endogenous somatostatin induces secretion of growth hormone-releasing factor in rats. J Endocrinol. 1988;117:245–252. doi: 10.1677/joe.0.1170245. [DOI] [PubMed] [Google Scholar]
- 17.Sugihara H, Minami S, Wakabayashi I. Post-somatostatin rebound secretion of growth hormone is dependent on growth hormone-releasing factor in unrestrained female rats. J Endocrinol. 1989;122:583–591. doi: 10.1677/joe.0.1220583. [DOI] [PubMed] [Google Scholar]
- 18.Farhy LS, Straume M, Johnson ML, Kovatchev BP, Veldhuis JD. A construct of interactive feedback control of the GH axis in the male. Am J Physiol. 2001;281:R38–R51. doi: 10.1152/ajpregu.2001.281.1.R38. [DOI] [PubMed] [Google Scholar]
- 19.Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD. Unequal autonegative feedback by GH models the sexual dimorphism in GH secretory dynamics. Am J Physiol. 2002;282:R753–R764. doi: 10.1152/ajpregu.00407.2001. [DOI] [PubMed] [Google Scholar]
- 20.Farhy LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1240–R1249. doi: 10.1152/ajpregu.00086.2003. [DOI] [PubMed] [Google Scholar]
- 21.Farhy LS, Veldhuis JD. Putative GH pulse renewal: periventricular somatostatinergic control of an arcuate-nuclear somatostatin and GH-releasing hormone oscillator. Am J Physiol. 2004;286:R1030–R1042. doi: 10.1152/ajpregu.00473.2003. [DOI] [PubMed] [Google Scholar]
- 22.Gevers E, Pincus SM, Robinson ICAF, Veldhuis JD. Differential orderliness of the GH release process in castrate male and female rats. Am J Physiol. 1998;274:R437–R444. doi: 10.1152/ajpregu.1998.274.2.R437. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson LMS, Clark RG, Robinson ICAF. Sex difference in growth hormone feedback in the rat. J Endocrinol. 1990;126:27–35. doi: 10.1677/joe.0.1260027. [DOI] [PubMed] [Google Scholar]
- 24.Maiter DM, Gabriel SM, Koenig JI, Russell WE, Martin JB. Sexual differentiation of growth hormone feedback effects on hypothalamic growth hormone-releasing hormone and somatostatin. Neuroendocrinology. 1990;51:174–180. doi: 10.1159/000125334. [DOI] [PubMed] [Google Scholar]
- 25.Veldhuis JD, Bowers CY. Three-peptide control of pulsatile and entropic feedback-sensitive modes of growth hormone secretion: modulation by estrogen and aromatizable androgen. J Pediatr Endocrinol Metab. 2003;16(Suppl 3):587–605. [PubMed] [Google Scholar]
- 26.Pincus SM, Gevers E, Robinson ICAF, van den Berg G, Roelfsema F, Hartman ML, Veldhuis JD. Females secrete growth hormone with more process irregularity than males in both human and rat. Am J Physiol. 1996;270:E107–E115. doi: 10.1152/ajpendo.1996.270.1.E107. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg G, Veldhuis JD, Frolich M, Roelfsema F. An amplitude-specific divergence in the pulsatile mode of GH secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab. 1996;81:2460–2466. doi: 10.1210/jcem.81.7.8675561. [DOI] [PubMed] [Google Scholar]
- 28.Veldhuis JD, Roemmich JN, Rogol AD. Gender and sexual maturation-dependent contrasts in the neuroregulation of growth hormone secretion in prepubertal and late adolescent males and females—a general clinical research center-based study. J Clin Endocrinol Metab. 2000;85:2385–2394. doi: 10.1210/jcem.85.7.6697. [DOI] [PubMed] [Google Scholar]
- 29.Winer LM, Shaw MA, Baumann G. Basal plasma growth hormone levels in man: new evidence for rhythmicity of growth hormone secretion. J Clin Endocrinol Metab. 1990;70:1678–1686. doi: 10.1210/jcem-70-6-1678. [DOI] [PubMed] [Google Scholar]
- 30.Engstrom BE, Karlsson FA, Wide L. Marked gender differences in ambulatory morning growth hormone values in young adults. Clin Chem. 1998;44:1289–1295. [PubMed] [Google Scholar]
- 31.Ho KKY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64:51–58. doi: 10.1210/jcem-64-1-51. [DOI] [PubMed] [Google Scholar]
- 32.Asplin CM, Faria AC, Carlsen EC, Vaccaro VA, Barr RE, Iranmanesh A, Lee MM, Veldhuis JD, Evans WS. Alterations in the pulsatile mode of growth hormone release in men and women with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1989;69:239–245. doi: 10.1210/jcem-69-2-239. [DOI] [PubMed] [Google Scholar]
- 33.Hindmarsh PC, Dennison E, Pincus SM, Cooper C, Fall CH, Matthews DR, Pringle PJ, Brook CG. A sexually dimorphic pattern of growth hormone secretion in the elderly. J Clin Endocrinol Metab. 1999;84:2679–2685. doi: 10.1210/jcem.84.8.5915. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Patrie J, Wideman L, Patterson M, Weltman JY, Weltman A. Contrasting negative-feedback control of endogenously driven and exercise-stimulated pulsatile growth hormone secretion in women and men. J Clin Endocrinol Metab. 2004;89:840–846. doi: 10.1210/jc.2003-031081. [DOI] [PubMed] [Google Scholar]
- 35.Iranmanesh A, Grisso B, Veldhuis JD. Low basal and persistent pulsatile growth hormone secretion are revealed in normal and hyposomatotropic men studied with a new ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1994;78:526–535. doi: 10.1210/jcem.78.3.8126122. [DOI] [PubMed] [Google Scholar]
- 36.Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Mulligan T, Johnson ML, Pincus SM, Straume M, Iranmanesh A. Differential impact of age, sex-steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1995;80:3209–3222. doi: 10.1210/jcem.80.11.7593428. [DOI] [PubMed] [Google Scholar]
- 37.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 38.Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- 39.Faria ACS, Veldhuis JD, Thorner MO, Vance ML. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab. 1989;68:535–541. doi: 10.1210/jcem-68-3-535. [DOI] [PubMed] [Google Scholar]
- 40.Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci USA. 1987;84:7686–7690. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veldhuis JD, Johnson ML 1995 Specific methodological approaches to selected contemporary issues in deconvolution analysis of pulsatile neuroendocrine data. In: Johnson M, Veldhuis J, Conn PM, eds. Quantitative Neuroendocrinology. Vol 28. New York: Academic Press; 25–92
- 42.Richmond E, Rogol AD, Basdemir D, Veldhuis OL, Clarke W, Bowers CY, Veldhuis JD. Accelerated escape from GH autonegative feedback in midpuberty in males: evidence for time-delimited GH-induced somatostatinergic outflow in adolescent boys. J Clin Endocrinol Metab. 2002;87:3837–3844. doi: 10.1210/jcem.87.8.8770. [DOI] [PubMed] [Google Scholar]
- 43.Anderson SM, Wideman L, Patrie JT, Weltman A, Bowers CY, Veldhuis JD. Estradiol supplementation selectively relieves GH’s autonegative feedback on GH-releasing peptide-2-stimulated GH secretion. J Clin Endocrinol Metab. 2001;86:5904–5911. doi: 10.1210/jcem.86.12.8076. [DOI] [PubMed] [Google Scholar]
- 44.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartman ML, Pincus SM, Johnson ML, Matthews DH, Faunt LM, Vance ML, Thorner MO, Veldhuis JD. Enhanced basal and disorderly growth hormone secretion distinguish acromegalic from normal pulsatile growth hormone release. J Clin Invest. 1994;94:1277–1288. doi: 10.1172/JCI117446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pincus SM, Hartman ML, Roelfsema F, Thorner MO, Veldhuis JD. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol. 1999;277:E948–E957. doi: 10.1152/ajpendo.1999.277.5.E948. [DOI] [PubMed] [Google Scholar]
- 47.Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus S. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol. 2001;281:R1975–R1985. doi: 10.1152/ajpregu.2001.281.6.R1975. [DOI] [PubMed] [Google Scholar]
- 48.Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe CA, Barkan A, Johnson ML, Pincus SM. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol. 2001;280:R721–R729. doi: 10.1152/ajpregu.2001.280.3.R721. [DOI] [PubMed] [Google Scholar]
- 49.Iranmanesh A, South S, Liem AY, Clemmons D, Thorner MO, Weltman A, Veldhuis JD. Unequal impact of age, percentage body fat, and serum testosterone concentrations on the somatotrophic, IGF-I, and IGF-binding protein responses to a three-day intravenous growth hormone-releasing hormone pulsatile infusion in men. Eur J Endocrinol. 1998;139:59–71. doi: 10.1530/eje.0.1390059. [DOI] [PubMed] [Google Scholar]
- 50.Shah N, Evans WS, Bowers CY, Veldhuis JD. Tripartite neuroendocrine activation of the human growth-hormone (GH) axis in women by continuous 24-hour GH-releasing peptide (GHRP-2) infusion: pulsatile, entropic, and nyctohemeral mechanisms. J Clin Endocrinol Metab. 1999;84:2140–2150. doi: 10.1210/jcem.84.6.5687. [DOI] [PubMed] [Google Scholar]
- 51.Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J Clin Endocrinol Metab. 2004;89:2290–2300. doi: 10.1210/jc.2003-031799. [DOI] [PubMed] [Google Scholar]
- 52.Bray MJ, Vick TM, Shah N, Anderson SM, Rice LW, Iranmanesh A, Evans WS, Veldhuis JD. Short-term estradiol replacement in postmenopausal women selectively mutes somatostatin’s dose-dependent inhibition of fasting growth hormone secretion. J Clin Endocrinol Metab. 2001;86:3143–3149. doi: 10.1210/jcem.86.7.7647. [DOI] [PubMed] [Google Scholar]
- 53.Diggle PJ, Liang KY, Zeger SL 1994 Analysis of longitudinal data. New York: Oxford University Press
- 54.Kuehl RO, ed. 1994 Split-plot designs. In: Statistical principles of research design and analysis. Belmont, CA: Duxbury Press; 473–498
- 55.Veldhuis JD, Evans WS, Iranmanesh A, Weltman AL, Bowers CY. Short-term testosterone supplementation relieves growth hormone autonegative feedback in men. J Clin Endocrinol Metab. 2004;89:1285–1290. doi: 10.1210/jc.2003-031017. [DOI] [PubMed] [Google Scholar]
- 56.Hartman ML, Faria AC, Vance ML, Johnson ML, Thorner MO, Veldhuis JD. Temporal structure of in vivo growth hormone secretory events in man. Am J Physiol. 1991;260:E101–E110. doi: 10.1152/ajpendo.1991.260.1.E101. [DOI] [PubMed] [Google Scholar]
- 57.Martha Jr PM, Goorman KM, Blizzard RM, Rogol AD, Veldhuis JD. Endogenous growth hormone secretion and clearance rates in normal boys as determined by deconvolution analysis: relationship to age, pubertal status and body mass. J Clin Endocrinol Metab. 1992;74:336–344. doi: 10.1210/jcem.74.2.1730812. [DOI] [PubMed] [Google Scholar]
- 58.Sheppard MC, Kronheim S, Pimstone BL. Stimulation by growth hormone of somatostatin release from the rat hypothalamus in vitro. Clin Endocrinol (Oxf) 1978;9:583–586. doi: 10.1111/j.1365-2265.1978.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 59.Conway S, McCann SM, Krulich L. On the mechanism of growth hormone autofeedback regulation: possible role of somatostatin and growth hormone-releasing factor. Endocrinol. 1985;117:2284–2292. doi: 10.1210/endo-117-6-2284. [DOI] [PubMed] [Google Scholar]
- 60.Bertherat J, Timsit J, Bluet-Pajot M-T, Mercadier J-J, Gourdji D, Kordon C, Epelbaum J. Chronic growth hormone (GH) hypersecretion induces reciprocal and reversible changes in mRNA levels from hypothalamic GH-releasing hormone and somatostatin neurons in the rat. J Clin Invest. 1993;91:1783–1791. doi: 10.1172/JCI116389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alba-Roth J, Muller OA, Schopohl J, Von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab. 1988;67:1186–1189. doi: 10.1210/jcem-67-6-1186. [DOI] [PubMed] [Google Scholar]
- 62.Ghigo E, Arvat E, Valente F. Arginine reinstates the somatotrope responsiveness to intermittent growth hormone-releasing hormone administration in normal adults. Neuroendocrinology. 1991;54:291–294. doi: 10.1159/000125890. [DOI] [PubMed] [Google Scholar]
- 63.Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumbach WR, Carrick TA, Pausch MH, Bingham B, Carmignac D, Robinson ICAF, Houghten R, Eppler CM, Price LA, Zysk JR. A linear hexapeptide somatostatin antagonist blocks somatostatin activity in vitro and influences growth hormone release in rats. Mol Pharmacol. 1998;54:864–873. doi: 10.1124/mol.54.5.864. [DOI] [PubMed] [Google Scholar]
- 65.McCartney CR, Bellows AB, Gingrich MB, Hu Y, Evans WS, Marshall JC, Veldhuis JD. Exaggerated 17-hydroxyprogesterone response to intravenous infusions of recombinant human LH in women with polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2004;286:E902–E908. doi: 10.1152/ajpendo.00415.2003. [DOI] [PubMed] [Google Scholar]
- 66.Veldhuis JD, Anderson SM, Patrie JT, Bowers CY. Estradiol supplementation in postmenopausal women doubles rebound-like release of growth hormone (GH) triggered by sequential infusion and withdrawal of somatostatin: evidence that estrogen facilitates endogenous GH-releasing hormone drive. J Clin Endocrinol Metab. 2004;89:121–127. doi: 10.1210/jc.2003-031291. [DOI] [PubMed] [Google Scholar]
- 67.Rosner W, Smith RN. Isolation and characterization of the testosterone-estradiol-binding globulin from human plasma: use of a novel affinity column. Biochemistry. 1975;14:813. doi: 10.1021/bi00693a006. [DOI] [PubMed] [Google Scholar]
- 68.Veldhuis JD, Evans WS, Bowers CY. Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab. 2003;88:5484–5489. doi: 10.1210/jc.2003-030410. [DOI] [PubMed] [Google Scholar]
- 69.Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86:551–560. doi: 10.1210/jcem.86.2.7240. [DOI] [PubMed] [Google Scholar]
- 70.Pincus SM. Irregularity and asynchrony in biologic network signals. Methods Enzymol. 2000;321:149–182. doi: 10.1016/s0076-6879(00)21192-0. [DOI] [PubMed] [Google Scholar]
- 71.Evans WS, Anderson SM, Hull LT, Azimi PP, Bowers CY, Veldhuis JD. Continuous 24-hour intravenous infusion of recombinant human growth hormone (GH)-releasing hormone-(1,44)-amide augments pulsatile, entropic, and daily rhythmic GH secretion in postmenopausal women equally in the estrogen-withdrawn and estrogen-supplemented states. J Clin Endocrinol Metab. 2001;86:700–712. doi: 10.1210/jcem.86.2.7195. [DOI] [PubMed] [Google Scholar]


