Abstract
The present study examines the thesis that pulsatile GH secretion is controlled simultaneously by three principal signals; viz., GHRH, GH-releasing peptide (GHRP, ghrelin), and somatostatin (SS). According to this ensemble notion, no single regulatory peptide acts alone or can be interpreted in isolation. Therefore, to investigate gender-specific control of pulsatile GH secretion, we designed dual-effector stimulation paradigms in eight young men and six women as follows: 1) l-arginine/GHRH (to clamp low SS and high GHRH input); 2) l-arginine/GHRP-2 (to clamp low SS and high GHRP drive); 3) GHRH/GHRP-2 (to clamp high GHRH and high GHRP feed-forward); vs. 4) saline (unclamped). Statistical comparisons revealed that: 1) fasting pulsatile GH secretion was 7.6-fold higher in women than men (P < 0.001); 2) l-arginine/GHRH and l-arginine/GHRP-2 evoked, respectively, 4.6- and 2.2-fold greater burst-like GH release in women than men (P < 0.001 and P = 0.015); and 3) GHRH/GHRP-2 elicited comparable GH secretion by gender. In the combined cohorts, estradiol concentrations positively predicted responses to l-arginine/ GHRP-2 (r2 = 0.49, P = 0.005), whereas testosterone negatively predicted those to l-arginine/GHRH (r2 = 0.56, P = 0.002). Based upon a simplified biomathematical model of three-peptide control, the current outcomes suggest that women maintain greater GHRH potency, GHRP efficacy, and opposing SS outflow than men. This inference upholds recent clinical precedence and yields valid predictions of sex differences in self-renewable GH pulsatility.
Abbreviations: ANCOVA, Analysis of covariance; CV, coefficients of variation; E2, estradiol; GHRP, GH-releasing peptide; IGFBP, IGF binding protein; SS, somatostatin; Te, testosterone
Recent analyses of mechanisms that regulate the somatotropic, gonadotropic, and corticotropic axes indicate that multisignal interactions mediate developmental, adaptive, and gender-specific responses (1–5). This network-like perspective predicts that no individual component of an interlinked neuroendocrine system operates alone or may be viewed in isolation. In the case of GH secretion, rare human gene mutations and transgenic murine models establish that GHRH, GH-releasing peptide (GHRP, ghrelin), and somatostatin (SS) each contributes a key regulatory input (6–10). A major practical implication of this integrative concept is that the amount of GH secreted after the injection of any one of GHRH, GHRP, or SS would be determined by the aggregate effects of the injected peptide and the other two (unobserved) signals (3, 11–14). This concept predicts variable individual responses to GHRH, GHRP/ghrelin, or l-arginine (a putative inhibitor of hypothalamic SS release), as observed in clinical practice (15–17).
Gender and sex-steroid hormones direct pulsatile GH secretion and govern tissue-specific responses to this trophic hormone (1, 18–22). For example, although GH half-lives and pulse frequencies are comparable, women exhibit 2-fold higher amplitude and mass of GH secretory bursts than men (23–27). The hypothalamo-pituitary mechanisms that mediate this sex difference have not been elucidated. Regulation of the episodic mode of GH release is important, because pulsatile secretion impacts somatic growth, lipolysis, IGF-I synthesis, glucose homeostasis, and protein turnover (1, 18).
To investigate the basis for gender contrasts in pulsatile GH secretion in healthy young adults, we designed complementary combined secretagogue infusions. The objective of simultaneous stimuli was to clamp any two of three regulatory pathways, thereby leaving only one endogenous signal unmanipulated. In the case of assessing all three (GHRH, GHRP, and SS), this requires evaluating each of three pairs of effectors. Given the complexity of this schema, we compared clinical outcomes with forecasts of a minimal ensemble formulation of three-peptide control of GH secretion (3, 11, 28, 29).
Subjects and Methods
Subjects
Healthy young men (n = 8) and women (n = 6) each completed four study sessions. Mean body mass index values did not differ by gender, and the absolute range was 23–28 (men) and 21–26 (women) kg/m2. Participants provided written informed consent approved by the Mayo Institutional Review Board. The protocol was reviewed by the U.S. Food and Drug Administration under an investigator-initiated new drug number. Exclusion criteria were pregnancy, recent transmeridian travel (within 2 wk) or night-shift work; significant weight change (≥2 kg in 1 month); body mass index ≥ 30 kg/m2; acute or chronic systemic, inflammatory, or organic illness; use of anabolic steroids or glucocorticoids, antihypertensive or neuroactive medications; known or suspected hypothalamic-pituitary, adrenal, gonadal, and thyroid disease or diabetes mellitus; psychiatric treatment or substance abuse; and unwillingness or incompetence to provide voluntary, written, witnessed, informed consent. Volunteers exercised recreationally, but not competitively. None participated in another study concurrently. There were no dropouts after enrollment. Each subject had an unremarkable medical history and physical examination and normal screening laboratory tests of hepatic, renal, endocrine, metabolic, and hematologic function.
Mean (± sem) ages were 22 ± 0.9 and 23 ± 0.8 yr in men and women, respectively. Corresponding body mass indices were 27 ± 1.1 and 23 ± 1.4 kg/m2, respectively. Premenopausal women described a normal menarchal and menstrual history. Men reported normal pubertal onset, sexual development, and sexual function. Women were studied in the early-to-mid follicular phase (within 12 d of onset of menses), after a negative blood pregnancy test. Oral contraceptives, if used, were stopped at least 1 month before study.
Sampling paradigm
The study was a parallel-cohort, double-blind, placebo-controlled design comparing responses in men and women. Saline replaced peptide at each time, whether infused by bolus or continuously. The order of infusions was prospectively randomized and scheduled at least 2 d apart. Volunteers were admitted to the General Clinical Research Center on the morning of study. To obviate food-related confounds, subjects were given a constant meal (turkey sandwich or vegetarian alternative) of 8 kcal/kg containing 50% carbohydrate, 20% protein, and 30% fat the night before study. Participants then remained fasting, alcohol-abstinent, and caffeine-free overnight until 1300 h the next day. On the day of simultaneous sampling and infusion, iv catheters were inserted in contralateral forearm veins at 0700 h. Blood was withdrawn for later assay of serum estradiol (E2), testosterone (Te), IGF binding protein (IGFBP)-1, IGFBP-3, and IGF-I concentrations. Beginning at 0800 h, plasma samples (1.5 ml) were collected every 10 min for 5 h in chilled plastic tubes containing EGTA. Plasma was separated on ice and frozen at −70 C within 30 min of collection. Lunch was provided before discharge from the unit.
Infusions
Infusion studies were performed on separate mornings, after fasting, at least 2 d apart. As schematized in Fig. 1, the four protocols comprised iv delivery of: 1) combined GHRH and GHRP-2 at a constant rate of 1 μg/kg·h each for 3 h (1000 and 1300 h); 2) l-arginine 30 g over 30 min (0930–1000 h) followed immediately by bolus GHRH (1 μg/kg, GRF; Serono, Norwalk, MA); 3) l-arginine followed by bolus GHRP-2 (3 μg/kg); and 4) saline (0800–1300 h). The indicated doses of l-arginine and peptides are maximally stimulatory (30–32).
Fig. 1.
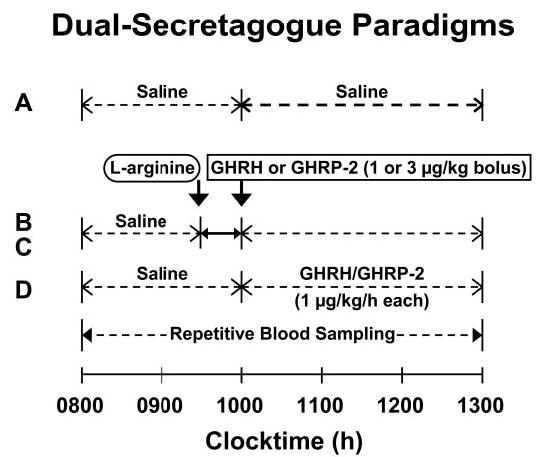
Experimental paradigm comprising separate-day, randomly ordered, fasting iv infusions of saline (A), l-arginine/GHRH (in tandem) (B), l-arginine/GHRP-2 (in tandem) (C), and constant combined GHRH/GHRP-2 (D) in healthy young adults. A maximally effective dose of each secretagogue was used to “clamp” correspondingly paired receptor-effector pathways (Subjects and Methods).
Hormone assays
Plasma GH concentrations were measured in duplicate by automated ultrasensitive double-monoclonal immunoenzymatic, magnetic particle-capture chemiluminescence assay using 22-kDa recombinant human GH as assay standard (Sanofi Diagnostics Pasteur Access, Chaska, MN). All samples (n = 148) from any given subject were analyzed together. Sensitivity is 0.010 μg/liter (defined as 3 sd above the zero-dose tube). Interassay coefficients of variation (CVs) were 7.9 and 6.3%, respectively, at GH concentrations of 3.4 and 12.1 μg/liter. The intraassay CVs were 4.9% at 1.12 μg/liter and 4.5% at 20 μg/liter. No values fell less than 0.020 μg/liter. Cross-reactivity with 20-kDa GH is less than 5% on a molar basis. Te concentrations were quantitated by automated competitive chemiluminescent immunoassay (ACS Corning, Bayer, Tarrytown, NY) (33, 34). Mean intra- and interassay CVs were 6.8 and 8.3%, with an assay sensitivity of 8 ng/dl (multiply by 0.0347 for nanomoles per liter) (35). E2 was measured by double-antibody RIA (Diagnostic Products Corp., Los Angeles, CA). Intraassay CVs are 18.3% at 3.6 pg/ml, 3.8% at 40.4 pg/ml, and 7.2% at 297 pg/ml. Interassay CVs are 8.1, 4.7, and 4.9% at 16.0, 31.1, and 119 pg/ml, respectively. IGFBP-1, IGFBP-3, and total IGF-I concentrations were measured by immunoradiometric assay (after extraction) (Diagnostic Systems Laboratories, Webster, TX) (33–35). Interassay CVs were 9% at 64 μg/liter and 6.2% at 157 μg/liter. Intraassay CVs were 3.4% at 9.4, 3% at 55.4, and 1.5% at 264 μg/liter.
Deconvolution analyses of stimulated GH secretion
Basal (time-invariant) and pulsatile (burst-like) modes of GH secretion were estimated simultaneously using published estimates of biexponential GH disappearance (viz., 3.5 and 20.8 min apportioned as 63% slow decay) (36, 37), conditional on prior pulse-time identification by validated Cluster analysis (38, 39). The principal analytical outcomes of the deconvolution procedure are: 1) basal, pulsatile, and total GH secretion during saline infusion (micrograms per liter per 5 h); and 2) the summed mass of GH secreted in bursts after stimulation by saline or dual secretagogues (micrograms per liter per 3 h).
Simplified ensemble feedback model
The simplified ensemble model incorporates assumed interactions among the four principal peptides that regulate pulsatile GH secretion (3, 11, 29, 40). In brief, the overall construction assumes that: 1) GHRH and SS stimulate and inhibit GH secretion, respectively; 2) GHRH and SSergic neurons interact reciprocally in the arcuate nucleus; 3) feedback by a GH pulse induces SS outflow from the periventricular nucleus, which inhibits both pituitary GH and hypothalamic GHRH release; and 4) ghrelin acts via three primary mechanisms: 1) antagonism of SS’s inhibition of the neuronal release and pituitary effect of GHRH; 2) stimulation of hypothalamic GHRH secretion; and 3) stimulation of somatotrope GH secretion directly.
Other statistical comparisons
Two-way analysis of covariance (ANCOVA) in a 2 (gender) × 3 (secretagogue) factorial design was used to contrast logarithmically transformed responses by gender and secretagogue pair. This model assumes that the response to saline is the covariate, thereby accommodating anticipated within-subject correlations. Post hoc comparisons were made by Tukey’s honestly significantly different test at protected experiment-wise P < 0.05 (41). An unpaired two-tailed Student’s t test was used to compare baseline hormone concentrations by gender. Linear regression analysis was applied to examine the relationship between GH secretory-burst mass and E2 or Te concentrations in the combined cohorts (42).
Data are presented as the arithmetic mean ± sem.
Results
Primary outcomes
Table 1 summarizes mean fasting (0800 h) concentrations of GH, total IGF-I, IGFBP-1, IGFBP-3, Te, and E2. Sex differences included higher Te concentrations in men and higher GH and E2 and a trend toward higher IGFBP-1 concentrations in women. IGF-I and IGFBP-3 values did not differ by gender.
TABLE 1.
Baseline hormone concentrations
| Measure | Men (n = 8) | Women (n = 6) |
|---|---|---|
| GH (μg/liter) | 2.2 ± 0.46 | 4.4 ± 0.64a |
| IGF-I (μg/liter) | 368 ± 24 | 396 ± 15 |
| Te (ng/dl) | 586 ± 62 | 44 ± 2.1b |
| E2 (pg/ml) | 28 ± 1.9 | 74 ± 18a |
| IGFBP-1 (μg/liter) | 19 ± 4.0 | 30 ± 3.8c |
| IGFBP-3 (mg/liter) | 4617 ± 198 | 4516 ± 171 |
Data are the mean ± sem (n is the number of subjects). To convert Te and E2 concentrations to SI units, multiply by 0.0347 and 3.67, respectively.
P ≤ 0.013;
P < 0.001; and
P = 0.061 by gender.
Figure 2 presents mean (±sem) plots of GH concentrations. Profiles are shown in relation to iv infusion of saline, l-arginine/GHRH, l-arginine/GHRP-2, and combined GHRH-GHRP-2 (Subjects and Methods). Mean GH concentrations were greater in women than men during saline infusion (Table 1) and after each stimulus pair, except for combined GHRH/GHRP-2.
Fig. 2.
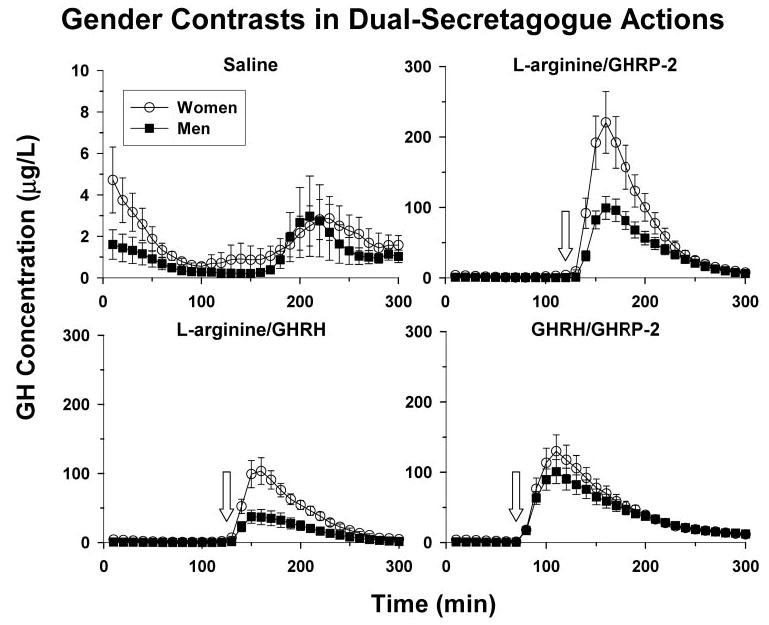
Mean (±sem) GH concentrations monitored every 10 min for 5 h in young men (n = 8) and women in the early follicular phase of the menstrual cycle (n = 6) during iv infusion of saline (top left) or the paired secretagogues: l-arginine/GHRP-2 (top right), L-arginine/GHRH (bottom left), and GHRH/ GHRP-2 (bottom right). l-Arginine and peptide delivery began at, respectively, 100 and 130 min (x-axis, where zero min is 0800 h).
Quantitative assessment of GH secretion was carried out by deconvolution analysis. In the saline control session, GH secretion in women differed from that in men by way of: 1) higher GH secretory-burst mass (P = 0.026) and thereby greater pulsatile (P = 0.016) [and total (P = 0.022)] GH secretion (each P < 0.001 vs. saline); 2) higher basal GH secretion (P = 0.036); and 3) longer-duration GH secretory bursts (P = 0.042). These outcomes were selective, inasmuch as GH interburst intervals (median, 93 min) and half-lives (median, 17 min) did not differ in the two cohorts.
Figure 3 highlights salient gender contrasts in stimulated GH secretory-burst mass (micrograms per liter per 3 h) in relation to gender and individual secretagogue pair. ANCOVA revealed significant effects of secretagogue pair (P < 0.001), gender (P < 0.001), and their interaction (P = 0.030). The rank order of stimulus efficacy in both men and women was l-arginine/GHRP-2 > l-arginine/GHRH ≥ GHRH/GHRP-2 ≫ saline. Post hoc comparisons by gender disclosed the following primary distinctions: 1) 7.6-fold more pulsatile GH secretion in women than men during saline infusion (P < 0.001); 2) 4.6-fold greater burst-like GH secretion in women than men after tandem l-arginine/GHRH injection (P < 0.001); 3) 2.2-fold higher pulsatile GH secretion in women than men after sequential l-arginine/GHRP-2 stimulation (P = 0.015); and 4) statistically similar secretory responses in men and women during combined continuous infusion of GHRH/GHRP-2. Subsequent post hoc statistical comparisons among secretagogue pairs indicated that all three active interventions differed from one another in women (0.001 < P = 0.042), whereas in men only l-arginine/GHRP-2 and l-arginine/ GHRH differed (P < 0.001).
Fig. 3.
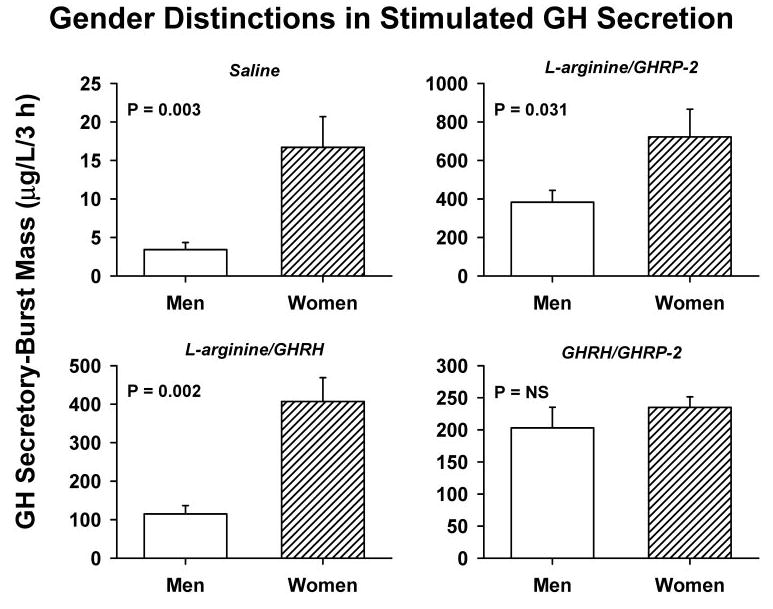
Mass of GH secreted in bursts over the 3-h interval after iv infusion of saline or paired secretagogues, as defined in Fig. 1. Data are the mean ± sem (P = NS denotes P > 0.05). The cohorts comprised eight men and six women. Statistical contrasts reflect ANCOVA followed by Tukey’s post hoc comparisons (Subjects and Methods).
Figure 4 illustrates linear regression analyses of GH secretory-burst mass on sex-steroid concentrations in the combined cohorts. Principal findings were: 1) E2 concentrations correlated positively with l-arginine/GHRP-2-induced GH secretion (r2= 0.49, P = 0.005); and 2) Te correlated negatively with burst-like GH release stimulated by l-arginine/ GHRH (r2 = 0.56, P = 0.002). Neither sex steroid correlated with the effects of paired l-arginine/GHRP-2 or combined GHRH/GHRP-2. A negative correlation of Te with fasting pulsatile GH secretion after saline infusion was considered borderline significant for multiple-comparison protected P < 0.01 (r2 = 0.40, P = 0.015). Bivariate regression analysis corroborated the individual rather than joint nature of the correlations noted above.
Fig. 4.
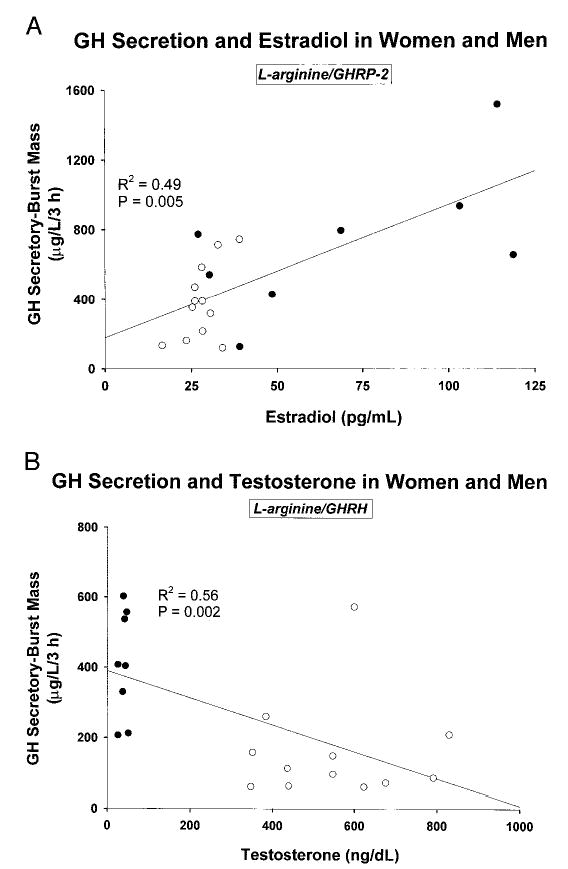
Linear regression analysis of the relationship between dual secretagogue-stimulated GH secretory-burst mass (micrograms per liter per 3 h) and concentrations of E2 (A) or Te (B) in the combined cohorts of men (n = 8) and women (n = 6). The square of the correlation coefficient (r) and P value are shown. To convert the indicated Te and E2 concentrations to SI units, multiply by 0.0347 and 3.67, respectively.
Model-assisted interpretations
Structure of three-peptide ensemble
For modeling purposes, the assumed interactions among GHRH, GHRP/ghrelin, and SS include the expectation that: 1) GHRP releases hypothalamic GHRH (43, 44); 2) a submaximal amount of GHRP synergizes with a maximally stimulatory dose of GHRH (45, 46); 3) cyclical SS withdrawal evokes rebound-like secretion of GHRH and GH (3, 11, 28, 29, 47); and 4) GHRP antagonizes central SSergic inhibition of GHRH release, but not hypothalamic secretion of SS to the pituitary gland (12, 48).
Hypotheses
The interpretative question is why women secrete more GH than men during presumptive simultaneous SS withdrawal (by l-arginine) and maximal stimulation with either GHRH or GHRP-2, but not during combined drive by GHRH/GHRP-2 (Fig. 3). Minimal explanatory hypotheses examined were: 1) GHRH potency is greater in women than men; 2) GHRP-2/ghrelin efficacy is higher in women than men; 3) both 1) and 2) are true; and 4) both 1) and 2) are true and cyclical GH-induced initial SS release is higher and interpulse pituitary inhibition by SS is lower in women than men (17). The first three notions reflect the reported capability of exogenous E2 in women to: 1) increase GHRH potency [but not efficacy] (32); and 2) augment GHRP-2 efficacy (49). And, the fourth addresses the observations that: 1) women exhibit greater fractional feedback inhibition by a pharmacological dose of recombinant human GH than men, possibly implying greater SS release during maximal negative feedback (50); and 2) E2 supplementation attenuates the inhibitory potency (but not efficacy) of infused SS by 50% (51).
Interpretations
The direct output of biomathematical simulations is highlighted in Fig. 5. Based upon the four minimal models tested, we infer that a tripartite mechanism could account for the observed gender differences. In particular, in this construction, women would be more responsive than men to each of: 1) a submaximal GHRH stimulus; 2) a maximal GHRP/ghrelin stimulus; and 3) GH as a feedback signal driving SS release. The concept of high-amplitude GH pulse-renewal in women is that GH-induced SS release serves to quench an evolving GH secretory burst, and subsequent SS withdrawal evokes a burst of GHRH and GH secretion (3, 11).
Fig. 5.
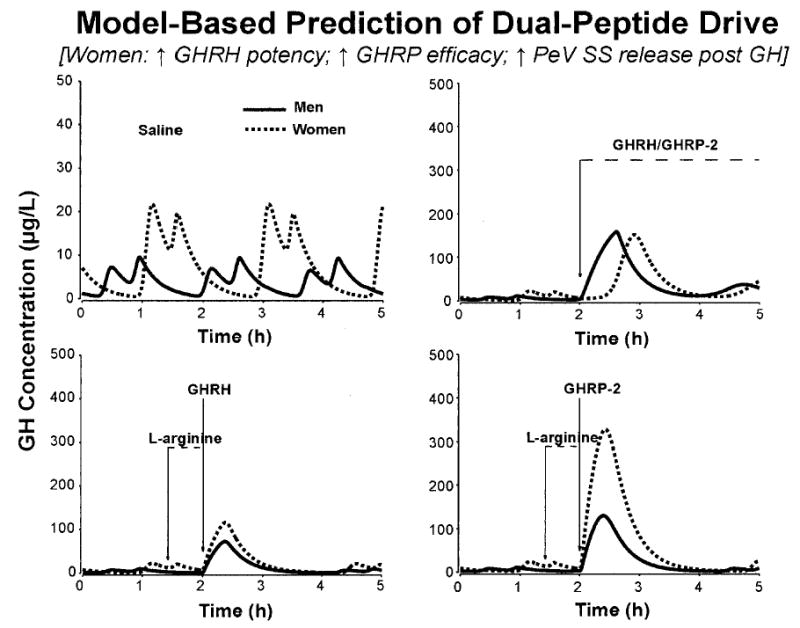
Output of a simplified ensemble model that links GHRH, GHRP/ghrelin, and GHRH via GH-negative feedback (Subjects and Methods). Model simulations predicted that women compared with men exhibit all three of: 1) enhanced GHRH potency (lower one-half maximally stimulatory concentration); 2) higher ghrelin efficacy (greater maximal stimulatory effect); 3) accentuated time-delayed GH-induced SS release, which quenches high-amplitude GH pulses and evokes more prominent post-SS rebound-like release of GHRH and GH. The paired curves in each panel apply to model predictions in men (solid line) and women (interrupted line). The four separate panels illustrate the corresponding four interventions studied; viz., iv infusion of saline (top left) or combined GHRH/GHRP-2 (top right), l-arginine/GHRH (bottom left), and l-arginine/GHRP-2 (bottom right).
Discussion
The present clinical investigation is unique to our knowledge in using mechanistically complementary pairs of secretagogues and an objective model of three-peptide interactions to examine the basis of gender differences in pulsatile GH secretion. Women were studied in the early-to-mid follicular phase of the menstrual cycle to ensure representation of a range of physiological E2 concentrations. Experimentally, we observed that: 1) unstimulated GH secretory bursts are larger in women than men; 2) combined infusion of maximally effective l-arginine/GHRH or l-arginine/ GHRP-2, but not GHRH/GHRP-2, is significantly more stimulatory in women than men; and 3) E2 positively determines GH secretion driven by l-arginine/GHRP-2, whereas Te negatively determines that stimulated by l-arginine/GHRH. Interpretatively, the foregoing set of outcomes is concordant with a unifying mechanistic postulate, in which women maintain greater submaximal GHRH drive, maximal GHRP stimulation and peak GH-induced SS release than men.
By evaluating GH responses to three complementary pairs of secretagogues via a simplified interactive three-peptide model (3, 11, 28, 29, 40), we were able to: 1) account for inferred gender differences in spontaneous GH release (Subjects and Methods) (3, 23–27, 40, 52, 53); 2) explicate the sex distinctions recognized here in dual-secretagogue actions (Fig. 5); and 3) build upon the inferences of recent clinical studies that used single effectors. In the first context, model-based analyses predict that the generation of high-amplitude GH pulses requires cycles of: 1) GH feedback-evoked hypothalamic SS outflow, which suppresses GH and GHRH secretion within ongoing bursts; 2) SS withdrawal-induced rebound release of GHRH, which induces GH secretion in the next pulse; and 3) ghrelin-dependent facilitation of GHRH stimulation and opposition to SS inhibition (3, 11, 28, 29, 40, 47, 54–57). According to these assumptions, larger spontaneous GH pulses in women than men may reflect more prominent feedforward by GHRH and ghrelin, and greater fractional suppression of an evolving GH secretory burst by SSergic feedback. Similar interactions are able to account for gender differences observed here in response to dual-secretagogue paradigms.
In relation to the impact of gender or sex steroids on the stimulatory effect of GHRP administered alone, clinical studies have reported greater GHRP-stimulated GH secretion in pubertal girls than boys, prepubertal girls administered estrogen, boys treated with an aromatizable androgen, and postmenopausal women supplemented with E2 compared with placebo (30, 58–60). Based on the notion of 3-peptide control of pulsatile GH secretion (3, 11, 28, 29, 40), our finding of higher GHRP efficacy in women than men monitored during putatively reduced SS outflow would predictively: 1) amplify synergistic GH stimulation by GHRP and GHRH, as inferred in a recent comparison in older men and women (16); and 2) antagonize negative feedback on the GHRP stimulus, as reported after E2 supplementation (49). In principle, higher ghrelin/GHRP drive in women than men might be explained by E2-dependent transcriptional up-regulation of the human ghrelin-receptor gene, which is demonstrable in vitro (61). In laboratory studies, pituitary expression of the ghrelin receptor is greater in the adult female than male rat (62); and partial transgenic silencing of the CNS cat-echolaminergic neuronal GHRP/ghrelin receptor reduces pulsatile GH secretion and IGF-I concentrations in the female but not male animal (10). In the present cohort of young adults, E2 concentrations correlated positively with maximal stimulation by combined l-arginine/GHRP-2, thereby accounting for about 50% of the variability in induced GH secretion. Viewed collectively, these data allow the hypothesis that women maintain greater responsiveness to GHRP than men due to relatively greater availability of E2 over Te.
Combined maximal GHRH and GHRP-2 stimulation, as implemented here, was intended to assess hypothalamic SS restraint indirectly via an ensemble 3-peptide model of effector interactions (3, 11, 28, 29, 40). This design is valid, if the efficacy (maximal effect) of the joint stimulus is clearly less than maximal somatotrope secretory capacity. The latter condition was met, because the absolute effect of GHRH/ GHRP-2 was about 40% that of l-arginine/GHRP-2. In this setting, we found comparable combined GHRH/GHRP-2 drive of GH secretion in young men and women. At first glance, similar responsiveness could mean that SSergic inhibition does not differ by gender. However, this interpretation would apply only if gender also did not affect stimulation by GHRH and/or GHRP-2 during putative SS withdrawal. In fact, women secreted 2.2- to 4.6-fold more GH than men in response to GHRH and GHRP-2 stimulation after l-arginine infusion. Moreover, other studies indicate that E2 facilitates each of: 1) submaximal stimulation by GHRH after l-arginine infusion [increases potency by decreasing the one half-maximally stimulatory dose of GHRH]; 2) maximal stimulation by GHRP-2 [agonist efficacy], which putatively antagonizes central SS action; and 3) post-SS rebound GH release, which is dependent upon endogenous GHRH drive (30, 32, 60, 63). Thus, the model-assisted prediction is that higher GH feedback-induced SSergic inhibition would be necessary to yield similar (rather than greater) GH secretion in estrogen-sufficient women in the face of potentiated stimulation by GHRP-2. A countervailing SSergic mechanism could also explain why: 1) E2 fails to amplify a maximal GHRH stimulus, which might be expected to synergize with endogenous ghrelin (32); 2) E2 is able to augment maximal GHRP-2 stimulation, which uniquely antagonizes hypothalamo-pituitary effects of SS (30, 49); and 3) a maximally inhibitory pulse of recombinant human GH suppresses GH secretion by a higher percentage in women than in men (50). Although not obligatory for pulse renewal, reported blunting of submaximal SS inhibition at the pituitary level by an estrogen-enriched milieu (51) would predict higher basal (nonpulsatile) GH secretion and elevated inter-pulse GH concentrations, as observed here in women.
Greater stimulation of GH secretion by combined l-arginine/GHRH in women than men was the most striking gender contrast unveiled (viz. 4.6-fold). The sex difference was explained in part by a strong negative correlation between the concentration of Te and the stimulatory effects of l-arginine/GHRH in the combined cohorts (r2 = 0.56, P = 0.002). The basis for this negative association is not known. However, assuming that l-arginine limits hypothalamic SS outflow, then higher efficacy of l-arginine/GHRH stimulation in women would point to greater feedforward drive by endogenous ghrelin. This inference reflects: 1) the capability of a small amount of (exogenous) GHRP-2 or ghrelin to synergize with a pharmacological dose of GHRH (16, 45, 46, 64); 2) present use of a maximally effective GHRH stimulus, which is not modulated by either E2 or Te administration (32, 65); and 3) prominent facilitative actions of E2 on GHRP stimulation (30, 31, 49, 60).
By way of qualifications, we assumed that l-arginine suppresses SS outflow to a significant, albeit not necessarily complete, degree in both sexes. The assumption appears to be valid in the rat and human, but does not exclude additional (unknown) actions of this amino acid (1, 13, 66, 67). Although the current bolus dose of GHRP-2 (3 μg/kg) is the most effective one tested experimentally in the human, whether higher doses are potentially more stimulatory is not known. Thus, strictly defined, the facilitative effect of E2 is to enhance the potency and/or efficacy of the GHRP stimulus (30). Further clinical investigations will be required to ensure validity of our inferences, albeit sufficient by construction to account for available observations.
In summary, women secrete severalfold more GH in bursts than men in the fasting state and after combined stimulation with a maximally effective combination of l-arginine/GHRH or l-arginine/GHRP-2, but not GHRH/ GHRP-2. The key points predicted by the ensemble construct are that available data (present results and earlier studies) can be harmonized by the hypotheses that SSergic outflow in women is opposed by greater feedforward by GHRH (increased potency) and GHRP/ghrelin (increased efficacy).
Acknowledgments
We thank Kris Nunez for excellent support of manuscript preparation; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo Research Unit nursing staff for conducting the protocol.
Footnotes
This work was supported in part by the General Clinical Research Center Grant MO1 RR00585 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01 NIA AG 14799 and AG 19695 and K25 HD01474 from the National Institutes of Health (Bethesda, MD).
References
- 1.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 2.Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farhy LS, Veldhuis JD. Putative GH pulse renewal: periventricular somatostatinergic control of an arcuate-nuclear somatostatin and GH-releasing hormone oscillator. Am J Physiol. 2004;286:R1030–R1042. doi: 10.1152/ajpregu.00473.2003. [DOI] [PubMed] [Google Scholar]
- 4.Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements in interglandular signaling. Proc Natl Acad Sci USA. 2004;101:6740–6745. doi: 10.1073/pnas.0300619101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann G, Maheshwari H. The Dwarfs of Sindh: severe growth hormone (GH) deficiency caused by a mutation in the GH-releasing hormone receptor gene. Acta Paediatr Suppl. 1997;432:33–38. doi: 10.1111/j.1651-2227.1997.tb18366.x. [DOI] [PubMed] [Google Scholar]
- 7.Frohman LA. New insights into the regulation of somatotrope function using genetic and transgenic models. Metabolism. 1996;45:1–3. doi: 10.1016/s0026-0495(96)90067-0. [DOI] [PubMed] [Google Scholar]
- 8.Roelfsema F, Biermasz NR, Veldman RG, Veldhuis JD, Frolich M, Stokvis-Brantsma WH, Wit J-M. Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. J Clin Endocrinol Metab. 2000;86:2459–2464. doi: 10.1210/jcem.86.6.7536. [DOI] [PubMed] [Google Scholar]
- 9.Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest. 2002;109:1429–1436. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhy LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1240–R1249. doi: 10.1152/ajpregu.00086.2003. [DOI] [PubMed] [Google Scholar]
- 12.Bowers CY, Granda-Ayala R. GHRP-2, GHRH and SRIF interrelationships during chronic administration of GHRP-2 to humans. J Pediatr Endocrinol Metab. 1996;9(Suppl 3):261–270. [PubMed] [Google Scholar]
- 13.Mueller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- 14.Baumbach WR, Carrick TA, Pausch MH, Bingham B, Carmignac D, Robinson ICAF, Houghten R, Eppler CM, Price LA, Zysk JR. A linear hexapeptide somatostatin antagonist blocks somatostatin activity in vitro and influences growth hormone release in rats. Mol Pharmacol. 1998;54:864–873. doi: 10.1124/mol.54.5.864. [DOI] [PubMed] [Google Scholar]
- 15.Merimee TJ, Bergess JA, Rabinowitz D. Sex-determined variation in serum insulin and growth hormone response to amino acid stimulation. J Clin Endocrinol Metab. 1966;26:791–793. doi: 10.1210/jcem-26-7-791. [DOI] [PubMed] [Google Scholar]
- 16.Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J Clin Endocrinol Metab. 2004;89:2290–2300. doi: 10.1210/jc.2003-031799. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuis JD, Bowers CY. Three-peptide control of pulsatile and entropic feedback-sensitive modes of growth hormone secretion: modulation by estrogen and aromatizable androgen. J Pediatr Endocrinol Metab. 2003;16(Suppl 3):587–605. [PubMed] [Google Scholar]
- 18.Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–146. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 19.Merimee TJ, Fineberg SE. Studies of the sex-based variation of human growth hormone secretion. J Clin Endocrinol Metab. 1971;33:896–902. doi: 10.1210/jcem-33-6-896. [DOI] [PubMed] [Google Scholar]
- 20.Jansson JO, Eden S, Isaksson OGP. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 21.Vahl N, Moller N, Lauritzen T, Christiansen JS, Jorgensen JO. Metabolic effects and pharmacokinetics of a growth hormone pulse in healthy adults: relation to age, sex, and body composition. J Clin Endocrinol Metab. 1997;82:3612–3618. doi: 10.1210/jcem.82.11.4388. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe CA, Turgeon DK, Lown K, DeMott-Friberg R, Watkins PB. Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab. 2002;283:E1008–E1015. doi: 10.1152/ajpendo.00513.2001. [DOI] [PubMed] [Google Scholar]
- 23.Stolar MW, Baumann G. Secretory patterns of growth hormone during basal periods in man. Metab Clin Exp. 1986;35:883–888. doi: 10.1016/0026-0495(86)90233-7. [DOI] [PubMed] [Google Scholar]
- 24.Winer LM, Shaw MA, Baumann G. Basal plasma growth hormone levels in man: new evidence for rhythmicity of growth hormone secretion. J Clin Endocrinol Metab. 1990;70:1678–1686. doi: 10.1210/jcem-70-6-1678. [DOI] [PubMed] [Google Scholar]
- 25.Engstrom BE, Karlsson FA, Wide L. Marked gender differences in ambulatory morning growth hormone values in young adults. Clin Chem. 1998;44:1289–1295. [PubMed] [Google Scholar]
- 26.van den Berg G, Veldhuis JD, Frolich M, Roelfsema F. An amplitude-specific divergence in the pulsatile mode of GH secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab. 1996;81:2460–2466. doi: 10.1210/jcem.81.7.8675561. [DOI] [PubMed] [Google Scholar]
- 27.Veldhuis JD, Roemmich JN, Rogol AD. Gender and sexual maturation-dependent contrasts in the neuroregulation of growth hormone secretion in prepubertal and late adolescent males and females—a general clinical research center-based study. J Clin Endocrinol Metab. 2000;85:2385–2394. doi: 10.1210/jcem.85.7.6697. [DOI] [PubMed] [Google Scholar]
- 28.Farhy LS, Straume M, Johnson ML, Kovatchev BP, Veldhuis JD. A construct of interactive feedback control of the GH axis in the male. Am J Physiol. 2001;281:R38–R51. doi: 10.1152/ajpregu.2001.281.1.R38. [DOI] [PubMed] [Google Scholar]
- 29.Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD. Unequal autonegative feedback by GH models the sexual dimorphism in GH secretory dynamics. Am J Physiol. 2002;282:R753–R764. doi: 10.1152/ajpregu.00407.2001. [DOI] [PubMed] [Google Scholar]
- 30.Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86:551–560. doi: 10.1210/jcem.86.2.7240. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis JD, Evans WS, Bowers CY. Impact of estradiol supplementation on dual peptidyl drive of growth-hormone secretion in postmenopausal women. J Clin Endocrinol Metab. 2002;87:859–866. doi: 10.1210/jcem.87.2.8251. [DOI] [PubMed] [Google Scholar]
- 32.Veldhuis JD, Evans WS, Bowers CY. Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab. 2003;88:5484–5489. doi: 10.1210/jc.2003-030410. [DOI] [PubMed] [Google Scholar]
- 33.Iranmanesh A, South S, Liem AY, Clemmons D, Thorner MO, Weltman A, Veldhuis JD. Unequal impact of age, percentage body fat, and serum testosterone concentrations on the somatotrophic, IGF-I, and IGF-binding protein responses to a three-day intravenous growth hormone-releasing hormone pulsatile infusion in men. Eur J Endocrinol. 1998;139:59–71. doi: 10.1530/eje.0.1390059. [DOI] [PubMed] [Google Scholar]
- 34.Shah N, Evans WS, Veldhuis JD. Actions of estrogen on the pulsatile, nyctohemeral, and entropic modes of growth hormone secretion. Am J Physiol. 1999;276:R1351–R1358. doi: 10.1152/ajpregu.1999.276.5.R1351. [DOI] [PubMed] [Google Scholar]
- 35.Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- 36.Faria ACS, Veldhuis JD, Thorner MO, Vance ML. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab. 1989;68:535–541. doi: 10.1210/jcem-68-3-535. [DOI] [PubMed] [Google Scholar]
- 37.Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci USA. 1987;84:7686–7690. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 39.Evans WS, Faria AC, Christiansen E, Ho KKY, Weiss J, Rogol AD, Johnson ML, Blizzard RM, Veldhuis JD, Thorner MO. Impact of intensive venous sampling on characterization of pulsatile GH release. Am J Physiol. 1987;252:E549–E556. doi: 10.1152/ajpendo.1987.252.4.E549. [DOI] [PubMed] [Google Scholar]
- 40.Farhy LS, Veldhuis JD, Hypothalamo-pituitary mechanisms mediating the effects of ghrelin on the somatotrope axis: a deterministic model. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004 (Abstract 843A)
- 41.Kuehl RO 1994 Split-plot designs. In: Statistical principles of research design and analysis. Belmont, CA: Duxbury Press; 473–498
- 42.Fisher LD, van Belle G 1996 Descriptive statistics. In: Biostatistics: a methodology for the health sciences. New York: John Wiley, Sons; 58–74
- 43.Guillaume V, Magnan E, Cataldi M, Dutour A, Sauze N, Renard M, Razafindraibe H, Conte-Devolx B, Deghenghi R, Lenaerts V. Growth hormone (GH)-releasing hormone secretion is stimulated by a new GH-releasing hexapeptide in sheep. Endocrinology. 1994;135:1073–1076. doi: 10.1210/endo.135.3.7915227. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher TP, Thomas GB, Clarke IJ. Growth hormone-releasing and somatostatin concentrations in the hypophysial portal blood of conscious sheep during the infusion of growth hormone-releasing peptide-6. Domest Anim Endocrinol. 1996;13:251–258. doi: 10.1016/0739-7240(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 45.Hataya Y, Akamizu T, Takaya K, Kanamoto N, Ariyasu H, Saijo M, Moriyama K, Shimatsu A, Kojima M, Kangawa K, Nakao K. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans. J Clin Endocrinol Metab. 2001;86:4552. doi: 10.1210/jcem.86.9.8002. [DOI] [PubMed] [Google Scholar]
- 46.Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–1174. doi: 10.1210/jcem.86.3.7314. [DOI] [PubMed] [Google Scholar]
- 47.Sato M, Chihara K, Kita T, Kashio Y, Okimura Y, Kitajima N, Fujita T. Physiological role of somatostatin-mediated autofeedback regulation for growth hormone: importance of growth hormone in triggering somatostatin release during a trough period of pulsatile growth hormone release in conscious male rats. Neuroendocrinology. 1989;50:139–151. doi: 10.1159/000125213. [DOI] [PubMed] [Google Scholar]
- 48.Fairhall KM, Mynett A, Robinson IC. Central effects of growth hormone-releasing hexapeptide (GHRP-6) on growth hormone release are inhibited by central somatostatin action. J Endocrinol. 1995;144:555–560. doi: 10.1677/joe.0.1440555. [DOI] [PubMed] [Google Scholar]
- 49.Anderson SM, Wideman L, Patrie JT, Weltman A, Bowers CY, Veldhuis JD. Estradiol supplementation selectively relieves GH’s autonegative feedback on GH-releasing peptide-2-stimulated GH secretion. J Clin Endocrinol Metab. 2001;86:5904–5911. doi: 10.1210/jcem.86.12.8076. [DOI] [PubMed] [Google Scholar]
- 50.Veldhuis JD, Patrie J, Wideman L, Patterson M, Weltman JY, Weltman A. Contrasting negative-feedback control of endogenously driven and exercise-stimulated pulsatile growth hormone secretion in women and men. J Clin Endocrinol Metab. 2004;89:840–846. doi: 10.1210/jc.2003-031081. [DOI] [PubMed] [Google Scholar]
- 51.Bray MJ, Vick TM, Shah N, Anderson SM, Rice LW, Iranmanesh A, Evans WS, Veldhuis JD. Short-term estradiol replacement in postmenopausal women selectively mutes somatostatin’s dose-dependent inhibition of fasting growth hormone secretion. J Clin Endocrinol Metab. 2001;86:3143–3149. doi: 10.1210/jcem.86.7.7647. [DOI] [PubMed] [Google Scholar]
- 52.Devesa J, Lois N, Arce V, Diaz MJ, Lima L, Tresguerres JA. The role of sexual steroids in the modulation of growth hormone (GH) secretion in humans. J Steroid Biochem Mol Biol. 1991;40:165–173. doi: 10.1016/0960-0760(91)90179-9. [DOI] [PubMed] [Google Scholar]
- 53.Hindmarsh PC, Dennison E, Pincus SM, Cooper C, Fall CHD, Matthews DR, Pringle PJ, Brook CGD. Sexually dimorphic pattern of growth hormone secretion in the elderly. J Clin Endocrinol Metab. 1999;84:2679–2685. doi: 10.1210/jcem.84.8.5915. [DOI] [PubMed] [Google Scholar]
- 54.Magnan E, Cataldi M, Guillaume V, Conte-Devolx B, Graziani N, Figaroli JC, Thomas F, Chihara K, Oliver C. Acute changes in growth hormone-releasing hormone secretion after injection of BIM 23014, a long acting somatostatin analog, in rams. Life Sci. 1992;51:831–838. doi: 10.1016/0024-3205(92)90610-2. [DOI] [PubMed] [Google Scholar]
- 55.Maiter DM, Gabriel SM, Koenig JI, Russell WE, Martin JB. Sexual differentiation of growth hormone feedback effects on hypothalamic growth hormone-releasing hormone and somatostatin. Neuroendocrinology. 1990;51:174–180. doi: 10.1159/000125334. [DOI] [PubMed] [Google Scholar]
- 56.Sugihara H, Minami S, Wakabayashi I. Post-somatostatin rebound secretion of growth hormone is dependent on growth hormone-releasing factor in unrestrained female rats. J Endocrinol. 1989;122:583–591. doi: 10.1677/joe.0.1220583. [DOI] [PubMed] [Google Scholar]
- 57.Clark RG, Carlsson LMS, Rafferty B, Robinson ICAF. The rebound release of growth hormone (GH) following somatostatin infusion in rats involves hypothalamic GH-releasing factor release. J Endocrinol. 1988;119:397–404. doi: 10.1677/joe.0.1190397. [DOI] [PubMed] [Google Scholar]
- 58.Loche S, Colao A, Cappa M, Bellone J, Aimaretti G, Farello G, Faedda A, Lombardi G, Deghenghi R, Ghigo E. The growth hormone response to hexarelin in children: reproducibility and effect of sex steroids. J Clin Endocrinol Metab. 1997;82:861–864. doi: 10.1210/jcem.82.3.3795. [DOI] [PubMed] [Google Scholar]
- 59.Bellone J, Aimaretti G, Bartolotta E, Benso L, Imbimbo BP, Lenhaerts V, Deghenghi R, Camanni F, Ghigo E. Growth hormone-releasing activity of hexarelin, a new synthetic hexapeptide, before and during puberty. J Clin Endocrinol Metab. 1995;80:1090–1094. doi: 10.1210/jcem.80.4.7714074. [DOI] [PubMed] [Google Scholar]
- 60.Shah N, Evans WS, Bowers CY, Veldhuis JD. Oral estradiol administration modulates continuous intravenous growth hormone (GH)-releasing peptide-2 driven GH secretion in postmenopausal women. J Clin Endocrinol Metab. 2000;85:2649–2659. doi: 10.1210/jcem.85.8.6729. [DOI] [PubMed] [Google Scholar]
- 61.Petersenn S, Rasch AC, Penshorn M, Beil FU, Schulte HM. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology. 2001;142:2649–2659. doi: 10.1210/endo.142.6.8184. [DOI] [PubMed] [Google Scholar]
- 62.Kamegai J, Wakabayashi I, Kineman RD, Frohman LA. Growth hormone-releasing hormone receptor (GHRH-R) and growth hormone secretagogue receptor (GHS-R) mRNA levels during postnatal development in male and female rats. J Neuroendocrinol. 1999;11:299–306. doi: 10.1046/j.1365-2826.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- 63.Veldhuis JD, Anderson SM, Patrie JT, Bowers CY. Estradiol supplementation in postmenopausal women doubles rebound-like release of growth hormone (GH) triggered by sequential infusion and withdrawal of somatostatin: evidence that estrogen facilitates endogenous GH-releasing hormone drive. J Clin Endocrinol Metab. 2004;89:121–127. doi: 10.1210/jc.2003-031291. [DOI] [PubMed] [Google Scholar]
- 64.Bowers CY 1998 Synergistic release of growth hormone by GHRP and GHRH: scope and implication. In: Bercu BB and Walker RF, eds. Growth hormone secretagogues in clinical practice. New York: Marcel Dekker, Inc; 1–25
- 65.Veldhuis JD, Mielke K, Bradford K, Miles JM, Bowers CY, Impact of short-term testosterone supplementation in healthy older men on maximal dual secretagogue drive of burst-like GH secretion: comparison with estrogen action in women. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004 (Abstract 421A)
- 66.Alba-Roth J, Muller OA, Schopohl J, Von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab. 1988;67:1186–1189. doi: 10.1210/jcem-67-6-1186. [DOI] [PubMed] [Google Scholar]
- 67.Gianotti L, Maccario M, Lanfranco F, Ramunni J, Di Vito L, Grottoli S, Mueller EE, Ghigo E, Arvat E. Arginine counteracts the inhibitory effect of recombinant human insulin-like growth factor I on the somatotroph responsiveness to growth hormone-releasing hormone in humans. J Clin Endocrinol Metab. 2000;85:3604–3608. doi: 10.1210/jcem.85.10.6872. [DOI] [PubMed] [Google Scholar]


