Abstract
Tendon pathology has many manifestations, from spontaneous rupture to chronic tendinitis or tendinosis; the etiology and pathology of each are very different, and poorly understood. Tendon is a comparatively poorly vascularised tissue that relies heavily upon synovial fluid diffusion to provide nutrition. During tendon injury, as with damage to any tissue, there is a requirement for cell infiltration from the blood system to provide the necessary reparative factors for tissue healing. We describe in this review the response of the vasculature to tendon damage in a number of forms, and how and when the revascularisation or neovascularisation process occurs. We also include a section on the revascularisation of tendon during its use as a tendon graft in both ligament reconstruction and tendon–tendon grafting.
Keywords: tendinopathy, tendon grafts, tendon repair, tendon rupture, tendon vascularisation
Introduction
Evidence exists that tendon repair can occur either intrinsically via the resident tenocytes [1,2] or via extrinsic mechanisms, whereby cells from the surrounding sheath or synovium invade the tissue [3]. It seems likely that both mechanisms occur, although the involvement of external cells depends on the site of injury and vascular perfusion. Many in vivo animal studies have been performed on the ability of tendon to repair following laceration, transection and other models of injury [4-7]. A large number of in vitro studies on animal and human tendon have also been performed [1,2,8,9]. The roles of many factors in tendon repair have been examined, including cell proliferation and DNA synthesis [10,11], collagen [11], noncollagenous protein [12] and proteoglycan synthesis [13]. Intratendinous degenerative change is strongly associated with both chronic tendinopathies and spontaneous tendon rupture [14-17].
Degeneration and subsequent rupture of tendons has been associated with hypovascularity of specific regions within certain tendons [18,19]. A permanent disruption of the central blood vessels to normal tendons has been shown to cause cellular death and disintegration of collagen bundles [20]. Contradictory to this, however, would appear to be the finding that in many cases of chronic tendinopathy that have been examined histologically, there is an 'angiofibroblastic' response, and the presence of many large blood vessels [21-23]. The presence of blood vessels was not considered to be an indication of tissue repair [22]. What then is the role of a proliferative vasculature in the damaged tendon?
The aim of this review is to discuss the importance of the vasculature in tendon damage and repair, and what is known about factors that regulate changes in the vasculature of what is normally a sparsely vascularised tissue.
Tendon vasculature
During development, tendons are highly cellular and metabolically active, and are thus supplied with a rich capillary network [20]. Mature tendons, however, are poorly vascularised [24-26]; tendon nutrition is more reliant on synovial fluid diffusion than vascular perfusion [27], although they do have more blood vessels than is commonly accepted. Like any other connective tissue, tendon does not undergo neovascularisation under normal circumstances. The vascular supply of specific tendons, and the sources of the vasculature within each tendon, has been described in detail elsewhere [18-20,24-26,28-35].
The vascular supply to the tendon has been shown to arise from three distinct areas: the musculotendinous junction; the osseotendinous junction; and vessels from various surrounding connective tissue such as the paratenon, mesotenon and vincula [24,26,30,32]. Vessels are generally arranged longitudinally within the tendon, passing around the collagen fibre bundles in the endotenon, a sheet of loose connective tissue contiguous with the external layer or epitenon [20,31,32] (Fig. 1). In the hand tendons, very few straight vessels are seen [24], and these tend to be curved allowing them to straighten out during tendon movement [32]. Vessels at the bone insertion do not pass directly through from the bone into the tendon due to a cartilage layer between the tendon and bone [25], but they anastomose with those of the periosteum, forming an indirect link with the osseous circulation [31].
Figure 1.
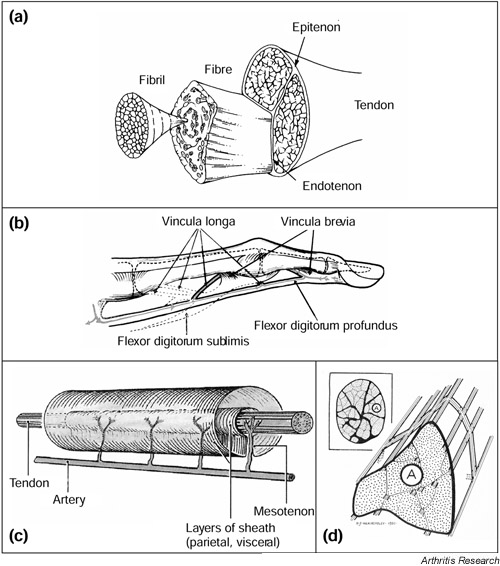
The structure and vascular supply of tendon. (a) The hierarchical structure of a typical tendon, composed of bundles of collagen fibrils bound together into fibres by the endotenon and surrounded by the epitenon. Adapted from Connective Tissue Research [94]. (b) Diagram showing the vincula supply to the sheathed digital flexor tendons (grey line with arrowheads). Published with permission from Journal of Bone and Joint Surgery [32]. (c) The supplying artery forms branches (mesotenon) into the tendon, passing through the sheath at a number of sites. Published with permission from Grant's Atlas of Anatomy [95]. (d) Blood vessels are arranged longitudinally in the epitenon, with frequent cross-anastomoses, and pass through the endotenon surrounding the fibre bundles (fascicles). Published with permission from Journal of Bone and Joint Surgery [32].
A number of arteries may be responsible for contributing to the blood supply of a tendon. For instance, six arteries supply blood to the rotator cuff tendons [30], branches of the peroneal and posterior tibial arteries supply the Achilles tendon [25], and four artery branches have been shown to supply the digital flexors [28]. There are, however, significant differences between the vascular supplies of different tendons; for instance each of the four tendons of the rotator cuff complex show a different vascular pattern [19]. There are also differences between 'round' tendons, such as the Achilles tendon, and those of the rotator cuff, and 'flat' tendons such as the patella tendon [19]. In particular, there is a significant difference between tendons that are contained within a sheath, and those that are unsheathed [32,33]. In unsheathed tendons, vessels may pass through the surrounding paratenon into the tendon at any point along the tendon. Sheathed tendons, however, have a much better defined, vincular supply; the blood vessels enter the tendon only at specific points along the tendon [32,33] (Fig. 1).
A number of tendons (but not all) appear to have regions of reduced vascularity, including the supraspinatus [19,34], biceps tendon [19], Achilles tendon [25,35], patella tendon [36] and posterior tibial tendon [18]. These avascular zones are commonly associated with degeneration and rupture, although there is no direct evidence to suggest that hypovascularity is a primary cause of tendon rupture. Degeneration of the supraspinatus has been attributed to ageing [37], although this does not explain why other rotator cuff tendons, also with hypovascular regions, do not share a similar predisposition to pathology.
The region of avascularity has been particularly studied in the supraspinatus, which is termed 'Codmans critical zone' after first being described by Codman and Akerson in 1931 [38]. However, this zone is not essentially avascular, and indeed intrinsic vessels of this zone have been shown to become filled with fluid once the tendon is removed from the compression applied by the humeral head [34]. Zones of avascularity within tendons may not be as clear cut as it would seem; the lack of blood penetration to these areas may be due to extrinsic factors such as regions of compression, as opposed to a simple lack of blood vessels. It seems likely that a combination of factors is responsible for predisposing a tendon to degeneration and/or rupture.
Neovascularisation of tendon
Tendon graft
Although not essentially a pathological condition, the neovascularisation of tendon during its use as a tendon graft has been widely studied in both human and animal models, and this model allows us to study the process of tendon neovascularisation per se. The patellar tendon is often used as an autograft to reconstruct or replace the anterior cruciate ligament (ACL) or posterior cruciate ligament [36,39-43]. The properties required for a tissue to replace the ACL have been discussed [44], one of these was concluded to be the need for a vascular supply. The advantages of using the patellar tendon as a cruciate ligament graft have been described [45] and the patellar tendon graft is the only autogenic transplant shown to have the strength and mechanical properties similar to those of normal cruciate ligaments [46]. The long-term survival of a patellar tendon graft is thought to be dependent on revascularisation of the graft [47,48]. Paulos et al. discussed that as much of the blood supply of the patellar tendon as possible should be preserved to maintain viability and facilitate the revascularisation process [45]. Studies have shown that the vascularisation of patellar tendon transplants occurs in relation to time in a number of models, including rabbit [48], dog [49,50], sheep [42,43], monkey [36] and human [47,51]. The vascularisation of patellar tendons used as cruciate ligament grafts has been examined over lengthy time periods [42,43,52], and up to 6.5 years in one human study [51].
The stages of graft revascularisation have been charted and described by a number of investigators [42,43,49]. Initially nutrition of the graft is thought to occur through diffusion of nutrients from the adjacent synovial tissue and synovial fluid [48,53]. The primary stage of graft vascularisation, which occurs in the first two weeks, is a surrounding of the graft material by a thick, richly vascularised loose connective tissue. Vascular invasions are present in the periphery of the graft, however regions of ischemic necrosis in the mid-substance of the grafts were also seen [42,43]. By 6 to 10 weeks, the graft had become revascularised [42,43,49], with intrinsic vessels seen originating in the proximal and distal portions of the patellar tendon graft, and migrating centrally. Areas of necrosis could still be seen at this stage [49]. Complete revascularisation of grafts was seen to occur by 16–20 weeks, and by 26 weeks the vascular response had subsided [49]. Longer term analysis up to 52 and 104 weeks showed a paucity of blood vessels, as is usually seen in normal cruciate ligaments [42,43]. The main sources of vessels for the revascularisation process are the endosteal vessels of both the femur and the tibia [36], the infrapatellar fat pad, and the synovial tissues [49]. The revascularisation of a patellar tendon graft is considered to be one of the four transitional phases of tendon patellar graft healing, as described by Kasperczyk et al.[42]. The revascularisation, or 'revitalisation', stage is the second of the four stages, and is considered to start at six weeks post surgery.
Original work by Whiston and Walmsley [54] showed that grafted tendon left for lengthy periods of time becomes histologically identical to the original tendon. Amiel et al.[48] described that a patellar tendon placed in the anatomical and environmental milieu of the ACL becomes 'ligamentised', and this has been supported by Rougraff et al.[51]. However, Bosch et al.[43] claim that this autografted tissue does not in fact undergo 'ligamentisation', but only develops into a partially organised replacement tissue. Even after two years, the tissue was structurally and mechanically different from a normal ligament.
The use of auto- or allografts makes very little difference to the cellular repopulation and revascularisation of tendon grafts when used in cruciate ligament reconstruction. Allografts are freeze-thawed to destroy potential immunologic complications before grafting. These grafts showed a similar revascularisation rate as studies described using autografts [36,39,42,43,49].
Along with the use of tendon as a graft for the ACL and/or posterior cruciate ligament, tendons have also been used as grafts to replace other tendons [55-57]. Much of the experimental work has investigated the best source of tendon for grafting. The source of donor tendon has been shown to affect the state of new blood vessel growth and infiltration into the graft [58-60]. Intrasynovial (peroneus longus) and extrasynovial (flexor digitorum profundus [FDP]) tendon donors were grafted into the synovial sheaths in the forepaws of dogs [59]. The extrasynovial grafts were shown to vascularise along with the ingrowth of vascular adhesions, while intrasynovial grafts healed with a minimum of vascular ingrowth. It was concluded that the ability of flexor tendon grafts to heal without adhesions is at least partially donor tissue specific. Similar work performed by Seiler et al.[58], also in dogs, showed the formation of vascular adhesions by extrasynovial grafts, while intrasynovial grafts healed with a minimum of adhesion, with neovascularisation originating from the proximal and distal sites of repair. Work by Eiken and Lundborg [61] has also stressed the importance of the tendon sheath in tendon grafting procedures. The profundus tendon of the third chicken toe was transferred into the fourth toe in either an intact or an excised sheath. The tendons placed into intact sheaths did not form adhesions while those in the excised sheath did. The vascular pattern seen in the tendon of the intact sheath reflected that of a normal profundus tendon by 16 weeks. It was concluded that an intact digital sheath nourishes the graft and prevents the need for adhesion formation.
Differences between sources of vascularised and nonvascularised tendon grafts have also been compared. Singer et al.[60] compared seven vascularised and eight nonvascularised tendon grafts in monkeys. Although the vascularised tendons appeared to show the formation of fewer adhesions, there was generally little difference between the two tendon sources. However, experimental numbers were small.
The vascularisation of autogenic tendon grafts is vital to allow the tendon to function as a ligamentous structure or as an alternative tendon. Although the source of blood vessels and the timescale of vascularisation have been examined, the cellular factors that are responsible for mediating the process have not been investigated, and there is a large gap in our knowledge regarding how the revascularisation of such grafts is controlled.
Acute injury
Much of the experimental work on tendon repair has concentrated on various methods of repairing complete or partial tendon lacerations. However there is actually little mention of changes in vascularisation associated with the repair process. Matthews [6] attempted to address the lack of information about circulation in repairing tendon using an injection technique. In a New Zealand white rabbit model that had undergone a closed tenotomy of the FDP tendon, he showed a plexus of fine new vessels forming at the tip of lacerated tendons. Adhesions that formed between tendons and the surrounding connective tissues were also shown to be highly vascularised. Increased vascularity at the tendon stump following tendon division and suture has also been shown in the flexor tendon of the canine forefoot [3]. Capillary buds were seen to invade the tendon at focal breaks made by the suturing process. Gelberman et al.[10] showed an initial vascular response in tendon injury of the dog FDP that was profuse and haphazard, and stated that the early stages of repair were characterised by a marked increase in vascularity. Kakar et al.[62] noted neovascularisation in the tendon sheath and epitenon of the FDP in the New Zealand white rabbit model after partial plantar laceration, although there was no mention of any vascularisation within the endotenon. Richards [63] showed that within a few hours of digital flexor tendon injury or division, the mesotenon could be seen carrying leashes of dilated blood vessels to the injured tendon. He also showed that impairment of tendon healing occurs if there is a diminution of the blood supply. The proximal end of the divided tendon stump showed cell and tissue necrosis.
Chronic tendinopathy
Chronic painful tendon pathology (tendinopathy), often referred to as a tendinitis, is perhaps more accurately described as a tendinosis, reflecting the absence of an inflammatory process [14-17,22]. The cause of chronic tendinopathy is unknown, although alterations in the histology, histochemistry and biochemistry of such lesions have been extensively studied [12-17,22,64,65]. A number of studies have shown an increase in vascularity at the site of chronic painful tendon lesions [22,23]. However, these lesions were found consistently at a point where the blood supply in the normal tendon is poorest [66]. Indeed rotator cuff disease of the shoulder, particularly of the supraspinatus tendon, has generally been considered a consequence of its avascular region [67,68], although this is not necessarily the case (as discussed earlier). The changes seen during chronic tendon degeneration were shown to be the same as those that occur when the blood supply in the Achilles tendon is disrupted [67]. It was hypothesised that, due to an impoverished vascular supply to the insertion region, a portion of the tissue may die, resulting in tissue degeneration [67].
A study by Astrom and Rausing [22] on biopsies from 163 patients with Achilles tendinopathy, demonstrated hypervascularity of the tendon, with groups of thick walled vessels distributed unevenly compared to biopsy specimens of macroscopically normal tissue from a nonsympto-matic area. This hypervascularity, however, was not thought to be associated with tissue repair. A study in a rabbit model of Achilles paratenonitis and tendinosis [69], using radiolabeled microspheres, showed that blood flow is increased in the damaged tendon compared to control tendons [70]. It was concluded that the degenerative alterations within the tendon and paratenon were not due to a chronic circulatory impairment, although measurements of blood flow during actual exercise were not performed. Two histopathologic studies of the patellar tendon in chronic patellar tendinosis or 'jumpers knee' showed capillary proliferation and prominent angiogenesis in the degenerate region [21,71]. The abnormal tissue was shown to correlate well with magnetic resonance findings [21]. The paratenon is also a site of chronic pain, and has been shown to undergo marked neovascularisation and degenerative vascular changes [23,70]. It was suggested that vascular alterations of the paratenon disturb the blood supply and cause ischemic pain during exercise [23]. Chronic tendon pathology would therefore seem to be a highly active process in terms of neovascularisation of the tissue. Why this hypervascular state (and the associated tendinopathy) fails to resolve is unknown, and the invasion and proliferation of new blood vessels may be a contributory factor to the pain and chronicity of the disease.
Spontaneous tendon rupture
Spontaneous tendon rupture (the sudden rupture of a tendon without preceding clinical symptoms) is a relatively common occurrence in sports, particularly in the recreational athlete [72]. Studies have shown the presence of tendon degeneration at the sites of tendon rupture, at time points soon after the rupture, which suggests that the degeneration was not a secondary event [73,74]. Indeed it is now generally accepted that tendon degeneration causes the rupture and does not result from it [17,75]. However, there are some studies that question the relationship between tendon degeneration and tendon rupture. A study by Williams [76] for instance found no complete ruptures of the Achilles tendon in patients with chronic Achilles tendinopathy, suggesting that degeneration does not inevitably lead to rupture. Barfred [77] summarised much of the early work on tendon ruptures, some of which claims that degeneration occurs prior to rupture, others that did not show prior degeneration. A rat model, described by Barfred, showed that after rupture there was no alteration in the blood vessels either within or around the tendon. He further showed that prior degeneration was not a necessary precondition for tendon rupture [78]. There is, however, strong evidence that partial tear and ruptures of a tendon are a consequence of tendon matrix degeneration; tendon degeneration is seen in partially ruptured tendons [67,79]. A study by Kannus and Jozsa [17] of 891 spontaneously ruptured tendons showed the presence of degeneration in all tendons, with none showing a normal tendon structure. After examining two cases of tendon rupture taken immediately postrupture, Davidsson and Salo [73] showed degenerative and necrobiotic changes along with inflammatory and regenerative changes, which were considered to have occurred prior to the rupture. It was suggested that vascular changes due to age and activity are an influencing factor on the degeneration of tendon that ultimately led to rupture. Arner and Lindholm [80] also saw vascular changes in some ruptured tendons, although samples were taken at more than two weeks postrupture, and these changes were considered to be a secondary result of an inflammatory, regenerative process.
In summary, there is uncertainty regarding the role of the vasculature in the processes underlying tendon rupture and tendon degeneration. However, there are generally differences in vascularity between chronic tendinopathy and those tendons that rupture 'spontaneously'. Chronic painful lesions show degeneration coinciding with blood vessel infiltration and cell proliferation without much indication of tissue repair [22]. Spontaneous ruptures do not show much evidence of vascularisation or cell proliferation, although they are also associated with degeneration of the tissue [17].
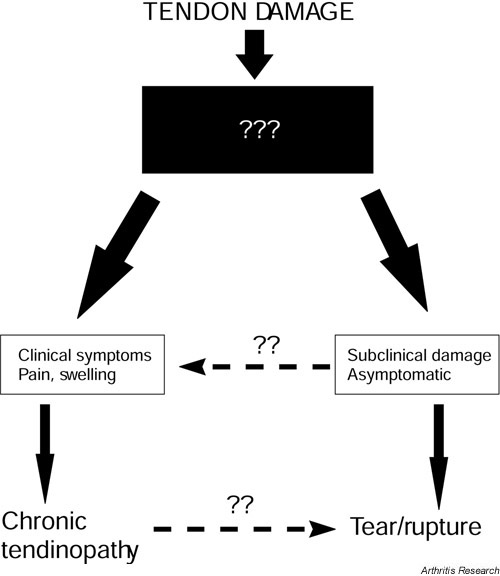
We suggest two possible consequences of tendon damage, which are summarised in Figure 2. The clinical condition of chronic tendinopathy, which manifests primarily as pain, shows vascular infiltration and cell proliferation within the tendon. In contrast, in the subclinical condition preceding spontaneous tendon rupture, there is a failure to elicit a vascular response. Indeed the lack of vascularity is possibly one of the causative factors. The tendon degenerates and ultimately results in weakened tissue, which subsequently ruptures. The factors influencing the response of the tendon are unknown and are represented by the black box in Figure 2.
Figure 2.
Schematic diagram illustrating two basic consequences of tendon damage. We postulate that the abundant neovascularisation in chronic tendinopathy is responsible for the clinical symptoms (pain, swelling). In the absence of a vascular response, the degenerative process is asymptomatic, and may lead directly to tendon rupture. Subclinical degeneration may also lead to the clinical condition of tendinopathy. Painful tendinopathy, however, does not necessarily lead to tendon rupture. The black box signifies our lack of understanding of the processes that distinguish these two potentially different pathways.
Angiogenic factors and tendon injury
Very little is known about growth factors and cytokines expressed in tendon wound healing and repair, although there have been a few studies [81-85]. Chang et al.[84] used immunolocalisation and in situ hybridisation to quantify the presence of basic fibroblast growth factor (bFGF) in a rabbit model of flexor tendon wound healing. They showed a significantly elevated number of cells in both the tendon substance and the epitenon expressing this angiogenic growth factor. It was suggested that the bFGF upregulation may represent an early, sustained signal for angiogenesis. This growth factor has also been shown to be present in uninjured canine flexor tendons through its binding to heparin-sepharose columns [85] and an increase in staining for bFGF has been shown in the early postoperative period after autogenous patellar tendon grafting used to reconstruct the ACL [86].
Platelet derived growth factor, epidermal growth factor and transforming growth factor (TGF)-β1 have also been shown to be upregulated during tendon wound repair and grafting [82,83,85]. Their roles are most likely to be involved in the revascularisation of the tendon [82,84]. Vascular endothelial growth factor (VEGF), the prototypic angiogenic growth factor, has also been localised to cells in the early stage of tendon wound healing. Bidder et al.[81], using in situ hybridisation in a canine model of tendon injury, identified cell populations within the repair site expressing message for VEGF, suggesting their potential for organising the angiogenic response during the early postoperative phase of wound healing. The distribution of growth factors in tendon healing, however, has not been thoroughly researched and many more questions need to be answered regarding the growth factor and cytokine profile of both normal and pathological tendon. It should also be noted that there is a paucity of literature describing the distribution of receptors for potential angiogenic growth factors, such as those for VEGF (VEGFR-1, VEGFR-2 and VEGFR-3), and receptors for those growth factors that may be involved in tendon repair, such as bFGF and platelet derived growth factor. The latter have been shown to regulate αvβ3 and α5β1 integrin expression in tenocytes, which have a function in angiogenesis [87]. An understanding of when and where these growth factors and their receptors are expressed would help us to further determine the role that they play in tendon injury and pathology. We suggest the need for similar investigations to those that have been performed in ligament, where the profile of growth factor induction and expression during injury and repair have been more extensively investigated [88,89].
Conclusion
The main points of this review are summarised in Table 1.
Table 1.
Summary of the main points of the tendon vasculature in repair and pathology
| Condition | Observations |
| Tendon grafts | Neovascularisation is essential for long term survival of graft Potential model for study of neovascularisation in tendon injury and pathology |
| Acute injury | Lack of knowledge of timing and control of the vascular response Increased vascularity at tendon stump following resection Initial vascular response is profuse and haphazard Diminution of blood supply results in impairment of repair process |
| Chronic tendinopathy | Increased vascularity is associated with chronic (painful) tendon lesions Hypervascularity is apparently not associated with signs of attempted repair Hypervascularity may be a contributory factor to pain and chronicity of tendon lesions |
| Spontaneous tendon rupture | Degeneration occurs before rupture without preceding clinical symptoms There is usually no evidence of a vascular response Absence of blood vessels is responsible for absence of pain and symptoms |
Although the relative roles of vascular perfusion and synovial fluid diffusion as mechanisms of tendon nutrition during the healing of tendon is still not clear [85], it is thought that flexor tendons are nourished to a greater extent by synovial fluid diffusion [27]. However, this review illustrates not only the importance of vascularisation of tendons during their attempts at healing and integration when used as grafts, but also brings into question its role in chronic tendon lesions and tendon degeneration. It seems apparent that, despite an increase in the number of blood vessels in chronic tendon pathologies [22,23], there is very little suggestion of a healing response [22]. The role that the vasculature plays in certain tendon pathologies is therefore an open question and sheds doubt onto whether vascular invasion of tendon is, in fact, a desirable thing under some circumstances.
In many patients the degenerative processes does not manifest clinically, with no symptoms such as pain or swelling. In a survey of 891 'spontaneous' ruptures, Kannus and Jozsa found that two-thirds of the patients had no symptoms prior to rupture [17]. Why there is a lack of clinical symptoms is unknown, but may be related to the absence of nerve endings in the (relatively) avascular tendon. We postulate that the initial insult does not always trigger a vascular or fibroproliferative response, resulting in an absence of inflammation and/or pain. It may be that there is a limited cellular response in these tissues, perhaps because the cells are terminally differentiated, quiescent, senescent or possibly dead. There may be no release of factors that are necessary to promote cell activity. If there is a vascular response, perhaps because trauma or damage reaches an area where vessels are present, then painful tendon symptoms may develop, with ingrowth of blood vessels and fibroblast proliferation. The source of the pain in tendinopathy is unknown, whether derived from sensitised nerve endings within the tendon or in the surrounding peritendinous tissues. In other tissues, such as the intervertebral disc, ingrowth of blood vessels is associated with ingrowth of nerves [90], but very little is known about the innervation of degenerate and painful tendons.
It is apparent that many etiological factors and pathways may be responsible for tendon degeneration [72]. One potentially important factor is hypoxia in the tendon, and how this relates to vascularisation. As tendon is a relatively sparsely vascularised tissue, it has been suggested that hypoxia may be a cause of tissue degeneration and subsequent rupture in tendons [14-19,67,68,91]. The ability to measure oxygen tensions within tendon [92] may help us to further understand the role that local hypoxia has in tendon degeneration. With respect to neovascularisation as part of this process, VEGF has been shown to function as a hypoxia-inducible factor [93], potentially providing a link between ischaemia/hypoxia and tendon neovascularisation.
The angiogenic process is mediated by a vast milieu of growth factors and cytokines, yet very little work has been done to investigate the presence and role of these growth factors and cytokines in normal, injured and healing tendon. Although the presence of angiogenic factors has been shown in healing tendon [81,84], there have been few attempts made to examine the growth factor profile of normal and pathological tendons. There is, therefore, a large gap in our knowledge concerning how the vascularisation process in tendon is controlled. This knowledge is necessary if we are to understand why a vascular response in chronic tendinopathy apparently does not lead to repair or resolution of the condition, while revascularisation of tendon grafts allows normal function of the tissue.
Abbreviations
ACL = anterior cruciate ligament; bFGF = basic fibroblast growth factor; FDP = flexor digitorum profundus; TGF = transforming growth factor; VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor.
Acknowledgments
Acknowledgements
We would like to thank the Arthritis Research Campaign (ARC) and the Cambridge Arthritis Research Endeavour (CARE) for their financial support.
References
- Manske PR, Lesker PA. Biochemical evidence of flexor tendon participation in the repair process – an in vitro study. J Hand Surg. 1984;9B:117–120. [PubMed] [Google Scholar]
- Gelberman RH, Manske PR, Van de Berg JS, Lesker PA, Akeson WH. Flexor tendon repair. in-vitro: 1984;a comparitive histologic study of the rabbit, chicken, dog and monkey:39–48. doi: 10.1002/jor.1100020107. [DOI] [PubMed] [Google Scholar]
- Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg. 1962;44A:49–64. [PubMed] [Google Scholar]
- Gelberman RH, Manske PR, Akeson WH, Woo SL, Lundborg G, Amiel D. Flexor tendon repair. J Orthop Res. 1986;4:119–128. doi: 10.1002/jor.1100040116. [DOI] [PubMed] [Google Scholar]
- Li BH, Hong GX, Zu TB. A histological observation on the flexor tendon healing within intact sheath. J Tongji Med Univ. 1991;11:169–173. doi: 10.1007/BF02888130. [DOI] [PubMed] [Google Scholar]
- Matthews JP. Vascular changes in flexor tendons after injury and repair: an experimental study. Injury. 1977;8:227–233. doi: 10.1016/0020-1383(77)90136-x. [DOI] [PubMed] [Google Scholar]
- Kain CC, Russell JE, Burri R, Dunlap J, McCarthy J, Manske PR. The effect of vascularization on avian flexor tendon repair. A biochemical study. Clin Orthop. 1988;233:295–303. [PubMed] [Google Scholar]
- Mass DP, Tuel R. Human flexor tendon participation in the in vitro repair process. J Hand Surg Am. 1989;14:64–71. doi: 10.1016/0363-5023(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Packer DL, Dombi GW, Yu PY, Zidel P, Sullivan WG. An in vitro model of fibroblast activity and adhesion formation during flexor tendon healing. J Hand Surg Am. 1994;19:769–776. doi: 10.1016/0363-5023(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Amifl D, Gonsalves M, Woo S, Akeson WH. The influence of protected passive mobilization on the healing of flexor tendons: a biochemical and microangiographic study. Hand. 1981;13:120–128. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Vande-Berg JS, Lundborg GN, Akeson WH. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg Am. 1983;65:70–80. [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Cawston TE, Hazleman BL, Mackie EJ. Tenascin-C and human tendon degeneration. Am J Pathol. 1996;149:933–943. [PMC free article] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1994;53:367–376. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Josza L, Reffy A, Kannus P, Demel S, Elek E. Pathological alterations in human tendons. Arch Orthop Trauma Surg. 1990;110:15–21. doi: 10.1007/BF00431359. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports. 1997;7:113–118. doi: 10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Frey C, Shereff M, Greenidge N. Vascularity of the posterior tibial tendon. J Bone Joint Surg Am. 1990;72:884–888. [PubMed] [Google Scholar]
- Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52:540–553. [PubMed] [Google Scholar]
- Peacock EE. A study of the circulation in normal tendons and healing grafts. Ann Surg. 1959;149:415–428. doi: 10.1097/00000658-195903000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Popp JE, Kaeding CC, Lucas J. Correlation of MR imaging and pathologic findings in athletes undergoing surgery for chronic patellar tendinitis. Am J Roentgenol. 1995;165:115–118. doi: 10.2214/ajr.165.1.7785569. [DOI] [PubMed] [Google Scholar]
- Astrom M, Rausing A. A survey of surgical and histopathologic findings. Clin Orthop. 1995;316:151–164. [PubMed] [Google Scholar]
- Kvist M, Jozsa L, Jarvinen MJ, Kvist H. Chronic Achilles paratenonitis in athletes: a histological and histochemical study. Pathology. 1987;19:1–11. doi: 10.3109/00313028709065127. [DOI] [PubMed] [Google Scholar]
- Takasugi H, Akahori O, Nishihara K, Tada K. Three-dimensional architecture of blood vessels of tendons demonstrated by corrosion casts. Hand. 1978;10:9–15. doi: 10.1016/s0072-968x(78)80020-5. [DOI] [PubMed] [Google Scholar]
- Schmidt-Rohlfing B, Graf J, Schneider U, Niethard FU. The blood supply of the Achilles tendon. Int Orthop. 1992;16:29–31. doi: 10.1007/BF00182980. [DOI] [PubMed] [Google Scholar]
- Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK. Blood supply of the Achilles tendon. J Orthop Res. 1998;16:591–596. doi: 10.1002/jor.1100160511. [DOI] [PubMed] [Google Scholar]
- Gelberman RH. Flexor tendon physiology: tendon nutrition and cellular activity in injury and repair. Instr Course Lect. 1985;34:351–360. [PubMed] [Google Scholar]
- Ochiai N, Matsui T, Miyaji N, Merklin RJ, Hunter JM. Vascular anatomy of flexor tendons. I. Vincular system and blood supply of the profundus tendon in the digital sheath. J Hand Surg Am. 1979;4:321–330. doi: 10.1016/s0363-5023(79)80068-4. [DOI] [PubMed] [Google Scholar]
- Zhang ZZ, Zhong SZ, Sun B, Ho GT. Blood supply of the flexor digital tendon in the hand and its clinical significance. Surg Radiol Anat. 1990;12:113–117. doi: 10.1007/BF01623335. [DOI] [PubMed] [Google Scholar]
- Chansky HA, Iannotti JP. The vascularity of the rotator cuff. Clin Sports Med. 1991;10:807–822. [PubMed] [Google Scholar]
- Schatzker J, Branemark PI. Intravital observations on the microvascular anatomy and microcirculation of the tendon. Acta Orthop Scand. 1969;126(suppl):1–23. doi: 10.3109/ort.1969.40.suppl-126.01. [DOI] [PubMed] [Google Scholar]
- Brockis JG. The blood supply of the flexor and the extensor tendons of the fingers in man. J Bone Joint Surg Br. 1953;35B:131–138. doi: 10.1302/0301-620X.35B1.131. [DOI] [PubMed] [Google Scholar]
- Chaplin DM. The vascular anatomy within normal tendons, divided tendons, free tendon grafts and pedicle tendon grafts in rabbits. A microradioangiographic study. J Bone Joint Surg Br. 1973;55:369–389. [PubMed] [Google Scholar]
- Ling SC, Chen CF, Wan RX. A study on the vascular supply of the supraspinatus tendon. Surg Radiol Anat. 1990;12:161–165. doi: 10.1007/BF01624517. [DOI] [PubMed] [Google Scholar]
- Stein V, Laprell H, Tinnemeyer S, Petersen W. Quantitative assessment of intravascular volume of the human Achilles tendon. Acta Orthop Scand. 2000;71:60–63. doi: 10.1080/00016470052943919. [DOI] [PubMed] [Google Scholar]
- Clancy-WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wis-nefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63:1270–1284. [PubMed] [Google Scholar]
- Brewer BJ. Aging of the rotator cuff. Am J Sports Med. 1979;7:102–110. doi: 10.1177/036354657900700206. [DOI] [PubMed] [Google Scholar]
- Codman EA, Akerson IB. The pathology associated with rupture of the supraspinatus tendon. Ann Surg. 1931;93:348–359. doi: 10.1097/00000658-193101000-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland EG, Morrey BF, An KN, Wood MB. The relationship of vascularity and water content to tensile strength in a patellar tendon replacement of the anterior cruciate in dogs. Am J Sports Med. 1986;14:436–448. doi: 10.1177/036354658601400602. [DOI] [PubMed] [Google Scholar]
- Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24:698–701. doi: 10.1177/036354659602400524. [DOI] [PubMed] [Google Scholar]
- Zanotti RM, Freiberg AA, Matthews LS. Use of patellar allograft to reconstruct a patellar tendon-deficient knee after total joint arthroplasty. J Arthroplasty. 1995;10:271–274. doi: 10.1016/s0883-5403(05)80173-1. [DOI] [PubMed] [Google Scholar]
- Kasperczyk WJ, Bosch U, Oestern HJ, Tscherne H. Staging of patellar tendon autograft healing after posterior cruciate ligament reconstruction. A biomechanical and histological study in a sheep model. Clin Orthop. 1993;286:271–282. [PubMed] [Google Scholar]
- Bosch U, Kasperczyk WJ. Healing of the patellar tendon autograft after posterior cruciate ligament reconstruction – a process of ligamentization? An experimental study in a sheep model. Am J Sports Med. 1992;20:558–566. doi: 10.1177/036354659202000513. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Butler DL, Paulos LE, Grood ES. Intra-articular cruciate reconstruction. I: Perspectives on graft strength, vascularization, and immediate motion after replacement. Clin Orthop. 1983;172:71–77. [PubMed] [Google Scholar]
- Paulos LE, Butler DL, Noyes FR, Grood ES. Intra-articular cruciate reconstruction. II: Replacement with vascularized patellar tendon. Clin Orthop. 1983;172:78–84. [PubMed] [Google Scholar]
- Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66:344–352. [PubMed] [Google Scholar]
- Alm A. Survival of part of patellar tendon transposed for reconstruction of anterior cruciate ligament. Acta Chir Scand. 1973;139:443–447. [PubMed] [Google Scholar]
- Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14:449–462. doi: 10.1177/036354658601400603. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64:217–224. [PubMed] [Google Scholar]
- Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14:348–360. doi: 10.1177/036354658601400502. [DOI] [PubMed] [Google Scholar]
- Rougraff B, Shelbourne KD, Gerth PK, Warner J. Arthroscopic and histologic analysis of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:277–284. doi: 10.1177/036354659302100219. [DOI] [PubMed] [Google Scholar]
- Alm A, Stromberg B. Transposed medial third of patellar ligament in reconstruction of the anterior cruciate ligament. A surgical and morphologic study in dogs. Acta Chir Scand. 1974;445(suppl):37–49. [PubMed] [Google Scholar]
- Ginsburg JH, Whiteside LA, Piper TL. Nutrient pathways in transferred patellar tendon used for anterior cruciate ligament reconstruction. Am J Sports Med. 1980;8:15–18. doi: 10.1177/036354658000800103. [DOI] [PubMed] [Google Scholar]
- Whiston TB, Walmsley R. Some observations of bone and tendon after tunnelling of bone and insertion of tendon. J Bone Joint Surg Am. 1960;42A:377–386. doi: 10.1302/0301-620X.42B2.377. [DOI] [PubMed] [Google Scholar]
- Liu TK, Yang RS. Flexor tendon graft for late management of isolated rupture of the profundus tendon. J Trauma. 1997;43:103–106. doi: 10.1097/00005373-199707000-00024. [DOI] [PubMed] [Google Scholar]
- Wehbe MA, Mawr B, Hunter JM, Schneider LH, Goodwyn BL. Two-stage flexor-tendon reconstruction. Ten-year experience. J Bone Joint Surg Am. 1986;68:752–763. [PubMed] [Google Scholar]
- McClinton MA, Curtis RM, Wilgis EF. One hundred tendon grafts for isolated flexor digitorum profundus injuries. J Hand Surg Am. 1982;7:224–229. doi: 10.1016/s0363-5023(82)80170-6. [DOI] [PubMed] [Google Scholar]
- Seiler JG, Gelberman RH, Williams CS, Woo SL, Dickersin GR, Sofranko R, Chu CR, Rosenberg AE. Autogenous flexor-tendon grafts. A biomechanical and morphological study in dogs. J Bone Joint Surg Am. 1993;75:1004–1014. doi: 10.2106/00004623-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Chu CR, Williams CS, Seiler JG, Amiel D. Angiogenesis in healing autogenous flexor-tendon grafts. J Bone Joint Surg Am. 1992;74:1207–1216. [PubMed] [Google Scholar]
- Singer DI, Morrison WA, Gumley GJ, O'Brien BM, Mitchell GM, Barton RM, Frykman GK. Comparative study of vascularized and nonvascularized tendon grafts for reconstruction of flexor tendons in zone 2: an experimental study in primates. J Hand Surg Am. 1989;14:55–63. doi: 10.1016/0363-5023(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Eiken O, Lundborg G. Experimental tendon grafting within intact tendon sheath. Scand J Plast Reconstr Surg. 1983;17:127–131. doi: 10.3109/02844318309013107. [DOI] [PubMed] [Google Scholar]
- Kakar S, Khan U, McGrouther DA. Differential cellular response within the rabbit tendon unit following tendon injury. J Hand Surg Br. 1998;23:627–632. doi: 10.1016/s0266-7681(98)80017-x. [DOI] [PubMed] [Google Scholar]
- Richards HJ. Repair and healing of the divided digital flexor tendon. Injury. 1980;12:1–12. doi: 10.1016/0020-1383(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Bank RA, TeKoppele JM, Oostingh G, Hazleman BL, Riley GP. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1999;58:35–41. doi: 10.1136/ard.58.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53:359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagergren C, Lindholm A. Vascular distribution in the Achilles tendons: an angiographic and micro-angiographic study. Acta Chir Scand. 1959;116(suppl):491–495. [PubMed] [Google Scholar]
- Macnab I. Rotator cuff tendinitis. Ann R Coll Surg Engl. 1973;53:271–287. [PMC free article] [PubMed] [Google Scholar]
- Cofield RH. Rotator cuff disease of the shoulder. J Bone Joint Surg Am. 1985;67:974–979. [PubMed] [Google Scholar]
- Backman C, Boquist L, Friden J, Lorentzon R, Toolanen G. Chronic achilles paratenonitis with tendinosis: an experimental model in the rabbit. J Orthop Res. 1990;8:541–547. doi: 10.1002/jor.1100080410. [DOI] [PubMed] [Google Scholar]
- Backman C, Friden J, Widmark A. Blood flow in chronic Achilles tendinosis. Radioactive microsphere study in rabbits. Acta Orthop Scand. 1991;62:386–387. doi: 10.3109/17453679108994477. [DOI] [PubMed] [Google Scholar]
- Khan KM, Bonar F, Desmond PM, Cook JL, Young DA, Visentini PJ, Fehrmann MW, Kiss ZS, O'Brien PA, Harcourt PR, Dowling RJ, O'Sullivan RM, Crichton KJ, Tress BM, Wark JD. Patellar tendinosis (jumper's knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology. 1996;200:821–827. doi: 10.1148/radiology.200.3.8756939. [DOI] [PubMed] [Google Scholar]
- Kannus P, Natri A. Etiology and pathophysiology of tendon ruptures in sports. Scand J Med Sci Sports. 1997;7:107–112. doi: 10.1111/j.1600-0838.1997.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Davidsson L, Salo M. Pathogenesis of subcutaneous tendon ruptures. Acta Chir Scand. 1969;135:209–212. [PubMed] [Google Scholar]
- Fox JM, Blazina ME, Jobe FW, Kerlan RK, Carter VS, Shields-CL J, Carlson GJ. Degeneration and rupture of the Achilles tendon. Clin Orthop. 1975;107:221–224. doi: 10.1097/00003086-197503000-00025. [DOI] [PubMed] [Google Scholar]
- Gibson W. Are 'spontaneous' Achilles tendon ruptures truly spontaneous? [letter]. Br J Sports Med. 1998;32:266. [PubMed] [Google Scholar]
- Williams JG. Achilles tendon lesions in sport. Sports Med. 1986;3:114–135. doi: 10.2165/00007256-198603020-00003. [DOI] [PubMed] [Google Scholar]
- Barfred T. Achilles tendon rupture. Aetiology and pathogenesis of subcutaneous rupture assessed on the basis of the literature and rupture experiments on rats. Acta Orthop Scand. 1973;152(suppl):3–126. [PubMed] [Google Scholar]
- Barfred T. Histology of the rat Achilles tendon before and after tendon rupture. Acta Pathol Microbiol Scand A. 1971;79:287–292. doi: 10.1111/j.1699-0463.1971.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Kalebo P, Goksor LA, Sward L, Peterson L. Soft-tissue radiography, computed tomography, and ultrasonography of partial Achilles tendon ruptures. Acta Radiol. 1990;31:565–570. [PubMed] [Google Scholar]
- Arner O, Lindholm A. Subcutaneous rupture of the Achilles tendon: a study of 92 cases. Acta Chir Scand. 1959;239(suppl):1–51. [PubMed] [Google Scholar]
- Bidder M, Towler DA, Gelberman RH, Boyer MI. Expression of mRNA for vascular endothelial growth factor at the repair site of healing canine flexor tendon. J Orthop Res. 2000;18:247–252. doi: 10.1002/jor.1100180212. [DOI] [PubMed] [Google Scholar]
- Kuroda R, Kurosaka M, Yoshiya S, Mizuno K. Localization of growth factors in the reconstructed anterior cruciate ligament: immunohistological study in dogs. Knee Surg Sports Traumatol Arthrosc. 2000;8:120–126. doi: 10.1007/s001670050198. [DOI] [PubMed] [Google Scholar]
- Chang J, Most D, Stelnicki E, Siebert JW, Longaker MT, Hui K, Lineaweaver WC. Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: evidence for dual mechanisms of repair. Plast Reconstr Surg. 1997;100:937–944. doi: 10.1097/00006534-199709001-00016. [DOI] [PubMed] [Google Scholar]
- Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lin-eaweaver WC. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. J Hand Surg Am. 1998;23:1052–1058. doi: 10.1016/S0363-5023(98)80015-4. [DOI] [PubMed] [Google Scholar]
- Duffy-FJ Jr, Seiler JG, Gelberman RH, Hergrueter CA. Growth factors and canine flexor tendon healing: initial studies in uninjured and repair models. J Hand Surg Am. 1995;20:645–649. doi: 10.1016/S0363-5023(05)80284-9. [DOI] [PubMed] [Google Scholar]
- Kuroda R, Kurosaka M, Yoshiya S, Mizuno K. Localization of growth factors in the reconstructed anterior cruciate ligament: immunohistological study in dogs. Knee Surg Sports Traumatol Arthrosc. 2000;8:120–126. doi: 10.1007/s001670050198. [DOI] [PubMed] [Google Scholar]
- Harwood L, Goomer RS, Gelberman RH, Silva MJ, Amiel D. Regulation of αvβ5 and α5β1 integrin receptors by basic fibro-blast growth factor and platelet-derived growth factor-BB in intrasynovial flexor tendon cells. Wound Repair Regen. 1999;7:381–388. doi: 10.1046/j.1524-475X.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Harwood FL, Akeson WH, Amiel D. Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments. Iowa Orthop J. 1998;18:19–25. [PMC free article] [PubMed] [Google Scholar]
- Panossian V, Liu SH, Lane JM, Finerman GA. Fibroblast growth factor and epidermal growth factor receptors in ligament healing. Clin Orthop. 1997;342:173–180. [PubMed] [Google Scholar]
- Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, Hughes SP, Polak JM. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147–153. doi: 10.1302/0301-620x.79b1.6814. [DOI] [PubMed] [Google Scholar]
- Birch HL, Rutter GA, Goodship AE. Oxidative energy metabolism in equine tendon cells. Res Vet Sci. 1997;62:93–97. doi: 10.1016/s0034-5288(97)90127-2. [DOI] [PubMed] [Google Scholar]
- Stone KR, Bowman HF, Boland A, Steadman JR. Ligament and tendon oxygenation measurements using polargraphic oxygen sensors. Arthroscopy. 1987;3:187–195. doi: 10.1016/s0749-8063(87)80064-6. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Agur AMR, Lee MJ. In Grant's Atlas of Anatomy. 10th. Philadelphia: Lippincott Williams & Wilkins;; 1999. The Upper Limb. pp. 414–517. [Google Scholar]