Abstract
Sorting endosomes and the endocytic recycling compartment are critical intracellular stores for the rapid recycling of internalized membrane receptors to the cell surface in multiple cell types. However, the molecular mechanisms distinguishing fast receptor recycling from sorting endosomes and slow receptor recycling from the endocytic recycling compartment remain poorly understood. We previously reported that Rab15 differentially regulates transferrin receptor trafficking through sorting endosomes and the endocytic recycling compartment, suggesting a role for distinct Rab15-effector interactions at these endocytic compartments. In this study, we identified the novel protein Rab15 effector protein (REP15) as a binding partner for Rab15-GTP. REP15 is compartment specific, colocalizing with Rab15 and Rab11 on the endocytic recycling compartment but not with Rab15, Rab4, or early endosome antigen 1 on sorting endosomes. REP15 interacts directly with Rab15-GTP but not with Rab5 or Rab11. Consistent with its localization, REP15 overexpression and small interfering RNA-mediated depletion inhibited transferrin receptor recycling from the endocytic recycling compartment, without affecting receptor entry into or recycling from sorting endosomes. Our data identify REP15 as a compartment-specific protein for receptor recycling from the endocytic recycling compartment, highlighting that the rapid and slow modes of transferrin receptor recycling are mechanistically distinct pathways.
INTRODUCTION
Early endosomes comprise two distinct compartments identified primarily through trafficking studies using the transferrin receptor (TfR). At steady state, internalized TfR resides on sorting endosomes (SEs) and the endocytic recycling compartment (ERC) (Maxfield and McGraw, 2004). In SEs, the TfR is sorted for direct recycling back to the plasma membrane or transported to lysosomes via late endosomes for down-regulation (Yamashiro et al., 1984; Yamashiro and Maxfield, 1987a,b; Schmid et al., 1988; Ghosh et al., 1994). A second slower route for receptor recycling occurs from the ERC, after receptor transit through SEs (Sheff et al., 1999; Maxfield and McGraw, 2004). An emerging model suggests that in addition to sorting desensitized receptors for down-regulation, SEs and the ERC provide a local reserve of intracellular receptors for rapid delivery to the cell surface (Szekeres et al., 1998; Lin et al., 2000; Park et al., 2004).
Rab GTPases are potent regulators of membrane trafficking events (reviewed in Somsel Rodman and Wandinger-Ness, 2000; Stein et al., 2003). They are small monomeric GTPases that cycle between a GTP-bound and GDP-bound state, which is regulated through molecular interactions with guanine nucleotide exchange factors and GTPase activating proteins (reviewed in Pfeffer and Aivazian, 2004). Furthermore, rabs are generally compartment specific, functioning through effector molecules to regulate vesicle fusion, vesicle budding, receptor sorting, and cytoskeletal interactions (Somsel Rodman and Wandinger-Ness, 2000; Segev, 2001). Distinct endocytic rabs regulate the rapid and slow modes of TfR recycling from SEs and the ERC, respectively. Rab4 preferentially regulates rapid receptor recycling from the SEs (van der Sluijs et al., 1992), although some Rab4 is also associated with the ERC (Sheff et al., 1999; de Renzis et al., 2002). Conversely, Rab11 has been shown to regulate protein transport from the ERC to the trans-Golgi network and the plasma membrane. Moreover, these transport pathways are differentially affected in cells expressing Rab11 mutants with altered guanine nucleotide binding activities (Ullrich et al., 1996; Ren et al., 1998; Wilcke et al., 2000), suggesting that numerous transport pathways could originate from the ERC.
We previously reported that Rab15 is a novel endocytic GTPase that colocalizes with Rab4, Rab5, and the TfR on SEs as well as Rab11 on the ERC (Zuk and Elferink, 1999, 2000), suggesting that Rab15 may link these distinct endosomal compartments. Consistent with this tenet, overexpression of constitutively inactive Rab15 mutants differentially regulated transport through SEs and the ERC. Expression of the GDP-bound mutant Rab15-T22N promoted the recycling of the TfR from both SEs and the ERC. Conversely, overexpression of the guanine nucleotide free mutant Rab15-N121I increased the indirect or slower mode of TfR recycling from the ERC, without affecting rapid recycling from SEs (Zuk and Elferink, 2000). However, the mechanistic differences controlling Rab15-mediated receptor trafficking through SEs and the ERC remain poorly understood.
Rabs function via specific effectors to regulate distinct membrane transport steps (Somsel Rodman and Wandinger-Ness, 2000; Pfeffer, 2001; Deneka et al., 2003). Thus, the differential effects of Rab15 mutants on TfR transit through SEs and the ERC could involve unique sets of Rab15–effector interactions or an interacting protein with highly specialized properties. In this study, we identified Rab15 effector protein (REP15) as a novel binding partner for Rab15-GTP. When overexpressed in HeLa cells, REP15 did not localize to SEs; rather, REP15 colocalized with a subset of Rab15 and Rab11 on the ERC. Moreover, REP15 did not interact directly with Rab5 or Rab11, consistent with a role for REP15 as a compartment specific effector for Rab15. Consistent with its subcellular localization, overexpression and small interfering RNA (siRNA)-mediated depletion of REP15 attenuated the recycling of internalized TfR from the ERC without affecting receptor trafficking through SEs or TfR entry into the ERC. Together, our data identify REP15 as a novel component for receptor recycling from the ERC, further highlighting that SEs and the ERC are mechanistically distinct endosomal compartments.
MATERIALS AND METHODS
Reagents and Plasmids
Cell culture and general reagents were obtained from Invitrogen (Carlsbad, CA), Fisher Scientific (Hampton, NH), and Sigma-Aldrich (St. Louis, MO) unless specified otherwise. Restriction enzymes were purchased from New England Biolabs (Beverly, MA). Plasmids encoding amino-terminal hemag-glutinin (HA)-tagged wild-type and mutant Rab15 in pCI-Neo and pLexA, and a HeLa cell library pretransformed into EGY187 (Mat α) have been described previously (Strick et al., 2002). Plasmids encoding the Rab11 mutants Q70L and S25N, and green fluorescent protein (GFP)-labeled Rab7 were generous gifts from R. Prekeris (University of Colorado Health Sciences Center, Denver, CO) and A. Wandinger-Ness (University of New Mexico, Albuquerque, NM), respectively. For yeast two-hybrid analysis, cDNAs encoding Rab11-Q70L and Rab11-S25N were amplified by PCR using specific primer sets described previously and subcloned directly into the EcoR1 site of pLexA. Human REP15 lacking an amino-terminal tag was PCR amplified (upper primer, 5′-GAAATGGGGCAGAAAGCATCGCAA-3′; lower primer, 5′-GGCTCTAGATCAGAGAATGCTGATATAAAC-3′) and subcloned directly into pCR3.1 (Invitrogen). Human REP15 containing an amino-terminal cMyc (MEQKLISEEDL) or HA (MYPYDVPDYA) epitope were PCR amplified using the lower primer described above in combination with the appropriate upper primer (cMyc: 5′-GCTGTAGAATGGAACAAAAATTAATCTCAGAAGAAGATCTGGGGCAGAAAGCATCGCAAC-3′ or HA (5′-GATATCATGTACCCTTATGATGTGCCAG-3′), and the resulting PCR products were subcloned into pCR3.1 (Invitrogen).
Antibodies
HA monoclonal antibody (mAb) 12CA5 (Roche Diagnostics, Indianapolis, IN), monoclonal human TfR H68.4, polyclonal Rab11 antibody (Zymed Laboratories, South San Francisco, CA), cMyc monoclonal 9B11 antibody (Cell Signaling Technology, Beverly, MA), early endosome antigen 1 (EEA1) mAb (BD Transduction Laboratories, San Diego, CA), and heat shock cytosolic 70 protein (Hsc70) polyclonal antibody (Stressgen Biotechnologies, Victoria, British Columbia, Canada) were purchased from sources as indicated. Alexa594-labeled transferrin (Tfn), goat anti-mouse and anti-rabbit secondary antibodies coupled to Alexa488 or Alexa594 were purchased from Invitrogen. A Rab4 polyclonal antibody was a generous gift from P. van der Sluijs (University Medical Center Utrecht, The Netherlands). A Syntaxin 13 polyclonal antibody was a gift from R. Prekeris (University of Colorado Health Sciences Center). An anti-glutathione S-transferase (GST) polyclonal antibody was generous gift from Peter McPherson (McGill University, Montreal, Quebec, Canada). Rabbit polyclonal antiserum for Rab15 was generated against the synthetic peptide (NH2-CQAHRKELDGLRTC-COOH) (Covance, Denver, PA). To prepare an antibody against REP15, human REP15 (residues 3–236) was cloned directly into pGEX-4T (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) expressed as a GST-fusion and purified by affinity chromatography using glutathione agarose beads (Sigma-Aldrich). When thrombin cleaved, recombinant REP15 devoid of GST was insoluble; therefore, insoluble REP15 was used as an antigen to prepare a rabbit polyclonal antisera (Covance). The resulting REP15 anti-sera was purified by initial passage over GST immobilized on AminoLink coupling gel, and the flow through was subsequently purified against GST-REP15 immobilized to AminoLink coupling gel (Pierce Chemical, Rockville, IL).
Confocal Microscopy and Colocalization Studies
For confocal analysis, HeLa cells were seeded onto Matrigel (BD Biosciences, San Jose, CA)-coated coverglasses (Fisher Scientific, Pittsburgh, PA) and transfected with the appropriate plasmid DNA for 48 h. Cells were fixed in 4% paraformaldehyde (Ted Pella, Redding, CA) in phosphate-buffered saline (PBS) for 10 min, blocked and permeabilized with 10% goat sera (Hyclone Laboratories, Logan, UT), 0.02% saponin, and 1% bovine serum albumin (BSA) in PBS, and then incubated with the appropriate antibody in PBS supplemented with 1% BSA, 0.02% saponin overnight at 4°C. Cells were washed three times with PBS and incubated with the appropriate secondary antibody coupled to Alexa488 or Alexa594 in PBS containing 1% BSA, 0.02% saponin for 1 h at room temperature. The coverglasses were mounted onto glass slides using FluorSave reagent (Calbiochem, San Diego, CA). Images were obtained using a BX50 epifluorescent microscope (Olympus, Tokyo, Japan) equipped with a Plan-Apo 100×/1.35 oil immersion objective and argon and krypton lasers with excitations at 488 and 568 nm, respectively. Images were then processed using Fluoview 2.0 imaging software (Olympus). To avoid unbiased selection, the two channels were imaged separately and not merged until acquisition was complete. Before acquisition, PMT and the Laser power adjustments were optimized for each channel to avoid saturation of a particular channel. Images were processed using Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA), saved as TIFF files, then imported into MetaMorph 4.6 (Molecular Devices, Sunnyvale, CA) and analyzed using the Measure Colocalization Application as follows. Individual cells were selected using the region of interest selection function in MetaMorph 4.6, and the threshold values were empirically determined to ensure that background signals were not contributing to the quantification. Values were calculated as the percentage of pixels for signal A colocalizing with the percentage of pixels for signal B, and then normalized to the total area of a given cell. The final values represent the mean ± SE for six to 10 randomly selected cells from three independent experiments.
Cell Culture, Transfections, TfR Trafficking Assays, and Organelle Immunoisolations
All cell culture, transfections, uptake studies, and organelle immunoisolations have been described previously (Zuk and Elferink, 1999, 2000; Strick et al., 2002). Membrane fractions enriched in early or late endosomes were prepared by sucrose flotation gradient fractionation as described previously (Prekeris et al., 1998; Zuk and Elferink, 1999, 2000; Yan et al., 2004). Crude membrane fractions enriched in early, recycling, and late endosomes were prepared as described previously (Bache et al., 2003). Cell surface biotinylation assays for measuring internalized TfR were performed as described previously (Schmidt et al., 1997). Data points were collected in triplicate and were expressed as the ratio of internalized TfR versus surface TfR. Internalization rate constants for TfR were determined as described previously (Li et al., 2005), and the kinetic parameters were obtained by fitting the data with a linear regression over time. siRNA depletion experiments were performed using commercial control (D-001210-01-2) and REP15 (M-030132-00) siRNAs (Dharmacon, Lafayette, CO) and transfected into cells using LipofectAMINE 2000 reagent (Invitrogen).
Biotinylated Transferrin Enzyme-linked Immunosorbent Assays (ELISAs)
Tfn-depleted cells were incubated in DMEM with 5 μg/ml biotinylated Tfn (B-Tfn; Pierce Chemical) in DMEM containing 2 mg/ml BSA for 1 h at 16°C to load B-Tfn into SEs. Noninternalized ligand was removed by three alternating washes with ice-cold PBS, pH 4.2, and PBS (2 mg/ml BSA). The cells were chased at 37°C for 0–10 min in DMEM with a 100-fold excess of unlabeled Tfn, to promote ligand recycling from the SEs. In studies measuring Tfn recycling from the ERC, the cells were subjected to a second 10 min chase at 37°C followed washes with PBS, pH 4.2, and PBS containing 2 mg/ml BSA, to deplete the cell of SE-associated B-Tfn and to promote ligand transport to the ERC. One set of cells was incubated at 4°C and represented the amount of B-Tfn internalized into the ERC. The cells were then incubated at 37°C for increasing time (10–60 min) to promote recycling from the ERC to the cell surface. The amount of B-Tfn recycled into the chase media was quantified by ELISA, using anti-Tfn antibody-coated plates according to manufacturer's instructions (Bethyl Laboratories, Montgomery, TX). Bound B-Tfn was detected using 0.1 μg/ml streptavidin-horseradish peroxidase followed by QuantaBlue fluorogenic peroxidase substrate (Pierce Chemical) and then measured in a Spectromax Gemini fluorescent microplate reader (Molecular Devices) and expressed as relative fluorescent units (Rfu) ± SE per microgram of protein.
RESULTS
REP15, a Novel Effector for Rab15
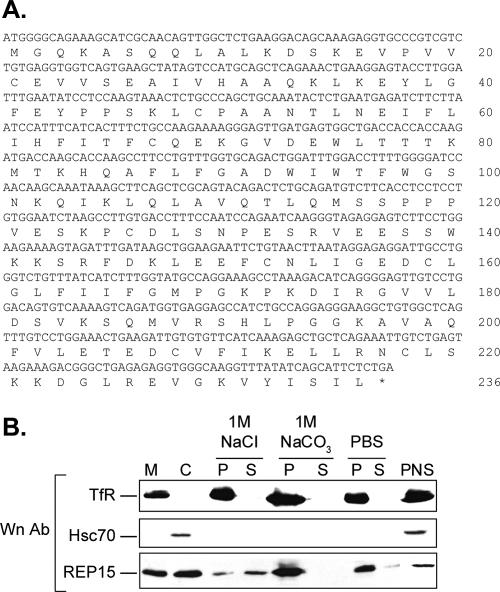
We previously reported that Rab15 mutants differentially regulated TfR trafficking through SEs and the ERC (Zuk and Elferink, 1999, 2000), raising the possibility that Rab15 function could be regulated in part via interactions with compartment-specific effectors. We recently identified mammalian suppressor of Sec4 (Mss4) as a binding partner for Rab15 in a yeast two-hybrid screen using a HeLa cell cDNA library. Moreover, interactions between Rab15 and Mss4 modulated the inhibitory effect of Rab15 on TfR trafficking in HeLa cells and homotypic SE fusion in vitro (Strick et al., 2002). To identify additional effectors for Rab15, we screened the yeast two-hybrid HeLa cell cDNA library using the GTP-bound mutant Rab15-Q67L as bait. Of the six clones isolated, DNA sequence analysis confirmed that four of the clones encoded novel proteins of unknown function that will be characterized and reported elsewhere. However, two clones encoded an identical open reading frame of 233 amino acids and hence were analyzed further. BLAST searches against the human and mouse genomes revealed that the open reading frame shared 100 and 73.8% identity, respectively, with amino acids 3–236 of a hypothetical protein of unknown function (accession nos. XP370686 and NP079896, respectively) (Figure 1A). Subsequent analysis using the SMART database detected no putative functional or membrane domains and revealed no similarity with other Rab effectors identified to date. Accordingly, we named the predicted protein Rab15 effector protein or REP15 (GenBank accession no. AY662682). Subsequent reverse transcription-PCR analysis confirmed that the mRNAs for REP15 and Rab15 are coexpressed in HeLa cells (Figure S1A).
Figure 1.
REP15 binds to membranes. (A) The cDNA and predicted amino acid sequence for human REP15 are shown. (B) Post-nuclear supernatants (PNS) prepared from HeLa cells transiently overexpressing HA-tagged REP15 were fractionated into high-speed membrane (M) and cytosol (C) fractions and analyzed by Western analysis (Wn) for TfR, Hsc70, and HA-tagged REP15 (REP15). Membrane pellets were washed with 1 M NaCl, 1 M NaCO3, pH 11.0, or PBS, and the washed membranes (P) and the supernatants (S) were examined by Western analysis.
As a potential binding partner for Rab15, we predicted that REP15 would also enrich in membrane fractions. Because REP15 did not contain apparent transmembrane or lipid modification motifs for membrane association, we examined the putative membrane binding properties of REP15 using biochemical criteria. A postnuclear supernatant was prepared from HeLa cells transiently expressing HA-tagged REP15, fractionated into membrane and cytosolic fractions by centrifugation and examined by Western analysis (Figure 1B). When overexpressed in HeLa cells, REP15 distributes with the TfR, a marker for endocytic membranes and with Hsc70, a cytosolic marker, consistent with our previous report on Rab15 (Zuk and Elferink, 2000). Subsequent biochemical analysis of the membrane fraction revealed that REP15 was partially extracted from salt-washed membranes (NaCl and PBS) but not from membranes after a high pH wash (Figure 1B). These data suggest that the membrane binding properties of REP15 likely involve protein–protein interactions rather than binding via neutral phospholipids.
REP15 Specifically Binds to Rab15-GTP
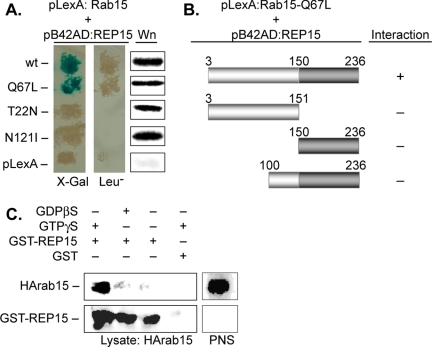
We next examined the guanine nucleotide dependence of REP15 binding to Rab15 using LacZ and Leu2 as reporter genes in a yeast two-hybrid binding assay. High levels of β-galactosidase activity were detected in strains coexpressing REP15 with GTP-bound forms of Rab15 (wild type or the GTP-bound mutant Q67L) but not the GDP-bound (T22N) or nucleotide-free (N121I) mutants of Rab15 (Figure 2A and Table 1). Western analysis confirmed that comparable levels of Rab15 were expressed in the diploid strains, indicating that the observed differences in binding were not due to altered levels of wild-type or mutant Rab15 (Figure 2A). Similarly, no interaction was observed with REP15 and wild-type or mutant Rab5, a GTPase associated with SEs, confirming the specificity of the results (Table 1).
Figure 2.
REP15 binding to Rab15 is guanine nucleotide dependent. (A) Yeast strains expressing REP15 fused to the activation domain of B42 (pB42AD:REP15) were mated with strains expressing wild type (wt) or the indicated mutants of Rab15 fused to LexA (pLexA:Rab15), and the resulting diploids were examined for protein interactions. Blue colonies on 5-bromo-4-chloro-3-indolyl-β-d-galactoside or growth on Leu-plates indicate specific interactions between REP15 and Rab15. Western analysis (Wn) confirmed comparable levels of Rab15 expression in all diploid strains. (B) The indicated amino- and carboxy-terminal truncations of REP15 were fused to the activation domain of B42, mated with a yeast strain expressing Rab15-Q67L, and the resulting diploids were examined for the presence (+) or absence (-) of protein interactions using a yeast two-hybrid approach as described above. (C) A postnuclear supernatant (PNS) prepared from HeLa cells expressing wild-type HA-tagged Rab15 (HArab15) was incubated in the absence (-) or presence (+) of GTPγS or GDPβS before incubation with GST-REP15. Complexes were isolated and examined by Western analysis using antibodies for HARab15 and GST-REP15.
Table 1.
REP15 interacts with GTP-bound Rab15 but not Rab5 or Rab11. β-Galactosidase activity in Rfu was determined in yeast diploid strains coexpressing the indicated bait constructs in pLexA and prey constructs in pB42AD
| pLexA Construct | pB42AD Construct | Rfu | Interaction |
|---|---|---|---|
| Rab15 WT | REP15 (3-236) | 121,884.6 ± 6679.9 | + |
| Rab15 Q67L | REP15 (3-236) | 113,834.0 ± 6361.9 | + |
| Rab15 T22N | REP15 (3-236) | 8629.2 ± 180.7 | – |
| Rab15 N1211 | REP15 (3-236) | 17,743.8 ± 2410.8 | – |
| Rab15 Q67L | REP15 (3-151) | 20,699.7 ± 342.9 | – |
| Rab15 Q67L | REP15 (151-236) | 12,720.6 ± 164.4 | – |
| Rab15 Q67L | REP15 (100-236) | ND | – |
| Rab5 WT | REP15 (3-236) | 4072.0 ± 623.7 | – |
| Rab5 Q79L | REP15 (3-236) | 3224.0 ± 1015.2 | – |
| Rab5 S34N | REP15 (3-236) | 501.7 ± 500.2 | – |
| Rab5 N1331 | REP15 (3-236) | 9503.7 ± 1333.2 | – |
| Rab11 Q70L | REP15 (3-236) | 26,407.3 ± 1193.1 | – |
| Rab11 S25N | REP15 (3-236) | 1825.3 ± 563.6 | – |
ND, not determined.
Values represent the means ± SE of three independent studies. Statistically significant interactions (+) between proteins are indicated (p < 0.001; analysis of variance [ANOVA]).
To identify the Rab15 binding domain(s) in REP15, we prepared different amino- and carboxy-terminal deletion mutants of REP15 and examined their ability to interact with Rab15-Q67L in a yeast two-hybrid binding assay. No interaction was observed in diploid strains coexpressing comparable levels of Rab15-Q67L and REP15 lacking the amino terminal (residues 151–236) or carboxy-terminal (residues 3–151) domains (Figure 2B and Table 1). Similarly, no interaction was detected between Rab15-Q67L and a REP15 mutant lacking 100 amino acids from the amino terminus. Thus, Rab15-GTP likely interacts through multiple sites of REP15 or via a highly ordered structural motif requiring full-length REP15.
We verified the guanine nucleotide dependence of Rab15 binding to REP15 by performing pull-down studies. We previously reported that endogenous Rab15 was not readily detected in baby hamster kidney, Chinese hamster ovary (CHO), Trvb-1, or HeLa cells using our existing Rab15 antibodies (Zuk and Elferink, 1999; Strick et al., 2002). Thus, we performed binding studies using cell lysates prepared from HeLa cells transiently overexpressing wild-type, HA-tagged Rab15 (HArab15) and full-length REP15 expressed as a recombinant GST-fusion protein. Before incubation with recombinant REP15, cell lysates were then incubated in the absence or presence of guanosine 5′-O-(2-thio)diphosphate (GDPβS) or the nonhydrolysable analog GTPγS, to lock wild type Rab15 into a GDP or GTP-bound state respectively. The lysates were incubated with GST-REP15 immobilized on glutathione agarose beads and the resulting REP15-HArab15 complexes isolated and examined by Western analysis (Figure 2C). REP15 bound efficiently to wild type Rab15 preloaded with guanosine 5′-O-(3-thio)triphosphate. Conversely, no REP15 binding was detected with wild-type Rab15 in the presence or absence of GDPβS, or beads containing GST only. Thus, consistent with our yeast two-hybrid data, REP15 preferentially interacts with GTP-bound Rab15.
REP15 Localizes with Rab15 on Endosomal Membranes
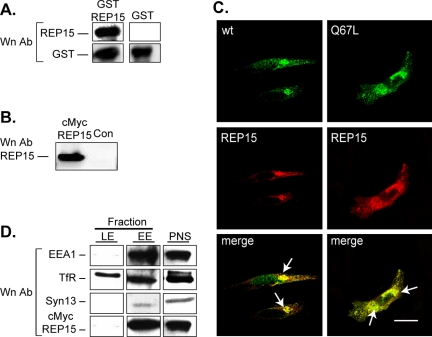
To determine the subcellular localization of REP15, we prepared a polyclonal antibody against recombinant REP15 and confirmed its specificity by Western analysis. As shown in Figure 3A, the anti-REP15 antibody reacted with GST-REP15, but not with GST only. Conversely, Western analysis using the REP15 antibody detected overexpressed cMycREP15, but not endogenous REP15 protein in HeLa cell lysates (Figure 3B) under these conditions. Similarly, endogenous REP15 expression in untransfected or mock-transfected HeLa cells was not readily detected using the REP15 polyclonal antibody in immunofluorescence microscopy studies (our unpublished data).
Figure 3.
REP15 colocalizes with Rab15-GTP on early endosomal membranes. (A) Equal amounts of REP15 expressed as a fusion protein with GST (GST-REP15) or GST only were examined by Western analysis using a REP15 polyclonal antibody. (B) Lysates prepared from control untransfected HeLa cells or cells transiently expressing cMycREP15 were examined by Western analysis using an REP15 polyclonal antibody. (C) Representative examples of HeLa cells transiently coexpressing REP15 with wild-type (wt) or GTP-bound (Q67L) Rab15 were analyzed by confocal microscopy. (D) Fractions enriched in late endosomes (LE) and early endosomes (EE) were prepared by sucrose flotation gradient centrifugation, trichloroacetic acid precipitated, and analyzed by Western analysis for EEA1, TfR, cMycREP15, and Syntaxin 13 (Syn13) immunoreactivity as indicated.
To confirm that REP15 is endogenously expressed in HeLa cells, a crude membrane fraction enriched in SEs and the ERC was prepared from untransfected HeLa cells and cells overexpressing cMycREP15. Before fractionation, the cell lysates were incubated with 1 mM GTPγS for 30 min at 4°C, to lock endogenous Rabs in their GTP bound state, because REP15 preferentially binds to GTP-bound Rab15. Under these conditions, Western analysis of the endosomal membrane fractions detected low levels of REP15 in untransfected control HeLa cells (Figure S1B). Conversely, high levels of REP15 and cMyc immunoreactivity were detected in fractions prepared from cells overexpressing cMyc-tagged REP15. TfR was readily detected in endosomal membrane fractions from control and REP15 overexpressing cells, consistent with the presence of SEs and the ERC in these fractions. These data indicate that endogenous REP15 is likely expressed at a low level in HeLa cells.
Therefore, to examine the subcellular localization of REP15, it was necessary to perform confocal microscopy using HeLa cells transiently cotransfected with REP15 and wild-type Rab15 or Rab15-Q67L. Punctate Rab15 staining was observed in the periphery of the cytoplasm as well as the perinuclear region of the cell, consistent with the distribution of Rab15 between SEs and the ERC, respectively. Conversely, strong REP15 staining was detected principally in the perinuclear region of transfected cells coinciding partially with Rab15 staining (Figure 3C). Quantitative analysis of the confocal micrographs revealed that 63 ± 3.4 and 70 ± 4.4% of REP15 colocalized with wild-type Rab15 and Rab15-Q67L, respectively. Comparable patterns of colocalization with Rab15 were also detected in HeLa cells coexpressing REP15 tagged at the amino terminus with a cMyc epitope (cMycREP15). In these cells, cMycREP15 preferentially colocalized with wild-type Rab15 and Rab15-Q67L in the perinuclear region (54 ± 5.2 and 56 ± 4.4%, respectively (Figure S2). Thus REP15 preferentially colocalizes with a perinuclear pool of Rab15 in HeLa cells. Moreover, the presence of an amino terminal cMyc epitope does not interfere with the perinuclear localization of overexpressed REP15.
To confirm the endosomal location of REP15, membrane fractions enriched in early endosomal membranes (both SEs and the ERC) or late endosomes were prepared by sucrose flotation gradients from HeLa cells transiently expressing cMycREP15, and they were examined by Western analysis. REP15 was detected in early endosomal membranes enriched in EEA1, the TfR, and Syntaxin 13, a t-SNARE that resides on SE membrane (Figure 3D) (Trischler et al., 1999). Conversely, REP15 immunoreactivity was not detected with down-regulated TfR in late endosomal membrane fractions. Thus, REP15 localizes to early endosomal membranes, consistent with a role as a Rab15 binding protein.
REP15 Localizes to the ERC
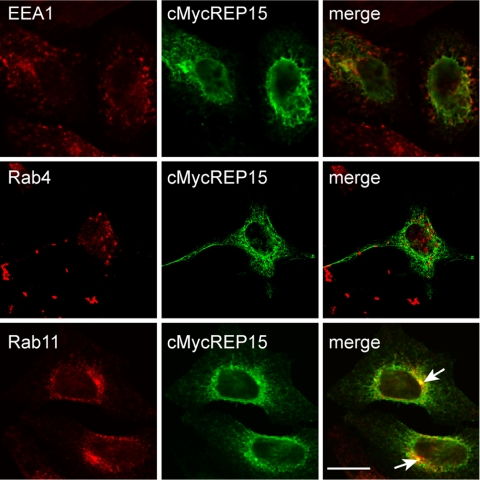
We previously reported that Rab15 localized to both peripheral SEs as well as the ERC (Zuk and Elferink, 1999), where it functioned to differentially regulate TfR transport (Zuk and Elferink, 2000). Our studies showing that REP15 colocalized with Rab15 in a perinuclear distribution in HeLa cells suggested that REP15 might be a compartment specific effector for Rab15 at the ERC. To address this issue, we examined the localization of transiently expressed cMycREP15 on early endosomal membranes using confocal microscopy. No significant overlap in cMycREP15 staining was observed with the SE-specific markers EEA1 or Rab4 (Figure 4) or the late endosomal marker Rab7 (our unpublished data) (Barbieri et al., 1994; Bottger et al., 1996; Rybin et al., 1996; Simonsen et al., 1998; Trischler et al., 1999). Similarly, cMycREP15 staining did not overlap with Calnexin or mannosidase II, markers for the endoplasmic reticulum and the cis/medial-Golgi, respectively (Lippincott-Schwartz et al., 1991; Wada et al., 1991) (our unpublished data). Conversely, cMycREP15 staining overlapped with endogenous Rab11, an established marker for the ERC (Ullrich et al., 1996) (Figure 4). Quantitative analysis of the confocal micrographs showed that 56 ± 7.1% of cMycREP15 overlapped with endogenous Rab11 in the perinuclear region of the cell.
Figure 4.
REP15 associates with the ERC and not SEs. Representative examples of HeLa cells transiently expressing cMycREP15 were stained with antibodies against endogenous EEA1 and Rab4 (markers for SEs) or the ERC specific marker Rab11. Arrows indicate areas of colocalization (yellow) in the merged (merge) image. Scale, 10 μm.
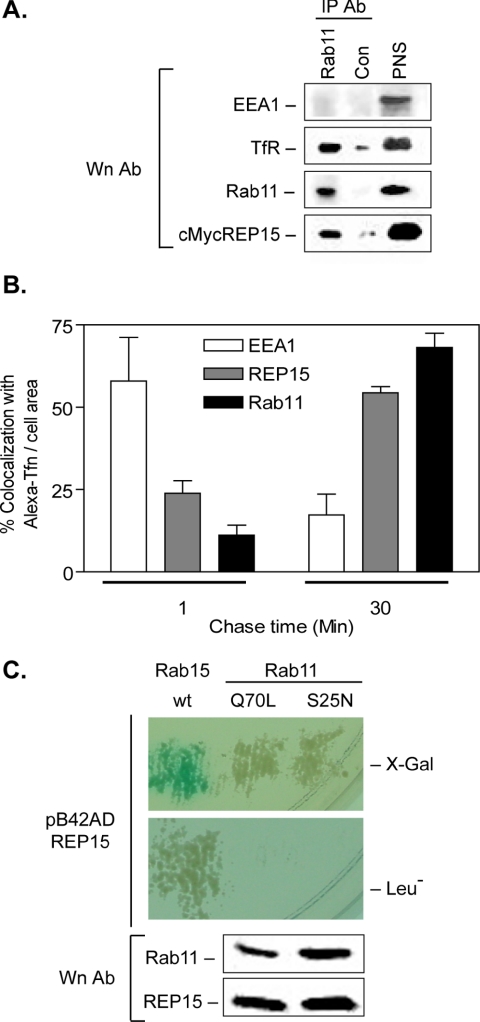
To determine whether the perinuclear distribution of REP15 corresponded to the ERC, we performed organelle immunoprecipitation studies. Postnuclear supernatants were prepared from HeLa cells transiently expressing cMycREP15, and immunoprecipitated under nondenaturing conditions using an antibody against the ERC marker Rab11. The resulting immune complexes were examined by Western analysis. REP15 coprecipitated with Rab11 and the TfR but not with the early endosomal marker EEA1 (Figure 5A). To verify the association of REP15 with the ERC, we examined the colocalization of REP15 with internalized Tfn under chase conditions that promoted Tfn transport from SEs to the ERC. Duplicate sets of HeLa cells stably expressing REP15 were allowed to internalize Alexa594-labeled transferrin (Alexa-Tfn) at 16°C for 1 h, conditions that specifically load Alexa-Tfn into the SEs and decrease transport to subsequent endosomal compartments, including the ERC (Ren et al., 1998; Zuk and Elferink, 2000). Control studies confirmed that the intracellular distribution of REP15 in cells loaded at 16°C was indistinguishable from that detected in cells loaded at 37°C, indicating that incubation at 16°C does not alter the expression and/or targeting of REP15 (our unpublished data). After internalization, the cells were washed and then chased at 37°C for 1 or 30 min in media containing unlabeled Tfn to promote Alexa-Tfn recycling from SEs and transport to the ERC. The cells were then fixed, costained for cMycREP15 with endogenous EEA1 or Rab11, and examined by confocal microscopy (Figure 5B). After a 1-min chase, high amounts of Alexa-Tfn colocalized with EEA1 in SEs (57.9 ± 13.6%). The lower levels of ligand colocalizing with Rab11 and REP15 (11.1 ± 3.0 and 23.8 ± 3.8%, respectively), likely represent an incomplete block in TfR transport from SEs to the ERC in HeLa cells under these conditions. Conversely, a 30-min chase at 37°C resulted in decreased colocalization of Alexa-Tfn with EEA1, and a concomitant increase in Alexa-Tfn costaining with REP15 and Rab11 (54.4 ± 1.8 and 68.1 ± 4.3%, respectively), consistent with ligand transport to the ERC. The increased colocalization of REP15 with internalized Alexa-Tfn at longer chase periods, confirms that REP15 associates with the ERC rather than SEs.
Figure 5.
REP15 localizes to the Rab11-positive ERC. (A) A PNS prepared from HeLa cells transiently expressing cMycREP15 was immunoprecipitated (IP) under nondenaturing conditions with antibodies (antibody) against Rab11 or by using a control IgG antisera and examined by Western analysis (Wn) using antibodies for EEA1, TfR, Rab11, and cMyc. (B) HeLa cells overexpressing REP15 were allowed to internalize Alexa-Tfn at 16°C for 1 h to load SEs and then chased for the indicated times to promote ligand transport from the SEs to the ERC. Cells were fixed, stained for REP15, endogenous EEA1 or Rab11, and the amount of internalized Alexa-Tfn colocalizing with each of these proteins was quantified by confocal microscopy. Values are expressed as the percentage of colocalization ± SE normalized to area from three independent studies (p < 0.001; ANOVA). (C) Diploid strains coexpressing REP15 fused to the activation domain of B42 (pB42AD:REP15) and wild-type Rab15, Rab11-Q70L, or Rab11-S25N were examined for protein interactions as described in the legend to Figure 2A. No interaction was observed between REP15 and the indicated Rab11 mutants. Wn confirmed comparable levels of Rab11 and REP15 expression in all diploid strains.
The majority of Rab-GTP effectors identified to date bind specifically to their cognate Rab. However, some Rab effectors have been shown to be multivalent, interacting with Rabs involved in sequential transport steps. For example, Rabaptin-5 and Rabenosyn-5 interact with GTP-bound Rabs 4 and 5 on SEs (Vitale et al., 1998; de Renzis et al., 2002). The Rab11 effector FIP2 binds Rabs 11 and 8, possibly linking membrane transport between the ERC and the cis-Golgi (Hattula and Peranen, 2000; Lindsay and McCaffrey, 2002). Because REP15 colocalized with Rab11 and Rab15 on the ERC, we used a yeast two-hybrid binding assay to determine whether REP15 is a shared effector for these endosomal Rabs. No direct interaction was observed between REP15 and constitutively active, GTP-bound Rab11 (Q70L) or the GDP-bound mutant Rab11-S25N (Figure 5C and Table 1). Western analysis confirmed that the absence of binding was not due to differences in the expression levels of REP15 or the Rab11 mutants (Figure 5C). These data indicate that REP15 colocalizes with Rab11 and Rab15 on the ERC and not on SEs.
Effects of REP15 Overexpression on TfR Trafficking
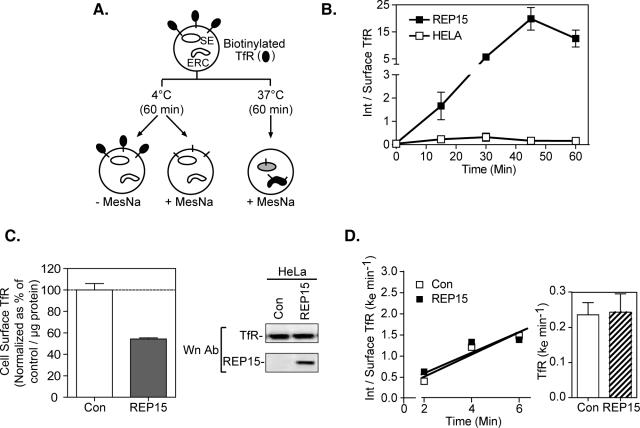
Since REP15 colocalizes with Rab15 and Rab11 on the ERC, we examined the effect of REP15 overexpression on TfR trafficking through this compartment. Cell surface proteins were biotinylated at 4°C with sulfo-NHS-SS-biotin, which is cleaved by washing the cells with cell-impermeable reducing agents such as sodium 2-mercaptoethanesulfonate (MesNa) (Schmidt et al., 1997) (Figure 6, A and B). After biotinylation, the cells were incubated at 37°C from 0 to 60 min to allow biotinylated proteins to endocytose and traffic to SEs and the ERC, respectively. Internalized TfR was distinguished from surface receptor by washing the cells at 4°C with MesNa. Biotinylated proteins were isolated from cell lysates using streptavidin-agarose, and the amount of surface and internalized TfR was quantified by Western analysis coupled with spot densitometry. The data were graphed as a ratio of internalized TfR versus surface TfR levels at any given time point. In control mock-transfected cells, the level of internalized TfR was maximal at 30 min, decreasing to lower levels at longer chase times (45 and 60 min), consistent with internalized TfR recycling back to the cell surface from peripheral SEs and the ERC (Figure 6B). Conversely, higher levels of internalized TfR were detected in cells stably expressing REP15, reaching maximal levels at 45–60 min. Therefore, overexpression of REP15 in HeLa cells regulates the trafficking of internalized TfR.
Figure 6.
Internalized TfR accumulates in cells overexpressing REP15. (A) Schematic diagram of the cell surface biotinylation assay for TfR internalization. (B) HeLa cells stably overexpressing cMycREP15 (REP15) or mock-transfected cells (Con) were surface biotinylated. The cells were then incubated at 4°C (-) to retain TfR on the cell surface or at 37°C for 0–60 min to allow TfR internalization. Endocytosis was stopped by rapid cooling to 4°C and noninternalized TfR was stripped of biotin (+) with MesNa washes (MesNa). Control plates were washed in washing buffer lacking MesNa (-) to measure total biotinylated TfR. Biotinylated proteins were isolated by Streptavidin pull-downs (PD) and analyzed by Western analysis using antibodies against TfR. Values represent the ratio of internalized TfR versus surface TfR ± SE three independent experiments. (C) Left, mock-transfected (Con) HeLa cells and HeLa cells overexpressing cMycREP15 were surface biotinylated at 4°C, surface TfR was isolated by streptavidin-agarose, and identified by Western analysis. Experimental values are normalized as a percentage of surface TfR levels in control cells and represent the mean ± SE of surface associated TfR from three independent assays. Right, Western analysis of equal amounts of protein confirmed negligible differences in the expression level of endogenous TfR in control (Con) and cMycREP15-expressing HeLa cells. (D) Surface biotinylated HeLa cells overexpressing cMycREP15 or mock-transfected cells (Con) were incubated at 37°C for 2, 4, or 6 min, and the amount of internalized TfR quantified by streptavidin-agarose pull-downs and Western analysis. The internalization rate constants were calculated as a linear regression coefficient and graphed. Ke values of 0.2630 ± 0.035 and 0.2435 ± 0.052 min-1 were determined for HeLa cells overexpressing cMycREP15 and mock-transfected (Con) cells, respectively.
Given the accumulation of TfR in REP15-expressing cells, we proposed that REP15 overexpression would result in reduced levels of TfR on the cell surface. To test this, intact mock-transfected HeLa cells and cells overexpressing REP15 were biotinylated with NHS-SS-biotin at 4°C for 30 min. Biotinylated cell surface proteins were isolated from cell lysates using streptavidin-agarose, and the level of biotinylated TfR was examined by Western analysis and spot densitometry. Under these conditions, we routinely detected a 50% reduction in the amount of biotinylated TfR on the surface of REP15-overexpressing cells relative to mock-transfected cells (Figure 6C). Western analysis of the cell lysates confirmed that the differences in surface levels of TfR were not a consequence of discernible differences in the expression level of the receptor (Figure 6C). Thus, the accumulation of internalized TfR in REP15 overexpressing cells corresponded to decreased cell surface levels of TfR.
Using surface biotinylation assays, we next examined whether REP15 overexpression altered the rate of TfR internalization from the cell surface. As shown in Figure 6D, the first order rate constants for TfR internalization were directly comparable in cells stably overexpressing REP15 and mock-transfected control cells, indicating that REP15 does not regulate the internalization of the TfR from the cell surface.
REP15 Regulates TfR Recycling from the ERC
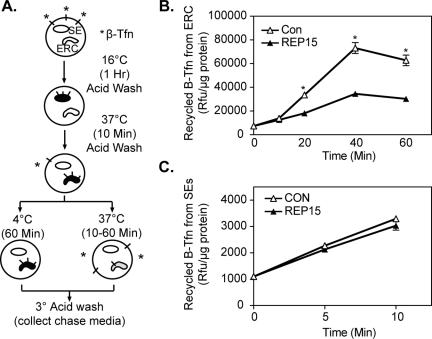
The observed decrease in cell surface TfR levels coupled with the intracellular retention of TfR in REP15-overexpressing cells, suggested a role for REP15 in receptor recycling. To test this directly, we developed an ELISA using B-Tfn to measure the recycling of internalized B-Tfn into the cell media. In these studies, mock-transfected HeLa cells and HeLa cells stably expressing REP15 were incubated with B-Tfn for 1 h at 16°C to load the SEs. The cells were shifted to 37°C in media containing a 100-fold excess of unlabeled Tfn for 10 min to promote B-Tfn recycling from SEs and to chase the B-Tfn into the ERC. The cells were then acid washed to remove any recycled B-Tfn that could associate with receptor on the cell surface. One set of cells was retained at 4°C as a measure of B-Tfn internalized into the ERC (Figure 7A). Under these conditions, comparable levels of B-Tfn were loaded into the ERC of control cells versus REP15-expressing HeLa cells (Figure S3). The acid washed cells were then chased for increasing times to promote B-Tfn recycling from the ERC. The medium was collected and the amount of recycled B-Tfn in the chase media measured by ELISA assay (see Materials and Methods). Under these conditions, increasing levels of recycled B-Tfn were detected in the media of control and REP15-expressing cells, reaching a maximum at 40 min (Figure 7B). However, we detected a 47–51% decrease in the relative amount of B-Tfn recycled into the chase media of REP15-expressing cells at 40 and 60 min, respectively, relative to control cells, consistent with a role for REP15 in TfR recycling.
Figure 7.
Overexpression of REP15 delayed recycling of B-Tfn from the ERC and not the SEs. (A) Schematic of the TfR recycling assay from the ERC. (B) HeLa cells stably expressing cMycREP15 (REP15) and mock-transfected cells (Con) were labeled with B-Tfn for 1 h at 16°C and then acid washed and chased at 37°C to label the ERC. After labeling the ERC, the cells were incubated at 37°C as indicated, and the amount of B-Tfn recycled into the chase media was measured using a B-Tfn ELISA. Values are expressed as the mean Rfu per microgram of protein ± SE of three independent assays. Significant differences (*p < 0.01; ANOVA) were observed between experimental and control conditions. (C) HeLa cells stably expressing REP15 (REP15) and mock-transfected cells (Con) were labeled with B-Tfn at 16°C for 1 h, washed, and chased for 5 and 10 min at 37°C in media containing unlabeled Tfn to promote B-Tfn recycling from the SEs. The amount of B-Tfn recycled into the chase media from SEs was quantified using a B-Tfn ELISA as described above.
We verified that REP15 overexpression decreased TfR recycling by examining recycling of the TfR from the ERC in transiently transfected cells using a cell surface biotinylation assay. In biotinylated, mock-transfected control cells, the amount of internalized TfR decreased during successive recycling incubations (Figure S4, A and B), consistent with efficient receptor recycling from the ERC to the cell surface. Conversely, the level of TfR in the ERC was unaltered in cells transiently overexpressing REP15, consistent with a block in receptor recycling. Therefore, our ligand and receptor recycling studies indicate that REP15 overexpression results in decreased TfR recycling from the ERC.
We next verified that the effect of REP15 overexpression was specific for TfR recycling from the ERC and not fast TfR recycling from the SE. In these studies, cells were labeled with B-Tfn at 16°C for 1 h to load B-Tfn into the SEs, acid washed to remove surface-bound B-Tfn, and then chased for 5 and 10 min at 37°C in media containing unlabeled Tfn. The amount of B-Tfn recycled into the media from the SEs was quantified using a B-Tfn ELISA. Comparable levels of B-Tfn were detected in the chase media from REP15-expressing and untransfected control HeLa cells, confirming that REP15 does not regulate B-Tfn recycling from the SEs (Figure 7C).
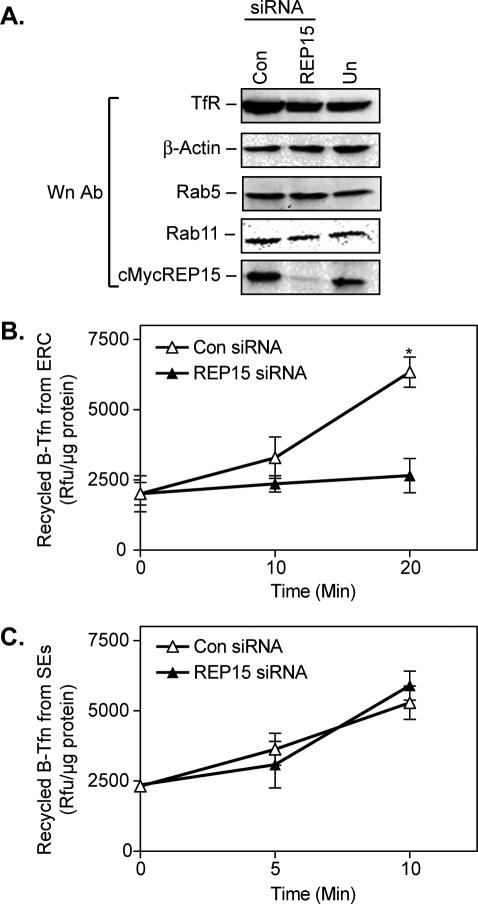
To confirm the effect of REP15 on TfR recycling from the ERC, we used siRNAs to deplete HeLa cells of endogenous REP15. Given the difficulties detecting endogenous REP15 using our REP15 antibody (Figures 3 and S2), the specificity of the REP15 siRNA was verified using HeLa cells stably expressing cMycREP15. Western analysis confirmed that transfection with REP15 siRNA depleted 80–90% of cMycREP15, with no effect on the level of endogenous TfR, β-actin, EEA1, and the endocytic GTPases Rab11 and Rab5. Comparable levels of REP15, TfR, β-Actin, EEA1, Rab11, and Rab5 were detected in untransfected cells and cells transfected with a control siRNA, confirming the specificity of the REP15 siRNA (Figure 8A). We next examined the effect of depleting endogenous REP15 on TfR recycling from the ERC. HeLa cells were transfected with a control siRNA or REP15 siRNA. Seventy-two hours post-transfection, the cells were incubated with B-Tfn for 1 h at 16°C to load the SEs, washed, and chased for 20 min to promote recycling from the SEs and ligand transport to the ERCs. The cells were then washed again and incubated for 10 or 20 min at 37°C to promote recycling from the ERC. The relative amount of recycled B-Tfn in the media was measured using a B-Tfn ELISA (see Materials and Methods). B-Tfn recycling from the ERC in REP15-depleted cells was blocked after a 20-min chase, consistent with our REP15 overexpression studies (Figure 6B). Conversely, B-Tfn recycling from the ERC was unaffected in HeLa cells transfected with a control siRNA (Figure 8B). Additional recycling studies confirmed that siRNA-mediated depletion of REP15 had no effect on B-Tfn recycling from SEs (Figure 8C). Together, these data identify REP15 as a novel Rab15 effector important for TfR recycling from the ERC.
Figure 8.
REP15 depletion by siRNA specifically reduces B-Tfn recycling from the ERC. (A) HeLa cells stably expressing cMycREP15 were transfected with REP15 or control (Con) siRNA duplexes for 72 h and then examined by Western analysis as indicated. Untransfected (Un) cells stably expressing cMycREP15 were used as an additional control. (B) HeLa cells were transfected with control (Con) or REP15 siRNAs and used to measure B-Tfn recycling from the ERC using a B-Tfn ELISA. All values are the mean of triplicate values and are expressed as Rfu per microgram of protein. Statistical differences between experimental and control sets were observed (*p < 0.01; ANOVA). (C) Control and REP15 siRNAs were transfected into HeLa cells as described in B, and B-Tfn recycling from the SEs was measured using a B-Tfn ELISA. No difference in B-Tfn recycling from the SEs was detected after REP15 depletion.
DISCUSSION
In this study, we present functional evidence that REP15 is a novel protein important for TfR recycling from the ERC. When overexpressed in HeLa cells, REP15 is compartment specific, colocalizing with Rab15 and Rab11 on the ERC but not with Rab15, Rab4, or EEA1 on SEs. Consistent with its localization, siRNA-mediated depletion of REP15 resulted in retention of the TfR in the ERC, without affecting receptor transport to the ERC or fast receptor recycling from SEs. TfR recycling from the ERC was also defective in cells overexpressing wild-type REP15. Similar inhibitory phenotypes have been reported in overexpression and siRNA-mediated knockdown studies on other endocytic proteins. For example, overexpression and siRNA silencing of the EH domain (EHD) protein has also been reported to inhibit TfR endocytosis (Guilherme et al., 2004). Similarly, epidermal growth factor receptor down-regulation was disrupted in HeLa cells overexpressing full-length Hrs, and in cells transfected with siRNA specific for Hrs (Bache et al., 2003; Morino et al., 2004). Thus, the inhibition in TfR recycling detected in cells overexpressing REP15, could be due to the titration of partner proteins involved in this endocytic step. Our data showing that REP15 overexpression did not alter the delivery of internalized TfR to SEs, receptor access to the ERC, or the fast mode of TfR recycling from the SE further supports our contention that REP15 specifically regulates TfR recycling from the ERC.
Several lines of evidence indicate that REP15 is a binding partner for GTP-bound Rab15 at the ERC. First, REP15 interacts with Rab15 in a guanine nucleotide manner. Second, consistent with our localization data, REP15 did not interact with Rab5, an SE-specific GTPase (Barbieri et al., 1994; Rybin et al., 1996; Roberts et al., 1999). Moreover, despite their colocalization to the ERC, we could not detect an interaction between REP15 and Rab11 in a yeast two-hybrid binding assay. Finally, REP15 colocalized preferentially with a pool of Rab15 on the ERC of HeLa cells, rather than with Rab15 on EEA1-positive peripheral SEs. Although we cannot rule out the possibility that small amounts of REP15 transiently reside on SEs, REP15 overexpression and siRNA-mediated depletion did not affect TfR trafficking through SEs, suggesting that REP15 primarily associates with and regulates TfR recycling from the ERC.
How can we reconcile the inhibitory effect of REP15 overexpression and siRNA-depletion of REP15 on TfR recycling from the ERC with Rab15-GTP function? We previously reported that Rab15 distributes between SEs and the ERC in a variety of cell types and that it differentially regulates TfR transport through these endosomal compartments (Zuk and Elferink, 1999, 2000). In these studies, expression of the guanine nucleotide-deficient Rab15 mutant N121I, stimulated the slow recycling of TfR from the ERC, without affecting rapid receptor recycling from SEs. Conversely, overexpression of Rab15-GTP had no effect on TfR recycling from the SEs or the ERCs, indicating that Rab15-Q67L does not phenocopy the inhibitory effect of REP15 on TfR recycling. However, in these studies wild-type Rab15 and Rab15-Q67L expression inhibited TfR entry into SEs, primarily at the level of SE fusion (Zuk and Elferink, 2000; Strick et al., 2002). Thus, the inhibition in TfR trafficking through SEs caused by wild-type Rab15 and Rab15-Q67L would be expected to mask any potential effects of Rab15-GTP on trafficking events downstream of SEs, including TfR recycling from the RE. Thus, given the opposing effects of REP15 and the nucleotide deficient Rab15 mutant N121I expression on TfR recycling from the ERC, it seems likely that REP15 could functionally interact with Rab15 to regulate this transport step. The ultimate test for the involvement of this interaction would be to examine the effect of REP15 mutants deficient in Rab15 binding on TfR recycling. However, low transfection levels and toxic effects of existing truncated REP15 mutants were routinely observed in our experiments, thus limiting their use in these types of studies.
Our results demonstrating that overexpression of REP15 resulted in TfR accumulation in the ERC are similar to previous functional studies on Rab11 and receptor trafficking through this compartment. Overexpression of the GTP-bound mutant Rab11-Q70L causes accumulation of the β2-adrenergic receptor as well as the TfR in the ERC (Moore et al., 2004). Interestingly, Rab11-induced accumulation of the β2-adrenergic receptor was associated with impaired β2-adrenergic receptor trafficking to lysosomes. Similarly, interactions between Rab11 and the Rab coupling protein were recently reported to regulate TfR sorting from the degradative pathway to the slow recycling pathway via the ERC (Peden et al., 2004), suggesting a role for Rab11 and possibly the ERC in receptor sorting, rather than receptor recycling per se. In this context, the slow recycling pathway and the degradative pathway would be competitive, such that a reduction in receptor down-regulation could result in receptor accumulating in the ERC.
A key finding presented here is the identification of REP15 as a novel regulator of TfR recycling from the ERC. To date, Rabs 11, 15, and 25a represent the only endocytic GTPases with roles for regulating receptor transport through the ERC (Ullrich et al., 1996; Wang et al., 2000; Zuk and Elferink, 2000; Lindsay and McCaffrey, 2002). In contrast to Rab11 and Rab15, Rab25a is expressed specifically in polarized epithelial cells where it functions to regulate apical recycling and transcytosis (Goldenring et al., 1993; Wang et al., 2000). The precise role of Rab11 in receptor recycling is less clear, since its distribution varies between cell types (Ullrich et al., 1996; Green et al., 1997; Ren et al., 1998; Brown et al., 2000; van Ijzendoorn et al., 2003). In nonpolarized cells, Rab11 is associated with the ERC and plays a role in slow TfR recycling. Conversely in polarized Madin-Darby canine kidney (MDCK) cells, Rab11 has been shown to be a marker for apical recycling and does not seem to regulate TfR recycling from the ERC (Wang et al., 2000). Moreover, protein transport to the trans-Golgi network and recycling to the plasma membrane is affected in cells expressing Rab11 mutants (Ullrich et al., 1996; Ren et al., 1998; Wilcke et al., 2000), suggesting a role for this GTPase in multiple transport steps from the ERC. Thus, how could Rab15 and Rab11 function in nonpolarized cells to regulate TfR recycling from the ERC? Rab15 and REP15 could act in concert with Rab11 via yet unidentified partner proteins. In this model, our data showing that REP15 partially colocalizes with Rab15 and Rab11 on the ERC could denote a distinct sorting domain or sub-compartment within the ERC that regulates TfR availability for recycling. Alternatively, this region may correspond to specific domains or regions linking two distinct and differentially regulated recycling pathways in the ERC. Two kinetically distinct recycling routes leading from the ERC to the plasma membrane were recently characterized in CHO cells (Lampson et al., 2001). In these studies, the insulin-regulated amino peptidase is sorted from the TfR in the ERC and is returned to the plasma membrane via distinct transport vesicles, consistent with the existence of different recycling routes from the ERC. Similarly, Rab11 and Rab25a have been shown to regulate distinct recycling pathways through the apical recycling system in MDCK cells (Wang et al., 2000), supporting a multilevel organization for receptor recycling from the ERC in multiple cell types. Future experiments will be required to distinguish which recycling pathway from the ERC is regulated by REP15.
Our studies are consistent with a role for REP15 regulating the exit of the TfR from the ERC and not receptor delivery to this compartment. Overexpression of REP15 resulted in decreased amounts of TfR on the cell surface when measured using a cell surface biotinylation assay. However, comparable levels of internalized TfR were detected in control and cells stably expressing REP15 when the ERC of the cells were loaded at 16°C. Therefore, REP15 overexpressing cells recycled less TfR than control cells, yet with similar kinetics, consistent with a model in which REP15 functions to determine the size of the TfR recycling pool. In this context, REP15 could function to regulate either TfR sorting within the ERC or the generation/maturation of recycling vesicles emanating from the ERC. Notwithstanding, the above-mentioned results identifying REP15 as an ERC-specific regulator of TfR recycling make a significant step toward our understanding of the mechanistic components essential for receptor recycling from this unique endosomal compartment.
Supplementary Material
Acknowledgments
This work was supported in part by National Science Foundation Grant IBN-0343739 and National Institutes of Health Grant R21 CA112605 (to L.A.E.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0204) on September 29, 2005.
Abbreviations used: Alexa-Tfn, Alexa594-labeled transferrin; B-Tfn, biotinylated transferrin; EEA1, early endosome antigen 1; ERC, endocytic recycling compartment; Mss4, mammalian suppressor of Sec4; REP15, Rab15 effector protein 15; SE, sorting endosome; Tfn, transferrin; TfR, transferrin receptor.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bache, K. G., Raiborg, C., Mehlum, A., and Stenmark, H. (2003). STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 278, 12513-12521. [DOI] [PubMed] [Google Scholar]
- Barbieri, M. A., Li, G., Colombo, M. I., and Stahl, P. D. (1994). Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J. Biol. Chem. 269, 18720-18722. [PubMed] [Google Scholar]
- Bottger, G., Nagelkerken, B., and van der Sluijs, P. (1996). Rab4 and Rab7 define distinct nonoverlapping endosomal compartments. J. Biol. Chem. 271, 29191-29197. [DOI] [PubMed] [Google Scholar]
- Brown, P. S., Wang, E., Aroeti, B., Chapin, S. J., Mostov, K. E., and Dunn, K. W. (2000). Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic 1, 124-140. [DOI] [PubMed] [Google Scholar]
- de Renzis, S., Sonnichsen, B., and Zerial, M. (2002). Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4, 124-133. [DOI] [PubMed] [Google Scholar]
- Deneka, M., Neeft, M., and van der Sluijs, P. (2003). Regulation of membrane transport by rab GTPases. Crit. Rev. Biochem. Mol. Biol. 38, 121-142. [DOI] [PubMed] [Google Scholar]
- Ghosh, R. N., Gelman, D. L., and Maxfield, F. R. (1994). Quantification of low density lipoprotein and transferrin endocytic sorting HEp2 cells using confocal microscopy. J. Cell Sci. 107, 2177-2189. [DOI] [PubMed] [Google Scholar]
- Goldenring, J. R., Shen, K. R., Vaughan, H. D., and Modlin, I. M. (1993). Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J. Biol. Chem. 268, 18419-18422. [PubMed] [Google Scholar]
- Green, E. G., Ramm, E., Riley, N. M., Spiro, D. J., Goldenring, J. R., and Wessling-Resnick, M. (1997). Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem. Biophys. Res. Commun. 239, 612-616. [DOI] [PubMed] [Google Scholar]
- Guilherme, A., Soriano, N. A., Bose, S., Holik, J., Bose, A., Pomerleau, D. P., Furcinitti, P., Leszyk, J., Corvera, S., and Czech, M. P. (2004). EHD2 and the novel EH domain binding protein EHBP1 couple endocytosis to the actin cytoskeleton. J. Biol. Chem. 279, 10593-10605. [DOI] [PubMed] [Google Scholar]
- Hattula, K., and Peranen, J. (2000). FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr. Biol. 10, 1603-1606. [DOI] [PubMed] [Google Scholar]
- Lampson, M. A., Schmoranzer, J., Zeigerer, A., Simon, S. M., and McGraw, T. E. (2001). Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles. Mol. Biol. Cell 12, 3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N., Xiang, G. S., Dokainish, H., Ireton, K., and Elferink, L. A. (2005). The listeria protein internalin B mimics hepatocyte growth factor-induced receptor trafficking. Traffic 6, 459-473. [DOI] [PubMed] [Google Scholar]
- Lin, J. W., Ju, W., Foster, K., Lee, S. H., Ahmadian, G., Wyszynski, M., Wang, Y. T., and Sheng, M. (2000). Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat. Neurosci. 3, 1282-1290. [DOI] [PubMed] [Google Scholar]
- Lindsay, A. J., and McCaffrey, M. W. (2002). Rab11-FIP2 functions in transferrin recycling and associates with endosomal membranes via its COOH-terminal domain. J. Biol. Chem. 277, 27193-27199. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Glickman, J., Donaldson, J. G., Robbins, J., Kreis, T. E., Seamon, K. B., Sheetz, M. P., and Klausner, R. D. (1991). Forskolin inhibits and reverses the effects of brefeldin A on Golgi morphology by a cAMP-independent mechanism. J. Cell Biol. 112, 567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield, F. R., and McGraw, T. E. (2004). Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5, 121-132. [DOI] [PubMed] [Google Scholar]
- Moore, R. H., Millman, E. E., Alpizar-Foster, E., Dai, W., and Knoll, B. J. (2004). Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. J. Cell Sci. 117, 3107-3117. [DOI] [PubMed] [Google Scholar]
- Morino, C., Kato, M., Yamamoto, A., Mizuno, E., Hayakawa, A., Komada, M., and Kitamura, N. (2004). A role for Hrs in endosomal sorting of ligand-stimulated and unstimulated epidermal growth factor receptor. Exp. Cell Res. 297, 380-391. [DOI] [PubMed] [Google Scholar]
- Park, M., Penick, E. C., Edwards, J. G., Kauer, J. A., and Ehlers, M. D. (2004). Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972-1975. [DOI] [PubMed] [Google Scholar]
- Peden, A. A., Schonteich, E., Chun, J., Junutula, J. R., Scheller, R. H., and Prekeris, R. (2004). The RCP-Rab11 complex regulates endocytic protein sorting. Mol. Biol. Cell 15, 3530-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S., and Aivazian, D. (2004). Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell. Biol. 5, 886-896. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487-491. [DOI] [PubMed] [Google Scholar]
- Prekeris, R., Klumperman, J., Chen, Y. A., and Scheller, R. H. (1998). Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 143, 957-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, M., Xu, G., Zeng, J., De Lemos-Chiarandini, C., Adesnik, M., and Sabatini, D. D. (1998). Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 95, 6187-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R. L., Barbieri, M. A., Pryse, K. M., Chua, M., Morisaki, J. H., and Stahl, P. D. (1999). Endosome fusion in living cells overexpressing GFP-rab5. J. Cell Sci. 112, 3667-3675. [DOI] [PubMed] [Google Scholar]
- Rybin, V., Ullrich, O., Rubino, M., Alexandrov, K., Simon, I., Seabra, M. C., Goody, R., and Zerial, M. (1996). GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature 383, 266-269. [DOI] [PubMed] [Google Scholar]
- Schmid, S. L., Fuchs, R., Male, P., and Mellman, I. (1988). Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell 52, 73-83. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., Hannah, M. J., and Huttner, W. B. (1997). Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J. Cell Biol. 137, 445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev, N. (2001). Ypt/Rab GTPases: regulators of protein trafficking. Sci. STKE 2001, RE11. [DOI] [PubMed] [Google Scholar]
- Sheff, D. R., Daro, E. A., Hull, M., and Mellman, I. (1999). The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., Lippe, R., Christoforidis, S., Gaullier, J. M., Brech, A., Callaghan, J., Toh, B. H., Murphy, C., Zerial, M., and Stenmark, H. (1998). EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494-498. [DOI] [PubMed] [Google Scholar]
- Somsel Rodman, J., and Wandinger-Ness, A. (2000). Rab GTPases coordinate endocytosis. J. Cell Sci. 113, 183-192. [DOI] [PubMed] [Google Scholar]
- Stein, M. P., Dong, J., and Wandinger-Ness, A. (2003). Rab proteins and endocytic trafficking: potential targets for therapeutic intervention. Adv. Drug. Deliv. Rev. 55, 1421-1437. [DOI] [PubMed] [Google Scholar]
- Strick, D. J., Francescutti, D. M., Zhao, Y., and Elferink, L. A. (2002). Mammalian suppressor of Sec4 modulates the inhibitory effect of Rab15 during early endocytosis. J. Biol. Chem. 277, 32722-32729. [DOI] [PubMed] [Google Scholar]
- Szekeres, P. G., Koenig, J. A., and Edwardson, J. M. (1998). Involvement of receptor cycling and receptor reserve in resensitization of muscarinic responses in SH-SY5Y human neuroblastoma cells. J. Neurochem. 70, 1694-1703. [DOI] [PubMed] [Google Scholar]
- Trischler, M., Stoorvogel, W., and Ullrich, O. (1999). Biochemical analysis of distinct Rab5- and Rab11-positive endosomes along the transferrin pathway. J. Cell Sci. 112, 4773-4783. [DOI] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbe, S., Zerial, M., and Parton, R. G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs, P., Hull, M., Webster, P., Male, P., Goud, B., and Mellman, I. (1992). The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70, 729-740. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn, S. C., Mostov, K. E., and Hoekstra, D. (2003). Role of Rab proteins in epithelial membrane traffic. Int. Rev. Cytol. 232, 59-88. [DOI] [PubMed] [Google Scholar]
- Vitale, G., Rybin, V., Christoforidis, S., Thornqvist, P., McCaffrey, M., Stenmark, H., and Zerial, M. (1998). Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 17, 1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, I., Rindress, D., Cameron, P. H., Ou, W. J., Doherty, J. J., 2nd, Louvard, D., Bell, A. W., Dignard, D., Thomas, D. Y., and Bergeron, J. J. (1991). SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 266, 19599-19610. [PubMed] [Google Scholar]
- Wang, X., Kumar, R., Navarre, J., Casanova, J. E., and Goldenring, J. R. (2000). Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 275, 29138-29146. [DOI] [PubMed] [Google Scholar]
- Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B., and Salamero, J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151, 1207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro, D. J., and Maxfield, F. R. (1987a). Acidification of morphologically distinct endosomes in mutant and wild-type CHO cells. J. Cell Biol. 105, 2723-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro, D. J., and Maxfield, F. R. (1987b). Kinetics of endosome acidification in mutant and wild-type CHO cells. J. Cell Biol. 105, 2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro, D. J., Tycko, B., Fluss, S. R., and Maxfield, F. R. (1984). Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell 37, 789-800. [DOI] [PubMed] [Google Scholar]
- Yan, Q., Sun, W., McNew, J. A., Vida, T. A., and Bean, A. J. (2004). Ca2+ and N-ethylmaleimide-sensitive factor differentially regulate disassembly of SNARE complexes on early endosomes. J. Biol. Chem. 279, 18270-18276. [DOI] [PubMed] [Google Scholar]
- Zuk, P. A., and Elferink, L. A. (1999). Rab15 mediates an early endocytic event in CHO cells. J. Biol. Chem. 274, 22303-22312. [DOI] [PubMed] [Google Scholar]
- Zuk, P. A., and Elferink, L. A. (2000). Rab15 differentially regulates early endocytic trafficking. J. Biol. Chem. 275, 26754-26764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.