Abstract
Rcd1, initially identified as a factor essential for the commitment to nitrogen starvation-invoked differentiation in fission yeast, is one of the most conserved proteins found across eukaryotes, and its mammalian homolog is expressed in a variety of differentiating tissues. Here we show that mammalian Rcd1 is a novel transcriptional cofactor and is critically involved in the commitment step in the retinoic acid-induced differentiation of F9 mouse teratocarcinoma cells, at least in part, via forming complexes with retinoic acid receptor and activation transcription factor-2 (ATF-2). In addition, antisense oligonucleotide treatment of embryonic mouse lung explants suggests that Rcd1 also plays a role in retinoic acid-controlled lung development.
Keywords: development/differentiation/F9/retinoic acid/transcription
Introduction
Differentiation is a fundamental attribute of the cells of multicellular organisms which is required for the formation of their bodies. Such an attribute, however, is not specific to the cells of multicellular organisms, and the cells of many unicellular organisms including yeast differentiate in order to survive hostile environments. From a regulatory point of view, the process of cell differentiation conceptually could be composed of two steps: the commitment to differentiation and the subsequent expression of genes that determine the phenotype of differentiated cells. The control of commitment is crucial for the timing of differentiation, whereas the control of subsequent gene expression is crucial for the determination of the particular differentiated phenotype. Our recent studies have suggested that the mechanism and the factors involved therein to control a cell’s commitment to differentiation may not be very cell type specific and even less organism specific (Yamamoto et al., 1999).
The fission yeast Schizosaccharomyces pombe resembles higher eukaryotes in terms of gene structure, cell cycle control and a variety of other cellular processes, and undergoes a differentiation called sexual development consisting of conjugation, meiosis and sporulation. In this organism, the commitment to sexual development is controlled mainly by two external signals, nutrient starvation and mating pheromone availability, and is executed by Ste11, a transcriptional factor with an HMG box that activates a set of genes required for conjugation and meiosis (Kelly et al., 1988; Watanabe et al., 1988; Hughes et al., 1990; Sugimoto et al., 1991; Willer et al., 1995). Several distinct signal cascades control the commitment process by regulating the expression and action of the ste11+ gene or the mating pheromone signal cascades. The cAMP–Pka1 cascade mediates mainly glucose signals and inhibits the onset of differentiation by repressing the ste11+ gene (Watanabe and Yamamoto, 1996). Recently, our laboratory identified five factors controlling this commitment process. A factor with a helix–loop–helix structure that has a limited homology to MyoD promotes differentiation by modulating sensitivity to cAMP (Benton et al., 1993). The stress mitogen-activated protein (MAP) kinase encoded by phh1+/sty1+/spc1+ (Kato et al., 1996) is required for nutrient starvation-invoked ste11+ induction via activation of activation transcription factor 1 (Atf1) (Takeda et al., 1995). Rcd1, a novel factor highly conserved among eukaryotes, is required for nitrogen starvation-invoked, but not carbon starvation-invoked, ste11+ induction (Okazaki et al., 1998). On the other hand, Nrd1, an RNA-binding protein, represses a subset of ste11+-regulated genes until cells reach a critical level of nutrient starvation (Tsukahara et al., 1998). Pas1-associated Pef1, comprising a cyclin and its partner kinase, promotes the onset of the cell cycle and additionally represses the onset of differentiation by inhibiting the mating pheromone signaling (Tanaka and Okayama, 2000).
Interestingly, mammalian cells contain the structural and/or functional homologs of many of these factors. Tcf-1/Lef-1 is a Ste11-like factor essential for the terminal differentiation of T cells (Seidensticker and Behrens, 2000). The cAMP–Pka1 cascade is well known to regulate differentiation negatively in hematopoietic cells (Halvorson and Cligan 1995). MyoD promotes myogenic differentiation (Cossu and Borello, 1999). The activity of p38, a homolog of the Phh1/Sty1/Spc1 stress MAP kinase, critically influences hematopoietic cell differentiation (Nagata et al., 1998). Rod1, isolated on the basis of functional suppression of the lethal haploid meiosis of a thermolabile pat1 mutant, is a mammalian counterpart of Nrd1, and its overexpression blocks differentiation of the K562 human leukemia cell, irrespective of the type of inducers used, just as anticipated from the function of its yeast counterpart (Yamamoto et al., 1999). Activation transcription factor 2 (ATF-2), the closest homolog of fission yeast Atf1, contains an intrinsic histone acetylase activity (Kawasaki et al., 2000) and regulates genes including those encoding tumor necrosis factor-α, transforming growth factor-β, cyclin A, E-selectin, DNA polymerase β and c-Jun, some of which are critically involved in cell differentiation (Min and Pober, 1997; Jain et al., 1999; Beier et al., 2000). Rcd1 is highly conserved among eukaryotes, and at least budding yeast, nematodes, fruit flies and mammals contain its homologs with >70% amino acid identity, though their function is not well known (Okazaki et al., 1998; Gregory et al., 2000). The budding yeast homolog was identified as being a component of the CCR4–NOT complex that is evolutionarily conserved up to mammals (Chen et al., 2001) and is involved in the deadenylation of mRNA as well as the regulation of TFIID activity (Chen et al., 2002).
F9 mouse teratocarcinoma cells differentiate to visceral endoderm cells upon treatment with retinoic acid (RA). Visceral endoderm cells are of an early embryonic cell type and often form embryoid bodies (EBs), which are considered to be reminiscent of early embryogenesis with the ordered appearance of primitive endoderm and their differentiated derivatives (Strickland and Mahdavi, 1978; Hogan and Taylor, 1981; Lake et al., 2000). These cells synthesize α-fetoprotein (AFP) typically produced in fetal and neonatal liver. One of the earliest differentiation marker genes responding to RA is c-jun, which is thought to play a critical role in RA-induced F9 cell differentiation because its constitutive expression induces differentiation. The c-jun promoter contains the sequence designated as the differentiation response element (DRE). This element is both necessary and sufficient for the induction of c-jun during RA-invoked F9 differentiation, and is recognized by the DRF complex, which reportedly is composed at least of p300/CBP and ATF-2, the latter as its DNA-binding subunit (Kitabayashi et al., 1992, 1995; Kawasaki et al., 1998; Ugai et al., 1999).
Mouse lung development initiates on day 9.5 post-coitum (p.c.) with bud formation from the laryngotracheal groove, and involves branching morphogenesis. The pseudoglandular stage (days 9.5–16.6 p.c.) in this process is characterized by the formation of bronchial and bronchiolar trees, which are lined with undifferentiated epithelial cells juxtaposed to splanchnic mesoderm. By day 12 p.c., branching of bronchial trees gives rise to the left lobe and the four right lobes of the lung. Branching morphogenesis during this stage involves mesenchymal– epithelial cell interactions including paracrine growth factor stimulation that induces cellular proliferation, migration and differentiation, with activation of lung-specific genes (Wellington et al., 1996; Costa et al., 2001). RA is known to play two distinct roles in this process. RA signaling is required for early bud formation, yet it inhibits subsequent branching morphogenesis (Malpel et al., 2000). At the onset of branching morphogenesis, RA is degraded and its receptor becomes downregulated. This decline in RA signaling triggers the expression of Fgf-10, a key molecule that induces branching morphogenesis (Bellusci et al., 1997).
To examine whether the functional similarity between yeast and mammalian differentiation factors can be extended further to Rcd1, we recently investigated the role of mammalian Rcd1 in F9 differentiation and embryonic mouse lung development, both of which are regulated by RA. We found that Rcd1 is critically involved in these differentiation processes. Here we present evidence showing that the mammalian counterpart of Rcd1, initially discovered as a factor essential for yeast differentiation, is a novel transcriptional cofactor and plays a critical role in RA-regulated cell differentiation and development.
Results
Rcd1 is expressed in actively differentiating tissues
Taking into consideration the role of Rcd1 in fission yeast and its striking amino acid conservation, we tentatively hypothesized that mammalian Rcd1 would also be involved in cell differentiation and first investigated whether or not Rcd1 expression was closely associated with differentiating tissues. To this end, polyclonal antibodies were raised against the N- and C-terminal sequences of human Rcd1. The high sensitivity and specificity of the antibodies, particularly that which was raised against the C-terminal sequence (αRcdC), were confirmed by detection of a GST–Rcd1 fusion protein produced in Escherichia coli. αRcdC showed virtually no cross-reaction with E.coli proteins, yet reacted highly with the fusion protein and its presumed in vivo degradation products.
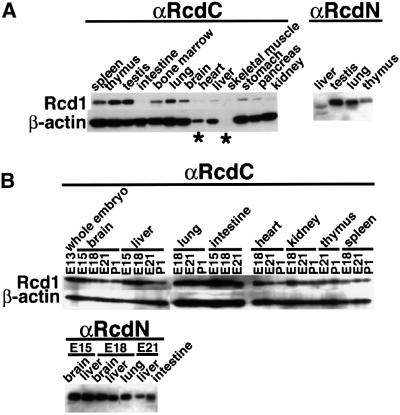
Using this antibody, we first analyzed the tissue-specific expression of Rcd1 in adult rat (5 weeks old) and rat embryo. Cell lysates were prepared from various tissues and examined by western blotting. Rcd1 was detected as a single band in various tissues, which was confirmed by detection of the same band with the antibody raised against the N-terminal sequence (αRcdN) (Figure 1A). Rcd1 was expressed most abundantly in the testis, thymus, spleen and lung of adult rat. The bone marrow, brain and stomach contained a medium level of Rcd1, whereas intestine, heart, skeletal muscle, liver, pancreas and kidney had a barely detectable level. On the other hand, Rcd1 was expressed in virtually all tissues of early rat embryo, but was particularly abundant in brain, liver, lung, intestine and heart (Figure 1B). However, its level in these tissues decreased during the later stages of embryogenesis, which was confirmed by detection with αRcdN. These results show that Rcd1 is expressed abundantly in many actively developing organs.
Fig. 1. Expression of Rcd1 in adult and embryonic rat organs. (A) Rcd1 expression in various rat organs at 5 weeks. Cell lysates (5 µg protein for αRcdC and 50 µg protein for αRcdN) prepared from various tissues were separated by 10% SDS–PAGE, and Rcd1 was detected by western blotting with αRcdC in the left panel and αRcdN in the right panel. As loading controls, β-actin was detected by re-immunoblotting of the same membrane filters as used for Rcd1 detection. The asterisks indicate the tissues expressing muscle-type (non-β) actin abundantly. (B) Rcd1 expression in various organs of rat embryo. Lysates (10 µg protein for αRcdC and 100 µg for αRcdN) prepared from various tissues were separated by 10% SDS–PAGE, and Rcd1 was detected by western blotting with αRcdC in the left panel and αRcdN in the right panel. The same membrane filters were re-probed for β-actin as loading controls.
Rcd1 is induced during in vitro differentiation in some cell lines
To investigate further a possible involvement of Rcd1 in cell differentiation, we next examined the level of Rcd1 in various differentiation-inducible hematopoietic and embryonal carcinoma cell lines. The cell lines examined include Jurkat, Daudi, K562, HL-60, U937, P19 and F9 cells (Yamamoto et al., 1999). Jurkat is a human acute lymphoblastic leukemia cell line and differentiates into interleukin-2 (IL-2)-producing T cell-like cells by co-stimulation with 12-O-tetradecanoylphorbol-13-acetate (TPA) and phytohemagglutinin (PHA). Daudi, a Burkitt lymphoma-derived human lymphoblastoid cell line, differentiates into immunoglobulin-producing cells. K562, a human pluripotent hematopoietic cell line, differentiates into megakaryocytes in response to TPA. HL-60, a human promyelocytic leukemia cell line, differentiates into granulocytes upon treatment with RA. U937, a human histiocytic lymphoma cell line, differentiates into macrophages upon TPA treatment. The mouse teratocarcinoma cell lines P19 and F9 differentiate into nervous tissue and primitive endoderm, respectively, upon treatment with RA (Hogan and Taylor, 1981; Jones-Villeneuve et al., 1982; Edwards and McBurney, 1983). Rcd1 was expressed in these blood cell and embryonal carcinoma cell lines, either constitutively or transiently with early induction and/or later repression during differentiation induction (data not shown). We were particularly interested in the transient induction of Rcd1 in F9 cells and chose this embryonal carcinoma cell line to investigate the biological role of Rcd1 in depth.
The earliest responding differentiation marker gene in F9 cells so far known is c-jun, and the induction of c-jun is thought to play an important role in RA-induced F9 cell differentiation because constitutive expression of this gene invokes differentiation of F9 cells (Kitabayashi et al., 1992, 1995; Kawasaki et al., 1998; Ugai et al., 1999). Accordingly, if Rcd1 were involved in the commitment to differentiation, its function would have to be executed at the same time as, or earlier than, the production of c-Jun. Rcd1 appears to fulfill this criterion. As shown in Figure 2A, Rcd1 was induced at almost the same time as c-Jun upon stimulation with RA in F9 cells, at least partly via transcriptional activation (Figure 2B).
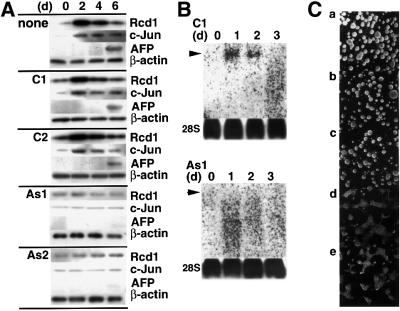
Fig. 2. RCD1 antisense oligonucleotides block RA-induced differentiation of F9 cells. (A) The effect of RCD1 antisense oligonucleotides on the level of Rcd1 in RA-treated F9 cells. F9 cells were treated with 50 nM RA in the absence (none) or presence of 1 µM antisense oligonucleotide 1 (As1) (CAAAAAAAGTGGGATGTGTGCTGC), As2 (TCCATGATGCGCAGACACAG), control oligonucleotide 1 (C1) (AACAAAGTGGGTGGATTGCATGAC) or C2 (AGACACATGGCTATCCACGG). Cells were harvested every other day and lysed. Lysates (2 µg protein for each lane) were separated by SDS–PAGE, and immunoblotted with αRcdC, anti-c-Jun or anti-AFP antibody. β-actin protein was detected as a loading control by re-immunoblotting of the same membrane filters. (B) Northern blot analysis of RCD1 mRNA in F9 cells treated with the antisense oligonucleotide As1 or the control oligonucleotide C1. F9 cells were treated with RA in the presence of 1 µM C1 or As1 for 1–3 days. Total RNA was extracted from the cells at each time point, treated with formaldehyde, separated by agarose gel electrophoresis and hybridized with an RCD1 probe (upper panel) or a 28S RNA control probe (lower panel). Arrows indicate the position of RCD1 mRNA. (C) Blocking of the RA-induced formation of EBs by RCD1 antisense oligonucleotides. F9 cells were incubated for 10 days with 50 nM RA in the absence (a) or presence of C1 (b), C2 (c), As1 (d) or As2 (e). The F9 cells incubated with no or control oligonucleotides (C1 or C2) formed EBs very well (a, b and c). On the other hand, almost no EBs were formed when F9 cells were incubated with As1 or As2 (d and e).
Rcd1 is required for RA-induced differentiation of F9 cells
The next question we addressed was whether Rcd1 was critically involved in RA-induced differentiation of F9 cells. To answer this question, we examined the effect of two independent RCD1 antisense oligonucleotides (As1 and As2) and two irrelevant control oligonucleotides (C1 and C2) on RA-induced F9 differentiation. Although the antisense strategy does not give 100% inhibition, treatment with either one of the RCD1 antisense oligonucleotides, but not with any one of the control oligonucleotides, effectively blocked the production of Rcd1 protein and its transcript (Figure 2A and B), with concomitant inhibition of the induction of c-Jun and AFP as well as the formation of EBs (spherical cell mass), three general markers for F9 differentiation (Figure 2A and C). The extent of blocking was roughly parallel to the extent of reduction in the amount of Rcd1 by the antisense oligonucleotide treatment. These results suggest that Rcd1 is required for RA-induced differentiation of F9 cells.
Ectopic expression of Rcd1 highly sensitizes F9 cells to RA and induces their spontaneous differentiation without exogenously adding RA
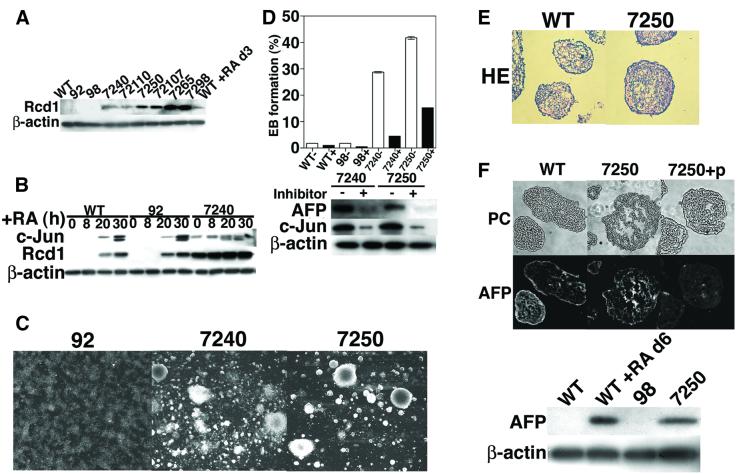
The early induction of Rcd1 by RA treatment and its requirement for F9 differentiation led us to examine the effect of constitutive expression of Rcd1 on this differentiation process. A human full-length RCD1 cDNA was isolated from a library, inserted into a cytomegalovirus (CMV) promoter-based expression vector and transfected into F9 cells followed by G418 selection. F9 transfectants stably expressing various levels of Rcd1 (clones 7240, 72110, 7250, 72107, 7265 and 7298) were obtained (Figure 3A). These Rcd1 expressors were similar to the original and empty vector-transfected F9 cells (clones 92 and 98) in proliferative ability (data not shown). However, they were found to express c-Jun constitutively prior to RA treatment (see clone 7240 versus wild type and clone 92 in Figure 3B) and spontaneously form EBs at 10- to 15-fold higher frequencies than the control (Figure 3C and open columns in Figure 3D), though RA treatment further increased both c-Jun expression (see clone 7240 in Figure 3B) and the frequencies of EB formation (data not shown). The EBs of the Rcd1 expressors were morphologically indistinguishable from those of RA-treated wild-type F9 cells (Figure 3E). The authenticity of the spontaneously formed EBs was confirmed by detection of AFP, a key marker of visceral endoderm, by immunohistochemical staining and western blotting (Figure 3F). The enhanced spontaneous differentiation of F9 cells by ectopic Rcd1 expression, however, still depended upon the RA presumably contained in the culture medium. Treatment of the Rcd1 expressors with the potent RA antagonist ER-27191 (Yoshimura et al., 1995) suppressed not only spontaneous formation of EBs (filled columns in Figure 3D, upper panel), but also expression of the differentiation marker proteins, AFP and c-Jun (Figure 3D, lower panel). These results suggest that ectopic or constitutive expression of Rcd1 by itself does not induce F9 cell differentiation, but rather sensitizes the cells to RA.
Fig. 3. Ectopic expression of Rcd1 induces spontaneous differentiation of F9 cells. (A) Level of Rcd1 in the stable RCD1 transfectants. A human full-length RCD1 cDNA inserted into a CMV promoter-based expression vector was transfected into F9 cells and stable transfectants were selected for G418 resistance. 7240 and 72110 cells are independent stable transfectants expressing an Rcd1 level comparable with those obtained in original F9 cells by RA treatment. 7250 and 72107 cells are those overexpressing a medium level of Rcd1, whereas 7265 and 7298 are those overexpressing a high level, and clones 92 and 98 are stable transfectants of an empty vector used as a negative control. (B) Ectopic expression of Rcd1 induces c-jun. Original F9 (WT), 92 and 7240 were cultured in medium containing 50 nM RA, harvested at the indicated times and lysed. Cell lysates (2 µg protein for each lane) were then separated by 10% SDS–PAGE, and Rcd1, c-Jun and β-actin were detected by western blotting with the corresponding antibodies. (C) The morphology of RCD1 transfectants and control transfectant. (D) Ectopic expression of Rcd1 induces the formation of EBs and the expression of AFP and c-Jun, but this induction still depends on RA in the culture medium. Original F9 (WT), 98, 7240 and 7250 cells were plated at 1000 cells on a 10 cm dish, cultured for 4 days in the presence (+) (filled column) or absence (–) (open column) of 100 nM ER-27191, a specific RA antagonist (Yoshimura et al., 1995), and examined for the formation of EBs by visually scanning >200 grown colonies under the microscope. The average values with standard deviation were calculated from the results of two independent experiments (upper panel). The amounts of AFP and c-Jun in these cells were analyzed by western blotting (lower panel). (E) The morphology of the EBs spontaneously formed from 7250 cells and formed from F9 cells by RA treatment. Cells were cultured for 6 days in the absence (for 7250) or presence (for F9 cells) of 50 nM RA, and thin sections of the EBs formed were prepared and stained with hematoxylin–eosin (HE). (F) Expression of AFP in the EBs. The EBs formed in (E) were stained with an anti-AFP antibody in the absence or presence of the blocking epitope peptide (7250 + P). The top panels are phase-contrast micrographs. The lower panel shows western blot detection of AFP produced in RA-treated F9 cells and spontaneously differentiating 7250 cells. Original F9 (WT), 98 and 7250 cells were cultured in normal medium for 6 days whereas as a positive control F9 cells were induced to differentiate in medium containing 50 nM RA. The cells were lysed, the lysates (30 µg protein for each lane) were separated by 10% SDS–PAGE and AFP was detected by western blotting with the same antibody as for immunostaining.
Rcd1 forms complexes with RARs and ATF-2 in an RA-dependent manner
The requirement for Rcd1 in RA-induced F9 differentiation and the sensitization of F9 to RA by ectopic Rcd1 expression suggest that Rcd1 might closely interact with retinoic acid receptors (RARs) and other co-acting transcriptional factors. We therefore analyzed the possible association of Rcd1 with RARs or its known interacting molecules RXRs, ATF-2 and p300/CBP by performing immunoprecipitation analysis. F9 cells were treated with RA for 0–3 days and cell lysates were prepared. Each subtype of RAR and RXR, and ATF-2 or Rcd1 were then precipitated with the corresponding antibodies and assayed for the amounts of co-precipitated Rcd1 and ATF-2 by western blotting (Figure 4, middle and right panels). In parallel, the amounts of RARs, RXRs, ATF-2 and Rcd1 in the whole lysates (left panel) and the amounts of ATF-2 and Rcd1 both in the immunoprecipitates and left in the supernatants (bottom section in both middle and right panels) were determined by western blotting in order to examine the effect of RA treatment on co-precipitation and roughly estimate the immunoprecipitation efficiencies for these two proteins, which we found to be >80%. Unfortunately, the immunoprecipitation efficiencies for RARs and RXRs could not be determined because of interference by the antibodies used for immunoprecipitation. In our experiments, p300/CBP could not be detected reliably in F9 cells with any of the three different antibodies that were available to us. Therefore, we did not pursue its analysis.
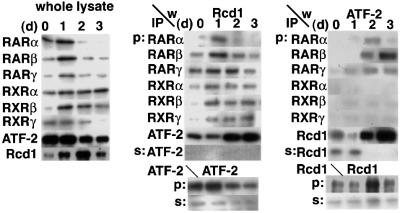
Fig. 4. Rcd1 forms complexes with RAR and ATF-2 in an RA-dependent manner. F9 cells were cultured for 0, 1, 2 and 3 days in medium containing 50 nM RA, and lysed. The amounts of ATF-2, Rcd1, RARs and RXRs in the whole-cell lysates were determined by western blotting of 5 µg protein of each lysate (left panel). Meanwhile, ATF-2, RARs and RXRs were immunoprecipitated from 100 µg of protein of the lysates with 0.1 µg each of mouse monoclonal anti-RARα (SC-551), anti-RARβ (SC-552), anti-RARγ (M-454, G-1), anti-RXRα (DN197), anti-RXRβ (SC-831), anti-RXRγ (SC-555) and anti-ATF-2 (C-19) antibodies, and co-precipitated Rcd1 and ATF-2 were detected with αRcdC (middle panel) and anti-ATF-2 (right panel) antibodies after separation by SDS–PAGE of 1/20 the precipitates (p). In parallel, in order to estimate the extent of complex formation, the amounts of ATF-2 (bottom in middle panel) and Rcd1 (bottom in right panel) both in the precipitates and left in the supernatants after ATF-2 and Rcd1 precipitations, respectively, as well as the amounts of Rcd1 (s in middle panel) and ATF-2 (s in right panel) left in the supernatants after these precipitations were determined by western blot of 1/20 of the precipitates and 5 µg of protein of the supernatants.
Upon RA stimulation, the amounts of most of the RAR subtypes were elevated transiently, like Rcd1, whereas ATF-2 expression was suppressed (Figure 4, left panel). Rcd1 co-precipitated with any one of the RAR and RXR subtypes, and RA treatment increased the amount of co-precipitated Rcd1 (middle panel). With a 1 day lag, ATF-2 co-precipitated with these RARs and Rcd1, but not with RXRs, and virtually all the ATF-2 molecules in the day 3 lysate co-precipitated with Rcd1 with no detectable ATF-2 left in the immunoprecipitation supernatant (s:Rcd1 in right panel). These results indicate that upon RA treatment, ATF-2 forms complexes with Rcd1 and RARs, but not with RXRs. The onset of the ATF-2 co-precipitation with these factors coincided with the induction of c-jun (Figure 2A).
The occurrence of the interaction between Rcd1 and ATF-2 is not specific to a particular cell line. When lysates from the brain, liver and heart of several stages of mouse embryo were immunoprecipitated with the anti-ATF-2 antibody, Rcd1 in noticeably higher amounts co-precipitated from the lysates of the early developmental stages of these organs (Figure 5).
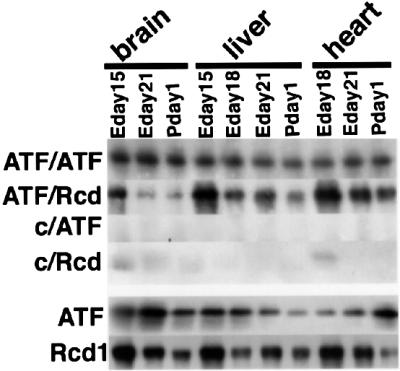
Fig. 5. Rcd1 co-precipitates with ATF-2 from embryonic tissue lysates. ATF-2 was immunoprecipitated from lysates (100 µg of protein) of embryo tissues with anti-ATF-2 antibody (ATF) or control IgG (C), and co-precipitated Rcd1 was detected with αRcdC after separation of 1/20 of the precipitates by SDS–PAGE.
ATF-2 co-precipitation with RARs depends upon Rcd1
Upon stimulation with RA, ATF-2 formed complexes with RARs and Rcd1 in F9 cells. In order to obtain insights into the role of Rcd1 in formation of these complexes, we investigated the effects of reduced Rcd1 expression on the co-immunoprecipitation of ATF-2 with RARs. F9 cells were stimulated with RA in the presence of the antisense oligonucleotide As1 or the control oligonucleotide C1 and lysed. The lysates were then immunoprecipitated for each subtype of RAR and detected for co-precipitated ATF-2 as performed in Figure 4. In parallel, the amounts of RARs, ATF-2 and Rcd1 in these cell lysates were estimated by direct immunoblotting with corresponding antibodies. As shown in the left panel of Figure 6, the induction of Rcd1 that occurred at days 2 and 3 post-RA treatment was completely suppressed in the As1-treated cells, as expected, whereas the levels of each RAR subtype and ATF-2, which were transiently induced or later repressed during RA stimulation, were not significantly influenced by the As1 treatment. Since RARs regulate their own promoters by directly binding to the RA response element therein (Leid et al., 1992), this result suggests that unlike RA-invoked c-Jun induction and subsequent F9 differentiation, Rcd1 is not essential for RA-invoked RAR induction.
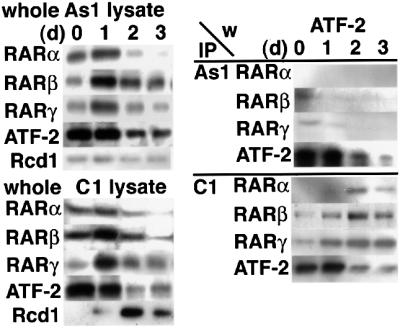
Fig. 6. Rcd1 is required for co-immunoprecipitation of ATF-2 with RARs. F9 cells were treated with RA together with the antisense oligonucleotide 1 (As1) (right upper panel) or the control oligonucleotide 1 (C1) (right lower panel) for 1, 2 and 3 days as in Figure 4 and lysed. The cell lysates (100 µg of protein each) were immunoprecipitated for each subtype of RARs. A 1/20 aliquot of each precipitate was subjected to SDS–PAGE and immunoblotted for ATF-2. A sample from each cell lysate (5 µg of protein) was also separated by SDS–PAGE and immunoblotted for RARs, ATF-2 and Rcd1 in order to show that there was no significant change in the levels of these components other than Rcd1.
When expression of Rcd1 was blocked by treatment with As1, the amount of the ATF-2 co-precipitated with each RAR subtype was markedly reduced (Figure 6, right panel). In the same set of experiments, ATF-2, used as a positive control, was immunoprecipitated efficiently and detected by western blotting. These results indicate that Rcd1 mediates RA-induced formation of the complex containing RARs and ATF-2.
Rcd1 is a component of the DRF transcriptional complex
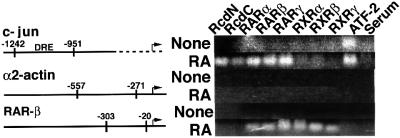
DRE is a differentiation-specific enhancer sequence present in the c-jun promoter, which is recognized by the DRF transcriptional complex containing ATF-2 as a DNA-binding subunit (Ugai et al., 1999). We therefore examined whether the DRE-bound DRF contains Rcd1 and RARs, by performing chromatin immunoprecipitation (ChIP) assays. F9 cells untreated or treated with RA for 2 days were incubated with formaldehyde to cross-link DNA and associated proteins, lysed and sonicated extensively for fragmentation of cross-linked DNA–protein fibers. Cross-linked DNA–protein fragments were then immunoprecipitated with antibodies for Rcd1, ATF-2 and RARs, and amplified by PCR after removal of cross-linked proteins. The primer sets for this analysis were designed to amplify the DRE-containing region of the c-jun promoter region (GenBank accession No. X17215). As a negative control, we used the α2-actin promoter region that contains neither DRE- nor steroid hormone receptor-binding sites, and as a positive control we used the RAR-β promoter that contains RAR-/RXR-binding sites. As shown in Figure 7, before RA treatment, only ATF-2 was detected at the c-jun promoter, but, after RA treatment, not only ATF-2 but also Rcd1 and RARs were found bound to the promoter. Furthermore, consistent with the immunoprecipitation assay results, none of the RXRs bound to the promoter. In these assays, the α2-actin promoter, used as a negative control, showed no co-precipitation with any of these antibodies, whereas the RAR-β promoter, used as a positive control for RAR/RXR binding, showed co-precipitation with all the RAR/RXR antibodies, as anticipated. However, the RAR-β promoter did not co-precipitate with any of the anti-Rcd1 antibodies. These data not only confirm the validity of the ChIP assay results, but also indicate that unlike the RARs in DRE-bound DRF, those bound to the RAR-β promoter were not associated with Rcd1, being consistent with no apparent suppression of RAR-β induction by antisense RCD1 treatment (Figure 6). We also performed band shift and supershift assays and obtained data totally consistent with the ChIP assay results (data not shown). These results indicate that at least a fraction of the DRE-bound DRF molecules was composed of ATF-2, RARs and Rcd1.
Fig. 7. DRE-bound DRF contains ATF-2, Rcd1 and RARs. F9 cells were cultured in medium containing 50 nM RA for 0 or 2 days. The F9 cell lysates before (None) or after RA stimulation (RA) were subjected to ChIP assays for the DRE region of the c-jun promoter, the α2-actin promoter (negative control) and the RAR-β promoter (positive control). The numbers in the figure are the positions at which the corresponding primers can hybridize.
Rcd1 plays a role in branching morphogenesis during mouse lung development
Branching morphogenesis of lung involves mesenchymal– epithelial signaling that induces cellular proliferation, migration and subsequent transcriptional activation of lung-specific genes (Costa et al., 2001). RA is known to play two distinct roles in lung pattern formation. RA stimulates formation of proximal tubules at an early stage, whereas a reduction in RA signaling resulting from degradation of RA and repression of its receptors is important for subsequent branching morphogenesis that leads to the formation of distal bud-like structures (Malpel et al., 2000).
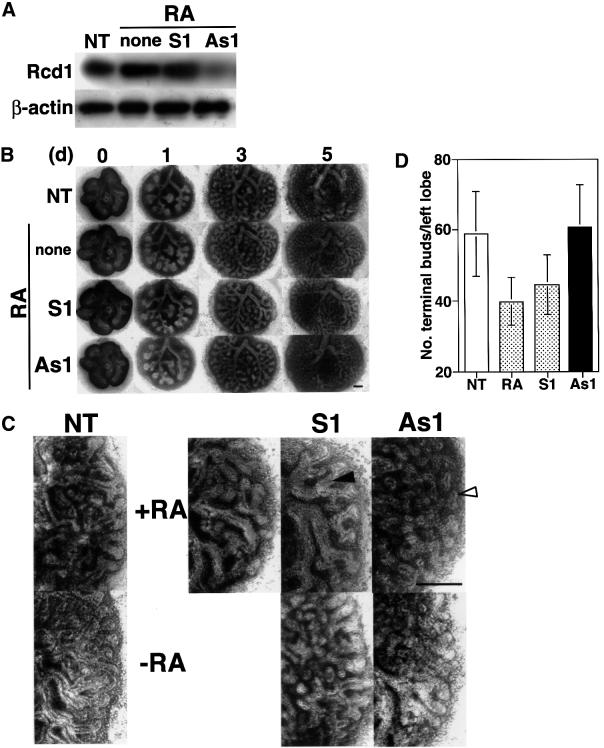
We investigated the role of Rcd1 in RA-regulated embryonic mouse lung development in an organ culture. Lung rudiments were dissected from day 11.5 p.c. embryonic mice and cultured in medium with or without 1 µM RA. The lungs, which had two buds in the left lung at the beginning of the culture experiment, started branching during culture (Figure 8B). Treatment with RA inhibited branching morphogenesis with manifestation of long proximal tubules (see filled arrow in Figure 8C). However, co-treatment with the Rcd1 antisense oligonucleotide As1 led to the disappearance of long proximal tubules and the formation of distal bud-like structures (Figure 8B–D), with a concurrent reduction in the level of Rcd1 protein (Figure 8A). This effect of the antisense oligonucleotide was actually produced via blocking the RA-induced inhibition of branching morphogenesis because the antisense oligonucleotide had no obvious effect on branching morphogenesis of the explants not treated with RA (Figure 8C). These results suggest that Rcd1 is also involved in RA-controlled mouse lung development.
Fig. 8. Treatment with an RCD1 antisense oligonucleotide suppresses RA-induced inhibition of embryonic lung branching morphogenesis. (A) The effect of RCD1 antisense oligonucleotide on the level of Rcd1 in embryonic lung. Day 11.5 p.c. embryonic mouse lung explants were cultured without (NT) or with 1 µM RA for 5 days in the absence (none) or presence of 1 µM control sense oligonucleotide 1 (S1) (GCAGCACACATCCCACTTTTTTTG) or 1 µM As1. The lung explants were lysed, and lysates (10 µg protein for each lane) were separated by SDS–PAGE and immunoblotted with αRcdC. β-actin was detected as a loading control by re-immunoblotting of the same membrane filters. (B) Development of explanted embryonic mouse lung during culture in the absence (NT) or presence of 1 µM RA only (none), 1 µM RA and 1 µM S1, or 1 µM RA and 1 µM As1. Bar = 0.25 mm. (C) Enlarged photographs of the left lobes of the 5 day cultured embryonic mouse lung explants in (B) and those in the same experiments but without RA treatment. The same experiments as in (B) but without RA treatment were carried out (photographs in the second row), and the effect of antisense oligonucleotide on branching morphogenesis was compared with those with RA treatment. The filled arrow shows a distal tubule; the open arrow shows a terminal bud. Bar = 0.25 mm. (D) The number of terminal buds formed in the left lobe of 5 day cultured embryonic mouse lung explants. A total of 11 or 12 lung explants for each experimental set were examined for the number of buds formed in the left lobe at day 5 in the same experiments as described in (B). Data are expressed as mean ± SD (n = 11 or 12).
Discussion
Cell differentiation is an important attribute of virtually all organisms, which was developed to survive hostile environments. From the mechanistic point of view, this unique cell function conceptually could be divided into two steps: the commitment to differentiation and subsequent expression of differentiated phenotypes. Our recent work with fission yeast differentiation regulators and their mammalian counterparts has raised the possibility that the mechanism and factors used to control a cell’s commitment to differentiation might not be very cell specific and even less organism specific (Yamamoto et al., 1999). Our present finding supports this possibility.
Rcd1 was discovered initially as a factor required for the commitment to nitrogen starvation-invoked, but not glucose starvation-invoked, differentiation in fission yeast. Cells lacking this factor have no apparent defects except for the inability to initiate differentiation upon starvation of nitrogen sources. Database searches revealed that its homologs are present as one of the most evolutionarily conserved proteins in a variety of eukaryotes ranging from yeast to mammals, although their biological role in other organisms is unknown (Okazaki et al., 1998). The experimental data presented here indicate that, just like the fission yeast counterpart, mammalian Rcd1 plays a role in the step of the cell’s commitment to differentiation. First, Rcd1 is expressed in a variety of developing organs in rat embryo, and its expression decreases at late stages of embryogenesis. Secondly, Rcd1 is essential for commitment to RA-induced visceral endodermal differentiation of the F9 embryonic carcinoma cell. Furthermore, Rcd1 is essential for RA-controlled lung development.
F9 mouse teratocarcinoma cells differentiate to visceral endoderm upon stimulation with RA. c-jun has been identified among the earliest genes responding to RA and is believed to play a key role in the commitment to this differentiation. The c-jun promoter contains an enhancer essential for its differentiation-associated activation. This enhancer called DRE is recognized by DRF, which contains p300/CBP and ATF-2 as the DNA-binding subunit and is activated by RA (Kawasaki et al., 1998; Ugai et al., 1999). However, the mechanism by which RA activates DRF remains unknown.
Nuclear hormone receptors represent a large family of ligand-dependent transcription factors. They are key regulators of cell growth and differentiation, homeostasis and development (Rossi et al., 2000; Zile, 2001). In the absence of hormone, nuclear receptors repress transcription of target genes via their association with corepressors that contain histone deacetylase activity. Hormone binding triggers release of corepressors and subsequent association of an array of coactivators, such as p300/CBP, PCAF, ACTR and SRC-1 that exhibit histone acetyltransferase activities (Torchia et al., 1998; Chen et al., 1999). However, neither RAR nor any of the cofactors acting for RARs seem to interact directly with ATF-2 (Torchia et al., 1998; Ugai et al., 1999) although p300/CBP reportedly is contained in DRF (Kawasaki et al., 1998). Thus, there was a missing link between RAR and ATF-2. All the properties of Rcd1 reported here seem to fit nicely the expected properties of the missing link.
Our data show Rcd1 to be a component of DRF. In F9 cells, Rcd1 is associated with the RAR, but upon stimulation with RA it forms a complex containing RAR and ATF-2, but not RXR. The data from the ChIP assays indicate that at least a fraction of DRE-bound DRF is composed of this complex. Interestingly, the RAR/RXR species bound to the RAR-β promoter were not associated with Rcd1 (Figure 7), and antisense RCD1 treatment did not block the RA-invoked RAR-β induction (Figure 6). Thus, RARs do not require Rcd1 for activation of the RAR-β promoter, which is elicited via their direct binding to the RA response element. Moreover, it appears that only the RAR/RXR molecules not bound to Rcd1 are able to activate the promoter. Whether this can be generalized to other RAR promoters or even any promoters containing the RA response element remains to be elucidated.
The nature of the interactions and the physical mechanism of the complex formation among RARs, Rcd1 and ATF-2 are unknown at present. However, the association of Rcd1 with RARs, at least, seems to be indirect. In vitro pull-down assays with E.coli-expressed Rcd1 and any of the RARs showed no significant interaction between them (our unpublished data), suggesting that it might be mediated by an additional factor. However, carefully controlled pull-down assays with proteins expressed in more relevant cells would certainly be required to conclude anything on the nature of the interactions between RARs and Rcd1, and between Rcd1 and ATF-2.
In fission yeast, Atf1, the closest homolog of mammalian ATF-2, is essential for nitrogen starvation-invoked induction of the ste11+ gene, whose action is absolutely required for the onset and progression of conjugation and meiosis (Takeda et al., 1995; Okazaki et al., 1998). Furthermore, Rcd1 and Atf1 are similar in that their deletion suppresses the pat1-114 mutation, which results in ste11+ induction and subsequent lethal haploid meiosis at the non-permissive temperature. In the light of our present findings, this functional similarity suggests that Atf1 may be an association partner for fission yeast Rcd1. In contrast, it is unlikely that RAR is also an association partner for Rcd1 in fission yeast because no gene encoding such a receptor or a related molecule has been found in this organism. This in turn raises the possibility that RAR might not be the sole upstream interacting factor for Rcd1. Interestingly, Rcd1 protein contains a consensus sequence (amino acids 198–201) for the binding of STAT3 protein that critically regulates embryonal stem cell differentiation (Matsuda et al., 1999). In this regard, it is noteworthy that Rcd1 is also involved in megakaryocytic and erythroid differentiations of K562 cells that are induced by TPA and NaB, respectively (N.Hiroi and H.Okayama, unpublished observation). In addition, the presence of a considerable amount of ATF-2-asscociated Rcd1 molecules at least in several organs during mouse embryogenesis (Figure 5) might also reflect its possible involvement as a critical cofactor in the regulation of other genes. Furthermore, in an erythropoietin-responsive erythroid cell, Rcd1 is highly induced by stimulation with erythropoietin, suggesting the possible involvement of Rcd1 also in erythropoietin-induced proliferation, survival and differentiation of erythroid cells (Gregory et al., 2000).
Materials and methods
Cell culture, DNA transfection and proliferation assay
The human leukemia cell lines K562 (JCRB 0019), U937 (JCRB 9021), Jurkat (ATCC TIB 152), Daudi (JCRB 9071) and HL-60 (JCRB 0085) were cultured in 5% CO2 at 37°C in RPMI 1640 medium containing l-glutamine (Sigma) supplemented with 10% heat-inactivated fetal calf serum (Equitech-bio Inc.). F9 cells (JCRB 0721) and P19 cells (ATCC CRL-1825) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum.
K562, HL60, U937, Daudi, Jurkat, F9 and P19 cells were induced to differentiate by culturing in medium containing 10 nM TPA, 10 µM all-trans-RA, 20 nM TPA, PHA and TPA at 2 µg and 20 ng per ml, 50 nM RA, and 300 nM RA, respectively.
pIRES-human RCD1 contains a full-length human RCD1 cDNA in a CMV promoter-based expression vector with the neo selection marker. Stable transfection was carried out by lipofection with Lipofectamine™ 2000 reagent. pIRES-human RCD1 (10 µg) and empty pIRES (10 µg) were transfected separately into 106 F9 cells followed by incubation at 37°C for 48 h. After incubation, cells were washed with growth medium and selected in the medium containing G418 at 100 µg/ml. G418-resistant cells were expanded and analyzed for protein expression and differentiation ability.
Western blot detection and immunoprecipitation
Western blot analysis was performed as described (Yamamoto et al., 1999). Various tissues of embryonic and post-natal rats were dissected and quickly frozen in liquid N2. These tissues were weighed and ground into powder while frozen in liquid N2. For western blotting, cells and powdered tissues were lysed with RIPA buffer. After 10 min incubation on ice, lysed cells and tissues were centrifuged at 20 000 g for 10 min at 4°C, and the supernatants were used for western blot analysis after adjustment of protein amounts. For immunoprecipitation, cells and powdered tissues were lysed with SDS-free RIPA buffer. Immuno precipitation was performed by incubation with the relevant antibodies and protein G–Sepharose beads in SDS-free RIPA buffer overnight at 4°C followed by two washes with 10× volumes of SDS-free RIPA buffer. Immunoprecipitated proteins were then analyzed by western blotting. The proteins in loading buffer were boiled for 5 min, separated by SDS–PAGE (10% polyacrylamide), and blotted onto Immobilon™-P membranes (Millipore). The filters were blocked with 5% skimmed milk in TBS-T for 1 h at room temperature. The anti-c-Jun/AP-1 (SC-45), anti-RARα (SC-551), anti-RARβ (SC-552), anti-RARγ (M-454, G-1), anti-RXRα (DN197), anti-RXRβ (SC-831), anti-RXRγ (SC-555) and anti-ATF-2 (C-19) antibodies were purchased from Santa Cruz Biotechnology, and the anti-β-actin (A5441) antibody from Sigma. The filters were then washed, incubated with the secondary antibody (sheep anti-mouse or donkey anti-rabbit) conjugated with horseradish peroxidase (Amersham Pharmacia) for 1 h at room temperature and washed in TBS-T. Immunoblotted bands were detected by using the ECL system (Amersham Pharmacia) with the same exposure time for detection with the same antibody.
Immunocytochemistry
Differentiated F9 cells in culture dishes were rinsed with phosphate-buffered saline (PBS), dehydrated with methanol and fixed at room temperature for 10 min with a solution of 1% acetic acid and 99% methanol. For immunostaining, cells were washed with ice-cold PBS and incubated with anti-AFP goat antibody for 30 min at 37°C, and then rinsed with PBS. The cells were incubated with anti-goat biotinylated donkey antibody for 30 min at 37°C followed by incubation with streptavidin–fluorescein for 30 min at 37°C and three washes with PBS. All antibodies are products of Amersham Pharmacia.
ChIP assay
The ChIP assay was performed as described (Shang et al., 2000). A total of 106 F9 cells were grown for 2 days with or without RA, washed twice with PBS and treated with 1% formaldehyde at room temperature for 10 min. The cells were then rinsed with ice-cold PBS twice, incubated for 15 min at 30°C in 100 mM Tris–HCl pH 9.4 containing 10 mM dithiothreitol (DTT), and sedimented by centrifugation at 400 g for 5 min. The cells were washed with 1 ml of ice-cold PBS and 1 ml each of buffer I and buffer II. The cells were then resuspended in 0.3 ml of lysis buffer containing protease inhibitors and sonicated with a Branson Sonifier 250 three times for 10 s each at the setting of output control at 5 and duty cycle 10%, followed by centrifugation for 10 min. Supernatants were collected and diluted in buffer followed by incubation with 2 µg of sheared salmon sperm DNA, 20 µl of pre-immune serum and protein G–Sepharose (rinsed with dilution buffer) for 2 h at 4°C in order to suppress non-specific interactions. Immunoprecipitation was then performed by incubation on ice for >2 h with specific antibodies. After immunoprecipitation, 25 µl of protein G–Sepharose and 200 ng of salmon sperm DNA were added and rotated at 4°C overnight. Precipitations were washed for 10 min with rotation sequentially with TSE I, TSE II and buffer III. Precipitates were then washed three times with TE buffer and extracted three times with 1% SDS, 0.1 M NaHCO3. Eluates were pooled and heated at 65°C for at least 6 h to reverse formaldehyde cross-links. For PCR, 0.5 µl from a 100 µl DNA extraction and 30 cycles of amplification were used. The primers used for amplification of DRE were GTCAGCCCACAATGCACCGG (forward primer), GCTACCAGTCAATCCCTAAA (reverse primer); those for amplification of the α2-actin promoter were CATGACTCCTCTGCATATT (forward primer), ACGAGCTGAGCTGCCTCCTG (reverse primer); and those for amplification of the RAR-β promoter were GCCGTGGGGAGCAGCCGGCGGCT (forward primer), GCGCACGGGAACTCTGGTCC (reverse primer).
Effect of antisense oligonucleotide on RA-treated fetal lung explants
Fourteen pregnant ICR mice were purchased from Japan SLC (Shizuoka, Japan). At embryonic day 11.5, the pregnant mice were anesthetized with an intra-abdominal pentobarbital injection, their gravid uteruses were excised, and all fetuses were removed. The fetal lungs with the trachea were taken from the fetuses aseptically under a stereo microscope for culture. The lungs with two branches from the left lobar bronchus were used for the morphogenesis study. In total, 69 pairs of the lungs with the trachea (11–12 pairs in each experimental group) were placed on a 6-well tissue culture plate formatted with an 8 µm pore size insert (Falcon/Beckon Dickinson, NJ). The lung explants were cultured for 5 days in chemically defined medium (Fitton–Jackson modification BGJb, Gibco, Grand Island, NY) containing 10% fetal bovine serum, 0.2 mg/ml ascorbic acid and 50 U/mg penicillin/streptomycin with additions of RA (Sigma) at a concentration of 10–6 M alone, RA and the RCD1 sense oligonucleotide at a concentration of 1 µM, or RA and the RCD1 antisense oligonucleotide at a concentration of 1 µM and without any other treatment (NT). The number of terminal buds in the left lungs was counted at days 0, 1, 3 and 5, and phase-contrast micrographs were taken. The data were analyzed by unpaired Student’s t-test. Results were determined to be significant if P < 0.05. At the end of cultivation, the cultured lung tissues were frozen for western blot analyses.
Acknowledgments
Acknowledgements
This work was supported by research grants from Ministry of Education, Science and Culture, Japan.
References
- Beier F., Taylor,A.C. and Lu Valle,P. (2000) Activation transcription factor 2 is necessary for maximal activity and serum induction of the cyclin A promoter in chondrocytes. J. Biol. Chem., 275, 12948–12953. [DOI] [PubMed] [Google Scholar]
- Bellusci S., Grindley,J., Emoto,H., Itoh,N. and Hogan,B.L.M. (1997) Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development, 124, 4867–4878. [DOI] [PubMed] [Google Scholar]
- Benton B.K., Reid,M.S. and Okayama,H. (1993) A Schizosaccharo myces pombe gene that promotes sexual differentiation encodes a helix–loop–helix protein with homology to MyoD. EMBO J., 12, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lin,R.J., Xie,W., Wilpitz,D. and Evans,M. (1999) Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell, 98, 675–686. [DOI] [PubMed] [Google Scholar]
- Chen J., Rappsilber,J., Chiang,Y.C., Russell,P., Mann,M. and Denis,C.L. (2001) Purification and characterization of the 1.0 MDa CCR4–NOT complex identifies two novel components of the complex. J. Mol. Biol., 314, 683–694. [DOI] [PubMed] [Google Scholar]
- Chen J., Chiang,Y.C. and Denis,C.L. (2002) CCR4, a 3′-5′ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J., 21, 1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G. and Borello,U. (1999) Wnt signaling and the activation of myogenesis in mammals. EMBO J., 18, 6867–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R.H., Kalinichenko,V.V. and Lim,L. (2001) Transcription factors in mouse lung development and function. Am. J. Physiol., 280, L823–L838. [DOI] [PubMed] [Google Scholar]
- Edwards M.K. and McBurney,M.W. (1983) The concentration of retinoic acid determines the differentiated cell types formed by a teratocarcinoma cell line. Dev. Biol., 98, 187–191. [DOI] [PubMed] [Google Scholar]
- Gregory R.C., Lord,K.A., Panek,L.B., Gaines,P., Dillon,S.B. and Wojchowski,D.M. (2000) Subtraction cloning and initial characterization of novel epo-immediate response genes. Cytokine, 12, 845–857. [DOI] [PubMed] [Google Scholar]
- Halvorson M.J., and Coligan,J.E. (1995) Enhancement of VLA integrin receptor function on thymocytes by cAMP is dependent on the maturation stage of the thymocytes. J. Immunol., 15, 4567–4574. [PubMed] [Google Scholar]
- Hogan B.L.M. and Taylor,A. (1981) Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature, 291, 235–237. [DOI] [PubMed] [Google Scholar]
- Hughes D.A., Fukui,Y. and Yamamoto,M. (1990) Homologous activators of ras in fission and budding yeast. Nature, 344, 80–83. [DOI] [PubMed] [Google Scholar]
- Jain R.G., Phelps,K.D. and Pekala,P.H. (1999) Tumor necrosis factor-α initiated signal transduction in 3T3-L1 adipocytes. J. Cell Physiol., 179, 58–66. [DOI] [PubMed] [Google Scholar]
- Jones-Villeneuve E.M., McBurney,M.W., Rogers,K.A. and Kalnins,V.I. (1982) Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J. Cell Biol., 94, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Okazaki,K., Murakami,H., Stettler,S., Fantes,P.A. and Okayama,H. (1996) Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett., 378, 207–212. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Song,J., Eckner,R., Ugai,H., Chiu,R., Taira,K., Shi,Y., Jones,N. and Yokoyama,K.K. (1998) P300 and ATF-2 are components of the DRF complex, which regulates retinoic acid- and E1A-mediated transcription of the c-jun gene in F9 cells. Genes Dev., 12, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Schiltz,L., Chiu,R., Itakura,K., Taira,K., Nakatani,Y. and Yokoyama,K.K. (2000) ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature, 405, 195–200. [DOI] [PubMed] [Google Scholar]
- Kelly M., Burke,J., Smith,M., Klar,A. and Beach,D. (1988) Four mating-type genes control sexual differentiation in the fission yeast. EMBO J., 7, 1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabayashi I. et al. (1992) Transcriptional regulation of the c-jun gene by retinoic acid and E1A during differentiation of F9 cells. EMBO J., 11, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabayashi I., Eckner,R., Arany,Z., Chiu,R., Gachelin,G., Livingston,D.M. and Yokoyama,K.K. (1995) Phosphorylation of the adenovirus E1A-associated 300 kDa protein in response to retinoic acid and E1A during the differentiation of F9 cells. EMBO J., 14, 3496–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J., Rathjen,J., Remiszewski,J. and Rathjen,P. (2000) Reversible programming of pluripotent cell differentiation. J. Cell Sci., 113, 555–566. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner,P. and Chambon,P. (1992) Multiplicity generates diversity in the retinoic acid signaling pathways. Trends Biochem. Sci., 17, 427–433. [DOI] [PubMed] [Google Scholar]
- Malpel S., Mendelsohn,C. and Cardoso,V. (2000) Regulation of retinoic acid signaling during lung morphogenesis. Development, 127, 3057–3067. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Nakamura,T., Nakano,K., Arai,T., Katsuki,M., Heike,T. and Yokota,T. (1999) STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J., 18, 4261–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min W. and Pober,J.S. (1997) TNF initiates E-selectin transcription in human endothelial cells through parallel TRAF-NF-κB and TRAF-RAC/CDC42-JNK-c-Jun/ATF2 pathways. J. Immunol., 159, 3508–3518. [PubMed] [Google Scholar]
- Nagata Y., Takahashi,N., Davis,R.J. and Todokoro,K. (1998) Activation of p38 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation. Blood, 92, 1859–1869. [PubMed] [Google Scholar]
- Okazaki N., Okazaki,K., Watanabe,Y., Kato-Hayashi,M., Yamamoto,M. and Okayama,H. (1998) Novel factor highly conserved among eukaryotes controls sexual development in fission yeast. Mol. Cell. Biol., 18, 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.A., McCaffery,P.J., Drager,U.C. and De Luca,L.M. (2000) Retinoids in embryonal development. Physiol. Rev., 80, 1021–1054. [DOI] [PubMed] [Google Scholar]
- Seidensticker M.J. and Behrens,J. (2000) Biochemical interactions in the wnt pathway. Biochim. Biophys. Acta, 1495, 168–182. [DOI] [PubMed] [Google Scholar]
- Shang Y., Hu,X., DiRenzo,J., Lazar,M.A. and Brown,M. (2000) Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell, 103, 843–852. [DOI] [PubMed] [Google Scholar]
- Strickland S. and Mahdavi,V. (1978) The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell, 15, 393–403. [DOI] [PubMed] [Google Scholar]
- Sugimoto A., Iino,Y., Maeda,T., Watanabe,Y. and Yamamoto,M. (1991) Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev., 5, 1990–1999. [DOI] [PubMed] [Google Scholar]
- Takeda T., Toda,T., Kominami,K., Kohnosu,A., Yanagida,M. and Jones,N. (1995) Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J., 14, 6193–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. and Okayama,H. (2000) A Pcl-like cyclin activates the Res2p–Cdc10p cell cycle ‘Start’ transcriptional factor complex in fission yeast. Mol. Biol. Cell, 11, 2845–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia J., Glass,C. and Rosenfeld, MG. (1998) Co-activators and co-repressors in the integration of transcriptional responses. Curr. Opin. Cell Biol., 10, 373–383. [DOI] [PubMed] [Google Scholar]
- Tsukahara K., Yamamoto,H. and Okayama,H. (1998) An RNA binding protein negatively controlling differentiation in fission yeast. Mol. Cell. Biol., 18, 4488–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugai H., Uchida,K., Kawasaki,H. and Yokoyama,K.K. (1999) The coactivators p300 and CBP have different functions during the differentiation of F9 cells. J. Mol. Med., 77, 481–494. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. and Yamamoto,M. (1996) Schizosaccharomyces pombe pcr1+ encodes a CREB/ATF protein involved in regulation of gene expression for sexual development. Mol. Cell. Biol., 16, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Iino,Y., Furuhata,K., Shimoda,C. and Yamamoto,M. (1988) The S.pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J., 7, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington V., Cardoso,S., Mitsialis,S.A., Brody,J.S. and Williams,M.C. (1996) Retinoic acid alters the expression of pattern-related genes in the developing rat lung. Dev. Dyn., 207, 47–59. [DOI] [PubMed] [Google Scholar]
- Willer M., Hoffmann,L., Styrkarsdottir,U., Egel,R., Davey,J. and Nielsen,O. (1995) Two-step activation of meiosis by the mat1 locus in Schizosaccharomyces pombe. Mol. Cell. Biol., 15, 4964–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Tsukahara,K., Kanaoka,Y., Jinno,S. and Okayama,H. (1999) Isolation of a mammalian homologue of a fission yeast differentiation regulator. Mol. Cell. Biol., 19, 3829–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H., Nagai,M., Hibi,S., Kikuchi,K., Abe,S., Hida,T., Higashi,S., Hishinuma,I. and Yamanaka,T. (1995) A novel type of retinoic acid receptor antagonist: synthesis and structure–activity relationships of heterocyclic ring-containing benzoic acid derivatives. J. Med. Chem., 38, 3163–3173. [DOI] [PubMed] [Google Scholar]
- Zile M.H. (2001) Function of vitamin A in vertebrate embryonic development. J. Nutr., 131, 706–708. [DOI] [PubMed] [Google Scholar]