Abstract
Tamoxifen, a potent anticancer agent known to interrupt the enhanced estrogen activity of malignant mammary gland cells, was recently approved by the Food and Drug Administration (FDA) for the treatment of breast cancer. In this investigation, the toxic effects of tamoxifen were evaluated through cell multiplication, and cytological, surface ultrastructural, and biochemical studies on human cervical carcinoma cells (HeLa) and/or murine erythroleukemic (MEL) cells (BB-88). Tamoxifen treatment demonstrated an inhibitory effect on HeLa cell multiplication at lower concentrations and toxicity at higher concentrations and longer treatment durations. The drug also triggered morphological and biochemical changes as revealed by light microscopy, scanning electron microscopy (SEM), fluorescence microscopy, Nucleosome ELISA, and the DNA smear pattern. Cytological observations showed nuclear condensation, cell shrinkage, multinucleation, and apoptotic bodies. Surface ultrastructure of tamoxifen treated cells examined under the SEM revealed abnormalities such as membrane blebbing, holes, and cytoplasmic extrusions, all of which are characteristics of programmed cell death (apoptosis). Redistribution of the membrane phospholipid phosphatidylserine (PS) from the protoplasmic surface of the plasma membrane to the cell surface was identified using annexin V-enhanced green fluorescent protein (EGFP) in tamoxifen treated MEL BB-88 cells, a general feature of cells undergoing apoptosis. Tamoxifen treated cells demonstrated internucleosomal damages of the genomic DNA and DNA fragmentations, evidenced by an increase in free nucleosomes, and distinctive DNA smear patterns on the agarose gel.
INTRODUCTION
Tamoxifen, or Nolvadex®, is a synthetic antiestrogen that has been used since the 1970s to treat advanced and early stage breast cancer [1]. The drug is classified as a selective estrogen receptor modulator (SERM) because it behaves like an estrogen agonist in some tissues and an antagonist in others [2]. The mechanism of tamoxifen's antitumor activity is primarily due to its antiestrogen action [3]. Estrogen, when acting as a harmful hormone, has the ability to promote cancer of the breast and uterine lining. Tamoxifen is a synthetic estrogen-like hormone that binds to the estrogen receptors and acts as a competitor of natural estrogen to inhibit the growth of malignant mammary tumors [2].
There are two types of cell death mechanisms: necrosis and apoptosis (programmed cell death). Necrosis is initiated by intense toxic stress, and its action is nonspecific to cells and tissues. Apoptosis is a normal mechanism of development and tissue homeostasis [4]. This “cell suicide” phenomenon is initiated by one or more external signals, such as the lack of a necessary hormone or by cellular exposure to chemotherapeutic chemicals, which activates a cascade of events that includes the activation of endonucleases [5]. Programmed cell death, or apoptosis, is defined by various morphological and biochemical changes. In the early stages, characteristics of apoptosis include cell shrinkage, nuclear condensation, DNA degradation, and breakdown of the nuclear material. Later stages of apoptosis are characterized by the formation of membrane blebs leading to ldquo;apoptotic bodies” and cell fragmentation that are phagocytized by macrophages [6, 7, 8, 9]. Most of these morphological changes can be detected with the light and scanning electron microscopes, while molecular modification of the cell membrane changes can be visualized by observing the redistribution of phosphatidylserine (PS) from the inner leaflet of the plasma membrane to the cell surface using the immunofluorescence annexin V-enhanced green fluorescent protein (EGFP) that has a strong affinity for PS [10, 11].
Limited in vitro studies on the effects of tamoxifen in non-breast cancer cell proliferation reported conflicting results. A study by Grenman et al. [12] found an inhibitory effect of the drug on several human cancer cell lines. Khar et al. [13], on the other hand, found no inhibitory action of tamoxifen in the presence of fetal calf serum on HeLa cells. In another study, Bonita et al. [14] reported the antiproliferative effect of the agent on both the HeLa and the murine erythroleukemic cells (MEL BB-88), however, the inhibitory influence of the drug was diminished in the presence of B-estradiol. Other studies suggested that tamoxifen induced programmed cell death in MCF-7 human breast cancer cells [15, 16]. The study performed by Grenman et al. [12] found the inhibitory effect of tamoxifen (1–10 mM) on HeLa cell proliferation after 6 days. However, the drug's effects at higher concentrations over shorter time periods (less than 72 hours) were not evaluated. Osborne et al. [17], on the other hand, found a growth inhibitory effect of the drug on human breast cancer cells (MCF-7).
In this investigation, the morphological and biochemical effects of tamoxifen on human cervical carcinoma cells (HeLa) and murine erythroleukemic cells (MEL BB-88) were examined. In vitro studies were conducted to determine the drug's role on HeLa cell multiplication and its involvement in cell death. To investigate the cell death mechanism, morphological characteristics of cells and cell membrane were studied using light and scanning electron microscopy. Assays including annexin V-EGFP, an immunofluorescense technique, Nucleosome ELISA, and DNA laddering technique were employed.
MATERIALS AND METHODS
Maintenance of cell lines
HeLa (human cervical carcinoma cells) and BB-88 (murine erythroleukemia cells MEL) cell lines were purchased from the American Type Culture Collection (Rockville, MD). BB-88 cells (MEL BB-88) grow in suspension, whereas HeLa are epithelial cells that grow as multilayers on the tissue culture flasks surface. Both cell lines were purchased from Sigma-Aldrich (St. Louis, MO) and maintained in Dulbecco's Modified Eagle's Medium, supplemented with 10% fetal calf serum (DME-10) and grown in 25 cm2 tissue culture flasks at 37°C in 7.5% CO2 in air (as outlined by Ahearn et al. [18] and Ruddy et al. [19]). Cells were recultured twice per week.
Drug treatment
Tamoxifen was purchased from Sigma-Aldrich in St. Louis, MO and dissolved in 0.2 ml dimethyl sulfoxide (DMSO) to a final concentration of 400 μg/ml in sterile deionized water. The solution for control treatments was made by dissolving 0.2 ml DMSO in the same amount of sterile deionized water. Both stock solutions were kept at 8°C.
Cell multiplication study
In contrast to our previous study [14], logarithmically growing HeLa cells were seeded at 1.3 × 104 cells/ml in DME-10 in six well culture plates. After 24 hours, the cells were treated with 0, 3, 5, 10, 15, and 20 μg/ml tamoxifen. To be sure that nutrient depletion would not be a factor in cell growth inhibition, the medium and drug were removed and replaced with fresh DME-10 and tamoxifen on each day of the study. Cell number was determined after 24, 48, 72, and 96 hours using the Trypan Blue exclusion method. Control and tamoxifen treated cells were photographed directly in the culture plate using an inverted light microscope equipped with a Nikon camera.
Light microscopy
Exponentially growing HeLa cells were treated with 5, 10, 15, and 20 μg/ml tamoxifen or the control solution for 24, 48, 72, and 96 hours. Cells were harvested and fixed with 0.075 M KCl hypotonic solution for 10 minutes at 8°C. Cells were then fixed four times in a 3 : 1 methanol : glacial acetic acid, and slides were prepared and stained with KaryoMAX Giemsa Stain (Gibco BRL, Grand Island, NY). Cells were examined and scored for enucleation, cell shrinkage, nuclear blebs, pyknotic fragments, nuclear condensation, and apoptotic bodies, characteristic features of apoptotic cells [6, 7, 8, 9, 20].
Scanning electron microscopy (SEM)
Approximately 25.000 cells/ml of resuspended HeLa cells were plated on glass coverslips in a petri dish and incubated for 24 hours. Petri plates were flooded with 4 ml DME-10 containing 5, 10, 15, or 20 μg/ml of tamoxifen for 24, 48, 72, or 96 hours. Control petri dishes received 4 ml of DME-10 containing a comparable concentration of DMSO in deionized water. Exponentially growing MEL BB-88 cells were treated with 0, 5, 10, or 15 μg/ml of tamoxifen in 25 cm2 tissue culture flasks and sampled after 24 and 48 hours of exposure.
Samples were fixed in 2% glutaraldehyde for 1 hour followed by 1% osmium tetroxide for 1 hour. Cells were dehydrated in an increasing acetone series, critically point dried (Polaron E 3000), coated with gold-palladium, and observed under an ISI Super III-A scanning electron microscope. Photographs obtained with a Mamiya camera (Polaroid B-52 and 55 films) were used to study the surface ultrastructural morphology.
Immunofluorescence staining (BioVision®, Palo Alto, CA)
Exponentially growing MEL BB-88 cells were treated with 5, 10, and 15 μg/ml of tamoxifen or the control solution for 4, 8, 12, and 24 hours. Cells were collected by centrifugation, resuspended in 1x binding buffer, and slide spreads were made. Samples were then fixed with annexin V-enhanced green fluorescent protein (EGFP) and propidium iodide (PI) for 5 minutes, and the slides were prepared following the method outlined by BioVision [21]. Treated and untreated cells were viewed under a fluorescence microscope with a dual filter set of FITC and rhodamine in order to detect cell membrane disruption in treated and untreated cells.
Nucleosome ELISA assay (Oncogene™ research products, Cambridge, MA)
HeLa cells were seeded at 100.000 cells/ml in 25 cm2 tissue culture flasks for 24 hours and then treated with 0 and 10 μg/ml tamoxifen for 4, 6, and 10 hours. Free nucleosomes were then detected using the Nucleosome ELISA assay steps outlined by Oncogene [22]. The absorbance of resulting yellow products was measured at 450 nm in a Bio-Tek Microplate Reader (EL312).
TACS™ ethidium bromide apoptotic DNA laddering kit (Trevigen, Inc., Gaithersburg, MD)
Exponentially growing BB-88 cells were exposed to 5, 10, and 15 μg/ml of tamoxifen for 12, 24, and 48 hours. Following the provided Trevigen protocol, cells were harvested by centrifugation and the pellet was resuspended in a sample buffer [23]. The DNA was stabilized by lysing the sample to obtain a purified aqueous layer. Sodium acetate and 2-propanol were added to the aqueous sample. The sample was then centrifuged, the pellet was washed with 70% ethanol, vacuum dried, resuspended in DNase-free water, and the concentration of the DNA was quantified spectrophotometrically (Milton Roy Spectromic 1201).
One μg of DNA sample was placed in each well and separated according to size by electrophoresis on a 0.8% agarose gel at 70 V for 1.5 hours. The DNA smear was stained with ethidium bromide, visualized under the UV transilluminator, and photographed.
RESULTS
Cell multiplication study
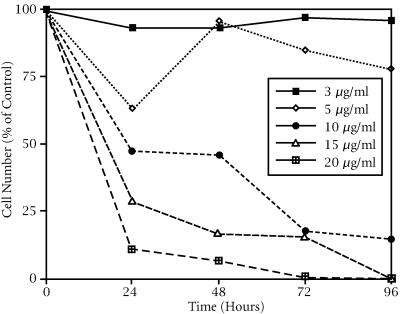
HeLa cell growth inhibition was both time and drug-concentration dependent in an inverse relationship in excess of 5 μg/ml (see Figure 1). A minimum growth inhibitory effect was seen at 3 and 5 μg/ml tamoxifen treatments. At 5 μg/ml, the number of cells/ml initially decreased (about 65% of the control) and then followed a trend similar to the 3 μg/ml treated group, but at a slower growth rate after 24 hours. The number of viable HeLa cells treated with 10 μg/ml, compared to the control, began to decrease (45%) after only 24 hours and continued to decrease to 20% of the control by 96 hours. A drastic reduction in viable cell number was seen by 24 hours of tamoxifen treatment at 15 and 20 μg/ml and resulted in complete death by 96 hours (see Figure 1).
Figure 1.
Growth inhibitory effect of tamoxifen on HeLa cells. Percent of control number of viable cells/ml as a function of time after tamoxifen treatment in 6-well culture plates.
Light microscopy
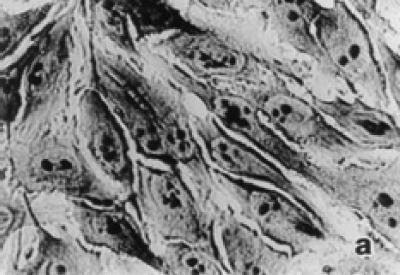
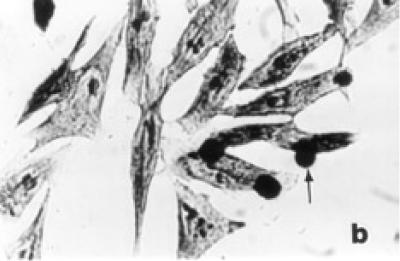
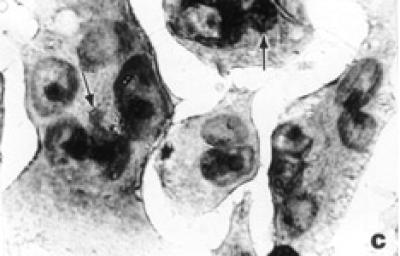
Untreated HeLa cells exhibited typical growth patterns and normal morphology (see Figure 2a). The leading edge of the cell is spread into a flattened “veil-like” projection called a lamellipodium, whereas the trailing end of the cell is narrowed into a tail. Each control cell had one distinct nucleus with several nucleoli. Light microscopic observations of treated HeLa cells showed shrinkage, nuclear condensation, and occasional enucleation (see Figure 2b). Multinucleation and apoptotic bodies, which are characteristic of programmed cell death (apoptosis), were also detected in treatment groups (see Figure 2c). Tamoxifen treatment at 10 μg/ml or higher exhibited elongated lamellipodia and many detached cells. Frequency of dead cells increased in abundance in 15 and 20 μg/ml tamoxifen treated groups even after 24 hours of exposure.
Figure 2.
Morphological characteristics of untreated and tamoxifen treated HeLa cells under the light microscope. (a) Confluent control cells at 72 hours showing normal morphology with lamellipodium, prominent nucleus, and nucleoli in each cell × 400. (b) Tamoxifen treated cells (10 μg/ml) at 48 hours showing nuclear condensation, cell shrinkage, and enucleation (arrow) × 400. (c) Cells treated with tamoxifen (15 μg/ml) at 48 hours showing multinucleation and apoptotic bodies (arrows) × 900.
Scanning electron microscopy (SEM)
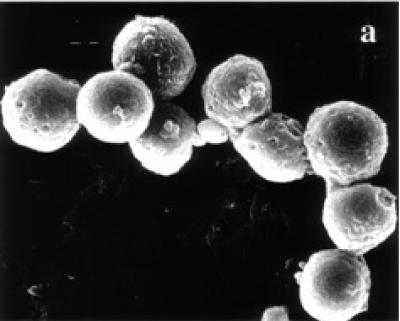
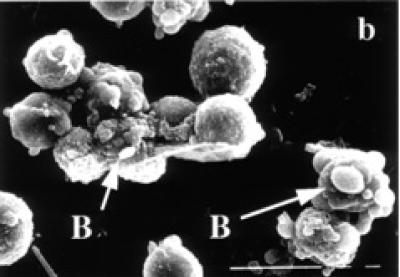
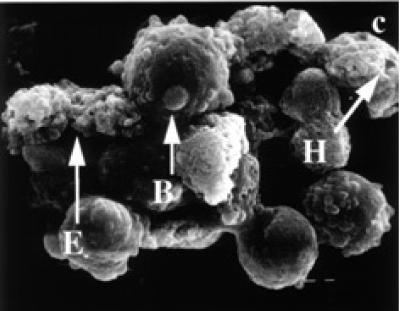
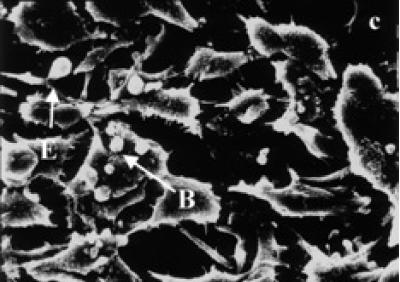
Surface ultrastructure of tamoxifen treated cells, examined with the scanning electron microscope, revealed distinct morphological changes when exposed to tamoxifen. Exponentially growing (24 and 48 hours) untreated BB-88 cells are spherical in shape with a few blebs scattered over the intact cell membrane (see Figure 3a). Treated BB-88 cells at 24 and 48 hours were distorted in shape and exhibited clumping. Abnormalities such as cell shrinkage, severe membrane blebbing (see Figure 3b), holes, and cytoplasmic extrusions (see Figure 3c) were detected in the treatment groups.
Figure 3.
Surface ultrastructural characteristics of BB-88 cells treated with or without tamoxifen as depicted under the SEM. (a) Control BB-88 cells at 48 hours (× 2400). (b) Tamoxifen treated BB-88 cells (24 hours, 10 μg/ml) showing clumping, cell shrinkage, and membrane blebbing (B) (× 3000). (c) BB-88 cells treated with tamoxifen (48 hours, 10 μg/ml) showing clumping, cell membrane blebbing (B), holes (H), and cytoplasmic extrusions (E) (× 3500).
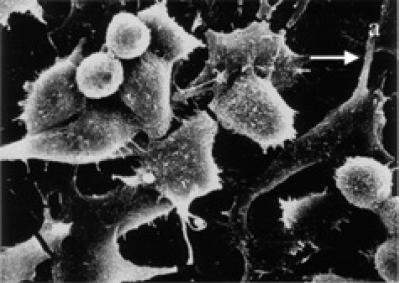
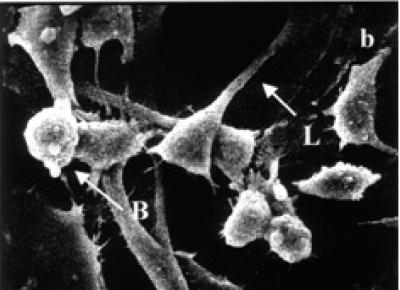
Control HeLa cells are flattened in shape and show numerous microvilli on the cell surface with extending lamellipodia and membrane connections (see Figure 4a). Occasional presence of rounded cells and blebbings on the cell surface are characteristics of HeLa cells in culture. In contrast, treated HeLa cells exhibited shrinkage and narrowing of lamellipodia with blunt microvilli. Compared to control cells, the tamoxifen treated cells produced many detached osmiophilic electron dense cells with conspicuous blebs, or apoptotic like bodies on the cell surface (see Figures 4b and 4c).
Figure 4.
SEM micrographs of surface ultrastructural characteristics of HeLa cells treated with and without tamoxifen. (a) Cell surface characteristics of 48 hours untreated HeLa cells showing numerous microvilli on the cell surface and lamellipodia extensions (L) (× 1500). (b) Tamoxifen treated HeLa cells (48 hours, 5 μg/ml) exhibiting cell surface blebbing (B) and narrowing of cells with elongated and shortened lamellipodia (L). Cell detachment is visible (× 1800). (c) 48 hours treated (15 μg/ml) HeLa cells depicting many blebs or apoptotic bodies (B) on the cell surface, broken lamellipodia, and cytoplasmic extrusions (E). Cell shrinkage and detachment are evident (× 1500).
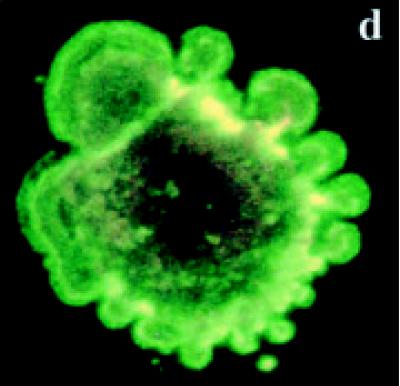
Detection of the bound annexin V-EGFP
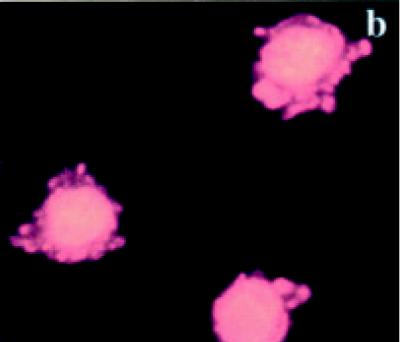
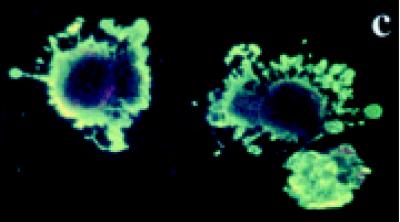
Untreated BB-88 cells were spherical in shape and both the nucleus and the plasma membrane appeared red due to the nuclear counterstain propidium iodide (PI) (see Figures 5a and 5b). On occasions, dying or dead cells were detected in the untreated group with blebs and bound annexin V-enhanced fluorescent green protein (EGFP) which stained the cell membrane green. A higher proportion of tamoxifen treated BB-88 cells showed green staining in the plasma membrane resulted from the bound annexin- V protein to the redistributed phosatidylserine (PS) translocated on the surface of cells possibly undergoing apoptosis (see Figures 5c and 5d). Apoptosis is indicated by a green staining (EGFP) plasma membrane surrounding the lightly stained (PI) nucleus, as seen in Figures 5c and 5d [21].
Figure 5.
Immunofluorascence staining with annexin V-enhanced green fluorescent protein (EGFP) and propidium iodide (PI). (a), (b) Control BB-88 cells (24 hours) showing red staining due to PI throughout the cell (× 400). (c), (d) Tamoxifen treated BB-88 cells (8 hours, 5 μg/ml) showing annexin V-EGFP green staining in the plasma membrane ((c) × 400, (d) × 900) and blebs on cells undergoing apoptosis.
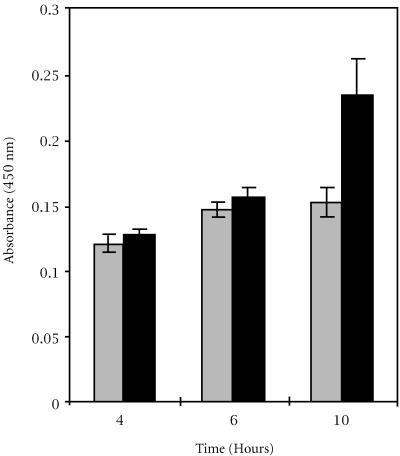
Nucleosome ELISA assay
Tamoxifen treatment (10 μg/ml) of HeLa cells for 4, 6, and 10 hours yielded a higher absorbance than their respective controls (see Figure 6). A trend was identified that showed an increase in absorbance among the tamoxifen treated lysates samples indicating an increase in the degradation of genomic chromatin into free nucleosomes. The increases in absorbance for the 4 and 6 hours tamoxifen treatments, compared to their corresponding controls, were not significantly different. However, the increase in absorbance for the 10 hours treatment group was statistically significant (ANOVA, P < 0.05).
Figure 6.
Degradation of chromatin into free nucleosomes of HeLa cells treated with 10 μg/ml tamoxifen (black bars) compared to the control (gray bars), detected by the Nucleosome ELISA assay. Absorbance (450 nm) is a function of time after the drug treatment. Ten hour treatment period is significantly different from the control.
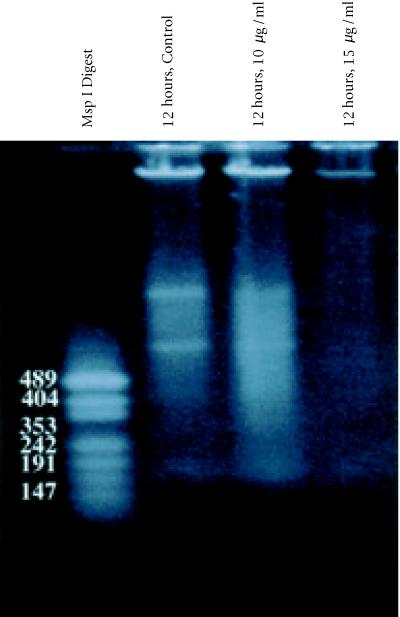
DNA laddering pattern
DNA was isolated from tamoxifen treated BB-88 cells and subjected to agarose gel electrophoresis to visualize the DNA laddering pattern. Tamoxifen induced degradation of genomic DNA was indicated by DNA smear patterns that are different from those of the controls. Tamoxifen treatment lanes revealed banding in the first half of the gel similar to the control, but visible DNA smearing patterns continued, contiguous to the smaller KB ladder fragments (see Figure 7). A faint DNA smearing observed in lane 4 (12 hours/ 15 μg/ml) was possibly due to the drastic effect of the drug on the cell viability which might have resulted in a severe degradation of nucleosomes.
Figure 7.
Agarose gel (0.8%) of DNA extracted from BB-88 cells: Lane 1, KB Ladder; Lane 2, Control (12 hours); Lane 3, tamoxifen treatment (12 hours, 10 μg/ml); Lane 4, tamoxifen treatment (12 hours, 15 μg/ml). Only a faint DNA smearing is visible in the 15 μg/ml treatment group.
DISCUSSION
Tamoxifen, a promising anticancer drug, is reported to exert antiproliferative and cytotoxic effects in breast and non-breast cancer cells [16]. A study performed by Khar and Ali [13] showed that tamoxifen had no effect on HeLa cells when they were grown in the presence of fetal calf serum and that tamoxifen's toxic effects were only seen under serum-free conditions. Our study, however, showed an impediment of HeLa cell multiplication with as little as 5 μg/ml tamoxifen grown in DME supplemented with 10% fetal calf serum. At higher concentrations, the agent exerted drastic effects on cell growth and viability. In addition, our cell multiplication study ensured that the cell growth inhibition was due to the drug and not a limited amount of nutrients or waste build-up because the drug and media were removed and replaced daily. Similar adverse effects of the agent were reported on murine erythroleukemic BB-88 cells [14, 24]. At lower tamoxifen concentrations (3 and 5 μg/ml), HeLa cell growth was minimally inhibited. At 5 μg/ml, however, the initial decrease in cell number and subsequent increase in cell growth could be due to a shock effect leading to a longer lag phase. After the lag phase, however, the cells in 5 μg/ml multiplied at a slower rate.
Disruption of normally integrated cell cycle progression can trigger a cascade of morphological and biological events. Morphological studies performed in this investigation through light microscopy and scanning electron microscopy lend evidence that an apoptotic pathway is occurring with tamoxifen treatment. Although a few cells from the control groups resembled those from treated groups, tamoxifen caused morphological alterations in a large proportion of cells. Other studies support these findings; for example, when cell injury was induced, cells undergoing apoptosis were characterized by shrinkage of cells, condensation of nuclear material, cell membrane blebbing, apoptotic bodies, and narrowing of lamellipodia [5, 16, 25]. Cytological results of Perry et al. [16] and Welsh [26] showed inhibitory effects of tamoxifen on breast cancer cells (MCF-7) and demonstrated evidence of apoptosis in treated cells. They documented incidences of enucleation, blebbing, pyknotic fragments, and apoptotic bodies; similar changes were also found in this study in treated HeLa and/or BB-88 cell lines.
Adherent epithelial cells characteristically produce lamellipodia with leading and trailing edges [27]. The lamellipodium involves an assembly of actin monomers into filaments and contains many actin, cross-linking, severing, and bundling proteins. Previous studies with human melanoma cells have shown that cells which failed to express the crosslinking protein ABP280 were unable to form lamellipodia and formed spherical blebs at the membrane surfaces [28]. The shape distortions and membrane blebbing are suggested to be stemmed from actin filament dysfunction [27]. The formation of blebs or apoptotic bodies is probably due to inactivation of focal adhesion kinase (FAK), which leads to a disruption of cell adhesion and a subsequent detachment of cells from the substratum and their neighbors [29].
Immunofluorescence studies further confirmed the evidence of apoptosis in tamoxifen treated murine erythroleukemic cells (BB-88). The plasma membrane is composed of a distribution of phospholipids, such as phosphatidyleserine (PS). Annexin V-EGFP is a protein that has a strong affinity for the exposed PS on the cell surface and stains the plasma membrane green when examined by fluorescence microscopy [14]. In this study plasma membranes of many tamoxifen treated cells appeared green possibly due to the binding of annexin V-EGFP to the exposed PS on the cell surface. The redistribution of the plasma membrane PS is one of several events in cells undergoing apoptosis [10]. Studies have suggested that PS may serve as a “flag” or identifying surface marker that help the removal of apoptotic cells by macrophages [10, 11].
Our finding is in agreement with the study made by Martin et al. [10] and Verhoven et al. [11], who observed fluorescent bound PS translocation in apoptotic Jurkat cells and T lymphocytes, respectively. Cell membranes of many treated BB-88 cells were covered with spherical blebs. Annexin binding to apoptotic cells is normally concentrated in the membranes of cell surface blebs. It has been suggested that translocation of PS from the inner leaflet to the outer leaflet of the cell membrane can be attributed to enzymes such as flippase, scrambalase, or translocase which facilitate bidirectional bilayer movement of all phospholipids due to its strong affinity for them [11].
In previous investigations, DNA damage was observed in various cell types undergoing apoptosis [5, 30, 31, 32]. Many chemicals and anticancer drugs are known to induce apoptosis [15, 16, 32, 33]. During apoptosis, endonucleases are activated that cleave the genome at DNA linker regions between adjacent nucleosomes, causing the formation of mono- and oligonucleosomes [5, 31]. The Nucleosome ELISA is a quantatative assay that detects DNA fragments through a colorimetric reaction due to histone protein binding. In this study, a significant increase in DNA fragmentation was detected by Nucleosome ELISA at 10 hours of tamoxifen treatment, indicating an induction of apoptosis by tamoxifen. However, at 4 and 6 hours duration, the beginning stages of apoptosis might have resulted in limited chromatin degradation.
The DNA laddering is a hallmark of apoptosis [16, 34]. Apoptotic cleavage of genomic DNA can be identified as DNA fragments or smear on agarose gels. The smearing patterns\linebreak observed in this study occurred in the treated cell lanes towards the smaller KB fragments, as opposed to the control cell lane. The smearing is caused by the cleavage of DNA into low molecular weight fragments that travel according to size on an agarose gel. This is in agreement with a study performed by Perry et al. [16], who reported tamoxifen's DNA cleaving ability and subsequent apoptosis in the breast cancer cell line MCF-7. Our study showed DNA degradation ability of the agent in the non-breast cancer murine erythroleukemic cells (MEL BB-88).
Apoptosis is a complex system initiated by various internal signaling pathways. There are evidences which show that mitochondria play a central role in triggering the programmed cell death [35, 36]. On the other hand, numerous experimentations have shown that caspase activated DNase (CAD) degrades genomic DNA in the activation of apoptosis [25, 37, 38, 39, 40]. It is clear from these and other studies that apoptosis is under genetic control, such as by tumor suppressor genes (i.e., p53, BRAC1, and BRAC2) and oncogenes (i.e., myc and the Bcl-2 family), see [25, 34, 36, 41].
It is necessary that the basic mechanism of tamoxifen's action on various cell types be known in order to maximize its effects on cancer cells. The sequence of events leading to apoptosis induced by tamoxifen is still unclear. Many clinical studies have examined the chemotherapeutic nature of this chemical in breast cancer patients [42, 43]. In vitro studies with tamoxifen and its metabolite, 4-hydroxytamoxifen have been done utilizing estrogen receptor positive cell lines and, as in this study, estrogen receptor negative cell lines [16, 44]. Cytotoxic effects were documented in these investigations. Therefore, tamoxifen's anticancer action may not be solely dependent on the interference of estrogen receptor sites.
Despite these uncertainties, the cytostatic and cytotoxic effects of tamoxifen on various cancer cells are clear and support tamoxifen's role as a chemotherapeutic agent on certain cancer types. In this investigation, tamoxifen, depending on the concentrations and exposure duration, was found to be cytostatic or cytotoxic, and caused morphological changes and DNA degradation in the two non-breast cancer cell lines. The results suggest that tamoxifen induced cell death through an apoptotic pathway.
References
- Sterns V, Gelmann EP. Does tamoxifen cause cancer in human? J Clin Onco. 1998;16:779–792. doi: 10.1200/JCO.1998.16.2.779. [DOI] [PubMed] [Google Scholar]
- Jordan CV. Designer estrogens. Scien Amer. 1998;279:60–67. doi: 10.1038/scientificamerican1098-60. [DOI] [PubMed] [Google Scholar]
- Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45–52. doi: 10.3181/00379727-217-44204. [DOI] [PubMed] [Google Scholar]
- Fernandez P, Rotello R, Rangini Z, et al. Expression of a specific marker of avian programmed cell death in both apoptosis and necrosis. Proc Natl Acad Sci USA. 1994;91:8652–8655. doi: 10.1073/pnas.91.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton M. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992;11:105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35:297–303. doi: 10.1007/BF00689448. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Smith S, Jones M, et al. Do all programmed cell deaths occur via apoptosis? Proc Natl Acad Sci USA. 1993;90:980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomei LD, Shapiro JP, Cope FO. Apoptosis in C3H/10T mouse embryonic cells: evidence for internucleosomal DNA modification in the absence of double-strand cleavage. Proc Natl Acad Sci USA. 1993;90:853–857. doi: 10.1073/pnas.90.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CPM, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenman S, Shapira A, Carey TE. In vitro response of cervical cancer cell lines CaSki, HeLa, and ME-180 to the antiestrogen tamoxifen. Gynecol Oncol. 1988;30:228–238. doi: 10.1016/0090-8258(88)90029-7. [DOI] [PubMed] [Google Scholar]
- Khar A, Ali AM. Serum protects HeLa cells from antiestrogen effects in culture. Euro J Cancer and Clin Oncol. 1987;23:761–763. doi: 10.1016/0277-5379(87)90275-6. [DOI] [PubMed] [Google Scholar]
- Bonita DM, Eraydin NB, Majumdar SK. Response of non-breast cancer cells to tamoxifen in the presence and absence of β-estradiol. The Nucleus. 1999;42:49–55. [Google Scholar]
- Candi E, Melino G, De Laurenzi V, et al. Tamoxifen and somatostatin affect tumors by inducing apoptosis. Cancer Lett. 1995;96:141. doi: 10.1016/0304-3835(95)03924-l. [DOI] [PubMed] [Google Scholar]
- Perry RR, Kang Y, Greaves B. Effects of tamoxifen on growth and apoptosis of estrogen-dependent and independent human breast cancer cells. Ann Surg Oncol. 1995;2:238–245. doi: 10.1007/BF02307030. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Boldt D, Clark G, et al. Effects of tamoxifen on human breast cancer cell cycle kinetics: accumulation of cells in early G1 phase. Cancer Res. 1993;43:3583–3585. [PubMed] [Google Scholar]
- Ahearn GS, Daehler CC, Majumdar SK. Serum-free media for murine erythroleukemia cells still not as good as serum-supplemented media. In Vitro Cell Dev Bio. 1992;28:227–229. doi: 10.1007/BF02634236. [DOI] [PubMed] [Google Scholar]
- Ruddy JM, Marc C, Majumdar SK. In vitro cytotoxic evaluation of fumagillin, a potent anti-angiogenic chemical on mouse and human cancer cell lines. Pharmacol Toxicol. 2000;1(2):1–11. [Google Scholar]
- Oncor®, ApopTag® . Gaithersburg, MD: 1998. In situ apoptosis detection kits. [Google Scholar]
- Biovision Research Products . Palo Alto, Canada: 1999. Annexin V-EGFP Apoptosis Detection Kit. [Google Scholar]
- Oncogene™ Research Products . Cambridge, Massachusetts: 1999. Nucleosome ELISA kit. [Google Scholar]
- Trevigen, Inc. TACS™ . Gaithersburg, MD: Ethidium bromide apoptotic DNA laddering kit. [Google Scholar]
- Majumdar SK, Eraydin NB, Bonita DM, et al. Antiproliferative effect of tamoxifen on two non-breast cancer cell types. In Vitro Cell Dev Bio. 1999;35:33–33. [Google Scholar]
- Sakahira H, Masato E, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during Apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Welsh J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem Cell Biol. 1994;72:537–545. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- Karp G. John Wiley & Sons, Inc.; New York, NY: 1999. Cell and Molecular Biology; pp. 700–732. [Google Scholar]
- Stossel TP. The machinery of cell crawling. Science. 1993;260:1087. [Google Scholar]
- Varner JA, Cheresh DA. Integrins and Cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- McConkey DJ, Hartzell P, Orrenius S. Rapid turnover of endogenous endonuclease activity in thymocytes: effects of inhibitors of macromolecular synthesis. Arch Biochem Biophys. 1990;278:284–287. doi: 10.1016/0003-9861(90)90261-v. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Inter Rev Cytol. 1980;68:251–305. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Bachman CA, Bills DA, Majumdar SK. Evidence of p53-induced apoptosis in cancer cells exposed to taxol. In vitro Cell Dev Biol. 1997;34:434–435. doi: 10.1007/s11626-998-0072-3. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Shen L, Glazer R. Introduction of apoptosis in glioblastoma cells by inhibition of protein kinase C and its association with the rapid accumulation of p53 and induction of the insulin-like growth factor-1-binding protein-3. Biochem Pharmacol. 1998;55:1711–1719. doi: 10.1016/s0006-2952(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Finkel E. The mitochondrion: is it central to apoptosis? Science. 2001;292:624–626. doi: 10.1126/science.292.5517.624. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong W-X, Cheng EH-Y, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondria dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, et al. A caspase activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Kirsch G, Clem RJ, et al. Conversion of Bcl-2 to a bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Deshmukh M. Caspases: a treatment target for neurodegenerative disease? Nat Med. 1997;3:954–955. doi: 10.1038/nm0997-954. [DOI] [PubMed] [Google Scholar]
- Kothakota S. Caspase 3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Leslie P. Life or death in cells. The Scientist. 2001;15:18–19. [Google Scholar]
- Bradbury J. How tamoxifen induces apoptosis. Lancet. 1996;347:818. [Google Scholar]
- Takebayashi H, Oida H, Fujisawa K, et al. Hormone-induced apoptosis by Fas-nuclear receptor fusion proteins: novel biological tools for controlling apoptosis in vivo . Cancer Res. 1996;18:4164–4170. [PubMed] [Google Scholar]
- Taylor CM, Blancard B, Zava DT. Estrogen receptor-mediated cytotoxic effects of the antiestrogens tamoxifen and 4-hydroxytamoxifen. Cancer Res. 1984;44:1409–1414. [PubMed] [Google Scholar]