Abstract
Translesion DNA synthesis (TLS) and homologous DNA recombination (HR) are two major pathways that account for survival after post-replicational DNA damage. TLS functions by filling gaps on a daughter strand that remain after DNA replication caused by damage on the mother strand, while HR can repair gaps and breaks using the intact sister chromatid as a template. The RAD18 gene, which is conserved from lower eukaryotes to vertebrates, is essential for TLS in Saccharomyces cerevisiae. To investigate the role of RAD18, we disrupted RAD18 by gene targeting in the chicken B-lymphocyte line DT40. RAD18–/– cells are sensitive to various DNA-damaging agents including ultraviolet light and the cross-linking agent cisplatin, consistent with its role in TLS. Interestingly, elevated sister chromatid exchange, which reflects HR- mediated post-replicational repair, was observed in RAD18–/– cells during the cell cycle. Strikingly, double mutants of RAD18 and RAD54, a gene involved in HR, are synthetic lethal, although the single mutant in either gene can proliferate with nearly normal kinetics. These data suggest that RAD18 plays an essential role in maintaining chromosomal DNA in cooperation with the RAD54-dependent DNA repair pathway.
Keywords: genome instability/homologous DNA recombination/post-replication repair/RAD18/translesion DNA synthesis
Introduction
Various types of DNA lesions are generated continuously not only by environmental factors but also by naturally occurring damage during DNA synthesis. Estimates of the number of various types of DNA damage produced daily per human genome range from a few to several thousand (Lindahl, 1993; Kunkel et al., 1999). The majority of spontaneous and induced DNA damage appears to be repaired efficiently by excision repair pathways. However, some types of DNA damage that are known to arrest a DNA replication fork could cause a daughter strand gap, if excision repair does not eliminate such DNA lesions on mother strands before they encounter replication forks (Wyatt et al., 1999). This daughter strand gap may lead to a double-strand break (DSB) in the sister chromatid (Haber, 1999; Flores-Rozas and Kolodner, 2000). If a DSB is left unrepaired, a DNA damage checkpoint may be activated, leading to programmed cell death (reviewed in van Gent et al., 2001). Thus, DNA replication across an existing lesion can result in more severe damage, including gaps and chromatid breaks. Cells have evolved methods of post-replication DNA repair (PRR) to remove such secondary DNA lesions. It is believed that PRR is carried out mainly by two pathways: translesion DNA synthesis (TLS) and homologous recombination (HR) repair using the intact sister chromatid (reviewed in Friedberg et al., 1995). While the phenotypes of mutants in HR pathway genes in vertebrate cells are relatively well characterized (Sonoda et al., 2001), the role of TLS in maintenance of chromosomal DNA is not clear because of the absence of genetic studies in vertebrate cells. For the same reason, the functional relationship between TLS and HR in vertebrate cells needs to be elucidated.
Genetic studies of Saccharomyces cerevisiae revealed that all known components of PRR, including RAD6 (UBC2), RAD18, REV1, REV3 and REV7, belong to the RAD6/RAD18 epistasis group ((McDonald et al., 1997; reviewed in Lawrence, 1994; Broomfield et al., 2001). Consistent with a function in TLS, yeast rad6 and rad18 mutants are extremely sensitive to killing by DNA-damaging agents, including ultraviolet (UV) light, ionizing radiation (IR), DNA alkylating agents and DNA cross-linking agents such as mitomycin C and cisplatin (Lawrence and Christensen, 1976; Prakash, 1981; Fabre et al., 1989). It is known that Rad6 is a ubiquitin-conjugating enzyme (E2), forming a tight complex with the Rad18 protein. Rad18 has DNA-binding activity, and may recruit Rad6 protein to DNA lesions (Bailly et al., 1994). Rad18 contains a RING-finger motif, which has been shown to be common to E3 ubiquitin ligases. Thus, RAD18 may be involved in the transfer of a ubiquitin molecule from the Rad6 E2 enzyme onto its target proteins. It is unclear which target molecules are ubiquitylated in the RAD6/RAD18-dependent DNA repair pathway. Likewise, the mechanism by which the RAD6/RAD18 ubiquitin machinery regulates translesion DNA polymerases has still to be elucidated. In mammals, there are two homologs of the RAD6 gene, HR6A and HR6B, and a single RAD18 gene (Koken et al., 1991; Roest et al., 1996; Baarends et al., 1999; van der Laan et al., 2000). Human and mouse Rad18 proteins can interact with both HR6A and HR6B proteins (Tateishi et al., 2000; Xin et al., 2000), implying that mammalian RAD6 and RAD18 could work cooperatively, as in yeast. Mice deficient in the HR6B gene showed defective spermatogenesis but no obvious defects in DNA repair, probably because of the redundant function of HR6B and HR6A (Roest et al., 1996). However, the function of RAD18 in vertebrates remains to be elucidated due to the lack of genetic studies.
In order to investigate the role of RAD18 in vertebrate cells, we generated RAD18-deficient cells from the chicken B-lymphocyte line DT40 (Buerstedde and Takeda, 1991). Here we show that deletion of RAD18 leads to UV sensitization, consistent with a role in TLS. We also found that RAD18–/– RAD54–/– double mutant cells are unable to proliferate, suggesting that these two genes cooperatively play an essential role for cell survival. The functional relationship between RAD18 and the RAD54-dependent HR pathway is discussed.
Results
Isolation of the chicken RAD18 gene and generation of RAD18-deficient cells
We isolated the chicken RAD18 cDNA, which encodes a protein of 501 amino acids with a predicted mol. wt of 55 kDa (Figure 1). The chicken Rad18 protein shows ∼50% identity to the human and mouse Rad18 proteins, and ∼25% identity to the yeast Rad18 proteins (S.cerevisiae Rad18 and Schizosaccharomyces pombe Rhp18/Rad6). Sequence comparison between the homologs shows that Rad18 protein is highly divergent. Even mouse and human homologs show only ∼65% identity overall. However, sequence conservation was significant within the RING-finger domain located at the N-terminus, with ∼70% identity to human and mouse Rad18. All the cysteine and histidine residues that constitute the RING-finger motifs (C-C-C-H-C-C-C-C) were perfectly conserved at precise intervals from yeast to human (Figure 1, asterisks), suggesting an important role of the RING-finger for RAD18 function.
Fig. 1. Comparison of amino acid sequences of the Rad18 proteins. Amino acid sequence comparison between chicken, human, mouse, budding yeast (Sc) and fission yeast (Sp) Rad18 proteins. RING-finger motifs are marked with asterisks (*). The B-box is marked with +. Highlighted letters represent identical amino acids among more than three species. Shaded letters represent similar (P, A, G, S and T; E, D, N and Q; V, I, L and M; F, W and Y; R, K and H) amino acids conserved among more than three species. Numbers denote amino acid positions.
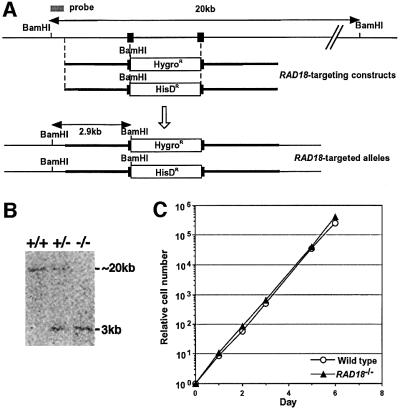
Genomic DNA fragments around the RAD18 coding region (Figure 2A) were amplified by PCR from DT40 cells. Gene targeting constructs were generated from the amplified genomic DNA. Gene targeting events are expected to insert a premature stop codon immediately after amino acid 162 and to delete the genomic sequences that encode amino acids 163–182 of the chicken Rad18 protein. Gene targeting of the RAD18 locus was confirmed by Southern blot analysis of BamHI-digested genomic DNA for the appearance of a 2.9 kb band as well as the disappearance of a >20 kb band, which corresponds to the intact allele (Figure 2B). Because all five RAD18–/– DT40 clones obtained showed essentially the same phenotype, we only show data from two representative clones hereafter. The proliferative properties of RAD18–/– cells were monitored using growth curves (Figure 2C), showing that RAD18–/– cells are able to proliferate with normal kinetics. We conclude that RAD18 is not essential for cell viability.
Fig. 2. Gene targeting of the RAD18 locus. (A) Schematic representation of part of the RAD18 locus, the gene disruption constructs and the configuration of the targeted alleles. Solid boxes indicate the positions of the exons. Only disrupted exons are indicated. (B) Southern blot analysis of BamHI-digested genomic DNA from cells with the indicated genotypes of the RAD18 gene, using the probe shown in (A). The positions and sizes of hybridizing fragments of the wild-type and targeted loci are indicated. (C) Growth curves of cells of the indicated genotypes. Similar results were obtained in at least three independent experiments.
RAD18–/– cells are highly sensitive to UV and cisplatin
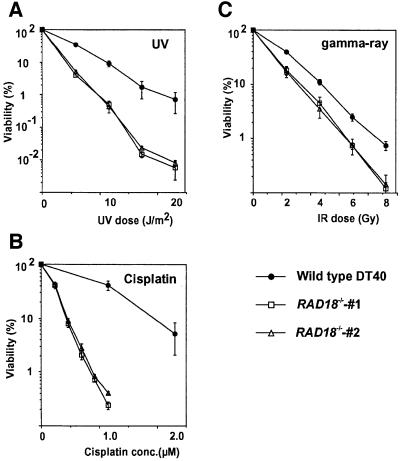
To analyze the DNA repair capacity of RAD18–/– cells, we examined the viability of wild-type and RAD18–/– cells after genotoxic treatment by colony survival assays. RAD18–/– cells were hypersensitive to cisplatin, moderately sensitive to UV and mildly sensitive to IR, when compared with wild-type DT40 cells (Figure 3). Elevated sensitivity to these DNA-damaging agents is reminiscent of the phenotype of yeast mutants of the RAD6/RAD18 epistasis group genes (McKee and Lawrence, 1980; Keszenman et al., 1992). To investigate the cause of cell death following exposure to DNA-damaging agents, we measured cytologically detectable chromosomal breaks, which are believed to reflect DSBs, as previously described (Sonoda et al., 1998). After the treatment with each DNA-damaging agent, RAD18–/– cells showed ∼2- to 4-fold more chromosome aberrations compared with wild-type cells (data not shown). We assume that the elevated chromosome aberrations account for the sensitivity of RAD18–/– cells to various types of DNA-damaging agents.
Fig. 3. Sensitivity of clones of the wild-type and RAD18–/– cells to DNA-damaging agents. The fractions of surviving colonies after the indicated treatment of cells compared with untreated controls of the same genotype are shown on the y-axis on a logarithmic scale. (A) UV; (B) cisplatin (CDDP; continuous exposure); (C) γ rays. The dose of 137Cs γ and UV, and concentrations of cisplatin are displayed on the x-axis on a linear scale in each graph. The data shown are means ± SE of at least three separate experiments.
RAD18–/– cells are defective in post-replication DNA repair of UV damage
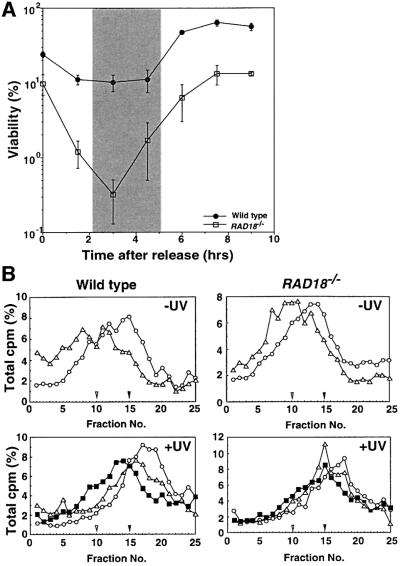
To address whether or not RAD18 is involved in PRR as in yeasts, we examined the cell cycle-specific sensitivity of RAD18–/– cells to UV damage. Cells were synchronized at G1 phase by an elutriation method (Takata et al., 1998), and released into fresh media. At various times after the release, the UV sensitivity of wild-type and RAD18–/– cells was measured by colony survival assay. Consistent with the idea that RAD18 is involved in PRR, the sensitivity of RAD18–/– cells to UV damage was most striking after the onset of S phase (Figure 4A).
Fig. 4. Defective PRR following UV irradiation in the absence of RAD18. (A) Cell cycle-specific sensitivity of RAD18–/– cells. Wild-type and RAD18–/– cells were synchronized at G1 phase by elutriation. Cell cycle phase was determined by the incorporation of BrdU followed by fluorescence-activated cell sorting analysis (data not shown) and is shown as a shaded box. At the time points indicated after release, cells were irradiated with UV at 2.5 J/m2 and cell survival was measured. RAD18–/– cells are most sensitive to UV irradiation after the onset of DNA replication. (B) Impaired post-replication repair of RAD18- deficient DT40 cells. Top panels: wild-type cells or RAD18-deficient cells were pulse labeled with [3H]thymidine (0.93 MBq/ml) for 15 min (open circles). In a pulse–chase experiment, the pulse-labeled cells were incubated further for 30 min (open triangles) in fresh medium containing 10 µM unlabeled thymidine and uridine. Samples were sedimented on 5–20% alkaline sucrose gradients from right to left. Bottom panels: wild-type and RAD18-deficient DT40 cells were irradiated with UV light (8 J/m2), incubated for 10 min, and then pulse-labeled with [3H]thymidine (0.93 MBq/ml) for 15 min (open circles). In a pulse– chase experiment, the pulse-labeled cells were incubated further for 30 min (open triangles) or 90 min (closed squares) in the chase medium. Left: wild-type cells. Right: RAD18-deficient cells. Closed and open arrowheads indicate the positions of bacteriophage λ DNA (42 kb) and T4GT7 DNA (165.6 kb), respectively.
To assess directly the ability of RAD18–/– cells to carry out PRR, we measured the size of newly replicated DNA following UV irradiation (Prakash, 1981). In the absence of UV irradiation (Figure 4B, top panels), the size of labeled DNA in RAD18–/– cells was increased to the same extent as that of wild-type cells at 30 min after pulse labeling (open triangles) when compared with 0 min (open circles), suggesting that both types of cells underwent constant DNA synthesis during the chase period. After UV irradiation, wild-type cells showed a constant increase in DNA size from 30 min (open triangles) to 90 min (closed squares) after pulse labeling (bottom left panel). In contrast, UV-irradiated RAD18–/– cells showed no significant increase in DNA size especially from 30 to 90 min after pulse labeling (bottom right panel), suggesting that RAD18–/– cells were not able to continue DNA synthesis after UV irradiation. This result is reminiscent of that seen in XP-V cells, and indicates that depletion of RAD18 results in an accumulation of DNA gaps. Taken together, we conclude that RAD18 is involved in PRR in vertebrate cells as well as in yeast.
Sister chromatid exchange is enhanced in RAD18–/– cells
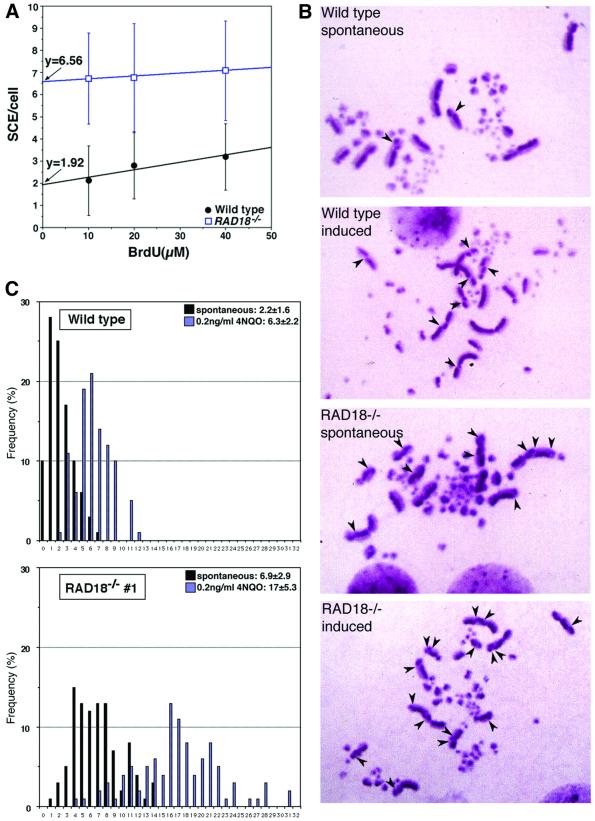
Since TLS and HR constitute post-replication repair pathways in yeast (Broomfield et al., 2001), we wished to know which pathway is mainly affected in RAD18–/–. To evaluate HR-mediated repair events, we determined the level of microscopically visible sister chromatid exchange (SCE) events (Sonoda et al., 1999; Dronkert et al., 2000). We first estimated the contribution of bromodeoxyuridine (BrdU), which is used for SCE staining (see Materials and methods), to the induction of SCE. We measured SCE levels with various concentrations of BrdU and assumed that the extrapolation of SCE events to the y-axis represents the spontaneous SCE level without BrdU (Figure 5A). This estimation indicates that the few SCEs observed in normally cycling wild-type DT40 cells are not solely induced by BrdU, but occur mostly spontaneously, as observed in human cells (Galloway and Evans, 1975; Crossen et al., 1977). Remarkably, RAD18–/– cells showed an ∼3-fold higher spontaneous SCE level than did wild-type cells (Figure 5B and C), indicating that a defect in RAD18 leads to enhancement of HR-mediated repair events even in the absence of genotoxic treatments.
Fig. 5. Sister chromatid exchange is elevated in RAD18–/– cells. (A) Wild-type DT40 and RAD18–/– cells were cultured with various concentrations of BrdU for two rounds of the cell cycle (16 h), and SCE levels were measured. The value of extrapolation to the y-axis is shown. (B) SCEs of wild-type DT40 and RAD18–/– cells. Sister chromatid stain of wild-type and RAD18–/– cells treated or not with 4NQO is shown as indicated. Arrowheads indicate the sites of SCE. (C) Level of SCE per cell in wild-type DT40 (top panel) and RAD 18–/– (bottom panel). Mean ± SE is shown at the top of each panel. Solid box, distribution of SCEs/cell without 4NQO treatment; shaded box, distribution of SCEs/cell with 0.2 ng/ml 4NQO treatment.
We next measured the level of SCE following exposure of cells to 4-nitroquinoline 1-oxide (4NQO), which damages base residues in a manner similar to UV irradiation. The level of induced SCE was 6.3 ± 2.2 SCEs/cell for wild-type cells and 17 ± 5.3 SCEs/cell for RAD18–/– cells (Figure 5B and C). Thus, a defect in RAD18 indeed elevated HR-mediated repair events.
We interpreted the increased level of SCE in RAD18–/– cells as preferential usage of the HR pathway due to the lack of the TLS pathway. However, it is also possible that the HR pathway is hyperactivated in RAD18–/– cells. To address this question, we evaluated the capability of the HR pathway by examining the frequency of targeted integration at the ovalbumin locus (Buerstedde and Takeda, 1991). The frequency of targeted integration was reduced in RAD18–/– cells compared with wild-type cells (Table I), indicating that hyperactivation of the HR pathway is not likely. Thus, we conclude that the elevated SCE in RAD18–/– cells may be caused by more frequent usage of HR-mediated repair in the mutant cells than in wild-type cells.
Table I. Targeted integration frequencies in RAD18–/– cells.
| Genotype analyzed | Targeted integration frequency at the ovalbumin locus |
|---|---|
| Wild type | 44/50 (88%) |
| RAD18–/– no. 1 | 22/54 (41%) |
| RAD18–/– no. 2 | 17/57 (30%) |
Wild-type and RAD18–/– cells were transfected with the targeting construct of the ovalbumin locus. Two independent clones of RAD18–/– cells were examined.
Double mutants of Rad18 and Rad54 are unable to proliferate
To investigate further the functional relationship between the RAD18-dependent PRR pathway and HR, we generated cells deficient in both RAD18 and RAD54. We previously showed that RAD54-deficient DT40 cells exhibit hypersensitivity to γ rays and marked reduction in gene targeting efficiencies, but are able to proliferate with nearly normal kinetics (Bezzubova et al., 1997; Takata et al., 1998). We have failed to generate cells deficient in both RAD18 and RAD54. Thus, we generated conditional RAD18–/–RAD54–/– double mutant cells using the tamoxifen-inducible Cre-loxP system (Zhang et al., 1998; Fujimori et al., 2001) as summarized in Figure 6A. Upon the addition of tamoxifen to the culture media, the RAD54 transgene (designated as loxP-RAD54 hereafter) is eliminated by Cre-recombinase, generating mutant cells that completely lost functional RAD18 and RAD54 genes. As we reported previously, the Cre-mediated recombination worked efficiently in DT40 cells; loxP-RAD54 was eliminated in virtually all the cells within 3 days after the addition of tamoxifen, as verified by western blot analysis of Rad54 as well as the loss of transgene marker [green fluorescent protein (GFP) fluorescence; data not shown]. Since the tamoxifen-inducible Cre-loxP system is known to have genotoxic effects to some extent (Loonstra et al., 2001; Silver and Livingston, 2001), we generated RAD18–/– and RAD54–/– clones that carry the Cre-recombinase (hereafter called RAD18–/– w/CRE and RAD54–/– w/CRE cells) to normalize the toxic effect of the Cre-loxP system in the experiments.
Fig. 6. RAD18–/– RAD54–/– double mutant cells are not viable. (A) Schematic representation of conditional RAD18–/– RAD54–/– double mutant cells. We generated RAD18–/–RAD54–/– cells that carry the chimeric Cre-recombinase and a transgene containing both the chicken RAD54 and GFP genes flanked by loxP sequences on both sides (loxP-RAD54) (upper panel). Upon addition of tamoxifen to the culture media, both GdRAD54 and GFP transgenes are expected to be eliminated (lower panel). The efficiency of loxP-RAD54 transgene deletion was verified by flow cytometric analysis of GFP fluorescence. (B) Growth curves of the indicated cell cultures in the absence and presence of tamoxifen (TAM). The data shown are the averaged results from two separate clones of each genotype. (C) Chromosomal breaks are accumulated in dying RAD18–/–RAD54–/– double mutant cells. Cells were exposed to tamoxifen for 3 days. More than 50 mitotic cells were analyzed in each case.
We analyzed the proliferative properties of RAD18–/– w/CRE and RAD18–/– RAD54–/– (w/loxP-RAD54, CRE) cells in the absence and presence of tamoxifen by measuring their growth rate (Figure 6B) as well as by colony formation assay (data not shown). Although continuous exposure of RAD18–/– cells to tamoxifen reduced their proliferation rates to some extent (Figure 6B), RAD18–/– cells could proliferate exponentially. Expression of the Cre-recombinase did not affect the viability of RAD18–/– and RAD54–/– cells irrespective of the absence or presence of tamoxifen (data not shown). In marked contrast to these populations, the RAD18–/– RAD54–/– (w/loxP-RAD54, CRE) cells exhibited a dramatic reduction in growth rate in the presence of tamoxifen and eventually ceased proliferation (Figure 6B), suggesting that RAD18–/– RAD54–/– cells are lethal. However, since tamoxifen is known to have genotoxic effects and indeed RAD18–/– cells show a slight reduction of growth rate in the presence of tamoxifen, it is possible that RAD18–/– RAD54–/– cells are viable but extremely sensitive to tamoxifen. To exclude this possibility, we carried out the following experiments: RAD18–/– RAD54–/– (w/loxP-RAD54, CRE) cells were treated with tamoxifen for only 24 h, when the amount of Rad54 protein is reduced by <50% (data not shown), and transferred to tamoxifen-free media. About 20% of these cells were able to form colonies, all of which were found to retain the loxP-RAD54 transgene as verified by the presence of the transgene marker, GFP fluorescence (data not shown). From this result, together with the fact that no RAD18–/– RAD54–/– clone was obtained by directly targeting the RAD54 gene of RAD18–/–RAD54+/– cells, we conclude that cells deficient in both RAD18 and RAD54 are unable to proliferate.
To investigate the cause of cell death, we analyzed chromosomal breaks in dying RAD18–/– RAD54–/– cells. RAD18–/– (w/CRE) and RAD54–/– (w/CRE) cells showed few chromosomal aberrations (Figure 6C). These values were not dependent on tamoxifen. Remarkably, in contrast to these control populations, the RAD18–/– RAD54–/– cells had ∼0.35 aberrations per cell at day 3 after adding tamoxifen (Figure 6C). We previously showed that the level of spontaneous chromosomal breaks of various HR-deficient DT40 clones is closely correlated with the rate of cell death during the cell cycle (reviewed in Morrison and Takeda, 2000; Sonoda et al., 2001). These observations indicate that the increased chromosomal breaks may account for the massive cell death of RAD18–/– RAD54–/– cells.
Discussion
Vertebrate RAD18 is involved in post-replication DNA repair
Our data show that RAD18 is involved in the repair of diverse types of DNA lesions, including UV-, IR- and cisplatin-induced DNA damage. In addition to the hypersensitivity to UV and cisplatin, RAD18–/– cells exhibited higher levels of UV-induced chromosomal breaks, when compared with wild-type cells (data not shown). Presumably, DNA gaps caused by UV damage and subsequent DNA replication were left unrepaired more frequently in RAD18–/– cells than in wild-type cells. Furthermore, after UV irradiation, the size of newly replicated DNA strands in RAD18–/– cells was significantly smaller than that of wild-type DT40 cells (Figure 4B). Similar results were obtained in murine embryonic cells deficient in RAD18 (S.Tateishi and M.Yamaizumi, submitted) as well as in human cells overexpressing a dominant-negative form of the human RAD18 gene (Tateishi et al., 2000). These observations demonstrate that RAD18 is indeed involved in PRR in vertebrate cells as well as in yeast.
It is unclear how RAD18 controls PRR. Two major pathways for PRR are TLS and HR. We favor the possibility that RAD18 is involved in PRR mainly by regulating the TLS pathway, for the following reasons. First, RAD18–/– cells showed elevated levels of SCE in the presence and the absence of genotoxic reagents, which indicates that the activity of HR is not abolished in RAD18–/– cells. Secondly, the double mutant RAD18–/– RAD54–/– showed synthetic lethality. If RAD18 mainly controls the HR pathway, depletion of the HR pathway gene RAD54 from RAD18–/– cells might not cause such a dramatic effect.
The possibility that RAD18 also controls HR and/or other PRR pathways in addition to TLS remains to be verified in a future study. Indeed, we observed the decreased frequency of targeted integration (Table I), which indicates that a certain type of HR is affected in RAD18–/– cells, although the majority of HR is not halted in RAD18–/–, as exemplified by the elevated SCE level. Involvement of RAD18 in a copy choice DNA synthesis mechanism (Broomfield et al., 2001) is also plausible. Recently, it has been proposed that error-free PRR genes, RAD5 and MMS2, are involved in a copy choice DNA synthesis mechanism (Torres-Ramos et al., 2002). Because RAD5 and MMS2 genes are epistatic to RAD6 and RAD18, RAD18 could control PRR through a copy choice mechanism by controlling RAD5 and/or MMS2. Future study, including the generation of mutants in TLS genes (i.e. TLS polymerases, RAD5 and MMS2, etc.) and double mutants between RAD18 and those genes, would establish whether and how RAD18 controls TLS and other PRR mechanisms in vertebrate cells.
RAD18 plays an important role in maintaining chromosomal integrity during the cell cycle, by cooperating with RAD54-dependent HR pathways
Synthetic lethality of RAD18–/– and RAD54–/– mutations is surprising, given the fact that either single mutant causes only a slight phenotypes in terms of cell proliferation. RAD18–/– cells can grow at a normal rate compared with wild type (Figure 2C). Among the genes involved in HR, the RAD54 gene, when mutated, causes a relatively mild phenotype in both murine embryonic stem cells and DT40 cells, i.e. normal mouse embryogenesis, nearly normal rates of cell proliferation and only moderately elevated IR sensitivity (Bezzubova et al., 1997; Essers et al., 1997). Synthetic lethality of RAD18–/– and RAD54–/– mutations demonstrates that a defect in RAD18 is fully substituted by the RAD54-dependent HR pathway, while a defect in RAD54 can be substituted by the RAD18 in maintenance of chromosomal DNA during the cell cycle. Thus, the roles of a RAD18-dependent PRR pathway and RAD54-depdendent HR pathways may be significantly redundant in repairing replication-associated DNA damage. Presum ably, a large number of gaps are generated during DNA replication and, in the absence of RAD18, even a minor defect in HR, i.e. a defect in RAD54, may be lethal to the cells.
Cisplatin sensitivity of RAD18–/– cells
RAD18–/– cells showed an extreme sensitivity to cisplatin (Figure 3B). The cross-linking agent cisplatin, which is widely used in chemotherapy of cancer, forms covalent adducts with chromosomal DNA. Cross-linking agents form a variety of DNA adducts: intrastrand cross-links, interstrand cross-links and protein–DNA cross-links (reviewed in Zamble and Lippard, 1995). A number of repair pathways could be involved in repairing DNA damage caused by cisplatin, depending on the type of damage (reviewed in Dronkert and Kanaar, 2001). While yeast mutants deficient in the nucleotide excision repair (NER) or HR pathway show mildly increased sensitivity to cisplatin, rad6 and rad18 mutants exhibit dramatically increased sensitivity (Lawrence and Christensen, 1976). Similarly, loss of RAD18 in DT40 cells dramatically increased sensitivity to cisplatin. RAD18–/– cells showed the same pattern of chromosomal breaks following UV radiation (data not shown). These observations suggest that RAD18 may be involved in repairing cisplatin-induced DNA damage mainly in the same manner as it is involved in repairing UV damage. Further investigation based on these findings would provide insight into the molecular mechanisms of the chemotherapeutic function of cisplatin.
Materials and methods
Isolation of the chicken RAD18 gene
To isolate the chicken RAD18 gene, we designed oligonucleotide PCR primers based on conserved sequences between the human and mouse RAD18 homologs (Tateishi et al., 2000; van der Laan et al., 2000; Xin et al., 2000). By using these PCR primers, chicken cDNA fragments were isolated and proved to encode a protein that is highly homologous to human and mouse RAD18. RACE PCR was carried out to isolate the 3′ and 5′ termini of the chicken RAD18 gene.
Cell culture, DNA transfection and irradiation
Cells were cultured in RPMI 1640 supplemented with 10–5 M β-mercaptoethanol, 10% fetal calf serum and 1% chicken serum (Sigma, St Louis, MO) at 39.5°C. Methods for DNA transfection and genotoxic treatments have been described previously (Takata et al., 1998).
Plasmid construction
Two RAD18 disruption constructs, RAD18-hisD and RAD18-hygro, were generated from genomic PCR products combined with hisD and hygro selection marker cassettes. Genomic DNA sequences were amplified using the primers 5′-CCCATAACTATTGTTCCCTTTGCATACGG-3′ and 5′-TTTGCAAACTTTCCAATCTGTTCCTGCCAGACC-3′ (for the left arm of the KO construct); and 5′-GCAGGATCAAGAGAGTGTTCTGAAGGC-3′ and 5′-GGGATTTAGAGAATCACACTGAGCATTATACACGTGC-3′ (for the right arm of the KO construct), Amplified PCR products were cloned into pCRII-TOPO vecotor (Invitrogen). The 2.9 kb NheI (in genomic DNA)–BamHI (in pCRII) fragment from the left arm and the 3.1 kb EcoRV (in pCRII)–ApaI (in genomic DNA) fragment were cloned into pBluescript (Stratagene). The BamHI site was used to clone marker gene cassettes. The 0.3 kb BamHI–NheI fragment from the left arm was used to make the probe for Southern blot analysis. RAD54-puro and RAD54-bsr disruption constructs were described previously (Takata et al., 1998; Fukushima et al., 2001). We constructed an expression vector pCR3-loxP-RAD54/IRES-EGFP-loxP (named loxP-RAD54), in which RAD54 and GFP (EGFP) genes are flanked by the loxP sequences, by inserting the RAD54 BamHI fragment into the BamHI site of pCR3-loxP-MCS-loxP (Fujimori et al., 2001).
Generation of RAD18–/– and RAD18–/– RAD54–/–(w/loxP-RAD54, CRE) cells
The wild-type DT40 cells were transfected sequentially with RAD18-hygro and RAD18-hisD targeting constructs to obtain RAD18–/– cells. RAD18–/– cells were transfected with the RAD54-puro targeting construct. The resulting RAD18–/– RAD54+/– clone was transfected with loxP- RAD54, which can be selected by a neo selection marker. RAD18–/– RAD54+/– (w/loxP-RAD54) cells subsequently were transfected with the RAD54-bsr targeting construct to produce RAD18–/– RAD54–/– (w/loxP- RAD54) cells. Then, the plasmid containing CreER chimeric recombinase (pANMerCreMer) (Zhang et al., 1998) was co-transfected with an Eco-gpt marker plasmid into RAD18–/– RAD54–/– (w/loxP-RAD54, CRE) cells. We also transfected the plasmid containing the CreER chimeric recombinase into RAD18–/– and RAD54–/– cells, and obtained RAD18–/– w/CRE and RAD54–/– w/CRE cells.
Chromosome aberration analysis
Karyotype analysis was performed as described previously (Sonoda et al., 1998). For the morphological analysis of chromosome aberrations, cells were treated with colcemid for 3 h to enrich mitotic cells.
Measurement of SCE levels
Measurement of SCE levels was performed as described previously (Sonoda et al., 1999). Briefly, cells were cultured in the presence of 10 µM BrdU for 18 h and pulsed with 0.1 µg/ml colcemid for the last 2 h. 4NQO (0.2 ng/ml) was added 8 h before harvest. Harvested cells were treated with 75 mM KCl for 15 min, and subsequently fixed with methanol:acetic acid (3:1) for at least 30 min. Cells were fixed onto wet (50% ethanol) glass slides and dried on a 40–42°C plate. Dried slides were incubated with 10 µg of Hoechst 33258/ml in phosphate buffer pH 6.8 for 20 min, followed by rinsing with MacIlvaine solution (164 mM Na2HPO4, 16 mM citric acid pH 7.0). Slides were irradiated with black light (λ = 352 nm) for 60 min and incubated in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) solution at 62°C for 1 h before staining with 3% Giemsa solution pH 6.8 and subsequent microscopic observation.
Measurement of the size of newly synthesized DNA strands following UV irradiation
Post-replication repair was analyzed by the sedimentation velocity method. In brief, actively growing cells (105 cells/dish) were irradiated with UV light at 8 J/m2, incubated for 10 min in 2 ml of pre-warmed medium, and then pulse-labeled with 0.93 MBq/ml [methyl-3H]thymidine for 15 min. In pulse–chase experiments, the pulse-labeled cells were incubated further for 30 or 90 min in fresh medium containing 10 µM unlabeled thymidine and uridine. As a control, cells were treated in the same way without UV irradiation. The cells were lysed in the dishes by addition of 0.1 ml of lysis solution containing 0.2 M NaOH and 20 mM EDTA, and the lysates were irradiated with 20 Gy of X-rays on ice. Aliquots of the cell suspension were layered on the top of 3.8 ml of 5–20% (w/v) alkaline sucrose density gradients containing 0.2 M NaOH, 0.5 M NaCl, 10 mM EDTA and 0.5% sodium n-dodecanoyl-salcosinate, and centrifuged at 25 000 r.p.m. for 6 h at 4°C in a P56ST rotor (Hitachi). After centrifugation, drop fractions were collected directly onto GF/C glass filters (Whatman), and the acid-insoluble radioactivities were counted using a liquid scintillation counter.
Acknowledgments
Acknowledgements
We would like to thank Y.Sato and M.Nagao for their technical assistance, and Drs Roger Woodgate (NIH, USA), Nicolaas G.J.Jaspers (Rotterdam, The Netherland), Carmen C.Robinett (Stanford, CA) and D.Leanne Jones (Stanford, CA) for critical reading and discussion. Financial support was provided in part by CREST.JST (Saitama, Japan), a center of excellence (COE) grant for Scientific Research from the Ministry of Education, Science and Culture of Japan and by grants from The Uehara Memorial Foundation and The Naito Foundation. This work was funded in part by grants from the Virtual Research Institute of Aging of Nippon Boehringer Ingelheim. Y.Y. is a recipient of the JSPS.
References
- Baarends W.M., Hoogerbrugge,J.W., Roest,H.P., Ooms,M., Vreeburg,J., Hoeijmakers,J.H. and Grootegoed,J.A. (1999) Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev. Biol., 207, 322–333. [DOI] [PubMed] [Google Scholar]
- Bailly V., Lamb,J., Sung,P., Prakash,S. and Prakash,L. (1994) Specific complex formation between yeast RAD6 and RAD18 proteins: a potential mechanism for targeting RAD6 ubiquitin-conjugating activity to DNA damage sites. Genes Dev., 8, 811–820. [DOI] [PubMed] [Google Scholar]
- Bezzubova O., Silbergleit,A., Yamaguchi-Iwai,Y., Takeda,S. and Buerstedde,J.M. (1997) Reduced X-ray resistance and homologous recombination frequencies in a RAD54–/– mutant of the chicken DT40 cell line. Cell, 89, 185–193. [DOI] [PubMed] [Google Scholar]
- Broomfield S., Hryciw,T. and Xiao,W. (2001) DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res., 486, 167–184. [DOI] [PubMed] [Google Scholar]
- Buerstedde J.M. and Takeda,S. (1991) Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell, 67, 179–188. [DOI] [PubMed] [Google Scholar]
- Crossen P.E., Drets,M.E., Arrighi,F.E. and Johnston,D.A. (1977) Analysis of the frequency and distribution of sister chromatid exchanges in cultured human lymphocytes. Hum. Genet., 35, 345–352. [DOI] [PubMed] [Google Scholar]
- Dronkert M.L. and Kanaar,R. (2001) Repair of DNA interstrand cross-links. Mutat. Res., 486, 217–247. [DOI] [PubMed] [Google Scholar]
- Dronkert M.L., Beverloo,H.B., Johnson,R.D., Hoeijmakers,J.H., Jasin,M. and Kanaar,R. (2000) Mouse RAD54 affects DNA double-strand break repair and sister chromatid exchange. Mol. Cell. Biol., 20, 3147–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J., Hendriks,R.W., Swagemakers,S.M., Troelstra,C., de Wit,J., Bootsma,D., Hoeijmakers,J.H. and Kanaar,R. (1997) Disruption of mouse RAD54 reduces ionizing radiation resistance and homologous recombination. Cell, 89, 195–204. [DOI] [PubMed] [Google Scholar]
- Fabre F., Magana-Schwencke,N. and Chanet,R. (1989) Isolation of the RAD18 gene of Saccharomyces cerevisiae and construction of rad18 deletion mutants. Mol. Gen. Genet., 215, 425–430. [DOI] [PubMed] [Google Scholar]
- Flores-Rozas H. and Kolodner,R.D. (2000) Links between replication, recombination and genome instability in eukaryotes. Trends Biochem. Sci., 25, 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E.C., Walker,G. and Siede,W. (1995) DNA damage tolerance and mutagenesis in eukaryotic cells. In DNA Repair and Mutagenesis. American Sociey for Microbiology, Washington, DC, pp. 523–593.
- Fujimori A. et al. (2001) Rad52 partially substitutes for the Rad51 paralog XRCC3 in maintaining chromosomal integrity in vertebrate cells. EMBO J., 20, 5513–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T. et al. (2001) Genetic analysis of the DNA-dependent protein kinase reveals an inhibitory role of Ku in late S–G2 phase DNA double-strand break repair. J. Biol. Chem., 276, 44413–44418. [DOI] [PubMed] [Google Scholar]
- Galloway S.M. and Evans,H.J. (1975) Sister chromatid exchange in human chromosomes from normal individuals and patients with ataxia telangiectasia. Cytogenet. Cell Genet., 15, 17–29. [DOI] [PubMed] [Google Scholar]
- Haber J.E. (1999) DNA recombination: the replication connection. Trends Biochem. Sci., 24, 271–275. [DOI] [PubMed] [Google Scholar]
- Keszenman D.J., Salvo,V.A. and Nunes,E. (1992) Effects of bleomycin on growth kinetics and survival of Saccharomyces cerevisiae: a model of repair pathways. J. Bacteriol., 174, 3125–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M.H., Reynolds,P., Jaspers-Dekker,I., Prakash,L., Prakash,S., Bootsma,D. and Hoeijmakers,J.H. (1991) Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc. Natl Acad. Sci. USA, 88, 8865–8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T., Niu,Q.W., Chan,Y.S. and Chua,N.H. (1999) Inducible isopentenyl transferase as a high-efficiency marker for plant transformation. Nat. Biotechnol., 17, 916–919. [DOI] [PubMed] [Google Scholar]
- Lawrence C. (1994) The RAD6 DNA repair pathway in Saccharomyces cerevisiae: what does it do and how does it do it? BioEssays, 16, 253–258. [DOI] [PubMed] [Google Scholar]
- Lawrence C.W. and Christensen,R. (1976) UV mutagenesis in radiation-sensitive strains of yeast. Genetics, 82, 207–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. (1993) Instability and decay of the primary structure of DNA. Nature, 362, 709–715. [DOI] [PubMed] [Google Scholar]
- Loonstra A., Vooijs,M., Beverloo,H.B., Allak,B.A., van Drunen,E., Kanaar,R., Berns,A. and Jonkers,J. (2001) Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl Acad. Sci. USA, 98, 9209–9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J.P., Levine,A.S. and Woodgate,R. (1997) The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics, 147, 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee R.H. and Lawrence,C.W. (1980) Genetic analysis of γ-ray mutagenesis in yeast. III. Double-mutant strains. Mutat. Res., 70, 37–48. [DOI] [PubMed] [Google Scholar]
- Morrison C. and Takeda,S. (2000) Genetic analysis of homologous DNA recombination in vertebrate somatic cells. Int. J. Biochem. Cell Biol., 32, 817–31. [DOI] [PubMed] [Google Scholar]
- Prakash L. (1981) Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol. Gen. Genet., 184, 471–478. [DOI] [PubMed] [Google Scholar]
- Roest H.P. et al. (1996) Inactivation of the HR6B ubiquitin-conjugating DNA repair enzyme in mice causes male sterility associated with chromatin modification. Cell, 86, 799–810. [DOI] [PubMed] [Google Scholar]
- Silver D.P. and Livingston,D.M. (2001) Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol. Cell, 8, 233–243. [DOI] [PubMed] [Google Scholar]
- Sonoda E., Sasaki,M.S., Buerstedde,J.M., Bezzubova,O., Shinohara,A., Ogawa,H., Takata,M., Yamaguchi-Iwai,Y. and Takeda,S. (1998) Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J., 17, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E., Sasaki,M.S., Morrison,C., Yamaguchi-Iwai,Y., Takata,M. and Takeda,S. (1999) Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol. Cell. Biol., 19, 5166–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E., Takata,M., Yamashita,Y.M., Morrison,C. and Takeda,S. (2001) Homologous DNA recombination in vertebrate cells. Proc. Natl Acad. Sci. USA, 98, 8388–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Sasaki,M.S., Sonoda,E., Morrison,C., Hashimoto,M., Utsumi,H., Yamaguchi-Iwai,Y., Shinohara,A. and Takeda,S. (1998) Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J., 17, 5497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi S., Sakuraba,Y., Masuyama,S., Inoue,H. and Yamaizumi,M. (2000) Dysfunction of human Rad18 results in defective postreplication repair and hypersensitivity to multiple mutagens. Proc. Natl Acad. Sci. USA, 97, 7927–7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ramos C.A., Prakash,S. and Prakash,L. (2002) Requirement of RAD5 and MMS2 for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell. Biol., 22, 2419–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan R., Roest,H.P., Hoogerbrugge,J.W., Smit,E.M., Slater,R., Baarends,W.M., Hoeijmakers,J.H. and Grootegoed,J.A. (2000) Characterization of mRAD18Sc, a mouse homolog of the yeast postreplication repair gene RAD18. Genomics, 69, 86–94. [DOI] [PubMed] [Google Scholar]
- van Gent D.C., Hoeijmakers,J.H. and Kanaar,R. (2001) Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet., 2, 196–206. [DOI] [PubMed] [Google Scholar]
- Wyatt M.D., Allan,J.M., Lau,A.Y., Ellenberger,T.E. and Samson,L.D. (1999) 3-Methyladenine DNA glycosylases: structure, function and biological importance. BioEssays, 21, 668–676. [DOI] [PubMed] [Google Scholar]
- Xin H., Lin,W., Sumanasekera,W., Zhang,Y., Wu,X. and Wang,Z. (2000) The human RAD18 gene product interacts with HHR6A and HHR6B. Nucleic Acids Res., 28, 2847–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamble D.B. and Lippard,S.J. (1995) Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem. Sci., 20, 435–439. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wienands,J., Zurn,C. and Reth,M. (1998) Induction of the antigen receptor expression on B lymphocytes results in rapid competence for signaling of SLP-65 and Syk. EMBO J., 17, 7304–7310. [DOI] [PMC free article] [PubMed] [Google Scholar]