Abstract
Three gene products that form independent mechanosensitive channel activities have been identified in Escherichia coli. Two of these, MscL and MscS, play a vital role in allowing the cell to survive acute hypotonic stress. Much less is known of the third protein, MscK (KefA). Here, we characterize the MscK channel activity and compare it with the activity of its structural and functional homologue, MscS. While both show a slight anionic preference, MscK appears to be more sensitive to membrane tension. In addition, MscK, but not MscS activity appears to be regulated by external ionic environment, requiring not only membrane tension but also high concentrations of external K+, NH4+, Rb+ or Cs+ to gate; no activity is observed with Na+, Li+ or N-methyl-d-glucamine (NMDG). An MscK gain-of-function mutant gates spontaneously in the presence of K+ or similar ions, and will gate in the presence of Na+, Li+ and NMDG, but only when stimulated by membrane tension. Increased sensitivity and the highly regulated nature of MscK suggest a more specialized physiological role than other bacterial mechanosensitive channels.
Keywords: MscL/MscS/osmoregulation/stretch-activated channels/turgor maintenance
Introduction
Essentially all organisms possess the ability to detect mechanical forces. The human senses of touch, hearing and balance, as well as the capacity to regulate blood pressure, are all forms of this ability. In addition, plants detect the forces of wind and gravity, and microbes detect membrane tension evoked by a sudden shift to a hypotonic environment. One of the ways cells detect these forces is by the gating of mechanosensitive (MS) channels (for reviews see Hamill and McBride, 1996; Sukharev et al., 1997; Tavernarakis and Driscoll, 1997; Hamill and Martinac, 2001). Because of their complicated nature and possible requirements for other proteins (Tavernarakis and Driscoll, 1997), heterologous expression of putative eukaryotic MS channel candidate molecules has rarely led to any measurable MS channel activity (but see Goodman et al., 2002), and thus far none have been reconstituted functionally in synthetic lipids. Hence, the possible role that auxiliary proteins, membrane tension and protein– lipid interactions play in eukaryotic MS channel gating is difficult to assess. In contrast, several bacterial proteins that directly or indirectly sense changes in the osmotic environment, including MS channels, have been purified and reconstituted (Poolman et al., 2002). The study of functionally reconstituted prokaryotic osmotic sensor activities has begun to reveal their molecular mechanisms, including the ability to detect the biophysical properties of the membrane bilayer, protein–lipid interactions and ionic concentrations, the latter of which evoke a response either by binding to the protein or through modulation of interactions with charged lipids (Poolman et al., 2002). Hence, bacterial mechano-sensors serve as the de facto model systems for determining the general principles underlying the ability of biological systems to detect osmotic and mechanical forces.
The electrophysiological characterization of Escherichia coli membrane patches reveals three discrete MS channel activities: MscL (mechanosensitive channel of large conductance), MscS (smaller conductance) and MscM (mini-conductance). These channels gate at different membrane tensions, with more tension required to gate the MS channels with larger conductance (Berrier et al., 1996). The molecular identity of MscM, its regulation and function are unknown. Furthermore, MscM activity appears to be dependent upon assay conditions and is, therefore, sometimes difficult to observe. In contrast, MscL has been well characterized. A crystal structure of a closed conformation of the Mycobacterium tuberculosis orthologue has been solved to 3.5 Å resolution (Chang et al., 1998), a second gate and possible conformations of the open and transition states have been predicted (Sukharev et al., 2001a,b; Kong et al., 2002), and the primary events in channel gating (Moe et al., 1998, 2000) derived from mutagenesis studies (Ou et al., 1998; Yoshimura et al., 1999, 2001) have been proposed. The MscS activity, however, recently has been revealed to be more complex, and appears actually to consist of two similar, but distinguishable, activities resulting from two independent though homologous gene products: YggB and KefA (KefA is also called AefA or, as below, MscK) (Levina et al., 1999).
KefA (MscK) was discovered originally as a gain-of-function mutant E.coli strain, RQ2, which failed to grow in a medium containing a high concentration of K+ while in the presence of betaine (McLaggan et al., 2002). The mutations leading to this phenotype were found to be L565Q and G922S in the kefA open reading frame; the latter mutation correlated with the phenotype. The lack of an obvious phenotype for a kefA-null established that the RQ2 strain is a gain-of-function mutant. A genetic correlation between kefA and an MS channel activity that resembles, but is distinct from MscS was noted (Levina et al., 1999). Further investigation of kefA homologues by generation of null mutants and subsequent reconstitution into lipid membranes led to the discovery that yggB not only correlates with MscS activity (Levina et al., 1999) but also encodes the pore-forming unit (Okada et al., 2002; Sukharev, 2002). KefA and YggB are 1120 and 286 amino acids, respectively, but it is the C-terminal 300 residues of KefA that exhibit structural and sequence similarity to YggB. Because of the homology between YggB and KefA, the demonstration that YggB, independent of other proteins, encodes MscS activity, and the correlation of KefA expression with channel activity, it seems likely that KefA also encodes the pore-forming unit of a channel.
The phenotype of the RQ2 strain is only observed in K+-based media; in media where Na+ replaces K+, the mutant grows in a manner essentially indistinguishable from the parent (McLaggan et al., 2002). Because these observations were based on whole-cell physiology, it is difficult to determine whether the phenotype is due to ionic preference of the channel, regulation of channel gating or to indirect regulation through physiological changes (e.g. increased cell turgor in the high K+ medium). In addition, because only the mutated KefA protein led to a measurable phenotype, it is unclear whether the wild-type KefA channel is also regulated by ionic environment.
Here we have studied the KefA channel activity and the influence of different ionic conditions on its gating by patch-clamp recording from E.coli spheroplasts. Our data strongly suggest that K+ and monovalent cations with similar or slightly larger ionic radii regulate channel gating of both the mutated and wild-type KefA channels. Our data demonstrate that KefA is highly regulated, requiring specific ionic conditions as well as membrane tension to gate.
Because yggB encodes MscS activity while kefA encodes a channel activity that is regulated by K+, we propose, and hereafter utilize, the convention of designating yggB as mscS, and kefA as mscK (for K+ regulated).
Results
Whereas MscS activity appears unaffected by K+ and Na+ ions, MscK activity is observed only when the primary permeant cation is K+, not Na+
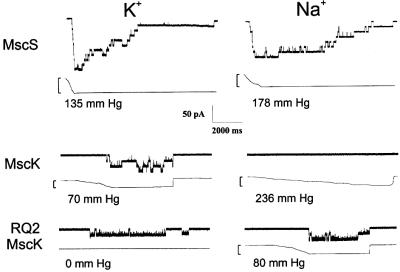
As described in Materials and methods, spheroplasts were generated from cells that lack MscK but express both MscS and MscL channels. When native membranes from these giant cells were assayed for channel activity using standard patch–clamp techniques and suction within the electrode as a stimulus (Blount et al., 1999), MscS and MscL activities were observed in virtually every patch that did not rupture upon addition of sufficient stimulus. MscS activity was observed in NaCl as well as KCl salts (Figure 1, top). Note that for these experiments, the NaCl in the bath and electrode buffer was simply substituted with an equimolar amount of KCl. For simplicity, we will refer to these and other buffers varying the major cation as buffers where Na+ (or other cation) is the primary permeant cation. Desensitization, closure upon a sustained pressure stimulus, was observed under both ionic conditions. No significant differences in the rate of desensitization were observed consistently in the different buffer conditions.
Fig. 1. Typical traces of MscS (top) and wild-type MscK (middle) and the RQ2 MscK in ionic conditions in which K+ (left) or Na+ (right) is the primary permeant cation. Recordings were generated as described in Materials and methods at –20 mV; channel openings are downward. The bottom trace of each set depicts the pressure in mm Hg; the maximum pressures achieved across the membrane patch are indicated.
Bacterial MS channels appear to gate in response to tension within the membrane rather than the pressure across it (Gustin et al., 1988; Sokabe et al., 1991; Sukharev et al., 1999; Moe and Blount, 2002; Sukharev, 2002). Hence, the amount of pressure required for channel gating measured by patch–clamp can vary between independent traces in accordance with the geometry of the patched membrane. Therefore, for the study of MscL mutants and homologues, we have developed a method by which MscS is used in native membrane patches as an internal standard (Blount et al., 1996a,c, 1999; Moe et al., 1998, 2000). Here we use the complementary approach, employing MscL as an internal control for the characterization of MscK in an mscS-null background. Consistent with previous studies, we found here that 1.5 ± 0.1 times the amount of pressure required for MscS gating was required to gate MscL (Table I). This threshold ratio was maintained even in the Na+-based buffer, suggesting that neither channel is affected by the change in monovalent cations, or that both channels are affected to the same extent. We favour the former interpretation because it is the most parsimonious, and because no significant differences were observed in the amount of pressure stimulus needed to gate the channels, as compared with standard buffer conditions.
Table I. Threshold ratios demonstrate that wild-type and RQ2 MscK channels are more sensitive to membrane tension than MscL or MscS and can gate spontaneously when the primary permeant cation is K+ (left) but not Na+ (right).
| Strain | K+ |
Na+ |
||
|---|---|---|---|---|
| Spontaneous activitya | Threshold ratiob | Spontaneous activitya | Threshold ratioc | |
| MscS | No | 1.5 ± 0.1 (21) | No | 1.5 ± 0.1 (17) |
| Wild-type MscK | No | 2.2 ± 0.2 (12) | No | NDd |
| RQ2 MscK | Yes | 2.6 ± 0.1 (5) | No | 2.5 ± 0.2 (9) |
aSpontaneous activity was defined as observing channel activity under the indicated buffer conditions without inducing pressure across the membrane to increase tension.
bThreshold ratios were determined as described in Materials and methods and in the text. The number of observations is in parentheses. Wild-type and RQ2 MscK activities were significantly different from MscS as determined by Student’s t-test (P < 0.001), while wild-type MscK did not deviate significantly from the RQ2 MscL activity that required membrane tension (P > 0.3).
cThreshold ratios were determined as described in Materials and methods and in the text. The number of observations is in parentheses. MscS activity in Na+ was not significantly different from MscS in K+ as determined by Student’s t-test (P > 0.3), while the RQ2 activity deviated from MscS in either buffer (P < 0.001) but did not differ from wild-type or RQ2 MscK in K+ buffer (P > 0.1).
dChannel activity was not detected.
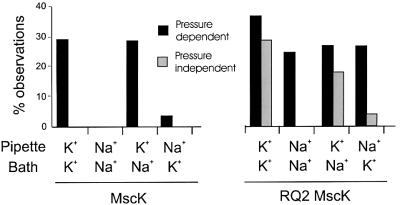
As described in Materials and methods, a strain that is null for mscS but wild type for mscK and mscL was used to assay MscK activity. MscK activity was observed when K+ was the primary permeant cation (Figure 1, middle left), but was only observed in ∼30% of the traces in which MscL was recorded (23 of 79 independent traces; Figure 2). When observed, two or more active units were often seen. Although MscK and MscS channel activities are similar in conductance (∼1 nS), the rapid desensitization seen with MscS (above) was absent with MscK activities, as is consistent with previous observations (Levina et al., 1999). The MscK activity also appeared to have more frequent rapid partial closures from the open state. Utilizing wild-type MscL activity as an internal standard, we found that 2.2 ± 0.2 times the pressure to gate MscK was required to open MscL. This threshold ratio is greater than the 1.5 ± 0.2 ratio observed for MscS (Table I), indicating that MscK gates at membrane tensions lower than that required to activate MscS. Additionally, in contrast to MscS, no MscK activity was observed in 51 independent MscL activity-containing traces in which Na+ was the primary permeant cation (Figure 1, middle right; Figure 2). In support of previous observations (Levina et al., 1999), no activity of this 1.0 nS conductance was observed in 50 MscL-containing traces from the PB113 strain, which is null for mscS and mscK, demonstrating that the latter gene indeed correlates with the activity observed.
Fig. 2. The activities of wild-type MscK (left) and the RQ2 MscK (right) are dependent upon the ionic composition of the pipette (periplasmic) solution. The major permeant cation within the pipette or bath is as labelled. The histogram shows the percentage of MscL-containing traces where activities were observed in a spontaneous, pressure- independent (grey) or pressure-dependent (black) manner for each condition. Each percentage is derived from ≥50 MscL-containing traces.
An MscK mutant that evokes a gain-of-function phenotype gates spontaneously when the primary permeant cation is K+, but requires membrane tension when the primary permeant cation is Na+
A previous study characterized the gain-of-function mutant E.coli strain RQ2 that failed to grow in a medium containing high concentrations of K+ while in the presence of betaine (McLaggan et al., 2002). The lesions lie within the mscK gene and are predicted to yield L565Q and G922S substitutions within the protein (McLaggan et al., 2002). Here, in order to assay the L565Q/G922S MscK channel activity, we generated an mscS-null of the RQ2 strain. The RQ2 MscK activity was similar to that of wild type in conductance and kinetics when assayed in a K+-based buffer (Figure 1, bottom left) and was only observed in a subset of traces in which MscL was observed (23 of 63 independent traces; Figure 2). However, in contrast to wild-type MscK, in 18 of the 23 independent observations of the RQ2 MscK activity, pressure across the membrane was not required to actuate the channel; once the gigaohm seal was generated, spontaneous activities were observed. In the five traces that did require additional pressure to gate, the pressure ratios centred at 2.6 ± 0.1, similar to the 2.2 ± 0.2 value (Table I) obtained for MscK when K+ was the primary permeant cation. These data suggest the possibility that the RQ2 MscK channels, when in the presence of K+, may be in one of two conformations: the majority would be in a conformation that allows spontaneous gating, while a minority of channels, not unlike the K+-stimulated wild-type channel, would be primed to gate upon sensing membrane tension. Unlike the wild-type MscK channel, activity of the RQ2 mutant was observed when Na+ was the primary permeant cation (21 of 86 traces; Figure 1, bottom right; Figure 2). However, under these conditions, pressure was required to gate the channel. Comparing the threshold ratios (2.5 ± 0.2 for RQ2 MscK in Na+, 2.2 ± 0.2 for MscK in K+) indicates that the membrane tension required to gate the mutated channel under these conditions is similar to that required to gate the wild-type MscK when the primary permeant ion is K+. The amount of tension to gate the mutated channel under these conditions is also similar to that required to gate the wild-type MscK when the primary permeant ion is K+ (threshold ratios of 2.5 ± 0.2 for RQ2 MscK in Na+, 2.2 ± 0.2 for MscK in K+; Table I).
The ionic environment of the pipette (periplasm), not the bath (cytoplasm), determines MscK channel behaviour
To test whether the influence of monovalent cations on wild-type MscK gating was due to ionic interactions occurring on a particular face of the membrane, we assayed MscK activity by patch–clamp using asymmetric buffer conditions. As seen in Figure 2, MscK activity was observed consistently only when K+ was in the pipette, even if Na+ was placed in the bath (15 of 53 MscL-containing traces). Because the traces were obtained from excised inside-out patches of membrane, the pipette solution bathes the periplasmic face of the membrane. When Na+ was in the pipette, and K+ in the bath, MscK activity was observed in only two of 61 traces. Similarly, spontaneous activity of the RQ2 MscK mutant was observed when K+ was in the pipette (10 of 56 MscL-containing traces), but not when Na+ was in the pipette, even if K+ was in the bath (only two of 53 MscL-containing traces exhibited MscK activity). These data strongly suggest that the ionic environment on the periplasmic, not cytoplasmic, side of the membrane is important for MscK gating.
Ionic preferences: K+ and Na+ equally permeate MscK; MscS and MscK both have a slight anionic preference
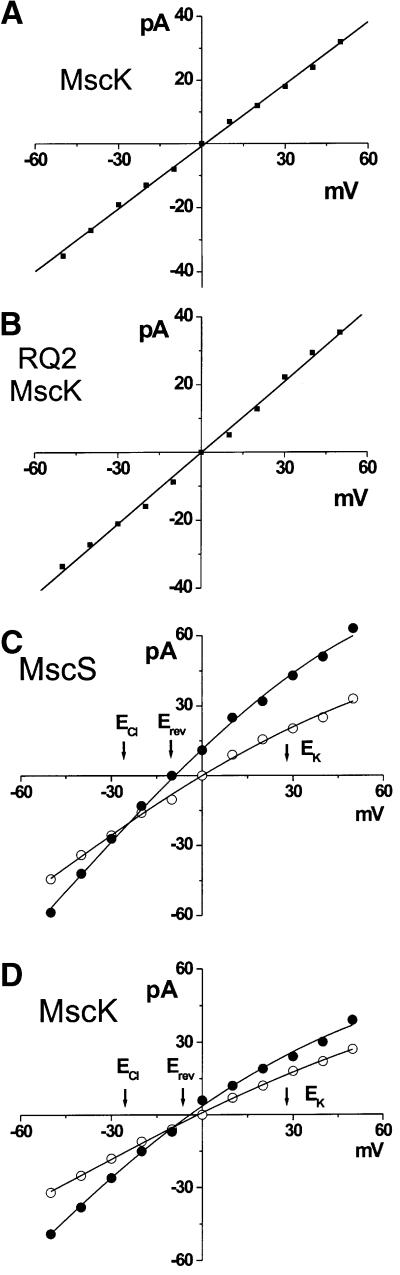
To survey the ionic preference of MscK, the channel activity was assayed by patch–clamp under asymmetric ionic conditions. First, to test whether K+ was more permeant than Na+, a current–voltage (I–V) relationship was established under conditions in which K+ was placed in the pipette, while Na+ was in the bath. Under these conditions, the K+ within the pipette will allow channel gating. If there were a differential permeation for the two monovalent cations, then the I–V relationship would be non-linear and displaced from the origin because of the inability to pass one of the ions efficiently. However, as shown in Figure 3A and B, no such rectification or current offset was observed for either wild-type MscK or the RQ2 MscK; both plots are linear. These data demonstrate that, once open, the MscK channel passes Na+ as easily as K+.
Fig. 3. Ionic preferences of wild-type MscK, RQ2 MscK and MscS. (A) The wild-type MscK does not discriminate between K+ or Na+ cations as indicated by the current–voltage relationship of single channel activities when the major permeant cation is K+ (200 mM) within the pipette and Na+ (200 mM) within the bath. (B) In an experiment analogous to that shown in (A), the current–voltage relationship of the RQ2 MscK demonstrates that it also does not discriminate between K+ or Na+ cations. (C) MscS shows an anionic preference as indicated by the current–voltage relationship. Single-channel activities are plotted under symmetrical conditions in which both the pipette and bath contained 200 mM KCl (open circles), then subsequent replacement of the bath with the same buffer containing 600 mM KCl (closed circles) (D) In an analogous experiment to that shown in (C), MscK shows a very slight but measurable anionic preference.
Previously, before it was recognized that two independent genes encode MscS-like activities, some studies had noted a slight preference for anionic conductance. Hence, it was of interest to determine whether this preference was a function of MscS, MscK or both. Therefore, I–V relationships were evaluated under asymmetric conditions in which the KCl concentrations were 200 mM in the pipette, and 600 mM in the bath. Under these conditions, the I–V relationship for MscS failed to run through the origin, approaching the equilibrium potential of Cl– rather than K+, demonstrating that this channel has a slight anionic preference (Figure 3C). This ionic preference is similar to that previously characterized (Martinac et al., 1987). Although MscK also showed some anionic preference (Figure 3D), it is slight, even when compared with MscS. In these experiments, when channel activity was measured under symmetrical ionic conditions, the relationship did not deviate consistently or significantly from linear. However, under asymmetric conditions, a slight apparent rectification was seen consistently as in previous studies (Martinac et al., 1987), suggesting that ionic or osmotic environments may influence channel permeation. Also, under these buffer conditions at high positive potentials, a stabilized subconducting state at one-half unitary conductance was often observed in both MscS and MscK channels (data not shown).
The ionic influence on MscK depends upon the ionic radius
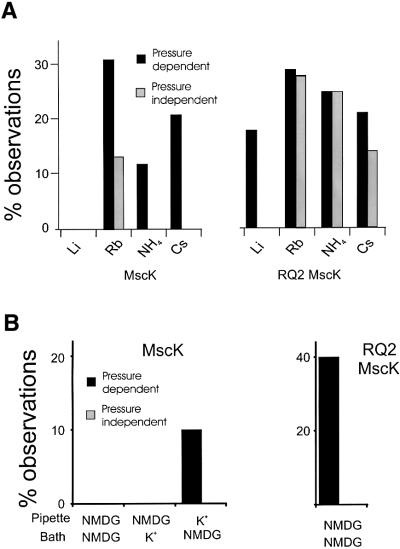
The inorganic cations Li+, Rb+, NH4+ and Cs+ were also tested for their influence on the activity of the wild-type MscK and the RQ2 mutant. The ions can be divided roughly into two categories (compare Figure 2 with 4A): Li+ and Na+, both of small ionic radius, fall into one category. No wild-type MscK activity was detected in either ionic environment. Activity of the RQ2 mutant was observed under these ionic conditions, but only upon the addition of a significant membrane tension stimulus. The second category includes K+, NH4+, Rb+ and Cs+, all of larger ionic radius than Na+ or Li+. In the presence of these ions, there is a dramatic increase in the probability of observing activity of the wild-type and mutant MscK. The one notable variation is Rb+, which not only allows wild-type MscK to gate in response to membrane tension, but also induces spontaneous activity in a small subset of the patches where activity is observed.
Fig. 4. The influence of different ions on the activities of wild-type and RQ2 MscK. In each of the experiments shown, the buffers were as described in Materials and methods and the primary permeant ion is indicated; all were at 200 mM final concentration. (A) The ionic influence on wild-type MscK (left) and the RQ2 MscK (right) activities depends upon the ionic radius of the inorganic cation. The major permeant cation is as labelled. The histogram shows the percentage of MscL-containing traces where activities were observed in a spontaneous, pressure-independent (grey) or pressure-dependent (black) manner for each condition. Each percentage is derived from ≥50 MscL-containing traces. The smaller Li+ and Na+ ions decrease activity, while K+, Rb+, NH4+ and Cs+ increase activity (see Figure 2). Note that Rb+ has the added effect of inducing spontaneous gating of MscK. (B) NMDG has an influence on channel activity similar to that of Na+ and Li+. The major permeant cation within the pipette and bath is as labelled. The histograms show the percentage of MscL-containing traces for wild-type MscK (left) and RQ2 MscK (right) where activities were observed in a spontaneous, pressure-independent (grey) or pressure-dependent (black) manner for each condition. Each percentage is derived from ≥50 MscL-containing traces. Note that, although activity is reduced with this ion, activity of normal conductance can be measured if K+ is the major permeant cation in the pipette while measuring wild-type MscK and the RQ2 MscK, demonstrating that the channels can conduct this large cation.
MscK activity is activated by K+, rather than inhibited by Na+
From the experiments above, it is impossible to distinguish whether K+ and similar ions are activating channel activity, or whether Na+ is inhibiting it. We reasoned that the use of a large but permeant cation, such as N-methyl-d-glucamine (NMDG), which probably would not fit into an inorganic ion-binding site, may allow us to determine the innate properties of the channel independently of inorganic monovalent cation binding. Hence, we assayed activity under conditions in which NMDG was the primary permeant cation. As seen in Figure 4B (left), no wild-type MscK channel activity was observed in 53 MscL-containing traces under these conditions. However, when K+ was placed on the pipette (periplasmic) side of the patched membrane, and NMDG remained on the bath (cytoplasmic) side, MscK channel activity was observed (five of 50 MscL-containing patches). This channel behaviour is similar to that observed when Na+ is the primary permeant cation (above). Consistently, the RQ2 MscK activity was observed in a percentage of MscL-containing patches, but only when pressure was applied across the membrane (Figure 4B, right). When they were observed, the wild-type and RQ2 MscK channels exhibited a unitary conductance similar to that observed in other ionic conditions, demonstrating that NMDG can permeate when the channel is open.
NMDG is significantly different from Na+, yet in the presence of either of these ionic conditions we observe a lack of channel activity. It seems unlikely that these disparate cations bind a common site or have similar activities, especially since cations of intermediate ionic radius, such as K+, have an opposing effect on channel behaviour. A more likely interpretation is that the ‘default’ or quiescent state of the channel is observed in the presence of Na+ or NMDG. Hence, the most parsimonious interpretation of these data is that K+, and cations of similar ionic radius, specifically interact with the protein or a protein–lipid interface and thus stimulate the channel.
Discussion
Recently, it has become clear that the previously characterized 1 nS MS channel activity in E.coli is actually comprised of two similar, but distinguishable, activities derived from two homologous proteins. A major portion of this channel activity is due to MscS, which is seen in virtually every trace obtained from native membranes. In contrast, MscK activity is observed in only a fraction of electrophysiological traces. However, when MscK activity is observed, two or more active units often are detected. One interpretation posits that the channels are clustered in the bacterial membrane. However, the giant cells that are utilized in these electrophysiological experiments represent multiple cells, and the amount of membrane within a normal membrane patch is greater than that which comprises a single bacterial cell. This fact makes subcellular clustering a less unlikely explanation. An alternative interpretation is that, similar to mechanisms proposed for some eukaryotic MS channels, interactions with periplasmic and/or cytoskeletal proteins are required for MscK channel activity. Such interactions have been proposed previously for MscK (McLaggan et al., 2002). In this scenario, proteins that directly associate with MscK and are required for function are stripped away in the majority of patched membranes during seal formation, and activity is observed only in the minority of patches where these protein associations remain. Consistent with this hypothesis, we have thus far failed to reconstitute MscK activity (data not shown) even when utilizing protocols identical to those successful for MscL and MscS reconstitution (Blount and Moe, 1999; Moe et al., 2000; Okada et al., 2002). MscS is projected to contain three transmembrane domains (S.Miller, W.Bartlett, S.Chandrasekaran, S.Simpson, M.Edwards and I.R.Booth, submitted). A portion, residues 845–1120, of MscK shares sequence and organizational similarity with the entire MscS protein, but the full-length protein contains eight additional proximal transmembrane domains and a large periplasmic region (McLaggan et al., 2002). These additional protein domains may be involved in interactions with regulatory proteins that would have no influence on MscS activity. Gain-of-function mutations in the Salmonella typhimurium MscK cluster to the 845–1120 region of the protein (H.Jeong and J.Roth, personal communication), which is consistent with a role for this region as the pore-forming domain.
The E.coli RQ2 mutant strain was isolated originally by its inability to grow well in a medium containing high concentrations of K+ while in the presence of betaine (McLaggan et al., 2002). The mutations leading to this phenotype were found to be in the mscK gene. The lack of an obvious phenotype for the mscK-null established that the mutations within the RQ2 strain evoked a gain-of-function phenotype. One of the more interesting features of this mutant strain is that it grows well when the excess K+ in the medium is substituted with Na+. McLaggan et al. (2002), in a titration experiment, demonstrated that the RQ2 strain grows normally at 20 mM K+, but is inhibited by 50% at 120 mM K+, suggesting that the affinity is in the several tens of mM. Early studies using patch–clamp to study the RQ2 strain utilized the whole-cell patch mode on entire protoplasts (Cui et al., 1995; Cui and Adler, 1996). A general increase in MS channel activity and dwell time, including that of MscL, was noted. However, because independent protoplast preparations were generated necessarily for each strain, and the amount of residual cell wall material could vary between preparations, the interpretation is complicated. In addition, no significant differences were observed in MS channel activity between KCl and NaCl buffers (Cui and Adler, 1996). At the time of these studies, however, the distinction between MscS and MscK had not been realized, so differences due to MscK activity, which represents the minority of 1 nS conducting channels, presumably were missed. Here, using excised patches of strains lacking MscS, we show that the mutated MscK activity, in strain RQ2, gates spontaneously in the presence of K+. In addition, K+ regulates wild-type MscK channel activity. Because there is no preference for K+ permeation, this regulation must be at a site independent of the pore. Such regulation is found in other osmotic sensors including ProP (Koo et al., 1991), BetP (Rubenhagen et al., 2001) and OpuA (van der Heide et al., 2001). The latter study is of unique importance in that it suggests that in some cases ions are sensed through charged membrane lipid head groups (for a more complete discussion of ion-sensing mechanisms of reconstituted osmosensors see Poolman et al., 2002). Perhaps the best-documented case of an osmosensor that senses ions independently of osmolarity involves the modulation of the osmotic sensor KdpD, which regulates the transcription of the E.coli Kdp potassium pump. This sensor is stimulated by high osmolarity, but inhibited by internal K+ concentrations (Sugiura et al., 1994; Jung et al., 2000). Here we demonstrate that for normal MscK channel gating, both membrane tension and an environment in which external K+ is in high concentration are required; no activity is seen when Na+ is substituted for K+. Because no channel activity is observed with external NMDG, which is structurally quite dissimilar to inorganic ions, we suspect that the channel is stimulated by K+, rather than inhibited by Na+. Consistent with the whole-cell physiological data, it appears that it is the ionic conditions on the periplasmic side of the membrane (in the pipette) that are important for channel activity.
Similar to many other enzymes and channels, the influence of ions on MscK activity is dependent upon the ionic radius. Ions of smaller radius, such as Li+ and Na+, do not stimulate the MscK channel, while ions of larger radius, including K+, Rb+ and Cs+, do. Interestingly, Rb+ cannot only substitute for K+, but it can stimulate a portion of wild-type MscK channels independent of additional membrane tension. For E.coli, and some other enteric bacteria, Rb+ does not substitute easily for K+; growth in Rb+ is much slower than in K+ (Rhoads et al., 1977). However, strains both null and wild type for mscK exibited the same degree of slowed growth when Rb+ was substituted for K+ (Y.Li and P.Blount, unpublished data). Hence, the inability of Rb+ to support E.coli growth when substituted for K+ apparently is not due simply to aberrant flux of solutes through the MscK channel, but probably also involves the ability of K+ pumps to distinguish between these two cations, as proposed previously (Rhoads et al., 1977).
Mutagenesis studies have helped to identify functional domains of bacterial MS channels. A random mutagenesis study (Ou et al., 1998), combined with site-directed mutagenesis (Yoshimura et al., 1999, 2001) and the M.tuberculosis crystal structure of the closed channel (Chang et al., 1998), have led to a hypothesis for the primary events in MscL channel gating. According to this theory, hydrophobic amino acids and glycines are located toward the constriction site of the channel, and the primary energy barrier for channel gating is breaking of this ‘hydrophobic lock’ and transient exposure of these residues to the lumen of the opening channel (Chang et al., 1998; Moe et al., 1998, 2000). A recent random mutagenesis study of MscS found only one mutant, but the phenotype, character and properties of the random substitution, and phenotypic properties of site-directed substitutions at this site were all reminiscent of the MscL study, suggesting that MscS is also subject to the ‘hydrophobic lock’ hypothesis (Okada et al., 2002). If true, and invoking its homology with MscS, it seems likely that MscK also shares this mechanism. The RQ2 strain harbours two mutations within the mscK gene that are predicted to lead to L565Q and G922S substitutions within the channel protein. Recombination analysis strongly suggests that the G922S mutation, which is in the last transmembrane domain, plays the major role in evoking the phenotype (McLaggan et al., 2002). The lesion in the gain-of-function MscS mutant protein lies in the first of three predicted transmembrane domains (analogous to the third from last in MscK). Hence, these data suggest that the last three transmembrane domains of MscK are important for channel opening, and may encode the channel pore. Consistent with this hypothesis, expression, in trans, of the last 300 amino acids of MscK effects the rescue of an MscL– MscS– double mutant from hypo-osmotic shock; imposing the G922S mutation onto this fragment elevates this restoration (S.Miller, W.Bartlett, S.Chandrasekaran, S.Simpson, M.Edwards and I.R.Booth, submitted). In addition, the MscK fragment appears to evoke this rescue in both Na+- and K+-based media, suggesting that the absent region contains the domain responsible for K+ regulation. Channel activity has not yet been associated with the MscK fragment, suggesting that channel leakage, rather than gating, may be responsible; perhaps the absence of domains responsible for protein–protein interactions and K+ regulation leads to a partially open channel. Regardless, combined, the data above suggest that the pore for MscS and MscK lies within the last three transmembrane domains. Because we currently have no firm structural data, and none of the mutations affect conductance and may simply work through a general change in conformation, it is uncertain currently which of the three transmembrane domains may form the constriction point of the closed channel. It may be worth noting, however, that the greatest conservation of the extended gene family is seen in the last transmembrane domain and the subsequent cytoplasmic region (Touze et al., 2001; Okada et al., 2002; S.Miller, W.Bartlett, S.Chandrasekaran, S.Simpson, M.Edwards and I.R.Booth, submitted; C.D.Pivetti, S.Miller, M.-R.Yen, W.Busch, Y.-H.Tseng, I.R.Booth and M.H.Saier,Jr, submitted). In addition, the data above suggest that the first several domains of MscK, which are not present in MscS, impose the K+ regulation on the channel. How then does the G922S mutation obviate the dependence on K+ for MscK gating? One interpretation is that when K+ binds the regulatory domain, the channel achieves a conformation that is primed for gating in response to membrane tension. Most of the RQ2 channels appear to be in a similar conformation when in the presence of Na+. Hence, this mutant has, in essence, separated the normally tight K+ regulation from ion permeation. However, the mutant still contains the K+-binding site, as indicated by the spontaneous channel activity observed in buffers containing high concentrations of this ion. Hence, it appears that both the G922S mutation and K+ binding to a regulatory domain increase the probability of gating. When both are present, they act synergistically to allow spontaneous activity.
While it is generally accepted that MscL and MscS are required for protection against acute hypotonic shifts, thus serving as ‘emergency release valves’, the physiological role of MscK has been more elusive (Levina et al., 1999). Our studies suggest that MscK is regulated by the external K+ concentration, allowing it to gate at lower membrane tensions than MscL or MscS. Hence, under certain environmental conditions, gating of this channel may obviate gating of the unregulated MS channels. The kinetics and preferences of MscK are subtly different from those of MscS, suggesting that it may have a slightly different role. However, under what environmental conditions might MscK gate? K+ is not normally found in high concentrations within the environment. One exception is in animal urine, which also contains relatively high concentrations of NH4+. Preliminary experiments have suggested that NH4+ and K+ are about equal in their ability to activate the MscK channel in vivo (Y.Li and P.Blount, unpublished data), and such an effect could be additive. One common milieu for E.coli is within the intestines of animals. Perhaps, then, the MscK channel assists in bacterial survival in small aqueous pools or soil environments where animal wastes may accumulate or be concentrated. Another study suggests that a proposed orthologue of MscK in Pseudomonas aeruginosa may also play a role in pathogenesis since it is associated with the ability of this organism to be virulent in Caenorhabditis elegans and mice (Tan et al., 1999). One possibility is that MscK plays a role in toxin secretion. Finally, the ability of the MscK channel to gate upon changes in osmolarity and environments of high K+ and NH4+ suggests that it could play a role in the cell’s survival in the kidney upon infecting that organ. Although these proposed functional roles for MscK are enticing, they will require additional study for verification.
Materials and methods
Bacterial strains and growth
For the study of MscS activity independent of MscK, we utilized PB113, a ΔrecA variant of MJF429 ΔmscS, ΔmscK::Kan) (Levina et al., 1999) that was generated utilizing P1 transduction as previously described for PB104 (Sukharev et al., 1994; Blount et al., 1996b) to host an expression construct containing a polyhistidine-tagged wild-type mscS gene (Okada et al., 2002). As in previous studies (Blount et al., 1996a,c, 1999), no difference in channel activity or permeability was noted in Tn10-containing (ΔrecA) strains. Strain MJF451 (ΔyggB) was used to study MscK independently of MscS. Strain MJF480 (RQ2, ΔyggB, speA::Tn10), used to assay the RQ2 MscK, was constructed in two steps, using the close proximity of the yggB and speA genes on the E.coli chromosome. First, strain MJF451 (ΔyggB) was transduced to TetR using a P1 lysate of CAG12168 (speA::Tn10) and the transductants screened by PCR for retention of the ΔyggB allele. The resultant strain MJF452 (ΔyggB, speA::Tn10) was used to make a further P1 lysate and used to transduce strain RQ2 (McLaggan et al., 2002) to TetR. Transductants were screened for acquisition of the yggB deletion by PCR using flanking primers. The resultant strain MJF480 (kefA2, ΔyggB, speA::Tn10) was purified and stored at –80°C. All cultures were grown at 37°C in Luria– Bertani medium (LB) with orbital shaking at 250 r.p.m. For the PB113 plasmid-bearing strain, ampicillin (100 µg/ml) was added; expression was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) (1 mM) during the last 20 min of growth.
Spheroplast preparation and electrophysiological analysis
Escherichia coli giant spheroplasts were generated and used in patch– clamp experiments as described previously (Martinac et al., 1987), with modifications (Sukharev et al., 1994; Blount et al., 1996c, 1999). Excised, inside-out patches were examined at room temperature under symmetrical conditions using a buffer comprised of 200 mM KCl, 90 mM MgCl2, 10 mM CaCl2 and 5 mM HEPES adjusted to pH 6.0. For experiments in which K+ was replaced with other permeant ions, the chloride form of the salt substituted the KCl in the above recipe; all were at 200 mM final concentration. Records were gathered at –20 mV (bath or cytoplasmic, by convention) for channel pressure response experiments, and +50 mV to –50 mV for determination of the I–V relationship and conductance. For the I–V relationship utilizing asymmetric solutions, the bath buffer was replaced with a buffer identical to that above but containing a final concentration of 600 mM KCl. All data were acquired at a sampling rate of 50 kHz with a 10 kHz filtration using an AxoPatch 200B amplifier in conjunction with Axoscope software (Axon). The traces shown were filtered additionally in Clampfit 8.0 software (Axon) with the low pass Boxcar filter with smoothing point 7. A piezoelectric pressure transducer (World Precision Instruments) was used to measure the pressure response of the channels. The pressure threshold for activation of the MscS and MscK channels, with respect to the activation threshold of MscL, was determined as described previously (Blount et al., 1996c, 1999). Briefly, the opening threshold for MscS or MscK was defined as the pressure required to gate, simultaneously, within 7 s, two or more channels. The MscL opening threshold was defined as the pressure at which single channel openings were readily observed every 0.5–2 s. For all histograms, the percentage of times MscK was observed was derived from a minimum of 50 independent traces in which MscL was observed.
Acknowledgments
Acknowledgements
The authors would like to acknowledge Dr Michelle Edwards for constructive comments on the manuscript. This research was supported by a Robert A.Welch Foundation Grant I-1420, US Air Force Grant F49620-01-1-0503, NIH grants GM61028 and DK60818, Wellcome Trust Visiting Fellowship 055277 and Programme Grant 040174.
References
- Berrier C., Besnard,M., Ajouz,B., Coulombe,A. and Ghazi,A. (1996) Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol., 151, 175–187. [DOI] [PubMed] [Google Scholar]
- Blount P. and Moe,P. (1999) Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol., 7, 420–424. [DOI] [PubMed] [Google Scholar]
- Blount P., Sukharev,S.I., Moe,P.C., Nagle,S.K. and Kung,C. (1996a) Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria. Biol. Cell, 87, 1–8. [PubMed] [Google Scholar]
- Blount P., Sukharev,S.I., Moe,P.C., Schroeder,M.J., Guy,H.R. and Kung,C. (1996b) Membrane topology and multimeric structure of a mechanosensitive channel protein of Escherichia coli. EMBO J., 15, 4798–805. [PMC free article] [PubMed] [Google Scholar]
- Blount P., Sukharev,S.I., Schroeder,M.J., Nagle,S.K. and Kung,C. (1996c) Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl Acad. Sci. USA, 93, 11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P., Sukharev,S.I., Moe,P.C., Martinac,B. and Kung,C. (1999) Mechanosensitive channels of bacteria. Methods Enzymol., 294, 458–482. [DOI] [PubMed] [Google Scholar]
- Chang G., Spencer,R.H., Lee,A.T., Barclay,M.T. and Rees,D.C. (1998) Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science, 282, 2220–2226. [DOI] [PubMed] [Google Scholar]
- Cui C. and Adler,J. (1996) Effect of mutation of potassium-efflux system, KefA, on mechanosensitive channels in the cytoplasmic membrane of Escherichia coli. J. Membr. Biol., 150, 143–152. [DOI] [PubMed] [Google Scholar]
- Cui C., Smith,D. and Adler,J. (1995) Characterization of mechano sensitive channels in Escherichia coli cytoplasmic membrane by whole-cell patch clamp recording. J. Membr. Biol., 144, 31–42. [DOI] [PubMed] [Google Scholar]
- Goodman M.B., Ernstrom,G.G., Chelur,D.S., O’Hagan,R., Yao,C.A. and Chalfie,M. (2002) MEC-2 regulates C.elegans DEG/ENaC channels needed for mechanosensation. Nature, 415, 1039–1042. [DOI] [PubMed] [Google Scholar]
- Gustin M.C., Zhou,X.L., Martinac,B. and Kung,C. (1988) A mechano sensitive ion channel in the yeast plasma membrane. Science, 242, 762–765. [DOI] [PubMed] [Google Scholar]
- Hamill O. and Martinac,B. (2001) Molecular basis of mechanotrans duction in living cells. Physiol. Rev., 81, 685–740. [DOI] [PubMed] [Google Scholar]
- Hamill O.P. and McBride,D.W. (1996) The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev., 48, 231–252. [PubMed] [Google Scholar]
- Jung K., Veen,M. and Altendorf,K. (2000) K+ and ionic strength directly influence the autophosphorylation activity of the putative turgor sensor KdpD of Escherichia coli. J. Biol. Chem., 275, 40142–40147. [DOI] [PubMed] [Google Scholar]
- Kong Y., Shen,Y., Warth,T.E. and Ma,J. (2002) Conformational pathways in the gating of Escherichia coli mechanosensitive channel. Proc. Natl Acad. Sci. USA, 99, 5999–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S.P., Higgins,C.F. and Booth,I.R. (1991) Regulation of compatible solute accumulation in Salmonella typhimurium: evidence for a glycine betaine efflux system. J. Gen. Microbiol., 137, 2617–2625. [DOI] [PubMed] [Google Scholar]
- Levina N., Totemeyer,S., Stokes,N.R., Louis,P., Jones,M.A. and Booth,I.R. (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J., 18, 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Buechner,M., Delcour,A.H., Adler,J. and Kung,C. (1987) Pressure-sensitive ion channel in Escherichia coli. Proc. Natl Acad. Sci. USA, 84, 2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaggan D., Jones,M.A., Gouesbet,G., Levina,N., Lindey,S., Epstein,W. and Booth,I.R. (2002) Analysis of the kefA2 mutation suggests that KefA is a cation-specific channel involved in osmotic adaptation in Escherichia coli.Mol. Microbiol., 43, 521–536. [DOI] [PubMed] [Google Scholar]
- Moe P.C. and Blount,P. (2002) A novel approach for probing protein–lipid interactions of MscL, a membrane-tension-gated channel. In Leatherbarrow,R. and Templer,R. (eds), Modelling Membranes and Proteins. Royal Society of Chemistry, London, UK, in press.
- Moe P.C., Blount,P. and Kung,C. (1998) Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol. Microbiol., 28, 583–592. [DOI] [PubMed] [Google Scholar]
- Moe P., Levin,G. and Blount,P. (2000) Correlating a protein structure with function of a bacterial mechanosensitive channel. J. Biol. Chem., 275, 31121–31127. [DOI] [PubMed] [Google Scholar]
- Okada K., Moe,P.C. and Blount,P. (2002) Functional design of bacterial mechanosensitive channels: comparisons and contrasts illuminated by random mutagenesis. J. Biol. Chem., 277, 27682–27688. [DOI] [PubMed] [Google Scholar]
- Ou X.R., Blount,P., Hoffman,R.J. and Kung,C. (1998) One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl Acad. Sci. USA, 95, 11471–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Blount,P., Folgering,J.H.A., Friesen,R.H.E., Moe,P.C. and van der Heide,T. (2002) How do membrane proteins sense water stress? Mol. Microbiol., 44, 889–902. [DOI] [PubMed] [Google Scholar]
- Rhoads D., Woo,A. and Epstein,W. (1977) Discrimination between Rb+ and K+ by Escherichia coli. Biochim. Biophys. Acta, 469, 45–51. [DOI] [PubMed] [Google Scholar]
- Rubenhagen R., Morbach,S. and Kramer,R. (2001) The osmoreactive betaine carrier BetP from Corynebacterium glutamicum is a sensor for cytoplasmic K+. EMBO J., 20, 5412–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M., Sachs,F. and Jing,Z. (1991) Quantitative video microscopy of patch clamped membranes stress, strain, capacitance and stretch channel activation. Biophys. J., 59, 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Hirokawa,K., Nakashima,K. and Mizuno,T. (1994) Signal-sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Mol. Microbiol., 14, 929–938. [DOI] [PubMed] [Google Scholar]
- Sukharev S. (2002) Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction and gating characteristics in liposomes. Biophys. J., 83, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S.I., Blount,P., Martinac,B., Blattner,F.R. and Kung,C. (1994) A large-conductance mechanosensitive channel in E.coli encoded by mscL alone. Nature, 368, 265–268. [DOI] [PubMed] [Google Scholar]
- Sukharev S.I., Blount,P., Martinac,B. and Kung,C. (1997) Mechano sensitive channels of Escherichia coli—the MscL gene, protein and activities. Annu. Rev. Physiol., 59, 633–657. [DOI] [PubMed] [Google Scholar]
- Sukharev S.I., Sigurdson,W.J., Kung,C. and Sachs,F. (1999) Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol., 113, 525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S., Betanzos,M., Chiang,C. and Guy,H. (2001a) The gating mechanism of the large mechanosensitive channel MscL. Nature, 409, 720–724. [DOI] [PubMed] [Google Scholar]
- Sukharev S., Durell,S. and Guy,H. (2001b) Structural models of the MscL gating mechanism. Biophys. J., 81, 917–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M., Rahme,L., Sternberg,J., Tompkins,R. and Ausubel,F. (1999) Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P.aeruginosa virulence factors. Proc. Natl Acad. Sci. USA, 96, 2408–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N. and Driscoll,M. (1997) Molecular modeling of mechanotransduction in the nematode Caenorhabditis elegans. Annu. Rev. Physiol., 59, 659–689. [DOI] [PubMed] [Google Scholar]
- Touze T., Gouesbet,G., Bolangiu,C., Jebbar,M., Bonnassie,S. and Blanco,C. (2001) Glycine betaine loses its osmoprotective activity in a bspA strain of Erwinia chrysanthemi. Mol. Microbiol., 42, 87–99. [DOI] [PubMed] [Google Scholar]
- van der Heide T., Stuart,M. and Poolman,B. (2001) On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J., 20, 7022–7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Batiza,A., Schroeder,M., Blount,P. and Kung,C. (1999) Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys. J., 77, 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Batiza,A. and Kung,C. (2001) Chemically charging the pore constriction opens the mechanosensitive channel MscL. Biophys. J., 80, 2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]