Abstract
Following ectodomain shedding, Notch-1 undergoes presenilin (PS)-dependent constitutive intramembranous endoproteolysis at site-3. This cleavage is similar to the PS-dependent γ-secretase cleavage of the β-amyloid precursor protein (βAPP). However, topological differences in cleavage resulting in amyloid β-peptide (Aβ) or the Notch-1 intracellular domain (NICD) indicated independent mechanisms of proteolytic cleavage. We now demonstrate the secretion of an N-terminal Notch-1 Aβ-like fragment (Nβ). Analysis of Nβ by MALDI-TOF MS revealed that Nβ is cleaved at a novel site (site-4, S4) near the middle of the transmembrane domain. Like the corresponding cleavage of βAPP at position 40 and 42 of the Aβ domain, S4 cleavage is PS dependent. The precision of this cleavage is affected by familial Alzheimer’s disease-associated PS1 mutations similar to the pathological endoproteolysis of βAPP. Considering these similarities between intramembranous processing of Notch and βAPP, we conclude that these proteins are cleaved by a common mechanism utilizing the same protease, i.e. PS/γ-secretase.
Keywords: Notch signaling/Notch-1-β peptide/presenilin/γ-secretase/site-4 cleavage
Introduction
The Notch cell surface receptors are type I transmembrane domain (TM) proteins that are critically required for a variety of signaling events during embryogenesis and in adulthood (reviewed in Mumm and Kopan, 2000). Notch receptors undergo a cascade of endoproteolytic cleavages required for Notch signaling (reviewed in Mumm and Kopan, 2000). Upon binding of membrane-anchored ligands from the DSL (Delta/Serrate/Lag-2) family, Notch receptors undergo consecutive cleavages at site-2 (S2) and site-3 (S3) (reviewed in Mumm and Kopan, 2000). Cleavage of mouse Notch-1 at S2 occurs in its ectodomain by TACE [tumor necrosis factor-α (TNF-α)-converting enzyme], a member of the ADAM (a disintegrin and metalloprotease domain) family ∼12 amino acids distant from the TM. This ‘ectodomain shedding’ event results in the generation of NEXT (Notch extracellular truncation; Brou et al., 2000; Mumm et al., 2000), that is cleaved subsequently at S3 within the TM close to the cytoplasmic border (Schroeter et al., 1998). Cleavage of Notch at S3 liberates NICD (Notch intracellular domain) that translocates to the nucleus, where it is involved in target gene transcription (reviewed in Mumm and Kopan, 2000). S3 cleavage strictly depends on the biological activity of the presenilin (PS) proteins (reviewed in Steiner and Haass, 2000), which may contribute the catalytic site of γ-secretase, an unusual intramembrane-cleaving aspartyl protease complex (Wolfe et al., 1999; Li et al., 2000; Steiner et al., 2000; Esler et al., 2002a).
Beside the Notch-1–4 receptors (De Strooper et al., 1999; Mizutani et al., 2001; Saxena et al., 2001), several other type I TM proteins have been identified as sub strates for PS-dependent endoproteolysis, including the Alzheimer’s disease (AD)-associated β-amyloid protein precursor (βAPP) (De Strooper et al., 1998), ErbB-4 (Ni et al., 2001; Lee et al., 2002), E-cadherin (Marambaud et al., 2002) and LRP (May et al., 2002). These proteins undergo ‘ectodomain shedding’ in their large extracellular domains, prior to the consecutive PS-dependent cleavage within the TM. In the case of βAPP, these cleavages are mediated by α-secretase and β-secretase (reviewed in Esler and Wolfe, 2001). Cleavage of βAPP by α- and β-secretase (BACE) results in the generation of the respective βAPP C-terminal fragments (CTFs), CTFα and CTFβ, which are the direct substrates for γ-secretase cleavage. Cleavage of CTFβ and CTFα by γ-secretase occurs in the middle of the TM and leads to the liberation of Aβ and p3 peptides (Haass and Selkoe, 1993), respectively. Aβ is deposited in the brain of AD patients in ‘senile plaques’, an invariant pathological hallmark of AD (reviewed in Selkoe, 2001). Recently, the elusive C-terminal cleavage product of γ-secretase, AICD (βAPP intracellular domain), has been identified and characterized. Surprisingly, AICD results from PS-dependent γ-secretase cleavage of βAPP–CTFs predominantly after Leu49 (Aβ numbering). This cleavage is almost identical to the S3 cleavage of Notch-1 (Gu et al., 2001; Sastre et al., 2001; Yu et al., 2001; Weidemann et al., 2002) and does not occur after Val40 and Ala42 (Aβ numbering) as predicted. Thus, γ-secretase cleaves the βAPP TM at several sites: one in the middle after position 40 (γ40) and 42 (γ42) (with major γ40 and minor γ42 cleavage) and one close to the cytoplasmic border after position 49 (γ49) of the Aβ domain. Interestingly, AICD may translocate to the nucleus where it could have a role in transcriptional regulation (Cao and Südhof, 2001; Cupers et al., 2001; Kimberly et al., 2001; Gao and Pimplikar, 2002) similar to NICD.
Because of these striking similarities between Notch and βAPP endoproteolysis, we hypothesized that an Aβ/p3-like species (called Notch β-peptide, Nβ) derived from NEXT intramembranous proteolysis may be secreted into the extracellular space. Here we report the identification and characterization of secreted Nβ peptides derived from endoproteolysis of NEXT derivatives. Sequence analysis revealed that Nβ is derived from endoproteolytic cleavage near the middle of the Notch-1 TM at site-4 (S4), which is 12 amino acid residues upstream of S3. Like S3 cleavage, S4 cleavage occurs in a PS- and γ-secretase-dependent manner. Strikingly, familial AD (FAD)-associated PS mutants known to cause the increased production of C-terminally elongated pathogenic Aβ42 also affect the generation of C-terminally elongated Nβ variants, supporting a direct role for PS in the proteolytic cleavage of Notch-1 and βAPP.
Results
Detection of a secreted Notch-1 Aβ-like peptide
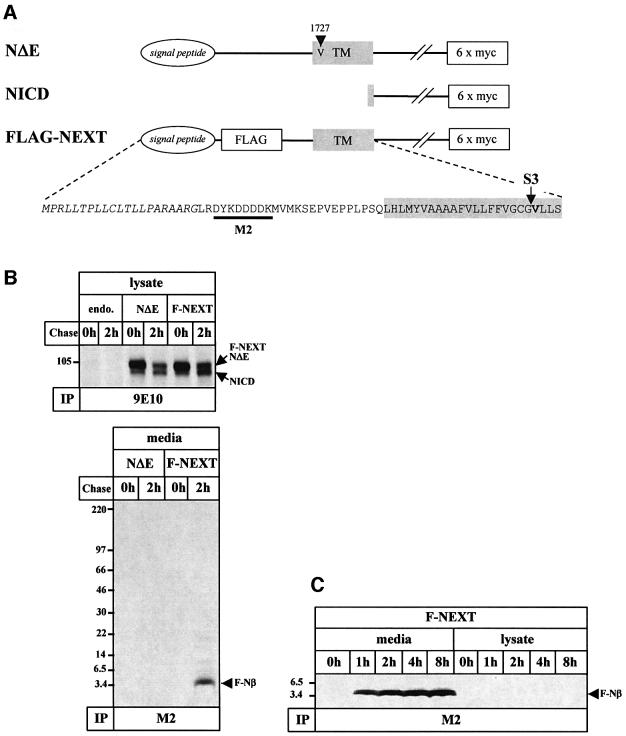
Given the similarities between the endoproteolytic processing of βAPP and Notch, we hypothesized that Aβ-like peptides derived from an S2-cleaved membrane-retained NEXT fragment might be secreted into the extracellular space. In order to investigate this, we stably transfected human embryonic kidney 293 (K293) cells with F-NEXT, an N-terminally FLAG-tagged NΔE variant (Figure 1A). We first investigated whether F-NEXT undergoes constitutive S3 endoproteolysis like the well-characterized NΔE variant (Kopan et al., 1996). F-NEXT- and NΔE-expressing cells were pulse labeled with [35S]methionine for 1 h and chased for 2 h. Immunoprecipitation with anti-myc antibody 9E10 revealed robust amounts of F-NEXT and NΔE (Figure 1B). NICD was produced during the pulse labeling and the chase period and accumulated after 2 h of chase (Figure 1B), consistent with previous results (Steiner et al., 1999a). To analyze secretion of Notch-1 peptides, the corresponding conditioned media were immunoprecipitated with the anti-FLAG antibody M2. Strikingly, robust amounts of a peptide of a molecular mass of ∼4 kDa were observed in the conditioned media of F-NEXT-expressing cells after a 2 h chase period (Figure 1B). This peptide is secreted in a time-dependent manner (Figure 1C) similar to Aβ (Haass et al., 1993) and did not accumulate inside the cells (Figure 1C). As expected, no M2-precipitable peptides were detected in the corresponding media of NΔE-expressing cells, demonstrating the specificity of the isolation procedure (Figure 1B). Untagged NΔE was also processed into a secreted peptide (data not shown). We conclude from these data that NEXT undergoes constitutive endoproteolysis resulting in the secretion of a peptide, which we term Notch-1-β peptide (Nβ) in analogy to Aβ.
Fig. 1. Detection of a secreted Notch-1 fragment. (A) Schematic representation of NΔE, NICD and F-NEXT. All three mouse Notch-1 variants contain a hexametric myc tag at the C-terminus to facilitate detection. F-NEXT is a NΔE variant that contains an insertion of the FLAG epitope and two adjacent methionine residues after the signal peptide sequence to facilitate the detection of secreted Notch-1 fragments. An arrowhead indicates the M1727V mutation present in NΔE (Kopan et al., 1996). An arrow shows S3, and the critical valine is shown in bold. The recognition site of the anti-FLAG antibody M2 is represented by the black bar. (B) Detection of a secreted FLAG-tagged Notch-1 fragment (F-Nβ) derived from F-NEXT. Untransfected K293 cells or K293 cells stably expressing NΔE or F-NEXT were pulse labeled with [35S]methionine for 1 h and chased for 0 and 2 h. Upper panel: cell lysates were immunoprecipitated with anti-myc antibody 9E10. F-NEXT and NΔE undergo S3 cleavage with similar efficiency. Note that in this pulse–chase paradigm, NICD generation is detectable after pulse labeling for 1 h without chase. Lower panel: conditioned media from K293 cells stably expressing NΔE or F-NEXT were immunoprecipitated with anti-FLAG M2 agarose. (C) Time-dependent secretion of F-Nβ. K293 cells stably expressing F-NEXT were pulse labeled with [35S]methionine for 1 h and chased for the indicated times. F-Nβ was analyzed in conditioned media, and cell lysates by immunoprecipitation with anti-FLAG M2 agarose. A longer exposure revealed that very low amounts of F-Nβ were also detectable in the cell lysates (data not shown). Identical results were obtained with F-NEXT M1727V (data not shown).
Nβ is derived from intramembranous proteolysis at a novel cleavage site
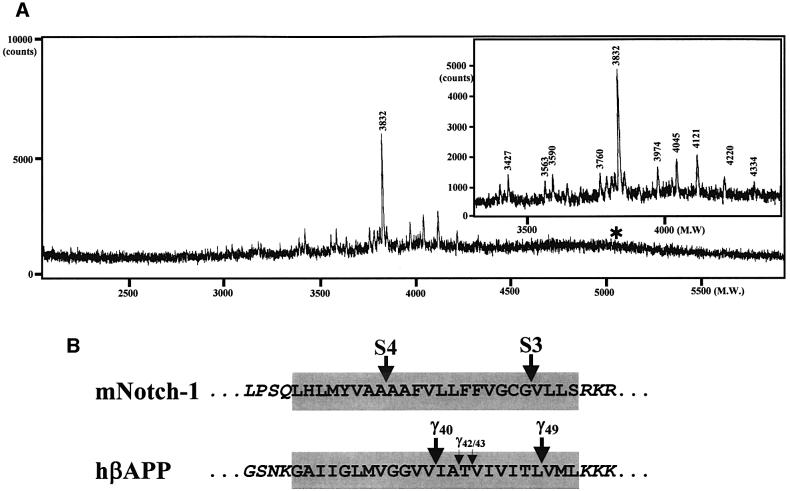
We hypothesized that Notch-1 may undergo a γ40 analogous cleavage further N-terminal of S3, which could result in Nβ secretion. To determine the exact cleavage site of F-NEXT, we performed matrix-associated laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis of the secreted FLAG-tagged Nβ (F-Nβ). MALDI-TOF MS analysis of M2-immunoprecipitated F-Nβ revealed a major peak of a molecular mass of 3832 Da among several minor peaks, indicating heterogeneous processing of the Notch-1 TM (Figure 2A, inset; see Table I for the F-Nβ peptides identified in this study). Interestingly, the heterogeneity of the F-Nβ C-termini (see Table I) is similar to the C-terminal heterogeneity observed in secreted Aβ species (Wang et al., 1996). Strikingly, the major F-Nβ peak corresponded to a peptide ending with Ala1731 (mouse Notch-1 numbering, Figure 2), indicating that F-Nβ is derived by a predominant cleavage at a novel site between Ala1731 and Ala1732, and not by cleavage at S3 between Gly1743 and Val1744 as predicted. This is consistent with the lack of any detectable F-Nβ species with a C-terminus corresponding to Gly1743 (see asterisk in Figure 2A). Identical results were obtained when Neuro2a, COS or CHO cells were used in this analysis (data not shown). Thus, these data strongly suggest that Notch-1, like βAPP, undergoes several distinct intramembranous cleavages: one close to the cytoplasmic border (S3) of the TM and a novel heterogeneous cut near the middle of the TM, which we now term site-4 (S4) cleavage (Figure 2B).
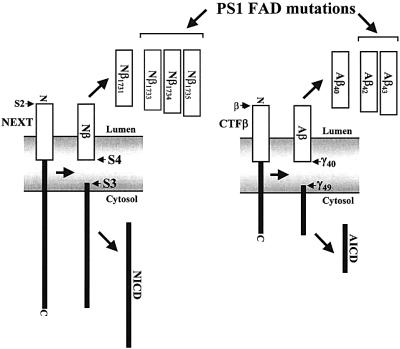
Fig. 2. Characterization of F-Nβ by IP/MS. (A) MALDI-TOF MS spectrum of F-Nβ. Conditioned medium from K293 cells stably expressing F-NEXT was immunoprecipitated with anti-FLAG M2 agarose, and F-Nβ was analyzed by MALDI-TOF MS (see Materials and methods). Molecular masses in Daltons of the individual peaks are indicated. Multiple peaks including a major peak at 3832 Da were observed at the mass range from 3400 to 4400 Da (inset). Peaks higher than 4400 Da including the theoretically predicted peak of ∼5058 Da corresponding to F-Nβ derived from S3 cleavage (asterisk, see B) were not observed. The major F-Nβ peak of 3832 Da was also identified by IP/MS analysis from conditioned media of K293 cells stably transfected with F-NEXT M1727V (data not shown). (B) Schematic representation of intramembranous cleavages of the mouse Notch-1 and human βAPP TMs. S4 cleavage of F-NEXT derivatives occurs predominantly between Ala1731 and Ala1732.
Table I. List of F-Nβ species found in conditioned media.
| Mr (observed) | F-Nβ | Mr (calculated) | Amino acid sequence |
|---|---|---|---|
| 4334 | F-Nβ1736 | 4333.81 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLMYVAAAAFVL1736 |
| 4220 | F-Nβ1735 | 4220.66 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLMYVAAAAFV1735 |
| 4121 | F-Nβ1734 | 4121.53 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLMYVAAAAF1734 |
| 3974 | F-Nβ1733 | 3974.36 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLMYVAAAA1733 |
| 4045 | F-Nβ1731 | 4045.45 | RGLRDYKDDDDKMVMKSEPVEPPLPSQ LHLMYVAA1731 |
| 3832 | F-Nβ1731 | 3832.22 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLMYVAA1731 |
| 3563 | F-Nβ1731 | 3562.89 | DYKDDDDKMVMKSEPVEPPLPSQ LHLMYVAA1731 |
| 3760 | F-Nβ1730 | 3761.15 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLMYVA1730 |
| 3590 | F-Nβ1728 | 3590.95 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLMY1728 |
| 3427 | F-Nβ1727 | 3427.78 | LRDYKDDDDKMVMKSEPVEPPLPSQ LHLM1727 |
Bold letters indicate a peptide sequence and its properties of the major species.
S4 cleavage is PS/γ-secretase dependent
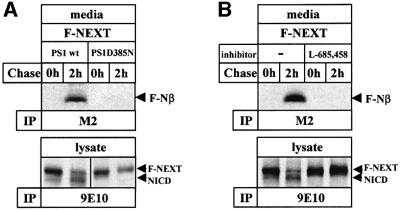
Since γ40/42 cleavage of βAPP has been shown to require PS activity (reviewed in Steiner and Haass, 2000), we next investigated whether S4 cleavage is also PS dependent. K293 cells expressing biologically inactive PS1 D385N (Steiner et al., 1999b), which lacks one of the two putative active site aspartates (Wolfe et al., 1999), were stably transfected with F-NEXT and analyzed for the generation of F-Nβ. Strikingly, F-Nβ generation was almost completely inhibited in cells expressing PS1 D385N (Figure 3A, upper panel). Consistent with previous results (Capell et al., 2000), NICD production was also significantly reduced (Figure 3A, lower panel). To confirm further the PS/γ-secretase dependence of F-Nβ generation, we treated cells with the γ-secretase inhibitor L-685,458 (Shearman et al., 2000). When L-685,458 was added to the pulse–chase experiment, an almost complete inhibition of F-Nβ generation was observed (Figure 3B, upper panel). As expected, NICD generation (Martys-Zage et al., 2000; Beher et al., 2001) was also blocked (Figure 3B, lower panel). Taken together, these results demonstrate that F-Nβ generation occurs by cleavage at S4 in a PS/γ-secretase-dependent manner similar to the generation of Aβ.
Fig. 3. F-Nβ is generated in a PS- and γ-secretase-dependent manner. (A) PS dependence of F-Nβ generation. K293 cells stably expressing wild-type PS1 or mutant PS1 D385N were stably transfected with the F-NEXT cDNA. Upper panel: F-Nβ generation was analyzed from conditioned media of metabolically labeled cells as described in Figure 1B. Lower panel: corresponding cell lysates were analyzed for NICD generation as described in Figure 1B. (B) γ-secretase dependence of F-Nβ generation. Upper panel: F-Nβ generation was analyzed as described in Figure 1B from conditioned media of K293 cells stably co-expressing F-NEXT and wild-type PS1 that were metabolically labeled in the presence or absence of γ-secretase inhibitor L-685,458 (1 µM). Lower panel: corresponding cell lysates were analyzed for NICD generation as described in Figure 1B.
FAD-associated PS mutations affect S4 cleavage
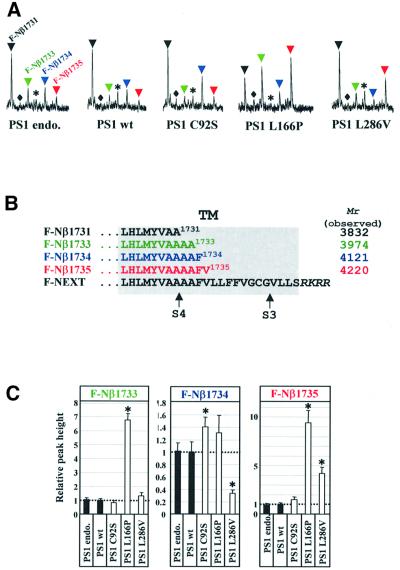
All FAD-associated PS mutations analyzed so far result in the increased secretion of Aβ42 (reviewed in Steiner and Haass, 2000). We therefore investigated whether the PS-dependent S4 cleavage is also influenced by FAD-associated PS mutants. F-NEXT was stably transfected into K293 cells expressing wild-type PS1 or the FAD-associated PS1 mutants PS1 C92S, PS1 L166P and PS1 L286V (Kulic et al., 2000; Okochi et al., 2000; Moehlmann et al., 2002). In particular, the L166P mutation was chosen because it not only causes one of the strongest increases in Aβ42 generation, but also significantly inhibits S3 cleavage (Moehlmann et al., 2002). Thus, this mutation allows investigation of whether a PS mutant that inhibits S3 cleavage also affects S4 cleavage. We analyzed the conditioned media of cells co-expressing the PS1 derivatives and F-NEXT by MALDI-TOF MS for putative alterations in the C-termini of F-Nβ. Strikingly, this revealed significant changes in the C-terminal cleavage pattern of Nβ produced in cells expressing wild-type or FAD mutant PS1 (Figure 4A). Specifically, the PS1 L166P mutation, which causes an extremely strong increase of Aβ42 generation (Moehlmann et al., 2002), also produced strongly increased levels of elongated F-Nβ peptides. These include F-Nβ species elongated by two and four amino acids (F-Nβ1733 and F-Nβ1735; Figure 4B). In addition, levels of F-Nβ terminating at amino acid 1734 (Figure 4B) were also slightly but reproducibly elevated (Figure 4A). Thus, inhibition of S3 cleavage by the PS1 L166P mutant does not cause a block of S4 cleavage, but rather shifts the S4 cleavage in a manner similar to the effects caused by FAD mutant PS on γ40/γ42 cleavage. Interestingly, the two other FAD mutants analyzed produced individual changes in C-terminal heterogeneity of the S4 cleavage. While PS1 C92S elevated levels of a peptide terminating after amino acid 1734, PS1 L286V increased peptides terminating after amino acid 1735 and decreased the levels of F-Nβ1734 (Figure 4A). Therefore, the FAD mutants analyzed apparently cause individual and characteristic cleavage patterns. They all have in common the increased production of elongated species. Like Aβ42 production, the levels of elongated F-Nβ are most affected significantly by the very aggressive PS1 L166P mutation, which causes FAD in early adulthood (Moehlmann et al., 2002). These effects are not restricted to K293 cells, since we also observed similar effects of FAD-associated PS2 mutations in Neuro2a cells (data not shown). To substantiate these findings further, we performed multiple independent experiments followed by a semi-quantitative analysis (see Materials and methods). This fully confirmed the primary observations. All FAD mutants analyzed affected the generation of the F-Nβ C-terminus in a highly reproducible and quantitative manner (Figure 4C).
Fig. 4. PS1 FAD mutations affect the relative levels of elongated F-Nβ species. (A) MALDI-TOF MS spectra of F-Nβ species secreted from cells co-expressing F-NEXT and the indicated PS1 derivatives. Conditioned media were analyzed by IP/MS as described in Figure 2B. The mass range from ∼3750 to ∼4250 Da is shown. Black arrowhead, F-Nβ1731; colored arrowheads, F-Nβ1733 (green), F-Nβ1734 (blue) and F-Nβ1735 (red). The rhombus indicates the peak corresponding to F-Nβ1732 (molecular mass 3903 Da), and the asterisk indicates an F-Nβ1731 (molecular mass 4045 Da) species with a different N-terminus from that of the major F-Nβ1731 (molecular mass 3832 Da) species (compare Figure 2B and Table I). Note that FAD-associated PS1 mutations, in particular PS1 L166P, show an altered production of the various F-Nβ species. (B) C-termini of F-Nβ species affected by FAD-associated PS1 mutants. Sequences within the putative TM are shown. Black, F-Nβ1731; green, F-Nβ1733; blue, F-Nβ1734; red, F-Nβ1735. Arrows indicate S3 and S4. (C) Semi-quantitative analysis of elongated F-Nβ species. Conditioned media from cells expressing the indicated PS1 FAD-associated mutants (each medium amount for immunoprecipitation was normalized to contain the same level of F-Nβ1731; see Materials and methods) were analyzed by IP/MS. Peak heights corresponding to secreted F-Nβ1733, F-Nβ1734 and F-Nβ1735 were measured and are expressed relative to the peak height of the internal control (1 pmol bovine insulin β-chain; see Materials and methods). Asterisks indicate the significance of the increase and decrease of the various F-Nβ species relative to both endogenous/wild-type PS1-expressing cells (P < 0.0001; Students t-test). The data are the mean from five independent measurements.
Discussion
We have examined whether membrane-retained Notch fragments such as NEXT secrete peptides similar to Aβ upon NICD generation. Here we demonstrate that the NEXT derivative F-NEXT indeed undergoes an additional and unexpected cleavage at a novel site near the middle of the membrane, which results in the secretion of an Aβ-like peptide (Figure 5). Upon overexpression of F-NEXT, we identified several F-Nβ species with heterogeneous C-termini similar to Aβ. The major cleavage site was identified in the center of four sequential alanine residues between Ala1731 and Ala1732 in all cell lines analyzed so far (data herein and data not shown). Since it has been shown previously that NICD is generated by cleavage at S3 between Gly1743 and Val1744 (Schroeter et al., 1998), one would have expected to detect a corresponding Nβ peptide terminating at Gly1743. However, in our study, we identified robust levels of peptides terminating at amino acid 1731, but failed to detect the secreted peptide corresponding to S3 cleavage in cultured media. Therefore, the intramembranous cleavage resulting in the immediate secretion of Nβ must be biochemically distinct from the previously identified S3 cleavage. Based on our findings, Nβ generation is therefore the result of a novel cleavage, and we consequently introduce the term S4 for this intramembranous cut.
Fig. 5. Similar intramembranous cleavages of Notch-1 and βAPP. Following S2 cleavage, the Notch fragment NEXT is cleaved in a PS- and γ-secretase-dependent manner within the membrane at two major sites, S3 and S4. S3 cleavage occurs close to the cytosolic membrane border and leads to the liberation of NICD, whereas S4 cleavage occurs near the middle of the TM and causes the release of Nβ peptides. S3 and S4 cleavages are strikingly similar to γ49 and γ40 cleavages of CTFβ of βAPP, respectively. The generation of longer forms of Nβ and Aβ peptides (Aβ42,43) are affected in a similar manner at least by some FAD-associated PS mutants.
Like AICD and Aβ production, generation of NICD and the novel Nβ are both dependent on PS-mediated γ-secretase processing. This is demonstrated by the significant reduction of Nβ generation upon the expression of a biologically inactive PS1 variant. Moreover, a highly potent γ-secretase inhibitor also blocked Nβ production. However, at present, we do not know the order of these cleavages. As discussed for AICD/Aβ generation (Sastre et al., 2001), it is most likely that all the intramembranous γ-secretase cleavages occur simultaneously, since neither longer Aβ/Nβ nor AICD/NICD fragments have been identified so far.
Several PS-dependent γ-secretase substrates have been described. These include βAPP (De Strooper et al., 1998), ErbB-4 (Ni et al., 2001; Lee et al., 2002), E-cadherin (Marambaud et al., 2002), LRP (May et al., 2002) and Notch-1–4 (De Strooper et al., 1999; Mizutani et al., 2001; Saxena et al., 2001), and probably also CD44 (Okamoto et al., 2001). Apparently, all these type I TM proteins release their C-terminal tails into the cytoplasm. Under in vivo conditions, some of the proteolytically liberated ICDs translocate to the nucleus (Schroeter et al., 1998; Struhl and Adachi, 1998; Cao and Südhof, 2001; Ni et al., 2001; Okamoto et al., 2001; Lee et al., 2002) where they do or may regulate transcription of target genes (reviewed in Steiner and Haass, 2001). The cleavage, which results in the liberation of ICDs, takes place very close to the cytoplasmic border of these proteins (Schroeter et al., 1998; Gu et al., 2001; Okamoto et al., 2001; Sastre et al., 2001; Yu et al., 2001; Marambaud et al., 2002; Weidemann et al., 2002). In contrast, the γ-secretase cleavage of βAPP, which liberates Aβ, occurs in the middle of the TM. This apparent discrepancy has been used as an argument for the existence of two independent proteolytic enzymes involved in NICD generation and Aβ liberation (Yu et al., 2001; reviewed in Sisodia and St George-Hyslop, 2002). Indeed, this idea may be supported by the selective inhibition of Aβ production without affecting Notch cleavage (Petit et al., 2001). The consequence of these findings would be that Aβ generation and NICD (or even ICD production in general) is mediated by independent and distinct intramembrane cleaving proteases. However, our findings strongly support the idea that an evolutionarily conserved PS-dependent mechanism is involved in the two γ-secretase cleavages of Notch-1 and βAPP. Both result in the generation of the respective ICD (via cleavage close to the cytoplasmic border of the membrane) as well as the secretion of a small and hydrophobic peptide (via cleavage within the middle of the TM). The additional S4 intramembranous cleavage is also in agreement with recent findings demonstrating that an exchange of the βAPP TM with the Notch TM allows intramembranous γ-secretase processing at a position homologous to the site of Aβ40 generation (Zhang et al., 2002). However, in the latter case, the cleavage was obtained several amino acids C-terminal of the S4 cleavage site determined in this study at a position corresponding to F-Nβ1735. The reason for this discrepancy is probably due to insertion of the Notch TM into an artificial βAPP backbone (Zhang et al., 2002). In contrast, we used constitutively processed authentic Notch-1 derivatives mimicking NEXT. Finally, the selective inhibition of γ-secretase-mediated Aβ production (Petit et al., 2001) has been challenged recently and it was clearly demonstrated that the inhibitors used do not directly affect PS-dependent γ-secretase cleavage and Aβ production (Esler et al., 2002b).
Intramembranous endoproteolysis (S4/S3) of Notch-1 and βAPP shares many common features: (i) substrates of both S4/S3 and γ40/γ49 cleavages are truncated extracellularly by shedding enzymes (reviewed in Mumm and Kopan, 2000; Steiner and Haass, 2000); (ii) S4/S3 (Figure 3) as well as γ40/γ49 cleavages were found to be PS dependent; (iii) both the γ49 and S3 cleavages occur at analogous sites very close to the cytoplasmic border of the membrane (Schroeter et al., 1998; Gu et al., 2001; Sastre et al., 2001; Yu et al., 2001; Weidemann et al., 2002); (iv) upon S4/S3 and γ40/γ49 cleavages, the resulting fragments, Nβ/NICD (Figure 1) and Aβ/AICD, are released into the extracellular space or into the cytoplasm, respectively; (v) the extracellularly released fragments (Nβ/Aβ) do not correspond directly to the intracellularly released fragments (NICD/AICD), i.e. so far neither Nβ1743/Aβ49 nor NICD1732 (NICD starting from Ala1732, mouse Notch-1 numbering)/AICD59 (AICD starting from Ile41, Aβ numbering) have been identified; (vi) the C-termini of both peptides are heterogeneous; and, finally, (vii) FAD-associated mutants of PS1 cause the generation of C-terminally elongated Nβ fragments very similar to Aβ42 generated in the presence of FAD mutants (Figure 4). Due to these extensive similarities, it is very likely that the intramembraneous cleavages of both proteins occur by a common mechanism and are not mediated by different βAPP- and Notch-1-cleaving enzymes as proposed previously (Petit et al., 2001, 2002a,b; Yu et al., 2001; Taniguchi et al., 2002; reviewed in Sisodia and St George-Hyslop, 2002). The conservation of this mechanism strongly suggests that at least Notch-1 and βAPP are cleaved twice by the same PS-dependent enzymatic mechanism. It certainly remains to be shown if the other γ-secretase substrates also undergo an additional intramembranous cleavage.
Finally, our data also support the hypothesis that PS harbors the catalytic site of γ-secretase and executes the cleavage of both βAPP and Notch-1. Interestingly, a very aggressive FAD-associated PS1 mutant (PS1 L166P), which causes an extreme increase of Aβ42 production, also dramatically shifts the corresponding cleavage of Notch-1. The most parsimonious explanation for this finding is a direct contact of PS with its substrates. Together with the finding that γ-secretase inhibitors bind to PS (Esler et al., 2000; Li et al., 2000), the observation of the ‘critical aspartates’ (Wolfe et al., 1999), the similarity around the active site aspartate in TM7 of PSs to type 4 prepilin peptidases (Steiner et al., 2000) and the finding that mutations of Leu166 of PS1 affect the generation of AICD and NICD generation in a similar manner (Moehlmann et al., 2002) suggest that PS is indeed the proteolytic subunit within the multicomponent γ-secretase complex. Such a complex requires additional factors for its assembly, stability and activity. One of these components is nicastrin (Yu et al., 2000), which is required for PS expression (Edbauer et al., 2002; Hu et al., 2002; Lopez-Schier and St Johnston, 2002), γ-secretase activity (Edbauer et al., 2002) and Notch S3 cleavage (Chung and Struhl, 2001; Hu et al., 2002; Lopez-Schier and St Johnston, 2002).
Materials and methods
Antibodies and reagents
The monoclonal antibodies 9E10 against the c-myc epitope and M2 against the FLAG epitope were obtained from Sigma (St Louis, MO). The γ-secretase inhibitor L-685,458, ([(2R,4R,5S)-2-benzyl-5-(Boc-amino)-4-hydroxy-6-phenyl-hexanoyl]-Leu-Phe-NH2) was purchased from Bachem.
cDNA constructs
The cDNAs encoding the mouse Notch-1 variants NΔE and NICD carrying a C-terminal hexametric myc tag (Schroeter et al., 1998) in pcDNA3-hygro (+) vector were described previously (Steiner et al., 1999a). These Notch-1 variants contain the M1727V mutation (Kopan et al., 1996). Mouse Notch-1 F-NEXT variants either containing or lacking the M1727V mutation were obtained by PCR-mediated mutagenesis. First, F-NEXT M1727V was generated using the ExSite PCR-based site-directed mutagenesis kit (Stratagene) using NΔE as the template and the primers 5′-P-ATCGTCGTCCTTGTAGTCTCTCAAGCCTCTTGCGCCGAGCGCGGGCAGCAGCGTTAG-3′ and 5′-P-GACAAGATGGTGATGAAGAGTGAGCCGGTGGAGCCTCCGCTGCCC TCGCAGCTG-3′. Subsequently, F-NEXT cDNA was generated by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene) using F-NEXT M1727V cDNA as the template and the primers 5′-CCTCGCAGCTGCACCTCATGTACGTGGCAGCG-3′ and 5′-CGCGCCACGTACATGAGGTGCAGCTGCGAGG-3′. Each mutant was sequenced to verify successful mutagenesis.
Cell culture, cell lines and cDNA transfection
K293 cells stably expressing either wild-type PS1 (Okochi et al., 2000), PS1 C92S (Okochi et al., 2000), PS1 L166P (Moehlmann et al., 2002), PS1 L286V (Kulic et al., 2000) or PS1 D385N (Steiner et al., 1999b) were generated and cultured as described. Stable transfections with NΔE, NICD and F-NEXT cDNA constructs were carried out using Lipofectamine 2000 (Invitrogen) according to the supplier’s instructions.
Analysis of Notch-1 metabolites
Confluent cells in a 10 cm dish were analyzed for Notch-1 metabolites in pulse–chase experiments. Following starvation in methionine- and serum-free minimal essential medium (MEM) for 40 min, cells subse quently were metabolically labeled with 400 µCi of [35S]methionine/cysteine (Redivue Promix, Amersham) for 1 h in methionine- and serum-free MEM and chased for 2 h in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) and excess amounts of unlabeled methionine. Conditioned media were collected, immediately put on ice and, after a clarifying spin at 3000 g and addition of a protease inhibitor cocktail (1:1000; Sigma) and 0.025% of sodium azide, subjected to immunoprecipitation with anti-FLAG M2 agarose (Sigma). Immuno precipitates were separated on 10–20% Tris-tricine gels (Invitrogen) and analyzed for F-Nβ species by fluorography. Cell lysates were prepared as described (Okochi et al., 2000) and analyzed for NΔE, F-NEXT and derivatives thereof, and NICD by immunoprecipitation with antibody 9E10 as described (Steiner et al., 1999a). γ-Secretase dependence of Notch-1 endoproteolysis was analyzed using γ-secretase inhibitor L-685,458 (1 µM; Shearman et al., 2000), which was added to the culture media 2 h before starvation and present throughout the starvation, pulse and chase periods.
Combined immunoprecipitation/MALDI-TOF MS (IP/MS) analysis of Nβ species
Cell lines stably expressing F-NEXT derivatives were grown in 20 cm dishes. After reaching confluence, the culture media were replaced with 24 ml of 10% FCS/DMEM and media were incubated for 3 h. A 20 ml aliquot of the conditioned media was collected, immediately put on ice and subjected to a clarifying spin. Following addition of a protease inhibitor mix (1:1000, Sigma) and 0.025% sodium azide, conditioned media were immunoprecipitated with M2 agarose for 4 h at 4°C. Immunoprecipitates were washed three times for 10 min at 4°C with wash buffer 1 (0.1% N-octylglucoside, 140 mM NaCl, 10 mM Tris pH 8.0, 0.025% sodium azide) and once with wash buffer 2 (10 mM Tris pH 8.0, 0.025% sodium azide). Immunoprecipitated peptides were eluted with trifluoroacetic acid/acetonitrile/water (1:20:20) saturated with α-cyano-4-hydroxy cinnamic acid. The dissolved samples were dried on a stainless plate and subjected to MALDI-TOF MS analysis. The MS peak heights and molecular masses were calibrated with angiotensin (Sigma) and bovine insulin β-chain (Sigma).
Semi-quantitative analysis of F-Nβ species
Conditioned media from the respective K293 cells co-expressing F-NEXT/PS were collected and aliquots of the conditioned media were subjected to IP/MS analysis. The peak heights of F-Nβ1731 in the MS spectra were measured and its peak heights relative to the peak height of 1 pmol bovine insulin β-chain (internal control) were calculated. These relative peak heights were used to calculate the relative levels of the F-Nβ1731 species contained in each conditioned medium of the respective F-NEXT/PS-transfected cells. Subsequently, the amounts of the conditioned media were adjusted to contain the same levels of F-Nβ1731 using a standard curve for F-Nβ1731, and again subjected to IP/MS analysis. After confirming that the F-Nβ1731 peak has the same height as the peak of the internal control, peak heights corresponding to C-terminally longer F-Nβ species (F-Nβ1733, F-Nβ1734 or F-Nβ1735) were measured and its peak heights relative to the internal control were calculated. Relative peak heights of F-Nβ species obtained from endogenous/wild-type PS1 and PS1 FAD-associated mutants were compared.
Acknowledgments
Acknowledgements
We wish to thank Drs Junji Takeda, Hiroshi Mori and Shinji Tagami for critically reading the manuscript, Yumi Satoh, Nuripa Aidaralieva and Gabi Basset for technical assistance, and Dr Raphael Kopan for the NΔE and NICD constructs. This work was supported by grants from the Ministry of Health and Welfare (14121601 to M.T. and M.O., and 13080101 to M.T.), the Ministry of Education, Science, Culture and Sports (14017060 and 14770499 to M.O.), the Deutsche Forschungs gemeinschaft (Priority program on ‘Cellular Mechanisms of Alzheimer’s Disease’ to C.H. and H.S.) and the American Health Association Foundation (AHAF to C.H. and H.S).
References
- Beher D., Wrigley,J.D., Nadin,A., Evin,G., Masters,C.L., Harrison,T., Castro,J.L. and Shearman,M.S. (2001) Pharmacological knock-down of the presenilin 1 heterodimer by a novel γ-secretase inhibitor: implications for presenilin biology. J. Biol. Chem., 276, 45394–45402. [DOI] [PubMed] [Google Scholar]
- Brou C. et al. (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell, 5, 207–216. [DOI] [PubMed] [Google Scholar]
- Cao X. and Sudhof,T.C. (2001) A transcriptionally active complex of APP with Fe65 and histone acetyltransferase Tip60. Science, 293, 115–120. [DOI] [PubMed] [Google Scholar]
- Capell A., Steiner,H., Romig,H., Keck,S., Baader,M., Grim,M.G., Baumeister,R. and Haass,C. (2000) Presenilin-1 differentially facilitates endoproteolysis of the β-amyloid precursor protein and Notch. Nat. Cell Biol., 2, 205–211. [DOI] [PubMed] [Google Scholar]
- Chung H.M. and Struhl,G. (2001) Nicastrin is required for presenilin-mediated transmembrane cleavage in Drosophila. Nat. Cell Biol., 3, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Cupers P., Orlans,I., Craessaerts,K., Annaert,W. and De Strooper,B. (2001) The amyloid precursor protein (APP)-cytoplasmic fragment generated by γ-secretase is rapidly degraded but distributes partially in a nuclear fraction of neurones in culture. J. Neurochem., 78, 1168–1178. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Saftig,P., Craessaerts,K., Vanderstichele,H., Guhde,G., Annaert,W., Von Figura,K. and Van Leuven,F. (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature, 391, 387–390. [DOI] [PubMed] [Google Scholar]
- De Strooper B. et al. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature, 398, 518–522. [DOI] [PubMed] [Google Scholar]
- Edbauer D., Winkler,E., Haass,C. and Steiner,H. (2002) Presenilin and nicastrin regulate each other and determine amyloid β-peptide production via complex formation. Proc. Natl Acad. Sci. USA, 99, 8666–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler W.P. and Wolfe,M.S. (2001) A portrait of Alzheimer secretases—new features and familiar faces. Science, 293, 1449–1454. [DOI] [PubMed] [Google Scholar]
- Esler W.P. et al. (2000) Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nat. Cell Biol., 2, 428–434. [DOI] [PubMed] [Google Scholar]
- Esler W.P. et al. (2002a) Amyloid-lowering isocoumarins are not direct inhibitors of γ-secretase. Nat. Cell Biol., 4, 110–111. [DOI] [PubMed] [Google Scholar]
- Esler W.P., Kimberly,W.T., Ostaszewski,B.L., Ye,W., Diehl,T.S., Selkoe,D.J. and Wolfe,M.S. (2002b) Activity-dependent isolation of the presenilin–γ-secretase complex reveals nicastrin and a γ-substrate. Proc. Natl Acad. Sci. USA, 99, 2720–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. and Pimplikar,S.W. (2001) The γ-secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc. Natl Acad. Sci. USA, 98, 14979–14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Misonou,H., Sato,T., Dohmae,N., Takio,K. and Ihara,Y. (2001) Distinct intramembrane cleavage of the β-amyloid precursor protein family resembling γ-secretase-like cleavage of Notch. J. Biol. Chem., 276, 35235–35238. [DOI] [PubMed] [Google Scholar]
- Haass C. and Selkoe,D.J. (1993) Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell, 75, 1039–1042. [DOI] [PubMed] [Google Scholar]
- Haass C., Hung,A.Y., Schlossmacher,M.G., Teplow,D.B. and Selkoe,D.J. (1993) β-amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J. Biol. Chem., 268, 3021–3024. [PubMed] [Google Scholar]
- Hu Y., Ye,Y. and Fortini,M.E. (2002) Nicastrin is required for γ-secretase cleavage of the Drosophila Notch receptor. Dev. Cell, 2, 69–78. [DOI] [PubMed] [Google Scholar]
- Kimberly W.T., Zheng,J.B., Guenette,S.Y. and Selkoe,D.J. (2001) The intracellular domain of the β-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a Notch-like manner. J. Biol. Chem., 276, 40288–40292. [DOI] [PubMed] [Google Scholar]
- Kopan R., Schroeter,E.H, Weintraub,H. and Nye,J.S. (1996) Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc. Natl Acad. Sci. USA, 93, 1683–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulic L., Walter,J., Multhaup,G., Teplow,D.B., Baumeister,R., Romig,H., Capell,A., Steiner,H. and Haass,C. (2000) Separation of presenilin function in amyloid β-peptide generation and endoproteolysis of Notch. Proc. Natl Acad. Sci. USA, 97, 5913–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Jung,K.M., Huang,Y.Z., Bennett,L.B., Lee,J.S., Mei,L. and Kim,T.W. (2002) Presenilin-dependent γ-secretase-like intramem brane cleavage of ErbB4. J. Biol. Chem., 277, 6318–6323. [DOI] [PubMed] [Google Scholar]
- Li Y.M. et al. (2000) Presenilin 1 is linked with γ-secretase activity in the detergent solubilized state. Proc. Natl Acad. Sci. USA, 97, 6138–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Schier H. and St Johnston,D. (2002) Drosophila nicastrin is essential for the intramembranous cleavage of Notch. Dev. Cell, 2, 79–89. [DOI] [PubMed] [Google Scholar]
- Marambaud P. et al. (2002) A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J., 21, 1948–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martys-Zage J.L., Kim,S.H., Berechid,B., Bingham,S.J., Chu,S., Sklar,J., Nye,J. and Sisodia,S.S. (2000) Requirement for presenilin 1 in facilitating Jagged 2-mediated endoproteolysis and signaling of Notch 1. J. Mol. Neurosci., 15, 189–204. [DOI] [PubMed] [Google Scholar]
- May P., Reddy,Y.K. and Herz,J. (2002) Proteolytic processing of LRP mediates regulated release of its intracellular domain. J. Biol. Chem., 277, 18736–18743. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Taniguchi,Y., Aoki,T., Hashimoto,N. and Honjo,T. (2001) Conservation of the biochemical mechanisms of signal transduction among mammalian Notch family members. Proc. Natl Acad. Sci. USA, 98, 9026–9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehlmann T. et al. (2002) Presenilin-1 mutations of leucine 166 equally affect the generation of Notch and APP intracellular domains independent of their effect on Aβ42 production. Proc. Natl Acad. Sci. USA, 99, 8025–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm J.S. and Kopan,R. (2000) Notch signaling: from the outside in. Dev. Biol., 228, 151–165. [DOI] [PubMed] [Google Scholar]
- Mumm J.S., Schroeter,E.H., Saxena,M.T., Griesemer,A., Tian,X., Pan,D.J., Ray,W.J. and Kopan,R.A. (2000) A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch1. Mol. Cell, 5, 197–206. [DOI] [PubMed] [Google Scholar]
- Ni C.Y., Murphy,M.P., Golde,T.E. and Carpenter,G. (2001) γ-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science, 294, 2179–2181. [DOI] [PubMed] [Google Scholar]
- Okamoto I., Kawano,Y., Murakami,D., Sasayama,T., Araki,N., Miki,T., Wong,A.J. and Saya,H. (2001) Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J. Cell Biol., 155, 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M., Eimer,S., Bottcher,A., Baumeister,R., Romig,H., Walter,J., Capell,A., Steiner,H. and Haass,C. (2000) A loss of function mutant of the presenilin homologue SEL-12 undergoes aberrant endoproteolysis in Caenorhabditis elegans and increases Aβ 42 generation in human cells. J. Biol. Chem., 275, 40925–40932. [DOI] [PubMed] [Google Scholar]
- Petit A., Bihel,F., Alves da Costa,C., Pourquie,O., Checler,F. and Kraus,J.L. (2001) New protease inhibitors prevent γ-secretase-mediated production of Aβ40/42 without affecting Notch cleavage. Nat. Cell Biol., 3, 507–511. [DOI] [PubMed] [Google Scholar]
- Petit A., Dumanchin-Njock,C., Andrau,D., Da Costa,C.A. and Checler,F. (2002a) Amyloid-lowering isocoumarins are not direct inhibitors of γ-secretase. Nat. Cell Biol., 4, 111–112. [DOI] [PubMed] [Google Scholar]
- Petit A., St George-Hyslop,P., Fraser,P. and Checler,F. (2002b) γ-secretase-like cleavages of Notch and βAPP are mutually exclusive in human cells. Biochem. Biophys. Res. Commun., 290, 1408–1410. [DOI] [PubMed] [Google Scholar]
- Sastre M., Steiner,H., Fuchs,K., Capell,A., Multhaup,G., Condron,M.M., Teplow,D.B. and Haass,C. (2001) Presenilin-dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO rep. 2, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena M.T., Schroeter,E.H., Mumm,J.S. and Kopan,R. (2001) Murine notch homologs (N1–4) undergo presenilin-dependent proteolysis. J. Biol. Chem., 276, 40268–40273. [DOI] [PubMed] [Google Scholar]
- Schroeter E.H., Kisslinger,J.A. and Kopan,R. (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature, 393, 382–386. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J. (2001) Alzheimer’s disease: genes, proteins and therapy. Physiol. Rev., 81, 741–766. [DOI] [PubMed] [Google Scholar]
- Shearman M.S. et al. (2000) L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid β-protein precursor γ-secretase activity. Biochemistry, 39, 8698–8704. [DOI] [PubMed] [Google Scholar]
- Sisodia S.S. and St George-Hyslop,P.H. (2002) γ-secretase, Notch, Aβ and Alzheimer’s disease: where do the presenilins fit in? Nat. Rev. Neurosci., 3, 281–290. [DOI] [PubMed] [Google Scholar]
- Song W., Nadeau,P., Yuan,M., Yang,X., Shen,J. and Yankner,B.A. (1999) Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc. Natl Acad. Sci. USA, 96, 6959–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H. and Haass,C. (2000) Intramembrane proteolysis by presenilins. Nat. Rev. Mol. Cell. Biol., 1, 217–224. [DOI] [PubMed] [Google Scholar]
- Steiner H. and Haass,C. (2001) Nuclear signaling: a common function of presenilin substrates? J. Mol. Neurosci., 17, 193–198. [DOI] [PubMed] [Google Scholar]
- Steiner H. et al. (1999a) A loss of function mutation of presenilin-2 interferes with amyloid β-peptide production and Notch signaling. J. Biol. Chem., 274, 28669–28673. [DOI] [PubMed] [Google Scholar]
- Steiner H., Romig,H., Pesold,B., Baader,M., Citron,M., Loetscher,H., Jacobsen,H. and Haass,C. (1999b) Amyloidogenic function of Alzheimer’s disease associated presenilin-1 in the absence of endoproteolysis. Biochemistry, 38, 14600–14605. [DOI] [PubMed] [Google Scholar]
- Steiner H. et al. (2000) Glycine 384 is required for presenilin-1 function and is conserved in bacterial polytopic aspartyl proteases. Nat. Cell Biol., 2, 848–851. [DOI] [PubMed] [Google Scholar]
- Struhl G. and Adachi,A. (1998) Nuclear access and action of Notch in vivo. Cell, 93, 649–660. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y. et al. (2002) Notch receptor cleavage depends on but is not directly executed by presenilins. Proc. Natl Acad. Sci. USA, 99, 4014–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Sweeney,D., Gandy,S.E. and Sisodia,S.S. (1996) The profile of soluble amyloid β protein in cultured cell media. Detection and quantification of amyloid β protein and variants by immunoprecipitation–mass spectrometry. J. Biol. Chem., 271, 31894–31902. [DOI] [PubMed] [Google Scholar]
- Weidemann A., Eggert,S., Reinhard,F.B., Vogel,M., Paliga,K., Baier,G., Masters,C.L., Beyreuther,K. and Evin,G.A. (2002) Novel ε-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry, 41, 2825–2835. [DOI] [PubMed] [Google Scholar]
- Wolfe M.S., Xia,W., Ostaszewski,B.L., Diehl,T.S., Kimberly,W.T. and Selkoe,D.J. (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature, 398, 513–517. [DOI] [PubMed] [Google Scholar]
- Yu C., Kim,S.H., Ikeuchi,T., Xu,H., Gasparini,L., Wang,R. and Sisodia,S.S. (2001) Characterization of a presenilin-mediated amyloid precursor protein carboxyl-terminal fragment γ. Evidence for distinct mechanisms involved in γ-secretase processing of the APP and Notch1 transmembrane domains. J. Biol. Chem., 276, 43756–43760. [DOI] [PubMed] [Google Scholar]
- Yu G. et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature, 407, 48–54. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ye,W., Wang,R., Wolfe,M.S., Greenberg,B.D. and Selkoe,D.J. (2002) Proteolysis of chimeric β-amyloid precursor proteins containing the Notch transmembrane domain yields amyloid β-like peptides. J. Biol. Chem., 277, 15069–15075. [DOI] [PubMed] [Google Scholar]