Abstract
The nf-kb2 gene encodes the cytoplasmic NF-κB inhibitory protein p100 from which the active p52 NF-κB subunit is derived by proteasome-mediated proteolysis. Ligands which stimulate p100 processing to p52 have not been defined. Here, ligation of CD40 on transfected 293 cells is shown to trigger p52 production by stimulating p100 ubiquitylation and subsequent proteasome-mediated proteolysis. CD40-mediated p52 accumulation is dependent on de novo protein synthesis and triggers p52 translocation into the nucleus to generate active NF-κB dimers. Endogenous CD40 ligation on primary murine splenic B cells also stimulates p100 processing, which results in the delayed nuclear translocation of p52–RelB dimers. In both 293 cells and primary splenic B cells, the ability of CD40 to trigger p100 processing requires functional NF-κB-inducing kinase (NIK). In contrast, NIK activity is not required for CD40 to stimulate the degradation of IκBα in either cell type. The regulation of p100 processing by CD40 is likely to be important for the transcriptional regulation of CD40 target genes in adaptive immune responses.
Keywords: Aly/CD40/NF-κB2/NIK/p100
Introduction
CD40 is a member of the tumour necrosis factor receptor (TNFR) family which plays a central role in adaptive immune responses (Calderhead et al., 2000). CD40 is expressed on B cells and certain accessory cells. The ligand for CD40 is CD154, which is expressed on activated CD4+ T cells and triggers clonal expansion and differentiation of B lymphocytes. CD40–CD154 interactions are required for the development of thymus-dependent humoral immunity (Calderhead et al., 2000).
CD40 ligation activates NF-κB transcription factors (Berberich et al., 1994) which are important in the regulation of immune and inflammatory responses (Karin and Ben-Neriah, 2000). NF-κB is composed of dimeric complexes of members of the Rel/NF-κB family of polypeptides (Baeuerle and Henkel, 1994). In mammals, this family comprises Rel-A, c-Rel, Rel-B, NF-κB1/p50 and NF-κB2/p52. NF-κB dimers in unstimulated cells interact with one of a family of cytoplasmic inhibitory proteins (IκBs) which prevent nuclear entry (Karin and Ben-Neriah, 2000). This family includes IκBα, IκBβ and IκBε together with the precursor forms of NF-κB1 (p105) and NF-κB2 (p100). p105 and p100 are proteolytically processed by the proteasome to produce p50 and p52, respectively. Following agonist stimulation, IκBα, IκBβ and IκBε and p105 are phosphorylated by the IκB kinase (IKK) complex, triggering their ubiquitylation and degradation by the proteasome (Karin and Ben-Neriah, 2000). Associated NF-κB dimers are then released to translocate into the nucleus and modulate gene expression. Proteolysis of NF-κB2 p100 is regulated by the IKK1 (IKKα) subunit of the IKK complex, which triggers proteasome-mediated processing to generate p52 which can then undergo nuclear translocation (Senftleben et al., 2001). In contrast, processing of p105 to p50 occurs constitutively and is not significantly affected by IKK-mediated phosphorylation which promotes p105 degradation (Karin and Ben-Neriah, 2000).
CD40 activates the IKK complex, inducing rapid IκBα degradation and subsequent nuclear translocation of associated NF-κB dimers, containing predominantly p50, Rel-A and c-Rel (Berberich et al., 1994; Kosaka et al., 1999). Activation of the IKK complex involves phosphorylation of two serine residues in the activation loops of the two component kinases, IKK1 (IKKα) and IKK2 (IKKβ) (Ling et al., 1998; Delhase et al., 1999). Analysis of the naturally occurring alymphoplasia (aly/aly) mutant mouse strain, which contains a mutation in NF-κB-inducing kinase (NIK) (Shinkura et al., 1999), has suggested that this mitogen-activated protein (MAP) 3-kinase is required for CD40-induced IκBα phosphorylation in splenic B cells (Garceau et al., 2000). NIK phosphorylates and activates the IKK complex (Lin et al., 1998; Ling et al., 1998) and may function as an IKK kinase in CD40 signalling.
aly/aly and nik–/– mice are characterized by the systemic absence of lymph nodes and Peyer’s patches, disorganized splenic and thymic architectures and immunodeficiency (Koike et al., 1996; Yin et al., 2001). Similar defects are observed in nfkb2–/– mice (Caamano et al., 1998; Franzoso et al., 1998), and recent data have indicated that NIK regulates NF-κB2 p100 processing (Xiao et al., 2001b). Introduction of the aly mutation into NIK blocks its ability to promote p100 processing in transfected cells (Xiao et al., 2001b), suggesting that the phenotype of aly/aly mice results from defective NIK-induced p100 processing. Aberrant development of peripheral lymphoid organs has also been observed in lymphotoxin (LT) β receptor (LTβR)–/– mice (Futterer et al., 1998). Similarities in phenotypes of LTβR, NIK and NF-κB2 p100/p52 knockout mice suggest that these proteins act in a common signalling pathway in LTβR-positive stromal cells.
Two studies have described cell-autonomous defects in aly/aly B-cell function (Karrer et al., 2000; Yamada et al., 2000). Since B cells do not express LTβR (Ware et al., 1995), this implies that NIK activity is essential for signalling via a distinct receptor on these cells. In an effort to identify such receptors on B cells, the potential role of CD40 in regulating p100 processing was investigated. These experiments demonstrate that CD40 stimulates the production of p52 via proteasome-mediated proteolysis of p100, triggering nuclear translocation of p52–RelB dimers.
Results
CD40 induces NF-κB2 p52 production in transfected 293 cells
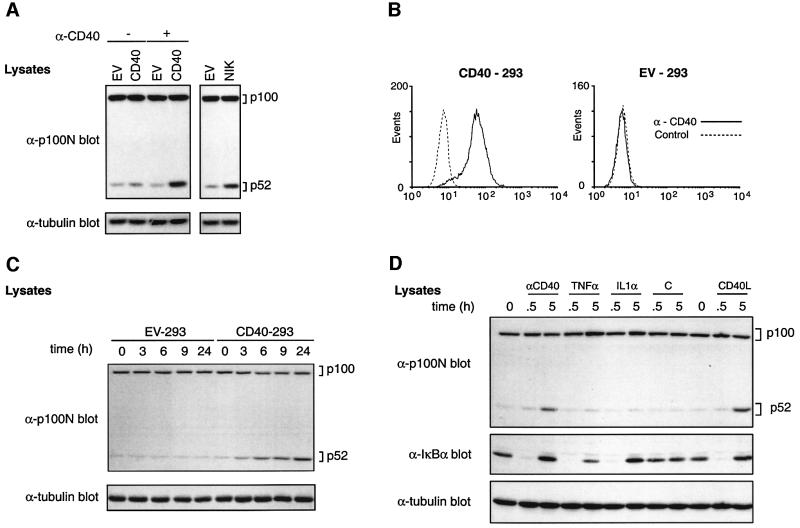
To investigate whether CD40 regulates the processing of p100 to p52, 293 cells were transiently transfected with plasmids encoding CD40 or with no insert (empty vector; EV) and western blots probed for endogenous p100/p52. In EV-transfected cells, very little p52 was detected although p100 was clearly evident (Figure 1A). As expected, NIK expression induced a dramatic increase in p52 levels (Xiao et al., 2001b). Transfection of CD40 also modestly increased p52 levels, which were increased further by cross-linking CD40 with anti-CD40 monoclonal antibody (mAb). p100 levels were not affected by CD40 expression. Cross-linked CD40 is therefore able to induce p52 accumulation, although it was unclear whether this was due to increased p100 processing.
Fig. 1. CD40 induces p52 production in transfected 293 cells. (A) 293 cells were transfected with the indicated vectors. After 24 h, anti-CD40 (+) or control (–) mAbs were added and cells incubated for a further 14 h. Cell lysates were western blotted. (B) 293 cells were transfected with a plasmid encoding CD40 or with empty vector (EV), and clones were isolated after G418 selection. CD40 expression of a representative CD40-transfected clone (CD40-293, clone B4) or EV-transfected 293 cells (EV-293) was determined by flow cytometry. (C) CD40-293 or EV-293 cells were stimulated for the indicated times with anti-CD40 mAb (10 µg/ml) and lysates western blotted. Similar kinetics of p52 induction were detected over a range of anti-CD40 mAb concentrations (see Supplementary figure 1 available at The EMBO Journal Online). (D) CD40-293 cells were stimulated with anti-CD40 mAb, TNF-α, IL-1α or CD40L for the indicated times and lysates western blotted.
293 cells were stably transfected with a plasmid encoding murine CD40 to facilitate subsequent biochemical analyses. A representative clone (B4), which expressed high levels of transfected CD40 (Figure 1B), was selected for the following experiments. Similar results were obtained with an independently isolated CD40-293 clone (data not shown). Stably expressed CD40 had little effect on the levels of endogenous p100 or p52 compared with control cells transfected with EV (Figure 1C). However, cross-linking of CD40 with anti-CD40 mAb induced a clear increase in p52 expression which was evident after 3 h stimulation and continued to increase up to 24 h (Figure 1C). Up to 9 h, the level of p100 decreased in parallel with increased p52, suggesting that p52 was indeed being processed from p100. However, after more prolonged stimulation, p100 levels rose again, presumably due to increased NF-κB-dependent p100 gene transcription (Sun et al., 1994). Stimulation with recombinant CD40 ligand (CD40L) also clearly induced p52 production (Figure 1D), whereas stimulation with TNF-α or interleukin-1 (IL-1) did not affect p52 levels, although p100 levels were up-regulated and IκBα was degraded as expected (Sun et al., 1994). Thus, the ability of CD40 to trigger p52 production is not common to all receptors which activate NF-κB.
CD40 ligation induces p100 processing to p52
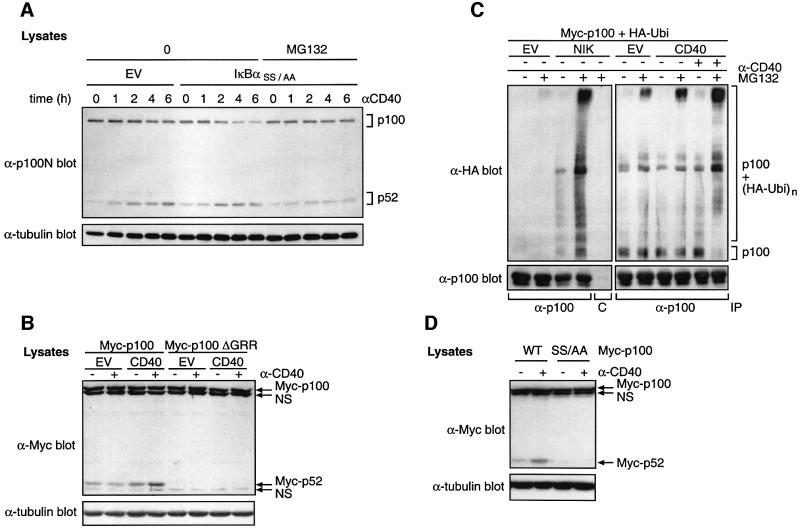
To determine whether NF-κB-dependent gene expression is required for CD40-induced p52 production, CD40-293 cells were transfected with a super-repressor mutant of IκBα (IκBαSS/AA), which blocks NF-κB activation (Roff et al., 1996), or with EV. Expression of IκBαSS/AA did not block the ability of CD40 ligation to induce p52 (Figure 2A). However, the CD40-induced decrease in p100 was much more apparent in IκBαSS/AA-transfected cells and matched the corresponding increase in p52. Pre-treatment of IκBαSS/AA-transfected cells with MG132 proteasome inhibitor prevented both the decrease in p100 and the increase in p52 triggered by CD40 ligation. Thus CD40-induced p52 production requires proteasome activity but is independent of NF-κB activity. Further more, correlation between the reduction in p100 levels with increased p52 suggests that CD40 induces p52 production via proteasome-mediated proteolysis of p100.
Fig. 2. CD40 induces ubiquitylation and proteasome-mediated processing of p100 to generate p52. (A) CD40-293 cells were transfected with a plasmid encoding IκBαSS/AA or with no insert as a control. Cells were cultured for 24 h, pre-cultured for 30 min with MG132 or control vehicle and then stimulated for the indicated times with anti-CD40 or left unstimulated. Western blots of cell lysates were probed as shown. IκBαSS/AA was expressed at similar levels in all transfections as determined by western blot analysis (data not shown). (B) 293 cells were co-transfected with plasmids encoding CD40 or EV and Myc-p100 or Myc-p100ΔGRR. Cells were stimulated with anti-CD40 mAb for the last 15 h of a 48 h culture and lysates western blotted. The positions of non-specific bands (NS) are indicated. (C) 293 cells were co-transfected with plasmids encoding Myc-p100 and HA-ubiquitin (HA-Ubi) together with plasmids encoding CD40, NIK or with no insert (EV). After 48 h culture, cells were incubated for 15 min with 50 µM MG132 and then stimulated for 1 h with anti-CD40 or control mAb. p100 was immunoprecipitated from cell lysates with anti-hp100 antibody and then resolved on a 6% SDS–polyacrylamide gel and western blotted. Pre-immune serum (C) was used a as a control for immunoprecipitations. (D) 293 cells were co-transfected with vectors encoding CD40 and Myc-p100 or Myc-p100(S866A,S870A) (SS/AA). Cells were incubated with anti-CD40 or control mAb for the last 15 h of a 48 h culture period and lysates western blotted. Similar levels of CD40 expression in each transfection were confirmed by immunoblotting with anti-CD40 antibody (data not shown).
A glycine-rich region (GRR; residues 346–377) in p100 is required for proteasome-mediated processing to p52 (Heusch et al., 1999). To determine the role of the GRR in CD40-triggered p52 production, 293 cells were co-transfected with plasmids encoding CD40 or EV and either wild-type (Myc-p100) or GRR-deleted (Myc-p100ΔGRR) p100. CD40 co-transfection increased Myc-p52 levels compared with EV, and a further increase was evident after CD40 cross-linking (Figure 2B). However, no Myc-p52 was produced from Myc-p100ΔGRR with or without CD40 expression. Thus the GRR is required for CD40-induced p52 production, consistent with p100 processing.
To investigate whether CD40 induces p100 ubiquitylation, 293 cells were co-transfected with plasmids encoding Myc-p100 and hemagglutinin (HA)-ubiquitin together with CD40 or EV plasmids. Polyubiquitylation of Myc-p100 was demonstrated by the appearance of high molecular weight bands in western blots of immunoprecipitated p100 probed for HA-ubiquitin. In the presence of MG132, a basal level of p100 polyubiquitylation was observed in EV-transfected cells (Figure 2C). However, a significant increase in p100 polyubiquitylation was evident when MG132-treated cells were co-transfected with CD40, which was enhanced further by anti-CD40 mAb. Transfected NIK also induced p100 ubiquitylation, as expected (Xiao et al., 2001b). CD40-induced p52 production therefore correlates with increased p100 ubiquitylation.
Two serines (S866 and S870) in the C-terminus of p100 are required for NIK-induced processing to p52 (Xiao et al., 2001b). To investigate whether these residues are important for CD40-induced p100 processing, a vector encoding Myc-p100 containing serine to alanine mutations at these sites, Myc-p100(S866A,S870A), was co-transfected with a plasmid encoding CD40 into 293 cells. CD40 ligation increased Myc-p52 levels in Myc-p100-transfected but not Myc-p100(S866A,S870A)-transfected cells (Figure 2D). Thus, serines 866 and 870 in the p100 PEST region are required for CD40-induced p52 production.
CD40 induction of p52 requires de novo protein synthesis
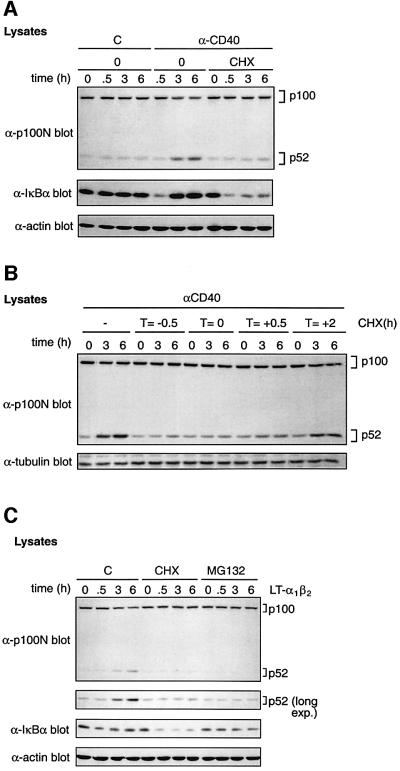
Since the induction of p52 by CD40 ligation occurred over several hours (Figure 1C), a possible requirement for protein synthesis was investigated. Surprisingly, cycloheximide (CHX) pre-treatment dramatically reduced CD40-induced p100 proteolysis and p52 production (Figure 3A; upper panel). However, CHX had no effect on the ability of CD40 ligation to trigger IκBα degradation, although IκBα resynthesis was blocked as expected (Figure 3A; middle panel). CHX was also added at different times relative to anti-CD40 mAb. CHX treatment inhibited p52 production when added at 0.5 h prior to, simultaneously with or 0.5 h after anti-CD40 mAb stimulation. However, when added 2 h after anti-CD40 mAb, p52 was induced similarly to control cells (Figure 3B). Thus new protein synthesis is required during the first 2 h after CD40 ligation to induce p100 processing efficiently.
Fig. 3. CD40 induction of p52 is dependent on de novo protein synthesis. (A) CD40-293 cells were pre-treated with cycloheximide (CHX) or vehicle control (0) for 30 min. Cells were then stimulated with anti-CD40 or control (C) mAb for the indicated times and lysates western blotted. (B) CHX was added to cultures of CD40-293 cells at the indicated times relative to anti-CD40 mAb. Cell lysates were western blotted. (C) 293 cells were pre-treated for 30 min with MG132, CHX or control medium (C). Cells were then stimulated for the times shown with LTα1β2, and lysates western blotted.
Consistent with published data with transfected LTβR (Xiao et al., 2001b), stimulation of endogenous LTβRs on 293 cells with recombinant LTα1β2, a ligand for LT-βR, induced a slow increase in endogenous p52 levels (Figure 3C; Supplementary figures 1 and 2). A small decrease in p100 levels was also evident compared with control cells, suggesting that elevated p52 levels were due to increased p100 processing. Both of these changes were blocked by pre-treatment of cells with MG132, demonstrating that proteolysis was mediated by the proteasome. Similarly to CD40, LTα1β2-induced p52 production was blocked substantially by CHX pre-treatment but was not dependent on NF-κB activation. Thus both CD40 and LT-βR require de novo protein synthesis to induce efficient p52 production via the proteasome.
CD40 ligation induces nuclear translocation of active p52 in CD40-293 cells
To determine whether CD40-induced p52 translocates into the nucleus, cytoplasmic and nuclear fractions were prepared from CD40-293 cells and western blotted for endogenous p100 and p52. Immunoblotting for tubulin (cytoplasmic marker) and SP1 (nuclear marker) confirmed cell fractionation (Figure 4A, lower panels). In unstimulated cells, very little p52 was detected in the nuclear fraction. However, after anti-CD40 mAb stimulation, there was a dramatic increase in nuclear p52 which was detectable after 3 h and continued to increase up to 24 h (Figure 4A, upper panel). A portion of CD40-induced p52 remained in the cytoplasm, presumably retained by complexing with IκBs. LTα1β2 stimulation also promoted nuclear p52 translocation. CD40-induced nuclear translocation of p52 occurred with noticeably slower kinetics than those of RelA (Figure 4A, lower panel) and was blocked by pre-treatment of cells with MG132 or CHX (data not shown).
Fig. 4. CD40 ligation induces nuclear translocation of p52 dimers. (A) Nuclear and cytoplasmic fractions were prepared from CD40-293 cells stimulated as indicated and western blotted. (B) Nuclear fractions were prepared from CD40-293 cells stimulated as shown. NF-κB DNA-binding activity was analysed by EMSA. (C) EMSAs were carried out on nuclear extracts from CD40-293 cells stimulated for 0.5 or 6 h with anti-CD40 mAb. Extracts were supershifted with the indicated antibodies to different Rel proteins or pre-immune serum (PI). The position of a p52-containing supershifted NF-κB complex is shown (asterisk). Specificity of κB binding was determined by competition with 100-fold unlabelled κB oligonucleotide (Oligo, NF-κB) or control Oct-1 oligonucleotide (Oligo, Oct-1). (D) Nuclear extracts from CD40-293 cells were supershifted with anti-p100N antibody and analysed by EMSA. The position of supershifted complexes is shown (arrow).
Electrophoretic mobility shift assays (EMSAs) confirmed that CD40 rapidly stimulated NF-κB activity in the CD40-293 cells (Figure 4B). Supershift analyses revealed that the majority of detected binding activity comprised dimers of p50 and RelA (Figure 4C). However, a minor p52-containing complex was clearly detected by supershift analysis with anti-p100 antibody in nuclear extracts from cells stimulated for 6 h with anti-CD40 mAb (Figure 4C). The kinetics of appearance of this band paralleled the appearance of p52 in the nuclear fraction (Figure 4D, compare with A). Furthermore, p52-containing NF-κB complex formation triggered by anti-CD40 stimulation was blocked by MG132 pre-treatment of the cells (data not shown). Control mAb failed to induce any p52 DNA-binding activity (Figure 4D). Thus CD40-induced p52 translocates into the nucleus and can bind to specific κB sites in target DNA.
CD40 triggers nuclear translocation of p52 in splenic B cells
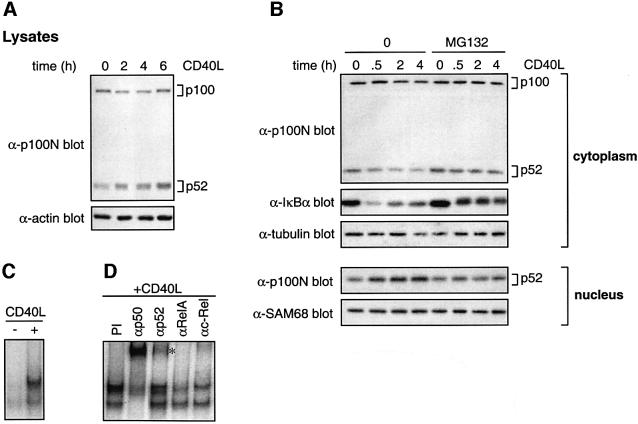
To confirm that results obtained with transfected CD40 reflected physiological CD40 activity, the ability of endogenous CD40 to stimulate p100 processing was investigated. Splenic B cells from C57BL/6 mice were stimulated with CD40L, and total p100/p52 determined by western blotting. In contrast to 293 cells, unstimulated splenic B cells expressed relatively high levels of p52 (Figure 5A). However, after 2 h CD40 ligation, the level of p52 increased in parallel with a small decrease in p100. More prolonged stimulation induced a further increase in p52, and p100 returned to control levels by 6 h. Thus CD40 stimulation of splenic B cells induces proteolysis of p100 and increased p52.
Fig. 5. CD40 ligation induces p100 proteolysis and nuclear translocation of p52 in splenic B cells. (A and B) Splenic B cells were stimulated in vitro with CD40L for the times shown or left unstimulated. Cell lysates (A) or cytoplasmic and nuclear fractions (B) were western blotted. (C) NF-κB EMSAs were carried out on nuclear fractions from primary splenic B cells stimulated with CD40L for 5 h or left unstimulated. (D) Nuclear extracts prepared from splenic B cells stimulated for 5 h with CD40L were supershifted with the indicated anti-Rel antibodies or pre-immune serum (PI). The position of the p52-containing supershifted NF-κB complex is shown (asterisk).
Cytoplasmic and nuclear fractions were also prepared from splenic B cells. After 2–4 h of CD40 ligation, decreased levels of cytoplasmic p100 were observed (Figure 5B). Prior to this and coincident with IκBα degradation, cytoplasmic p52 also decreased and then remained constant. Elevated levels of nuclear p52 were detected after 0.5 h CD40L stimulation and increased further after more prolonged stimulation. Decreases in cytoplasmic p100/p52 and the increase in nuclear p52 triggered by CD40 ligation were blocked by pre-treatment of cells with MG132. The role of protein synthesis could not be determined due to CHX toxicity.
Analysis of nuclear fractions from B cells by EMSA revealed two NF-κB-binding complexes that were induced by 5 h CD40L stimulation (Figure 5C). Supershift analyses indicated that the upper of these complexes contained predominantly p50, RelA and cRel, whereas the lower complexes contained p50 (Figure 5D). CD40-induced NF-κB activity also contained p52, as revealed by supershifting with anti-p100N antiserum. p52-containing NF-κB complexes were also detected after 0.5 h CD40L stimulation (data not shown).
These data suggest that endogenous CD40 on splenic B cells stimulates nuclear translocation of p52 both from existing cytoplasmic stores and as a consequence of p100 processing.
CD40-stimulated IκBα degradation promotes nuclear translocation of pre-existing p52
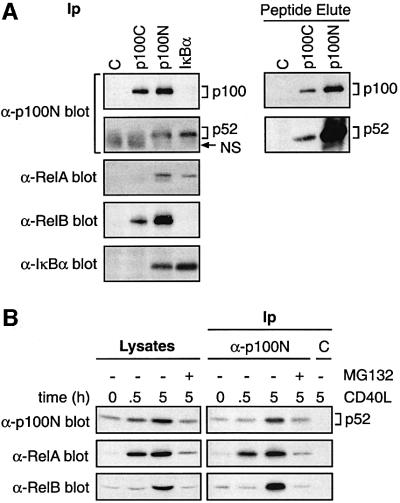
The decrease in cytoplasmic p52 after CD40 ligation occurs in parallel with IκBα degradation (Figure 5B), suggesting that cytoplasmic p52 might be released from proteolysed IκBα. Consistent with this possibility, p52, but not p100, was isolated specifically in anti-IκBα immunoprecipitates of splenic B-cell lysates, in addition to RelA. Conversely, IκBα co-immunoprecipitated with anti-p100N (p100/p52) antibody but not anti-p100C (p100) antibody (Figure 6A). Very low levels of p52 also co-purified in anti-p100C antibody immunoprecipitates (Figure 6A, right hand panel) but not IκBβ (data not shown). Thus splenic B cells contain at least two separate pools of cytoplasmic p52 complexed to distinct IκB molecules.
Fig. 6. CD40 induces nuclear accumulation of p52–RelA and p52– RelB dimers in splenic B cells. (A) Lysates of unstimulated splenic B cells were immunoprecipitated with the indicated antibodies and western blotted. In the right hand panels, specific peptide was used to elute bound antigen to decrease background Ig. (B) Nuclear extracts were prepared from splenic B cells stimulated with CD40L for 0.5 or 5 h or left unstimulated. MG132 was added 15 min prior to stimulation. p52 was then immunoprecipitated and associated Rel subunits identified by western blotting. C indicates control IgG immunoprecipitations.
Immunoprecipitation of nuclear extracts from splenic B cells with anti-p100N antibody demonstrated that 30 min CD40L stimulation triggered a large increase in nuclear p52–RelA dimers (Figure 6B) coincident with IκBα degradation (Figure 5B). CD40L stimulation for 30 min did not alter levels of p100 (Figure 5B) or p105 (data not shown). These data suggest that CD40 stimulation of splenic B cells induces the rapid nuclear translocation of pre-existing p52 complexed with RelA, which is released predominantly from degraded IκBα.
CD40 ligation on splenic B cells promotes nuclear translocation of p52–RelB dimers
In HeLa cells, p100 specifically retains RelB in the cytoplasm, and processing of p100 promotes nuclear translocation of p52–RelB dimers (Solan et al., 2001). RelB specifically co-purified with p100 in anti-p100C immunoprecipitates from unstimulated splenic B-cell lysates. Furthermore, immunodepletion of p100/p52 substantially removed RelB from B-cell lysates (data not shown). In contrast, RelB was not associated with IκBα (Figure 6A) or IκBβ (data not shown). These data indicate that RelB is retained in the cytoplasm of unstimulated B cells by p100.
Since RelB nuclear translocation is regulated by p100 (Solan et al., 2001), CD40 ligation was predicted to induce nuclear RelB in splenic B cells (Neumann et al., 1996) as a consequence of p100 processing. Western blotting of nuclear extracts confirmed that CD40L stimulation for 5 h increased nuclear RelB (Figure 6B). Moreover, at this time point, there was a large increase in the level of RelB detected in anti-p52 immunoprecipitates of nuclear extracts, which was blocked by MG132 pre-treatment (Figure 6B). No change in nuclear RelB was detected after 30 min CD40L stimulation in either lysates or immunoprecipitates. Thus CD40 stimulates proteasome-mediated proteolysis of p100 to generate p52–RelB dimers which translocate into the nucleus.
CD40 induction of p100 processing requires NIK activity
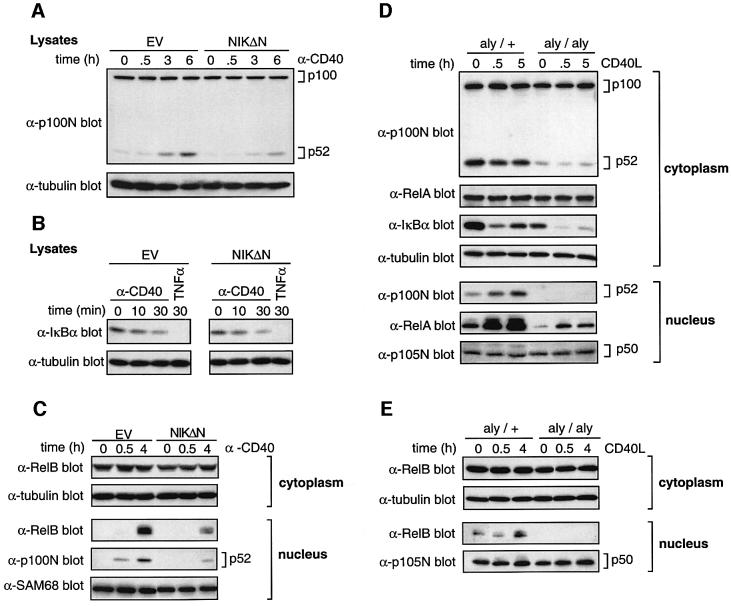
NIK regulates p100 processing and is essential for p52 production in vivo in splenocytes (Xiao et al., 2001b). To investigate whether CD40-induced processing of p100 requires NIK, CD40-293 cells were transfected with the C-terminal portion of NIK (NIKΔN; residues 624–947), which functions as a potent inhibitor of wild-type NIK (Lin et al., 1998), or EV. Expression of NIKΔN resulted in a clear inhibition of CD40-induced p52 production compared with EV (Figure 7A). However, both CD40- and TNF-α-induced IκBα degradation was unaffected by NIKΔN expression (Figure 7B). NIKΔN expression also substantially blocked CD40-induced nuclear translocation of both p52 and RelB (Figure 7C). Thus NIK activity is required for CD40 induction of p100 processing to p52 and subsequent nuclear translocation of p52 and RelB in CD40-293 cells.
Fig. 7. CD40 stimulation of p100 processing requires NIK activity. (A–C) CD40-293 cells were transfected with plasmids encoding Myc-NIKΔN (NIKΔN) or with no insert as a control (EV). Cells were stimulated as indicated. Lysates (A and B) or cytoplasmic and nuclear fractions (C) were western blotted. Equivalent expression of Myc-NIKΔN was confirmed by western blotting (data not shown). (D and E) Splenic B cells were isolated from aly/aly and aly/+ mice. Cells were stimulated with CD40L for 0.5 or 4 or 5 h, or left unstimulated (0). Cytoplasmic and nuclear fractions were western blotted.
To investigate the role of NIK in stimulation of p100 processing by endogenous CD40, splenic B cells were purified from aly/aly mice which contain a point mutation in NIK that abrogates its ability to trigger p100 processing (Shinkura et al., 1999; Xiao et al., 2001b). Consistent with published data (Xiao et al., 2001b), resting levels of p52 were severely reduced in aly/aly B cells compared with aly/+ B cells (Figure 7D), although p100 levels were similar. Stimulation of aly/aly B cells did not alter p52 levels in either cytoplasm or nucleus. In contrast, a clear increase in nuclear p52 was evident after CD40L stimulation of aly/+ B cells, as expected. CD40-induced nuclear translocation of RelB was also completely blocked in the aly/aly B cells, although cytoplasmic levels were similar to those of aly/+ B cells (Figure 7E). CD40 ligation triggered IκBα degradation and RelA nuclear translocation in B cells from mice of both genotypes (Figure 7D). However, absolute levels of IκBα expression were significantly reduced in aly/aly compared with aly/+ B cells, similar to IKK1-deficient B cells which are also defective in p100 processing (Senftleben et al., 2001). RelA was also expressed at slightly lower levels in the cytoplasm of aly/aly compared with aly/+ B cells, as reported previously (Yamada et al., 2000), contributing to a decreased CD40-induced nuclear translocation of RelA in aly/aly B cells. However, nuclear p50 levels were very similar in aly/aly and aly/+ B cells (Figure 7D and E), indicating that there was no generalized defect in nuclear translocation of Rel subunits in aly/aly B cells.
CD40 triggering of p100 processing and nuclear translocation of p52 and RelB therefore require functional NIK. In contrast, NIK activity is dispensable for CD40 stimulation of IκBα degradation.
Efficient CD40-induced p100 processing requires the TRAF2/3-binding site on CD40
A crucial initiating event in signalling from CD40 is the recruitment of TNFR-associated factors (TRAFs) to the CD40 cytoplasmic tail (Grammer and Lipsky, 2001). Five of the six known TRAFs (TRAF1, 2, 3, 5 and 6) associate with CD40, and genetic studies have indicated that TRAFs 2, 5 and 6 are required for efficient NF-κB activation by CD40 in B cells (Lomaga et al., 1999; Nakano et al., 1999; Nguyen et al., 1999).
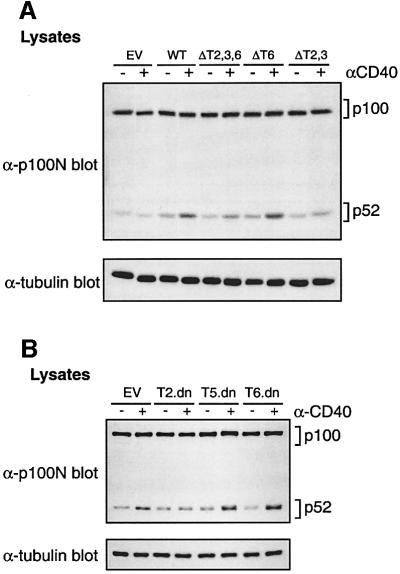
To investigate the role of TRAFs in CD40 induction of p100 processing, 293 cells were transiently transfected with vectors encoding chimeric CD40 proteins in which the TRAF-binding sites were ablated by point mutation (Ahonen et al., 2002). The ΔT2,3 chimera cannot bind TRAF2/3, which also indirectly blocks binding of TRAF1/5 which associate with TRAF2, whereas TRAF6 binding is ablated in the ΔT6 chimera. The ΔT2,3,6 mutant cannot bind any of the known TRAFs. Expression of wild-type CD40 chimera induced endogenous p52 levels, which were increased further by anti-CD40 mAb stimulation (Figure 8A). Similar results were obtained with the ΔT6 chimera. However, the level of p52 induced by the ΔT2,3 and ΔT2,3,6 chimeras with or without CD40 ligation was reduced consistently, although not completely ablated. These data suggest that the TRAF2/3-binding site, but not the TRAF6-binding site, is required for efficient CD40-induced p100 processing.
Fig. 8. Efficient p52 induction by CD40 requires its TRAF2/3-binding site. (A) 293 cells were transiently transfected with vectors encoding the indicated CD40 chimeras. Cells were stimulated with anti-CD40 mAb for the last 16 h of a 48 h culture and lysates western blotted. Each of the CD40 chimeras was expressed at similar levels (data not shown). (B) 293 cells were co-transfected with vectors encoding CD40 and dominant-negative TRAFs or EV. Cells were stimulated for the last 16 h of a 48 h culture and lysates western blotted. Similar levels of CD40 were expressed in all transfections, and each of the TRAF dominant-negative proteins was expressed at comparable levels (data not shown).
To explore this further, 293 cells were transiently co-transfected with vectors encoding wild-type CD40 and dominant-negative mutants of TRAFs 2, 5 and 6. These contain TRAF domains which mediate receptor binding but lack RING finger effector domains (Ishida et al., 1996a,b; Aizawa et al., 1997). Expression of dominant-negative TRAF5 or TRAF6 had little effect on CD40-induced p52 induction (Figure 8B). However, CD40 induction of p52 was reduced by expression of dominant-negative TRAF2, confirming the importance of the TRAF2/3-binding site in CD40 induction of p52.
Discussion
NIK and IKK1 are required for p52 generation in splenocytes and splenic B cells, respectively, suggesting that p100 proteolysis is regulated by receptors expressed on these cells (Yamada et al., 2000; Senftleben et al., 2001; Xiao et al., 2001b). In this study, CD40 is identified as one receptor on splenic B cells that regulates the physiological production of p52 via proteasome-mediated proteolysis of p100, resulting in delayed nuclear translocation of p52–RelB dimers. CD40 also induces rapid nuclear translocation of p52–RelA dimers from pre-existing cytoplasmic stores which are released predominantly from degraded IκBα. However, p52 levels in splenic B cells from CD40-deficient mice are very similar to those of wild-type B cells (data not shown). As p100 processing is blocked in B cells from both aly/aly mice (Figure 7D) and IKK1-deficient mice (Senftleben et al., 2001), another receptor presumably maintains p52 levels in these cells. A likely candidate for this function is BAFF-R, which has already been suggested to stimulate p100 processing in B cells (Karin and Lin, 2002).
p52-containing NF-κB dimers represent only a small fraction of total NF-κB activity (see Figures 4D and 5D). However, analyses of NF-κB2 knockout mice indicate that p52 performs functions that cannot be compensated by other Rel subunits (Caamano et al., 1998; Franzoso et al., 1998). Adoptive transfer experiments with aly/aly or NF-κB2–/– bone marrow have indicated cell-autonomous contributions for NIK and NF-κB2 in antibody responses to T-dependent antigens, in addition to their major functions in stromal cells (Franzoso et al., 1998; Yamada et al., 2000). Since CD40 is essential for T-dependent antibody responses (Calderhead et al., 2000), abrogation of CD40 regulation of p52-containing complexes in B cells may contribute to these phenotypes. Differences in fine κB-binding site specificity (Baeuerle and Henkel, 1994) and kinetics of induction by CD40 (Figure 6) suggest that p52–RelA and p52–RelB dimers may control the transcription of distinct CD40 target genes in B-cell responses.
It has been proposed that CD40 regulation of IκBα degradation is dependent on functional NIK expression based on reduced IκBα phosphorylation detected after CD40L stimulation of aly/aly B cells compared with aly/+ B cells (Garceau et al., 2000). However, this probably reflected the markedly reduced levels of IκBα expression in the mutant B cells, which were not assessed in this study (Garceau et al., 2000), rather than a defect in the regulation of IκBα phosphorylation. The present study demonstrates that NIK activity is not actually required for CD40 regulation of IκBα degradation in either CD40-293 cells or primary splenic B cells (Figure 7). Consistent with these data, CD40 activation of total NF-κB is only slightly reduced in B cells isolated from NIK-deficient mice (Yin et al., 2001). Thus NIK is either not involved in CD40 regulation of IκBα proteolysis or is redundant with another related MAP 3-kinase for this function. In contrast, NIK activity is essential for CD40 to trigger p100 processing to p52 and is also required for LTβR-mediated activation of IκBα phosphorylation in fibroblasts (Matsushima et al., 2001).
Deregulated p52 production is associated with abnormal lymphocyte proliferation and oncogenic transformation. Mice lacking the C-terminal (IκB-like) half of p100, while still expressing p52, develop gastric and lymphoid hyperplasia (Ishikawa et al., 1997). In many human lymphomas, chromosomal translocations and deletions affect the nfkb2 locus, generating p100 mutants which are constitutively processed to p52 (Xiao et al., 2001a). Thus p52 may play an important role in promoting lymphocyte proliferation. CD40 stimulation of B-cell proliferation therefore may involve p52 up-regulation. Consistent with this hypothesis, NF-κB2-deficient B cells have a diminished proliferative response to CD40 cross-linking compared with wild-type (Caamano et al., 1998). RelB-deficient B cells also display decreased CD40-induced proliferation, suggesting that p52–RelB dimers may be particularly important in regulating B-cell division (Snapper et al., 1996). aly/aly B cells display a more pronounced decrease in CD40-induced proliferation compared with NF-κB2–/– B cells (Garceau et al., 2000; Yamada et al., 2000). This may result both from the inability to trigger p100 processing to p52 in aly/aly B cells and from unprocessed p100 acting as an IκB and preventing nuclear translocation of other NF-κB subunits after agonist stimulation. Induction of p52 by CD40 may also be involved in rescuing B cells from apoptosis (Caamano et al., 2000; Calderhead et al., 2000).
Analysis of CD40 point mutants and expression of dominant-negative TRAFs (Figure 8) indicates that the TRAF2/3-binding site plays an important role in CD40-induced p100 processing. It is possible that TRAF2 directly links CD40 to the activation of NIK by recruitment of the kinase to the plasma membrane (Malinin et al., 1997). However, although overexpression of TRAF2 in 293 cells is known to activate IKK (Grammer and Lipsky, 2001), it does not induce processing to p52 (data not shown). TNFR1 stimulation was also found not to affect p100 processing in 293 cells (Figure 1D), consistent with some previous studies (Dejardin et al., 1999; Xiao et al., 2001a), although its cytoplasmic tail binds to TRAF2 and TRAF5 (Tada et al., 2001). This suggests that TRAF2 may act in conjunction with TRAF3, which does not bind NIK (Malinin et al., 1997), or with TRAF-independent CD40 signalling pathways (Ahonen et al., 2002) to stimulate p100 processing. In addition, since TNF-α stimulates p100 processing in HeLa cells (Naumann and Scheidereit, 1994), ligands may induce p100 processing in a cell-type specific fashion.
IKK1, a component kinase of the IKK complex (Karin and Ben-Neriah, 2000), is essential for germinal centre formation and normal splenic microarchitecture, similar to NF-κB2 and NIK (Kaisho et al., 2001). Wild-type NIK, but not NIK containing the aly point mutation, binds to IKK1 (Luftig et al., 2001; Matsushima et al., 2001). Furthermore, wild-type NIK activates the IKK complex (Lin et al., 1998; Ling et al., 1998). Thus NIK may trigger p100 phosphorylation via the IKK complex. Consistent with this hypothesis, p100 processing induced by transfected NIK is blocked in IKK1–/– embryonic fibroblasts, and recombinant IKK1 directly phosphorylates the C-terminus of p100 (Senftleben et al., 2001). Interest ingly, stimulation of p100 proteolysis by NIK is independent of NEMO (IKKγ) (Xiao et al., 2001a), a structural component of the prototypic IKK complex which regulates IκBα phosphorylation (Karin and Ben-Neriah, 2000). Thus the subunit composition of IKK1-containing complexes which phosphorylate p100 after CD40 stimulation may be distinct from the prototypic IKK complex. In addition, several agonists known to activate IKK1 and IKK2, including TNF-α and phorbol esters, do not trigger p100 processing (Figure 1D) (Sun et al., 1994; Xiao et al., 2001a). Activation of IKK1, therefore, is not sufficient to promote p100 proteolysis. It is possible that agonist triggering of p100 processing also requires binding of IKK1 to p100 similarly to Tax-induced p100 processing (Xiao et al., 2001a). Since NIK can bind independently to both IKK1 and p100 (Regnier et al., 1997; Xiao et al., 2001a), it may recruit IKK1 to p100 after CD40 stimulation.
The atypical NF-κB activation pathway (Senftleben et al., 2001), which stimulates the production of NF-κB2 p52 from its precursor p100, plays an important but ill defined role in B-cell biology (Ishikawa et al., 1997; Caamano et al., 1998; Franzoso et al., 1998). The demonstration that CD40 is a receptor that regulates p100 processing will help in the functional characterization of this signalling pathway.
Materials and methods
cDNA constructs and antibodies
Murine CD40 cDNA in the pcDSRα vector (Ed Clark, University of Washington, Seattle) was used for transient transfection experiments and was subcloned into the pMX1 vector (Ingenius) for stable transfection of 293 cells. Addition of an N-terminal Myc epitope tag to the cDNA encoding wild-type human p100, and generation of Myc-p100(S866A,S870A), Myc-p100ΔGRR (residues 346–377 deleted) Myc-NIK and Myc-NIKΔN (residues 624–947 of human NIK) were performed by PCR. All constructs were verified by DNA sequencing and subcloned into the pcDNA3 expression vector (Invitrogen). HA-tagged ubiquitin was cloned in the pCMV vector (Dirk Bohmann, EMBL, Heidelberg).
1C10 anti-CD40 mAb (10 µg/ml) was used to cross-link murine CD40 on CD40-expressing 293 cells (Johnson-Leger et al., 1998). A murine CD8α–CD154 fusion protein was used as CD40L (2 µg/ml; Marilyn Kehry, Boehringer Ingelheim Pharmaceuticals, Inc., USA). Experiments with B cells utilized CD40L rather than anti-CD40 mAb to avoid engaging Fc receptors. CD40 chimeras were cross-linked with G28.5 anti-CD40 mAb (10 µg/ml; ATCC).
Endogenous human p100/p52 was detected using a commercial anti-p100 mAb (UBI 05-361). Anti-peptide antisera were raised in rabbits to synthetic peptides corresponding to residues 1–15 of human p100 (anti-p100), 1–17 of murine p100 (anti-p100N), 861–873 of human p100 (anti-p100C) and 1–17 of murine NF-κB1 p105 (anti-p105N). Santa Cruz antibodies were used to detect Myc (c-789), IκBα (sc-21), SP1 (sc-59), RelA (sc-372), RelB (sc-226) and SAM68 (sc-333) on western blots. Anti-actin antibody was obtained from Sigma-Aldrich.
293 cells
For stable transfection with murine CD40, 7 × 105 cells were plated in a 90 mm dish (Life Technologies, Inc.) and, after 18 h in culture, transfected using LipofectAMINE (Life Technologies, Inc.). Cells were cultured for a further 48 h and then selected for neomycin resistance with 1 mg/ml G418 (Life Technologies, Inc.). After 3–4 weeks, clones were picked manually and then expanded. Expression of CD40 was determined by flow cytometry.
MG132 proteasome inhibitor (Biomol Research Labs; 20 µM) and CHX (Sigma-Aldrich; 10 µg/ml) were added 15–30 min prior to stimulation with anti-CD40 mAb or LTα1β2. Recombinant TNF-α (20 ng/ml), IL-1α (4 ng/ml) and LTα1β2 (100 ng/ml) were obtained from R & D systems.
Primary B cells
Specific pathogen-free C57/BL6 mice were bred at the National Institute for Medical Research (London, UK). Alymphoplasia (aly) mutant mice (Miyawaki et al., 1994) were kindly provided by Dr Hengartner (University of Zurich, Switzerland). These mice were bred locally under specific pathogen-free conditions by mating heterozygous (aly/+) females with homozygous (aly/aly) males. aly/+ mice were distinguished from aly/aly littermates by measuring serum IgA levels (Karrer et al., 2000). For experiments using B cells from aly/aly mice, sex-matched aly/+ littermates were used as controls. All mice were used at an age of 2–3 months.
Highly enriched populations of small dense B cells were prepared from spleens of C57BL/6 wild-type, aly/+ and aly/aly mice as described previously (Johnson-Leger et al., 1998). Isolated splenic B cells from C57BL/6, aly/+ and aly/aly mice all expressed comparable CD40 levels as assessed by flow cytometry (data not shown). Preparation of cytoplasmic and nuclear fractions from primary B cells was performed as described (Alkalay et al., 1995). To facilitate Rel detection in western blots, 5-fold more nuclear extract was loaded relative to cytoplasmic extract. Nuclear extracts were diluted in buffer A plus 0.1 mg/ml bovine serum albumin (Sigma) and 10% (v/v) glycerol prior to immunoprecipitation (Beinke et al., 2002) with anti-p52 antibody. MG132 treatment was carried out as for CD40-293 cells.
Protein analyses
293 cells (5 × 105 cells per 60 mm dish) were transiently transfected using LipofectAMINE (Life Technologies, Inc.) and cultured for a total of 48 h. CD40-293 cells (5 × 105) were plated 18 h prior to stimulation (60 mm dishes). Cells were stimulated as indicated in the figure legends. Cell lysates were prepared using buffer A (Beinke et al., 2002). Cytoplasmic and nuclear fractions were prepared as for B cells. For p100 ubiquitylation experiments, transiently transfected 293 cells were lysed in buffer A supplemented with 0.5% deoxycholate and 0.1% SDS (RIPA buffer), and p100 isolated by immunoprecipitation for 4 h. Immunoprecipitates were washed twice in RIPA buffer and three times in RIPA buffer plus 1 M urea prior to western blotting (Xiao et al., 2001b).
NF-κB EMSAs
EMSAs and antibody supershifting were performed as described (Alkalay et al., 1995). A radiolabelled double-stranded oligonucleotide (Promega) was used to detect NF-κB complexes, which corresponds to the NF-κB-binding site in the mouse immunoglobulin enhancer.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Dirk Bohmann, Ed Clark, David Gray, Ron Hay, Hans Hengartner, Jun-ichiro Inoue, Marilyn Kehry, Gary Nabel, Hiroyasu Nakano, Randolf Noelle, Matt Robinson, David Wallach and Toshiki Watanabe for reagents used in this study. Members of the Ley laboratory are also gratefully acknowledged for help during this study. This work was supported by the Medical Research Council, UK.
References
- Ahonen C.L., Manning,E.M., Erickson,L.D., O’Connor,B.P., Lind,E.F., Pullen,S.S., Kehry,M.R. and Noelle,R.J. (2002) The CD40–TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat. Immunol., 3, 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa S. et al. (1997) Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NF-κB activation. J. Biol. Chem., 272, 2042–2045. [DOI] [PubMed] [Google Scholar]
- Alkalay I., Yaron,A., Hatzubai,A., Jung,S., Avraham,A., Gerlitz,O., Pashut-Lavon,I. and Ben-Neriah,Y. (1995) In vivo stimulation of IκB phosphorylation is not sufficient to activate NF-κB. Mol. Cell. Biol., 15, 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P.A. and Henkel,T. (1994) Function and activation of NF-κB in the immune system. Annu. Rev. Immunol., 12, 141–179. [DOI] [PubMed] [Google Scholar]
- Beinke S., Belich,M.P. and Ley,S.C. (2002) The death domain of NF-κB1 p105 is essential for signal-induced p105 proteolysis. J. Biol. Chem., 277, 24162–24168. [DOI] [PubMed] [Google Scholar]
- Berberich I., Shu,G.L. and Clark,E.A. (1994) Cross-linking CD40 on B cells rapidly activates nuclear factor κB. J. Immunol., 153, 4357–4366. [PubMed] [Google Scholar]
- Caamano J.H., Rizzo,C.A., Durham,S.K., Barton,D.S., Raventos-Suarez,C., Snapper,C.M. and Bravo,R. (1998) Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J. Exp. Med., 187, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamano J., Tato,C., Cai,G., Villegas,E.N., Speirs,K., Craig,L., Alexander,J. and Hunter,C.A. (2000) Identification of a role for NF-κB2 in the regulation of apoptosis and in maintainance of T cell-mediated immunity to Toxoplasma gondii. J. Immunol., 165, 5720–5728. [DOI] [PubMed] [Google Scholar]
- Calderhead D.M., Kosaka,Y., Manning,E.M. and Noelle,R.J. (2000) CD40–CD154 interactions in B-cell signaling. Curr. Top. Microbiol. Immunol., 245, 73–99. [DOI] [PubMed] [Google Scholar]
- Dejardin E., Deregowski,V., Chapelier,M., Jacobs,N., Gielen,J., Merville,M.-P. and Bours,V. (1999) Regulation of NF-κB activity by IκB-related proteins in adenocarcinoma cells. Oncogene, 18, 2567–2577. [DOI] [PubMed] [Google Scholar]
- Delhase M., Hayakawa,M., Chen,Y. and Karin,M. (1999) Positive and negative regulation of IκB kinase activity through IKK-β subunit phosphorylation. Science, 284, 309–313. [DOI] [PubMed] [Google Scholar]
- Franzoso G. et al. (1998) Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions and splenic microarchitecture. J. Exp. Med., 187, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer A., Mink,K., Luz,A., Kosco-Vilbois,M.H. and Pfeffer,K. (1998) The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissue. Immunity, 9, 59–70. [DOI] [PubMed] [Google Scholar]
- Garceau N., Kosaka,Y., Masters,S., Hambor,J., Shinkura,T., Honjo,T. and Noelle,R.J. (2000) Lineage-restricted function of nuclear factor κB-inducing kinase (NIK) in transducing signals via CD40. J. Exp. Med., 191, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer A.C. and Lipsky,P.E. (2001) CD40-mediated regulation of immune responses by TRAF-dependent and TRAF-independent signaling mechanisms. Adv. Immunol., 76, 61–178. [DOI] [PubMed] [Google Scholar]
- Heusch M., Lin,L., Geleiunas,R. and Greene,W.C. (1999) The generation of nfkb2 p52: mechanism and efficiency. Oncogene, 18, 6201–6208. [DOI] [PubMed] [Google Scholar]
- Ishida T.K. et al. (1996a) Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J. Biol. Chem., 271, 28745–28748. [DOI] [PubMed] [Google Scholar]
- Ishida T.K., Tojo,T., Aoki,T., Kobayashi,N., Ohishi,T., Watanabe,T., Yamamoto,T. and Inoue,J. (1996b) TRAF5, a novel tumor necrosis receptor-associated factor family protein, mediates CD40 signaling. Proc. Natl Acad. Sci. USA, 93, 9437–9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Carrasco,D., Claudio,E., Ryseck,R.-P. and Bravo,R. (1997) Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-κB2. J. Exp. Med., 186, 999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Leger C., Christenson,J.R., Holman,M. and Klaus,G.G. (1998) Evidence for a critical role for IL-2 in CD40-mediated activation of naive B cells by primary CD4 T cells. J. Immunol., 161, 4618–4626. [PubMed] [Google Scholar]
- Kaisho T., Takeda,K., Tsujimura,T., Kawai,T., Nomura,F., Terada,N. and Akira,S. (2001) IκB kinase α is essential for mature B cell development and function. J. Exp. Med., 193, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. and Ben-Neriah,Y. (2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol., 18, 621–663. [DOI] [PubMed] [Google Scholar]
- Karin M. and Lin,A. (2002) NF-κB at the crossroads of life and death. Nat. Immunol., 3, 221–227. [DOI] [PubMed] [Google Scholar]
- Karrer U., Althage,A., Odermatt,B., Hengartner,H. and Zinkernagel,R.M. (2000) Immunodeficiency of alymphoplasia mice (aly/aly) in vivo: structural defect in secondary lymphoid organs and functional B cell defect. Eur. J. Immunol., 30, 2799–2807. [DOI] [PubMed] [Google Scholar]
- Koike R., Nishimura,T., Yasumizu,R., Tanaka,H., Hataba,Y., Watanabe,T., Miyawaki,S. and Miyasaka,M. (1996) The splenic marginal zone is absent in alymphoplastic aly mutant mice. Eur. J. Immunol., 26, 669–675. [DOI] [PubMed] [Google Scholar]
- Kosaka Y., Calderhead,D.M., Manning,E.M., Hambor,J.E., Black,A., Geleziunas,R., Marcu,K.B. and Noelle,R.J. (1999) Activation and regulation of the IκB kinase in human B cells by CD40 signaling. Eur. J. Immunol., 29, 1353–1362. [DOI] [PubMed] [Google Scholar]
- Lin X., Mu,Y., Cunningham,E.T., Marcu,K.B., Geleziunas,R. and Greene,W.C. (1998) Molecular determinants of NF-κB-inducing kinase action. Mol. Cell Biol., 18, 5899–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L., Cao,Z. and Goeddel,D.V. (1998) NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc. Natl Acad. Sci. USA, 95, 3792–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomaga M.A. et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40 and LPS signaling. Genes Dev., 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig M.A., Cahir-McFarland,E., Mosalios,G. and Kieff,E. (2001) Effects of the NIK aly mutation on NF-κB activation by the Epstein–Barr virus latent infection membrane protein, lymphotoxin β receptor and CD40. J. Biol. Chem., 276, 14602–14606. [DOI] [PubMed] [Google Scholar]
- Malinin N.L., Boldin,M.P., Kovalenko,A.V. and Wallach,D. (1997) MAP 3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature, 385, 540–544. [DOI] [PubMed] [Google Scholar]
- Matsushima A., Kaisho,T., Rennert,P.D., Nakano,H., Kurosawa,K., Uchida,D., Takeda,K., Akira,S. and Matusumoto,M. (2001) Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor 1. J. Exp. Med., 193, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki S., Nakamura,Y., Suzuka,H., Koba,M., Ikehara,S. and Shibata,Y. (1994) A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol., 24, 429–434. [DOI] [PubMed] [Google Scholar]
- Nakano H. et al. (1999) Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc. Natl Acad. Sci. USA, 96, 9803–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M. and Scheidereit,C. (1994) Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J., 13, 4597–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Wohlleben,G., Chuvpilo,S., Kistler,B., Wirth,T., Serfling,E. and Schimpl,A. (1996) CD40, but not lipopolysaccharide and anti-IgM stimulation of primary B lymphocytes, leads to a persistent nuclear accumulation of RelB. J. Immunol., 157, 4862–4869. [PubMed] [Google Scholar]
- Nguyen L.T. et al. (1999) TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity, 11, 379–389. [DOI] [PubMed] [Google Scholar]
- Regnier C.H., Song,H.Y., Gao,X., Goeddel,D.V., Cao,Z. and Rothe,M. (1997) Identification and characterization of an IκB kinase. Cell, 90, 373–383. [DOI] [PubMed] [Google Scholar]
- Roff M., Thompson,J., Rodriguez,M.S., Jacque,J.-M., Baleux,F., Arenzana-Seisdedos,F. and Hay,R.T. (1996) Role of IκB-α ubiquitination in signal-induced activation of NF-κB in vivo. J. Biol. Chem., 271, 7844–7850. [DOI] [PubMed] [Google Scholar]
- Senftleben U. et al. (2001) Activation by IKKα of a second evolutionary conserved, NF-κB signaling pathway. Science, 293, 1495–1499. [DOI] [PubMed] [Google Scholar]
- Shinkura R., Kitada,K., Matsuda,F., Tashiro,K., Ikuta,K., Suzuki,M., Kogishi,K., Serikawa,T. and Honjo,T. (1999) Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nat. Genet., 22, 74–77. [DOI] [PubMed] [Google Scholar]
- Snapper C., Zelazowski,P., Rosas,F.R., Kehry,M.R., Tian,M., Baltimore,D. and Sha,W.C. (1996) B cells from p50/NF-κB knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription and Ig class switching. J. Immunol., 156, 183–191. [PubMed] [Google Scholar]
- Solan N.J., Miyoshi,H., Bren,G.D. and Paya,C.V. (2001) RelB cellular regulation and transcriptional activity are regulated by p100. J. Biol. Chem., 277, 1405–1418. [DOI] [PubMed] [Google Scholar]
- Sun S.-C., Ganchi,P.A., Beraud,C., Ballard,D.W. and Greene,W.C. (1994) Autoregulation of the NF-κB transactivator RelA (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc. Natl Acad. Sci. USA, 91, 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K. et al. (2001) Critical roles of TRAF2 and TRAF5 in TNF-induced NF-κB activaiton and protection from cell death. J. Biol. Chem., 276, 36530–36534. [DOI] [PubMed] [Google Scholar]
- Ware C.F., VanArsdale,T.L., Crowe,P.D. and Browning,J.L. (1995) The ligands and receptors of the lymphotoxin system. Curr. Top. Microbiol. Immunol., 198, 175–218. [DOI] [PubMed] [Google Scholar]
- Xiao G., Cvijic,M.E., Fong,A., Harhaj,E.W., Uhlik,M.T., Waterfield,M. and Sun,S.-C. (2001a) Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: evidence for the involvement of IKKα. EMBO J., 20, 6805–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G., Harhaj,E.W. and Sun,S.-C. (2001b) NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell, 7, 401–409. [DOI] [PubMed] [Google Scholar]
- Yamada T., Mitani,T., Yorita,K., Uchida,D., Matsushima,A., Iwamasa,K., Fujita,S. and Matsumoto,M. (2000) Abnormal immune function of hematopoietic cells from Alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J. Immunol., 165, 804–812. [DOI] [PubMed] [Google Scholar]
- Yin L., Wu,L., Wesche,H., Arthur,C.D., White,J.M., Goeddel,D.V. and Schreiber,R.D. (2001) Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science, 291, 2162–2165. [DOI] [PubMed] [Google Scholar]