Abstract
Microinjection in mouse eggs of tr-kit, a truncated form of the c-kit tyrosine kinase present in mouse spermatozoa, causes resumption of meiosis through activation of phospholipase Cγ1 (PLCγ1) and Ca2+ mobilization from intracellular stores. We show that the Src-like kinase Fyn phosphorylates Tyr161 in tr-kit and that this residue is essential for tr-kit function. Fyn is localized in the cortex region underneath the plasma membrane in mouse oocytes. Using several approaches, we demonstrate that Fyn associates with tr-kit and that the interaction requires Tyr161. The interaction between tr-kit and Fyn triggers activation of the kinase as monitored by both autophosphorylation and phosphorylation of PLCγ1. Co-injection of tr-kit with the SH2 domain of Fyn, or pre-treatment with a Fyn inhibitor, impairs oocyte activation, suggesting that activation of Fyn by tr-kit also occurs in vivo. Finally, microinjection of constitutively active Fyn triggers oocyte activation downstream of tr-kit but still requires PLC activity. We suggest that the mechanism by which tr-kit triggers resumption of meiosis of mouse eggs requires a functional interaction with Fyn and phosphorylation of PLCγ1.
Keywords: Fyn/metaphase II arrest/oocyte activation/PLCγ1/tyrosine kinases
Introduction
The c-kit gene encodes a tyrosine kinase receptor essential for the establishment and the maintenance of the stem cell lineages from which melanocytes, hematopoietic cells and germ cells originate (Sette et al., 2000). In the postnatal mouse testis, c-kit is expressed in mitotic spermatogonia and its activation is required for both proliferation and survival of spermatogonia (Blume-Jensen et al., 2000; Kissel et al., 2000; Dolci et al., 2001). Expression of c-kit is restricted to proliferating diploid spermatogonia; it decreases in primary spermatocytes and it is absent in post-meiotic cells (Sorrentino et al., 1991). However, round spermatids express an alternative messenger driven by a cell-specific promoter in the 16th intron of the mouse c-kit gene (Rossi et al., 1992; Albanesi et al., 1996). The alternative transcript encodes a truncated c-kit protein of ∼28 kDa, which lacks the whole extracellular and transmembrane regions, and the ATP-binding site in the intracellular portion of the receptor. The truncated protein, named tr-kit, contains 12 hydrophobic amino acids deriving from translation of intronic sequences, the last 190 amino acids of c-kit, accounting for the phosphotransferase catalytic site, and the C-terminal tail, which mediates the interaction of c-kit with signaling molecules such as Grb2 and Grb7 (Thommes et al., 1999).
Tr-kit is expressed during differentiation of round spermatids into spermatozoa and it localizes in the mid-piece and subacrosomal region of mature sperm (Sette et al., 1997). Microinjection experiments have shown that recombinant tr-kit is able to trigger the activation of metaphase II (MII)-arrested mouse oocytes following the physiological route described for sperm-induced activation at fertilization: exocytosis of cortical granules; resumption of meiosis and extrusion of the second polar body; inactivation of the MAPK pathway; pronucleus formation; and onset of mitotic embryonic divisions (Sette et al., 1997). As in the case of fertilization, all these events required inositol trisphosphate (IP3)-dependent mobilization of Ca2+ from intracellular stores, since they were suppressed by Ca2+ chelators or phospholipase C (PLC) inhibitors (Sette et al., 1997). Furthermore, tr-kit was shown to activate PLCγ1 and stimulate IP3 production in transfected cells. Remarkably, the isolated SH3 domain of the phospholipase inhibited tr-kit function in the oocyte, even though a direct interaction was not observed (Sette et al., 1998).
In mammals, the ovulated oocyte is arrested at metaphase of the second meiotic division. Fusion with the sperm precedes the oscillations of intracellular Ca2+ levels that are considered the hallmark of a successful fertilization (Lawrence et al., 1997). It is generally accepted that an increase in IP3 intracellular concentration is responsible for opening of IP3 receptor-coupled Ca2+ channels on the endoplasmic reticulum and the onset of the Ca2+ signal (Yanagimachi, 1994). Indeed, Ca2+ oscillations are required both for early events, such as exocytosis of cortical granules and the block of polyspermy, and for late events, such as inactivation of the cyclin B–cdc2 complex, known as MPF, and completion of the meiotic cell cycle (Kline and Kline, 1992). Recent evidence, gathered using sperm extracts from different species, indicates that soluble factors released by the sperm could trigger Ca2+ release and metabolic activation of eggs at fertilization (Stricker, 1999). However, it remains unknown what the sperm factors are that trigger the release of Ca2+ from the intracellular stores. Since IP3 is involved, the activation of a PLC, the enzyme that catalyzes the hydrolysis of phosphatidylinositol bisphosphate (PIP2) with the production of IP3 and diacylglycerol (DAG), has been inferred.
Studies using echinoderm eggs have pointed to a role for a PLCγ isoform during fertilization (Runft et al., 2002). In the PLCγ subfamily (PLCγ1 and γ2 in mammals), the X and Y catalytic domain are separated by a Src-homology (SH) region containing two SH2 domains, an SH3 domain and a split PH domain. This intervening region seems to regulate the activity of the enzyme negatively. Upon binding of activators to either the SH2 or SH3 domains, and phosphorylation of Tyr783 in the SH region and Tyr1254 near the C-terminus, PLCγ is activated (Carpenter and Ji, 1999). The SH region has been used successfully as a specific dominant-negative inhibitor in vivo, probably because it prevents the association of activators with the endogenous enzymes (Roche et al., 1995). Indeed, injection of the tandem SH2 domains of PLCγ into echinoderm eggs blocks or delays the rise in Ca2+ at fertilization and causes polyspermy, indicating that PLCγ is important in this process (Carroll et al., 1997). Accordingly, it was demonstrated that PLCγ translocates to the plasma membrane and is activated within minutes in response to fertilization of sea urchin eggs (Rongish et al., 1999). More recently, a number of studies have suggested that a Src-like kinase is activated at fertilization in several non-mammalian organisms. Association of this kinase with PLCγ would favor its phosphorylation and activation, with the production of intracellular IP3. The consequent release of intracellular Ca2+ would trail most of the events required for egg activation and the onset of embryogenesis (Runft et al., 2002). However, the mechanisms by which fusion of the gametes induces activation of a Src-like tyrosine kinase remain to be elucidated.
Src is the prototype of a family of soluble tyrosine kinases, which are characterized by a myristoylation site at the N-terminus, followed by a unique region showing most of the heterogeneity in sequence between the members, an SH3 domain that mediates interactions with proteins containing proline-rich sequences, an SH2 domain that interacts with phosphorylated tyrosines in substrates and activators, and a catalytic domain (Thomas and Brugge, 1997). It is known that Src-like kinases are kept in a low activity conformation by phosphorylation of Tyr527 (according to the numbering of the prototype avian c-Src), which is catalyzed by the C-terminus Src kinase Csk. The phosphorylated tyrosine takes part in an intramolecular interaction with the SH2 domain, and this conformational modification stabilizes the binding of the SH3 domain to a polyproline helix in the linker region between the SH2 domain and the catalytic domain (Xu et al., 1997; Young et al., 2001). These intramolecular interactions keep the active site of the kinase poorly accessible to substrates (Sicheri and Kuriyan, 1997). Upon dephosphorylation of Tyr527 or displacement of the SH2 and SH3 intramolecular interactions by other proteins, Src-like kinases acquire a relaxed conformation that allows autophosphorylation of Tyr416 in the catalytic site and stabilizes the high activity state (Young et al., 2001).
In this report, we demonstrate that tr-kit-induced mouse oocyte activation requires a functional interaction with the Src-like tyrosine kinase Fyn. Tr-kit physically interacts with Fyn and this interaction stimulates the ability of Fyn to phosphorylate PLCγ1 both in vitro and in vivo. These results describe a new mechanism of activation of a Src-like kinase and suggest a possible pathway for mammalian egg activation at fertilization.
Results
Tyr161 in tr-kit is required for oocyte activation
We have shown previously that a GST–PLCγ1SH3 fusion protein interferes with tr-kit-induced egg activation, despite the fact that a direct interaction with tr-kit does not occur (Sette et al., 1998). To gain more insight into the function of tr-kit, we performed site-specific mutagenesis of regions of possible interest (Figure 1A). The 12 hydrophobic amino acids at the N-terminus, deriving from translation of intronic sequences and unique to tr-kit (Rossi et al., 1992), were deleted because they contain a PFLP motif, a putative SH3-binding site (Kroiher et al., 2001). We mutated the aspartate residue D16 into asparagine in the phosphotransferase domain (tr-kitD16N) because this mutation abolishes the catalytic activity of full-length c-kit (Tan et al., 1990). Finally, we substituted Tyr161 into phenylalanine (tr-kitY161F), since the corresponding residue in c-kit mediates the interaction with SH2 domains of the adaptor proteins Grb2 and Grb7 (Thommes et al., 1999).
Fig. 1. Tyr161 is essential for tr-kit-induced egg activation. (A) Schematic representation of tr-kit: the residues affected by site- directed mutagenesis are highlighted in bold. The sequence of the C-terminal regions of tr-kit, mimicked by the synthetic peptides, and of the 12 N-terminal amino acids, encoded by c-kit intronic sequences, are reported. (B) Western blot analysis of the expression of wild-type tr-kit and of Δ12, D16N and Y161F tr-kit mutants microinjected into eggs. A 30 µg aliquot of total protein extracts from transfected COS cells was loaded in each lane. (C) Summary of the results of microinjection experiments using 5 pl of tr-kit-transfected COS cell extracts (0.1–0.2 mg/ml) alone or together with 10 mg/ml of C or Y161 peptide. At least 40 eggs were injected for each experimental group. Pronuclear formation was monitored 6–7 h after microinjection. Data are the mean ± SD of at least three separate experiments for each group. (D) A representative example showing that most of the tr-kit-microinjected eggs have extruded the second polar body and formed a partheno genetic pronucleus.
Wild-type and mutant tr-kit proteins were expressed in COS cells and an amount comparable with 0.5–0.8 sperm equivalents (Figure 1B; see also Sette et al., 1998) was microinjected into mouse MII oocytes. As reported previously (Sette et al., 1997), microinjection of tr-kit caused activation of 60–70% of the oocytes as monitored by both polar body extrusion and pronucleus formation 6–7 h after injection (Figure 1C and D). Interestingly, tr-kitΔ12 and tr-kitD16N were still able to elicit oocyte activation significantly (Figure 1C), although to a minor extent (∼60 and 55%, respectively), indicating that neither the 12 hydrophobic amino acids nor the putative catalytic activity of tr-kit are essential for egg activation. On the contrary, we found that substitution of Tyr161 dramatically inhibited egg activation (15%). Furthermore, we observed that co-injection of tr-kit with a 13 amino acid peptide encompassing Tyr161 strongly decreased the percentage of activated oocytes (13%), whereas co-injection of a control peptide containing the last 13 amino acids of tr-kit had no effect (58%) (Figure 1C). As previously reported (Sette et al., 1997, 1998), non-injected or mock-injected eggs showed a 5–8% spontaneous acti vation under the culture conditions used. Thus, Tyr161 is critical for tr-kit-induced egg activation.
Src-like kinases phosphorylate Tyr161 of tr-kit
Tyr161 in tr-kit lays in a sequence that matches more closely the consensus for phosphorylation by soluble tyrosine kinases rather than by receptor tyrosine kinases (Songyang et al., 1995). Since the involvement of Src-like kinases at fertilization has been hypothesized in both invertebrates and vertebrates (Runft et al., 2002), we investigated whether they could phosphorylate tr-kit on Tyr161. Src and Fyn immunoprecipitated from testis extracts readily phosphorylated the Y161 peptide (Figure 2A). These results were confirmed using immunoprecipitates from Hek 293 cell extracts transfected with Src and Fyn (data not shown). Next, we expressed tyrosine kinases belonging to three different subfamilies, Abl, Tec and Fyn, in Hek 293 cells and performed an immunokinase assay using purified GST–tr-kit as substrate. We found that Fyn strongly phosphorylates GST–tr-kit, whereas a constitutively active form of Abl phosphorylates it very weakly (Figure 2B). Tec did not phosphorylate GST– tr-kit, even though it could phosphorylate other protein substrates in this assay (see Figure 7). Western blot analysis indicated that similar amounts of kinases were immunoprecipitated selectively from transfected cells (data not shown). Fyn specifically phosphorylates tr-kit in this assay, because GST alone was not phosphorylated (Figure 2C). To examine tr-kit phosphorylation in vivo by Fyn and whether Tyr161 in tr-kit is the specific target of the kinase, Hek 293 cells were co-transfected with Fyn and tr-kit wild-type or the Y161F mutant in different combinations, proteins were immunoprecipitated with an anti-phosphotyrosine antibody and the immunoprecipitates tested for the presence of tr-kit. As shown in Figure 2D, wild-type tr-kit, but not tr-kitY161F, was phosphorylated on tyrosines in a Fyn-dependent manner, indicating that Fyn specifically promotes phosphorylation of Tyr161 in tr-kit in vivo.

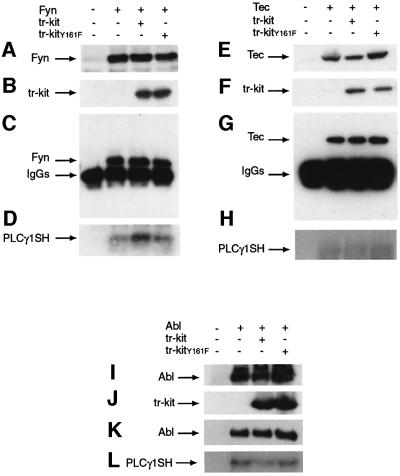
Fig. 2. Src-like kinases phosphorylate Tyr161 in tr-kit. (A) Mouse testis extracts (1 mg) were immunoprecipitated using 1 µg of pre-immune rabbit IgGs, rabbit α-Fyn or mouse α-Src antibodies, and the phosphoryl ating activity of the immunoprecipitates was tested using 10 µg of the synthetic of Y161 peptide as substrate (sequence in Figure 1A). Data correspond to c.p.m. incorporated per µg of total protein used for immunoprecipitation. Values are the mean ± SD of three independent experiments. (B) Autoradiography of an immunokinase assay using a purified GST–tr-kit (5 µg) as substrate for recombinant Abl, Tec and Fyn expressed in Hek 293 cells and immunoprecipitated using specific antibodies. Similar amounts of each kinase were used as judged by western blot analysis of the immunoprecipitates (data not shown). (C) Autoradiography of an immunokinase assay of recombinant Fyn as described in (B) using either purified GST (5 µg) or GST–tr-kit (5 µg) as substrates. (D) Western blot analysis of Fyn (upper panel) and tr-kit (central panel) expressed in transfected Hek 293 cells, and of tr-kit immunoprecipitated from cell extracts using an anti-phosphotyrosine antibody (lower panel).
Fig. 7. Tr-kit specifically stimulates phosphorylation of PLCγ1 by Fyn. COS cells were transfected with tr-kit or tr-kitY161F and Fyn (A–D), Tec (E–H) or Abl (I–L) and, after 24 h, cell extracts were immunoprecipitated with the α-Fyn antibody (C), α-Tec antibody (G) or α-Abl antibody (K). Immunoprecipitates were assayed for kinase activity towards the purified SH region of PLCγ1 (D, H and L) as described in Materials and methods. Similar amounts of Fyn (A), Tec (E) and Abl (I), and of tr-kit and tr-kitY161F (B, F and J) were expressed in the cell extracts, and equal amounts of tyrosine kinases were immunopre cipitated and assayed for enzymatic activity (C, G and K).
Physical interaction between tr-kit and Fyn
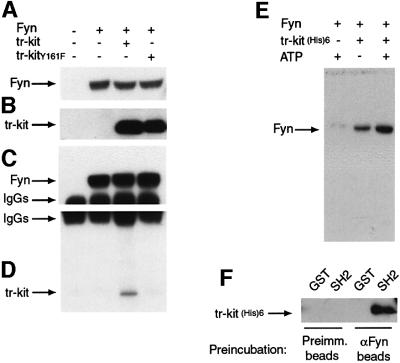
To study whether tr-kit and Fyn could physically interact, Hek 293 cells were transfected with the appropriate expression vectors (Figure 3A and B) and cell extracts were immunoprecipitated with an α-Fyn antibody (Figure 3C). Western blot analysis of the immunopre cipitates revealed that wild-type tr-kit co-immunoprecipitated with Fyn, whereas tr-kitY161F did not interact with the kinase (Figure 3D). The interaction between tr-kit and Fyn was confirmed with a pull-down experiment using a partially purified tr-kitHis6 protein expressed in bacteria and cell extracts from Hek 293 cells transfected with Fyn in the presence or absence of exogenous ATP. Figure 3E shows that Fyn is pulled down by the α-His6 antibody only when the beads had been pre-adsorbed to tr-kitHis6 and that the interaction is improved by exogenous ATP. Next we tested whether the interaction requires phosphorylation of tr-kit and the SH2 domain of Fyn. Extracts expressing recombinant Fyn were immunoprecipitated with either pre-immune or αFyn IgGs and the immunoprecipitates were incubated with purified tr-kit(His)6. Following the incubation, tr-kitHis6 was passed over beads pre-adsorbed to either GST or GST–FynSH2. As shown in Figure 3F, tr-kitHis6 was able to interact directly with GST–FynSH2 only after being pre-incubated with Fyn. These data demonstrate that tr-kit and Fyn physically interact and that Tyr161 is necessary for such interaction, indicating a correlation between tr-kit–Fyn association and biological activity of tr-kit.
Fig. 3. Physical association with Fyn requires Tyr161 in tr-kit. Western blot analysis of extracts (20 µg) from transfected Hek 293 cells was performed using the α-Fyn (A) or the α-c-kit antibody (B). Fyn was immunoprecipitated from cell extracts (500 µg) using 1 µg of α-Fyn, and immunoprecipitated proteins were analyzed by western blot using the α-Fyn (C) or the α-c-kit antibody (D). This experiment indicates that tr-kit, but not the Y161F mutant, co-immunoprecipitates with Fyn. (E) Association between Fyn and a bacterially expressed tr-kit protein. Protein A/G–Sepharose beads pre-adsorbed to 1 µg of α-His6 antibody were incubated for 1 h at 4°C with cell extracts from Hek 293 cells transfected with Fyn in the presence or absence of 100 µM ATP and purified tr-kitHis6 (2 µg). After three washes with homogenization buffer, proteins bound to the beads were eluted in sample buffer and analyzed by western blotting using the α-Fyn antibody. (F) Cell extracts from Hek 293 cells transfected with FynY528F were immunoprecipitated with either pre-immune or α-Fyn IgGs, and immunoprecipitates were incubated with 5 µg of tr-kitHis6 for 30 min at room temperature in the presence of 100 µM ATP to allow phosphorylation. Then, immunoprecipitates were separated by centrifugation and soluble tr-kitHis6 was incubated with either GST or GST–FynSH2 pre-adsorbed to glutathione–agarose beads. Proteins bound to GST fusions were eluted with 10 µM reduced glutathione and analyzed by western blotting using the α-c-kit antibody.
The SH region of Fyn inhibits tr-kit-induced oocyte activation
Western blot analysis demonstrates that Fyn is expressed in mouse eggs (Figure 4A), and we found that it localizes in the cortex region (Figure 4B) as described previously in rat eggs (Talmor et al., 1998). We set out to investigate whether the interaction between Fyn and tr-kit plays a role in oocyte activation. The SH region of Src-like kinases (Figure 5A) plays a crucial role in both autoinhibition of catalytic activity and interaction with activators and substrates (Thomas and Brugge, 1997), and a GST– FynSH3SH2 fusion protein exerts a dominant-negative action on Fyn in live cells (Roche et al., 1995). Interestingly, we observed that co-injection of tr-kit and GST–FynSH3SH2 (Figure 5B) into MII oocytes strongly inhibited resumption of the cell cycle triggered by tr-kit (from 52 to 9% in this set of experiments) (Figure 5C). We next asked whether the SH2 domain is sufficient for inhibition, because purified tr-kit interacts with the SH2 domain of Fyn after being phosphorylated by the kinase on Tyr161. Indeed, the isolated SH2 domain of Fyn acted as a strong inhibitor of tr-kit function in oocytes (Figure 5C), even though a weak inhibition was also exerted by the isolated SH3 domain. Accordingly, pull-down experiments using GST–Fyn fusion proteins indicated that the SH2 domain is sufficient for the interaction with tr-kit, albeit that a weak interaction with the SH3 domain was also detected (Figure 5D). The SH2 domain of a related kinase (Src) also inhibited tr-kit action in eggs, whereas that of Abl was ineffective (Figure 5C). Together, these results indicate that a specific interaction between tr-kit and the SH2 domain of a Src-like kinase is necessary for egg activation.

Fig. 4. Fyn is expressed in mouse MII oocytes. (A) Western blot analysis of Fyn expressed in 100 mouse ovulated oocytes. For comparison, 30 µg of cell extracts from mock- or Fyn-transfected Hek 293 cells were loaded on the gel. (B) Immunofluorescence analysis of Fyn expressed in mouse oocytes: cells were co-stained with α-Fyn antibody and the DNA dye Hoechst 3332.
Fig. 5. The SH region of Fyn interferes with tr-kit-induced egg activation. (A) Schematic representation of the organization of the Src-homology domains in Fyn. (B) Coomassie blue staining of an SDS–polyacrylamide gel with purified bacterial fusion proteins containing the Src-homology domains used for the microinjection experiments. (C) Summary of the co-injection experiments using tr-kit and 500 µg/ml of GST, or GST–FynSH3, GST–FynSH2, GST–FynSH3SH2, GST–AblSH2 or GST–SrcSH2. Data are the mean ± SD of at least three separate experiments for each experimental group (total of at least 30 eggs). (D) A representative pull-down experiment using 4 µg of GST–Fyn fusion proteins and cell extracts (500 µg) from Hek 293 cells transfected with tr-kit and Fyn.
Tr-kit stimulates the catalytic activity of Fyn
Displacement of the intramolecular interaction between Tyr527 (528 in Fyn) and the SH2 domain by another protein activates Src-like kinases. Interestingly, co-expression of Fyn and tr-kit in Hek 293 cells elicited a 2- to 3-fold stimulation of the catalytic activity of the kinase (Figure 6A). This activation was similar to that achieved by constitutive disruption of the intramolecular interaction between Tyr528 and the SH2 domain in the hyperactive FynY528F (Figure 6A). Activation of Fyn by Tyr528 substitution or by tr-kit was also confirmed by looking at the autophosphorylation levels of the kinase (Figure 6B), which correlate with its catalytic activity.
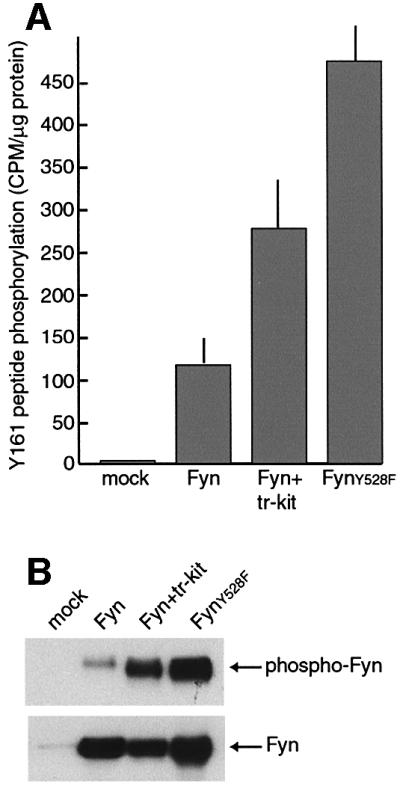
Fig. 6. Tr-kit stimulates the catalytic activity of Fyn. Fyn activity was measured in an immunokinase assay using extracts from Hek 293 cells that were transfected with wild-type Fyn, FynY528F or Fyn and tr-kit. After immunoprecipitation, beads were divided into two equal amounts. (A) Fyn activity was measured in one half of the beads using the Y161 peptide (10 µg) and 100 µM [γ-32P]ATP (0.1 µCi/µl) as substrates, and the assay was carried out as described in Materials and methods. (B) The other half of the beads were incubated as above in 25 µl of kinase buffer supplemented with 100 µM ATP and, at the end of the incubation, immunoprecipitated proteins were eluted in sample buffer and analyzed by western blot using the anti-phosphotyrosine (upper panel) or the α-Fyn antibody (lower panel).
Tr-kit stimulates phosphorylation of PLCγ1 by Fyn
Since tr-kit-induced egg activation requires PLCγ1 (Sette et al., 1998), and PLCγ1 is activated by phosphorylation of Tyr783 in the SH region (Kim et al., 1991), we investigated whether Fyn phosphorylates PLCγ1 and whether the co-expression of tr-kit affects this reaction. COS cells were co-transfected with the appropriate plasmids and, after immunoprecipitation of cell extracts with the α-Fyn antibody, the kinase activity was assayed using a GST–PLCγ1SH fusion protein as substrate. Interestingly, we found that Fyn directly phosphorylates GST–PLCγ1SH in vitro and that co-expression of tr-kit stimulates PLCγ1 phosphorylation (Figure 7A–D). On the other hand, tr-kitY161F, which is unable to interact with Fyn and to trigger egg activation, did not affect the activity of the kinase (Figure 7A–D). Activation of Fyn by tr-kit was specific, because in similar sets of experiments we demonstrated that tr-kit was unable to activate Tec (Figure 7E–H) or Abl (Figure 7I–L). Interestingly, both Tec and Abl lack the C-terminal tyrosine that plays an autoinhibitory role in Src-like kinases (Smith et al., 2001; Pluk et al., 2002).
Next, we investigated the ability of Fyn to phosphoryl ate PLCγ1 in vivo. To this end, Hek 293 cells were co-transfected in various combinations with PLCγ1, Fyn and tr-kit, PLCγ1 was then immunoprecipitated from cell extracts with a specific antibody, and the immunoprecipitates were analyzed by western blotting. When expressed alone, PLCγ1 (Figure 8D) was weakly phosphorylated on tyrosine residues (Figure 8E) whereas co-expression of Fyn promoted tyrosine phosphorylation of the phospho lipase. Moreover, expression of Fyn and tr-kit in the same cells dramatically increased phosphorylation of PLCγ1 (Figure 8E), confirming in vivo the effect exerted by tr-kit on the phosphorylation of PLCγ1 by Fyn in vitro. Interestingly, a band of ∼70 kDa was also detected in the immunoprecipitates, and its phosphorylation was strongly increased in the presence of tr-kit. Furthermore, western blot analysis of the α-PLCγ1 immunoprecipitates with the α-Fyn antibody showed that Fyn co-precipitates with PLCγ1 and that tr-kit promotes this association (Figure 8F). Again, tr-kitY161F was unable to stimulate either association of Fyn with PLCγ1 or its level of tyrosine phosphorylation, indicating that an interaction between tr-kit and Fyn through Tyr161 in tr-kit is necessary for such events.

Fig. 8. Co-expression of tr-kit stimulates Fyn-dependent phosphorylation of PLCγ1 in vivo. Hek 293 cells were transfected with PLCγ1, Fyn, tr-kit and tr-kitY161F in different combinations (A–C). After 24 h, cells were harvested, lysed, and clarified extracts were immunoprecipitated for 2 h using 1 µg of α-PLCγ1 antibody adsorbed to protein A–Sepharose beads. Immunoprecipitated proteins were analyzed by western blotting using the α-PLCγ1 (D), the α-phosphotyrosine (E) or the α-Fyn (F) antibody.
Activation of Fyn by tr-kit is sufficient to trigger oocyte activation
We next tested whether tr-kit induces egg activation by sequentially stimulating Fyn and PLCγ1 activation. Oocytes were pre-incubated for 30 min with the Src-like kinase inhibitor PP2 or its inactive analog PP3 before microinjection of tr-kit. PP2 completely suppressed tr-kit action, whereas PP3 was ineffective (Figure 9A), demonstrating the requirement for activation of a Src-like kinase. Furthermore, microinjection of constitutively active FynY528F triggered parthenogenetic egg activation mimicking the effect exerted by tr-kit, as demonstrated by pronuclear formation after 6–8 h and cell division after 18–24 h (Figure 9C and D). Furthermore, as in the case of tr-kit-induced egg activation, FynY528F action required PLC activity, since egg activation was inhibited when oocytes were pre-incubated with the PLC inhibitor U73122 (Figure 9B). These results suggest that tr-kit-induced oocyte activation is triggered by the sequential activation of Fyn and PLCγ1.
Fig. 9. Src-like kinase activity is required for tr-kit-induced egg activation. (A) Eggs were pre-incubated with either the vehicle DMSO, 5 µM PP2 or 5 µM PP3 for 30 min before microinjection with tr-kit. Injected eggs were returned to M16 medium containing the appropriate drug and scored for activation after 6 h. (B) Eggs were pre-incubated with either DMSO or 5 µM U73122 for 30 min before microinjection with FynY528F. Egg activation was scored after 6 h as pronucleus formation (C) or after 24 h as cell division (D).
Discussion
An association between tr-kit and Fyn is required for oocyte activation
Tr-kit triggers resumption of meiosis and activation of MII-arrested oocytes when microinjected into their cytoplasm. This effect requires activation of PLCγ1 and mobilization of Ca2+ from intracellular stores (Sette et al., 1997, 1998). Here we demonstrate that activation of PLCγ1 is mediated by a functional interaction between tr-kit and a Src-like tyrosine kinase. Several observations obtained using as prototype the Src-like kinase Fyn, highly expressed in mouse oocytes, support this conclusion. (i) We have identified a mutant, tr-kitY161F, which is unable to associate with Fyn and to induce resumption of oocyte meiosis. (ii) Fyn phosphorylates Tyr161 of tr-kit in vitro and in transfected cells, suggesting that it could also promote phosphorylation of this crucial residue in the oocyte. (iii) The SH2 domain of Fyn, which binds in vitro to tr-kit, or the Src-like kinase inhibitor PP2, suppress tr-kit-induced egg activation. (iv) Constitutively active Fyn is sufficient to trigger egg activation. Since the SH2 domain of Src also blocks tr-kit-induced egg activation, whereas that of the unrelated tyrosine kinase Abl does not, the interaction appears to be specific for Src-like kinases. Therefore, we hypothesize that upon entry into the oocyte cytoplasm, tr-kit is phosphorylated by Fyn (or other Src-like kinase) on Tyr161, which promotes its interaction with the kinase, and that this complex triggers oocyte activation. The interaction between tr-kit and Fyn requires phosphorylation of Tyr161 and the SH2 domain of the kinase, as demonstrated by pull-down experiments with purified proteins and cell extracts.
Tr-kit acts as a molecular trigger of the catalytic activity of Fyn
Why is the interaction of tr-kit with Fyn functionally important? We report that co-expression of tr-kit and Fyn causes activation of Fyn as monitored by autophosphorylation of the kinase and phosphorylation of exogenous substrates. Since displacement of the intramolecular interaction between Tyr527 and the SH2 domain activates Src-like kinases (Thomas and Brugge, 1997), we suggest that, once phosphorylated on Tyr161, tr-kit acts as a molecular trigger to change the conformation of Fyn from a low to a high activity state (Figure 10). Indeed, we observed that co-expression of tr-kit stimulates the activity of Fyn 2- to 3-fold, eliciting an activation that is approximately half of that obtained with disruption of the intramolecular interaction in FynY528F. Our model is also supported by the observation that tr-kitY161F, which is unable to interact with Fyn, does not induce enzyme activation and that purified tr-kit binds to the SH2 domain of Fyn only after being phosphorylated. Since tr-kitY161F is also unable to trigger oocyte activation, a correlation exists between tr-kit–Fyn interaction and tr-kit biological activity.
Fig. 10. Hypothetical model of tr-kit-induced activation of Fyn. Fyn is maintained in a low activity state by the intramolecular interaction between the SH2 domain and phosphorylated Tyr528 in the C-terminus. Tr-kit is phosphorylated on Tyr161 by the ‘low activity’ Fyn; this allows interaction with the SH2 domain of Fyn and displacement of the intramolecular inhibition. Activated Fyn autophosphorylates in the activation loop, and interacts with PLCγ1, thereby promoting phosphorylation and activation of the lipase.
Tr-kit stimulates phosphorylation of PLCγ1 by Fyn
Tr-kit induces resumption of meiosis of mouse oocytes by promoting phosphorylation and activation of PLCγ1 (Sette et al., 1998). Here we show that a Src-like kinase mediates activation of PLCγ1 in microinjected eggs. PLCγ1 is activated through phosphorylation of Tyr783 in the SH region (Kim et al., 1991; Carpenter and Ji, 1999). Accordingly, we observed that Fyn directly phosphoryl ates GST–PLCγ1SH2SH2SH3 and that tr-kit stimulates this reaction. Moreover, co-expression in the same cell of tr-kit, Fyn and PLCγ1 brings about a massive increase in the phosphotyrosine content of PLCγ1. We also observed that tr-kit expression promotes the association of Fyn with PLCγ1. Taken together, our results are consistent with a model in which tr-kit activates Fyn by direct interaction and promotes its association with PLCγ1, and the consequent phosphorylation of the phospholipase brings about its activation (Figure 10). In agreement with such a model, soluble tyrosine kinases such as Tec and Abl, which lack the phosphotyrosine-SH2 domain autoinhibition mechanism typical of Src-like kinases (Smith et al., 2001; Pluk et al., 2002), are not activated by tr-kit.
A possible pathway operating at fertilization
Since tr-kit is expressed specifically in male haploid cells and mature sperm (Sette et al., 1997), it is possible that it participates in egg activation at fertilization. In mammalian oocytes, the signal that triggers resumption of meiosis is provided by oscillatory increases in intracellular Ca2+ levels triggered by production of IP3 (Yanagimachi, 1994), and it has been demonstrated that fusion of the gametes precedes the onset of Ca2+ oscillations (Lawrence et al., 1997). Although it is unknown how the sperm triggers Ca2+ mobilization, it is plausible that soluble sperm factors are released into the oocyte cytoplasm at fertilization, because microinjection of extracts obtained from sperm of invertebrates and vertebrates, including mammals, is sufficient to elicit Ca2+ mobilization and egg activation (Stricker, 1999). The ‘sperm factor’ hypothesis is also supported by the evidence that large molecular weight molecules can transit from the sperm to the oocyte before the onset of Ca2+ oscillations (Jones et al., 1998). Since IP3 production is involved, it is conceivable that a PLC isoform is activated by a such sperm factor(s).
In echinoderm eggs, activation of a Src-like kinase, which associates with the SH2 domains of PLCγ, occurs within minutes after sperm–egg fusion (Kinsey, 1996; Giusti et al., 1999). The kinase involved has not been identified, even though microinjection of active mammalian Src triggers egg activation (Giusti et al., 2000) and the SH2 domain of Src-like kinases can delay or block the Ca2+ wave at fertilization (see Runft et al., 2002). These data suggest that in echinoderms, the sperm sequentially activates a Src-like kinase and PLCγ, thereby causing an increase in IP3, Ca2+ mobilization and egg activation. In the vertebrate Xenopus laevis, activation of the Src-like kinase Xyk occurs at fertilization (Sato et al., 1999), and tyrosine phosphorylation and activation of PLCγ are required for Ca2+ release (Sato et al., 2000). However, activation of PLCγ does not appear to involve the tandem SH2 domains of the enzyme, suggesting the involvement of a different mechanism for PLCγ activation (Runft et al., 1999). Nevertheless, in all the cases reported above, it remains unsolved how the sperm elicits the activation of the Src-like kinase.
Our results in mouse eggs fit well into the incomplete scenario of fertilization. Tr-kit is a sperm protein that interacts with Fyn and causes activation of the kinase and phosphorylation of PLCγ1, leading to resumption of oocyte meiosis. Since we show that constitutively active Fyn is sufficient to trigger egg activation, a signaling pathway similar to that activated by the sperm in other organisms is potentially functional in mouse oocytes. Similarly to what was observed in Xenopus eggs, Mehlmann et al. (1998) demonstrated that the tandem SH2 domains of PLCγ1 did not block the onset of Ca2+ oscillations at fertilization in mouse oocytes. This observation is in agreement with the pathway activated by microinjection of tr-kit because we found that oocyte activation is inhibited by co-injection of the SH3 domain of PLCγ1 but not by the tandem SH2 domains (Sette et al., 1998). One possibility is that the SH3 domain is required for the interaction with a proline-rich adaptor protein that mediates a correct recruitment of PLCγ1 at the plasma membrane (Sette et al., 1998). In support of this hypothesis, we found that a 70 kDa protein phosphorylated at its tyrosine residues co-immunoprecipitated with antibodies directed against PLCγ1 (Figure 8). More interestingly, its phosphotyrosine content was increased dramatically by co-expression of tr-kit and Fyn (Figure 8). Since a phosphotyrosine protein of identical size was also found in Fyn immunoprecipitates in tr-kit-expressing cells (unpublished observations), this 70 kDa phosphoprotein may functionally link PLCγ1, Fyn and tr-kit. Although the nature and function of such protein(s) is still under investigation, it probably represents an additional com ponent taking part to this signaling pathway.
In conclusion, our work demonstrates the presence in mouse sperm of a protein capable of inducing activation of a Src-like kinase in the oocyte. It is noticeable that we have detected a tr-kit homolog in human and rat sperm (M.P.Paronetto and C.Sette, in preparation), suggesting that this mechanism is conserved during evolution. Although there is no indication of tr-kit homologs in frog or lower eukaryotes yet, it is possible that sperm factors with a similar function also operate to trigger Src activation (i.e. relief of the SH2 domain autoinhibitory constraint) at fertilization in lower organisms.
Materials and methods
PCR-based cloning and mutagenesis
Total RNA was isolated from mice thymus and brain using the Trizol reagent (Gibco-BRL) and following the manufacturer’s instructions. Mouse Fyn and Src were amplified by RT–PCR using RNA extracted from thymus and brain, respectively, and subcloned into the eukaryotic expression vector pCMV5. pCMV5-FynY528F was produced by PCR by inserting the mutation in the 3′ oligonucleotide.
Site-directed mutagenesis was carried out by using the PCR-based approach to generate tr-kitΔ12, tr-kitD16N and tr-kitY161F, and the resulting cDNAs were inserted into pCMV5. All amplifications were performed using Pfu polymerase (Stratagene), and the sequence of PCR-derived fragments was confirmed by DNA sequence analysis.
Expression and purification of GST fusion proteins
pGEX-PLCγ1SH2SH2SH3 and pGEX-AblSH2 were generous gifts of S.Courtneidge (Sugen Inc., Redwood City, CA) and Daniela Barilà (University of Rome ‘Tor Vergata’), respectively; DNA sequences encoding SH2, SH3 and SH3SH2 of Fyn and Src were amplified by PCR from the pCMV5-Fyn or pCMV5-Src plasmids using Pfu polymerase. Full-length tr-kit was amplified as above. All oligonucleotides used contained the BamHI site at the 5′ end and the EcoRI site at the 3′ end. The PCR products were subcloned in-frame with GST into the pGEX-3X vector (Pharmacia). Plasmids were transformed into the Escherichia coli BL21 strain, grown at 30°C in LB medium to an OD600 = 0.6, and induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Gibco-BRL) for 3 h at the same temperature to produce GST fusion proteins. The recombinant proteins were purified from bacterial lysates and analyzed as reported previously (Sette et al., 1998).
Expression and purification of His6-tagged tr-kit
A tr-kitHis6 construct was prepared by PCR using Pfu polymerase. The reverse primer contained the sequence for the six histidines before the stop codon of tr-kit. The resulting cDNA was cloned into the pT7/7 vector using the NdeI and EcoRI sites. To express tr-kitHis6, the plasmid was transformed into the BL21 strain, grown at 30°C in LB medium and induced with IPTG for 3 h at the same temperature. Bacterial lysates were purified on Ni-NTA–agarose (Qiagen) by standard procedures.
Oocyte collection, microinjection and in vitro culture
MII oocytes were collected from hormonally primed (Hogan et al., 1994) 6- to 7-week-old CD1 female mice (Charles River Italia) 16 h after human cortionic gonadotrophin injection. Ovulated oocytes were freed from cumulus by incubation with 0.5 mg/ml hyaluronidase (Sigma-Aldrich) in M2 medium (Hogan et al., 1994), washed in M2 medium and then transferred to 50 µl drops of M16 (Hogan et al., 1994) under liquid paraffin for 15–20 min before the microinjection. Microinjection manipulations were performed as described previously (Sette et al., 1997). Briefly, we injected into the oocyte cytoplasm 2–5 pl of a tr-kit-expressing cell extract diluted to a protein concentration of 0.1–0.2 mg/ml in injection buffer [20 mM HEPES pH 7.4, 120 mM KCl, 100 µM EGTA, 10 mM β-glycerophosphate, 1 mM dithiothreitol (DTT), 10 µg/ml leupeptin, 10 µg/ml pepstatin]. Quantification of the volume injected was performed as described previously (Sette et al., 1998). Oocytes were transferred to 50 µl drops of M2 under mineral oil and microinjected using an Olympus invertoscope (Olympus) equipped with Hoffman modulation contrast optics (Modulation Optics, Inc., Greenvale, NY) and two Leitz mechanical micromanipulators (Leica AG, Heerbrugg, Switzerland). After microinjections, oocytes were returned to M16 medium drops under mineral oil and cultured at 37°C under a humidified atmosphere of 5% CO2 in air, and scored for pronucleus formation 6–7 h after injection.
Cell culture and transfections
pCMV5-tr-kit for eukaryotic expression of tr-kit has been described previously (Sette et al., 1997); pSGT-Abl and pSGT-Abl PP, encoding a constitutively active Abl with a double mutation (P242E/P249E) in the linker region, pRK-PLCγ1, for PLCγ1 expression, and pSRα-Tec, for Tec expression, were generously provided by Dr Superti-Furga (EMBL, Heidelbergh, Germany), Dr A.Ullrich (Max Planck Institut, Martinsried, Germany) and Dr H.Mano (Jichi Medical School, Tochigi, Japan), respectively.
Subconfluent COS or Hek 293 cell monolayers were cultured in 90 mm dishes and processed for CaPO4 transfection with 10 µg of the appropriate plasmid. At 24–48 h post-transfection, cells were harvested in homogenization buffer [50 mM HEPES pH 7.5, 10 mM β-glycerophos phate, 2 mM EGTA, 15 mM MgCl2, 0.1 mM sodium orthovanadate, 1 mM DTT, 10 µg/ml leupeptin and 10 µg/ml pepstatin, 10 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF)], homogenized with 40 strokes in a glass homogenizer, or lysed by adding 1% Triton X-100 and 120 mM NaCl to the homogenization buffer (lysis buffer). The lysates were centrifugated for 10 min at 10 000 g at 4°C and used for further analysis. Protein concentration was determined according to Bradford (1976).
Pull-down assays
Cell extracts (500 µg of total proteins) were added to 2–4 µg of GST fusion protein adsorbed on glutathione–agarose (Sigma) in 250 µl (final volume) of homogenization buffer supplemented with 0.05% bovine serum albumin (BSA). For binding of tr-kitHis6 to Fyn, protein A/G–Sepharose beads were pre-adsorbed with 1 µg of α-His6 antibody before incubation with 500 µg of cell extracts from Hek 293 cells transfected with Fyn in the absence or presence of 2 µg of purified tr-kitHis6 in 250 µl of homogenization buffer with or without 100 µM ATP for 1 h at 4°C under constant shaking. All beads were washed three times with homogenization buffer, eluted in SDS sample buffer and adsorbed proteins were resolved on a 10% SDS–polyacrylamide gel (Laemmli, 1970) for subsequent western blot analysis.
Immunoprecipitation and immunokinase assay
Cell extracts (500 µg of total proteins) were incubated with 1 µg of the specific antibody (described below) for 2 h at 4°C under constant shaking. Immune complexes were collected by adsorption onto protein A–Sepharose (Sigma-Aldrich). To remove non-specificically bound materials, the Sepharose beads were washed three times with homogenization buffer. Beads were either eluted in SDS sample buffer for western blot analysis, or washed once with kinase buffer (homogenization buffer supplemented with 100 µm ATP) and used for the enzymatic reaction. The kinase reactions were carried out by incubating the beads with the substrates, 3 µg of purified GST– PLCγSH2SH2SH3 or GST–tr-kit, or 10 µg of tr-kit Y161 peptide, in 25 µl kinase buffer also containing 0.1 µCi of [γ-32P]ATP for 30 min at 30°C, and terminated either by the addition of SDS sample buffer followed by boiling for 5 min (for GST–tr-kit and GST–PLCγSH2SH2SH3) and separated on SDS–poyacrylamide gels, or by spotting the reaction mixture onto squares of P-81 phosphocellulose paper (Whatman) as previously described (Sette et al., 1997). Radioactivity incorporated was determined by scintillation counting of the paper squares or by autoradiography of the dried gels.
Western blot analysis
Proteins were separated on 10% SDS–polyacrylamide gels and transferred to polyvinylidene fluoride Immobilon-P membranes (Millipore) using a semi-dry blotting apparatus (Bio-Rad). Membranes were saturated with 5% non-fat dry milk in phosphate-buffered saline (PBS) containing 0.1% Tween-20 for 1 h at room temperature, and incubated with the following first antibodies (1:1000 dilution) overnight at 4°C: rabbit α-Fyn (SC-16, Santa Cruz Biotechnology); rabbit α-PLCγ1 (SC-081, Santa Cruz Biotechnology); rabbit α-Tec (gift of Dr Mano); rabbit α-c-kit (Albanesi et al., 1996); mouse antiphosphotyrosine PY20 (SC-508); or α-Abl (SC-23, Santa Cruz Biotechnology). Secondary anti-mouse or anti-rabbit IgGs conjugated to horseradish peroxidase (Amersham) were incubated with the membranes for 1 h at room temperature at a 1:10 000 dilution in PBS containing 0.1% Tween-20. Immunostained bands were detected by a chemiluminescent method (Santa Cruz Biotechnology).
Immunofluorescence analysis
Mouse MII oocytes were fixed for 15 min in 4% paraformaldehyde and permeabilized for 10 min in 0.1% Triton X-100 in M2 at room temperature. After incubation in blocking solution (M2 supplemented with 5% BSA) for 1 h, oocytes were incubated overnight at 4°C with rabbit polyclonal α-Fyn (1:200 dilution). Following five washes in M2, oocytes were incubated for 1 h at 37°C with rhodamine-conjugated secondary anti-rabbit IgGs (Calbiochem) and Hoechst dye (0.1 mg/ml, Sigma). Cells were washed five times in M2 drops and analyzed for immunofluorescence with an Olympus invertoscope by using a 40× objective. Images were collected with an RT-slider Spot camera (Diagnostic Instruments, Inc.) and digitally recorded using imaging software (IAS 2000) and Photoshop (Adobe Systems, Inc., Mountain View, CA).
Acknowledgments
Acknowledgements
We would like to thank Drs A.Ullrich, G.Superti-Furga, H.Mano, Daniela Barilà and S.Courtneidge for the generous gift of reagents. We acknowledge the support given to this study by Professor F.Mangia (University of Rome ‘La Sapienza’, Italy). This work was funded by grants from the Agenzia Spaziale Italiana (ASI), Consiglio Nazionale delle Ricerche (CNR), and by MURST 2000 Agency.
References
- Albanesi C., Geremia,R., Giorgio,M., Dolci,S., Sette,C. and Rossi,P. (1996) A cell- and developmental stage-specific promoter drives the expression of a truncated c-kit protein during mouse spermatid elongation. Development, 122, 1291–1302. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P., Jiang,G., Hyman,R., Lee,K.F., O’Gorman,S. and Hunter,T. (2000) Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat. Genet., 24, 157–162. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem., 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Carpenter G. and Ji,Q. (1999) Phospholipase C-γ as a signal-transducing element. Exp. Cell Res., 253, 15–24. [DOI] [PubMed] [Google Scholar]
- Carroll D.J., Ramarao,C.S., Mehlmann,L.M., Roche,S., Terasaki,M. and Jaffe,L.A. (1997) Calcium release at fertilization in starfish eggs is mediated by phospholipase Cγ. J. Cell Biol., 138, 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolci S., Pellegrini,M., Di Agostino,S., Geremia,R. and Rossi,P. (2001) Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J. Biol. Chem., 276, 40225–40233. [DOI] [PubMed] [Google Scholar]
- Giusti A.F., Carroll,D.J., Abassi,Y.A. and Foltz,K.R. (1999) Evidence that a starfish egg Src family tyrosine kinase associates with PLC-γ1 SH2 domains at fertilization. Dev. Biol., 208, 189–199. [DOI] [PubMed] [Google Scholar]
- Giusti A.F., Xu,W., Hinkle,B., Terasaki,M. and Jaffe,L.A. (2000) Evidence that fertilization activates starfish eggs by sequential activation of a Src-like kinase and phospholipase Cγ. J. Biol. Chem., 275, 16788–16794. [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington,R., Costantini,F. and Lacy,E., (1994) Manipulating the Mouse Embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 389–399.
- Jones K.T., Soeller,C. and Cannell,M.B. (1998) The passage of Ca2+ and fluorescent markers between the sperm and egg after fusion in the mouse. Development, 125, 4627–4635. [DOI] [PubMed] [Google Scholar]
- Kim H.K., Kim,J.W., Zilberstein,A., Margolis,B., Kim,J.G., Schlessinger,J. and Rhee,S.G. (1991) PDGF stimulation of inositol phospholipid hydrolysis requires PLC-γ1 phosphorylation on tyrosine residues 783 and 1254. Cell, 65, 435–441. [DOI] [PubMed] [Google Scholar]
- Kinsey W.H. (1996) Biphasic activation of Fyn kinase upon fertilization of the sea urchin egg. Dev. Biol., 174, 281–287. [DOI] [PubMed] [Google Scholar]
- Kissel H. et al. (2000) Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J., 19, 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D. and Kline,J.T. (1992) Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol., 149, 80–89. [DOI] [PubMed] [Google Scholar]
- Kroiher M., Miller,M.A. and Steele,R.E. (2001) Deceiving appearances: signaling by ‘dead’ and ‘fractured’ receptor protein-tyrosine kinases. BioEssays, 23, 69–76. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lawrence Y., Whitaker,M. and Swann,K. (1997) Sperm–egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development, 124, 233–241. [DOI] [PubMed] [Google Scholar]
- Mehlmann L.M., Carpenter,G., Rhee,S.G. and Jaffe,L.A. (1998) SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev. Biol., 203, 221–232. [DOI] [PubMed] [Google Scholar]
- Pluk H., Dorey,K. and Superti-Furga,G. (2002) Autoinhibition of c-Abl. Cell, 108, 247–259. [DOI] [PubMed] [Google Scholar]
- Roche S., Fumagalli,S. and Courtneidge,S.A. (1995) Requirement for Src family protein tyrosine kinases in G2 for fibroblast cell division. Science, 269, 1567–1569. [DOI] [PubMed] [Google Scholar]
- Rongish B.J., Wu,W. and Kinsey,W.H. (1999) Fertilization-induced activation of phospholipase C in the sea urchin egg. Dev. Biol., 215, 147–154. [DOI] [PubMed] [Google Scholar]
- Rossi P., Marziali,G., Albanesi,C., Charlesworth,A., Geremia,R. and Sorrentino,V. (1992) A novel c-kit transcript, potentially encoding a truncated receptor, originates within a kit gene intron in mouse spermatids. Dev. Biol., 152, 203–207. [DOI] [PubMed] [Google Scholar]
- Runft L.L., Watras,J. and Jaffe,L.A. (1999) Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCγ or G(q)-mediated activation of PLCβ. Dev. Biol., 214, 399–411. [DOI] [PubMed] [Google Scholar]
- Runft L.L., Jaffe,L.A. and Mehlmann,L.M. (2002) Egg activation at fertilization: where it all begins. Dev. Biol., 245, 237–254. [DOI] [PubMed] [Google Scholar]
- Sato K., Iwao,Y., Fujimura,T., Tamaki,I., Ogawa,K., Iwasaki,T., Tokmakov,A.A., Hatano,O. and Fukami,Y. (1999) Evidence for the involvement of a Src-related tyrosine kinase in Xenopus egg activation. Dev. Biol., 209, 308–320. [DOI] [PubMed] [Google Scholar]
- Sato K., Tokmakov,A.A., Iwasaki,T. and Fukami,Y. (2000) Tyrosine kinase-dependent activation of phospholipase Cγ is required for calcium transient in Xenopus egg fertilization. Dev. Biol., 224, 453–469. [DOI] [PubMed] [Google Scholar]
- Sette C., Bevilacqua,A., Bianchini,A., Mangia,F., Geremia,R. and Rossi,P. (1997) Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development, 124, 2267–2274. [DOI] [PubMed] [Google Scholar]
- Sette C., Bevilacqua,A., Geremia,R. and Rossi,P. (1998) Involvement of phospholipase Cγ1 in mouse egg activation induced by a truncated form of the c-kit tyrosine kinase present in spermatozoa. J. Cell Biol., 142, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette C., Dolci,S., Geremia,R. and Rossi,P. (2000) The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int. J. Dev. Biol., 44 (6 Spec. No.), 599–608. [PubMed] [Google Scholar]
- Sicheri F. and Kuriyan,J. (1997) Structures of Src-family tyrosine kinases. Curr. Opin. Struct. Biol., 7, 777–785. [DOI] [PubMed] [Google Scholar]
- Smith C.I., Islam,T.C., Mattsson,P.T., Mohamed,A.J., Nore,B.F. and Vihinen,M. (2001) The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. BioEssays, 23, 436–446. [DOI] [PubMed] [Google Scholar]
- Songyang Z. et al. (1995) Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature, 373, 536–539. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Giorgi,M., Geremia,R., Besmer,P. and Rossi,P. (1991) Expression of the c-kit proto-oncogene in the murine male germ cells. Oncogene, 6, 149–151. [PubMed] [Google Scholar]
- Stricker S.A. (1999) Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol., 211, 157–176. [DOI] [PubMed] [Google Scholar]
- Talmor A., Kinsey,W.H. and Shalgi,R. (1998) Expression and immunolocalization of p59c-fyn tyrosine kinase in rat eggs. Dev. Biol., 194, 38–46. [DOI] [PubMed] [Google Scholar]
- Tan J.C., Nocka,K., Ray,P., Traktman,P. and Besmer,P. (1990) The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science, 247, 209–212. [DOI] [PubMed] [Google Scholar]
- Thomas S.M. and Brugge,J.S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol., 13, 513–609. [DOI] [PubMed] [Google Scholar]
- Thommes K., Lennartsson,J., Carlberg,M. and Ronnstrand,L. (1999) Identification of Tyr-703 and Tyr-936 as the primary association sites for Grb2 and Grb7 in the c-Kit/stem cell factor receptor. Biochem. J., 341, 211–216. [PMC free article] [PubMed] [Google Scholar]
- Xu W., Harrison,S.C. and Eck,M.J. (1997) Three-dimensional structure of the tyrosine kinase c-Src. Nature, 385, 595–602. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. (1994) Mammalian fertilization. In Knobil,E. and Neill,J.D. (eds), The Physiology of Reproduction. Vol. 1. Raven Press, New York, NY, pp. 189–317.
- Young M.A., Gonfloni,S., Superti-Furga,G., Roux,B. and Kuriyan,J. (2001) Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell, 105, 115–126. [DOI] [PubMed] [Google Scholar]