Abstract
TPX2, a microtubule-associated protein, is required downstream of Ran-GTP to induce spindle assembly. TPX2 activity appears to be tightly regulated during the cell cycle, and we report here one molecular mechanism for this regulation. We found that TPX2 protein levels are cell cycle regulated, peaking in mitosis and declining sharply during mitotic exit. TPX2 is degraded in mitotic extracts, as well as in HeLa cells exiting from mitosis. This instability depends, both in vitro and in vivo, on the anaphase-promoting complex/cyclosome (APC/C), a ubiquitin ligase that controls mitotic progression. In a reconstituted system, TPX2 is efficiently ubiquitinated by APC/C that has been activated by Cdh1. Two discrete elements in TPX2 are required for recognition by APC/CCdh1: a KEN box and a novel element in amino acids 1 to 86. Interestingly, the latter element, which has no known APC/C recognition motifs, is required for the ubiquitination of TPX2 by APC/CCdh1 in vitro and for its degradation in vivo. We conclude that APC/CCdh1 controls the stability of TPX2, thereby ensuring accurate regulation of the spindle assembly in the cell cycle.
The ubiquitin-proteasome pathway in eukaryotic cells plays essential roles in the control of diverse cellular processes, such as cell cycle regulation, cell signaling, apoptosis, transcription, and development (14, 16, 17, 28, 29). In this system, ubiquitin chains are attached to target proteins in an ATP-dependent manner, thus marking them for degradation by the proteasome. Ubiquitination of a target protein requires three enzymes, a ubiquitin-activating enzyme (E1), a conjugating enzyme (E2), and a ubiquitin ligase (E3). In this cascade of enzymatic reaction, ubiquitin is first activated by E1 via a thioester linkage; the activated ubiquitin is then transferred to E2 and finally to a substrate through the action of a substrate-specific E3. The polyubiquitinated substrate is then degraded by the proteasome (17). This irreversible process of protein destruction has the defining characteristic of the switch-like control for cellular transitions, which is essential for many biological processes, such as cell cycle regulation.
The anaphase-promoting complex/cyclosome (APC/C), a multisubunit E3 ubiquitin ligase essential for mitotic progression, was originally identified as a ubiqutin ligase for cyclin B (14, 15, 29, 30). Subsequent studies showed that APC/C is not only active at anaphase, where it ubiquitinates cyclin B, but its activity persists until the end of G1, targeting a large group of cell cycle regulators for degradation (2, 8, 20). Cell cycle-specific activation of APC/C can be attributed to mitosis-specific phosphorylation of APC/C subunits (24) and to the binding of Fizzy family proteins, Cdc20 and Cdh1 (8). Genetic and biochemical studies indicate that Cdc20 and Cdh1 are essential regulators of APC/C activity through their direct binding to and activation of APC/C. Cdc20 activates APC/C at the onset of anaphase to trigger chromosome separation, whereas Cdh1 replaces Cdc20 from APC/C at late anaphase and Cdh1 remains associated with APC/C until late G1 (7, 8, 14, 36).
APC/C controls mitosis and G1 by targeting an array of substrates for destruction at different cell cycle stages (14). Examples of APC/C substrates include Securin, Cdc20, Aurora A, Plk1, Kid, and Geminin (29, 30). APC/C recognizes either a KEN box (K-E-N) or a destruction box (D box, R-X-X-L, where X is any amino acid) in substrates (10, 31, 32). The D box is recognized by either APC/CCdc20 or APC/CCdh1, whereas KEN box-containing substrates appear to be ubiquitinated only by APC/CCdh1. In addition, a unique sequence in Aurora A, termed the A box (RXLXPSN), is also required for its efficient ubiquitination by APC/CCdh1 (26). Although it is not clear whether the A box sequence in Aurora A is directly recognized by APC/CCdh1, this sequence is required to activate the silent D box in the protein (26).
Proper segregation of chromosomes in the cell cycle requires the efficient formation of a bipolar microtubule spindle. It has recently been reported that the small GTPase Ran controls the spindle assembly in Xenopus egg extracts, since addition of Ran-GTP to extracts was sufficient to stimulate spindle assembly (4, 21, 37). The chromosome-localized Ran guanine nucleotide exchange factor, RCC1, provides a gradient of Ran-GTP around mitotic chromosomes (22). A key target of the Ran-GTP pathway for spindle assembly is TPX2, a microtubule-associated protein that promotes spindle assembly. TPX2 is normally sequestered through its association with importin β in the absence of Ran-GTP. During mitosis, Ran-GTP, generated by RCC1 around chromosomes, disrupts the TPX2-importin β complex, releasing active TPX2 to initiate bipolar spindle assembly (12, 35). Similarly, human TPX2 also plays a role in spindle formation and in spindle pole organization, since knockdown of TPX2 by RNA interference blocks spindle formation and generates multiple spindle poles (9, 13). A downstream target of TPX2 is a protein kinase, Aurora A, which is recruited to the spindle poles during mitosis in a TPX2-dependent manner (25). Ran-GTP-dependent activation of Aurora A requires TPX2 in Xenopus egg extracts (35). In fact, the first 43 amino acids from the N terminus of TPX2 directly bind to and activate Aurora A kinase (1, 5, 6, 11). Thus, TPX2, through activation of Aurora A, is an essential regulator of Ran-GTP-mediated spindle assembly.
The activity of TPX2 is confined to mitosis. We demonstrate here that one mechanism for this regulation is through control of its protein stability. We observed that TPX2 is an unstable protein in mitotic extracts and that TPX2 is a substrate for the APC/CCdh1 ubiquitin ligase, both in vitro and in vivo. Surprisingly, ubiquitination of TPX2 by APC/CCdh1 requires both a KEN box and amino acids 1 to 86, which contain no previously characterized APC/C recognition motifs. Regulation of TPX2 stability by APC/C is a physiological mechanism for control of spindle assembly in the cell cycle, as we observed that overexpression of a stable TPX2 variant causes an abnormal spindle and leads to prometaphase arrest, consistent with a previous report (13).
MATERIALS AND METHODS
Plasmids, antibodies, and reagents.
Human TPX2 cDNA was amplified by PCR using a clone from Open Biosystems as a template and subcloned into the pCS2-FA, pCS2-HA-FA, and pCS2-eGFP-FA vectors. Deletions and point mutations of TPX2 were constructed by PCR and/or by digestions with restriction enzymes. To construct a dominant-negative mutant of Cdh1 (Cdh1N), DNA encoding amino acids 1 to 125 of human Cdh1 was amplified by PCR and subcloned into pCS2 Flag-FA. pSuper and pSuper-Cdh1b were kindly provided by R. Agami (Netherlands Cancer Institute) (3).
Mouse TPX2 antibody was a gift from H. J. Heidebrecht (University of Kiel, Kiel, Germany), and rabbit TPX2 antibody was kindly provided by D. Compton (Dartmouth University). Cdc20, APC2, Cdc27, and rabbit Cdh1 antibodies have been described previously (8). p38 MAPK, cyclin A, and cyclin B antibodies were purchased from Santa Cruz Biotechnology; mouse Cdh1 antibody was from Neomarkers; phosphohistone H3 antibody was from Upstate Biotechnology; and hemagglutinin (HA) antibody was from Covance.
Cell culture, cell synchronization, and transfection.
HeLa S3 and HeLa cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Invitrogen) and antibiotics. The cells were synchronized at either the G1/S boundary by a double-thymidine treatment or at prometaphase by a thymidine-nocodazole treatment (8). Transfections were performed using Effectene as instructed by the manufacturer (QIAGEN). To determine the stability of wild-type and mutant TPX2 in vivo, transfected cells were arrested at prometaphase by a thymidine-nocodazole treatment, followed by release into fresh medium containing 10 μg/ml cycloheximide. The cells were harvested by incubation with trypsin, lysed, and subjected to Western blot analysis.
Recombinant protein expression and purification.
Human Cdc20 and Cdh1 were expressed and purified from baculovirus-infected Sf9 cells as described previously (8). Human UbcX and wheat E1 were expressed in Escherichia coli and purified by nickel affinity chromatography (QIAGEN) and by ubiquitin affinity chromatography, respectively. Human Emi1 was expressed in E. coli and purified by nickel affinity chromatography. Ubiquitin aldehyde was purchased from Boston Biochemicals, and ubiquitin was purchased from Sigma.
In vitro degradation assay.
HeLa S3 cells were treated either with 2 mM hydroxyurea or with thymidine-nocodazole, followed by a 1-hour release into fresh medium. Cells were harvested and then rinsed twice in ice-cold phosphate-buffered saline and once in hypotonic lysis buffer (20 mM HEPES-KOH, pH 7.6, 5 mM KCl, 1.5 mM MgCl2 containing 1 mM dithiothreitol, a protease inhibitor cocktail [Complete; Roche], 0.5 μM microcystin, and an energy mix). The cell pellet was then resuspended in 1 volume of hypotonic lysis buffer. After swelling on ice for 30 min, the cells were lysed by freezing them in liquid nitrogen and thawing them at 37°C. The cell lysates were then passed through a 21-gauge needle 20 times and centrifuged at maximum speed in a microcentrifuge at 4°C. The clarified supernatant was collected, aliquoted, frozen in liquid nitrogen, and stored at −80°C.
For degradation assays, extracts were thawed at 37°C, immediately transferred to ice, and supplemented with ubiquitin (1.25 mg/ml, final concentration), a protease inhibitor cocktail (Roche), and an energy mix. Nine microliters of the extract was then added to 1 microliter of 35S-labeled substrates synthesized by in vitro translation (Promega). Reactions proceeded at room temperature for the indicated times, and the extent of degradation was determined and quantified by phosphorimaging (Molecular Dynamics). In the Emi1 inhibition assay, extracts were preincubated with 100 μg/ml Emi1 for 2 h on ice prior to addition of the substrate. In the immunodepletion assay, anti-Cdc27 or control antibody beads were added to the extract for 2 h at 4°C. The beads were removed by centrifugation, and the supernatant was used for degradation assays. A portion of the beads and supernatant was processed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis as indicated in the figure legends.
Ubiquitination assays.
Interphase extracts from Xenopus eggs (27) were immunoprecipitated with anti-Cdc27 antibody-protein A beads for 2 h at 4°C to purify APC/C. The APC/C beads were collected by centrifugation and washed five times in XB buffer (10 mM HEPES-KOH, pH 7.8, 100 mM KCl, 1 mM MgCl2, 0.1 mM CaCl2, 50 mM sucrose) containing 500 mM KCl and 0.5% NP-40 and five times in XB buffer. The purified APC/C beads were then incubated with recombinant Cdh1 or Cdc20 for 1 h at 25°C, followed by washing in XB buffer three times. Ubiquitination reactions were initiated by mixing 35S-labeled substrate with E1 (50 μg/ml), E2 (50 μg/ml), ubiquitin (1.25 mg/ml), ubiquitin aldehyde (1 μM), and an energy regeneration mix. Reactions were performed at 25°C and stopped at various times by addition of the SDS sample buffer. Samples from each time point were then analyzed by SDS-PAGE and phosphorimaging (Molecular Dynamics).
Immunoprecipitation, in vitro binding, and Western blotting.
Cells were lysed in the lysis buffer (20 mM HEPES-KOH, pH 7.6, 150 mM KCl, 0.1 mM EDTA, 0.5% NP-40, 10% glycerol containing a protease inhibitor cocktail [Complete; Roche], 0.5 μM microcystin, and 1 mM dithiothreitol) by incubation on ice for 30 min, followed by centrifugation at maximum speed in a microcentrifuge for 30 min at 4°C. Clarified extracts were immunoprecipitated overnight at 4°C with TPX2 or Cdh1 antibody or total rabbit immunoglobulin Gs (IgGs) covalently coupled to Affi-Prep protein A beads (Bio-Rad). Immune complexes were collected by centrifugation, washed three times in the lysis buffer at 4°C, and processed for SDS-PAGE and Western blotting.
For in vitro binding studies, 10 μl of in vitro-translated TPX2 samples were mixed with 200 ng recombinant Cdh1 protein in the presence or absence of 500 ng affinity-purified polyclonal anti-Cdh1 antibody that had been coupled to the protein A beads. Binding was allowed to proceed at room temperature for 1 hour, and then the beads were collected by gentle centrifugation and washed twice in the lysis buffer and twice in XB buffer. The beads were boiled in SDS sample buffer and subjected to SDS-PAGE and phosphorimaging.
For Western blotting, whole-cell extracts or immunoprecipitates in SDS sample buffer were separated by SDS-PAGE; electroblotted to polyvinylidene difluoride membranes; blocked in 10% nonfat dry milk in 10 mM Tris-Cl, pH 7.8, 150 mM NaCl, 0.1% Tween 20; and probed overnight with the indicated antibodies in the blocking buffer. The blots were developed using Enhanced Chemiluminescence Plus (Amersham), followed by scanning with a PhosphorImager (Molecular Dynamics).
Flow cytometry and immunofluorescence staining.
For flow cytometry, cells were fixed 30 h posttransfection in 1% paraformaldehyde in phosphate-buffered saline for 30 min on ice, followed by 70% ethanol fixation and propidium iodide-RNase incubation. Equal numbers of cells were subjected to flow cytometry using asynchronous untransfected HeLa cells to distinguish between green fluorescent protein (GFP)-positive and GFP-negative cells, as well as to standardize the DNA content. For immunofluorescence staining, transfected cells were fixed in methanol, permeabilized with detergent, and incubated with either HA or GFP antibodies, together with β-tubulin antibodies and DAPI (4′,6′-diamidino-2-phenylindole) to visualize DNA. The mitotic index was determined by scoring DAPI-stained mitotic structures in transfected cells.
RESULTS
TPX2 protein levels fluctuate in the cell cycle.
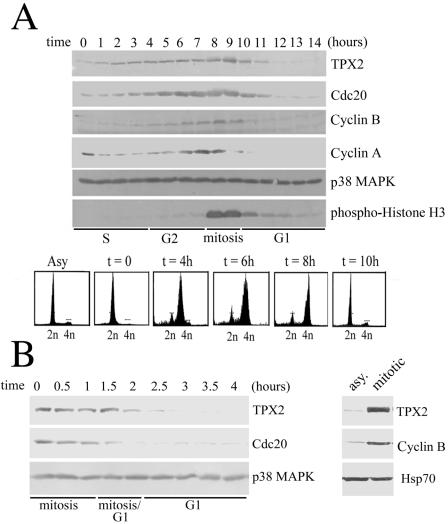
To investigate the regulation of TPX2, we directly examined levels of the TPX2 protein across the cell cycle and compared it to a group of key cell cycle regulators. HeLa S3 cells were arrested at the G1/S boundary by a double-thymidine treatment and then released into fresh medium to allow the cells to progress synchronously from the G1/S boundary to the next G1. The cell cycle profile of released cells was analyzed by flow cytometry, and the mitotic time points were determined by levels of phosphorylated histone H3 (Fig. 1A). Whole-cell extracts were prepared, and protein levels were analyzed by Western blotting. The level of TPX2 was relatively low at the G1/S boundary but increased as cells progressed into S and G2, peaked at mitosis (9 h after release), and then rapidly decreased as cells exited mitosis and entered G1 (Fig. 1A), consistent with a previous report (13). Notably, TPX2 levels mimicked those of cyclin B and Cdc20, two known mitotic substrates of APC/C. In contrast, levels of another APC/C substrate, cyclin A, peaked significantly before TPX2. To further address the kinetics of TPX2 down-regulation during mitotic exit, HeLa cells were synchronized at prometaphase using a thymidine-nocodazole block, followed by release into fresh medium, thus allowing the cells to synchronously exit mitosis and enter G1. We observed that the TPX2 levels rapidly decreased as the cells completed mitosis and that the profile of TPX2 levels again mimicked that of Cdc20 (Fig. 1B, left). Furthermore, like cyclin B, TPX2 levels in asynchronously growing cells were drastically lower than prometaphase cells arrested by nocodazole (Fig. 1B, right). Thus, TPX2 protein levels fluctuate in the cell cycle in a manner similar to that of known substrates of APC/C, such as cyclin B and Cdc20, suggesting that the stability of TPX2 is regulated during mitotic exit.
FIG. 1.
Levels of the TPX2 protein fluctuate in the cell cycle. (A) HeLa S3 cells were arrested at the G1/S boundary by a double-thymidine treatment. Cells were released into fresh medium and harvested every hour. Cell lysates were Western blotted with TPX2, Cdc20, cyclin B, cyclin A, p38 MAPK, and phosphohistone H3 antibodies. The p38 MAPK Western blot served as a loading control. Cell cycle stages were determined by propidium iodide staining and flow cytometry. (B) HeLa S3 cells were arrested at prometaphase through a thymidine-nocodazole treatment. Cells were released into fresh medium and harvested at the indicated times (left). Extracts from asynchronous cells (asy) and prometaphase-arrested cells (mitotic) were also directly compared (right). Cell lysates were Western blotted with TPX2, Cdc20, cyclin B, Hsp70, and p38 MAPK antibodies as indicated. Cell cycle stages were determined by propidium iodide staining and flow cytometry, as well as by DAPI staining and microscopy (data not shown).
TPX2 is an unstable protein in mitotic extracts.
We next turned to an in vitro system to directly evaluate the stability of TPX2 during mitotic exit. Concentrated hypotonic extracts were prepared from HeLa S3 cells that were synchronized either at S phase by hydroxyurea treatment or at anaphase-telophase by a thymidine-nocodazole arrest, followed by a 1-hour release into fresh medium. We found that TPX2 was stable in S-phase extracts, with a half-life of greater than 100 min (Fig. 2A). In contrast, TPX2 is rapidly degraded in mitotic extracts, with a half-life of 10 min (Fig. 2A, short exposure). Furthermore, we reproducibly detected a high-molecular-weight smear of TPX2 in mitotic extracts (Fig. 2A, long exposure). Such a high-molecular-weight smear is usually correlated with protein ubiquitination.
FIG. 2.
TPX2 is unstable in mitotic extracts and is ubiquitinated by APC/C in vitro. (A) HeLa S3 cells were synchronized in S phase with hydroxyurea or at late anaphase/telophase with a thymidine-nocodazole block, followed by a 1-hour release in fresh medium. The cells were lysed in a hypotonic buffer, and the stability of the 35S-labeled TPX2 was analyzed in the two extracts. TPX2 remaining at each time point was quantified, and half-lives (t1/2) were determined. (B) TPX2 is a substrate of APC/CCdh1 in vitro. APC/C was immunopurified from Xenopus interphase extracts and activated with recombinant Cdh1. APC/C-dependent ubiquitination of 35S-labeled TPX2 was analyzed in the presence of E1, E2, ubiquitin (Ub), ubiquitin-aldehyde, and an energy mix. (C) Comparison of APC/CCdh1 and APC/CCdc20 activities toward TPX2. APC/C was immunopurified from Xenopus interphase extracts and activated with recombinant Cdh1 or Cdc20. APC/C-dependent ubiquitination of 35S-labeled TPX2 or Plk-1 was analyzed in the presence of E1, E2, ubiquitin, ubiquitin-aldehyde, and an energy mix. Plk-1, a physiological target of APC/C Cdh1, was included as a substrate under identical experimental conditions for comparative purposes.
TPX2 is a substrate of APC/CCdh1 in vitro.
We investigated whether TPX2 is degraded through a ubiquitin-dependent pathway. Given that APC/C is a key ubiquitin ligase functioning in mitosis, we reasoned that the instability of TPX2 in mitotic extracts could result from its degradation by the APC/C pathway. To directly analyze the role of APC/C in the stability of TPX2, we reconstituted an APC/C-mediated ubiquitination assay in vitro (8) and directly tested the ability of TPX2 to serve as a substrate for APC/C. Inactive Xenopus APC/C was first immunoprecipitated from interphase egg extracts under stringent wash conditions using an antibody to the Cdc27 subunit of APC/C. Recombinant Cdh1 was then added to activate APC/C, and the ubiquitination assay was initiated by incubating the active APC/CCdh1 with E1, E2 (UbcX), ubiquitin, ATP, and 35S-labeled TPX2 substrates. TPX2 was efficiently ubiquitinated by APC/CCdh1, as indicated by the formation of high-molecular-weight TPX2-ubiquitin conjugates that appeared as a smear (Fig. 2B). We also tested the other form of active APC/C, APC/CCdc20, in the same reconstituted ubiquitination assay. Under the conditions used, APC/CCdc20 ubiquitinated TPX2, though weakly compared to APC/CCdh1 (Fig. 2C). Similarly, the Xenopus homolog of TPX2, xTPX2, was also efficiently ubiquitinated by APC/CCdh1, but weakly by APC/CCdc20 (data not shown). Notably, the differential ubiquitination of TPX2 by APC/CCdh1 versus APC/CCdc20 is very similar to that of Plk-1, a bona fide APC/CCdh1 substrate (Fig. 2C). These data suggest that TPX2 is a specific substrate of APC/CCdh1.
TPX2 and Cdh1 form a complex in vivo during mitosis.
We next determined whether the endogenous TPX2 is a bona fide APC/CCdh1 target in vivo. We first tested whether endogenous TPX2 interacts with Cdh1. HeLa cells were arrested at prometaphase by a thymidine-nocodazole block and then released for 2 h into fresh medium. The association between TPX2 and APC/C was analyzed by immunoprecipitation from cell lysates with control rabbit IgG, Cdc20, or Cdh1 antibody, followed by Western blotting with a TPX2 antibody. The anti-Cdc20 and Cdh1 antibodies were generated and affinity purified against their unique N-terminal 200 amino acids, not the WD-40 repeats, of the respective proteins. The specificities of the antibodies were confirmed in Cdc20 and Cdh1 knockdown experiments, which demonstrated no cross-reactivity against each other (data not shown). We observed that, although Cdc20 and Cdh1 precipitated similar amounts of APC/C, only Cdh1 was found specifically associated with TPX2 (Fig. 3A). We also reproducibly detected a specific association between Cdc20 and Cdh1 under these experimental conditions, consistent with the fact that Cdc20 is a substrate of APC/CCdh1. To examine the cell cycle specificity of the TPX2-Cdh1 association, we analyzed cells collected at various time points after release from the thymidine-nocodazole block. Interestingly, we observed a peak of TPX2-Cdh1 complex at 30 to 90 min postrelease, a time window during which the cells undergo anaphase and cytokinesis (Fig. 3B). These results indicate that TPX2 and Cdh1 are specifically associated as cells exit from mitosis. We noticed that levels of both TPX2 and Cdh1 are declining as cells exit from mitosis and into G1. Therefore, the inability to detect association at later time points may partially reflect this decline in protein levels. In contrast, however, we did not detect any association between Cdc20 and TPX2 during mitotic exit (Fig. 3A and data not shown), indicating that TPX2 is specifically recognized by Cdh1, not by Cdc20.
FIG. 3.

Cell-cycle-dependent association of TPX2 and Cdh1 (A) Cdh1, not Cdc20, associates with TPX2. Extracts were prepared from cells after a 2-hour release from a thymidine-nocodazole block and subsequently immunoprecipitated with Cdc20, Cdh1 antibodies, or control rabbit IgG. Immune complexes were then analyzed by Western blotting with the indicated antibodies. APC2 is a subunit of APC/C. (B) Cdh1 and TPX2 associate in late mitosis. HeLa S3 cells were synchronized by a thymidine-nocodazole block and released for the times indicated. Cdh1 and TPX2 were immunoprecipitated (IP) with respective antibodies, and the immune complexes were analyzed by Western blotting with the indicated antibodies. Cell cycle stages were determined by propidium iodide staining and flow cytometry. Total levels of TPX2 in these extracts are shown in Fig. 1B.
APC/C and Cdh1 are required for TPX2 degradation in vitro and in vivo.
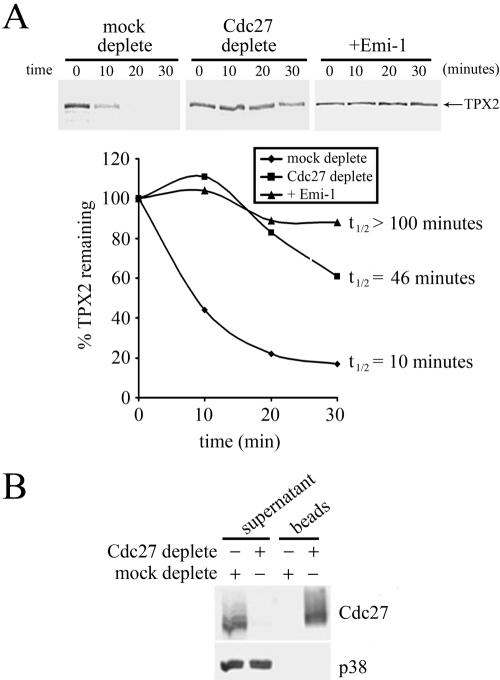
We directly examined whether APC/C is required for TPX2 degradation in mitotic extracts by depleting APC/C from extracts with an antibody against Cdc27, a subunit of APC/C, prior to the TPX2 degradation assay. We observed that TPX2 was greatly stabilized in extracts depleted with the Cdc27 antibody, but not in mock-depleted (control rabbit IgG) extracts (Fig. 4A, top). Depletion of Cdc27 resulted in a >4-fold increase in the half-life of TPX2 (Fig. 4A, bottom). Furthermore, we failed to observe any high-molecular-weight TPX2-ubiquitin conjugates in extracts depleted of APC/C (data not shown). Western blot analysis indicated that treatment of extracts with the Cdc27 antibody removes greater than 95% of Cdc27 from extracts (Fig. 4B). Since Cdc27 is a core subunit of APC/C, we infer that our Cdc27 antibody depletes the majority of APC/C activity from these extracts under our experimental conditions. Thus, APC/C is required for the ubiquitination and degradation of TPX2 in mitotic extracts. This conclusion was independently confirmed by the use of a specific inhibitor of APC/C, Emi1, which binds to and inhibits both Cdc20 and Cdh1 (19, 33, 34). When mitotic extracts were preincubated with recombinant Emi1, the ubiquitination and degradation of TPX2 in such extracts were prevented (Fig. 4A, right). Indeed, the half-life of TPX2 in Emi1-treated extracts was increased by more than 10-fold (see Fig. 6A, bottom), indicating that the APC/C activity is indeed necessary for the instability of TPX2.
FIG. 4.
Degradation of TPX2 in mitotic extracts is dependent on APC/C. (A) Mitotic extracts were prepared from cells harvested 1 hour post-thymidine-nocodazole release. Extracts were first incubated with control or α-Cdc27 antibodies or with 100 μg/ml recombinant Emi1 and then assayed for degradation of TPX2 (top). The extent of degradation was quantified as in Fig. 2 (bottom). (B) Western blot demonstrating the extent and specificity of depletion of Cdc27. “Supernatant” contains 5 μl of depleted or mock-depleted extracts, and “beads” contain immune complexes from 5 μl of extracts. The Cdc27 Western blot shows efficient removal of Cdc27 from extracts by the Cdc27 antibody. The p38 MAPK Western blot illustrates that p38 MAPK was not depleted by either antibody.
FIG. 6.
Recognition of TPX2 by APC/CCdh1. (A) Schematic representation of wild-type TPX2 and summary of ubiquitination assays and half-life (t1/2) measurement for various TPX2 mutants. Reactions were performed as described in the legend to Fig. 2. The extent of ubiquitination was semiquantified by assigning a value of either “+++” (full activity), “++” (moderate activity), “+” (detectable activity), or “−” (no detectable activity), examples of which are provided in panel B. 1-704 K12 is a TPX2 mutant lacking the KEN box and all three D boxes. Half-lives were determined as in Fig. 2 and are indicated in minutes. nd, not determined. (B) The amino-terminal region of TPX2 is recognized by APC/CCdh1. TPX2-N (amino acids 1 to 324), TPX2-C (amino acids 325 to 747), and TPX2 1-86 were analyzed in the reconstituted ubiquitination reaction described in the legend to Fig. 2B. (C) Binding of Cdh1 to TPX2 and TPX2-N. TPX2, TPX2-N, and TPX2-C were in vitro translated and then incubated with or without recombinant Cdh1. The Cdh1 complexes were then immunoprecipitated by anti-Cdh1 antibodies, washed, and analyzed by SDS-PAGE. The input lane contained 2% of the in vitro-translated sample used in each binding reaction. TPX2-C failed to specifically associate with Cdh1 above nonspecific background. (D) Stabilities of TPX2 mutants in mitotic extracts. The stabilities of the deletion and point mutants of TPX2 were analyzed in mitotic extracts as described in the legend to Fig. 2A. Ub, ubiquitin.
The amino-terminal region of Cdh1 (Cdh1N, amino acids 1 to 125) can act as a dominant-negative mutant when expressed in mammalian cells, leading to stabilization of APC/CCdh1 substrates through competitive binding of the substrates (23). We utilized this mutant to demonstrate that TPX2 is degraded by the APC/CCdh1 pathway in vivo. Flag-Cdh1N was transfected with HA-TPX2, followed by arresting cells at prometaphase with a thymidine-nocodazole block. When cells were released from prometaphase arrest into fresh medium in the presence of cycloheximide, an inhibitor of protein synthesis, we found that the low level of HA-TPX2 expressed was greatly stabilized by Flag-Cdh1N cotransfection (Fig. 5A), confirming that Cdh1 controls the stability of TPX2. This stabilization of the TPX2 protein is not due to a mitotic arrest as a result of expression of Flag-Cdh1N, as the majority of cells at 6 h postrelease were in G1, as determined by DAPI staining of chromosomes (data not shown).
FIG. 5.
The stability of TPX2 is under the control of the APC/C pathway in vivo. (A) Stabilization of TPX2 and TPX2 KEN by dominant-negative Cdh1. HeLa cells were transfected with expression vectors for HA-TPX2 or HA-TPX2 KEN in the presence or absence of Flag-Cdh1N. Cells were synchronized by a thymidine-nocodazole block and released for the times indicated. Total cell lysates were analyzed by Western blotting. HA-tagged TPX2 proteins were detected with an HA antibody, and Cdh1N was detected with a Flag antibody. The p38 MAPK Western blot served as a loading control. At T = 0, all cells were arrested at prometaphase, and at T = 6 h, all cells had progressed into G1 with similar kinetics (data not shown). (B) Knockdown of Cdh1 stabilizes TPX2. Expression of Cdh1 was blocked by transfection of pSuper-Cdh1b into HeLa cells. Levels of TPX2, Cdh1, cyclin A, and p38 MAPK were analyzed 60 h posttransfection by Western blotting.
To independently confirm that TPX2 is targeted for APC/CCdh1-dependent ubiquitination in vivo, we reduced the levels of Cdh1 in HeLa cells by RNA interference. Reduction of Cdh1 resulted in elevated levels of TPX2, as determined by Western blotting (Fig. 5B). Upon partial knockdown of Cdh1, both cyclin A levels (Fig. 5B) and the mitotic index (data not shown) were normal under our experimental conditions, indicating that partial reduction of Cdh1 did not change the cell cycle profile. Furthermore, TPX2 was stable in Cdh1 knockdown cells released from a prometaphase arrest, even though the knockdown cells exited mitosis and entered G1 with normal kinetics (data not shown). Thus, stabilization of TPX2 in knockdown cells is not a secondary effect caused by a change in the distribution of cells across cell cycle stages. We conclude that TPX2 is under the control of the APC/CCdh1 pathway in vivo.
Recognition of TPX2 by APC/CCdh1.
We next determined which region(s) of TPX2 is required for ubiquitination by APC/CCdh1 in vitro. It has been established that APC/C recognizes a KEN-box (K-E-N) motif and/or a D-box (R-X-X-L) motif in substrates, since mutations in these motifs in a substrate render them resistant to ubiquitination by APC/C (31, 32). Sequence analysis revealed that TPX2 contains one KEN box starting at amino acid 87 and three D boxes (DB1, DB2, and DB3) at positions 119, 341, and 708 (Fig. 6A).
To determine the region of TPX2 recognized by APC/C, we generated N-terminal (TPX2-N; amino acids 1 to 324) and C-terminal (TPX2-C; amino acids 325 to 747) fragments of TPX2. In an in vitro ubiquitination assay, TPX2-N was ubiquitinated by APC/CCdh1 as efficiently as the full-length protein (Fig. 6B, left; cf. Fig. 2B). In contrast, TPX2-C was not ubiquitinated to any appreciable degree (Fig. 6B, middle). Thus, APC/C recognizes the N-terminal half of TPX2, possibly the KEN box, and the first D box. Furthermore, both TPX2 and TPX2-N were capable of directly binding to purified Cdh1 protein (Fig. 6C, top and middle). In contrast, no specific binding between TPX2-C and Cdh1 above the nonspecific background was detectable under identical conditions (Fig. 6C, bottom). Therefore, we conclude that Cdh1 directly recognizes TPX2-N.
To map the exact sequence of recognition, we generated point mutations and truncations in the regions of the KEN box and the first D box and tested the stability of these TPX2 mutants in mitotic extracts. Deletion of both the KEN box and DB1 (TPX2 123-747) rendered TPX2 stable and unable to be ubiquitinated by APC/CCdh1 (Fig. 6A and D). Although mutation of DB1 (TPX2 DB1; RXXL to AXXA) had no effect on TPX2 stability, mutation of the KEN box (TPX2 KEN; KEN to AAA) partially stabilized the protein with a twofold increase in half-life (Fig. 6D), suggesting that the KEN box is recognized by APC/C. Mutations of both the KEN box and DB1 (TPX2 KEN-DB1) did not further stabilize the mutant protein compared with TPX2 KEN (Fig. 6A), indicating that DB1 does not contribute significantly to recognition. Surprisingly, TPX2 KEN, TPX2 KEN-DB1, and TPX2 1-704 KEN, DB1, DB2 (labeled 1-704K12 in Fig. 6A) were all significantly ubiquitinated by APC/CCdh1 in the in vitro ubiquitination assay (Fig. 6A and data not shown), even though TPX2 1-704K12 contains neither a KEN box nor a D box. Thus, a sequence(s) other than the KEN box and the D box is also recognized by APC/CCdh1. Consistent with this prediction, deletion of the first 83 amino acids (TPX2 84- 747) also gave rise to a stable form of TPX2 in mitotic extracts, even though this truncated version of TPX2 still contained an intact KEN box (Fig. 6D). This observation suggests that the first 83 amino acids in TPX2 participate in recognition by APC/CCdh1 and that the KEN box is not sufficient for APC/CCdh1 recognition. In agreement with this, we found that amino acids 1 to 86 of TPX2 were ubiquitinated by APC/C in a Cdh1-dependent manner (Fig. 6B). It is worth noting that, when TPX2 1-86 was used as a substrate, primarily lower-molecular-weight conjugates were formed, likely corresponding to mono-, di-, and triubiquitin conjugates. However, TPX2 1-86 was stable in mitotic extracts (Fig. 6A), consistent with its inability to form high-molecular-weight ubiquitin conjugates, a prerequisite for degradation by the proteasome. Given that there are 8 lysine residues in the first 86 amino acids of TPX2, the lack of polyubiquitination is unlikely to be due to a lack of lysine receptors for ubiquitin, although we cannot exclude the possibility that the spatial placement of these 8 lysine residues may be suboptimal for ubiquitin conjugation.
Extensive efforts, through both terminal truncations and internal deletions on the wild-type protein, as well as on a KEN-box mutant, to further delineate this novel APC/CCdh1 recognition element in the first 86 amino acids of TPX2 have not been conclusive (A. Seki, S. Stewart, and G. Fang, unpublished results). It therefore appears likely that APC/CCdh1 recognizes a complex element or a folded structural domain in the N-terminal region of TPX2, rather than a small stretch of several amino acids. Additionally, TPX2 1-86, when fused to either the N or C terminus, could not confer APC/C-dependent instability on a generic protein, such as GFP or glutathione S-transferase (data not shown). Thus, amino acids 1 to 86 have to act together with the KEN box for efficient ubiquitination of the full-length TPX2.
In summary, TPX2 contains two elements that are recognized by APC/CCdh1: the KEN box and a novel element in amino acids 1 to 86. The N-terminal region of TPX2 seems to play as important a role as the KEN box in recognition by APC/C, since deletion of this sequence increases the half-life of the protein (TPX2 84-747) by 4.6-fold, while mutating the KEN box only stabilizes the protein with a 2-fold increase in the half-life (Fig. 6A and D).
Stabilities of TPX2 mutants in vivo.
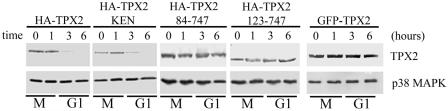
We analyzed the stabilities of various TPX2 proteins in a more physiological setting in vivo. HeLa cells were transiently transfected with expression vectors encoding various HA-tagged TPX2 mutants. We measured the half-lives of ectopically expressed proteins by arresting cells at prometaphase and then releasing them into fresh medium containing cycloheximide, an inhibitor of protein synthesis. Cells were collected at various times during their exit from mitosis into G1, during which the APC/CCdh1 became active. The levels of mutant proteins were determined by Western blot analyses. As expected, ectopically expressed wild-type HA-TPX2 was unstable and degraded in late mitosis/early G1 (Fig. 7). Interestingly, HA-TPX2 KEN was only partially stabilized during mitosis and G1 (Fig. 7). To determine whether degradation of HA-TPX2 KEN is mediated by APC/CCdh1 during mitotic exit, Flag-Cdh1N was transfected with HA-TPX2 KEN, followed by arresting cells at prometaphase with a thymidine-nocodazole block (Fig. 5A). When cells were released from prometaphase arrest into fresh medium in the presence of cycloheximide, we observed a stabilization of the TPX2 KEN mutant in the presence of Flag-Cdh1N, similar to that observed for wild-type HA-TPX2 (Fig. 5A). Given that HA-TPX2 KEN is efficiently degraded in the absence of Flag-Cdh1N (Fig. 5A), the stabilization of this mutant protein by Flag-Cdh1N suggests that a region(s) of TPX2 remains sensitive to APC/CCdh1 even in the absence of the KEN box. Based on the ubiquitination and degradation data in Fig. 6, this region is likely to reside in amino acids 1 to 83.
FIG. 7.
Stability of wild type and mutants of TPX2 in mitosis in vivo. HeLa cells were transiently transfected with the indicated HA-tagged TPX2 expression vector (0.2 μg/10-cm dish). Transfected cells were arrested by a thymidine-nocodazole block and then released into fresh medium containing 10 μg/ml cycloheximide. Cells were harvested at the times indicated, and the half-lives of expressed proteins were determined by Western blot analysis. The HA-tagged TPX2 proteins were detected with HA antibody, and GFP-TPX2 was detected with TPX2 antibody. The p38 MAPK Western blot served as a loading control. Cell cycle stages were determined by DAPI staining and microscopy. Low expression levels of TPX2 and TPX2 mutants in this experiment did not alter mitotic exit and progression into G1.
This conclusion is confirmed by analyzing the two deletion mutants HA-TPX2 84-747 and HA-TPX2 123-747, both of which remained stable even after cells had been in G1 for several hours (Fig. 7). Consistent with the importance of the N-terminal region of TPX2 in recognition by APC/CCdh1, fusion of GFP to the N terminus of TPX2 also stabilized the fusion protein (Fig. 7). Taken together, these results are consistent with our observations in the reconstituted ubiquitination assay in vitro and in the degradation assay in mitotic extracts (Fig. 6). We noted that a high level of expression of the nondegradable variants of TPX2, such as GFP-TPX2, led to a prometaphase arrest (see below). Therefore, we kept the levels of TPX2 expression in the current experiment very low by using limited amounts of DNA and found that a majority of cells transfected with different constructs exit from mitosis with similar kinetics (data not shown).
To rule out the possibility that the lack of ubiquitination/degradation of TPX2 mutants results from misfolding of the proteins, we compared the localization of GFP-TPX2, GFP-TPX2 KEN, GFP-TPX2 84-747, and GFP-TPX2 123-747 in transfected cells. We found that they all shared identical localization throughout the cell cycle (data not shown). Specifically, each was localized to the spindle at prometaphase and metaphase and then was found at the central spindle during anaphase and cytokinesis. This agrees well with published reports for both GFP-TPX2 and endogenous TPX2 (9, 13). Therefore, misfolding or mislocalization of TPX2 mutants is unlikely to be responsible for the observed stabilization of the TPX2 mutants.
Expression of a nondegradable variant of TPX2 results in accumulation of mitotic cells with monopolar spindles.
Fusion of green fluorescent protein to the N terminus of TPX2 stabilized the protein in mitotic extracts (Fig. 6A). Furthermore, GFP-TPX2 remained stable in vivo in cells exiting mitosis and entering G1, in contrast to HA-TPX2, which was degraded by an APC/CCdh1-dependent pathway (Fig. 7). These properties define GFP-TPX2 as a nondegradable variant of TPX2. We used GFP-TPX2, as opposed to stable TPX2 deletion mutants, to determine the physiological significance of degradation of TPX2 by APC/CCdh1 for the following reasons. First, GFP-TPX2 displays intracellular localization identical to that of endogenous TPX2 (13) (data not shown). Second, GFP-TPX2 can complement the function of xTPX2 in the Ran-GTP-mediated spindle assembly assay in Xenopus egg extracts (13), indicating that the stabilized GFP-TPX2 still maintains the full activity of the wild-type protein. Third, other stable mutants of TPX2, namely, TPX2 84-747 and TPX2 123-747, lack the Aurora A binding domain, rendering any phenotype generated by expressing these mutants difficult to interpret.
HeLa cells were transfected either with HA-TPX2 plus GFP or with the nondegradable GFP-TPX2 fusion, subjected to propidium iodide staining, and analyzed by flow cytometry to observe the relative DNA content in GFP-positive and GFP-negative cells (Fig. 8A). GFP-transfected cells and vector-transfected cells were used as controls. We observed a striking accumulation of cells with a 4N DNA content in GFP-positive cells that been transfected with GFP-TPX2 (Fig. 8A, first row). In contrast, the GFP-positive cells from the HA-TPX2 transfection exhibited a fairly normal cell cycle distribution with only a slight increase in the G2/M cells (Fig. 8A, second row). It is worth noting that, in both GFP-TPX2- and HA-TPX2-transfected samples, untransfected GFP-negative cells displayed a typical asynchronous cell cycle distribution.
FIG. 8.
Expression of GFP-TPX2 resulted in accumulation of prometaphase cells with abnormal spindles. (A) HeLa cells were transfected with GFP-TPX2 fusion (first row), with HA-TPX2 plus GFP (nine parts of HA-GFP with one part of GFP; second row), with GFP alone (third row), or with HA control vector (fourth row). The cells were fixed at 30 h posttransfection, stained with propidium iodide, and subjected to flow cytometry. Fluorescence-activated cell sorter profiles of the GFP-positive (right) and GFP-negative (left) cells are shown here. (B) HeLa cells were transfected with GFP-TPX2 or HA-TPX2, and the cells were fixed at 30 h posttransfection and stained with either HA or GFP antibodies, together with DAPI and an anti-β-tubulin antibody. The mitotic index and percentages of monopolar and bipolar cells were quantified in the transfected cells. (C) HeLa cells were transfected with GFP-TPX2, and the cells were fixed at 30 h posttransfection and stained with DAPI and β-tubulin antibodies. GFP-TPX2 is in green, DAPI is in blue, and β-tubulin is in red. Bar = 5 μm.
To determine whether the GFP-TPX2-expressing cells accumulate in G2 or in mitosis, a sample of the transfected cells was also stained with DAPI, together with an anti-HA or anti-GFP antibody, to determine the mitotic index (Fig. 8B). Cells expressing GFP-TPX2 accumulated in mitosis with a mitotic index of 47% (n = 601) compared to 13% (n = 594) for HA-TPX2 (Fig. 8B). Cells transfected with HA vector alone or GFP alone displayed mitotic indices of 3.5% and 3.2%, respectively, comparable to that of nontransfected cells (data not shown). We also stained GFP-TPX2-expressing cells with an anti-β-tubulin antibody. Consistent with a previous report (13), we found that the majority of the GFP-TPX2-transfected mitotic cells are arrested at prometaphase with a monopolar spindle, and only a small fraction of cells proceeded into metaphase with a bipolar spindle (Fig. 8B and C). This is in sharp contrast to GFP-transfected cells, in which no monopolar spindle was observed in 275 GFP-positive mitotic cells analyzed (data not shown). Finally, 5% of GFP-TPX2-expressing cells underwent apoptosis, while no apoptotic cells were observable in HA-TPX2-expressing cells (data not shown). We conclude that expression of nondegradable GFP-TPX2 leads to a prometaphase arrest with a monopolar spindle and to apoptosis. Although the exact cellular mechanism for this phenotype is not clear, it is unlikely to be due to the control of Aurora A localization by GFP-TPX2, as expression of GFP-TPX2 does not affect the localization of Aurora A (data not shown).
Given that the cellular phenotype for the expression of GFP-TPX2 occurred at prometaphase, a cell cycle stage prior to the APC/C-dependent degradation of endogenous TPX2, we speculate that this prometaphase arrest is a direct consequence of the high level of accumulation of the stable GFP-TPX2 protein in the cell cycle, rather than directly caused by the nondegradable nature of the GFP-TPX2 protein. The less severe phenotype in HA-TPX2-expressing cells likely results from a lower expression level of the protein due to its instability. These observations point to the importance of controlling the levels of the TPX2 protein in the cell cycle.
DISCUSSION
TPX2 controls the assembly of a bipolar spindle and hence contributes to the high fidelity of chromosome segregation during mitosis. It is thus expected that the activity of TPX2 would be tightly regulated during the cell cycle. Here, we provide a molecular mechanism that contributes to the exquisite regulation of TPX2 and, therefore, spindle assembly. The fluctuation of TPX2 levels is virtually identical to those of other APC/C substrates, such as cyclin B and Cdc20, peaking in mitosis and abruptly reduced to an almost undetectable level in G1. TPX2 is unstable in anaphase/telophase extracts but stable in S phase and in prometaphase (Fig. 2A and data not shown, respectively), demonstrating that TPX2 degradation activity is precisely regulated in the cell cycle. In a reconstituted ubiquitination assay with purified components, TPX2 directly binds to Cdh1 in vitro and is efficiently ubiquitinated by APC/CCdh1. In addition, endogenous Cdh1, but not Cdc20, associates with TPX2 at anaphase and during cytokinesis, at a time when TPX2 levels are subjected to down-regulation. Inactivation of the APC/C pathway either by expression of a dominant-negative mutant of Cdh1 or by RNA interference leads to stabilization of TPX2 in vivo. We conclude that TPX2 is a substrate of APC/CCdh1 during mitotic exit.
Recognition of TPX2 by APC/CCdh1.
The fact that the TPX2 1-704 K12 mutant, which contains neither a KEN box nor a D box, is still efficiently ubiquitinated in vitro and degraded in mitotic extracts indicates that TPX2 contains a novel element(s) for recognition by APC/C (Fig. 6A). Through deletion analyses, we identified two elements of TPX2 recognized by APC/CCdh1: a KEN box and the N-terminal region covering amino acids 1 to 86 (Fig. 6). Interestingly, this N-terminal fragment contains neither a KEN box nor a D box. Furthermore, in an in vitro ubiquitination assay reconstituted with purified components, this fragment in isolation can be directly ubiquitinated by APC/CCdh1, indicating that APC/CCdh1 recognizes a novel element in this region of the protein. These results underscore the idea that relying only on the presence of known APC/C recognition elements may overlook potential substrates. On the other hand, extensive attempts to further delineate this recognition element have proven unsuccessful, suggesting that APC/CCdh1 may recognize a complex sequence motif or a folded structure, rather than a small stretch of several amino acids.
Although we cannot exclude the possibility that TPX2 1-86 may only provide a required sequence context for the KEN box, for example, by controlling the conformational accessibility of the KEN box, this is unlikely, as in vitro biochemical analysis indicates that TPX2 1-86 is directly recognized and ubiquitinated by APC/CCdh1 (Fig. 6B). Whereas mutation of KEN increases the half-life of the protein (TPX2 KEN) by 2-fold, deletion of the N-terminal 84 amino acids (TPX2 84-747) stabilizes the protein and increases the half-life by 4.6-fold (Fig. 6A and D). These observations point to an important role for the N-terminal fragment in recognition by APC/CCdh1. Although ubiqutination of this fragment itself appears to mainly give rise to only mono-, di-, and triubiquitin conjugates, two lines of evidence suggest that recognition of this fragment by APC/CCdh1 is physiologically significant. First, TPX2 KEN, with mutations in the KEN box, is still efficiently ubiquitinated in vitro and degraded in a Cdh1-dependent manner in vivo (Fig. 5A, 6A, and 7). Second, TPX2 84-747, which lacks the N-terminal recognition sequence but retains the KEN box, is neither ubiquitinated in vitro nor degraded in vivo (Fig. 6A and D and 7). The lack of ubiquitination/degradation of TPX2 84-747 is unlikely to be due to a lack of ubiquitin receptors around the KEN box, since there are 12 lysine residues within 80 amino acids around the KEN sequence in TPX2 84-747. Thus, the N-terminal region of TPX2 is necessary for recognition by APC/CCdh1 in vivo. Given that TPX2 84-747 is correctly localized to the mitotic spindle in HeLa cells (data not shown), the lack of degradation of this mutant is unlikely to be due to a misfolding of the mutant protein. We concluded that the KEN box is not sufficient for recognition by APC/C and that the N-terminal element is an obligatory signal for APC/C-dependent degradation of TPX2. On the other hand, this N-terminal element (amino acids 1 to 86), although sufficient for mono-, di-, and triubiquitination, cannot be degraded in mitotic extracts. Thus, this element has to act together with the KEN box for efficient turnover of TPX2 in vivo.
Physiological function of TPX2 degradation.
What is the physiological significance of degradation of TPX2? TPX2 is a key regulator of the spindle assembly, and therefore, its activity is strictly confined to mitosis. Uncontrolled accumulation and activation of the TPX2 protein in interphase cells could alter the structure and dynamics of the cytoplasmic microtubule network, thereby interfering with the cytoskeletal structure in interphase cells. Thus, down-regulation of TPX2 protein levels through its degradation during mitotic exit could provide one mechanism to ensure that the activity of spindle assembly is restricted to mitosis.
During mitosis, the level of the TPX2 protein needs to be maintained within a tight physiological range in order for spindle structures to assemble and to function properly. Overexpression of nondegradable TPX2, such as GFP-TPX2, causes a drastic accumulation of cells in a prometaphase-like state with an abnormal spindle (Fig. 8), consistent with a previous report that excessive accumulation of TPX2, even the wild-type protein, in prometaphase cells interferes with normal spindle assembly, leading to a defect in chromosome congression and alignment (13). Thus, we speculate that the degradation of TPX2 by the APC/CCdh1 pathway contributes to the tight regulation of the TPX2 protein levels in mitosis.
Endogenous TPX2 is degraded at anaphase and during cytokinesis (Fig. 1B and 3B), at a time when the mitotic spindle undergoes a drastic reorganization in its structure and dynamics (18). Although we cannot directly analyze the effect of expressing nondegradable GFP-TPX2 on cytokinesis due to the arrest of GFP-TPX2-expressing cells at prometaphase, it is tempting to speculate that APC/C-mediated degradation of TPX2 may contribute to this global change in the mitotic-spindle structure at anaphase and during cytokinesis. It is interesting that although the overall level of the TPX2 protein is low in anaphase and telophase cells (Fig. 1B), the residual TPX2 protein remains associated with central-spindle and midbody microtubules in these cells (data not shown) (13), suggesting that degradation of TPX2 by the APC/C pathway is spatially regulated during mitotic exit. Given that the N-terminal region of TPX2 is an obligatory signal for recognition by APC/CCdh1 and that the same region is also involved in binding to and activation of Aurora A (1), association of TPX2 and Aurora A may protect TPX2 from degradation. Indeed, we found that TPX2 in the TPX2-Aurora A complex is resistant to degradation by the APC/C pathway in mitotic extracts (A. Seki and G. Fang, unpublished observation), indicating that the spatial regulation of substrate degradation during mitotic exit may be regulated by the accessibility of TPX2 to APC/CCdh1.
Acknowledgments
We thank Duane Compton (Dartmouth University) and H. J. Heidebrecht (University of Kiel) for providing anti-TPX2 antibodies, R. Agami (Netherlands Cancer Institute) for the gifts of pSuper and pSuper Cdh1b, P. Jackson (Stanford University) for human Emi1 construct, Wei-Meng Zhao and Jim Wong for reagents, A. Seki for deletion analysis of TPX2, and members of the Fang laboratory for valuable discussion.
S.S. was supported by a postdoctoral training grant from the National Cancer Institute (CA09151). This work was supported by a grant from the National Institutes of Health (GM062852) and by a Burroughs-Wellcome Career Award in Biomedical Sciences to G.F.
REFERENCES
- 1.Bayliss, R., T. Sardon, I. Vernos, and E. Conti. 2003. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12:851-862. [DOI] [PubMed] [Google Scholar]
- 2.Brandeis, M., and T. Hunt. 1996. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 15:5280-5289. [PMC free article] [PubMed] [Google Scholar]
- 3.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 4.Carazo-Salas, R. E., G. Guarguaglini, O. J. Gruss, A. Segref, E. Karsenti, and I. W. Mattaj. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400:178-181. [DOI] [PubMed] [Google Scholar]
- 5.Eyers, P. A., E. Erikson, L. G. Chen, and J. L. Maller. 2003. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13:691-697. [DOI] [PubMed] [Google Scholar]
- 6.Eyers, P. A., and J. L. Maller. 2004. Regulation of Xenopus Aurora A activation by TPX2. J. Biol. Chem. 279:9008-9015. [DOI] [PubMed] [Google Scholar]
- 7.Fang, G., H. Yu, and M. W. Kirschner. 1999. Control of mitotic transitions by the anaphase-promoting complex. Phil. Trans. R. Soc. Lond. B 354:1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang, G., H. Yu, and M. W. Kirschner. 1998. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2:163-171. [DOI] [PubMed] [Google Scholar]
- 9.Garrett, S., K. Auer, D. A. Compton, and T. M. Kapoor. 2002. hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr. Biol. 12:2055-2059. [DOI] [PubMed] [Google Scholar]
- 10.Glotzer, M., A. W. Murray, and M. W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349:132-138. [DOI] [PubMed] [Google Scholar]
- 11.Glover, D. M. 2003. Aurora A on the mitotic spindle is activated by the way it holds its partner. Mol. Cell 12:797-799. [DOI] [PubMed] [Google Scholar]
- 12.Gruss, O. J., R. E. Carazo-Salas, C. A. Schatz, G. Guarguaglini, J. Kast, M. Wilm, N. Le Bot, I. Vernos, E. Karsenti, and I. W. Mattaj. 2001. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104:83-93. [DOI] [PubMed] [Google Scholar]
- 13.Gruss, O. J., M. Wittmann, H. Yokoyama, R. Pepperkok, T. Kufer, H. Sillje, E. Karsenti, I. W. Mattaj, and I. Vernos. 2002. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4:871-879. [DOI] [PubMed] [Google Scholar]
- 14.Harper, J. W., J. L. Burton, and M. J. Solomon. 2002. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16:2179-2206. [DOI] [PubMed] [Google Scholar]
- 15.Hershko, A. 1996. Mechanisms and regulation of ubiquitin-mediated cyclin degradation. Adv. Exp. Med. Biol. 389:221-227. [DOI] [PubMed] [Google Scholar]
- 16.Hershko, A. 1997. Roles of ubiquitin-mediated proteolysis in cell cycle control. Curr. Opin. Cell Biol. 9:788-799. [DOI] [PubMed] [Google Scholar]
- 17.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi, T., and F. Uhlmann. 2005. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature 433:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu, J. Y., J. D. Reimann, C. S. Sorensen, J. Lukas, and P. K. Jackson. 2002. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat. Cell Biol. 4:358-366. [DOI] [PubMed] [Google Scholar]
- 20.Huang, J. N., I. Park, E. Ellingson, L. E. Littlepage, and D. Pellman. 2001. Activity of the APC(Cdh1) form of the anaphase-promoting complex persists until S phase and prevents the premature expression of Cdc20p. J. Cell Biol. 154:85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalab, P., R. T. Pu, and M. Dasso. 1999. The Ran GTPase regulates mitotic spindle assembly. Curr. Biol. 9:481-484. [DOI] [PubMed] [Google Scholar]
- 22.Kalab, P., K. Weis, and R. Heald. 2002. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295:2452-2456. [DOI] [PubMed] [Google Scholar]
- 23.Ke, P. Y., and Z. F. Chang. 2004. Mitotic degradation of human thymidine kinase 1 is dependent on the anaphase-promoting complex/cyclosome-CDH1-mediated pathway. Mol. Cell. Biol. 24:514-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraft, C., F. Herzog, C. Gieffers, K. Mechtler, A. Hagting, J. Pines, and J. M. Peters. 2003. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22:6598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kufer, T. A., H. H. Sillje, R. Korner, O. J. Gruss, P. Meraldi, and E. A. Nigg. 2002. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 158:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Littlepage, L. E., and J. V. Ruderman. 2002. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 16:2274-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray, A. W., and M. W. Kirschner. 1989. Cyclin synthesis drives the early embryonic cell cycle. Nature 339:275-280. [DOI] [PubMed] [Google Scholar]
- 28.Pagano, M. 1997. Cell cycle regulation by the ubiquitin pathway. FASEB J. 11:1067-1075. [DOI] [PubMed] [Google Scholar]
- 29.Peters, J. M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9:931-943. [DOI] [PubMed] [Google Scholar]
- 30.Peters, J. M. 1999. Subunits and substrates of the anaphase-promoting complex. Exp. Cell Res. 248:339-349. [DOI] [PubMed] [Google Scholar]
- 31.Pfleger, C. M., and M. W. Kirschner. 2000. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14:655-665. [PMC free article] [PubMed] [Google Scholar]
- 32.Pfleger, C. M., E. Lee, and M. W. Kirschner. 2001. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 15:2396-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimann, J. D., E. Freed, J. Y. Hsu, E. R. Kramer, J. M. Peters, and P. K. Jackson. 2001. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105:645-655. [DOI] [PubMed] [Google Scholar]
- 34.Reimann, J. D., B. E. Gardner, F. Margottin-Goguet, and P. K. Jackson. 2001. Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes Dev. 15:3278-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai, M. Y., C. Wiese, K. Cao, O. Martin, P. Donovan, J. Ruderman, C. Prigent, and Y. Zheng. 2003. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 5:242-248. [DOI] [PubMed] [Google Scholar]
- 36.Visintin, R., S. Prinz, and A. Amon. 1997. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278:460-463. [DOI] [PubMed] [Google Scholar]
- 37.Wilde, A., and Y. Zheng. 1999. Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284:1359-1362. [DOI] [PubMed] [Google Scholar]