Abstract
Resistance to the growth-inhibitory action of retinoic acid (RA), the bioactive derivative of vitamin A, is common in human tumors. One form of RA resistance has been associated with silencing and hypermethylation of the retinoic acid receptor β2 gene (RARβ2), an RA-regulated tumor suppressor gene. The presence of an epigenetically silent RARβ2 correlates with lack of the RA receptor α (RARα). Normally, RARα regulates RARβ2 transcription by mediating dynamic changes of RARβ2 chromatin in the presence and absence of RA. Here we show that interfering with RA signal through RARα (which was achieved by use of a dominant-negative RARα, by downregulation of RARα by RNA interference, and by use of RARα antagonists) induces an exacerbation of the repressed chromatin status of RARβ2 and leads to RARβ2 transcriptional silencing. Further, we demonstrate that RARβ2 silencing causes resistance to the growth-inhibitory effect of RA. Apparently, RARβ2 silencing can also occur in the absence of DNA methylation. Conversely, we demonstrate that restoration of RA signal at a silent RARβ2 through RARα leads to RARβ2 reactivation. This report provides proof of principle that RARβ2 silencing and RA resistance are consequent to an impaired integration of RA signal at RARβ2 chromatin.
Cells of different histotypes seem prone to lose the ability to respond to the growth-inhibitory action of retinoic acid (RA), the potent bioactive derivative of vitamin A. RA regulates fundamental cellular processes, such as growth, differentiation, and apoptosis (7). Previously, we and others showed a correlation between a common form of RA resistance and repressive epigenetic changes (at both the histone and DNA levels) in the RA receptor β2 gene (RARβ2) (5, 33, 34, 40).
RARβ2 is an RA-regulated tumor suppressor gene (19, 26, 32). Detection of aberrant RARβ2 methylation in tumors of different histotypes raised the question of whether this epigenetic change is critical for silencing this tumor suppressor gene. Previously, we proposed that aberrant RARβ2 inactivity might induce repressive epigenetic changes at RARβ2, leading to RARβ2 silencing and RA resistance (33, 34). RARβ2 transcription is normally regulated by dynamic histone changes in the presence and absence of RA (9, 14, 29, 41). Therefore, we hypothesized that the impaired integration of RA signal at RARβ2 can create a state of exacerbated-protracted RARβ2 transcriptional inactivity and attract chromatin-repressive changes, including DNA methylation. The conversion of RARβ2 from a state permissive for transcription into a stable state nonpermissive for transcription would cause biological RA resistance. Our hypothesis hinges on the original supposition of Ng and Bird (28) that chromatin inactivity, the prerequisite for epigenetic silencing of genes on chromosome X (18), could also lead to silencing of genes on other chromosomes. Thus, an aberrant inactive RARβ2 chromatin status would be the prerequisite for RARβ2 epigenetic silencing.
RARβ2 DNA methylation and silencing were shown to be induced by active recruitment of repressor proteins by an oncogenic fusion protein in leukemic cells (13). However, to our knowledge, this oncoprotein has not been demonstrated in epithelial cancer cells and tumors of the breast, prostate, colon, lung, and head and neck, where RARβ2 has also been found silenced (33, 34). In contrast, cancer epithelial cells and tumors appear to have either a low intracellular concentration of RA or a lack or derangement of proteins involved in either RA metabolism and homeostasis or RARβ2 transcriptional regulation. Thus, RA resistance might be the consequence of an exacerbated-protracted RARβ2 transcriptional repression caused by a defective integration of RA signal at RARβ2, which might be induced by genetic, epigenetic, metabolic, and environmental factors capable of shutting off the “communication” between RA and RARβ2 chromatin.
We identified and tested as a possible cause of aberrant RARβ2 inactivity the lack of functional RARα, the upper regulator of RARβ2 transcription. RARα has the role of keeping the chromatin of its direct target genes, such as RARβ2, poised for transcription yet inactive. Upon binding of RA to RARα, the chromatin status of the target genes is converted from inactive into active because of the exchange of corepressor complexes with coactivator complexes, which would rapidly induce histone changes, chromatin remodeling, and transcription activation (14, 29).
In the course of our studies of RA-resistant breast and prostate cancer cell lines, we observed the following: (i) the presence of low or negligible binding of RARα at RARβ2 in RA-resistant breast and prostate cancer epithelial cells carrying RARβ2 nonpermissive alleles (we define as nonpermissive the alleles that cannot be transcriptionally activated by RA and as permissive the alleles that are poised for transcription yet inactive in the absence of RA but capable of transcription in the presence of RA); (ii) the presence of RARβ2 unmethylated (U), permissive alleles in RARα-positive cells, which contain many other methylated (M) genes (20), pointing at RARα as a critical factor that can spare RARβ2 chromatin from falling into a nonpermissive status; and (iii) the presence of a minimal stretch of methylated CpGs in the first RARβ2 exon—corresponding to exon 5 of the RARβ locus (38)—in methylated alleles, suggesting that CpG methylation originates in a specific epicenter from unmethylated yet nonpermissive alleles.
In this study we simulated possible genetic, epigenetic, and metabolic scenarios that could impair the flow of RA signal at RARβ2 chromatin via RARα. Using three different strategies—a dominant-negative RARα lacking the RA-binding domain, downregulation of RARα by RNA interference, and RA antagonists acting specifically at RARα—we induced the conversion of RARβ2 permissive alleles into nonpermissive alleles in RA-sensitive human cells. The RARβ2 nonpermissive alleles developed a significant load of repressive histone tail modifications and failed to recruit RNA polymerase II at the region containing the transcription start site. Only a percentage of nonpermissive alleles developed CpG hypermethylation, thus showing that aberrant hypermethylation is not an absolute requirement for RARβ2 silencing. In this report we also demonstrate that restoring RA signal through RARα at an epigenetically silent RARβ2 is critical to reestablishing a RARβ2 status compatible with transcription. RARβ2 epigenetic silencing has been described as being associated with an RA-resistant phenotype (26, 32). Here we prove that RA resistance is the consequence of RARβ2 epigenetic silencing.
MATERIALS AND METHODS
Cell lines.
Breast and prostate cancer cell lines (ATCC, Manassas, VA) and COS-1 cells (ATCC) were grown using standard protocols.
Drugs.
All-trans-RA, 5-aza-2′-deoxycytidine (5-Aza), a demethylating agent (10), and trichostatin A (TSA), a histone deacetylase inhibitor (42), were all from Sigma (St. Louis, MO). These drugs were dissolved and stored as described previously (34). The RARα antagonist ER50891 was a kind gift of Kouichi Kikuchi, Discovery Research Laboratories, Ibaraki, Japan, and the RARα antagonist RO414253 was a kind gift from Salvatore Toma, National Cancer Institute, Genoa, Italy. Treatments with these drugs were all performed in medium supplemented with 5% charcoal-dextran-stripped serum.
Colony formation assay.
Exponentially growing cells were seeded at 5 × 102 cells/well in six-well plates in triplicate and allowed to attach to the substrate. Cells were left untreated or treated with drugs for 24 h, and then the medium was replaced with drug-free medium and the cells were grown for 14 to 21 days. Colonies were fixed with methanol, stained with Giemsa (Sigma), and counted to establish the colony formation index as described previously (34). The statistical significance was calculated by Student's t test for three independent experiments.
Cell transfections.
The RARα dominant-negative mutant RARα403 was subcloned by PCR into the FLAG-containing pCMV-tag vector (Stratagene, La Jolla, CA) with primers introducing EcoRI and XhoI restriction sites (sense, 5′-TATGAATTCATGGCCAGCAACAGCAGCTC-3′; antisense, 5′-ATACTCGAGGGGATCTCCATCTTCAGCGT-3′), and the empty pCMV-tag vector was transfected in T47D by using Lipofectamine Plus (Invitrogen, Carlsbad, CA). The LNasRARβ2VI vector, which harbors six copies of RARβ2 antisense (asRARβ2) and the control empty vector LNSX (kindly provided by S. Y. Sun, University of Texas M.D. Anderson Cancer Center, Houston, TX) (37) were also transfected in T47D by Lipofectamine Plus. Stable clones were selected and maintained with G418 (Invitrogen) at 0.8 mg/ml. Stable MDA-MB-231 clones overexpressing RARα 1 were obtained by cotransfecting cells with pSG5-hRARα 1 vector (kindly provided by Fausto Andreola, National Cancer Institute, Bethesda, MD) and G418-resistant pcDNA3.1(+) vector (Invitrogen) by use of Lipofectamine Plus. Control cells were cotransfected with the empty vector pSG5 (Promega, Madison, WI) and pcDNA3.1(+). Stably transfected cells, selected with increasing concentrations of G418 (0.5 to 2.5 mg/ml), were tested for expression of exogenous RARα 1 by Western blotting with the C-20 anti-RARα antibody (Santa Cruz Biotechnology).
Retroviral infections.
Supernatants containing either the RARα dominant-negative LXRARα403SN or the empty LXSN retroviral particles (kindly provided by Fausto Andreola, National Cancer Institute, Bethesda, MD) were used to infect T47D cells in the presence of 4 mg/ml Polybrene (Sigma) as described previously (39). Infected cells were selected with 0.8 mg/ml G418. Single clones were isolated after 14 to 21 days and screened for the presence of either the LXSN or the LXRARα403SN construct by reverse transcription-PCR (RT-PCR). Positive clones were maintained in 0.8 mg/ml G418.
RARα RNA interference.
The 19-nucleotide sequence (AGCGCACCAGGAAACCTTC) corresponding to nucleotides 680 to 699 of RARα exon 5 (GenBank accession no. NM_000964) was inserted into pSUPER-retro (OligoEngine, Seattle, WA) according to the manufacturer's instructions. The silencing efficiency of the resulting construct, pSUPER-RARα, was first tested on exogenous RARα by transiently cotransfecting COS-1 cells with pSG5-hRARα1, encoding the human RARα1 and pSUPER-RARα at different ratios. Exogenous RARα levels, normalized to GAPD (glyceraldehyde-3-phosphate dehydrogenase) expression levels, were estimated by Western blotting with the C-20 anti-RARα and anti-GAPD (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies and appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology; Amersham, Piscataway, NJ). Stable transfections with pSUPER-RARα and pSUPER-retro (control) were performed with Lipofectamine Plus. Transfected T47D clones were selected with 2 μg/ml puromycin (Sigma). Clones displaying significant silencing of RARα by real-time RT-PCR and Western blotting were chosen for further analysis.
RARα antagonist experiments.
Cells (5 × 102) were seeded in six-well plates in triplicate in medium containing 5% charcoal-dextran-stripped serum, allowed to attach to the plastic substrate, and treated with ER50891 (10 μM) and RA (0.1 μM), either alone or in combination, for 24 h. After this time, the medium was replaced by drug-free medium. Cells were grown until colonies became visible (14 to 21 days). Colonies were stained with Giemsa to establish the colony formation index (34). Pools of colonies derived from cells that survived each treatment (125 colonies from one well with no drugs, 118 clones from one well treated with ER50891 alone, and 115 clones from one well treated with ER50891 plus RA) were used to isolate genomic DNA that was used for methylation-specific PCR (MSP) analysis with M4 primers and U4 primers. In a parallel replica experiment, we instead used cloning cylinders to isolate six independent clones that survived the treatment with either ER50891 alone (which we designated ER clones) or ER50891 in combination with RA (which we designated ER/RA clones). All clones from each group that were expanded in drug-free medium did not show RA-induced RARβ2 transcription by real-time RT-PCR and were shown to be U or M by MSP. One clone from each of the groups ER-C6 and ER/RA-C5 was further expanded in drug-free medium.
Real-time RT-PCR.
Total RNA was obtained using Trizol (Invitrogen), treated with DNase I (Ambion, Austin, TX), retrotranscribed with a SuperScript first-strand synthesis system (Invitrogen), and amplified by real-time RT-PCR on an iCycler apparatus (Bio-Rad, Hercules, CA) by using iQ SYBR green Supermix (Bio-Rad) and specific primers for RARβ2 (sense, 5′-GACTGTATGGATGTTCTGTCAG-3′; antisense, 5′-ATTTGTCCTGGCAGACGAAGCA-3′), RARα (both isoforms 1 and 2) (sense, 5′-TGTGGACTTCGCCAAGCA-3′; antisense, 5′-CGTGTACCGCGTGCAGA-3′), RARα 1 (sense, 5′-GCCAGGCGCTCTGACCACTC-3′; antisense, 5′-CAGGCGCTGACCCCATAGTGGT-3′); and GAPD (sense, 5′-GAAGGTGAAGGTCGGAGTC-3′; antisense, 5′-GAAGATGGTGATGGGATTTC-3′), with the appropriate annealing temperature according to standard protocols. The RARα and RARβ2 transcription levels were normalized to the GAPD transcription level. The statistical significance was calculated using Student's t test for three independent determinations.
Luciferase assay.
The luciferase reporter assay was performed essentially as described previously using the luciferase reporter vector RARβ2-pGL2 (12) (kindly donated by K. Ozato, National Institutes of Health, Bethesda, MD) and the control vector pRL-TK (Promega). Vector DNAs were cotransfected with Lipofectamine Plus in cells grown in a 12-well plate; 24 h after transfection, the medium was replaced with medium with or without RA (1.0 μM). Luciferase activity was measured by a dual luciferase reporter assay system (Promega) according to the manufacturer's instructions. The values represent the averages (normalized to the control) of three independent experiments, each performed in triplicate.
ChIP.
Quantitative chromatin immunoprecipitation (ChIP) was performed using reagents purchased from Upstate (Lake Placid, NY) following the manufacturer's protocol. Chromatin was immunoprecipitated with antibodies against acetyl-histone H4 (Ac-H4) (Upstate) (1:400), acetyl-lysine (K) 9 histone H3 (H3-Ac-K9) (Upstate) (1:400), dimethyl-K4 histone H3 (H3-Me-K4) (Upstate) (1:400), trimethyl-K9 histone H3 (H3-Tri-Me-K9) (Upstate) (1:200), RARα C terminus (Santa Cruz Biotechnology) (1:60), RARα N terminus (Biolegend, San Diego, CA) (1:100), RARβ (Santa Cruz Biotechnology [1:70]; Active Motif, Carlsbad, CA [1:600]), RNA polymerase II (Upstate) (1:200), and FLAG epitope (Sigma) (1:350). Re-ChIP was performed with the anti-RARα C-terminus antibody on chromatin immunoprecipitated with an anti-Ac-H4 antibody after elution with re-ChIP elution buffer (10 mM EDTA, 50 mM Tris-HCl [pH 8.0], 0.7 M NaCl, 20 mM dithiothreitol). Control ChIPs were without the respective antibodies. The immunoprecipitated DNA was amplified by real-time PCR with either RARβ2 primers (sense, 5′-GGTTCACCGAAAGTTCACTCGCAT-3′; antisense, 5′-CAGGCTTGCTCGGCCAATCCA-3′) or the GAPD primers (sense, 5′-GGTGCGTGCCCAGTTGAACCA-3′; antisense, 5′-AAAGAAGATGCGGCTGACTGTCGAA-3′). The RARβ2 DNA relative enrichment was calculated by normalizing the RARβ2 PCR signal to the PCR signals obtained both from the input DNA (total chromatin fraction) and the GAPD DNA. Statistical significance was determined using the Student's t test on three independent determinations.
DNA methylation analysis.
Genomic DNA extracted with DNAzol (Invitrogen) was modified with sodium bisulfite as described previously (33). Modified DNA was used for both MSP and sequencing analyses. For sequencing analysis, a 635-bp region, encompassing 27 RARβ2 CpG sites in the RARβ2-regulatory region, was amplified by nested PCR (40) using first the primer set RARβ2 sense 1 (5′-GTATAGAGGAATTTAAAGTGTGGGTTGGG-3′) and RARβ2 antisense 1 (5′-CCTATAATTAATCCAAATAATCATTTACC-3′) and subsequently the primer set RARβ2 sense 2 (5′-GTAGG(C/T)GGAATATTGTTTTTTAAGTTAAG-3′) and RARβ2 antisense 2 (5′-AATCATTTACCATTTTCCAAACTTACTC-3′). The PCR products were either directly sequenced or sequenced after subcloning in the pCR4-TOPO plasmid vector (Invitrogen). In the latter case, we sequenced a minimum of 20 to a maximum of 50 clones corresponding to the sample specified in Results. MSP was performed by amplifying bisulfite-modified DNA with the first RARβ2 primer set (see above). Then, 2 μl of a 1:1,000 dilution of the first amplification product was reamplified with the U4 sense- and U4 antisense-specific primers or with the M4 sense- and M4 antisense-specific primers (33) or with the different combinations of U4 and M4 primers. RARβ2 alleles detected with both U4 primers were classified as U, and the alleles amplified with either both M4 primers (these products are the ones discussed in Results) or the sense M4 primer and the U4 antisense primer were classified as M alleles. No product was amplified with the U4 sense primer and the M4 antisense primer.
RESULTS
Lack of RARα correlates with RARβ2 epigenetic silencing in RA-resistant cancer cells.
Analysis of a panel of breast and prostate cell lines showed different RA-induced RARβ2 transcription levels in correlation with differential growth inhibition by RA after 24 h of treatment (Fig. 1A, top and bottom, respectively). DNA methylation analysis using two complementary techniques, bisulfite sequencing and MSP, identified cell lines (i) homozygous for RARβ2 M alleles (MCF7, MDA-MB-231, and LNCaP carry 100% of M alleles), (ii) homozygous for RARβ2 U alleles (T47D carries 100% of U alleles), and (iii) heterozygous for U and M alleles (DU145) (Fig. 1B). As determined on the basis of sequencing of 20 independent DU145 RARβ2 alleles, 30% were U alleles and 70% were M alleles. The distribution of methylated CpGs in the M alleles of all cell lines involved at least a common stretch of CpGs in the first RARβ2 exon (Fig. 1B, bottom). Lack of RA-induced RARβ2 transcription was also detected in cells (DU145) with U alleles, which must therefore be interpreted as nonpermissive. Moreover, RARβ2 was spared by methylation in T47D, a cell line with several other hypermethylated genes (20).
FIG. 1.
Lack of RARα correlates with RARβ2 epigenetic silencing in RA-resistant cells. (A) Real-time RT-PCR (top) and colony formation assay (bottom) on a panel of breast and prostate cell lines showed a positive correlation between RA-induced RARβ2 transcription and RA-induced growth inhibition. (B) Methylation-specific PCR (MSP, top) and bisulfite sequencing (bottom) show the presence of cells homozygous for RARβ2 M alleles (MCF-7, LNCaP, and MDA-MB-231), heterozygous for U and M alleles (DU145), and homozygous for U alleles (T47D) and the localization of methylated CpGs in M alleles. (C) ChIP analysis shows a significant increase in the levels of both histone Ac-H4 (P < 0.05) and H3-Ac-K9 (P < 0.01) in T47D RARβ2 chromatin but not in DU145 in response to RA. The levels of H3-Tri-Me-K9 and H3-Me-K4 were significantly higher (P < 0.01) and lower (P < 0.01), respectively, in DU145 relative to T47D. The dotted lines represent what we defined as “the histone modification threshold” required for RARβ2 transcription. (D) Schematic diagram showing that RARα binds the RARβ2 RARE region in the absence of RA and that transcription is induced in the presence of RA (top) (14). RARα transcription (bottom left) and binding of RARα protein at RARβ2 (bottom right) are significantly higher (P < 0.05) in cells with RARβ2-permissive alleles (T47D) than in cells with RARβ2 nonpermissive alleles (DU145 and MDA-MB-231).
Analysis of the chromatin associated with RARβ2 alleles enabled us to quantitate the level of a few histone modifications—considered a hallmark of either repressive or active chromatin (4, 22)—in the RARβ2 region encompassing the RA-responsive element (RARE), the TATA box, the transcription start site, and the common stretch of CpGs in the first exon. We detected significant differences in the levels of acetylation of both histone H4 (Ac-H4) and histone H3 at lysine (K) 9 (H3-Ac-K9) in response to RA treatment for 24 h in T47D RARβ2 chromatin but not in DU145 chromatin (Fig. 1C, top left and bottom left). Moreover, both in the absence (baseline) and in the presence of RA, the level of methylation of histone H3 on K4 (H3-Me-K4)—a parameter of chromatin activity—was higher in T47D than in DU145 chromatin (Fig. 1C, bottom right), while the opposite was true for H3-K9 methylation (H3-Tri-Me-K9)—a parameter of chromatin inactivity (Fig. 1C, top right). Thus, the overall quantity of histone modifications, which we define as the “histone modification threshold” (Fig. 1C, dotted lines), as well as the quality of histone modifications, seems to be critical in order to “switch on” RARβ2 transcription in response to RA. RA induced both qualitative and quantitative modifications in nonpermissive alleles as well but, apparently, unless a critical accumulation of histone changes hit the threshold, the transcription remained “off.”
Normally, RARα bound to RARβ2 keeps the chromatin poised for transcription (yet inactive) (14) (Fig. 1D, top left) and ready for activation by RA (Fig. 1D, top right). RA binding to RARα would induce histone modifications capable of activating RARβ2 very rapidly (29). Once RARβ2 is induced, it could regulate its own transcription (8). We observed that the presence of RARβ2 nonpermissive alleles (regardless of their DNA methylation status) always correlated with a lower level of RARα transcription (Fig. 1D, bottom left). Consistent with this observation, quantitative ChIP with anti-RARα antibodies, followed by RARβ2 DNA amplification of the region encompassing the RARE (schematic diagram in Fig. 1B), detected significantly more RARα associated with RARβ2 in T47D cells than in MDA-MB-231 and DU145 cells (Fig. 1D, bottom right). Of note, by ChIP with anti-RARβ antibodies we found RARβ2 binding only at the T47D RARβ2 promoter (data not shown).
Altogether, these observations made us hypothesize that a defective integration of RA signal at RARβ2 due to lack of functional RARα can convert RARβ2 into inactivity, marked by repressive epigenetic changes at histone and DNA level, and RA resistance.
Induction of RARβ2 epigenetic silencing by a dominant-negative RARα in RA-sensitive cells.
First, we simulated a genetic scenario whereby an RARα mutation with dominant-negative features occurs in RA-sensitive, RARα-positive cells homozygous for RARβ2-permissive alleles. We used a well-characterized dominant-negative RARα mutant, RARα403, which lacks the C-terminal RA-binding domain but retains the capacity to heterodimerize with the retinoid X receptor and bind to the RARE regions (Fig. 2A) (11, 15, 39). RARα 403 should compete with wild-type RARα in the heterodimerization with retinoid X receptor (23). It might also cause an impaired turnover of corepressor complexes at RARα target genes, because it lacks the C terminus (27, 29).
FIG. 2.
Functional RARα inhibition by a dominant-negative RARα in RA-sensitive cells leads to RARβ2 silencing marked by repressive epigenetic changes. (A) Schematic diagram representing the structure of the RARα-dominant-negative RARα403 (bottom) missing part of the RA-binding domain. (B) The FLAG-tagged RARα403, transiently transfected into T47D cells, binds the RARβ2 promoter concomitant with a significant (P < 0.05) decrease of RA-induced RARβ2 transcription (middle). ChIP experiments with antibodies (Ab) directed against the RARα C and N termini, showing that the C-Ab, but not the N-Ab, detects remarkably less wild-type RARα at RARβ2 in DN C8 cells, carrying both the dominant-negative and wild-type RARα isoforms, than LX C5 cells carrying only wild-type RARα (right). (C) In contrast to LX C5, DN C8 does not significantly express both luciferase from an exogenous RARβ2 promoter (P < 0.05) (left) and endogenous RARβ2 (P < 0.05) (middle) in response to the presence of RA, consistent with lack of recruitment of RNA polymerase II at the promoter (right). (D) RARβ2 ChIP analysis showing that RA significantly (P < 0.01) increases the level of activating histone modifications (Ac-H4, H3-K9-Ac, and H3-Me-K4) to the “threshold” (dotted line) associated with transcription activation (on) only in LX C5 chromatin and not in DN C8 chromatin. Consistently, the level of H3-Tri-Me-K9 is higher in DN C8 chromatin than in LX C5 chromatin. (E) MSP (left) and bisulfite sequencing (right) show that DN C8 is heterozygous for U and M RARβ2 alleles, with the methylated CpGs mostly localized in exon 1.
At 24 h after transient transfection with a FLAG-RARα403 construct, T47D breast cancer cells (which express mainly endogenous RARα1; data not shown) showed the presence of the dominant-negative mutant at the RARβ2 promoter (ChIP; Fig. 2B, bottom left), concomitant with significant RARβ2 transcriptional downregulation in response to the presence of RA (Fig. 2B, bottom middle). Thus, RARβ2 silencing seems to be initiated when RARα403 resides at the RARβ2 promoter region. The dominant-negative RARα was also found at the RARβ2 of a T47D clone expressing the dominant-negative protein (DN C8). This was deduced on the basis of a ChIP experiment with an antibody directed against the RARα C terminus; this antibody detected remarkably less wild-type RARα at RARβ2 than an antibody directed against the RARα N terminus in DN C8 relative to LX C5 (Fig. 2B, bottom right). RA treatment (24 h) failed to induce luciferase transcription from a transiently transfected RARβ2 promoter-luciferase construct (Fig. 2C, left), thus proving the dominant-negative effect of RARα403 on endogenous RARα. Finally, in response to the presence of RA, DN C8 did not show endogenous RARβ2 transcription (real-time RT-PCR; Fig. 2C, middle). This finding was mirrored by a lack of RNA polymerase II at RARβ2 (Fig. 2C, right). The repressed transcriptional status in the DN C8 clone was paralleled by repressive quantitative and qualitative histone modifications, which, in response to RA treatment, did not reach the threshold necessary for “switching on” RARβ2 transcription (Fig. 2D; dotted line). Interestingly, MSP analysis of DN C8 RARβ2 DNA with M4 and U4 primers (see Materials and Methods) showed U and M alleles. After bisulfite sequencing of 50 alleles, we found that DN C8 contained 60% U and 40% M alleles with a few methylated CpGs (Fig. 2E). In contrast to what was reported for cells carrying another dominant-negative RARα with an intact RA-binding domain (13), RA treatment (1 μM, 72 h) did not reverse RARβ2 DNA methylation. Only treatment with 5-Aza (0.8 μM for 72 h) led to demethylation and RA-induced RARβ2 transcription in DN C8 (data not shown).
RARβ2 epigenetic silencing confers an RA-resistant phenotype.
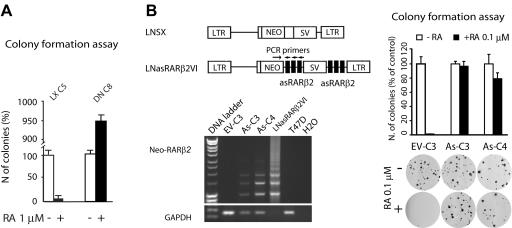
Concomitant with RARβ2 epigenetic silencing, DN C8 cells developed stable resistance to the growth-inhibitory effect of RA, as assessed by colony formation (Fig. 3A). Because RARα controls several RA-responsive target genes, we tested whether the observed RA resistance phenotype was indeed caused by RARβ2 silencing. To this end we targeted T47D RARβ2 transcription with an antisense RARβ2 (37). Stable T47D clones transfected with LNasRARβ2VI that carry multiple copies of the RARβ2 antisense, like the As-C3 and As-C4 clones (where “As” represents “antisense”) (Fig. 3B, left), were significantly less sensitive to RA-induced growth inhibition than the cognate control clone EV-C3 carrying the empty vector LNSX (Fig. 3B, right). Thus, it is conceivable that the RA-resistant phenotype developed by DN C8 cells is the consequence of RARβ2 epigenetic silencing.
FIG. 3.
RARβ2 epigenetic silencing confers an RA-resistant phenotype. (A) Colony formation assay showing that DN C8 cells, but not LX C5 cells, display RA resistance. (B) The RARβ2 antisense construct LNasRARβ2VI (top left) is detected in the T47D As-C3 and As-C4 clones, as shown by PCR (bottom left). Colony formation assay showing that As-C3 and As-C4, but not the control EV-C3 clone carrying the empty vector, display RA resistance (right).
RARβ2 DNA methylation is not necessary to confer a nonpermissive transcriptional status.
Because RA did not induce transcription from both RARβ2-U and RARβ2-M nonpermissive alleles in DN C8 cells, we hypothesized the existence of RA-resistant DN C8 cells homozygous for nonpermissive unmethylated alleles. We isolated 20 independent DN C8 subclones which were resistant to RA and unable to reexpress the RARβ2 transcript in response to RA. Screening for the presence of methylation in the first exon by MSP identified 18 clones heterozygous for both U and M alleles and 2 clones homozygous for U alleles (Fig. 4A). The CpG methylation-free status of the U alleles was confirmed by bisulfite sequencing (20 alleles of one of the two subclones are shown in Fig. 4B). The level of Ac-H4 did not increase significantly in response to RA in the RARβ2 chromatin of this subclone as it did in the RARβ2 chromatin of LX C5 (Fig. 4C, top) that is instead homozygous for RARβ2 U alleles that are permissive (Fig. 2E, top). The level of H3-Tri-Me-K9 in the DN C8 subclone chromatin was in the range observed in the parental DN C8 clone (Fig. 4C, bottom). Apparently, RARβ2 silencing also can be imposed in the absence of DNA methylation.
FIG. 4.
RARβ2 DNA methylation is not necessary to confer a nonpermissive RARβ2 transcriptional status. (A) RARβ2 methylation and transcription in 20 DN C8 subclones shows that 2 clones do not present CpG methylation and RA-induced RARβ2 transcription. (B) MSP and bisulfite sequencing analysis of one of the two DN C8 subclones homozygous for U alleles. (C) ChIP analysis shows that the chromatin of the DN C8 subclone homozygous for U alleles and the parental DN C8 is marked by comparable levels of Ac-H4 (top) and H3-Tri-Me-K9 (bottom) in the presence and absence of RA.
Knocking down RARα by RNA interference also triggers RARβ2 epigenetic silencing and RA resistance.
It can be argued that RARβ2 silencing in DN C8 cells is due to the recruitment of histone-modifying enzymes or DNA methyltransferases (DNMTs) at RARβ2 by the RARα403 protein, as shown for the PML-RARα protein (13). While nonrandom dominant-negative RARα mutations were never to our knowledge reported in cancer epithelial cells, RARα expression was reported to be low or absent in these cells (30). This could be due to loss of heterozygosity or RARα epigenetic silencing or both. Thus, we tested the effect of RARα knockdown on RARβ2 transcription by using RARα-specific RNA interference in T47D cells.
Stable expression in T47D cells of a short hairpin RNA (targeting a sequence common to both RARα1 and RARα2; Fig. 5A, top left), selected for efficient knockdown of exogenous RARα1 overexpressed into COS cells (Fig. 5A, bottom left), silenced the endogenous RARα in prototypic clones such as SIα C7 (Fig. 5A, top right). As a result, RA-induced RARβ2 transcription (Fig. 5A, bottom right) was abrogated in association with development of a RARβ2 chromatin status unable to integrate the RA signal (Fig. 5B). Indeed, RA failed to induce the level of both Ac-H4 and H3-Ac-K9 up to the threshold (Fig. 5B, left and middle; dotted line) associated with RARβ2 transcription. Moreover, SIα C7 chromatin showed a significantly higher level of H3-Tri-Me-K9 than the control pSC2 chromatin (Fig. 5B, right). MSP analysis showed the presence of M alleles. Bisulfite sequencing of 25 alleles evidenced, as it did in DN C8, either U alleles or M alleles methylated in just a few CpGs (Fig. 5C). Surprisingly, profound RARα downregulation seems sufficient to create a stable nonpermissive RARβ2 status, apparently marked by an accumulation of histone-repressive changes but not always by CpG methylation. We do not know yet how lack of RARα makes the RARβ2-chromatin a “prey” of repressor proteins. Also, in this case, when RARβ2 falls into silencing, cells apparently acquire resistance to RA-induced growth inhibition (Fig. 5D).
FIG. 5.
Stable RNA interference of RARα triggers RARβ2 epigenetic silencing and RA resistance. (A) The RARα targeting sequence (top left), cloned in the pSUPER-retro vector and selected for efficient knockdown of exogenous RARα (encoded by pSG-hRARα 1) in COS cells (bottom left), efficiently knocks down the endogenous RARα protein in the T47D clone SIα C7 (top right) consistent with significant transcriptional downregulation (P < 0.01) (bottom right). (B) Quantitative ChIP analysis showing that RA induces in SIα C7 RARβ2 chromatin significant lower levels of both Ac-H4 (P < 0.05) (left) and H3-Ac-K9 (P < 0.05) (middle) than in pS C2 chromatin, well below the threshold (dotted line) required for transcription. The level of H3-Tri-Me-K9 (right) is also significantly higher (P < 0.05) in SIα C7 RARβ2 chromatin than in pS C2 chromatin. (C) MSP (right) and bisulfite sequencing (left) show CpG hypermethylation in a few SIα C7 RARβ2 alleles and the localization of the CpGs. (D) Colony formation assay showing that SIα C7 cells are RA resistant.
Induction of RARβ2 epigenetic silencing by RARα antagonists.
It was reported that the intracellular RA level is lower in cancer epithelial cells than in their normal counterparts (17). Thus, we set out to test whether interfering with RA availability at endogenous RARα with RARα antagonists can force RARβ2 into a deep state of inactivity.
First we tested that the RARα-specific antagonist ER50891 (25) (Fig. 6A, top) was able to significantly inhibit the induction of RARβ2 transcription in response to RA in a time course experiment in which T47D cells were grown in the presence of ER50891 (10 μM) alone, RA (0.1 μM) alone, or ER50891 and RA in combination for 4, 8, and 24 h (Fig. 6A, bottom). By using ChIP analysis with a RARα-specific antibody we observed that the levels of endogenous RARα occupancy of the RARβ2 region containing the RARE did not differ significantly during the 24 h in cells grown in the presence of the antagonist (Fig. 6B, top). In contrast, we observed that in the same cell samples the levels of H3-Tri-Me-K9 (hallmark of repressive chromatin) and H3-Ac-K9 (hallmark of active chromatin) associated with RARβ2 DNA increased and decreased, respectively (Fig. 6B, bottom two panels). We also detected by both MSP analysis and bisulfite sequencing the appearance of M alleles (Fig. 6C, top), with methylation emerging once again in a few CpGs in the first exon (Fig. 6C). This experiment suggests that upon the RARα antagonist binding to endogenous RARα, a few histone and DNA-modifying enzymes might be rapidly recruited at RARβ2 to impose repressive changes. Our supposition is corroborated also by the observation that T47D cells treated up to 96 h with another RARα antagonist, RO415253 (10 μM) (1), developed DNA methylation within the first 4 h (Fig. 6D). This is consistent with previous reports showing that a dominant-negative RARα mutant transfected in cells carrying an unmethylated RARβ2 triggered the appearance of RARβ2 DNA methylation within a few hours of transfection (13). Thus, aberrant RARβ2-repressive changes seem to occur as rapidly as normal chromatin changes (29). However, we do not know yet which chromatin repressor proteins are recruited and in which order at RARβ2 in response to the RARα antagonist.
FIG. 6.
Induction of RARβ2 epigenetic silencing by RARα antagonists. (A and B) (A) Time course analysis showing that the RARα antagonist ER50891 (top) abrogates RA-induced RARβ2 transcription in T47D cells (bottom) concomitant with (B) induction of a significant (P < 0.05) increase of repressive H3-Tri-Me-K9 (middle) and a significant (P < 0.05) decrease of activating H3-Ac-K9 (bottom), while RARα resides at RARβ2 (top). (C) MSP (top) and bisulfite sequencing (bottom) show CpG methylation already at 4 h after treatment with ER50891. (D) Time course analysis of RARβ2 methylation with the RARα antagonist RO415253, showing rapid occurrence of CpG methylation (MSP, middle; bisulfite sequencing, bottom). (E) Colony formation assay showing that ER50891 can rescue T47D cells from RA-induced growth inhibition (top). MSP analysis of pools of clones derived from cells that survived treatment with ER50891 alone or in combination with RA detected both U and M alleles (bottom). (F) Stability of RARβ2 epigenetic silencing in ER-C6 and ER/RA-C5, showing heterozygosity for U and M alleles (top left), RA resistance (bottom left), lack of RA-induced RARβ2 transcription (top right), and histone H4 hypoacetylation (bottom right).
It can be argued that RARβ2 epigenetic silencing was observed because of the constant presence in the cell of inductive factors (such as the dominant-negative RARα, the RARα-RNA interference construct, and the RARα antagonists).
Here we show that RARβ2-repressive chromatin changes are retained by T47D cells after removal of the RARα antagonist. T47D cells treated for up to 24 h with ER50891 (10 μM), alone or in combination with RA (0.1 μM), were grown in drug-free medium until we observed the appearance of discrete colonies. A clonogenicity assay showed that ER50891 could rescue cells from RA-induced growth inhibition (Fig. 6E, top). DNA was extracted by the entire pool of clones that survived treatment with ER50891 alone or in combination with RA (for details, see Materials and Methods). MSP showed the presence in both pools of clones of RARβ2 alleles with and without CpG methylation (Fig. 6E, bottom). MSP analysis of two independent clones that survived ER50891 treatment (clone ER-C6) or combined ER50891 and RA treatment (clone ER/RA-C5) showed the presence of both U and M alleles (Fig. 6F, top left). The chromatin associated with the silent RARβ2 alleles in these clones was marked by histone H4 hypoacetylation (Fig. 6F, bottom right), consistent with lack of RA inducibility of RARβ2 transcription (Fig. 6F, top right) and RA resistance (Fig. 6F, bottom left). Apparently, RARα was no longer bound at RARβ2 (S. Pozzi, unpublished observations). We conclude that after removal of the inductive factor (RARα antagonist), the RARβ2-repressive epigenetic changes remain stable in at least some of the cells that were originally exposed to the inductive factor.
RARβ2 reactivation requires restoration of RA signal at a silent RARβ2 through RARα.
Our work, as well as the work of others, has shown that treatment of cells carrying a silent RARβ2 with either histone deacetylase inhibitors or demethylating agents, alone or in combination, can resensitize an epigenetically silent RARβ2 to RA (6, 24, 34). A few preliminary observations pointed at RARα as a critical factor for RA-induced RARβ2 reactivation from an epigenetically silent promoter. We observed specifically that (i) the occurrence of reactivation of RA-induced RARβ2 transcription in a RARα-negative SG5C1 clone (derived from MDA-MB-231) treated with TSA (330 nM) and/or 5-Aza (0.8 μM), alone or in combination (Fig. 7A, bottom left), was concomitant with reactivation of endogenous RARα (Fig. 7A, top left), (ii) the drug-induced endogenous RARα was found at the RARβ2 promoter in response to the presence of RA (Fig. 7A, middle), and (iii) RA-induced RARβ2 transcription after treatment with the different drugs was abrogated by the RARα antagonist ER50891 (Fig. 7A, right).
FIG. 7.
Restoring RA signal at a silent RARβ2 through RARα leads to RARβ2 reactivation. (A) Concomitant reactivation of RARα (top left) and RARβ2 (bottom left) with TSA and 5-Aza alone or in combination in MDA-MB-231 SG5C1 leads to a significant increase of RARα binding at RARβ2 (middle). The RARα antagonist ER50891 significantly abrogates RA-induced RARβ2 transcription after TSA and 5-Aza treatment alone (P < 0.05) or in combination (P < 0.001) (right). Ab, antibody. (B) Exogenous expression of RARα in MDA-MB-231 RARαC21 (top left) leads to significantly (P < 0.001) higher RA-induced RARβ2 transcription (top middle) concomitant with significant recruitment (P < 0.05) of RNA polymerase II at RARβ2 (top right) and a significant increase of Ac-H4 (P < 0.05) in the RARβ2 chromatin (bottom left). Apparently RARα associates significantly (P < 0.05) more with the reacetylated chromatin in the presence of RA (middle bottom). RARβ2 DNA associated with RARα is methylated (bottom right). (C) Real-time RT-PCR shows that the level of RA-induced RARβ2 transcription after treatment with TSA is significantly (P < 0.01) higher in RARαC21 than in SG5C1 (top left), and paralleled by a significant (P < 0.05) increase in Ac-H4 in the RARβ2 chromatin (middle left), as well as binding of both RARα and RNA polymerase II at RARβ2 (top right and bottom right, respectively), in the absence of demethylation of RARβ2 DNA (bottom left). Demethylation was observed only in the samples treated with 5-Aza (bottom left). (D). Schematic diagram based on the findings presented in panels A, B, and C showing that RARα—either drug-induced endogenous (en-RARα) or exogenous (ex-RARα)—plays an active role in reestablishing a RARβ2 status, which is permissive for transcription.
Next, we mechanistically tested the involvement of RARα in the reactivation of transcription from a silent RARβ2 in an MDA-MB-231 clone expressing exogenous RARα (RARα C21) (Fig. 7B, top left). We observed that RARβ2 transcriptional reactivation (Fig. 7B, top middle) and recruitment of RNA polymerase II at RARβ2 (Fig. 7B, top right) in response to the presence of RA (1 μM, 24 h) occurred with significant reacetylation of histone H4 (Fig. 7B, bottom left). By immunoprecipitating with anti-RARα antibodies the reacetylated chromatin, we found a significantly higher level of RARα bound at RARβ2 in response to RA (see Re-ChIP panel, Fig. 7B, bottom middle). MSP analysis of the RARβ2 DNA immunoprecipitated by RARα showed persistence of RARβ2 CpG methylation (Fig. 7B, bottom right). Apparently, reestablishing the RA signal at RARβ2 via exogenous RARα by inducing RARβ2 chromatin reacetylation enabled transcription from the methylated RARβ2.
Interestingly, the level of RA-induced RARβ2 transcription obtained with exogenous RARα was comparable to the level of RA-induced transcription obtained with the two drugs in SG5C1 (Fig. 7C, top left, arrows) and correlated with the increase of histone H4 reacetylation at RARβ2 up to the threshold enabling transcription (Fig. 7C, middle left, arrows) rather than with demethylation of RARβ2 DNA (Fig. 7C, bottom left). The increment in the level of drug-induced endogenous RARα in SG5C1 and drug-induced endogenous plus exogenous RARα in RARαC21 at RARβ2 (Fig. 7C, top right) was mirrored by an increment of RNA polymerase II recruitment at RARβ2 (Fig. 7C, bottom right). This latter increment, paralleled by the increment in RARβ2 transcription, might reflect an involvement of RARβ2 itself, which, once induced by RA, would contribute to its own transcription by a positive-feedback autoregulatory loop (8, 21, 36). However, we were not able to prove it by ChIP analysis with anti-RARβ2 antibodies as we did instead in control T47D cells (data not shown).
Based on the overall findings that we summarized in the schematic diagram in Fig. 7D, RARα appears to be critical in the restoration of a permissive transcriptional status at a silent RARβ2.
DISCUSSION
We proposed previously that hormone-regulated genes, whose transcription is normally regulated by dynamic changes of the chromatin status in the presence and absence of the specific hormone (9, 14, 29, 41), might be ideal models for testing the etiology of aberrant epigenetic silencing in cancer and aging (33). In particular, we hypothesized that an aberrant chromatin-repressive status, consequent to lack of either RA or a crucial component of the machinery necessary to mediate the integration of RA signal at RARβ2, could explain why RARβ2 is so frequently epigenetically silenced in RA-resistant cancer cells (33) and RA-resistant tumors (34).
In the first part of this report we prove that impairing the integration of RA signal through RARα at RARβ2 leads to RARβ2 epigenetic silencing. Conversely, we show that reintegration of RA-RARα signaling at a silent RARβ2 leads to transcriptional reactivation. RARα, the upper regulator of RARβ2 transcription, is expressed in RA-sensitive cells where RARβ2 can be induced by RA and is homozygous for unmethylated RARβ2 alleles (Fig. 1). When we interfered with RA signaling at RARβ2 by three alternative strategies, namely, a dominant-negative RARα lacking the RA-binding domain (Fig. 2), RARα-RNA interference (Fig. 5), and RARα antagonists (Fig. 6) in RA-sensitive cells, we always induced the conversion of RARβ2 alleles permissive for transcription into a nonpermissive (unresponsive to RA) status. The chromatin of nonpermissive alleles was marked by repressive histone modifications (H3-Tri-Me-K9, hypoacetylation of histone H4, and H3-K9), and also by CpG methylation, but in only a fraction of alleles, which remained unresponsive to RA. RA could not influence the level of critical histone modifications to reach what we define as the “threshold” of histone modifications required for transcription (as it happens instead in cognate control clones) even when used at a pharmacological concentration (1 μM). Remarkably, a nonpermissive RARβ2 status can be conferred without CpG methylation. Consistently, we identified RA-resistant cells, which were homozygous for RARβ2 unmethylated alleles yet nonpermissive for transcription in response to RA (Fig. 4).
We further demonstrated that the RARβ2 nonpermissive (nonresponsive to RA) status was stable also in the absence of the inductive factor. Specifically, we demonstrated that this is the case by attenuating the RA signal at RARβ2 with a RARα antagonist and showing that the RARβ2 nonpermissive status—marked by repressive histone H4 hypoacetylation and, in some alleles, also CpG methylation—was maintained for a long time after the RARα antagonist was removed (Fig. 6E and 6F).
It was beyond the scope of this study to show which repressor proteins (including histone-modifying enzymes and DNA methyltransferases) initiated the exacerbation of the RARβ2-repressed status in response to different inductive factors. However, from the initial repressive events induced by either the dominant-negative RARα or the RARα antagonist ER50891 we speculate that both histone and DNA-modifying enzymes might have been recruited at RARβ2 while there was persisting occupancy of the promoter by either the dominant-negative (Fig. 2B) or endogenous wild-type RARα (Fig. 6B). The order in which repressive changes at histone and DNA level accumulate at gene promoters has been addressed in a few studies (2, 35). In the case of RARβ2, the accumulation of repressive histone modifications appears to precede CpG methylation. We inferred that this might be the case because we found that only a fraction of nonpermissive alleles developed CpG methylation and in only a few CpGs in the first exon (Fig. 2, 5, and 6). It is conceivable that this region is the epicenter of CpG methylation. These findings suggest indirectly that DNMTs are recruited after other repressive critical proteins. This is because the nonpermissive status can also be achieved in the absence of CpG methylation.
We also demonstrated that induction of a silent, nonpermissive RARβ2 status is indeed the cause of biological RA resistance. Directly targeting RARβ2 transcription with a RARβ2 antisense in RA-sensitive T47D cells led to the same RA-resistant phenotype (Fig. 3) observed after induction of RARβ2 epigenetic silencing with any of the three strategies used to functionally inactivate RARα. In summary, impairment of RA signal at RARβ2 through RARα in RA-sensitive cells appears to lead to an exacerbation of the RARβ2 chromatin status, stable silencing, and, ultimately, RA resistance (Fig. 8).
FIG. 8.
The critical role of RA-RARα signaling in RARβ2 epigenetic silencing and RA resistance. (A) Normally, in the absence of RA, RARα keeps RARβ2 chromatin poised for transcription; upon RA binding at RARα the chromatin is converted to an active state (14). RARβ2 in turn enhances its own transcription (8, 21, 36). Induction of the RARβ2 tumor suppressor results in cell growth inhibition. (B) Impaired RA signal through RARα at RARβ2 apparently converts RARβ2 chromatin from a poised to a severely repressed, silenced state no longer compatible with transcription. This state is characterized by epigenetic repressive modifications at the histone and DNA level. The biological consequence of the conversion of RARβ2 into a silent state is the resistance to the growth-inhibitory action of RA. (C) Conversely, reconnecting RA signal at a silent RARβ2 chromatin through RARα restores a chromatin state again compatible with transcription. RARβ2 reactivation results in the reestablishment of responsiveness to the growth-inhibitory action of RA.
Conversely, we prove that reconnecting RA signal through an exogenous RARα at a silent, heavily hypermethylated RARβ2 in RA-resistant cells results in transcriptional reactivation concomitant with RARβ2 chromatin histone H4 reacetylation but not demethylation (Fig. 7C, left). Interestingly, the level of RA-induced RARβ2 reactivation in the presence of exogenous RARα was comparable to the level induced with TSA in the presence of endogenous RARα. Also, in this case RARβ2 reactivation was achieved without demethylation. Apparently, RA binding to RARα, which is known to actively recruit coactivator complexes with histone acetyltransferase activity (29), is sufficient to convert RARβ2 from a silent to a permissive state, as we show in the schematic diagram in Fig. 7D. A consequence of restoring RA-RARα signaling at RARβ2 likely is the reactivation of the RARβ2 receptor itself, which is expected to sustain its own transcription (8, 21, 36). Because we did not detect RA-induced RARβ2 reactivation after treatment with TSA and 5-Aza alone or in combination when we abrogated RARα function with the RARα antagonist (Fig. 7A, right), we conclude that RARβ2 can play a role in its own transcription but only after its transcription is triggered by RARα, as we show in the schematic diagram in Fig. 7D. In summary, reintegration of RA signal at an epigenetically silent RARβ2 through RARα in RA-resistant cells would restore a chromatin status enabling transcription of the tumor suppressor gene in response to the presence of RA and, consequently, sensitivity to the growth-inhibitory action of RA (Fig. 8).
Lack of functional integration of RA signal at RARβ2 through RARα might occur in vivo. RARα is not expressed in a high percentage of tumors (31). This leads to the hypothesis that in vivo RARβ2 silencing can be a consequence of RARα loss or silencing. In support of this hypothesis, we observed that reactivation of RARβ2 by TSA and 5-Aza also restored RARα (Fig. 7A, left). Interestingly, estrogen receptor alpha (ERα) is often epigenetically silenced in RARα-negative tumors (16). RARα is regulated, at least in part, by ERα (30). This makes us speculate that epigenetic silencing of a few hormonally regulated genes could occur in a “domino” fashion. We continue to assert (33, 34) that lack of intracellular RA availability at RARα could also trigger a repressive epigenetic domino effect. Recently we found that RARβ2 silencing leads to the silencing of downstream genes involved in RA metabolism, thus suggesting the existence of epigenetic networks (unpublished observations).
A few translational considerations come to mind at the end of this report. Detection of RARβ2 hypermethylation, a test for early breast cancer (3) and other cancers, apparently underestimates epigenetic RA resistance, because it may miss cells homozygous for silent, yet unmethylated, RARβ2 alleles (Fig. 4). Therapeutic strategies aimed at resensitizing RA-resistant cells to RA by restoring RARβ2 would require drugs powerful enough to reinduce simultaneously both RARβ2 and critical RARβ2 regulators. But more than anything else, this report highlights the importance of identifying the intrinsic and extrinsic factors that in vivo might force genes like RARβ2 into aberrant or protracted inactivity. Identification of these factors can lead to novel cancer prevention strategies.
Acknowledgments
We thank James Herman, David Kowalski, André Hoogeveen, and the anonymous reviewers for helpful suggestions and constructive criticism and Ellen Sanders for manuscript editing.
This work was supported by an AIRC grant (Italy) (N.S.), U.S. Army grant DAMD17-02-01-0432 (N.S.), a Roswell Park Cancer Institute Alliance grant (N.S.), the Graduate Program of Molecular Medicine, University of Milan (S.P., G.B., and G.S.), and the CISI Center of Excellence, University of Milan (S.R.).
REFERENCES
- 1.Apfel, C., F. Bauer, M. Crettaz, L. Forni, M. Kamber, F. Kaufmann, P. LeMotte, W. Pirson, and M. Klaus. 1992. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc. Natl. Acad. Sci. USA 89:7129-7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachman, K. E., B. H. Park, I. Rhee, H. Rajagopalan, J. G. Herman, S. B. Baylin, K. W. Kinzler, and B. Vogelstein. 2003. Histone modifications and silencing prior to DNA methylation of a tumor suppressor gene. Cancer Cell 3:89-95. [DOI] [PubMed] [Google Scholar]
- 3.Bean, G. R., V. Scott, L. Yee, B. Ratliff-Daniel, M. M. Troch, P. Seo, M. L. Bowie, P. K. Marcom, J. Slade, B. F. Kimler, C. J. Fabian, C. M. Zalles, G. Broadwater, J. C. Baker, Jr., L. G. Wilke, and V. L. Seewaldt. 2005. Retinoic acid receptor-beta2 promoter methylation in random periareolar fine needle aspiration. Cancer Epidemiol. Biomarkers Prev. 14:790-798. [DOI] [PubMed] [Google Scholar]
- 4.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 5.Bovenzi, V., N. L. Le, S. Cote, D. Sinnett, L. F. Momparler, and R. L. Momparler. 1999. DNA methylation of retinoic acid receptor beta in breast cancer and possible therapeutic role of 5-aza-2′-deoxycytidine. Anticancer Drugs 10:471-476. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, E. E., K. E. Bachman, S. Myohanen, J. G. Herman, and S. B. Baylin. 1999. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 21:103-107. [DOI] [PubMed] [Google Scholar]
- 7.Chambon, P. 1996. A decade of molecular biology of retinoic acid receptors. FASEB J. 10:940-954. [PubMed] [Google Scholar]
- 8.Chiba, H., J. Clifford, D. Metzger, and P. Chambon. 1997. Distinct retinoid X receptor-retinoic acid receptor heterodimers are differentially involved in the control of expression of retinoid target genes in F9 embryonal carcinoma cells. Mol. Cell. Biol. 17:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collingwood, T. N., F. D. Urnov, and A. P. Wolffe. 1999. Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J. Mol. Endocrinol. 23:255-275. [DOI] [PubMed] [Google Scholar]
- 10.Creusot, F., G. Acs, and J. K. Christman. 1982. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J. Biol. Chem. 257:2041-2048. [PubMed] [Google Scholar]
- 11.Damm, K., R. A. Heyman, K. Umesono, and R. M. Evans. 1993. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc. Natl. Acad. Sci. USA 90:2989-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey, A., S. Minucci, and K. Ozato. 1994. Ligand-dependent occupancy of the retinoic acid receptor beta 2 promoter in vivo. Mol. Cell. Biol. 14:8191-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Croce, L., V. A. Raker, M. Corsaro, F. Fazi, M. Fanelli, M. Faretta, F. Fuks, C. F. Lo, T. Kouzarides, C. Nervi, S. Minucci, and P. G. Pelicci. 2002. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science 295:1079-1082. [DOI] [PubMed] [Google Scholar]
- 14.Dilworth, F. J., and P. Chambon. 2001. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene 20:3047-3054. [DOI] [PubMed] [Google Scholar]
- 15.Durand, B., M. Saunders, C. Gaudon, B. Roy, R. Losson, and P. Chambon. 1994. Activation function 2 (AF-2) of retinoic acid receptor and 9-cis retinoic acid receptor: presence of a conserved autonomous constitutive activating domain and influence of the nature of the response element on AF-2 activity. EMBO J. 13:5370-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson, A. T., R. G. Lapidus, S. B. Baylin, and N. E. Davidson. 1995. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 55:2279-2283. [PubMed] [Google Scholar]
- 17.Guo, X., A. Ruiz, R. R. Rando, D. Bok, and L. J. Gudas. 2000. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis 21:1925-1933. [DOI] [PubMed] [Google Scholar]
- 18.Heard, E., P. Clerc, and P. Avner. 1997. X-chromosome inactivation in mammals. Annu. Rev. Genet. 31:571-610. [DOI] [PubMed] [Google Scholar]
- 19.Houle, B., C. Rochette-Egly, and W. E. Bradley. 1993. Tumor-suppressive effect of the retinoic acid receptor beta in human epidermoid lung cancer cells. Proc. Natl. Acad. Sci. USA 90:985-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, T. H., M. R. Perry, and D. E. Laux. 1999. Methylation profiling of CpG islands in human breast cancer cells. Hum. Mol. Genet. 8:459-470. [DOI] [PubMed] [Google Scholar]
- 21.Husmann, M., J. Lehmann, B. Hoffmann, T. Hermann, M. Tzukerman, and M. Pfahl. 1991. Antagonism between retinoic acid receptors. Mol. Cell. Biol. 11:4097-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, B. S., R. A. Chandraratna, R. A. Heyman, E. A. Allegretto, L. Mueller, and S. J. Collins. 1999. Retinoid X receptor (RXR) agonist-induced activation of dominant-negative RXR-retinoic acid receptor α403 heterodimers is developmentally regulated during myeloid differentiation. Mol. Cell. Biol. 19:3372-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, P. A., and S. B. Baylin. 2002. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3:415-428. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi, K., K. Tagami, S. Hibi, H. Yoshimura, N. Tokuhara, K. Tai, T. Hida, T. Yamauchi, and M. Nagai. 2001. Syntheses and evaluation of quinoline derivatives as novel retinoic acid receptor alpha antagonists. Bioorg. Med. Chem. Lett. 11:1215-1218. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y., M. O. Lee, H. G. Wang, Y. Li, Y. Hashimoto, M. Klaus, J. C. Reed, and X. Zhang. 1996. Retinoic acid receptor beta mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol. Cell. Biol. 16:1138-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molinari, E., M. Gilman, and S. Natesan. 1999. Proteasome-mediated degradation of transcriptional activators correlates with activation domain potency in vivo. EMBO J. 18:6439-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng, H. H., and A. Bird. 1999. DNA methylation and chromatin modification. Curr. Opin. Genet. Dev. 9:158-163. [DOI] [PubMed] [Google Scholar]
- 29.Perissi, V., A. Aggarwal, C. K. Glass, D. W. Rose, and M. G. Rosenfeld. 2004. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 116:511-526. [DOI] [PubMed] [Google Scholar]
- 30.Rishi, A. K., Z. M. Shao, R. G. Baumann, X. S. Li, M. S. Sheikh, S. Kimura, N. Bashirelahi, and J. A. Fontana. 1995. Estradiol regulation of the human retinoic acid receptor alpha gene in human breast carcinoma cells is mediated via an imperfect half-palindromic estrogen response element and Sp1 motifs. Cancer Res. 55:4999-5006. [PubMed] [Google Scholar]
- 31.Roman, S. D., C. J. Ormandy, D. L. Manning, R. W. Blamey, R. I. Nicholson, R. L. Sutherland, and C. L. Clarke. 1993. Estradiol induction of retinoic acid receptors in human breast cancer cells. Cancer Res. 53:5940-5945. [PubMed] [Google Scholar]
- 32.Seewaldt, V. L., B. S. Johnson, M. B. Parker, S. J. Collins, and K. Swisshelm. 1995. Expression of retinoic acid receptor beta mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 6:1077-1088. [PubMed] [Google Scholar]
- 33.Sirchia, S. M., A. T. Ferguson, E. Sironi, S. Subramanyan, R. Orlandi, S. Sukumar, and N. Sacchi. 2000. Evidence of epigenetic changes affecting the chromatin state of the retinoic acid receptor beta2 promoter in breast cancer cells. Oncogene 19:1556-1563. [DOI] [PubMed] [Google Scholar]
- 34.Sirchia, S. M., M. Ren, R. Pili, E. Sironi, G. Somenzi, R. Ghidoni, S. Toma, G. Nicolo, and N. Sacchi. 2002. Endogenous reactivation of the RARbeta2 tumor suppressor gene epigenetically silenced in breast cancer. Cancer Res. 62:2455-2461. [PubMed] [Google Scholar]
- 35.Stirzaker, C., J. Z. Song, B. Davidson, and S. J. Clark. 2004. Transcriptional gene silencing promotes DNA hypermethylation through a sequential change in chromatin modifications in cancer cells. Cancer Res. 64:3871-3877. [DOI] [PubMed] [Google Scholar]
- 36.Sucov, H. M., K. K. Murakami, and R. M. Evans. 1990. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc. Natl. Acad. Sci. USA 87:5392-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun, S. Y., H. Wan, P. Yue, W. K. Hong, and R. Lotan. 2000. Evidence that retinoic acid receptor beta induction by retinoids is important for tumor cell growth inhibition. J. Biol. Chem. 275:17149-17153. [DOI] [PubMed] [Google Scholar]
- 38.Toulouse, A., J. Morin, M. Pelletier, and W. E. Bradley. 1996. Structure of the human retinoic acid receptor beta 1 gene. Biochim. Biophys. Acta 1309:1-4. [DOI] [PubMed] [Google Scholar]
- 39.Tsai, S., S. Bartelmez, R. Heyman, K. Damm, R. Evans, and S. J. Collins. 1992. A mutated retinoic acid receptor-alpha exhibiting dominant-negative activity alters the lineage development of a multipotent hematopoietic cell line. Genes Dev. 6:2258-2269. [DOI] [PubMed] [Google Scholar]
- 40.Widschwendter, M., J. Berger, M. Hermann, H. M. Muller, A. Amberger, M. Zeschnigk, A. Widschwendter, B. Abendstein, A. G. Zeimet, G. Daxenbichler, and C. Marth. 2000. Methylation and silencing of the retinoic acid receptor-beta2 gene in breast cancer. J. Natl. Cancer Inst. 92:826-832. [DOI] [PubMed] [Google Scholar]
- 41.Xu, L., C. K. Glass, and M. G. Rosenfeld. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 9:140-147. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida, M., M. Kijima, M. Akita, and T. Beppu. 1990. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265:17174-17179. [PubMed] [Google Scholar]