Abstract
The growth factor independent 1 (Gfi1) transcriptional regulator oncoprotein plays a crucial role in hematopoietic, inner ear, and pulmonary neuroendocrine cell development and governs cell processes as diverse as self-renewal of hematopoietic stem cells, proliferation, apoptosis, differentiation, cell fate specification, and oncogenesis. However, the molecular basis of its transcriptional functions has remained elusive. Here we show that Gfi1 recruits the histone lysine methyltransferase G9a and the histone deacetylase 1 (HDAC1) in order to modify the chromatin of genes targeted for repression by Gfi1. G9a and HDAC1 are both in a repressive complex assembled by Gfi1. Endogenous Gfi1 colocalizes with G9a, HDAC1, and K9-dimethylated histone H3. Gfi1 associates with G9a and HDAC1 on the promoter of the cell cycle regulator p21Cip/WAF1, resulting in an increase in K9 dimethylation at histone H3. Silencing of Gfi1 expression in myeloid cells reverses G9a and HDAC1 recruitment to p21Cip/WAF1 and elevates its expression. These findings highlight the role of epigenetics in the regulation of development and oncogenesis by Gfi1.
The Gfi1 locus emerged in an insertional mutagenesis survey of mouse T-cell lymphomas acquiring interleukin 2 growth independence (13). Accumulating evidence confirms its oncogenic potential (25, 43, 44, 46, 49, 57, 63). Gfi1 is a frequent target of proviral insertion in T-cell (1, 13, 43, 44, 46) and splenic marginal zone (49) lymphomas induced by the murine leukemia virus. Gfi1 cooperates with the oncoproteins Pim-1 and c-Myc in T-cell lymphomagenesis (43-45, 63). Gfi1 is aberrantly expressed in lung tumors (25, 49, 57).
Gene targeting experiments reveal an essential role for Gfi1 in normal development (8, 18-20, 24, 25, 31, 55, 60). The most obvious and surprising phenotype of Gfi1-deficient mice is a lack of mature granulocytes (19, 24). The absence of Gfi1 in myeloid progenitor cells blocks their differentiation into granulocytes in vitro. Gfi1 mutations can cause human neutropenia and derepress Ela2, which is the most frequently mutated gene in leukemia-predisposing forms of human neutropenia (3, 37). Gfi1-deficient mice and humans also manifest defective lymphopoiesis. The thymic cellularity of mice lacking Gfi1 is about 10% of that of wild-type mice. T-cell development partially arrests at the stage from CD44+ CD25+ to CD44− CD25+, and mature B cells are reduced in Gfi1-deficient mice (19, 24, 59). In humans carrying dominant-negative Gfi1 mutations, both peripheral T- and B-lymphocyte numbers are reduced (37). Another phenotype in mice is loss of hearing, because Gfi1 is required for inner ear hair cell differentiation and survival (55), and one Gfi1 target is Pou4f3, encoding a transcription factor whose mutation leads to deafness in mice and humans (17).
More recently, mouse gene targeting studies have identified Gfi1 as one of the few intrinsic regulators of hematopoietic stem cell (HSC) self-renewal (18, 60). Gfi1−/− HSCs demonstrate functional impairment in long-term competitive repopulation and serial transplantation assays (18). Bromodeoxyuridine incorporation and cell cycle analysis reveal hyperproliferation of Gfi1−/− HSCs, apparently resulting from depletion of p21Cip/WAF1 and elevated levels of E2F5 and E2F6. Through an unknown mechanism, this leads to an exhaustion of HSCs (18, 60).
Gfi1 and its closely related family member Gfi1b contain six C2H2 zinc fingers and a unique 20-residue amino-terminal Snail/Gfi1 (SNAG) domain (15, 54, 64). Both proteins recognize virtually identical DNA-binding sequences (54, 64), and both require their SNAG domains in order to act as transcriptional repressors in reporter assays (15, 54). The functionally uncharacterized region intervening between the SNAG domain and the zinc fingers bears no homology and differentiates Gfi1 from Gfi1b.
Gfi1 and Gfi1b are generally viewed as transcriptional repressors; however, Gfi1b has been reported to function as a transcriptional activator in an artificial reporter system (18, 35, 42, 48, 60). Interestingly, elimination of the Gfi1 binding site in the promoter of the β1 soluble guanylyl cyclase gene significantly decreased its activity in reporter assays (48), and in Gfi1-deficient HSCs the expression of the target gene p21Cip/WAF1 is markedly decreased (18, 60). In contrast, overexpression of Gfi1 in Jurkat human T cells (23) and Gfi1b in myeloid cells (54) represses p21Cip/WAF1. Thus, it is currently unclear whether the activation of genes by Gfi1 or Gfi1b is a proximal or distal effect. In one case, Gfi1 is also able to relieve STAT3 from PIAS3-mediated inhibition of its transcriptional activator function by virtue of Gfi1's interaction with PIAS3 (42). These findings suggest that Gfi1 and Gfi1b activity may lead to transcriptional activation or repression, depending on the specific cellular and promoter context.
The molecular mechanisms through which Gfi1 and Gfi1b elicit their repressive effect remain elusive. Gfi1 recruits the cofactor Eto and histone deacetylases (HDACs) to its target promoter in order to repress transcription independently of the SNAG domain (30), indicating that it might act as a sequence-specific scaffold protein capable of recruiting chromatin-modifying enzymes and other cofactors to targeted promoters. Histone modifications, including phosphorylation, acetylation, methylation, and ubiquitination, have been implicated in transcriptional regulation of gene expression (4, 61), under the “histone code” rubric (21). Histone acetylation largely correlates with transcriptional activation, and its reversal is linked to gene repression (27). Hence, HDACs act as corepressors (53), with a few exceptions (29). Histone methylation, particularly at histone H3 lysines, has been linked to distinct effects on transcriptional regulation, depending on the site and particular (mono-, di-, or trimethylation) modification (26, 50). In mammalian cells, four enzymes (EuHMTase1, SETDB1, Suv39H1, and G9a) are known to methylate H3 lysine 9 (H3-K9) (34, 40, 51, 56). Suv39H1 specifically methylates H3-K9 at pericentric heterochromatin (38, 39, 41), though Suv39H1 may also direct gene repression within euchromatin (32). G9a methylates H3-K9 and weakly methylates H3-K27 (51). Deletion of G9a is embryonically lethal and results in loss of euchromatic mono- and dimethylation (38, 41, 52). Sequence-specific transcription factors recruit G9a to target genes (5, 16, 33). Here, we demonstrate that Gfi1 interacts with G9a and recruits G9a and HDAC1 to its target promoters, including p21Cip/WAF1 and other cell cycle regulators, in order to repress transcription through histone H3-K9 dimethylation.
MATERIALS AND METHODS
Cell culture.
HL-60 cells (generous gift of S. Collins) were maintained and cultured in Iscove's modified Dulbecco's medium containing 20% fetal bovine serum. HeLa cells (ATCC) were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Jurkat cells (ATCC) were maintained in RPMI 1640 medium containing 10% fetal calf serum. Cells were grown in a humidified incubator at 37°C with 5% CO2. All media (Invitrogen) were supplemented with 1% l-Gln and 1% antibiotic-antimycotic solution.
Plasmids.
The following plasmids were generous gifts: pCMV5-Gfi1 (P. N. Tsichlis), pcDNA3.1HA-G9a (K. L. Wright), (Myc)3-Suv39H1 (T. Jenuwein), pCDNA3.1(−)-HDAC1 (K. Robertson), and pCMX-hHDAC1-Flag (R. M. Evans). pCS2+Myc-Gfi1 was described previously (10). The plasmids containing various Gfi1 truncations were constructed by amplifying the corresponding regions of the human Gfi1 cDNA from pCS2+Gfi1 (37) and inserting them into the EcoRI/XbaI sites of the pCS2+Myc vector. The plasmids containing glutathione S-transferase (GST)-fused histone H3 (residues 1 to 84), either wild type or the noted lysine substitution mutants, were constructed by inserting the synthesized double-stranded oligonucleotides, which cover the corresponding region of human histone H3, into the EcoRI/XhoI sites of the pGEX-4T-3 vector (Amersham).
ChIP.
HeLa cells were transiently transfected using Lipofectamine Plus reagent (Invitrogen). At 48 h after transfection, the cells were subjected to chromatin immunoprecipitation (ChIP) assays, which were performed as described previously (10), with minor modifications. Briefly, the transfected HeLa cells were fixed with 1% formaldehyde for 10 min at 37°C and the reaction was terminated with 0.125 M glycine. Cells were sonicated in 1× radioimmunoprecipitation assay (RIPA) buffer (containing Complete proteinase inhibitor cocktail; Roche) on ice to generate soluble chromatin complexes with the length of DNA fragments less than 1 kb by using a Fisher Scientific model 500 ultrasonic dismembrator. The equivalent of ∼2 × 106 cells (1.5 ml soluble chromatin) was used per reaction with the following antibodies: anti-Gfi1 (N-20) and Gfi1 (N-20) (3 μg per reaction; sc-8558; Santa Cruz), anti-c-Myc (9E10) (3 μg per reaction; 1667203; Roche), antihemagglutinin (anti-HA; 3 μg per reaction; 1867423; Roche), H3 (dimethyl K9) antibody (0.5 μg per reaction; ab7312; Abcam; or 07-441; Upstate Biotechnology), and anti-acetyl-histone H3 (AcyH3; 3 μg per reaction; 06-599; Upstate Biotechnology). The corresponding normal goat, mouse, or rabbit immunoglobulins G (IgGs; Santa Cruz) were used in control experiments. Semiquantitative PCR was performed with the appropriate primer pairs which were designed based on the genomic sequences corresponding to each of the genes analyzed by using PRIMER3 software (http://www.genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). The sequences and product sizes of the primers for Ets2, p21Cip/WAF1, E2F5, c-Myc, and Gfi1 were described previously (10). The primers for PDE4D were 5′-TGAAACCCCACACAGTTGTCAC-3′(forward) and 5′-TGTTAGGGCTCCAGGACAAGCTTG-3′(reverse). Each experiment was performed at least three times, and typical data are shown.
Coimmunoprecipitation.
Coimmunoprecipitation assays were performed as described previously (11). Briefly, 40 hours after transient transfection, HeLa cells were harvested and lysed (7) in 1.5 ml of ice-cold RIPA buffer with Complete proteinase inhibitor cocktail. Cell lysates were cleared by centrifugation at 15,000 ×g for 30 min twice at 4°C. For each assay, 200 μl of the above cell lysates was incubated with 0.6 μg primary antibody, 140 μg bovine serum albumin, and 20 μl protein A or G Sepharose beads (Jackson Immunoresearch) in 1.4 ml RIPA buffer at 4°C overnight. Immunoprecipitates were collected and washed four times with 1.5 ml phosphate-buffered saline (PBS) and then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and detected by Western blotting. For endogenous coimmunoprecipitation assays, HL-60 cells (∼2 × 106 cells per reaction) were harvested and washed twice in PBS. Cells were then lysed by sonication in RIPA buffer and cleared as described above. Cell lysates were subjected to immunoprecipitation with 1 μg primary antibody, 140 μg bovine serum albumin, and 20 μl protein A or protein G Sepharose beads in 1.4 ml RIPA buffer with 4°C overnight incubation. G9a and Gfi1 coimmunoprecipitation studies were additionally performed with the Catch-and-Release spin column system (Upstate Biotechnology), following the manufacturer's protocol.
Expression and purification of GST fusion proteins.
GST-histone H3 (1-84, wild type and mutants) fusion proteins were expressed in Escherichia coli strain BL21(DE3) under 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) induction and purified using glutathione-Sepharose 4B beads (Amersham) according to the supplier's instructions.
Semiquantitative and real-time RT-PCR analysis.
Total RNA was prepared using the Absolutely RNA reverse transcription-PCR (RT-PCR) miniprep kit (Stratagene). One microgram of total RNA was used to produce cDNAs with oligo(dT)12 primer by superscript III RNA polymerase (Invitrogen). Primers were designed based on the cDNA sequences corresponding to each of the genes analyzed by using PRIMER3 software. The primer sequences for Ets2, p21Cip/WAF1, E2F5, c-Myc, and Gfi1 were described previously (10). The primers for PDE4D and β-actin are as follows: PDE4D forward, 5′-CGTGAATGGTACCAGAGCACAATC-3′; PDE4D reverse, 5′-ACTTGACTGCCACTGTCCTTTTCC-3′; β-actin forward, 5′-ACCCTTTCTTGACAAAACCTAACTT-3′; β-actin reverse, 5′-CTGTAACAATGCATCTCATATTTGG-3′. PCR conditions were determined previously to be in the linear range of amplification. The RT-PCR products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. Quantitative real-time RT-PCR was performed using an ABI 7300 real-time PCR system. TaqMan gene expression assays were purchased from Applied Bioystems (Ets2, HS00232009_ml; p21Cip/WAF1, HS00355782_ml; E2F5, HS00231092_ml; c-Myc, HS00509030_ml; PDE4D, HS00174805_ml; human 18S rRNA, 4319413E; human glyceraldehyde-3-phosphate dehydrogenase [GAPDH], 4326317E), and the relative quantification method with triplicate samples was used.
In vitro histone methyltransferase assay.
Cell extracts were prepared from HL-60 cells or HeLa cells 48 h posttransfection and were subjected to immunoprecipitation with anti-Gfi1 or control IgG (0.8 μg per sample in 1.5-ml volume) in RIPA buffer. After incubation overnight at 4°C, the immunoprecipitates were washed four times with PBS and subjected to in vitro histone methyltransferase assays, performed as described previously (16). Histone mixtures (including histone H1 and core histones; Roche), purified histone H3 and H4 (Roche or Upstate Biotechnology), synthesized H3(1-20) peptides (Upstate Biotechnology), or purified GST fusion proteins were incubated with 0.7 μCi per sample 3H-labeled S-adenosylmethionine (Amersham) in the presence of immunoprecipitated products or purified G9a (Upstate Biotechnology). The 40-μl reaction mixture was incubated at 30°C for 60 min in a reaction buffer containing 50 mM Tris-HCl and 0.5 mM dithiothreitol. Proteins were separated by SDS-PAGE and visualized by Coomassie blue staining and autoradiography.
Western blots.
Western blot assays were performed using the ECL Western blot analysis system (Amersham) or Visualizer detection kit (Upstate Biotechnology). The antibodies and their working concentrations were as follows: Gfi1 (N-20) (1:500; sc-5545; Santa Cruz), anti-Myc (9E10) (1:20,000; 1667203; Roche), anti-HA (1:1,000; 1867423; Roche), anti-G9a (1:500; 07-551; Upstate Biotechnology), and anti-HDAC1 (1:2,000; PA1-860; Affinity Bioreagents).
Cell cycle analysis.
Plasmids pCMV5-Gfi1 and pCS2+βGal were cotransfected with phrGFPII-N (Stratagene) into HeLa cells. Forty-eight hours after transfection, the cells were harvested, counted, and stained with 10 μg/ml Hoechst 33342 (Molecular Probes) for 45 min at 37°C. Green fluorescent protein (GFP) intensity and DNA content were analyzed with a Becton Dickinson LSR flow cytometer (University of Washington, Department of Immunology, Cell Analysis Facility) and Tree Star FlowJo 4.6.2 on an Apple Mac OS X computer. GFP staining was also confirmed by epifluorescence microscopy (not shown). Cell cycle analysis in the presence of expressed Gfi1 or β-galactosidase (β-Gal), but without GFP cotransfection, was independently confirmed with propidium iodide staining assays without coexpression of GFP (not shown).
RNA interference assays.
Predesigned short interfering RNAs (siRNAs) against Gfi1 (Gfi1-1 and Gfi1-2, corresponding to siRNA 3369 and 3465, respectively) and Silencer β-actin siRNA control (607; containing β-actin siRNA and a negative-control siRNA) were purchased from Ambion. (Both Gfi1-1 and Gfi1-2 behaved similarly [not shown], and Gfi1-2 was used throughout.) Alexa Fluor 555- and 488-labeled Gfi1-1 siRNA were synthesized by QIAGEN. (Both fluors behaved similarly [not shown], and Fluor 488 was used throughout.) Exponentially growing HL-60 cells were concentrated to 5 × 106 to 10 × 106 cells/ml in 200 μl siPORT buffer (Ambion). A 200 nM (or, with the same effect, 400 nM [not shown]) concentration of siRNAs was added immediately before the electroporation step. Electroporation was performed with a GenePulser XCell (Bio-Rad Laboratories) according to the manufacturer's instructions. siRNA uptake was confirmed 8 h after transfection with epifluorescence microscopy. Alexa Fluor 488-positive cells were sorted 20 h after transfection using a Becton Dickinson FACSVantage SE sorter (University of Washington, Department of Immunology, Cell Analysis Facility). Sorted cells were cultured for 2 more days and then subjected to total RNA preparation.
RESULTS
Ectopic expression of Gfi1 in HeLa cells decreases proliferation and retards cell cycle progression.
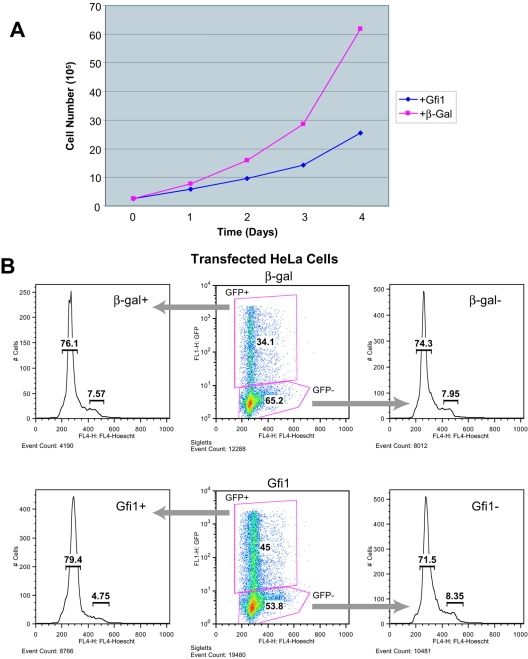
Gfi1 regulates cell proliferation and cell cycle progression in a cell context-specific manner. For instance, Gfi1 promotes proliferation of lymphoid cells (15, 18) but restricts proliferation of hematopoietic stem cells and myeloid cells (18, 60). Toward the goal of developing an in vitro model allowing us to experimentally define the molecular functions of Gfi1, we tested the effect of Gfi1 on HeLa human cervical carcinoma cells, in which it is not normally expressed. We found that transient expression of Gfi1 by transfection in HeLa cells results in significant inhibition of their rate of growth, compared to expression of an E. coli β-Gal control (Fig. 1A). To determine if Gfi1 affects the cell cycle progression of HeLa cells, we cotransfected Gfi1 or β-Gal expression vectors with a GFP-expressing plasmid and performed cell cycle analysis on GFP-positive and GFP-negative fractions (which, respectively, should mark populations expressing or not expressing Gfi1 or β-Gal). Expression of β-Gal had no effect on cell cycle progression of HeLa cells, but the Gfi1+/GFP+ population did have more nonproliferating (G0/G1) and fewer actively proliferating (S/G2/M) cells (Fig. 1B). Thus, expression of Gfi1 in HeLa cells alters cell cycle progression in a manner similar to its effects in HSCs and myeloid cells. Since several cell cycle-regulating genes have been identified as Gfi1 targets in myeloid and lymphoid cells (8, 10), it is possible that ectopically expressed Gfi1 also regulates their expression in HeLa cells.
FIG. 1.
Ectopic expression of Gfi1 in HeLa cells inhibits growth and retards cell cycle. (A) Growth curve of HeLa cells transiently transfected with vectors expressing Gfi1 or β-Gal, as a control. (B) Flow cytometric analysis of HeLa cells transiently cotransfected with either Gfi1 or β-Gal along with GFP expression vectors. Cells expressing or not expressing GFP (as a marker of transfection) were sorted and analyzed for DNA content by Hoechst 33342 staining.
Ectopically expressed Gfi1 binds to endogenous target genes and results in transcriptional repression in HeLa cells via histone H3-K9 dimethylation.
We have previously identified Gfi1 target genes in myeloid and T-lymphocyte cell lines (8, 10). Given that Gfi1 has the potential to regulate the cell cycle in HeLa cells, we next determined if it can recognize the same target genes in HeLa cells. We transiently transfected Gfi1 and tested the occupancy of Gfi1 at the promoters at a subset of genes involved in cell cycle regulation (Ets2, p21Cip/WAF1, E2F5, and c-Myc) by ChIP. As expected, Gfi1 binds to these known targets but not to the arbitrarily chosen, nontarget phosphodiesterase 4D (PDE4D) gene (Fig. 2A). Further, the recruitment of Gfi1 represses expression of these target genes but has no effect on the negative controls PDE4D, β-actin, and GAPDH (Fig. 2B). Since histone H3-K9 methylation is linked to gene repression, we checked the pattern of histone methylation at target promoters by using an antibody specific for dimethylated H3-K9 and found that the methylation of H3-K9 accumulates after Gfi1 binding (Fig. 2C). Based on these results, we hypothesize that Gfi1 recruits histone modifiers to its target promoters in order to repress gene expression through epigenetic mechanisms.
FIG. 2.
Gfi1 transcriptionally represses its target genes via histone H3-K9 dimethylation in HeLa cells. (A) ChIP assay demonstrating target promoter occupancy with Gfi1 overexpression compared to β-Gal-expressing negative control in transfected HeLa cells. Antibodies to Gfi1, but not normal IgG, immunoprecipitate (IP) chromatin fragments from which specific sequences within each target promoter can be PCR amplified and resolved by agarose gel electrophoresis with ethidium staining. (B) Semiquantitative RT-PCR (products resolved by ethidium-stained agarose gels, left) and real-time RT-PCR (TaqMan) assays (graphed results, right) confirm that Gfi1 promoter occupancy in transfected HeLa cells coincides with transcriptional repression, compared to nontarget β-actin, PDE4D, and GAPDH negative controls. ddH2O, double-distilled water as PCR control. (C) Recruitment of Gfi1 to target genes increases dimethylated histone H3-K9 (diMeH3K9), as shown by ChIP assay in transfected HeLa cells.
Gfi1 interacts in vivo with G9a predominately through its amino terminus.
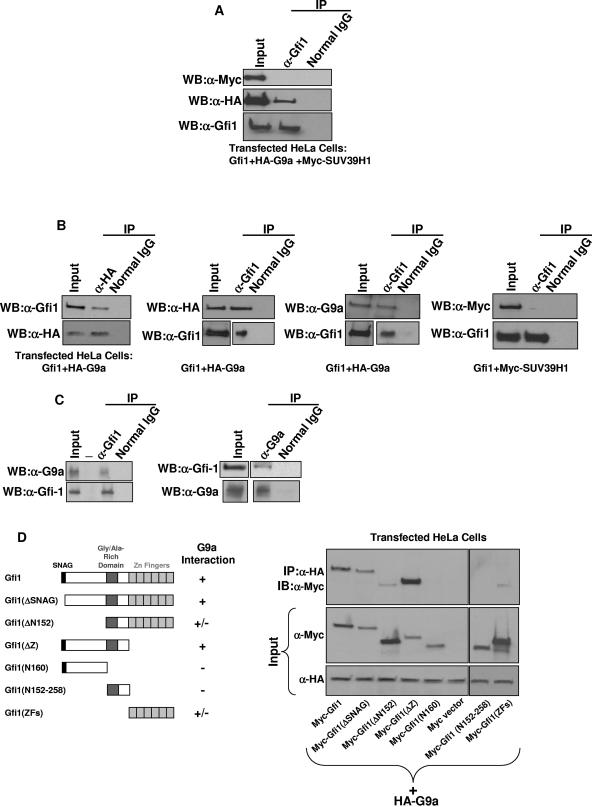
For at least two of the four known histone H3-K9 methyltransferases (34, 40, 51, 56), G9a and Suv39H1, it is the interaction with a DNA-binding transcription factor that directs them to target promoters (5, 16, 32, 33). To determine if Gfi1 has a role in the targeting of these methyltransferases, we cotransfected Gfi1 with both HA epitope-tagged G9a and Myc epitope-tagged Suv39H1 into HeLa cells, and the cell lysate was immunoprecipitated with an antibody against Gfi1. As shown in Fig. 3A, Western blot detection with antibodies to the corresponding epitope reveals that only HA epitope-tagged G9a, and not Myc epitope-tagged Suv39H1, coimmunoprecipitated with Gfi1. To exclude the possibility that a stronger association with G9a outcompetes detectable interactions between Gfi1 and Suv39H1, we cotransfected Gfi1 with HA-G9a or Myc-Suv39H1 separately and performed immunoprecipitation with antibodies against Gfi1, HA tag, G9a, or Myc tag. Again, we observed that Gfi1 specifically coimmunoprecipitates with G9a but not Suv39H1 (Fig. 3B). To examine whether the interaction between Gfi1 and G9a occurs in vivo, we attempted coimmunoprecipitation of the endogenous proteins in lysates from HL-60 human promyelocytes (which natively express both Gfi1 and G9a). We found that the Gfi1 antibody can specifically immunoprecipitate endogenous G9a and, vice versa, the G9a antibody can specifically immunoprecipitate endogenous Gfi1 (Fig. 3C).
FIG. 3.
Gfi1 associates with G9a in vivo. (A) Antibodies to Gfi1 coimmunoprecipitate Gfi1 with G9a, but not with Suv39H1, when plasmids expressing native Gfi1, HA epitope-tagged G9a, and Myc epitope-tagged Suv39H1 are cotransfected together into HeLa cells, as revealed by Western blots (WB) with detection by the indicated antibodies. (B) Gfi1 coimmunoprecipitates with HA-tagged G9a when both are transfected into HeLa cells, using either anti-HA or anti-Gfi1 antibodies for immunoprecipitation and anti-Gfi1 and anti-HA or anti-G9a antibodies (three left panels, respectively) for Western blot detection; however, Gfi1 does not coimmunoprecipitate with Myc-tagged Suv39H1 even when both are coexpressed in HeLa cells (as assayed by immunoprecipitation with anti-Gfi1 and Western blotting with anti-Myc, right panel). (C) Endogenous Gfi1 forms an immunoprecipitable complex with endogenous G9a in HL-60 cells, demonstrated by immunoprecipitation with either anti-Gfi1 or anti-G9a. Similar results were also observed in U937 promonocytes and Jurkat cells (not shown). (Dash denotes blank lane.) (D) Truncated forms of Gfi1 used to identify domains required for association with G9a. Myc-tagged Gfi1 mutants were cotransfected with HA-tagged G9a into HeLa cells; immunoprecipitation was performed with anti-HA, and Western blotting was performed with anti-Myc.
To identify the Gfi1 domains contributing to G9a interaction, we constructed a series of Gfi1 truncations and coexpressed them with HA-G9a in HeLa cells. Coimmunoprecipitation assays showed that deletion of the SNAG domain of Gfi1 did not abolish the interaction with G9a (Fig. 3D). In contrast, deletion of the N-terminal 152 amino acid residues, or everything but the zinc fingers (amino acids 1 to 257), however, severely diminished association with G9a. Conversely, the N-terminal non-zinc finger region alone (amino acids 1 to 258) is sufficient for the interaction with G9a, while the first 160 amino acids and the region between residues 152 and 258 are necessary, but each of themselves insufficient, for Gfi1 to coimmunoprecipitate with G9a. In conclusion, Gfi1 associates with G9a mainly through Gfi1's first 258 residues, though its zinc fingers can weakly contribute.
Gfi1 specifically recruits G9a-type H3-K9 methyltransferase activity in vivo.
We next tested whether Gfi1 can form a complex possessing histone lysine methyltransferase activity. We transfected Gfi1 or a control plasmid (expressing β-Gal) into HeLa cells and subjected the cell lysates to immunoprecipitation with a Gfi1-specific antibody or a control IgG. Each of the immunoprecipitated complexes was then assayed for methyltransferase activity with free histones (including H1 and core histones) as the substrate. None of the immunocomplexes derived from cells expressing the control plasmid or immunoprecipitated with control IgG possessed histone methyltransferase activity. In contrast, the immunoprecipitated Gfi1 complex induced tritium-labeled S-adenosylmethionine incorporation into primarily histone H3 (Fig. 4A). Moreover, coexpression of Gfi1 in HeLa cells with G9a, but not Suv39H1 or control vectors, increases the histone methyltransferase activity of the immunoprecipitated Gfi1 complex (Fig. 4B), indicating that G9a is responsible. To determine if the methyltransferase activity of the Gfi1 complex is mediated through the interaction with G9a, we coexpressed G9a in HeLa cells with either Myc-tagged full-length Gfi1 or just the N-terminal 160 residues of Gfi1 (which did not interact with G9a [Fig. 2D]) and immunoprecipitated with an anti-Myc antibody. Full-length Gfi1 recruited strong histone methyltransferase activity, but the N-terminal fragment of Gfi1 that is insufficient for G9a interaction did not (Fig. 4C). These observations indicate that recombinantly expressed Gfi1 recruits methyltransferase activity through its association with G9a. Similar immunoprecipitation experiments in HL-60 cells indicate that the endogenous Gfi1 complex is also able to specifically recruit histone methyltransferase activity in vivo (Fig. 4D).
FIG. 4.
Gfi1 complex recruits G9a-type histone methyltransferase activity. (A) Immunoprecipitated Gfi1-containing complex possesses histone methyltransferase activity. Vectors expressing either Gfi1 or β-Gal, as a negative control, were transfected into HeLa cells, and cell extracts were immunoprecipitated with anti-Gfi1 or nonspecific antibody, then incubated with histone mixtures (H1 and core histones) and S-[3H]adenosylmethionine, and resolved by SDS-PAGE with Coomassie blue staining (bottom panel). Autoradiography (top panel) demonstrates transfer of tritiated methyl to histones. (B) Coexpression of G9a in HeLa cells, but not Suv39H1, increases histone methyltransferase activity of the immunoprecipitated Gfi1 complex. G9a was HA tagged, Suv39 was Myc tagged, and immunoprecipitation was performed with anti-Gfi1. (Western blotting with the indicated antibodies was performed on the cell extract to confirm expected expression [bottom three panels].) (C) Histone methyltransferase activity recruitment by Gfi1 requires interaction with G9a. Immunoprecipitates of full-length Gfi1, but not the Gfi1(N160) fragment (which does not interact with G9a), demonstrate histone methyltransferase activity. [Gfi1 was Myc tagged, and anti-Myc was used for immunoprecipitation, because the Gfi1 antibody does not recognize the Gfi1(N160) fragment.] (D) Endogenous Gfi1 complex immunoprecipitated from HL-60 cells possesses histone methyltransferase activity, and the Gfi1 immunoprecipitate was similarly assayed. (E) The Gfi1 complex predominantly methylates histone H3, as demonstrated by comparing a mixture of histone substrates to those enriched for H3. (F) The Gfi1 complex methylates H3 but not H4. (G) The Gfi1 complex methylates H3-K9, but not H3-K4. A synthetic peptide corresponding to the amino-terminal 20 residues of H3 and one in which the K4 was predimethylated are both methylated, but predimethylation of K9 diminishes its acceptance as a substrate. (H) Gfi1 complex demonstrates the same site specificity as purified G9a on a series of recombinant histone H3 substrates. The first 84 amino acids of H3, in which lysines were each mutated to an arginine, were purified as GST fusion proteins and tested as substrates. Immunoprecipitates were prepared from HeLa cells transfected with the indicated vector, and their activity was compared to that of purified G9a. Cotransfection with a G9a expression vector does not add significant activity to the Gfi1 immunoprecipitate. Asterisks mark positions corresponding between Coomassie blue-stained and autoradiogram-detected proteins on SDS-polyacrylamide gels.
By using a mixture of free histones, or pure histone H3 or H4, as substrate for in vitro methyltransferase assays, we determined that the recombinant Gfi1 complex in HeLa cells specifically methylates H3 (Fig. 4E and F) and weakly methylates H1 (data not shown), which is consistent with the substrate specificity of G9a (16). We next determined the site of histone H3 methylation. The recombinant Gfi1 complex methylated a synthetic peptide corresponding to the first 20 residues of histone H3; it also methylated the same peptide with the lysine at position four (K4) predimethylated but only poorly methylated the peptide when the lysine at position nine (K9) was predimethylated (Fig. 4G), suggesting that the methyltransferase recruited by Gfi1 is specific for H3-K9. We next prepared a series of recombinant substrates (as GST fusion proteins) containing the N-terminal 84 amino acids of human histone H3 or mutants with a single lysine-to-arginine change at selected sites (Fig. 4H). The Gfi1 complex methylated the wild-type H3 substrate as well as the mutants replaced at K4, K36, and K79 (Fig. 4H). Substitution at K27 slightly reduced methylation of the H3 substrate. Strikingly, the substitution at K9 blocked methylation. These data confirm strong H3-K9 and weak H3-K27 specificity of the Gfi1 complex (Fig. 4H). We note that the methylation pattern of these recombinant substrates is unaffected by coexpression of exogenous G9a in HeLa cells prior to immunoprecipitation of the Gfi1 complex (Fig. 4H) and is similar to the pattern observed with purified recombinant G9a protein (Fig. 4H), indicating that Gfi1 specifically recruits G9a-type methyltransferase activity.
HDAC1 and G9a are in the same complex assembled by Gfi1.
It has been shown elsewhere that Gfi1 can associate with histone deacetylases (30). We confirmed that Gfi1, when expressed in HeLa cells, coimmunoprecipitates with endogenous HDAC1 (Fig. 5A). Similarly, in HL-60 cells (which natively express Gfi1), Gfi1 coimmunoprecipitates with endogenous HDAC1 (Fig. 5B). We then determined what domains are responsible for HDAC1 coimmunoprecipitation by utilizing a series of Gfi1 deletions (Fig. 5C): removal of the SNAG domain impaired coimmunoprecipitation, while the first 152 amino acids are insufficient. The region from residues 152 to 258 associates weakly. Fragments of Gfi1 containing either the first 258-amino-acid domain or the zinc finger region do, however, associate with HDAC1. Therefore, the N-terminal, non-zinc finger domain of Gfi1 associates with both G9a and HDAC1, while the zinc finger region can associate with HDAC1 but only weakly with G9a.
FIG. 5.
G9a and HDAC1 are in the same complex with Gfi1. (A) Transfected Gfi1 coimmunoprecipitates with endogenous HDAC1 in HeLa cells. (B) Endogenous Gfi1 forms an immunoprecipitable complex with endogenous HDAC1 in HL-60 cells. Immunoprecipitation was carried out with either anti-Gfi1 or anti-HDAC1 antibody. Similar results were also observed with U937 and Jurkat cells (not shown). (C) Gfi1 domains required for association with endogenous HDAC1 in HeLa cells are identified with truncations of Gfi1 (same approach as shown in Fig. 3D). (D) Both HDAC1 and G9a form stable complexes with Gfi1 that are resistant to washing at increasing salt concentrations following immunoprecipitation. (E) G9a and HDAC1 exist in the same complex recruited by Gfi1. Vectors expressing native HDAC1, HA-tagged G9a, and Myc-tagged Gfi1 were transfected into HeLa cells; immunoprecipitation was performed with anti-HDAC1; and Western blots were detected with antibodies specific for each of the three proteins. The Gfi1 expression vector was replaced with one containing β-Gal to serve as a negative control. (Ten percent of input is shown, to normalize signals.)
Gfi1 recruits both G9a and HDAC1; however, G9a and HDAC1 could be either in distinct complexes or in the same complex. Size-exclusion chromatography of lysates of Jurkat cells, which natively express Gfi1 and G9a, shows that G9a cofractionates with both Gfi1 and HDAC1 (not shown), suggesting that G9a and a portion of endogenous HDAC1 might be in a common complex assembled by Gfi1. To test this possibility, we first examined how strongly G9a and HDAC1 each associate with Gfi1. We cotransfected HeLa cells with Myc-tagged Gfi1 and either HDAC1 or G9a. Each cell extract was divided into eight aliquots. One aliquot was reserved as an “input” control; another was subjected to control immunoprecipitation with nonspecific normal IgG, and each of the remainder was immunoprecipitated with the specific antibody (anti-HDAC1 for Gfi1+HDAC1 or anti-G9a for Gfi1+G9a) and then washed with increasing NaCl concentrations. Finally, the washed complexes were detected by Western blot analysis with an anti-Myc antibody. As shown in Fig. 5D, both associations, Gfi1+HDAC1 and Gfi1+G9a, were resistant to dissociation at salt concentrations exceeding 500 mM (RIPA plus 400 mM NaCl). Thus, G9a and HDAC1 stably associate with Gfi1, implying that, if G9a and HDAC1 are in the same complex assembled by Gfi1, then they should be able to be coimmunoprecipitated with each other. To test this, we cotransfected HeLa cells with all three together (Myc-tagged Gfi1, HA-G9a, and HDAC1) and immunoprecipitated them with an HDAC1-specific antibody. (For a control, we replaced Gfi1 with β-Gal.) As predicted, in the presence of Gfi1, the anti-HDAC1 antibody was able to strongly immunoprecipitate G9a (Fig. 5E, left), while in the absence of Gfi1, the anti-HDAC1 antibody barely immunoprecipitated G9a (Fig. 5E, right). (The weak association between HDAC1 and G9a in the control experiment could have resulted from endogenous proteins that bring the two together or could indicate that the two weakly and directly associate with one another, even without a third party.) We conclude that G9a and HDAC1 exist in a repressive complex assembled by Gfi1.
Gfi1 recruits G9a and HDAC1 to its target gene promoters in vivo and consequently increases methylation of H3-K9.
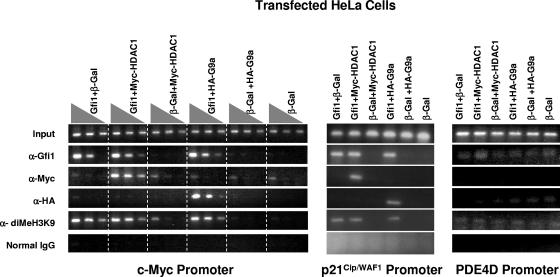
We have demonstrated that the transcriptional repressor Gfi1 forms a stable complex with G9a and HDAC1 that possesses H3-K9 methyltransferase activity and that targets of Gfi1 are subject to H3-K9 dimethylation. The most likely conclusion is that Gfi1 recruits G9a and HDAC1 to Gfi1 target promoters in vivo. To determine if this occurs, we cotransfected HeLa cells with vectors expressing either Gfi1 or a β-Gal control along with Myc-tagged HDAC1 or HA-tagged G9a and performed ChIP with antibodies against Gfi1 or the Myc tag or HA tag. Both Myc-tagged HDAC1 and HA-tagged G9a bound to the c-Myc promoter (Fig. 6), indicating that their recruitment is dependent on Gfi1. ChIP analysis of p21Cip/WAF1 (Fig. 6) and other Gfi1 target promoters (Ets2 and E2F5, not shown) produced similar results. The negative-control PDE4D promoter, which Gfi1 does not target, is not occupied by G9a or HDAC1. We then checked if H3-K9 dimethylation of the promoter correlates with occupancy by Gfi1, G9a, and HDAC1. H3-K9 dimethylation of the target promoters indeed requires expression and occupancy by Gfi1 (Fig. 6); therefore, expression of G9a and HDAC1 (either endogenously in HeLa cells or by overexpression through transfection) in the absence of Gfi1 is insufficient to achieve methylation of these repressible promoters. These results suggest that the demonstrated recruitment of HDAC1 and G9a to target genes is dependent upon Gfi1. Moreover, genes targeted for repression by Gfi1 are subject to epigenetic control through histone methylation and, by inference from Gfi1's association with HDAC1, probably also deacetylation.
FIG. 6.
Gfi1 recruits G9a and HDAC1 to the c-Myc and p21Cip/WAF1 promoters and increases H3-K9 methylation. HeLa cells were cotransfected with the noted combinations of plasmids and subjected to ChIP assays with antibodies against Gfi1, Myc tag, HA tag, dimethylated histone H3-K9, or control IgG. Each group of three lanes for the c-Myc promoter represents PCR analysis of threefold serial dilutions of the DNA templates. Analysis of the p21Cip/WAF1 promoter and the PDE4D promoter, included as a negative control, was performed at the highest of the three DNA concentrations.
Endogenous Gfi1 recruits G9a and HDAC1 to the p21Cip/WAF1 promoter and represses its expression in HL-60 cells.
We have shown that ectopic expression of Gfi1 in HeLa cells recruits G9a and HDAC1 to target genes and represses them through epigenetic mechanisms. However, in vivo, endogenous Gfi1 regulates its target genes in a promoter- and cell context-specific manner. To determine whether endogenous Gfi1 in HL-60 cells also represses its target genes through recruitment of G9a and HDAC1, we employed RNA interference assays to silence Gfi1 expression in vivo.
We found that an siRNA directed against Gfi1 effectively lowered quantities of Gfi1 protein in HL-60 cells and had no effect on expression of G9a, HDAC1, or β-actin, whereas a control siRNA against β-actin produced the expected and opposite effect, and a nonspecific siRNA had little effect on either Gfi1, G9a, HDAC1 or β-actin, as shown by Western blotting (Fig. 7A). Next, we treated HL-60 cells with versions of these siRNAs that were labeled with fluorescent dyes, so that individual cells taking up the siRNA could be tracked (Fig. 7B). We then used fluorescence-activated cell sorting to identify fluorescent and nonfluorescent populations that had either taken up or not taken up the siRNA, respectively (Fig. 7C). Finally, we measured transcriptional expression levels of Gfi1 and its target genes by real-time RT-PCR (Fig. 7D). The mRNA level of Gfi1 in the isolated Gfi1-siRNA fluorescently positive cells is about a third to a fifth lower than it is in either the fluorescently negative or similarly sorted β-actin-siRNA-treated cells; correspondingly, mRNA levels of the Gfi1 target gene, p21Cip/WAF1, were elevated by two- to threefold compared to the fluorescently negative or β-actin-siRNA-treated controls. In contrast, Gfi1 silencing actually reduced transcription of its targets c-Myc (Fig. 7D) and Ets2 and E2F5 (not shown). (Similar results appear in unsorted populations following siRNA treatment [not shown], although the effects are of less magnitude.) These gene silencing studies suggest that Gfi1 represses the expression of p21Cip/WAF1 in HL-60 cells. Given Gfi1's reliance on cellular context, it is perhaps not surprising that silencing of Gfi1 reduced transcription of c-Myc and other Gfi1 target genes, compared to our findings in HeLa cells.
FIG. 7.
Endogenous Gfi1 recruits G9a and HDAC1 to p21Cip/WAF1 promoter and transcriptionally represses the expression of p21Cip/WAF1 in HL-60 cells but does not have such effects on c-Myc. (A) Western blot demonstrating specificity of Gfi1, β-actin, and nonspecific control siRNAs in reducing protein levels in treated HL-60 cells. (B) Epifluorescence microscopy confirming uptake in HL-60 cells transfected with Alexa Fluor 488-labeled Gfi1 siRNA. Nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole). (C) Fluorescence-activated cell sorting of HL-60 cells electroporated with fluorescent Alexa Fluor 488-labeled siRNA to distinguish transfected from nontransfected populations. Left panel, side scatter profile for setting gates (pink); middle panel, cells not treated with siRNA; right panel, cells electroporated with Alexa Fluor 488-labeled Gfi1 siRNA with sorted populations identified. (D) Real-time RT-PCR (TaqMan) analysis of Gfi1 and p21Cip/WAF1 expression in control, untransfected, and transfected HL-60 cells sorted above. From left to right, HL-60 cells electroporated with unlabeled β-actin control siRNA (unsorted), electroporated with fluorescently labeled Gfi1 siRNA and sorted negatively for fluorescence (nontransfected), and electroporated with Gfi1 siRNA and sorted positively for fluorescence (transfected). (E) ChIP in HL-60 cells showing the presence of endogenous Gfi1, HDAC1, and G9a on p21Cip/WAF1 promoter and Gfi1 and HDAC1, but not G9a, on c-Myc promoter but neither Gfi1, HDAC1, nor G9a on control PDE4D promoter. (F) Specific silencing of Gfi1 by siRNA, but not nonspecific control siRNA (as shown by Western blotting, right panel), diminishes binding of G9a and HDAC1, diminishes incorporation of dimethylated histone H3-K9, and modestly increases incorporation of AcyH3, on the p21Cip/WAF1 promoter in HL-60 cells (as revealed by ChIP, left and middle panels). However, Gfi1 siRNA does not affect G9a or HDAC1 recruitment to the c-Myc promoter and correspondingly shows no changes in dimethylated histone H3-K9 and AcyH3.
Finally, we performed ChIP assays to determine if G9a and HDAC1 are present on the p21Cip/WAF1 and c-Myc promoters in HL-60 cells. Gfi1, as well as G9a and HDAC1, binds to the p21Cip/WAF1, but not the PDE4D control, promoter (Fig. 7E). However, only Gfi1 and HDAC1, not G9a, are present on c-Myc. When Gfi1 was silenced by siRNA, ChIP on unsorted cells reveals that the binding of G9a and HDAC1 to the p21Cip/WAF1 promoter was markedly diminished, in comparison to control treatment with a nonspecific siRNA that does not alter Gfi1 expression (Fig. 7F). There is corresponding reduction of p21Cip/WAF1 promoter H3-K9 dimethylation, but no apparent change in H3 acetylation. Contrastingly, on the c-Myc promoter, Gfi1 silencing has no effect on decreasing HDAC1 binding, G9a binding and H3-K9 dimethylation remain absent, and H3 acetylation is also unchanged. Thus, recruitment of G9a and HDAC1 to the p21Cip/WAF1 promoter in vivo depends on the presence of Gfi1 and this is reflected in the change in H3-K9 dimethylation. Gfi1 does not, however, recruit G9a to c-Myc, nor is it responsible for HDAC1 binding to c-Myc. The apparent absence of change in p21Cip/WAF1 promoter H3 acetylation with recruitment of HDAC1 could represent a limitation of ChIP, reflecting K9 methylation in a single nucleosome, while the neighboring nucleosomes remain acetylated and thus the overall ChIP signal is not significantly reduced. Alternatively, it may signify the contributions of other factors regulating acetylation at this locus, a possibility emphasized by the observation that HDAC1 binds to the c-Myc promoter but is G9a independent. The differences between the two promoters, with respect to Gfi1 recruitment of G9a and HDAC1, correlate with the opposite effects on expression that are induced by Gfi1 silencing in HL-60 cells.
DISCUSSION
Gfi1 is one of just a few known intrinsic regulators of HSCs (6, 9, 18, 60) and plays pivotal roles in hematopoiesis (18, 19, 24, 60, 62). Evidence of the complexity of its biological function includes not only observations that Gfi1 participates in distinct cellular events such as proliferation, apoptosis, differentiation, cell fate decisions, and tumorigenesis (20) but also the fact that Gfi1 functions in a cell context- and development-specific manner. For example, Gfi1 restricts proliferation of HSCs (18, 60) while promoting proliferation of lymphoid cells (19, 24, 37). Gfi1 target genes are similarly functionally diverse (10) and are further indicative of its involvement with varied processes at the molecular level (9). Moreover, Gfi1 regulates its target genes in a cell context-specific fashion. For instance, Gfi1 represses IL7Rα in CD8+ T cells but not in CD4+ cells (36). Gfi1 represses the expression of p21Cip/WAF1 in Jurkat cells (23), while Gfi1 is required for its expression in HSCs (18, 60). Therefore, it is of interest to investigate Gfi1's molecular mechanism. It had been previously shown that Gfi1 might recruit histone modifiers to repress gene expression (30). Here, we have demonstrated that Gfi1 associates with both G9a and HDAC1 in vivo and directs them to its target gene promoters to repress transcription.
The SNAG domain was initially thought to mediate the repressor function of Gfi1 (15), because deletion or point mutation of the SNAG domain diminishes its repressor activity in reporter assays. However, Gfi1's invertebrate homologs do not contain the SNAG domain but still act as repressors (20), and Gfi1 can maintain repressor activity in the absence of the SNAG domain (30). Here we have shown that deletion of the SNAG domain impaired but did not abolish the associations between Gfi1 and G9a and HDAC1 and that a considerable fraction of HDAC1 exists in the same G9a-containing complex that is recruited by Gfi1. Taken together, these findings point to at least three means through which Gfi1 acts as a transcriptional repressor: first, SNAG domain-mediated repression, the mechanism for which remains unclear but could relate to interference with the basal transcriptional apparatus; second, recruitment of G9a and HDAC1; and, third, recruitment of HDACs and other corepressors in the absence of G9a (30; D. Montoya-Durango etal., submitted). The SNAG domain could also participate in the latter two mechanisms. The functionally uncharacterized region of Gfi1 between the SNAG domains and the zinc fingers (residues 21 to 257) mediates association with both G9a and HDAC1, indicating that it contributes to Gfi1 repressor activity.
Histone H3-K9 modification is generally linked to gene repression. All of the four identified mammalian H3-K9 methyltransferases can be targeted to selected gene promoters by sequence-specific DNA-binding transcription factors. EuHMTase1 is recruited to E2F- and c-Myc-responsive genes (34). SETDB1 associates with the corepressor KAP-1 and is recruited to the Col11a2 gene promoter in NIH 3T3 cells (2, 47). Suv39H1 is recruited by Rb to the cyclin E promoter (32). G9a has been shown to interact with transcription factors PRDI-BF1, CDP/cut, and SHP and is recruited to their correspondingly targeted promoters (5, 16, 33).Interestingly, each of these three G9-associating transcription factors, as well as Gfi1, also interacts with HDACs. PRDI-BF1 recruits G9a and HDAC2 through distinct domains (16, 58), and the recruitment of HDAC is necessary for G9a-mediated repression (16). The domains of CDP/cut required for the interaction with HDAC1 overlap with those required for G9a interaction (33). The orphan nuclear receptor SHP interacts with G9a and HDAC1 through distinct domains, and G9a and HDAC1 act synergistically (5). Here we have shown that Gfi1 also associates with both G9a and HDAC1. The non-zinc finger region (residues 1 to 257) of Gfi1 is able to mediate association with both G9a and HDAC1, while the zinc fingers mediate strong association with HDAC1 and weakly with G9a. Our data and those of others (Montoya-Durango et al., submitted) indicate that a substantial proportion of HDAC1 is in the same Gfi1-assembled complex as G9a.
The development of multicellular organisms largely relies on epigenetic mechanisms to orchestrate the formation of distinct tissues and organs, since, with few exceptions, all cells in an individual contain the same genetic information. Specifically, many hematopoietic events occur under the control of epigenetic mechanisms. For instance, changes of histone modification patterns arise during B-cell development (14, 22). Gfi1 is an essential regulator of the self-renewal of HSCs and is involved in specification of multiple stages during hematopoiesis (9). Our finding that Gfi1 associates with histone H3-K9 methyltransferase G9a and HDAC1 in vivo provides new insight into the regulation of hematopoiesis. Furthermore, epigenetic inheritance is important during development, and epigenetic dysregulation is emerging as a major contributor to carcinogenesis (12). In particular, histone H3 methylation in myeloid leukemias differs from that of normal myeloid cells (28). Therefore, Gfi1's oncogenic potential could relate to its recruitment of histone modifiers and bears further investigation.
Acknowledgments
We thank M. Black and F. Lewis for assistance with cytometry and cell sorting and K. Williams for technical aid.
This work was supported by NIH grants HL79574 (H.L.G.), CA105152 (H.L.G.), DK58161 (M.H.), and HL79507 (M.H. and Z.D.) and Burroughs-Wellcome Fund SATR-1002189 (M.H.).
REFERENCES
- 1.Akagi, K., T. Suzuki, R. M. Stephens, N. A. Jenkins, and N. G. Copeland. 2004. RTCGD: retroviral tagged cancer gene database. Nucleic Acids Res. 32:D523-D527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyanathan, K., M. S. Lechner, P. Bell, G. G. Maul, D. C. Schultz, Y. Yamada, K. Tanaka, K. Torigoe, and F. J. Rauscher III. 2003. Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 17:1855-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson, K. F., F. Q. Li, R. E. Person, D. Albani, Z. Duan, J. Wechsler, K. Meade-White, K. Williams, G. M. Acland, G. Niemeyer, C. D. Lothrop, and M. Horwitz. 2003. Mutations associated with neutropenia in dogs and humans disrupt intracellular transport of neutrophil elastase. Nat. Genet. 35:90-96. [DOI] [PubMed] [Google Scholar]
- 4.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 5.Boulias, K., and I. Talianidis. 2004. Functional role of G9a-induced histone methylation in small heterodimer partner-mediated transcriptional repression. Nucleic Acids Res. 32:6096-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cellot, S., and G. Sauvageau. 2005. Gfi-1: another piece in the HSC puzzle. Trends Immunol. 26:68-71. [DOI] [PubMed] [Google Scholar]
- 7.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan, Z., and M. Horwitz. 2003. Gfi-1 oncoproteins in hematopoiesis. Hematology 8:339-344. [DOI] [PubMed] [Google Scholar]
- 9.Duan, Z., and M. Horwitz. 2005. Gfi-1 takes center stage in hematopoietic stem cells. Trends Mol. Med. 11:49-52. [DOI] [PubMed] [Google Scholar]
- 10.Duan, Z., and M. Horwitz. 2003. Targets of the transcriptional repressor oncoprotein Gfi-1. Proc. Natl. Acad. Sci. USA 100:5932-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan, Z., F. Q. Li, J. Wechsler, K. Meade-White, K. Williams, K. F. Benson, and M. Horwitz. 2004. A novel notch protein, N2N, targeted by neutrophil elastase and implicated in hereditary neutropenia. Mol. Cell. Biol. 24:58-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinberg, A. P., and B. Tycko. 2004. The history of cancer epigenetics. Nat. Rev. Cancer 4:143-153. [DOI] [PubMed] [Google Scholar]
- 13.Gilks, C. B., S. E. Bear, H. L. Grimes, and P. N. Tsichlis. 1993. Progression of interleukin-2 (IL-2)-dependent rat T-cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. Mol. Cell. Biol. 13:1759-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldmit, M., Y. Ji, J. Skok, E. Roldan, S. Jung, H. Cedar, and Y. Bergman. 2005. Epigenetic ontogeny of the Igk locus during B cell development. Nat. Immunol. 6:198-203. [DOI] [PubMed] [Google Scholar]
- 15.Grimes, H. L., T. O. Chan, P. A. Zweidler-McKay, B. Tong, and P. N. Tsichlis. 1996. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol. Cell. Biol. 16:6263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyory, I., J. Wu, G. Fejer, E. Seto, and K. L. Wright. 2004. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat. Immunol. 5:299-308. [DOI] [PubMed] [Google Scholar]
- 17.Hertzano, R., M. Montcouquiol, S. Rashi-Elkeles, R. Elkon, R. Yucel, W. N. Frankel, G. Rechavi, T. Moroy, T. B. Friedman, M. W. Kelley, and K. B. Avraham. 2004. Transcription profiling of inner ears from Pou4f3(ddl/ddl) identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum. Mol. Genet. 13:2143-2153. [DOI] [PubMed] [Google Scholar]
- 18.Hock, H., M. J. Hamblen, H. M. Rooke, J. W. Schindler, S. Saleque, Y. Fujiwara, and S. H. Orkin. 2004. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431:1002-1007. [DOI] [PubMed] [Google Scholar]
- 19.Hock, H., M. J. Hamblen, H. M. Rooke, D. Traver, R. T. Bronson, S. Cameron, and S. H. Orkin. 2003. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 18:109-120. [DOI] [PubMed] [Google Scholar]
- 20.Jafar-Nejad, H., and H. J. Bellen. 2004. Gfi/Pag-3/senseless zinc finger proteins: a unifying theme? Mol. Cell. Biol. 24:8803-8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, K., D. L. Pflugh, D. Yu, D. G. Hesslein, K. I. Lin, A. L. Bothwell, A. Thomas-Tikhonenko, D. G. Schatz, and K. Calame. 2004. B cell-specific loss of histone 3 lysine 9 methylation in the V(H) locus depends on Pax5. Nat. Immunol. 5:853-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karsunky, H., I. Mende, T. Schmidt, and T. Moroy. 2002. High levels of the onco-protein Gfi-1 accelerate T-cell proliferation and inhibit activation induced T-cell death in Jurkat T-cells. Oncogene 21:1571-1579. [DOI] [PubMed] [Google Scholar]
- 24.Karsunky, H., H. Zeng, T. Schmidt, B. Zevnik, R. Kluge, K. W. Schmid, U. Duhrsen, and T. Moroy. 2002. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat. Genet. 30:295-300. [DOI] [PubMed] [Google Scholar]
- 25.Kazanjian, A., D. Wallis, N. Au, R. Nigam, K. J. Venken, P. T. Cagle, B. F. Dickey, H. J. Bellen, C. B. Gilks, and H. L. Grimes. 2004. Growth factor independence-1 is expressed in primary human neuroendocrine lung carcinomas and mediates the differentiation of murine pulmonary neuroendocrine cells. Cancer Res. 64:6874-6882. [DOI] [PubMed] [Google Scholar]
- 26.Kouzarides, T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12:198-209. [DOI] [PubMed] [Google Scholar]
- 27.Kuo, M. H., and C. D. Allis. 1998. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 20:615-626. [DOI] [PubMed] [Google Scholar]
- 28.Lukasova, E., Z. Koristek, M. Falk, S. Kozubek, S. Grigoryev, M. Kozubek, V. Ondrej, and I. Kroupova. 2005. Methylation of histones in myeloid leukemias as a potential marker of granulocyte abnormalities. J. Leukoc. Biol. 77:100-111. [DOI] [PubMed] [Google Scholar]
- 29.Ma, J. 2005. Crossing the line between activation and repression. Trends Genet. 21:54-59. [DOI] [PubMed] [Google Scholar]
- 30.McGhee, L., J. Bryan, L. Elliott, H. L. Grimes, A. Kazanjian, J. N. Davis, and S. Meyers. 2003. Gfi-1 attaches to the nuclear matrix, associates with ETO (MTG8) and histone deacetylase proteins, and represses transcription using a TSA-sensitive mechanism. J. Cell. Biochem. 89:1005-10018. [DOI] [PubMed] [Google Scholar]
- 31.Moroy, T. 2005. The zinc finger transcription factor growth factor independence 1 (Gfi1). Int. J. Biochem. Cell Biol. 37:541-546. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen, S. J., R. Schneider, U. M. Bauer, A. J. Bannister, A. Morrison, D. O'Carroll, R. Firestein, M. Cleary, T. Jenuwein, R. E. Herrera, and T. Kouzarides. 2001. Rb targets histone H3 methylation and HP1 to promoters. Nature 412:561-565. [DOI] [PubMed] [Google Scholar]
- 33.Nishio, H., and M. J. Walsh. 2004. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc. Natl. Acad. Sci. USA 101:11257-11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa, H., K. Ishiguro, S. Gaubatz, D. M. Livingston, and Y. Nakatani. 2002. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296:1132-1136. [DOI] [PubMed] [Google Scholar]
- 35.Osawa, M., T. Yamaguchi, Y. Nakamura, S. Kaneko, M. Onodera, K. Sawada, A. Jegalian, H. Wu, H. Nakauchi, and A. Iwama. 2002. Erythroid expansion mediated by the Gfi-1B zinc finger protein: role in normal hematopoiesis. Blood 100:2769-2777. [DOI] [PubMed] [Google Scholar]
- 36.Park, J. H., Q. Yu, B. Erman, J. S. Appelbaum, D. Montoya-Durango, H. L. Grimes, and A. Singer. 2004. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21:289-302. [DOI] [PubMed] [Google Scholar]
- 37.Person, R. E., F. Q. Li, Z. Duan, K. F. Benson, J. Wechsler, H. A. Papadaki, G. Eliopoulos, C. Kaufman, S. J. Bertolone, B. Nakamoto, T. Papayannopoulou, H. L. Grimes, and M. Horwitz. 2003. Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat. Genet. 34:308-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters, A. H., S. Kubicek, K. Mechtler, R. J. O'Sullivan, A. A. Derijck, L. Perez-Burgos, A. Kohlmaier, S. Opravil, M. Tachibana, Y. Shinkai, J. H. Martens, and T. Jenuwein. 2003. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell 12:1577-1589. [DOI] [PubMed] [Google Scholar]
- 39.Peters, A. H., D. O'Carroll, H. Scherthan, K. Mechtler, S. Sauer, C. Schofer, K. Weipoltshammer, M. Pagani, M. Lachner, A. Kohlmaier, S. Opravil, M. Doyle, M. Sibilia, and T. Jenuwein. 2001. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107:323-337. [DOI] [PubMed] [Google Scholar]
- 40.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406:593-599. [DOI] [PubMed] [Google Scholar]
- 41.Rice, J. C., S. D. Briggs, B. Ueberheide, C. M. Barber, J. Shabanowitz, D. F. Hunt, Y. Shinkai, and C. D. Allis. 2003. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol. Cell 12:1591-1598. [DOI] [PubMed] [Google Scholar]
- 42.Rodel, B., K. Tavassoli, H. Karsunky, T. Schmidt, M. Bachmann, F. Schaper, P. Heinrich, K. Shuai, H. P. Elsasser, and T. Moroy. 2000. The zinc finger protein Gfi-1 can enhance STAT3 signaling by interacting with the STAT3 inhibitor PIAS3. EMBO J. 19:5845-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheijen, B., J. Jonkers, D. Acton, and A. Berns. 1997. Characterization of pal-1, a common proviral insertion site in murine leukemia virus-induced lymphomas of c-myc and Pim-1 transgenic mice. J. Virol. 71:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt, T., H. Karsunky, E. Gau, B. Zevnik, H. P. Elsasser, and T. Moroy. 1998. Zinc finger protein GFI-1 has low oncogenic potential but cooperates strongly with pim and myc genes in T-cell lymphomagenesis. Oncogene 17:2661-2667. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt, T., H. Karsunky, B. Rodel, B. Zevnik, H. P. Elsasser, and T. Moroy. 1998. Evidence implicating Gfi-1 and Pim-1 in pre-T-cell differentiation steps associated with beta-selection. EMBO J. 17:5349-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt, T., M. Zornig, R. Beneke, and T. Moroy. 1996. MoMuLV proviral integrations identified by Sup-F selection in tumors from infected myc/pim bitransgenic mice correlate with activation of the gfi-1 gene. Nucleic Acids Res. 24:2528-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz, D. C., K. Ayyanathan, D. Negorev, G. G. Maul, and F. J. Rauscher III. 2002. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 16:919-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharina, I. G., E. Martin, A. Thomas, K. L. Uray, and F. Murad. 2003. CCAAT-binding factor regulates expression of the beta1 subunit of soluble guanylyl cyclase gene in the BE2 human neuroblastoma cell line. Proc. Natl. Acad. Sci. USA 100:11523-11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin, M. S., T. N. Fredrickson, J. W. Hartley, T. Suzuki, K. Agaki, and H. C. Morse III. 2004. High-throughput retroviral tagging for identification of genes involved in initiation and progression of mouse splenic marginal zone lymphomas. Cancer Res. 64:4419-4427. [DOI] [PubMed] [Google Scholar]
- 50.Sims, R. J., III, K. Nishioka, and D. Reinberg. 2003. Histone lysine methylation: a signature for chromatin function. Trends Genet. 19:629-639. [DOI] [PubMed] [Google Scholar]
- 51.Tachibana, M., K. Sugimoto, T. Fukushima, and Y. Shinkai. 2001. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 276:25309-25317. [DOI] [PubMed] [Google Scholar]
- 52.Tachibana, M., K. Sugimoto, M. Nozaki, J. Ueda, T. Ohta, M. Ohki, M. Fukuda, N. Takeda, H. Niida, H. Kato, and Y. Shinkai. 2002. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 16:1779-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiagalingam, S., K. H. Cheng, H. J. Lee, N. Mineva, A. Thiagalingam, and J. F. Ponte. 2003. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann. N. Y. Acad. Sci. 983:84-100. [DOI] [PubMed] [Google Scholar]
- 54.Tong, B., H. L. Grimes, T. Y. Yang, S. E. Bear, Z. Qin, K. Du, W. S. El-Deiry, and P. N. Tsichlis. 1998. The Gfi-1B proto-oncoprotein represses p21WAF1 and inhibits myeloid cell differentiation. Mol. Cell. Biol. 18:2462-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallis, D., M. Hamblen, Y. Zhou, K. J. Venken, A. Schumacher, H. L. Grimes, H. Y. Zoghbi, S. H. Orkin, and H. J. Bellen. 2003. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development 130:221-232. [DOI] [PubMed] [Google Scholar]
- 56.Yang, L., L. Xia, D. Y. Wu, H. Wang, H. A. Chansky, W. H. Schubach, D. D. Hickstein, and Y. Zhang. 2002. Molecular cloning of ESET, a novel histone H3-specific methyltransferase that interacts with ERG transcription factor. Oncogene 21:148-152. [DOI] [PubMed] [Google Scholar]
- 57.Yap, Y. L., M. P. Wong, X. W. Zhang, D. Hernandez, R. Gras, D. K. Smith, and A. Danchin. 2005. Conserved transcription factor binding sites of cancer markers derived from primary lung adenocarcinoma microarrays. Nucleic Acids Res. 33:409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu, J., C. Angelin-Duclos, J. Greenwood, J. Liao, and K. Calame. 2000. Transcriptional repression by Blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol. Cell. Biol. 20:2592-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yucel, R., H. Karsunky, L. Klein-Hitpass, and T. Moroy. 2003. The transcriptional repressor Gfi1 affects development of early, uncommitted c-Kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J. Exp. Med. 197:831-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng, H., R. Yucel, C. Kosan, L. Klein-Hitpass, and T. Moroy. 2004. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J. 23:4116-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, Y., and D. Reinberg. 2001. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15:2343-2360. [DOI] [PubMed] [Google Scholar]
- 62.Zhu, J., L. Guo, B. Min, C. J. Watson, J. Hu-Li, H. A. Young, P. N. Tsichlis, and W. E. Paul. 2002. Growth factor independent-1 induced by IL-4 regulates Th2 cell proliferation. Immunity 16:733-744. [DOI] [PubMed] [Google Scholar]
- 63.Zornig, M., T. Schmidt, H. Karsunky, A. Grzeschiczek, and T. Moroy. 1996. Zinc finger protein GFI-1 cooperates with myc and pim-1 in T-cell lymphomagenesis by reducing the requirements for IL-2. Oncogene 12:1789-1801. [PubMed] [Google Scholar]
- 64.Zweidler-Mckay, P. A., H. L. Grimes, M. M. Flubacher, and P. N. Tsichlis. 1996. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol. Cell. Biol. 16:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]