Abstract
Scythe (BAT3 [HLA-B-associated transcript 3]) is a nuclear protein that has been implicated in apoptosis, as it can modulate Reaper, a central apoptotic regulator in Drosophila melanogaster. While Scythe can markedly affect Reaper-dependent apoptosis in Xenopus laevis cell extracts, the function of Scythe in mammals is unknown. Here, we report that inactivation of Scythe in the mouse results in lethality associated with pronounced developmental defects in the lung, kidney, and brain. In all cases, these developmental defects were associated with dysregulation of apoptosis and cellular proliferation. Scythe−/− cells were also more resistant to apoptosis induced by menadione and thapsigargin. These data show that Scythe is critical for viability and normal development, probably via regulation of programmed cell death and cellular proliferation.
Many key apoptotic regulators, such as members of the Bcl-2 and caspase families, are critical for normal development (11, 38). However, the interplay between apoptosis and development involves tissue-specific activation of apoptotic signaling pathways (12, 19). Therefore, animal models of gene inactivation often provide essential biological insights for understanding the specific context-dependent roles of apoptotic effectors (34). In the present study, we show important developmental requirements for the novel Reaper-binding apoptotic effector, Scythe (BAT3 [HLA-B-associated transcript 3]).
Scythe is a nuclear protein that contains several structural motifs, including an N-terminal ubiquitin homology region, a polyproline region, and a zinc finger-like domain (2). Notably, Scythe has been shown to interact with Reaper, the central regulator of developmental apoptosis in Drosophila melanogaster. This interaction is required for Reaper-induced apoptosis in Xenopus laevis egg extracts and leads to rapid mitochondrial cytochrome c release, caspase activation, and nuclear fragmentation (41, 42). In addition to Reaper, Scythe was also shown to bind to two other Drosophila apoptotic proteins, Hid and Grim (41). Further biochemical evidence that Scythe plays a role in apoptosis comes from the formation of a caspase-3-cleaved Scythe C-terminal fragment with proapoptotic activity after ricin treatment (49) and also the interaction of Scythe with immediate-early gene X-1, which is involved in the control of apoptosis and cellular growth (24). Scythe also possesses an amino acid sequence similar to that of members of the BAG family that can bind the ATPase domain of the HSP70 family of molecular chaperones (40). This region of Scythe has been shown to modulate Hsp70, a cytoprotective protein with dual roles as a regulator of protein conformation and a stress sensor (28), implying that Scythe may function through regulating the folding and activity of apoptotic signaling molecules (43).
To further investigate Scythe function, we used gene targeting to generate mice lacking this protein. Inactivation of Scythe in the mouse was incompatible with viability and resulted in either embryonic lethality consecutive to abnormal brain development or perinatal death associated with pronounced developmental defects in the lung and kidney. These developmental defects were associated with widespread aberrant apoptosis and proliferation. In addition, we found that Scythe was required for the normal apoptotic response after thapsigargin and menadione treatment. Thus, our data indicate Scythe is important for both apoptosis and cell proliferation and reveal a critical role for this protein during mammalian development.
MATERIALS AND METHODS
Generation of Scythe-deficient mice.
A mouse BAC (strain 129OLA) containing the Scythe genomic locus encompassing the 10-kb open reading frame (ORF) was obtained from Research Genetics. The targeting construct was designed to delete most of the ORF, including the reported ATG start (GenBank accession no. AAC82479) and an additional 1 kb of sequence upstream of this ATG, to ensure we had eliminated other potential start sites revealed by 5′ rapid amplification of cDNA end analysis (see Results). To do this, a 10-kb HindIII/XhoI genomic DNA fragment was cloned into the HindIII/XhoI site of NTK901 (Stratagene) to generate a region of homology at the 5′ end of the translation start site. A 2.3-kb EcoRV genomic fragment containing an exon encoding the final 26 amino acids of the Scythe ORF was linked with a ClaI site and then digested with XhoI to generate a 2.3-kb XhoI/ClaI fragment that was cloned into SalI/ClaI-cut NTK901 10-kb HindIII/XhoI. This targeting construct was linearized with NotI, electroporated into W9.5 embryonic stem (ES) cells, and grown in G418 (200 μg/μl) and fialurdine (FIAU)-containing media. Homologous recombinants (frequency of ∼1/30) were identified by Southern blotting of BamHI-digested genomic DNA from G418-resistant ES clones, using a 300-bp XhoI/EcoRV fragment 3′ to the 2.3-kb short homology region of the targeting construct as a probe. ES clones containing the targeted mutation were injected into 3.5-day C57BL/6 blastocysts, which were subsequently transferred into pseudopregnant foster mothers. Chimeric mice were crossed into strain C57BL/6 to produce heterozygous mutant mice. Germ line transmission of the mutation was verified by Southern blot analysis of tail DNA. Two independent Scythe strains were generated, and both had identical phenotypes. We also generated an inbred C57BL/6 Scythe strain and found lung and kidney phenotypes resulting from Scythe loss identical to those that occurred on an outbred (129SvJ × C57BL/6) background. Identification of Scythe−/− embryos was done using Southern analysis or PCR with the following primers: 5′CCGGAGCTCGGCCCCTGACAAGC (Scy32), 5′GTGGTCCCCTGGTGAGAAAGC (Scy21), and 5′CTTGTGTAGCGCCAAGTGC (GD-10). The wild-type (WT) (generated with Scy21 and Scy32) and Scythe mutant (generated with GD-10 and Scy21) alleles produced PCR products of 400 bp and 200 bp, respectively.
Scythe antibodies.
Rabbit antisera to Scythe were generated using the following two peptides, corresponding to COOH-terminal regions of Scythe, as an antigen: Scythe 23, (NH2)-RKVKPQPPLSDAYLSGMPAK; and Scythe 25, (NH2)-QRENASPAPGTTAEEAMSR. Keyhole limpet hemocyanin-conjugated peptides were injected with Freund's complete adjuvant and then boosted with peptide with incomplete adjuvant by Rockland, Inc. (Gilbertsville, PA). Antiserum specific for Scythe was obtained from each peptide, and sera were used without further purification.
Histology and immunohistochemistry.
Embryos were submersed in paraformaldehyde, and fixed tissues were cryoprotected in 25% buffered sucrose solution and cryosectioned at 12 μm by using an HM500M cryostat (Microm). For immunohistochemistry, the following antibodies were used: anti-Ki67 (1:1,000; Novocastra Labs), anti-β-tubulin III (Tuj1) (1:1,000; BAbCo), and rabbit anti-caspase-3 (1:1,500; Pharmingen). Antigen retrieval was used for all immunohistochemistry. Cryosections were incubated with antibodies overnight at room temperature after quenching of endogenous peroxidase by use of 0.6% hydrogen peroxide. Immunoreactivity was visualized with a VIP substrate kit (Vector Labs) according to the manufacturer's directions after tissues were treated with biotinylated secondary antibody and avidin DH-biotinylated horseradish peroxidase-H complex (Vectastain Elite kit; Vector Labs). Sections were counterstained with 0.1% methyl green (Vector Labs), dehydrated, and mounted in Permount. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was performed on cryosections by using Apoptag apoptosis detection systems (Chemicon International) according to the manufacturer's directions. For fluorescence detection, embryo sections were treated with blocking solution (5% donkey serum and 1% bovine serum) and then incubated with rabbit anti-Pax2 (1:200; Zymed), rat anti-uvomorulin (E-cadherin) (1:500; Sigma), rabbit anti-phospho-histone H3 (Ser 10) (1:50; Cell Signaling), goat anti-p57KIP2 (1:200; Santa Cruz), rat anti-laminin (1:200; Sigma), and rat anti-CD34 (1:500; Pharmingen).
Kidney organ culture and whole-mount immunohistochemistry.
Embryonic day 12.5 (E12.5) kidney rudiments were isolated and cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum on a Millicell culture plate insert (Millipore) at the medium/gas interface for 5 days. Kidneys were fixed in 100% methanol for 10 min and then washed in phosphate-buffered saline, 0.1 M, pH 7.4, 0.1% Tween 20 (PBST). They were then whole-mount stained with anti-uvomorulin (1/500; Sigma) and WT1 (1/200; Santa Cruz) antibodies at 4°C overnight, washed in PBST, incubated with Alexa Fluor 488 (1/400; Molecular Probes) or Cy3 (1/400; Jackson Immunoresearch) secondary antibody for 2 h at room temperature, washed in PBST, mounted in Mowiol-glycerol medium, and viewed using fluorescence with a Zeiss Axioscop microscope.
Protein extraction, immunoprecipitation, and Western blot analysis.
Cells or tissues were homogenized in 200 μl of lysis buffer (HEPES, pH 7.5, 50 mM; NaCl, 150 mM; EDTA, 1 mM; EGTA, 2.5 mM; Tween 20, 0.1%; glycerol, 10%; β-glycerophosphate, 10 mM; sodium fluoride, 1 mM; sodium orthovanadate, 0.1 mM; phenylmethylsulfonyl fluoride, 0.1 mM; and protease inhibitor cocktails [Roche]). Proteins were separated on 8% or 4% N,N-methylenebisacrylamide-12% Tris NuPAGE gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore). Antibodies used for Western blot analysis were rabbit anti-Scythe (1/5,000), mouse anti-WAF1 (Ab-6-p21) (1:200; Oncogene), rabbit anti-p27 (1:2,000; Santa Cruz), rabbit anti-poly(ADP-ribose) polymerase (PARP) (1:1,000; Cell Signaling), and goat anti-α actin (1:1,000; Santa Cruz).
Primary cell culture.
Cortical neurons were obtained from E13.5 WT and Scythe mutant embryos. After tissue isolation and disaggregation, neurons were plated in 6-well dishes or in chamber slides coated with poly-d-lysine in serum-free medium N2:Dulbecco's modified Eagle's medium (1:1) supplemented with 6 mg/ml d-glucose, 100 μg/ml transferrin, 25 μg/ml insulin, 20 nM progesterone, 60 μM putrescine, 30 nM selenium, 1 ng/ml glia-derived neurotrophic factor, and 10 ng/ml ciliary neurotrophic factor. Cell death was induced by addition of menadione (50 nM) or thapsigargin (50 nM). Apoptosis was quantified either by DEVD-caspase activity (using the fluorogenic substrate DEVD-7-amino-4-methylcoumarin) or TUNEL assay using Apoptag apoptosis detection systems (Chemicon International). Scythe cDNA was subcloned into pHA (pcDNA3.1 from Invitrogen modified by insertion of a hemagglutinin tag). For transfection, Lipofectamine 2000 (Invitrogen) was used for cortical neurons.
For immunocytochemistry, cells were grown on tissue culture glass slides (BD Falcon), treated and/or transfected, washed with phosphate-buffered saline (PBS), fixed with 1% formaldehyde for 15 min at room temperature, and washed several times in PBS. Cells were then incubated for 1 h in blocking solution (5% goat serum, 1% bovine serum albumin in PBS containing 0.1% Triton X-100), followed by specific antibody incubation (mouse antihemagglutinin at 1 μg/ml in blocking solution) overnight at 4°C. Cells were washed three times in PBST for 5 min and then incubated with secondary antibody (goat anti-mouse Cy3) for 1 h. After being washed three times in PBST for 5 min, cells were counterstained with Vectashield containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories) and mounted for analysis with a Zeiss Axioskop 2 fluorescence microscope.
RESULTS
Scythe is required for embryonic development.
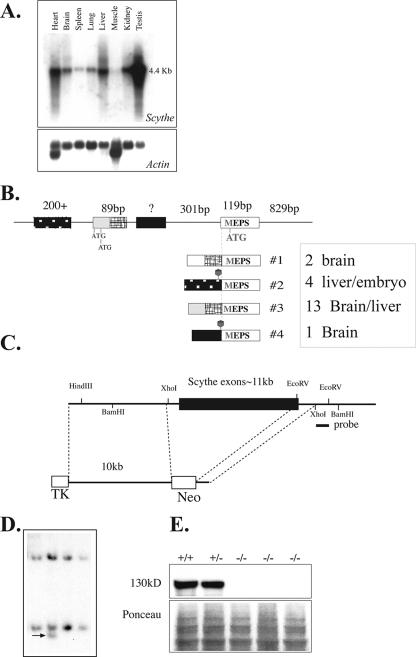
To determine the role of Scythe in embryonic development, we used gene targeting to generate Scythe-null mice. While Scythe mRNA is ubiquitously expressed with a transcript size of ∼4.4 kb, other Scythe transcripts are also present (Fig. 1A). For example, using reverse transcriptase PCR, we found a number of alternative 5′ regions that included different translation start sites and 5′ untranslated region (UTR) sequences (Fig. 1B), and we also found extensive splicing throughout the open reading frame (not shown). Therefore, the targeting construct was designed to delete almost the entire Scythe open reading frame (Fig. 1C). Targeted ES cells (Fig. 1D) were used to generate Scythe+/− animals. Interbreeding of Scythe+/− mice produced no viable Scythe−/− mice, indicating that deletion of this gene was embryonically or perinatally lethal (Table 1). We determined that Scythe loss caused lethality between E13.5 and E15.5, where Scythe−/− embryos often showed exencephaly or, more commonly, perinatal lethality (Table 1). By Western blot analysis of Scythe-null mouse embryo fibroblasts, using an antibody that recognizes Scythe, we confirmed that the targeted Scythe allele did not produce any protein (Fig. 1E).
FIG. 1.
Inactivation of mouse Scythe. (A) Northern blot analysis of Scythe mRNA expression in various adult tissues (top). Actin reprobing of the blot confirmed equal RNA loading (bottom). (B) Schematic representation of the Scythe 5′ UTR sequence and the alternative splices found by reverse transcriptase PCR for several tissues, including liver and brain. The sizes of some 5′ UTR and coding exons are shown schematically positioned on genomic DNA, and the spliced transcripts are indicated below the genomic DNA structure. The relative abundance of a particular transcript is indicated by the number of times it was isolated by PCR from a particular tissue, i.e., transcript no. 3 was the most common 5′ version of the Scythe. (C) Structure of the Scythe targeting vector that replaced almost the entire Scythe ORF. The probe used for the Southern blot analysis is indicated. TK, thymidine kinase. (D) Southern blot analysis after BamHI digestion of ES cells. (E) Scythe (130 kDa) is present in WT (+/+) and heterozygous (+/−) cells but is absent in Scythe-null (−/−) mouse embryo fibroblasts.
TABLE 1.
Embryonic survival in Scythe-null mice
| Time point | No. of mice of Scythe genotypea
|
No. of mice with:
|
|||
|---|---|---|---|---|---|
| +/+ | +/− | −/− | Resorptions | Exencephaly | |
| E12.5 | 8 | 12 | 5 | 8 | |
| E13.5 | 92 | 193 | 104 | 45 | 7 |
| E14.5 | 37 | 95 | 44 | 18 | 10 |
| E15.5 | 7 | 20 | 6 | 3 | 2 |
| E16.5 | 13 | 9 | 11 | 5 | |
| E17.5 | 6 | 11 | 8 | 2 | |
| E18.5 | 19 | 52 | 13 | 3 | 1 |
| E19.5 | 2 | 1 | 1 | ||
| >P0 | 313 | 518 | (31)b | ||
Genotyping of mice was determined by PCR analysis. The expected ratio of Scythe+/+ to Scythe+/− to Scythe−/− mice was 1:2:1.
Number in parentheses indicates dead mice. No Scythe-null mice were found alive.
Inactivation of Scythe leads to lung and kidney abnormalities.
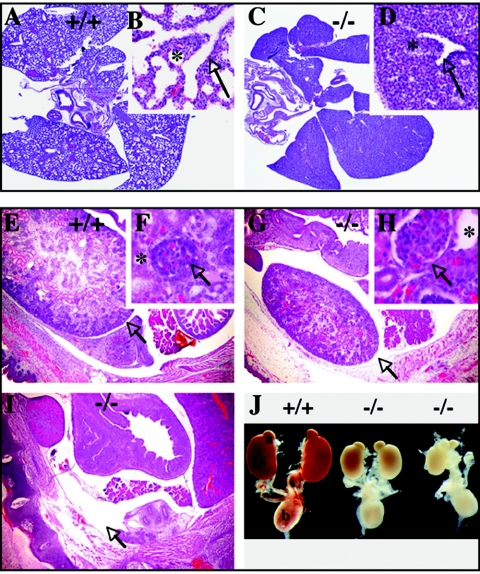
To determine the cause of the perinatal lethality, we examined embryos late in development. Analysis of E18.5 Scythe−/− embryos revealed pronounced defects in lung development, showing smaller and substantially hypoplastic lungs with decreased branching of terminal bronchioles and no alveolar formation compared to those of the WT (Fig. 2A to D). These lung defects were the likely cause of the perinatal death as at this stage; in contrast to WT embryos, Scythe−/− embryos died shortly after cesarean section because they failed to breathe (data not shown). In addition to defective lung development, Scythe−/− embryos often (>90%) exhibited bilateral hypoplastic kidneys (Fig. 2), or in some cases (2/31) unilateral agenesis was observed (Fig. 2J). Higher magnification of Scythe−/− kidneys showed that there were fewer glomeruli; these were often enlarged (Fig. 2G and H), and most tubules were dilated (Fig. 2F and H). As shown in Fig. 2J, isolated urogenital systems from E18.5 WT and Scythe−/− embryos showed examples of unilateral agenesis and hypoplastic kidneys but the remainder of the urogenital system, such as the adrenal glands and the bladder, formed normally. Thus, Scythe is required for normal development of the lung and kidney.
FIG. 2.
Lung and kidney abnormalities occur in E18.5 Scythe−/− embryos. (A to D) Compared to the WT (+/+) (A and B), abnormal lung development was present in Scythe mutants (−/−) (C and D). Lungs were analyzed at the saccular stage of development. In the WT, bronchial tree formation in the lungs was complete (arrow) and normal alveolization was observed (*) (B). Lungs were smaller and markedly less developed in Scythe−/− embryos (C and D), with decreased branching of terminal bronchioles (arrow) and nearly complete lack of alveolization (*). (Inset panels B and D show higher magnifications of panels A and C, respectively). (E to J) Abnormalities observed in E18.5 Scythe−/− kidneys. Scythe mutants showed smaller kidneys (G) or no kidney (I), with enlarged glomeruli (H, arrow) and dilated tubules (H, asterisk) compared to those of the WT (E and F, same symbols). Sections were stained with hematoxylin and eosin. Aside from kidney defects in Scythe−/− animals, the remainder of the urogenital system was normal (J). a, adrenal gland; k, kidney; b, bladder.
Analysis of metanephric development in Scythe mutants.
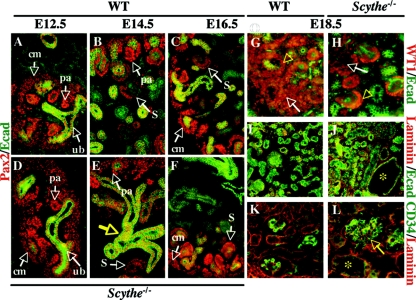
In situ hybridization analysis of developing kidney showed Scythe was expressed in the condensed mesenchyme, tubules, and podocytes (data not shown). Kidney organogenesis in mice begins at E11 and involves reciprocal inductive interactions between the ureteric bud epithelium and metanephric mesenchyme (26, 36). The metanephric mesenchyme condenses around the ureteric bud into pretubular aggregates that will undergo epithelialization to become comma-shaped bodies, S-shaped bodies, and eventually the epithelium component of the nephron (3, 10, 14, 20, 25). At E12.5, the ureteric buds were readily identified and surrounded by condensed mesenchyme in both WT and Scythe−/− kidneys (Fig. 3A and D). The inductive interactions between ureteric bud epithelium and mesenchyme proceeded normally in the absence of Scythe, as evidenced by the appearance of pretubular aggregates and comma- and S-shaped bodies in both WT and Scythe-null mice (Fig. 3B, C, E, and F). Therefore, although Scythe−/− kidneys are smaller, glomeruli number is relatively normal in proportion to kidney size.
FIG. 3.
Analysis of Scythe−/− kidneys. (A to F) Immunostaining of Pax2 (red) and E-cadherin (Ecad) (green) with control (A, B, and C) and mutant (D, E, and F) embryos was done at E12.5, E14.5, and E16.5. At E12.5, the ureteric bud (ub) was surrounded by condensed mesenchyme (A and D), and ureteric bud branching and formation of pretubular aggregates (pa) could be seen for both WT and Scythe−/− embryos. Pax2 was expressed in condensed mesenchymal cells (cm), pretubular aggregates, and comma- and S-shaped bodies (S) in both WT and Scythe−/− embryos at E14.5 and E16.5, but nephrogenic differentiation was delayed in Scythe−/− embryos (B, C, E, and F). The structure of the epithelium (Ecad staining) showed branching abnormalities (E, yellow arrow) in Scythe−/− kidneys. (G to L) At E18.5, WT1 (red) and E-cadherin (green) expression was detected in the condensing blastema (arrows), the proximal part of S-shaped bodies, and the podocytes of functional glomeruli (arrowheads) for both WT and Scythe−/− kidney (G and H) but to a lesser extent in Scythe−/− kidney. Laminin (red) distribution in Scythe−/− kidneys at E18.5 showed a pattern similar to that of the WT, while E-cadherin staining (green) showed enlarged tubules (asterisk) in Scythe−/− kidneys (I and J). The endothelial marker CD34 (green) is expressed similarly in the WT and in Scythe−/−, although enlarged glomeruli (arrow) and tubules (asterisk) are seen with Scythe−/− kidney (K and L).
Several ductal- and mesenchymal-derived molecules have been identified, and loss-of-function studies have established their crucial roles in nephrogenesis (5, 13, 46). To assess metanephric development in Scythe−/− embryos, we analyzed the expression of Pax2, a transcription factor important for multiple early steps of urogenital development (45). Normal Pax2 distribution was found with both WT and mutant embryos up to E16.5 (Fig. 3), whereas E-cadherin, a marker of epithelium, revealed branching defects during development of the Scythe-null kidneys and delayed maturation of structures in the cortex (Fig. 3D to F). Normally, at later stages of nephron formation, Pax2 expression is diminished and linked to increased expression of Wilms' tumor suppressor gene WT1 (35). Therefore, at E18.5 we checked for the expression of WT1, an essential regulator of kidney development that is expressed in the condensing blastema in the proximal part of S-shaped bodies and in the podocyte layer of functional glomeruli, and found less diffuse WT1 staining in Scythe−/− kidneys than in WT control tissue (Fig. 3G and H). This likely reflects less undifferentiated mesenchyme in the Scythe mutant, a scenario that may account for the perturbations of kidney growth. Other markers of kidney development, like laminin (Fig. 3I and J) and CD34 (Fig. 3K and L), were expressed normally at E18.5 but revealed enlarged glomeruli and dilated tubules in Scythe−/− kidneys (Fig. 3J and L).
Impaired renal branching morphogenesis and altered apoptosis and proliferation in Scythe−/− kidney explants ex vivo.
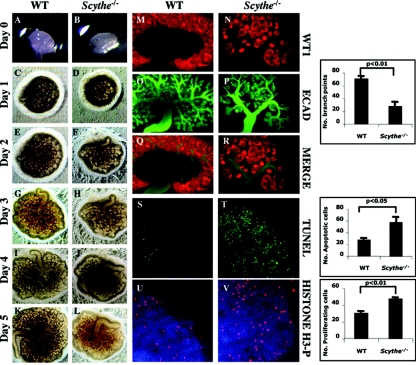
To further examine Scythe−/− kidneys, we followed the development of isolated Scythe−/− ureteric buds in explant cultures isolated from E12.5 embryos (Fig. 4). We found that the growth of kidney explants from mutant embryos was delayed and was smaller than that of the WT after 5 days in culture (Fig. 4A to L). Whole-mount kidneys were stained with WT1 (to identify nephrons) and E-cadherin (to identify ureteric bud branches) after 4 days of culture (Fig. 4M to R). Although Scythe−/− rudiments were able to develop, we observed substantial differences in branching between Scythe−/− and the WT, as the number of bifurcations was considerably reduced in Scythe−/− metanephroi (2.5-fold; P < 0.01) and bifurcations were less defined and elongation of the ureter was impaired, as shown by E-cadherin staining (Fig. 4N, P, and R).
FIG. 4.
Branching, apoptosis, and proliferation defects in Scythe−/− kidney explants. (A to L) Embryonic kidney rudiments were dissected from E12.5 WT and Scythe−/− embryos and grown in culture for 5 days (A to L). The growth and branching morphogenesis of Scythe−/− explants was impaired compared to that of the WT. (M to R) After 4 days in culture, explants were immunostained with E-cadherin (ECAD) (green) and WT1 (red) antibodies. E-cadherin stained ureteric bud branches, and WT1 is expressed in the condensed mesenchyme. (S and T) TUNEL analysis revealed a substantial increase in apoptosis for Scythe mutants (n = 3; t test, P of <0.05) compared to the WT. (U and V) Anti-phosphorylated histone H3 (H3-P) was used to identify mitotic cells and showed a 1.5-fold increase in Scythe−/− kidney explants (n = 3; t test, P of <0.01) compared to WT controls. Histograms correspond to the quantitative analysis of branch points, apoptotic cells, and proliferating cells for three different experiments.
To determine how Scythe loss impairs branching morphogenesis of the kidney, we evaluated apoptosis and proliferation in kidney cultures. Scythe−/− kidney cultures showed high levels of apoptosis as determined by TUNEL staining (twofold increase; P < 0.05) (Fig. 4S and T) and also a higher proliferative index as determined by the levels of phosphorylated histone H3, a marker for mitosis (1.5-fold increase; P < 0.01) (Fig. 4U and V). Thus, Scythe is important for normal ureteric branching morphogenesis, and this may occur via a mechanism that suppresses apoptosis and proliferation.
Altered apoptosis and proliferation in Scythe−/− kidneys in vivo.
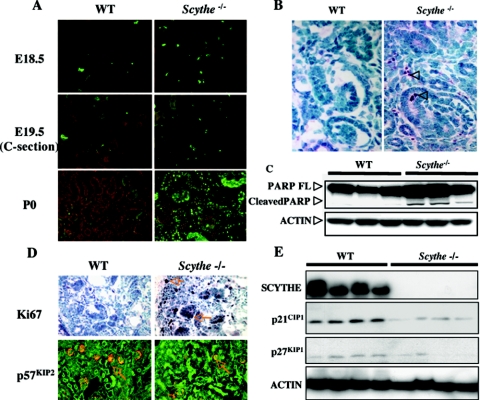
We also examined kidney development in vivo to determine levels of apoptosis and proliferation. Coincidently with relatively normal Scythe−/− kidney development before E14.5, TUNEL analysis showed little difference in cell death between Scythe−/− and WT kidneys at this stage (data not shown). However, between E18.5 and birth (P0), apoptosis was increased in the nephrogenic zones of Scythe−/− embryos compared to those of the WT (about threefold and fourfold increases at E18.5 and E19.5, respectively) (Fig. 5A), while fulminant apoptosis was observed in Scythe−/− kidneys at P0 compared to WT kidneys (Fig. 5A). To confirm these data, we checked E18.5 kidneys for activated caspase 3 by immunohistochemistry using anti-activated caspase 3 antibody (Fig. 5B) and found increased immunoreactivity for activated caspase 3 (1.5-fold increase in Scythe−/− versus WT) in the mesenchymal cells of E18.5 Scythe−/− kidneys compared to those of the WT. Finally, we checked for PARP cleavage as a measure of apoptosis and found higher levels of cleaved PARP in Scythe−/− kidneys than in WT kidneys (Fig. 5C).
FIG. 5.
Increased apoptosis and cellular proliferation occurs in Scythe−/− kidneys. (A) TUNEL assays of kidney sections at E18.5, E19.5, and P0 showed increased apoptosis in Scythe−/− kidneys. C-section, cesarean section. (B) Staining with anti-cleaved caspase 3 (arrowheads) showed increased apoptosis in cortical mesenchyme from E18.5 Scythe−/− kidneys. (C) Proteins were isolated from three WT and three Scythe−/− E18.5 kidneys, and PARP cleavage was analyzed by Western blot. Actin protein levels were used as a loading control. FL, full length. (D) Ki67 and p57KIP2 immunohistochemistry showed increased proliferation in Scythe−/− kidneys at E18.5 compared to the WT control. (E) Western blot analysis of cyclin-dependent kinase inhibitors p21CIP1 and p27KIP1 from multiple individual WT and Scythe−/− E18.5 kidneys showed expression patterns consistent with increased proliferation. Scythe and actin protein levels were used as controls.
In addition to increased apoptosis, we found increased proliferation in the Scythe−/− kidney. There was increased immunoreactivity for Ki67 (an S-phase marker; about a sixfold increase) in proliferative cell populations of the Scythe−/− kidney cortex but decreased immunoreactivity for the cyclin-dependent kinase inhibitor p57KIP2 in podocytes of glomeruli and in mesenchymal cells between renal tubules of the inner medulla (data not shown) of Scythe−/− kidneys (Fig. 5D). Western blot analysis also revealed decreased levels of the two CDK inhibitors p21CIP1 and p27KIP1 in Scythe−/− kidneys compared to kidneys of WT littermates (Fig. 5E). Thus, Scythe is important for modulating apoptosis and proliferation during kidney development, and disruption of these processes likely contributes to developmental defects in the Scythe−/− kidneys.
Inactivation of Scythe can affect brain development.
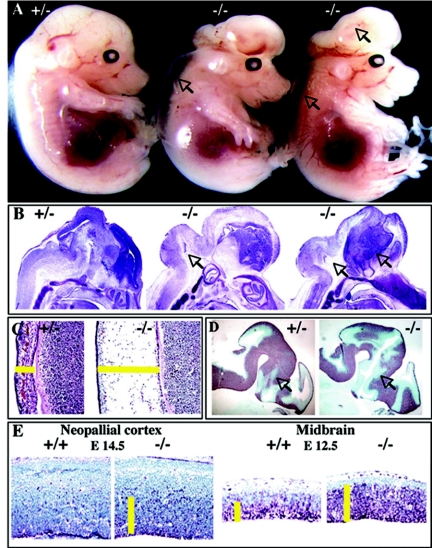
While all late-stage Scythe−/− embryos manifested branching morphogenesis defects, many Scythe−/− embryos at earlier developmental stages (E12.5 to E14.5) displayed exencephaly (Table 1). Histological examination of exencephalic Scythe−/− embryos showed abnormal expansion of the mesencephalon and telencephalon (Fig. 6A and B) and also edema (Fig. 6C). Exencephalic Scythe−/− embryos also showed extensive proliferation in the ventricular zone at different ages and in different brain regions, as judged by increased Ki67 immunoreactivity (Fig. 6E). Although Scythe loss led to excessive proliferation, Tuj1 immunoreactivity showed that embryos harbored neurons at the proper stage of differentiation (Fig. 6D).
FIG. 6.
Scythe loss affects neural development. (A) Excencephaly (arrows) was observed for Scythe−/− embryos at E14.5. (B) Improper expansion of the midbrain and the hindbrain (arrows) was observed for these E14.5 embryos, as indicated by Nissl staining. (C) Edema was also present. (D and E) Tuj1 and Ki67 staining showed an expanded ventricular zone at E12.5 in Scythe−/− embryos (D) and an increase in proliferating cells in the Scythe−/− neopallial cortex at E14.5 and midbrain at E12.5 (E).
Scythe modulates select apoptotic pathways.
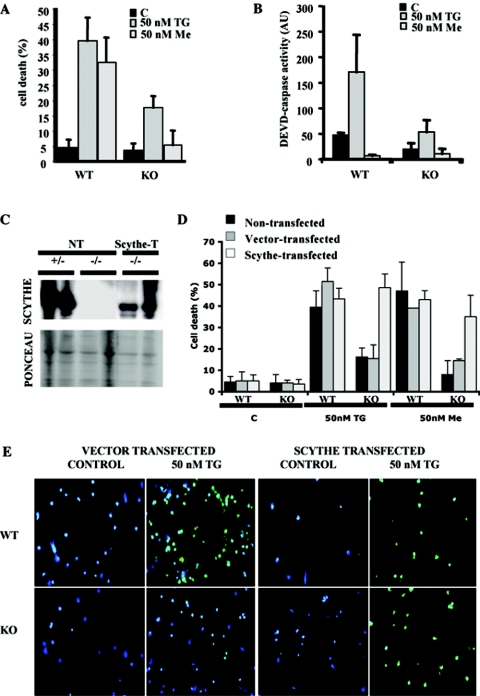
To illuminate the biochemical properties of Scythe that may be important for the developmental defects described above, we focused on isolated primary Scythe−/− cells. Therefore, we challenged Scythe−/− cortical neurons with various proapoptotic agents. We found no difference in apoptosis between WT and Scythe−/− cells by use of agents such as ionizing radiation, staurosporine, or hydrogen peroxide (data not shown). We also did not find a resistance of Scythe−/− cells to apoptosis resulting from ectopically expressed Reaper (data not shown). However, as judged by TUNEL staining (Fig. 7A) and caspase activation assays (Fig. 7B), Scythe−/− neurons were more resistant than WT controls to menadione and thapsigargin, agents that affect calcium flux, particularly in the endoplasmic reticulum (16, 48). To confirm a specific requirement for Scythe in thapsigargin/menadione-induced death, we reintroduced Scythe via transfection into Scythe−/− cortical neuron cells, confirmed Scythe expression (Fig. 7C), and found a restored sensitivity of Scythe−/− cells to these agents (Fig. 7D and E). Collectively, these data illustrate an important role for Scythe in the selective modulation of the apoptotic response.
FIG. 7.
Scythe−/− cortical neurons are resistant to certain apoptotic stimuli. Scythe−/− neurons are resistant to apoptosis after menadione or thapsigargin treatment, as determined using TUNEL assays (A) and caspase activity towards a DEVD substrate (B). Reintroduction of Scythe protein to Scythe−/− cells using pHA-Scythe or vector alone was confirmed by Western blot analysis (C) and restored susceptibility towards menadione and thapsigargin (D). TUNEL analysis of WT and Scythe−/− cortical neurons after treatment with thapsigargin and menadione in nontransfected, pHA-Scythe-transfected, or vector-alone-transfected cells (D and E). TUNEL-positive apoptotic cells are green, and DAPI-counterstained cells are blue. Me, menadione; TG, thapsigargin; KO, knockout; C, control; AU, arbitrary units; NT, nontransfected; Scythe-T, transfected.
DISCUSSION
Here we report that Scythe is critical for normal mammalian development. Inactivation of Scythe led to a variety of developmental defects associated with multiple organ systems, including the nervous system, lungs, and kidneys. We also found that in vitro, Scythe−/− cells were defective in specific apoptotic signaling pathways, as demonstrated by defective apoptosis after thapsigargin or menadione treatment. These are the first data ascribing a critical biological role to Scythe during mammalian development and in the selective regulation of apoptosis.
All Scythe−/− embryos died shortly after birth as a result of severe hypoplasia of the lung, reflected by decreased branching of terminal bronchioles and lack of alveolization and leading to embryo death at birth by failure to breathe. A range of kidney defects, including hypoplastic kidneys and renal agenesis, were also associated with Scythe loss. Molecular analysis of the requirements for lung and kidney development has identified many different molecules that affect specific stages of organ development (8, 37). In many cases, transcriptional programs drive organogenesis, and the kidney is quite well characterized in this regard (4, 5). Additionally, many secreted factors, such as transforming growth factor β1 and bone morphogenetic proteins 2 and 4, are also critical for the growth and development of these organs (1, 6). Similarly to Scythe, other proteins, such as the transcription factor Pod1, can affect both kidney and lung development (33). To better gauge the impact of Scythe loss during kidney development, we examined specific developmental events during kidney maturation by using Pax2 and WT1 expression. These two proteins are essential for early urogenital development, especially for mesenchymal-epithelial transformation. Pax2 is a transcription factor, and Pax2-null mice lack kidneys and ureters (45). WT1, another transcription factor, is involved in nephrogenesis and converts aggregated mesenchyme cells into nephrons (9), and WT1-null kidneys undergo extensive apoptosis resulting in renal agenesis (21). Interestingly, the heterozygous mutation of Pax2 and deletion of WT1 genes are both associated with increased apoptosis (21, 32) and play a role in regulating apoptosis (15, 27, 44). In Scythe−/− kidney bud explants from E12.5 embryos, Pax2 and WT1 expression levels were identical to those of WT controls, suggesting that early kidney development proceeds normally and is not affected by Scythe loss. However, from E14.5, developmental abnormalities, including branching and mesenchymal defects and altered glomerulus morphology, were found. As most branching occurs during early kidney development, before E16.5 (7), Scythe is probably important during early stages of kidney development. Furthermore, subsequent to this, widespread defects encompassing apoptosis and aberrant proliferation in the Scythe−/− kidney were observed. Together, these data suggest that Scythe functions during early kidney development to regulate apoptosis and proliferation and that disruption of this control leads to the developmental abnormalities observed. Notably, regulation of mitotic activity is critical for podocyte differentiation and final maturation of glomeruli (31), two aspects of kidney development that are perturbed in Scythe−/− kidneys.
Although proliferation and apoptosis normally decline at the end of kidney development and after birth, Scythe−/− kidneys exhibited a high level of apoptosis together with abnormal proliferation. Loss of the antiapoptotic protein Bcl-2 leads to polycystic kidney disease marked by increased apoptosis, substantially reduced size, fewer nephrons and hypertrophic tubules, and a fulminant apoptosis in the mesenchyme, as well as a failure to branch normally (29, 39, 47). Thus, in a manner reminiscent of Bcl-2 loss, the absence of Scythe also leads to altered apoptosis associated with renal dysplasia or agenesis during kidney development. Furthermore, the exencephaly of the Scythe−/− embryos was similar to that found with Caspase-3- or Caspase-9-deficient embryos, whereby decreased neuronal apoptosis led to an expanded ventricular zone (17, 22, 23). These observations together with the apoptotic resistance of Scythe cortical neurons support a contribution of Scythe to the regulation of apoptosis during development.
Prior to our current study, elegant biochemical analyses of a Xenopus cell-free system provided a strong link between Scythe and apoptosis (41-43). In those studies, Scythe was isolated as a binding partner for the Drosophila apoptotic regulator, Reaper. The binding of Scythe to Reaper released a sequestered apoptotic regulator that promoted cytochrome c release and thus induced apoptosis. Similarly, silencing the expression of endogenous Scythe prominently reduced the number of apoptotic cells after ricin treatment (49). These data suggested that Scythe is an important apoptotic regulator and that its loss would lead to dysregulation of apoptosis. However, our data show a selective involvement of Scythe in some apoptotic processes. Specifically, Scythe loss renders cells resistant to death induced by menadione or thapsigargin. While Scythe is a nuclear protein, motif analysis of the primary amino acid structure provides no evidence that it participates either directly or indirectly in transcription. However, the amino-terminal region of Scythe contains a ubiquitin-like domain, giving rise to the possibility it may bind to the proteasome (18). Notably, 26S proteasome function has been implicated in the normal control of apoptosis and proliferation via its ability to regulate degradation of signaling molecules important for these functions (30). Perhaps Scythe participates in the modulation of protein turnover.
Thus, our data show that Scythe function is critical for viability of the mouse, as its loss perturbs the development of the lung and other organs coincidently with defects in apoptosis and proliferation. Elucidating the mechanism that links Scythe to these developmental processes will provide new insights for understanding organogenesis.
Acknowledgments
We thank Shelly Self and Guillermo Oliver for help and advice regarding kidney cultures, Beatriz Sosa-Pineda for whole-mount immunostaining, and Troy Baudino, John Cleveland, and Evan Parganas for general advice. We also thank Jingfeng Zhao for technical assistance, the transgenic core unit for ES microinjection, and the Hartwell Center for Biotechnology for peptide synthesis and DNA sequence analysis.
These studies were supported by the NIH and by the American Lebanese and Syrian Associated Charities (ALSAC) of St. Jude Children's Research Hospital.
REFERENCES
- 1.Ball, E. M., and G. P. Risbridger. 2001. Activins as regulators of branching morphogenesis. Dev. Biol. 238:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Banerji, J., J. Sands, J. L. Strominger, and T. Spies. 1990. A gene pair from the human major histocompatibility complex encodes large proline-rich proteins with multiple repeated motifs and a single ubiquitin-like domain. Proc. Natl. Acad. Sci. USA 87:2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bard, J. B., A. Gordon, L. Sharp, and W. I. Sellers. 2001. Early nephron formation in the developing mouse kidney. J. Anat. 199:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard, M. 2004. Transcriptional control of kidney development. Differentiation 72:295-306. [DOI] [PubMed] [Google Scholar]
- 5.Brodbeck, S., and C. Englert. 2004. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr. Nephrol. 19:249-255. [DOI] [PubMed] [Google Scholar]
- 6.Bush, K. T., H. Sakurai, D. L. Steer, M. O. Leonard, R. V. Sampogna, T. N. Meyer, C. Schwesinger, J. Qiao, and S. K. Nigam. 2004. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev. Biol. 266:285-298. [DOI] [PubMed] [Google Scholar]
- 7.Cebrian, C., K. Borodo, N. Charles, and D. A. Herzlinger. 2004. Morphometric index of the developing murine kidney. Dev. Dyn. 231:601-608. [DOI] [PubMed] [Google Scholar]
- 8.Chuang, P. T., and A. P. McMahon. 2003. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 13:86-91. [DOI] [PubMed] [Google Scholar]
- 9.Clapp, W. L., and D. R. Abrahamson. 1993. Regulation of kidney organogenesis: homeobox genes, growth factors, and Wilms tumor. Curr. Opin. Nephrol. Hypertens. 2:419-429. [PubMed] [Google Scholar]
- 10.Clark, A. T., and J. F. Bertram. 1999. Molecular regulation of nephron endowment. Am. J. Physiol. 276:F485-F497. [DOI] [PubMed] [Google Scholar]
- 11.Cory, S., and J. M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 12.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 13.Davies, J. A., and A. W. Brandli. 2001. The kidney development database. [Online.] http://golgi.ana.ed.ac.uk/kidhome.html.
- 14.Dressler, G. 2002. Tubulogenesis in the developing mammalian kidney. Trends Cell Biol. 12:390-395. [DOI] [PubMed] [Google Scholar]
- 15.Englert, C., X. Hou, S. Maheswaran, P. Bennett, C. Ngwu, G. G. Re, A. J. Garvin, M. R. Rosner, and D. A. Haber. 1995. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 14:4662-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerasimenko, J. V., O. V. Gerasimenko, A. Palejwala, A. V. Tepikin, O. H. Petersen, and A. J. Watson. 2002. Menadione-induced apoptosis: roles of cytosolic Ca2+ elevations and the mitochondrial permeability transition pore. J. Cell Sci. 115:485-497. [DOI] [PubMed] [Google Scholar]
- 17.Hakem, R., A. Hakem, G. S. Duncan, J. T. Henderson, M. Woo, M. S. Soengas, A. Elia, J. L. de la Pompa, D. Kagi, W. Khoo, J. Potter, R. Yoshida, S. A. Kaufman, S. W. Lowe, J. M. Penninger, and T. W. Mak. 1998. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339-352. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann-Petersen, R., and C. Gordon. 2004. Integral UBL domain proteins: a family of proteasome interacting proteins. Semin. Cell Dev. Biol. 15:247-259. [DOI] [PubMed] [Google Scholar]
- 19.Hipfner, D. R., and S. M. Cohen. 2004. Connecting proliferation and apoptosis in development and disease. Nat. Rev. Mol. Cell Biol. 5:805-815. [DOI] [PubMed] [Google Scholar]
- 20.Kispert, A., S. Vainio, and A. P. McMahon. 1998. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125:4225-4234. [DOI] [PubMed] [Google Scholar]
- 21.Kreidberg, J. A., H. Sariola, J. M. Loring, M. Maeda, J. Pelletier, D. Housman, and R. Jaenisch. 1993. WT-1 is required for early kidney development. Cell 74:679-691. [DOI] [PubMed] [Google Scholar]
- 22.Kuida, K., T. F. Haydar, C. Y. Kuan, Y. Gu, C. Taya, H. Karasuyama, M. S. Su, P. Rakic, and R. A. Flavell. 1998. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325-337. [DOI] [PubMed] [Google Scholar]
- 23.Kuida, K., T. S. Zheng, S. Na, C. Kuan, D. Yang, H. Karasuyama, P. Rakic, and R. A. Flavell. 1996. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368-372. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, R., W. Lutz, E. Frank, and H. J. Im. 2004. Immediate early gene X-1 interacts with proteins that modulate apoptosis. Biochem. Biophys. Res. Commun. 323:1293-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechner, M. S., and G. R. Dressler. 1997. The molecular basis of embryonic kidney development. Mech. Dev. 62:105-120. [DOI] [PubMed] [Google Scholar]
- 26.Levinson, R., and C. Mendelsohn. 2003. Stromal progenitors are important for patterning epithelial and mesenchymal cell types in the embryonic kidney. Semin. Cell Dev. Biol. 14:225-231. [DOI] [PubMed] [Google Scholar]
- 27.Mayo, M. W., C. -Y. Wang, S. S. Drouin, L. V. Madrid, A. F. Marshall, J. C. Reed, B. E. Weissman, and A. S. Baldwin. 1999. WT1 modulates apoptosis by transcriptionally upregulating the bcl-2 proto-oncogene. EMBO J. 18:3990-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosser, D. D., and R. I. Morimoto. 2004. Molecular chaperones and the stress of oncogenesis. Oncogene 23:2907-2918. [DOI] [PubMed] [Google Scholar]
- 29.Nagata, M., H. Nakauchi, K. Nakayama, D. Loh, and T. Watanabe. 1996. Apoptosis during an early stage of nephrogenesis induces renal hypoplasia in bcl-2-deficient mice. Am. J. Pathol. 148:1601-1611. [PMC free article] [PubMed] [Google Scholar]
- 30.Naujokat, C., and S. Hoffmann. 2002. Role and function of the 26S proteasome in proliferation and apoptosis. Lab. Investig. 82:965-980. [DOI] [PubMed] [Google Scholar]
- 31.Pavenstadt, H., W. Kriz, and M. Kretzler. 2003. Cell biology of the glomerular podocyte. Physiol. Rev. 83:253-307. [DOI] [PubMed] [Google Scholar]
- 32.Porteous, S., E. Torban, N.-P. Cho, H. Cunliffe, L. Chua, L. McNoe, T. Ward, C. Souza, P. Gus, R. Giugliani, T. Sato, K. Yun, J. Favor, M. Sicotte, P. Goodyer, and M. Eccles. 2000. Primary renal hypoplasia in humans and mice with PAX2 mutations: evidence of increased apoptosis in fetal kidneys of Pax21Neu +/− mutant mice. Hum. Mol. Genet. 9:1-11. [DOI] [PubMed] [Google Scholar]
- 33.Quaggin, S. E., L. Schwartz, S. Cui, P. Igarashi, J. Deimling, M. Post, and J. Rossant. 1999. The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis. Development 126:5771-5783. [DOI] [PubMed] [Google Scholar]
- 34.Ranger, A. M., B. A. Malynn, and S. J. Korsmeyer. 2001. Mouse models of cell death. Nat. Genet. 28:113-118. [DOI] [PubMed] [Google Scholar]
- 35.Ryan, G., V. Steele-Perkins, J. F. Morris, F. J. Rauscher III, and G. R. Dressler. 1995. Repression of Pax-2 by WT1 during normal kidney development. Development 121:867-875. [DOI] [PubMed] [Google Scholar]
- 36.Sariola, H. 2002. Nephron induction revisited: from caps to condensates. Curr. Opin. Nephrol. Hypertens. 11:17-21. [DOI] [PubMed] [Google Scholar]
- 37.Shah, M. M., R. V. Sampogna, H. Sakurai, K. T. Bush, and S. K. Nigam. 2004. Branching morphogenesis and kidney disease. Development 131:1449-1462. [DOI] [PubMed] [Google Scholar]
- 38.Shi, Y. 2002. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9:459-470. [DOI] [PubMed] [Google Scholar]
- 39.Sorenson, C. M., S. A. Rogers, S. J. Korsmeyer, and M. R. Hammerman. 1995. Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am. J. Physiol. 268:F73-F81. [DOI] [PubMed] [Google Scholar]
- 40.Takayama, S., and J. C. Reed. 2001. Molecular chaperone targeting and regulation by BAG family proteins. Nat. Cell Biol. 3:E237-E241. [DOI] [PubMed] [Google Scholar]
- 41.Thress, K., E. K. Evans, and S. Kornbluth. 1999. Reaper-induced dissociation of a Scythe-sequestered cytochrome c-releasing activity. EMBO J. 18:5486-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thress, K., W. Henzel, W. Shillinglaw, and S. Kornbluth. 1998. Scythe: a novel reaper-binding apoptotic regulator. EMBO J. 17:6135-6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thress, K., J. Song, R. I. Morimoto, and S. Kornbluth. 2001. Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper. EMBO J. 20:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torban, E., M. R. Eccles, J. Favor, and P. R. Goodyer. 2000. PAX2 suppresses apoptosis in renal collecting duct cells. Am. J. Pathol. 157:833-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres, M., E. Gómez-Pardo, G. R. Dressler, and P. Gruss. 1995. Pax-2 controls multiple steps of urogenital development. Development 121:4057-4065. [DOI] [PubMed] [Google Scholar]
- 46.Vainio, S., and Y. Lin. 2002. Coordinating early kidney development: lessons from gene targeting. Nat. Rev. Genet. 3:533-543. [DOI] [PubMed] [Google Scholar]
- 47.Veis, D. J., C. M. Sorenson, J. R. Shutter, and S. J. Korsmeyer. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229-240. [DOI] [PubMed] [Google Scholar]
- 48.Wei, H., W. Wei, D. E. Bredesen, and D. C. Perry. 1998. Bcl-2 protects against apoptosis in neuronal cell line caused by thapsigargin-induced depletion of intracellular calcium stores. J. Neurochem. 70:2305-2314. [DOI] [PubMed] [Google Scholar]
- 49.Wu, Y. H., S. F. Shih, and J. Y. Lin. 2004. Ricin triggers apoptotic morphological changes through caspase-3 cleavage of BAT3. J. Biol. Chem. 279:19264-19275. [DOI] [PubMed] [Google Scholar]