Abstract
p38 mitogen-activated protein kinases (MAPKs) are activated primarily in response to inflammatory cytokines and cellular stress, and inhibitors which target the p38α and p38β MAPKs have shown potential for the treatment of inflammatory disease. Here we report the generation and initial characterization of a knockout of the p38β (MAPK11) gene. p38β−/− mice were viable and exhibited no apparent health problems. The expression and activation of p38α, ERK1/2, and JNK in response to cellular stress was normal in embryonic fibroblasts from p38β−/− mice, as was the activation of p38-activated kinases MAPKAP-K2 and MSK1. The transcription of p38-dependent immediate-early genes was also not affected by the knockout of p38β, suggesting that p38α is the predominant isoform involved in these processes. The p38β−/− mice also showed normal T-cell development. Lipopolysaccharide-induced cytokine production was also normal in the p38β−/− mice. As p38 is activated by tumor necrosis factor, the p38β−/− mice were crossed onto a TNFΔARE mouse line. These mice overexpress tumor necrosis factor, which results in development symptoms similar to rheumatoid arthritis and inflammatory bowel disease. The progression of these diseases was not however moderated by knockout of p38β. Together these results suggest that p38α, and not p38β, is the major p38 isoform involved in the immune response and that it would not be necessary to retain activity against p38β during the development of p38 inhibitors.
Mitogen-activated protein kinase (MAPK) cascades are important mediators of the cellular response to a wide variety of extracellular signals, including mitogens, growth factors, cytokines, and cellular stress (reviewed in references 22 and 30). There are 14 MAPKs in mammalian cells and these can be divided into four groups, the classical MAPKs (ERK 1 and ERK2), JNKs, p38s, and atypical MAPKs such as ERK3, ERK5, and ERK8 (22, 30, 34).
In mammalian cells, four isoforms of the p38 MAPKs have been described, p38α (also referred to as SAPK2a), p38β (SAPK2b), p38γ (SAPK3), and p38δ (SAPK4). The p38 MAPKs are activated primarily in response to proinflammatory cytokines and cellular stress such as UV irradiation and osmotic and chemical shock (reviewed in references 43, 47, and 58). Activation of p38 occurs by dual phosphorylation of a Thr-Xaa-Tyr motif, where Xaa is any amino acid, in the p38 kinase domain by an upstream MAPK kinase (MKK). Using MKK knockouts, it has been shown that in response to most stimuli MKK3 and MKK6 are the main MKKs activating p38α, although in some circumstances, such as UV-C stimulation, MKK4 may also play a role (3).
The major MKK required for p38 activation may also be affected by cell type and stimulus, for instance, MKK3 has been shown to be the major p38 activator in mesangial cells stimulated by transforming growth factor beta (53), while MKK6 appears to be the predominant isoform in thymocytes (52). Unlike p38α, the activation of p38 β, γ, and δ isoforms has not been extensively examined in MKK knockouts, however, it is probable that MKK6, and possibly MKK3, are required for the activation of these p38 isoforms (9, 10, 18, 38). MKK6 and MKK3 are in turn activated by phosphorylation by a MAPK kinase kinase (MKKK). The MKKK responsible for activating the p38 cascade appears to be cell type and stimulus specific, and several MKKKs have been implicated in the activation of p38. These MKKKs include MLKs, Ask, and Tak (4, 13, 20, 21, 37, 57).
p38 isoforms have been implicated in several processes including cell survival, apoptosis, and immune function (reviewed in references 8, 40, 43, 45, 47, 49, and 58). Much of the information on p38 function comes from the use of the relatively specific p38 inhibitors SB203580 and SB202190, members of a class of pyridinyl imidazoles. These compounds were originally identified as inhibitors that were able to suppress the bacterial lipopolysaccharide (LPS)-induced production of tumor necrosis factor (TNF) and have also been shown to be effective in animal models of chronic inflammatory disease (reviewed in references 29, 31, 32, and 44), findings that have stimulated a considerable amount of work on the role of p38 in the immune system.
SB203580 and SB202190 are able to inhibit both p38 α and β by binding to the ATP binding pocket of the p38 kinase domain and preventing ATP binding. SB203580 and SB202190 are able to bind here in p38 α and β due to the presence of a threonine side chain (Thr106 in p38α) in the ATP binding pocket of these kinases. Other MAPKs, including ERK1/2, p38γ, and p38δ, are not inhibited by SB203580 and SB202190 due to the presence of a large side chain, such as methionine or glutamine, in the equivalent position in the ATP binding pocket that prevents inhibitor binding (11, 12, 16). While these inhibitors were originally thought to be selective for p38 α and β, some other kinases have also been reported to be inhibited by SB203580 at concentrations similar to or lower than that required to inhibit p38α, including Raf (17), RICK, GAK, and CK1 (14).
While the use of p38 inhibitors has been critical in establishing roles for p38 in cellular function, they have not been able to distinguish between the functions of p38 α and β. One possible way in which this issue could be addressed is through the use of knockout or knockin mice. Knockout of p38α has been reported by four groups. In each case, p38α deficiency was found to result in embryonic lethality, although some variability in the age at which lethality occurred was seen (1, 2, 39, 51). These studies did however reveal a role for p38α in placental development (1, 39) and erythropoietin expression (51). Tetraploid rescue of the placental defect in p38α−/− embryos demonstrated that while p38α was essential for extraembryonic development, it was not essential for development of the embryo or survival of the adult mice. Consistent with the phenotype of p38α knockouts, double knockout of the p38 activators MKK 3 and 6 also resulted in embryonic lethality due to placental and vascular defects (3). Single knockouts of MKK 3 and 6 were however found to be viable, although defects in T-cell function and cytokine production were reported (33, 52, 56). Knockouts of p38γ or p38δ, as well as a double knockout of p38 γ and δ, have however been found to be viable (46). Here we report the generation and initial characterization of a p38β knockout.
MATERIALS AND METHODS
Antibodies.
Antibodies against ERK1/2, phosphorylated ERK1/2, phosphorylated p38, JNK, and phosphorylated JNK were from Cell Signaling. Antibodies against p38 γ and δ were generated in house and have been described previously (46). Antibodies against p38 α and β were raised in sheep against the peptides VPPPLDQEEMES and KPLEPSQLPGTHEIEQ, respectively, and the antibodies purified against their respective peptides before use.
Generation of p38β−/− mice.
Targeting vectors were constructed to generate a deletion of exons 2 to 7 of p38β, or to introduce an Asp168Ala mutation in the p38β gene (Fig. 1 and 2). The targeting vector for the knockout consisted of a 1.5-kb 5′ arm of homology, neomycin resistance cassette, 5.2-kb 3′ arm of homology followed by a thymidine kinase cassette. For the Asp168Ala mutation, the sequence around exons 2 to 7 was generated by PCR and mutated using the QuikChange PCR mutagenesis system (Stratagene) and inserted in the knockout vector. Arms of homology were generated by PCR using the primers CTGAGATGGGTAGGCTTGTTCTCGCG and CTGGCAGAGAACCGGTGATTCTAGC for the 5′ arm, TCATGCCTCTTCTCAGGACAGGG and AACGGGCTGCAGGTCTTGGTACACG for the 3′ arm, and AGAACGCTTTGTCCACTCAAAGGGG and GGACAGACACCCATCAGCTTAGAAGCC for the region encoding exons 2 to 7. Further details of the cloning can be provided on request. The correct cloning of the targeting vector was confirmed by DNA sequencing.
FIG. 1.
Generation of p38β kinase-dead knockin mice. To generate p38β knockin mice a targeting vector was made to introduce an Asp168Ala point mutation in exon 7 of the p38β/MAPK11 gene (A). This vector was used to target ES cells which were screened for homologous recombination using a probe 3′ to the targeting vector. The neomycin resistance cassette was removed from correctly targeted cells by transient expression of CRE recombinase. Removal of the neomycin resistance cassette was confirmed by Southern blotting after EcoRI/SspI digestion of genomic DNA with a probe 5′ to the targeting vector. Southern blotting with this probe resulted in a 10.5-kb band for the wild-type locus, and a 3.5-kb band for a correctly targeted p38b locus (B). Heterozygous ES cells were used to generate mice which were genotypes using a PCR-based strategy, described in the Materials and Methods, that generated a 236-bp fragment for the targeted genes and 100-bp fragment for the wild-type gene (C). To analyze expression of the Asp168Ala protein, primary MEF cells were cultured from p38β+/+, p38β−/− and p38βki/ki mice. MEF protein lysates were immunoblotted for expression of p38β (D). To analyze expression of p38β mRNA in the same cells, total RNA was extracted and p38β mRNA levels determined by quantitative reverse transcription-PCR using 18s rRNA levels as a loading control. Two primer sets were used, designated p38β1 (black bars) and p38β2 (white bars), for which sequences are given in Table 1. Error bars represent the standard error of duplicate assays on three separate plates of cells per genotype (E). To analyze the stability of D168A, C211R, and D168A/C211R mutations of p38β compared to the wild-type sequence, HA-tagged p38β was transfected into HeLa cells. Lysates were immunoblotted using an HA tag antibody to detect p38β, and a ribosomal protein S6 kinase (RSK) antibody as a loading control (F).
FIG. 2.
Generation of p38β knockout mice. To generate p38β knockout mice a targeting vector was constructed to delete exons 2 to 7 in the p38β/MAPK11 gene (A). This vector was used to target ES cells which were screened for homologous recombination using a probe 3′ to the targeting vector (A). Southern blotting after EcoR1/SspI digestion of genomic DNA with this probe resulted in a 10.5kb band for the wild-type locus, and a 7kb band for a correctly targeted p38β locus (B). Heterozygous ES cells were used to generate mice which were genotyped using a PCR based strategy, described in the Materials and Methods, that generated a 900-bp fragment for the targeted genes and 500-bp fragment for the wild-type gene (C).
Targeting vectors were introduced into E14 mouse embryonic stem (ES) cells by electroporation, and colonies were grown up and isolated as described (55). ES cells were maintained on growth inactivated mouse embryonic fibroblast (MEF) feeder layers in Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% fetal bovine serum (HyClone), 2 mM l-glutamine, 1 mM sodium pyruvate, 50 units/ml penicillin G, 50 μg/ml streptomycin (Invitrogen), 0.1 mM 2-mercaptoethanol, and 1,000 U/ml leukemia inhibitory factor. Targeted ES cells were identified by Southern analysis using probes external to the targeting vector. For the Asp168Ala knockin, the neomycin cassette was removed from the targeted ES cells by transient expression of CRE recombinase. ES cells heterozygous for the desired targeting were injected into blastocysts and the resulting chimeric mice were bred to C57BL/6 wild-type mice. Germ line transmission was determined by a combination of coat color and genotyping.
For routine genotyping of mice, PCR was used to genotype DNA isolated from ear biopsies. For genotyping of knockouts three primers were used (GCATCTCTGGAGCTCTGTGAGAGG, GGAGACTATCAGTCTGCCAACCC, and GGCGATGCCTGCTTGCCGAATATCATGG) which resulted in a product of 900 bp for the wild-type gene and 500 bp for the targeted gene. Mice targeted with the knockin mutation were genotyped using two primers (ATTCTCTCGCCTCTCCCTCTCTCC and CCGACCCTCCTGATCCTCCCTTAG), which generated a 373-bp wild-type product and a 567-bp knockin product. Mice were kept under specific-pathogen-free conditions and in accordance with local ethical guidelines and United Kingdom law.
Cell culture.
HeLa cells were maintained in DMEM containing 10% fetal bovine serum (Sigma), 2 mM l-glutamine, 50 units/ml penicillin G, and 50 μg/ml streptomycin (Invitrogen). Primary mouse embryonic fibroblast (MEF) cells were isolated as described (54) and cultured in DMEM containing 10% fetal bovine serum, 2 mM l-glutamine, 50 units/ml penicillin G, and 50 μg/ml streptomycin. HeLa and MEF cells were serum starved in DMEM containing 2 mM l-glutamine, 50 units/ml penicillin G, and 50 μg/ml streptomycin (Invitrogen) for 16 h before use.
To generate bone marrow derived macrophages, bone marrow cells were isolated from the femurs of mice. Cells were cultured on bacterial grade plastic in DMEM supplemented with 10% heat inactivated fetal bovine serum, 2 mM l-glutamine, 50 units/ml penicillin G 50 μg/ml streptomycin and 5 ng/ml recombinant CSF. After 7 days cells in suspension were discarded and adherent cells removed by washing in versene (Invitrogen). The adherent cells were then replated in DMEM supplemented with 10% heat inactivated fetal bovine serum, 2 mM l-glutamine, 50 units/ml penicillin G, 50 μg/ml streptomycin and 25 ng/ml recombinant colony-stimulating factor and used 24 h later.
Cells were stimulated with either anisomycin, arsenite or LPS using the concentrations and times indicated. For kinase assays and immunoblotting, cells were lysed in 50 mM Tris-HCl (pH 7.5), 1 mM EGTA, 1 mM EDTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1 mM sodium pyrophosphate, 0.27 M sucrose, 1% (vol/vol) Triton X-100, 0.1% (vol/vol) 2-mercaptoethanol, and complete proteinase inhibitor cocktail (Roche, East Sussex, United Kingdom). The lysates were centrifuged at 13,000 rpm for 5 min at 4°C, and the supernatants were removed, quick-frozen in liquid nitrogen, and stored at −80°C until use.
Immunoprecipitation kinase assay.
Kinases were immunoprecipitated from cell lysate using an appropriate antibody coupled to protein G-Sepharose. For assay of MAPKAP-K2 50 μg of cell lysate was used; however, for MSK1 1 mg of lysate was required in addition to a preclearing step by incubation with protein G-Sepharose for 30 min at 4°C to reduce nonspecific binding to the beads during immunoprecipitation (54). The immunoprecipitate was then washed with lysis buffer containing 0.5 M NaCl, with 50 mM Tris-HCl (pH 7.5), 0.1 mM EGTA, and 0.5 M NaCl, and twice with 50 mM Tris-HCl (pH 7.5) and 0.1 mM EGTA prior to assaying for protein kinase activity.
Protein kinases were assayed in 50-μl reactions by measuring the incorporation of radioactive [32P]phosphate from [γ-32P]ATP into substrate peptides. The assay was initiated by the addition of 35 μl of a mix of 50 mM Tris-HCl (pH 7.5), 100 μM EGTA, 2.5 μM PK1, 0.1% (vol/vol) 2-mercaptoethanol, 30 μM substrate peptide, 100 μM [γ-32P]ATP, and 10 mM MgCl2. For MSK1 Crosstide (GRPRTSSFAEG) was used as the substrate peptide, while for MAPKAP-K2 the substrate peptide used was KKLNRTLSVA. Reactions were stopped by pipetting 40 μl onto P81 paper followed by washing in 75 mM orthophosphoric acid. Incorporation of 32P was measured by Cerenkov counting in a Wallac 1409 liquid scintillation counter. One unit was defined as the incorporation of 1 nmol of phosphate into the substrate peptide in 1 min.
Immunoblotting.
Soluble protein extract (20 to 30 μg) was run on 4 to 12% polyacrylamide gels (Novex, Invitrogen) and transferred to nitrocellulose membranes. Proteins were detected using primary antibodies in combination with horseradish peroxidase (Pierce) and chemiluminescent substrate (Amersham).
Real-time PCR.
Cells were treated as indicated, then lysed and RNA isolated using the NucleoSpin RNA purification method (Macherey-Nagel). RNA was reverse transcribed (iScript; Bio-Rad) and real-time PCR carried out using Sybr green-based detection. 18s rRNA levels were used as normalization controls and relative mRNA levels calculated using the equation
 |
where E is the efficiency of the PCR, Ct is the threshold cycle, u is the mRNA of interest, r is the reference gene (18s RNA), s is the stimulated sample, and c is the unstimulated control sample. Primer sequences for the amplification of murine p38β, c-fos, junB, c-jun, nur77, interleukin-6, TNF-α, and 18s RNA are shown in Table 1.
TABLE 1.
Primers used for PCR
| Gene | Sense | Antisense |
|---|---|---|
| 18S rRNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| p38β1 | GCTGTCTCGCCCTTTCCAATC | CGTGCTTCAGGTGCTTGAGTAG |
| p38β2 | ATGAGGAGATGACCGGATATGTG | GCAGCAGTTCAGCCATGATG |
| c-jun | CGCCTCGTTCCTCCAGTC | ACGTGAGAAGGTCCGAGTTC |
| c-fos | TCTGGAACCTCCTCGCTCAC | GTTGGCACTAGAGACGGACAG |
| nur77 | CCTGTTGCTAGAGTCTGCCTTC | CAATCCAATCACCAAAGCCACG |
| IL-6 | TTCCATCCAGTTGCCTTCTTG | AGGTCTGTTGGGAGTGGTATC |
| TNF | CAGACCCTCACACTCAGATCATC | GCTACAGGCTTGTCACTCG |
Analysis of T cells.
Thymi and spleen were isolated from 5- to 6-week-old mice. Lymphocytes were isolated from these organs and total lymphocyte numbers were determined. Monoclonal antibodies raised against cell surface markers and conjugated to the fluorophores fluorescein isothiocyanate, phycoerythrin, allophoycocyanin, biotin, TriColor, or allophoycocyanin-indotricarbocyanine were used to analyze the development of T cells by flow cytometry. Cells were stained with saturating concentrations of antibody for the expression of the following markers using the antibodies indicated: CD4 (RM4-5), CD8 (53-6.7), CD25 (7D4), CD44 (IM7), Thy-1.2 (53-2.1), T-cell receptor β (H57-597), CD45.1 (A20), and CD45.2. Data were acquired on a FACSCalibur or BD LSRI (Becton Dickinson), events were collected and stored ungated with CellQuest software (Becton Dickinson) and analyzed with CellQuest or FlowJo software (Treestar). To analyze T-cell receptor-induced apoptosis in double-positive thymocytes, p38β+/+ and p38β−/− mice were injected with either phosphate-buffered saline or anti-CD3 antibody (50 ng). Mice were culled 48 h after injection and cell numbers for double-positive and CD4- and CD8-positive thymocytes determined by counting and fluorescence-activated cell sorting analysis.
Analysis of LPS-induced cytokine production.
Age-matched (10 to 12 weeks) mice were administered an intraperitoneal injection of 37.5 μg of LPS (Escherichia coli 055B5 Sigma L6529) dissolved in sterile saline solution. Control mice were given an intraperitoneal injection of the equivalent volume of saline. At the times indicated mice were culled by CO2 asphyxiation and blood was taken by cardiac puncture. Plasma levels of cytokines (TNF-α, interleukins 1α, 1β, 6, 12p40, and 13, Mip1a, Mip1b, and RANTES) were determined using a Luminex-based multiplex assay from Bio-Rad.
Analysis of p38β/TNFΔARE mice.
The generation and characterization of TnfΔARE mice has been previously described (27). TnfΔARE/+/p38β−/− mice were generated by breeding TnfΔARE/+ mice into a p38β-deficient background. Development of chronic inflammatory arthritis and Crohn's-like inflammatory bowel disease pathology was evaluated at 8, 12, and 16 weeks of age in TnfΔARE/+/p38β−/− compared to TnfΔARE/+/p38β+/+ littermates. Wild-type and p38β−/− mice were also included as additional control mice. All the mice used for assessment of pathology were kept in a mixed C57/129 background.
Clinical symptoms of arthritis were evaluated in ankle joints in a blind manner using a semiquantitative arthritis score ranging from 0 to 3, where 0 equated to no arthritis (normal appearance and grip strength), 1 equated to mild arthritis with some joint swelling, 2 equated to moderate arthritis (severe joint swelling and digit deformation, reduced grip strength), and 3 equated to severe arthritis (severe joint swelling and digit deformation, impaired movement, no grip strength).
For histological assessment of arthritis, paraffin-embedded joint tissue samples were sectioned and stained with hematoxylin and eosin and they were histologically evaluated in a blind fashion. Arthritic pathology was assessed separately for synovial hyperplasia, existence of inflammatory sites, cartilage destruction, and bone erosion and scored as 0 for normal, 1 for mild inflammation in periarticular tissue and/or mild edema, 2 for moderate inflammation and pannus formation with superficial cartilage and bone destruction, 3 for marked inflammation with pannus formation and moderate cartilage and bone destruction (depth to middle zone), and 4 for severe inflammation with pannus formation and marked cartilage and bone destruction (depth to tidemark).
For Inflammatory bowel disease, a histopathological score of inflammation in the terminal ileum was evaluated after hematoxylin/eosin staining of paraffin sections from the gastrointestinal tract of the mice, where 0 equated to no detectable pathology, 1 equated to isolated inflammatory cells in the stroma, 2 equated to the presence of inflammatory cells in groups (up to five cells) with focal infiltration to mucosa and submucosa layer (more than five groups of cells and less than 10 groups per 10 HPF), 3 equated to inflammatory cells in groups (up to five cells) with extensive mucosal and submucosal inflammation (more than 10 groups of cells per 10 HPF), and 4 equated to to transmural inflammation.
RESULTS
Generation of p38β gene-targeted mice.
In order to study the physiological function of p38β, we generated two lines of mice with alterations in the p38β (MAPK11) gene; a kinase-dead knockin mutation and a knockout. To generate p38β kinase-dead knockin (ki) mice, a targeting vector was used to insert an Asp168Ala mutation into the p38β gene using standard techniques (Fig. 1A, Materials and Methods). After positive embryonic stem cell colonies were identified, the neomycin gene was excised by transient expression of Cre recombinase in the ES cells (Fig. 1B). ES cells carrying the mutation but without the neomycin gene were used to generate mice, which were identified by PCR genotyping (Fig. 1C).
The homozygous knockin (p38βki/ki) mice were viable and fertile and showed no apparent health problems when maintained under specific-pathogen-free conditions. Surprisingly, when the expression of p38β was analyzed in the p38βki/ki mice, no p38β expression was seen in either the cortex (the tissue in which highest levels of p38β was seen in p38β+/+ animals) of these mice (data not shown), or in primary embryonic fibroblasts derived from these mice (Fig. 1D).
To ensure that the targeting had not disrupted the expression of the p38β gene, mRNA was isolated from wild-type, p38β−/− and p38βki/ki MEF cells and analyzed for p38β mRNA expression by real-time PCR. Levels of p38β mRNA in p38βki/ki cells were similar to that in wild-type cells, while as expected no p38β mRNA was seen in p38β−/− cells (Fig. 1E).
To determine if the Asp168Ala mutation affected the stability of p38β protein, hemagglutinin (HA)-tagged wild-type and Asp168Ala protein was transiently expressed in HeLa cells. Both proteins were expressed at similar levels, as judged by immunoblotting for the HA tag (Fig. 1F). The knockin mutation was generated in E14 ES cells, and further analysis of the p38β gene in the parent E14 cells, and also in the p38βki/ki mice, revealed a single nucleotide polymorphism that would result in the mutation of Cys211 to Arg. To determine the effect of single nucleotide polymorphism on p38β expression, Cys211Arg HA-p38β, and also Asp168Ala/Cys211Arg were expressed in HeLa cells. Mutation of Cys211 to Arg, either as a single mutation or as a double mutation with Asp168Ala, greatly reduced the expression of p38β in these cells (Fig. 1F). As this single nucleotide polymorphism may result in a natural knockout of p38β, this region of the p38β gene was sequenced in DNA from C57BL/6 or Sv129 mice. We have not however been able to establish the presence of this single nucleotide polymorphism in these strains of mice.
To generate p38β knockout mice, a targeting vector was used to delete exons 2 to 7 of the p38β (MAPK11) gene in embryonic stem cells (Fig. 2, Materials and Methods). These ES cells were used to obtain p38β knockout mice as described in the Materials and Methods. The mice were genotyped by PCR (Fig. 1C) and p38β−/− mice were found to be born at approximately the expected frequency from p38+/− matings (30% wild type, 48% heterozygous. and 22% knockout; n = 403). p38β−/− mice were fertile and of normal size, and had no apparent health problems or phenotype when maintained under specific-pathogen-free conditions.
To ensure that the knockout had been effective, the expression of p38β protein was examined in p38β+/+ and p38β−/− mice. To facilitate this, a p38β-specific antibody was generated by injection of a p38β-specific peptide (KPLEPSQLPGTHEIEQ) into sheep. The resulting antibody was found to recognize recombinant p38β but not p38α (data not shown). In p38β+/+ mice high levels of p38β were detected in the brain, thymus, and spleen, with lower levels found in the adrenals, lung, kidney, liver, pancreas, and heart (Fig. 3A). Little or no expression of p38β was seen in skeletal muscle. As expected, no p38β expression was seen in tissues from the p38β−/− mice.
FIG. 3.
Expression of p38 isoforms in p38β+/+ and p38β−/− tissues. Lysates were prepared from the total brain, cerebellum, cortex, adrenals, thymus, spleen, liver, pancreas, kidney, lung, heart, and skeletal muscle of p38β+/+ and p38β−/− mice; 30 μg of soluble protein was run on 4 to 12% polyacrylamide gels and immunoblotted for total p38β (A), p38α (B), p38γ (C), and p38δ (D).
The levels of the other p38 isoforms were also examined by immunoblotting in these tissues, to check for possible compensatory upregulation in the p38β−/− mice. Analysis of p38α expression showed it was more ubiquitously expressed than the p38β isoform in p38β+/+ mice, although lower levels of expression were seen in the brain, liver, and pancreas compared to other tissues. There was no upregulation of p38α in any of the tissues from the p38β−/− mice (Fig. 3B). Levels of p38γ were found to be highest in skeletal muscle, although expression was also seen in most other tissues. Interestingly, in some tissues, particularly the cortex and pancreas, there appeared to be a moderate increase in p38γ levels in the p38β−/− mice (Fig. 3C). In p38β+/+ mice, levels of p38δ were found to be highest in the adrenal gland, kidney, and heart, and these levels were not significantly changed in the p38β−/− mice (Fig. 3D).
Stress-activated signaling in MEF cells.
The role that p38β plays in downstream signaling pathways known to be regulated by p38 isoforms was investigated in primary MEF cells. Serum-starved MEF cells isolated from p38β+/+ and p38β−/− mice were stimulated with anisomycin to activate the p38 signaling pathway. Cells isolated from p38β+/+ and p38β−/− mice expressed comparable levels of p38α as well as ERK1/2, and JNK as judged by immunoblotting (Fig. 4A). Anisomycin strongly activated p38α and JNK, and neither of these activations was affected by knockout of p38β. ERK1/2 were only weakly activated in response to anisomycin, and this was also not affected by p38β knockout (Fig. 4A). Similar results were obtained for stimulation of the cells with arsenite, with the exception that a stronger activation of ERK1/2 was observed (data not shown).
FIG. 4.
Characterization of p38 dependent signaling in p38β−/− MEF cells. Primary MEF cells were isolated from p38β+/+ and p38β−/− mice. Cells were serum starved for 16 h and then left unstimulated (control), incubated with 5 μM SB 203580 for 1 h, and then stimulated with 10 μg/ml anisomycin (aniso + SB) for a further hour or stimulated with 10 μg/ml anisomycin for 1 h (A and B) or for the times indicated (C) without any addition of SB203580. (A) Cells were then lysed and protein extracts were immunoblotted for p38α, p38β, phosphorylated p38α, ERK1/2, phosphorylated ERK1/2, JNK, or phosphorylated JNK. (B) Cells were lysed and MAPKAP-K2 or MSK1 was immunoprecipitated and assayed for activity as described in the Materials and Methods. One unit was defined as the incorporation of 1 nmol of phosphate into the substrate peptide in 1 min. Black bars represent results from p38β+/+ cells, while white bars represent results from p38β−/− cells. Error bars represent the standard errors of duplicate assays on three independent stimulations per condition. (C) Total RNA was isolated from cells and quantitative reverse transcription-PCR carried out as described in the Materials and Methods. Levels of c-jun, c-fos, junB, and nur77 were determined relative to 18s rRNA and fold stimulation calculated relative to the levels of the mRNA in unstimulated p38β+/+ cells. Black bars represent results from p38β+/+ cells, while white bars represent results from p38β−/− cells. Error bars represent the standard errors of duplicate assays on three independent stimulations per condition.
The activity of the p38-activated kinases MSK1 and MAPKAP-K2 was determined by immunoprecipitation kinase assays. Anisomycin activated MSK1 and MAPKAP-K2 in wild-type MEF cells, and this activation was blocked by preincubation of the cells with SB203580. The activation of MAPKAP-K2 was slightly reduced in p38β−/− cells compared to p38β+/+ cells, however the activation of MSK1 was the same in both cell types. The activation of both MAPKAP-K2 and MSK1 in the p38β−/− cells was still found to be inhibited by SB203580 (Fig. 4B). Similar effects were observed following arsenite stimulation (data not shown).
Cellular stress has been shown to result in the induction of several immediate-early genes in MEF cells, including c-fos, junB, c-jun, and nur77, in a p38-dependent manner (19, 54). In p38β+/+ cells the transcription of these genes in response to anisomycin was blocked by SB203580, consistent with a role for p38α or p38β in the induction of these genes. The transcription of these genes in MEF cells was not however affected by the knockout of p38β (Fig. 4C).
T-cell development is normal in p38β−/− mice.
T-cell precursors can be developmentally identified by the expression of the cell surface molecules CD4, CD8, CD25, and CD44. Early T-cell progenitors lack both CD4 and CD8 and are referred to as double-negative (DN) cells. This stage of development can be further characterized by expression of CD25 and CD44; early precursors are CD25−CD44+ (DN1), CD25 is the upregulated (DN2) then CD44 downregulated (DN3) and in the DN4 stage of development cells are negative for both CD44 and CD25. Both CD4 and CD8 are then upregulated to give double-positive cells. Either CD4 or CD8 is then downregulated to give CD4+ or CD8+ single-positive thymocytes that then exit the thymus and move into the periphery.
Previous studies have shown that during T-cell development high levels of p38 activity can be found in CD4−CD8− T cells, and that this activity is down-regulated as the cells become CD44−CD25− cells (7, 48). Overexpression of MKK6 in T cells resulted in sustained activation of p38 and inhibited the CD25+CD44− to CD25−CD44− transition. Overexpression of dominant negative p38α in T cells resulted in decreased T-cell number, suggesting that p38 activity may help the survival of double-negative T cells (7).
To determine if p38β could play an important role in T-cell development, fluorescence-activated cell sorting analysis was performed on thymocytes from these mice. No significant differences were seen in total lymphocyte cell number in the thymus or spleen of p38β+/+ and p38β−/− mice (Fig. 5A). In the thymus of p38β−/− mice, the numbers of double-negative cells were normal, as was the development of double- and single-positive CD4+ and CD8+ cells (Fig. 5B and C). Normal ratios of CD4 and CD8 cells were also found in the spleens of p38β−/− mice.
FIG. 5.
p38β is not required for T-cell development. Thymi and spleens were isolated from 6-week-old p38β+/+ and p38β−/− mice and analyzed as described in the Materials and Methods. (A) Total cell numbers, determined by counting isolated cell suspensions, were determined for both the thymus and spleen of p38β+/+ and p38β−/− mice. (B) Fluorescence-activated cell sorting plot of CD4 and CD8 stained Thy1-positive cells in the thymus. Results are representative plots from the analysis of three p38β+/+ and p38β−/− mice. (C) Quantification of the total numbers of double-negative cells from p38β+/+ and p38β−/− thymi. (D) p38β+/+ and p38β−/− mice were injected with either PBS or anti-CD3 antibody (50 ng). Mice were culled 48 h after injection and numbers of double-positive and CD4 and CD8 single-positive thymocytes were determined by counting and fluorescence-activated cell sorting analysis.
Deletion of the p38 activator MKK6 in mice has also suggested a role for one or more of the p38 isoforms in T-cell death induced by anti-CD3 antibodies (52). As expected, injection of an anti-CD3 antibody resulted in a significant reduction (approximately fourfold decrease) in the numbers of double-positive thymocytes and a slight decrease in the number of single-positive CD4 thymoctes in p38β+/+ mice. This process did not however require p38β as similar reductions in double-positive and CD4 single-positive cells were also obtained in p38β−/− mice (Fig. 5D).
p38β is not required for cytokine production in vivo in response to LPS.
As p38 isoforms have been implicated in the expression of cytokines, including interleukins 1, 6, and 12 and TNF, in response to inflammatory agents through the use of p38α/β inhibitors (8, 29, 31, 33, 47), the ability of the p38β−/− macrophages or mice to produce cytokines in response to an LPS challenge was examined. Stimulation of bone marrow-derived macrophages from p38β+/+ and p38β−/− mice with LPS resulted in similar increases in the levels of mRNA for both interleukin-6 and TNF-α in both genotypes (Fig. 6A). Cytokine levels in blood were also examined following intraperitoneal injections of LPS into mice. LPS stimulated the transient production of TNF-α, which was upregulated at 1 h but not 4 h post injection. Interleukin-6, Mip1a, and Mip1b were also upregulated by 1 h, however the levels of these proteins were still high 4 h post injection. Interleukins 1a, 1b, 13, and 12p40 and RANTES were also induced, however these cytokines were found to be higher at 4 h than 1 h. No significant difference was seen in the levels of these cytokines between p38β+/+ and p38β−/− mice.
FIG. 6.
p38β is not required for cytokine production. A) Macrophages were derived from the bone marrow of p38β+/+ and p38β−/− mice. Cells were stimulated with 10 ng/ml of LPS for the times indicated and the induction of interleukin-6 (Il-6) and TNF-α mRNAs was measured by real-time PCR as described in the Materials and Methods. B) Mice were given an intraperitoneal injection of 37.5 μg of LPS in a sterile saline solution, or an injection equivalent volume of saline. Mice were sacrificed after 1 or 4 h and plasma levels of interleukins 1a, 1b, 6, 12p40, and 13, TNF-α, Mip1a, Mip1b, and RANTES were measured by a Luminex-based multiplex assay as described in the Materials and Methods. All mice were 10 to 12 weeks of age and error bars represent the standard deviation of measurement on five individual mice.
p38β deficiency does not modify TNF-driven chronic inflammatory arthritis and Crohn's-like inflammatory bowel disease pathology.
TNF is an important mediator of inflammatory diseases including rheumatoid arthritis and inflammatory bowel disease (reviewed in references 24 and 25). As TNF is able to activate p38 in vivo, we investigated the role p38β may play in the development of these pathologies downstream of TNF. To examine this, TnfΔARE mice were used as a model for TNF-induced pathology. These mice have a mutation in the 3′ untranslated region of the TNF gene that disrupts an AU-rich region, which results in an increased translation and stability of the TNF mRNA and elevated levels of TNF in these animals, leading to the development of both arthritis and inflammatory bowel disease (27).
p38β−/− mice were crossed to TnfΔARE mice and the development of arthritis and inflammatory bowel disease was determined in TnfΔARE/+/p38β+/+ and TnfΔARE/+/p38β−/− mice at 8, 12, and 16 weeks of age as described in the Materials and Methods (Fig. 7). Wild-type and p38β−/− mice were also analyzed as additional controls. Gross examination of TnfΔARE/+/p38β+/+ and TnfΔARE/+/p38β−/− mice showed no significant differences in disease onset, as at week 8 both genotypes exhibited moderate joint swelling and reduced grip strength. Over the following weeks of study, full-blown disease developed in all groups.
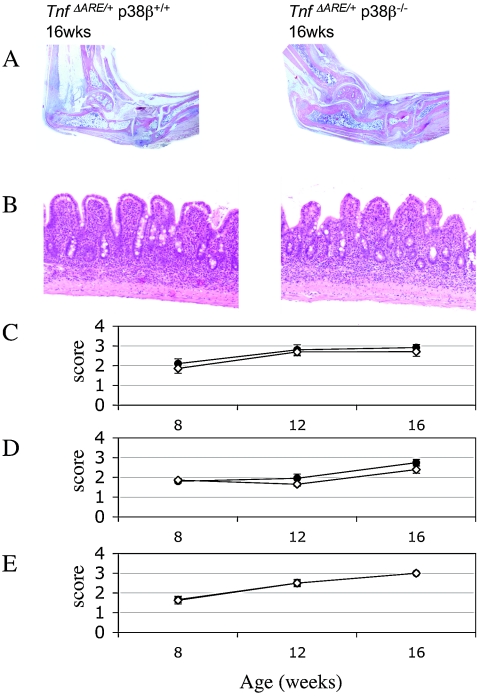
FIG. 7.
Inflammatory arthritis and inflammatory bowel disease in TnfΔARE/+/p38β+/+ and TnfΔARE/+/p38β−/− mice. Histologic examination (A) of ankle joints showed formation of the inflammatory pannus, and areas of cartilage and bone erosion are evident in both genotypes (B) of portions of ileum. Villous blunting and inflammatory infiltration are evident. Inflammatory bowel disease was evaluated histologically (C), while levels of arthritis were evaluated histologically (D) and clinically (E) as described in the Materials and Methods.
Histological examination was performed for both genotypes at 8, 12, and 16 weeks. At all time points, the severity score was identical for TnfΔARE/+/p38β+/+ and TnfΔARE/+/p38β−/− mice (Fig. 7) and in agreement with the clinical evaluation. In Fig. 7, both genotypes present all the hallmarks of full-blown arthritis: inflammation and pannus formation with superficial cartilage and bone destruction of ankle joints.
Crohn's-like inflammatory bowel disease developed by the TnfΔARE mice is restricted to the terminal ileum and occasionally to the proximal colon. For this, we histologically examined the terminal ileum and proximal colon of TnfΔARE/+/p38β+/+ and TnfΔARE/+/p38β−/− mice and based on the inflammation in the terminal ileum, inflammatory bowel disease development was evaluated as described in the Materials and Methods. Both genotypes developed inflammatory bowel disease with histopathological characteristics of villus blunting and infiltration of inflammatory cells to mucosal and submucosal gut layers (Fig. 7) becoming transmural with disease progression.
DISCUSSION
In contrast to the knockout of p38α, we report here that mice with knockouts of p38β are viable with no obvious health problems. One possible reason for this mild phenotype could be compensation between different p38 isoforms. This has been seen previously in p38γ knockouts, where the phosphorylation of the p38γ substrate SAP97 was insensitive to the p38α/β inhibitor SB203850 in wild-type cells but became sensitive to SB203580 in p38γ knockout cells (46). One possible way to reduce compensation would be to introduce point mutations to inactivate rather than delete p38β. As a result the kinase would still be expressed in cells from its endogenous promoter and be able to interact with but not phosphorylate its substrates. Similar knockin approaches have been used to successfully study other kinases, such as PDK1 and phosphatidylinositol 3-kinase (36, 42).
Mutation of the aspartic acid in the DGF motif of kinases to alanine has been used previously to generate inactive kinases. This mutation did not significantly the affect the stability of p38β as judged by transient expression in HeLa cells (Fig. 3) but did result in an inactive kinase (data not shown). Surprisingly, however, when the mutation was used to make p38βki/ki mice, these mice did not appear to express p38β protein, although expression of the p38β mRNA was normal. Further analysis revealed the presence of a single nucleotide polymorphism in the p38β gene in the wild-type and targeted E14 ES cells, and as would be expected, this single nucleotide polymorphism was also present in the p38βki/ki mice. The single nucleotide polymorphism would result in the mutation of cysteine 211 to arginine, and when the effect of this mutation was tested in transient transfection assays it destabilized the p38β protein. As a result the p38βki/ki mice were effectively similar to a knockout as they lacked detectable levels of the p38β protein, and the Cys211Arg single nucleotide polymorphism would need to be changed in the ES cells before an effective kinase-dead knockin could be made. The origin of the single nucleotide polymorphism is unclear, as while we were able to detect it in E14 ES cells, we have not yet been able to find the corresponding single nucleotide polymorphism in mice.
Using MEF cells we have shown that knockout of p38β does not affect the expression or activation of the ERK1/2 or JNK MAPK in response to cellular stress. Similarly knockout of p38β did not affect the expression or activation of p38α. As SB203580, which inhibits both p38 α and β, blocks the phosphorylation and activation of MSK1, MSK2, and MAPKAP-K2 in MEF cells in response to cellular stress (54), the activation of these kinases in the p38β−/− cells was also examined. Lack of p38β only slightly reduced the activation of MAPKAP-K2. Consistent with this, it has been reported that the activation of MAPKAP-K2 is significantly reduced, but not completely abolished in p38α−/− ES cells in response to arsenite stimulation (2). Together these results suggest that in MEF cells p38α is the main p38 isoform responsible for MAPKAP-K2 activation, but that there may be a minor contribution from p38β.
In vitro, MSK1 can be activated by both p38α and p38β but not p38 γ or δ (6, 35). We found that the activation of MSK1 was normal in p38β−/− MEF cells, suggesting that p38β is not responsible for MSK activation in vivo. Consistent with this, the activation of MSK1 by anisomycin has recently been reported to be greatly reduced in MEF cells from p38α−/− mice (5). Several immediate early genes have been suggested to be regulated by p38 in response to cellular stress, including c-fos, c-jun, junB, and nur77. The induction of these genes was not affected by knockout of p38β, although it was blocked by preincubation with SB203580. Consistent with this the induction of these genes is reduced or abolished in p38α−/− cells (5; V. Beardmore, unpublished observations). Together these results suggest that in MEF cells p38α is the main p38 isoform involved in the activation of downstream kinases and IE gene induction.
p38 has been implicated in T-cell development through the use of both specific inhibitors such as SB203580 and the expression of dominant negative or active p38α or MKK6 (7). These results have suggested that p38 activity is required to maintain normal numbers of CD4/CD8 double-negative thymocytes, but that p38 activity inhibits the formation of double-positive cells. Recently however it has been shown that p38α knockout does not result in defects in thymocyte development (23), suggesting that another isoform may be responsible. We show here that p38β knockout does also not result in major problems in thymocyte development. This suggests that either p38 α or β is dispensable for thymocyte development or that in T cells there is compensation by other p38 isoforms in the single knockouts.
Inhibitors of p38, such as SB203580, have been used extensively to study p38 function and have demonstrated a role for p38α or β in the production of pro-inflammatory cytokines, particularly TNF-α and interleukin-6. p38 inhibitors are also shown to have anti-inflammatory effects in animal models of inflammation, and clinical trials have been started for the use of some of these compounds to treat inflammatory disease (44). The role of p38 MAPKs in the immune system is likely to be complex, and p38 potentially plays roles both in the production of TNF and in mediating its downstream effects. p38α controls TNF production at least in part through the action of MAPKAP-K2, which is involved in the regulation of TNF translation via AU-rich regions in the TNF 3′ untranslated region (26, 28, 41, 50).
Consistent with the finding that p38α is the major activator of MAPKAP-K2 activation, the production of TNF was not compromised in p38β−/− mice or in LPS stimulated macrophages derived from these mice. The production of other cytokines including interleukins 1 and 6 and Mip1 was also not affected by the p38β knockout. TNF appears to play a central role in inflammatory disease, as mice with elevated TNF levels develop arthritis and inflammatory bowel disease. Consistent with this anti-TNF antibodies have been able to reduce the symptoms of both arthritis and inflammatory bowel disease (15). TNF can activate p38 in several cell types and has a potential role mediating the inflammatory effects of TNF. p38β however does not appear to be critical for these processes, as p38β knockout was found not to affect the development of arthritis or inflammatory bowel disease in a TnfΔARE mouse model of these diseases. This again suggests that p38α, or other MAPKs such as JNK or ERK, and not p38β are the critical MAPK signaling pathways downstream of TNF in the immune system. These results therefore suggest that maintaining potency against p38β is not necessary, and may even not be desirable, during the development of inhibitors of p38α for inflammatory disease.
In summary, p38β−/− mice have no apparent adverse phenotype and display normal stress-activated signaling in MEF cells as well as normal T-cell development and responses to TNF and LPS in the immune system. This suggests that p38β is not critical for these functions, however, the possibility that other p38 isoforms compensate for p38β in the knockout cannot be ruled out. Analysis of compound knockout or knockin mutations of p38 isoforms will be required to fully answer these questions.
Acknowledgments
We thank the antibody purification teams [Division of Signal Transduction Therapy (DSTT), University of Dundee] coordinated by Hilary McLauchlan and James Hastie for affinity purification of antibodies, the staff of the WBRU for maintenance of mouse colonies, the Sequencing Service (School of Life Sciences, University of Dundee) for DNA sequencing, and Alexia Giannakopoulou and Spiros Lalos (Biomedical Sciences Research Centre Al. Fleming) for technical assistance.
This research was supported by the United Kingdom Medical Research Council, Astra-Zeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck and Co., Merck KGaA, and Pfizer.
REFERENCES
- 1.Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. [PubMed] [Google Scholar]
- 2.Allen, M., L. Svensson, M. Roach, J. Hambor, J. McNeish, and C. A. Gabel. 2000. Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 191:859-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brancho, D., N. Tanaka, A. Jaeschke, J. J. Ventura, N. Kelkar, Y. Tanaka, M. Kyuuma, T. Takeshita, R. A. Flavell, and R. J. Davis. 2003. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 17:1969-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung, P. C., D. G. Campbell, A. R. Nebreda, and P. Cohen. 2003. Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J. 22:5793-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darragh, J., A. Soloaga, V. A. Beardmore, A. Wingate, G. R. Wiggin, M. Peggie, and J. S. Arthur. 2005. MSKs are required for the transcription of the nuclear orphan receptors Nur77, nurr1 and nor1 downstream of MAP kinase signalling. Biochem. J. 390:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deak, M., A. D. Clifton, L. M. Lucocq, and D. R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17:4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diehl, N. L., H. Enslen, K. A. Fortner, C. Merritt, N. Stetson, C. Charland, R. A. Flavell, R. J. Davis, and M. Rinc. 2000. Activation of the p38 mitogen-activated protein kinase pathway arrests cell cycle progression and differentiation of immature thymocytes in vivo. J. Exp. Med. 191:321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, C., R. J. Davis, and R. A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55-72. [DOI] [PubMed] [Google Scholar]
- 9.Enslen, H., D. M. Brancho, and R. J. Davis. 2000. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 19:1301-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enslen, H., J. Raingeaud, and R. J. Davis. 1998. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 273:1741-1748. [DOI] [PubMed] [Google Scholar]
- 11.Eyers, P. A., M. Craxton, N. Morrice, P. Cohen, and M. Goedert. 1998. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol. 5:321-328. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald, C. E., S. B. Patel, J. W. Becker, P. M. Cameron, D. Zaller, V. B. Pikounis, S. J. O'Keefe, and G. Scapin. 2003. Structural basis for p38alpha MAP kinase quinazolinone and pyridol-pyrimidine inhibitor specificity. Nat. Struct. Biol. 10:764-769. [DOI] [PubMed] [Google Scholar]
- 13.Gallo, K. A., and G. L. Johnson. 2002. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell. Biol. 3:663-672. [DOI] [PubMed] [Google Scholar]
- 14.Godl, K., J. Wissing, A. Kurtenbach, P. Habenberger, S. Blencke, H. Gutbrod, K. Salassidis, M. Stein-Gerlach, A. Missio, M. Cotten, and H. Daub. 2003. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc. Natl. Acad. Sci. USA 100:15434-15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldblatt, F., and D. A. Isenberg. 2005. New therapies for rheumatoid arthritis. Clin. Exp. Immunol. 140:195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gum, R. J., M. M. McLaughlin, S. Kumar, Z. Wang, M. J. Bower, J. C. Lee, J. L. Adams, G. P. Livi, E. J. Goldsmith, and P. R. Young. 1998. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J. Biol. Chem. 273:15605-15610. [DOI] [PubMed] [Google Scholar]
- 17.Hall-Jackson, C. A., M. Goedert, P. Hedge, and P. Cohen. 1999. Effect of SB 203580 on the activity of c-Raf in vitro and in vivo. Oncogene 18:2047-2054. [DOI] [PubMed] [Google Scholar]
- 18.Han, J., X. Wang, Y. Jiang, R. J. Ulevitch, and S. Lin. 1997. Identification and characterization of a predominant isoform of human MKK3. FEBS Lett. 403:19-22. [DOI] [PubMed] [Google Scholar]
- 19.Hazzalin, C. A., and L. C. Mahadevan. 2002. MAPK-regulated transcription: a continuously variable gene switch? Nat. Rev. Mol. Cell. Biol. 3:30-40. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison, M., K. S. Berman, and M. H. Cobb. 1998. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem. 273:28625-28632. [DOI] [PubMed] [Google Scholar]
- 21.Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90-94. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. M., J. M. White, A. S. Shaw, and B. P. Sleckman. 2005. MAPK p38 alpha is dispensable for lymphocyte development and proliferation. J. Immunol. 174:1239-1244. [DOI] [PubMed] [Google Scholar]
- 24.Kollias, G. 2004. Modeling the function of tumor necrosis factor in immune pathophysiology. Autoimmun. Rev. 3(Suppl. 1):S24-25. [PubMed] [Google Scholar]
- 25.Kollias, G., E. Douni, G. Kassiotis, and D. Kontoyiannis. 1999. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol. Rev. 169:175-194. [DOI] [PubMed] [Google Scholar]
- 26.Kontoyiannis, D., A. Kotlyarov, E. Carballo, L. Alexopoulou, P. J. Blackshear, M. Gaestel, R. Davis, R. Flavell, and G. Kollias. 2001. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 20:3760-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontoyiannis, D., M. Pasparakis, T. T. Pizarro, F. Cominelli, and G. Kollias. 1999. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10:387-398. [DOI] [PubMed] [Google Scholar]
- 28.Kotlyarov, A., A. Neininger, C. Schubert, R. Eckert, C. Birchmeier, H. D. Volk, and M. Gaestel. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1:94-97. [DOI] [PubMed] [Google Scholar]
- 29.Kumar, S., J. Boehm, and J. C. Lee. 2003. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2:717-726. [DOI] [PubMed] [Google Scholar]
- 30.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 31.Lee, J. C., S. Kumar, D. E. Griswold, D. C. Underwood, B. J. Votta, and J. L. Adams. 2000. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology. 47:185-201. [DOI] [PubMed] [Google Scholar]
- 32.Lee, J. C., and P. R. Young. 1996. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J. Leukoc. Biol. 59:152-157. [DOI] [PubMed] [Google Scholar]
- 33.Lu, H. T., D. D. Yang, M. Wysk, E. Gatti, I. Mellman, R. J. Davis, and R. A. Flavell. 1999. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18:1845-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 298:1912-1934. [DOI] [PubMed] [Google Scholar]
- 35.McCoy, C. E., D. G. Campbell, M. Deak, G. B. Bloomberg, and J. S. Arthur. 2005. MSK1 activity is controlled by multiple phosphorylation sites. Biochem. J. 387:507-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McManus, E. J., B. J. Collins, P. R. Ashby, A. R. Prescott, V. Murray-Tait, L. J. Armit, J. S. Arthur, and D. R. Alessi. 2004. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 23:2071-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriguchi, T., N. Kuroyanagi, K. Yamaguchi, Y. Gotoh, K. Irie, T. Kano, K. Shirakabe, Y. Muro, H. Shibuya, K. Matsumoto, E. Nishida, and M. Hagiwara. 1996. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J. Biol. Chem. 271:13675-13679. [DOI] [PubMed] [Google Scholar]
- 38.Moriguchi, T., F. Toyoshima, Y. Gotoh, A. Iwamatsu, K. Irie, E. Mori, N. Kuroyanagi, M. Hagiwara, K. Matsumoto, and E. Nishida. 1996. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-alpha, and H2O2. J. Biol. Chem. 271:26981-26988. [DOI] [PubMed] [Google Scholar]
- 39.Mudgett, J. S., J. Ding, L. Guh-Siesel, N. A. Chartrain, L. Yang, S. Gopal, and M. M. Shen. 2000. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. USA 97:10454-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 41.Neininger, A., D. Kontoyiannis, A. Kotlyarov, R. Winzen, R. Eckert, H. D. Volk, H. Holtmann, G. Kollias, and M. Gaestel. 2002. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J. Biol. Chem. 277:3065-3068. [DOI] [PubMed] [Google Scholar]
- 42.Okkenhaug, K., and B. Vanhaesebroeck. 2003. PI3K-signalling in B- and T cells: insights from gene-targeted mice. Biochem. Soc. Trans. 31:270-274. [DOI] [PubMed] [Google Scholar]
- 43.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 44.Pargellis, C., and J. Regan. 2003. Inhibitors of p38 mitogen-activated protein kinase for the treatment of rheumatoid arthritis. Curr. Opin. Investig. Drugs 4:566-571. [PubMed] [Google Scholar]
- 45.Rincon, M. 2001. MAP-kinase signaling pathways in T cells. Curr. Opin. Immunol. 13:339-345. [DOI] [PubMed] [Google Scholar]
- 46.Sabio, G., J. S. Arthur, Y. Kuma, M. Peggie, J. Carr, V. Murray-Tait, F. Centeno, M. Goedert, N. A. Morrice, and A. Cuenda. 2005. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24:1143-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saklatvala, J. 2004. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 4:372-377. [DOI] [PubMed] [Google Scholar]
- 48.Sen, J., R. Kapeller, R. Fragoso, R. Sen, L. I. Zon, and S. J. Burakoff. 1996. Intrathymic signals in thymocytes are mediated by p38 mitogen-activated protein kinase. J. Immunol. 156:4535-4538. [PubMed] [Google Scholar]
- 49.Shi, Y., and M. Gaestel. 2002. In the cellular garden of forking paths: how p38 MAPKs signal for downstream assistance. Biol. Chem. 383:1519-1536. [DOI] [PubMed] [Google Scholar]
- 50.Stoecklin, G., P. Stoeckle, M. Lu, O. Muehlemann, and C. Moroni. 2001. Cellular mutants define a common mRNA degradation pathway targeting cytokine AU-rich elements. RNA. 7:1578-1588. [PMC free article] [PubMed] [Google Scholar]
- 51.Tamura, K., T. Sudo, U. Senftleben, A. M. Dadak, R. Johnson, and M. Karin. 2000. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102:221-231. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka, N., M. Kamanaka, H. Enslen, C. Dong, M. Wysk, R. J. Davis, and R. A. Flavell. 2002. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 3:785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, L., R. Ma, R. A. Flavell, and M. E. Choi. 2002. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J. Biol. Chem. 277:47257-47262. [DOI] [PubMed] [Google Scholar]
- 54.Wiggin, G. R., A. Soloaga, J. M. Foster, V. Murray-Tait, P. Cohen, and J. S. Arthur. 2002. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol. Cell. Biol. 22:2871-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wurst, W. J., A.L. 1993. Production of targeted embryonic stem cell clones, p. 101-131. In Gene targeting. ILR Press, Oxford, England.
- 56.Wysk, M., D. D. Yang, H. T. Lu, R. A. Flavell, and R. J. Davis. 1999. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc. Natl. Acad. Sci. USA 96:3763-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi, K., K. Shirakabe, H. Shibuya, K. Irie, I. Oishi, N. Ueno, T. Taniguchi, E. Nishida, and K. Matsumoto. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 270:2008-2011. [DOI] [PubMed] [Google Scholar]
- 58.Zarubin, T., and J. Han. 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15:11-18. [DOI] [PubMed] [Google Scholar]