Abstract
Akt/protein kinase B (PKB) plays a critical role in the regulation of metabolism, transcription, cell migration, cell cycle progression, and cell survival. The existence of viable knockout mice for each of the three isoforms suggests functional redundancy. We generated mice with combined mutant alleles of Akt1 and Akt3 to study their effects on mouse development. Here we show that Akt1−/− Akt3+/− mice display multiple defects in the thymus, heart, and skin and die within several days after birth, while Akt1+/− Akt3−/− mice survive normally. Double knockout (Akt1−/− Akt3−/−) causes embryonic lethality at around embryonic days 11 and 12, with more severe developmental defects in the cardiovascular and nervous systems. Increased apoptosis was found in the developing brain of double mutant embryos. These data indicate that the Akt1 gene is more essential than Akt3 for embryonic development and survival but that both are required for embryo development. Our results indicate isoform-specific and dosage-dependent effects of Akt on animal survival and development.
In mammals, Akt1, Akt2, and Akt3 (also called protein kinase Bα [PKBα], PKBβ, and PKBγ) proteins have similar domain structures and can be activated by numerous growth factors in a phosphatidylinositol 3-kinase-dependent manner (1, 3, 4, 15, 23). Once activated, Akt phosphorylates and controls the activities of a diverse group of substrates involved in many cellular and physiological processes, such as cell survival, cell cycle progression, cell growth, metabolism, and angiogenesis (3, 10, 17, 23).
Although many proteins have been identified as Akt substrates, the challenge that remains is to show that they actually have an important impact on physiological processes in organisms. Recently, targeted deletion of specific isoforms of Akt in mice has proved to be a powerful tool for elucidating the physiological roles of Akt proteins (5, 7, 8, 13, 16, 25, 27, 28). Characterization of such knockout mice has yielded intriguing and surprising results. We and others found that ∼40% ofAkt1 knockout mice die at a neonatal stage with growth retardation, but the other ∼60% of Akt1 knockout mice survive apparently normally. This suggests that the other two remaining Akt isoforms can, in part, compensate for the loss of one Akt. Knockout of each single isoform gives rise to a distinct phenotype. In general, Akt1 null mice are growth retarded, which may result from placental insufficiency, while Akt2-deficient mice suffer from a type 2 diabetes-like syndrome and Akt3 null mice show impaired brain development (5, 7, 8, 13, 16, 25, 27, 28). These observations indicate that the three Akt isoforms have different nonredundant physiological functions. The relatively normal development and distinct physiological functions exhibited by single knockouts may be explained by differences in the tissue distribution and expression levels of these isoforms. We found that Akt1 is the major isoform in placenta and that placenta lacking this protein cannot form a proper vascular labyrinth; this may restrict nutrient supply to the fetus and impair growth (28). Similarly, in the major insulin-responsive tissues of fat, skeletal muscle, and liver, Akt2 is the predominant isoform (2, 7, 28). In the case of Akt3, which shows a more restricted level of expression with the brain containing the highest levels and has lower levels of expression in other tissues, ablation of this isoform affects postnatal brain development (13, 25).
We predicted that simultaneous inactivation of two Akt isoforms in mice would severely affect development and survival. To test this hypothesis, we crossed Akt1 with Akt3 knockout mice to generate compound (combined mutation of four alleles of Akt1 and Akt3) and double knockout (DKO) mice. Our results show that the Akt1 gene is more essential than the Akt3 gene for survival and that both proteins are required for normal embryo development. Our studies demonstrate isoform-specific and dosage-dependent effects of Akt genes on animal survival and development.
MATERIALS AND METHODS
Preparation of murine embryonic fibroblasts (MEFs) and Western analysis.
MEFs were prepared from embryonic day 10.5 (E10.5) to E13.5 embryos as previously described (14). Western analysis and the antibody that recognizes both mouse Akt1 and Akt3 have been described previously (25, 28). The isoform-specific antibodies for mouse Akt1, Akt2, and Akt3 have been described already (25). Antibodies for pSer473 were purchased from Cell Signaling Technology. Actin antibody was purchased from Neomarkers.
Mice.
Mice were housed in accordance with the Swiss Animal Protection Ordinance in groups with 12-h dark-light cycles and with free access to food and water. All procedures were conducted with relevant authority approval. Akt1−/− and Akt3−/− mice have been described previously (25, 28). All mice had a 129 Ola and C57BL/6 mixed background.
BrdU incorporation.
E10.5 to E11.5 pregnant mice were injected intraperitoneally with bromodeoxyuridine (BrdU) (1 mg per 20 g of body weight). The mice were sacrificed 2 h later, and embryos together with placenta were dissected (25, 28).
Hematoxylin-eosin (HE) and immunohistochemisty (IHC).
The HE and IHC protocols were as described previously (25, 28). For IHC, sections were incubated at 4°C overnight with antibodies for Akt1 and Akt3, BrdU (AbCam Ltd.), platelet endothelial cell adhesion molecule (PECAM) (Pharmingen), skin markers of keratin 10 (K10) and K14, and involucrin (BABCO). The sections were then processed as described in the protocol of the Vectastain ABC kit (Vector Laboratories).
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL staining).
Paraffin-embedded sections were treated with 20 μg/ml proteinase K for 10 min at 37°C (omitted for cryosections). Endogenous peroxidase was inactivated by treatment with 3% H2O2 in 100% methanol for 30 min at room temperature. The sections were then equilibrated with 1× terminal deoxynucleotidyl transferase (TdT) buffer for 15 min at room temperature and incubated with TdT and biotinylated dUTP (TdT reaction) for 1 h at 37°C. The reaction was stopped by washing with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). A Vectastain ABC kit (Vector Laboratories) was used as described by the manufacturer for color development.
Quantification and statistics.
Quantification of skin thickness and hair follicles was performed with three double-heterozygous (DH) (Akt1+/− Akt3+/−) and three Akt1−/− Akt3+/− mice as described previously (11). For the TUNEL assay of heart, five fields of each section were counted for apoptotic cells. The t test was used for statistical analysis. Similar scoring was performed for placental vasculature.
RESULTS
Tissue distribution of Akt isoforms in neonates and mid-gestation embryos.
We first investigated the expression patterns of the three isoforms in neonates and surprisingly show that the expression of the Akt3 isoform is more widely expressed than it is in adults (Fig. 1A). Similar to adult mice, Akt3 is highest in the brain and Akt2 is highest in fat as reported previously (7, 13, 25, 28). However, Akt1 and Akt3 are abundant in kidney and skeletal muscle, whereas they are nearly absent in adult mice (Fig. 1A). The ubiquitous expression pattern of the three Akt proteins in neonates indicates that all three isoforms are apparently required for early development.
FIG. 1.
Expression Akt isoforms in neonates, Akt1/Akt3 localization in E11.5 to E12.5 embryos, generation of Akt1/Akt3 compound, and double knockout mice. (A) Expression patterns of Akt isoforms in neonates. Four wild-type mice from two litters were sacrificed, and their tissues were pooled for lysate preparation. Forty micrograms of total protein was used for Western analysis with the indicated isoform-specific antibodies. (B) Akt1/Akt3 antibody specificity. Mouse Akt1, Akt2, and Akt3 cDNAs cloned in pCMV5 were expressed in HEK293 cells, and cell lysates were prepared for Western analysis. Immunoblot analysis was carried out with an affinity-purified Akt1/Akt3 antibody that recognizes Akt1 and Akt3. Actin levels were determined to control for equal loading. (C) Absence of Akt1 and Akt3 proteins in DKO mouse embryonic fibroblasts (MEFs). MEFs were prepared from wild-type, Akt1/Akt3 DKO, or Akt2/Akt3 DKO embryos, and cell lysates derived from these cells were used for Western analysis using the antibody as described in the legend to panel B with actin as control. (D) PCR genotyping for Akt1 and Akt3 loci. WT, wild type; DKO, double knockout. (E) Absence of Akt1 and Akt3 proteins in DKO mouse tissue (placenta). Total phospho-Akt is substantially decreased. (F to I) HE and immunohistochemical staining of E11.5 and E12.5 embryos with the Akt1/Akt3 antibody. Panels F and G are for the E11.5 embryo and H and I are for the E12.5 embryo; F and H show hematoxylin and eosin staining, and G and I show immunohistochemical staining. Akt1 or Akt3 (or both) is expressed in both the central and peripheral nervous systems of brain, neural tube, and heart. Arrowheads indicate various ganglia. Abbreviations: HB, hindbrain; MB, midbrain; FB, forebrain; BA, branchial arch; H, heart; NT, neural tube; CG, cranial ganglia; DRG, dorsal root ganglia; Li, liver; T, tongue.
These isoform-specific antibodies could not be used for immunohistochemistry (IHC) because of nonspecific background staining. Therefore, we used an antibody that specifically recognized both Akt1 and Akt3 (labeled Akt1/Akt3 antibody in Fig. 1B and C) that was originally developed against the carboxyl-terminal peptide sequence of porcine Akt1 (18). This affinity-purified antibody recognized mouse Akt1 and Akt3 but not Akt2 (Fig. 1B) and could be used for IHC.
At E11.5, the level of Akt1 or Akt3 protein (or both) is high in the brain, neural tube, and heart (Fig. 1F and G). At E12.5, the signals are predominantly localized in the developing brain and various ganglia, and the levels (indicated by the staining signal) increased compared to that at E11.5 (Fig. 1H and I). The tissue distribution patterns indicate that the two proteins are important for brain and cardiovascular development at E11.5 to E12.5.
Generation of Akt1 and Akt3 double mutant mice.
Akt1, Akt2, and Akt3 genes have been successfully targeted in our laboratory by homologous recombination (25, 28; B. A. Dümmler, unpublished data). In the present work, we first mated Akt1−/− males with Akt3−/− females to generate Akt1+/− Akt3+/− (DH) mice. Intercrossing of these DH mice gave rise to offspring with nine genotypes (Table 1). Genotyping of these mice was carried out by independent PCR for the Akt1 and Akt3 loci as previously described (25, 28) (Fig. 1D). Western blot analysis confirmed the absence of the two proteins in the MEFs and tissues from Akt1/Akt3 DKO mice (Fig. C and 1E).
TABLE 1.
Progeny of Akt1+/− Akt3+/− matingsa
| Genotype | 1+/+3+/+ | 1+/+3+/− | 1+/+3−/− | 1+/−3+/+ | 1+/−3+/− | 1+/−3−/− | 1−/−3+/+ | 1−/−3+/− | 1−/−3−/− |
|---|---|---|---|---|---|---|---|---|---|
| No. of animals | 27 | 34 | 20 | 31 | 74 | 37 | 11 | 3 | 0 |
| % | 11.3 | 14.3 | 8.4 | 13 | 31 | 15.5 | 4.6 | 1.3 | 0 |
| Theoretical % | 6.3 | 12.5 | 6.3 | 12.5 | 25 | 12.5 | 6.3 | 12.5 | 6.3 |
Total number analyzed, 237.
Akt1−/− Akt3+/− mice die at an early age and Akt1/Akt3 double knockout mice are embryonic lethal.
Among the nine genotypes, only Akt1−/− Akt3+/− and DKO mice had survival problems, whereas all others were produced in numbers consistent with the expected Mendelian ratio (Table 1). More than 90% of Akt1−/− Akt3+/− mice were lost by the time of genotyping, and we did not observe any DKO mice at the time of genotyping (Table 1). We next determined at what stage Akt1−/− Akt3+/− mice and DKO mice die. For Akt1−/− Akt3+/− mice, we set up matings between Akt1+/− Akt3−/− males and Akt1−/− females. Theoretically, offspring of this mating are half Akt1−/− Akt3+/− and half DH (1:1). Of the 20 newborns collected from four litters, 8 were DH and 12 were Akt1−/− Akt3+/−, which is consistent with the expected Mendelian ratio. Therefore, Akt1−/− Akt3+/− mice appear to develop to term. In a follow-up study, we found that the majority of Akt1−/− Akt3+/− mice died several days after birth.
We next determined the stage of DKO embryonic lethality. No double knockout embryos were found at E14.5. At E12.5, some double knockout embryos had already decayed, leaving only the placenta and body remnants (progeny of Akt1+/− Akt3−/− intercross mice were the following: for genotype Akt1+/+ Akt3−/−, 15 mice; for genotype Akt1+/− Akt3−/−, 12 mice; for genotype Akt1−/− Akt3−/−, 13 mice [7 of the Akt1−/− Akt3−/− mice were dead and partially decayed). DKO embryos dissected at E11.5 displayed severely retarded development (data not shown). When E10.5 embryos were dissected and genotyped, double knockout embryos were found with morphology comparable to that of Akt1+/− Akt3−/− littermates. These data indicate that double knockout mutants die around E11 to E12.
Thus, Akt3 appears to be redundant for the survival of Akt1 mutant mice. In contrast, Akt1 mutant mice (Akt1−/−) showed a 40% loss after birth, with the mortality of Akt1−/− Akt3+/− mice increased to more than 90%.
Multiple defects in the thymus, heart, and skin of Akt1−/− Akt3+/− mice.
Nearly all Akt1−/− Akt3+/− mice died within a few days after birth. To determine possible causes of death, we first performed systemic anatomic organ analysis of postnatal day 3 (P3) viable Akt1−/− Akt3+/− mice and their DH littermates (the latter mice survive normally and were, therefore, used as the control). Generally, most organs from Akt1−/− Akt3+/− mice analyzed were proportionally smaller than those of DH controls.
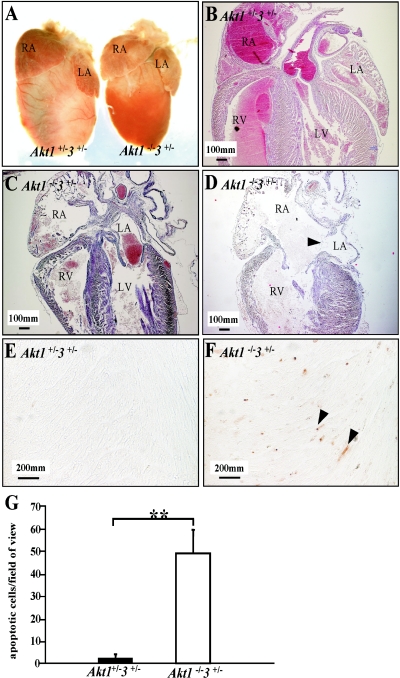
Strikingly, Akt1−/− Akt3+/− mice also displayed nonproportional hypotrophy of the thymus, heart, and skin. We have studied the six thymi from two litters of P3 mice (three Akt1−/− Akt3+/− and three DH). The thymus of Akt1−/− Akt3+/− mice is hypotrophic and much smaller than the control (Fig. 2A to C). There are many Hassall's corpuscle-like structures (arrowheads in Fig. 2E) formed in the thymus of Akt1−/− Akt3+/− mice, but they were not seen in the DH control (Fig. 2D).
FIG. 2.
Defects in the thymus from Akt1−/− Akt3+/− mice. (A) Thymi from P3 Akt1+/− Akt3+/− and Akt1−/− Akt3+/− littermates. (B to E) HE staining of the thymus from panel A. Panels B and C are lower-magnification images of panels D and E. Arrowheads in panel E indicate the Hassall's corpuscle-like structures in the Akt1−/− Akt3+/− mice which are not found in wild-type mouse thymus. Cap, capillary blood vessel. Magnification for panels B and C, ×40; for panels D and E, ×400.
To analyze heart development, we used eight Akt1−/− Akt3+/− and three Akt1−/− mice (similar numbers for control) from newborn to P8. The hearts of Akt1−/− Akt3+/− mice were smaller than those of controls, the difference being mainly in the ventricles (Fig. 3A). Further histological study of these P3 hearts and other P3 hearts from wild-type mice revealed that Akt1−/− Akt3+/− hearts had dilated (enlarged) atria and ventricles, especially on the right side (Fig. 3C and D). In addition, we found a range of defects in Akt1−/− Akt3+/− hearts, including atria septum defect, muscular ventricle septum defect, ventricle septum vascular anomaly, and thickened valves (data not shown). Small ventricle size and insufficient contractility of cardiomyocytes of Akt1−/− Akt3+/− hearts may lead to the retention of blood in the right side of the heart and cause the atria septum to open. One Akt1−/− Akt3+/− mouse that died after 8 days exhibited a significantly enlarged right atrium and ventricle (data not shown). Trichrome staining revealed a large area of necrosis (data not shown). TUNEL staining of the heart identified a large number of apoptotic cells outside of the necrotic area (Fig. 3F and G). Histological structure of the lung of Akt1−/− Akt3+/− mice was similar to that of controls (data not shown). The data presented are representative of the phenotype observed for these mice.
FIG.3.
Structural defects in the heart from Akt1−/− Akt3+/− mice. (A) The hearts of Akt1−/− Akt3+/− mice are smaller than those of the controls, but the atria are as big as those of the controls. (B to D) Sagittal sections of the hearts. (B to D) HE staining of hearts from panel A. (C and D) Enlargement of the right side of Akt1−/− Akt3+/− hearts and apparent atria septum defects in Akt1−/− Akt3+/− hearts (P3). The wall of the right atrium of the Akt1−/− Akt3+/− heart is thin due to enlargement (C and D). The arrowhead in panel D indicates atria septum defects. (E and F) TUNEL assay. Arrowheads indicate apoptotic cells. (G) Quantification of apoptosis. Error bars indicate standard deviation. **, P < 0.01. RA, right atrium; RV, right ventricle; LA, left atrium. Magnification for panels B to D, ×40; for panels E and F, ×400.
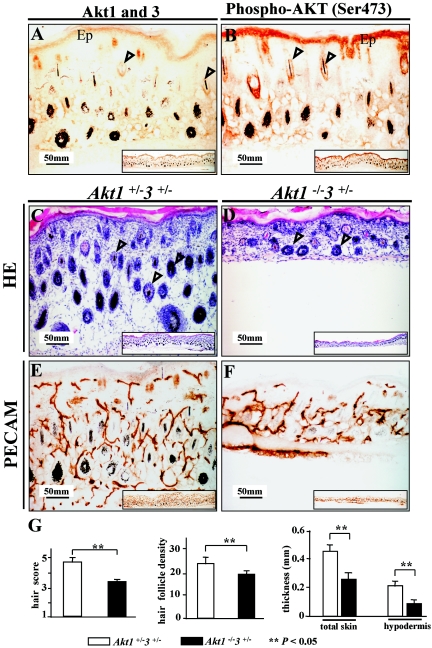
Previously, we observed impaired hair follicle development in Akt1−/− mice by HE staining (11). We performed immunohistochemical and HE analyses to evaluate the structure and development of the skin of Akt1−/− Akt3+/− mice. We have analyzed 11 Akt1−/− Akt3+/− mice (and a similar number for control) from E16.5 to P8 for skin development. The thin-skin phenotype observed was found in nearly all the mice examined.
Immunohistochemical staining showed Akt1 and Akt3 to be localized mainly in the keratinocytes of epidermis and follicles at P2 (Fig. 4A), which coincided with phospho-Ser473 staining (Fig. 4B). HE staining revealed a reduction in the thickness of Akt1−/− Akt3+/− skin at P2 and P8 (Fig. 4C). Thickness of the hypodermis and total skin, total number of hair follicles (or hair follicle density), and hair morphogenesis of skin samples from three Akt1−/− Akt3+/− mice and three controls (Aktα+/− Akt3+/−) at P3 were all significantly different (Fig. 4G). Cell differentiation is normal in Akt1−/− Akt3+/− skin based on keratin 14 (K14), keratin 10 (K10), and involucrin staining (data not shown). Although all skin layers were present in Akt1−/− Akt3+/− mice, individual skin layers were much thinner than in the wild type. Thus, the skin as a whole was much thinner. Hair follicles were distinctly reduced in size in Akt1−/− Akt3+/− animals, and there were fewer (black) hair fibers (data not shown). The impaired skin development of Akt1−/− Akt3+/− mice may be attributable to decreased vasculature revealed by PECAM (CD31) staining (24) (Fig. 4E and F). This provides further evidence for Akt1- and Akt3-dependent regulation of angiogenesis and vascular formation.
FIG.4.
Histology of Akt1−/− Akt3+/− skin. (A and B) Immunohistochemical staining of Akt1/Akt3 and phospho-Akt (Ser473) in control skin. (A) Akt1/Akt3 localization. The two proteins are mainly localized in the keratinocytes of the epidermis (Ep) and hair follicles (arrowheads). (B) Phospho-Akt (Ser473) in control skin. Localization of phospho-Akt is similar to that of Akt1/Akt3. (C and D) HE staining. Skin of Akt1−/− Akt3+/− mice is much thinner than that of control littermates and has fewer hair follicles (arrowheads). (E and F) PECAM (CD31) staining to display the vasculature. Akt1−/− Akt3+/− skin has fewer vessels than the control. Insets in all panels are lower magnification images showing a larger part of the skin. (G) Quantification of skin thickness and hair follicles. HE staining sections from three Akt1−/− Akt3+/− mice and three Akt1+/− Akt3+/− littermates (P3) were measured for thickness and hair follicle density. Error bars are standard deviation, and two asterisks indicate a significant difference between the two genotypes (P < 0.05). Magnification, ×200.
Abnormal development of DKO embryos.
At E11.5, some DKO embryos displayed apparent vascular defects and hemorrhages. E10.5 DKO embryos displayed morphology comparable to that of the control. Therefore, we studied embryonic development of E10.5 DKO embryos to understand the ensuing embryo lethality. We analyzed four to five mutant embryos (similar for control) at E10.5 and E12.5. The data presented are representative of the phenotype observed.
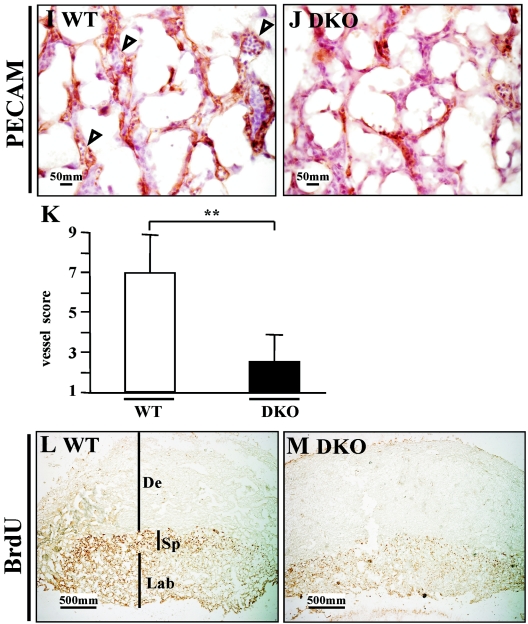
Vascular structure was studied using PECAM staining (24). We found a significant decrease in vasculature of DKO embryos compared with controls (Fig. 5A to H). Fine (capillary) vessels were abundant in the head (neuroepithelium) and body trunk of the control but were not apparent in DKO embryos (Fig. 5A to D). Furthermore, DKO embryos displayed hypoplastic branchial arches (Fig. 5G), fused or dilated arch arteries, and the absence of fine vessels (Fig. 5H).
FIG.5.
Analysis of the vascular system of DKO embryos. (A to H) Left panels are HE staining to show the morphology, and the right panels are PECAM staining to display vasculature. (A to D) Fine vessels (capillary) in the neuroepithelium of hindbrain. Arrowheads indicate the small vessels (capillary). Small vessels are abundant in the control and are evenly distributed, while these vessels are scarce in DKO embryos. (E to H) Branchial arches and their arteries. In the control, first, second, and third arch arteries can be seen (arrowheads), and small vessels are rich in mesenchymal tissue (F). However, the branchial arches of DKO mice are hypoplastic, with little mesenchymal tissue, and the large arch arteries cannot be distinguished (arrowhead). Small vessels are also scarce in DKO branchial arches. Abbreviations: NE, neuroepithelium; BA, branchial arch; WT, wild type. Magnification, ×100. (I to M) Histological analysis of DKO placenta. (I and J) E12.5 placentas. PECAM staining displays fetal vessels in the labyrinth. In the wild type (I), fetal vessels are well formed and filled with fetal nucleated erythroid cells (arrowheads), while in DKO mice (J) these vessels are rare, although the epithelial cells can be seen. (K) Quantification of fetal vessels in wild-type and DKO placenta. The double asterisks indicates a significant difference (P < 0.05). (L and M) E10.5 placentas. BrdU staining. DKO placenta shows a substantial reduction of proliferation, especially in the spongiotrophoblast layer (Sp). De, decidua; Lab, labyrinth. Magnification, ×40.
Previously, we observed reduced vasculature in Akt1−/− placenta (28), and a more significant vascular defect was found in DKO placenta (Fig. 5J and K). In addition, reduced cell proliferation was detected in DKO placenta (Fig. 5M).
The predominant expression of Akt1 or Akt3 (or both) in the nervous system at E11.5 to E12.5 suggests an important role of these two proteins in the maintenance of brain development. However, there was no significant difference between DKO and wild-type control mice with cell proliferation displayed by BrdU staining (data not shown).
An increase of apoptotic cells was detected using the TUNEL assay in the neuroepithelium and in the neurons of various ganglia in DKO embryos compared to the wild type (Fig. 6A to H). These results indicate that Akt1 and Akt3 contribute to the development of the nervous system by maintenance of cell survival.
FIG. 6.
Apoptosis in wild-type (WT) and DKO embryos. The left panels are HE staining, and the right panels are TUNEL staining. In the wild type, very few apoptotic cells can be seen in the neuroepithelium, but there are many in DKO neuroepithelium (arrowheads indicate apoptotic cells). (A to D) E10.5; (E to H) E9.5. Magnification for panels A and B, ×40; for panels C to F, ×200.
DISCUSSION
With neonatal mice, we found that the three Akt proteins are widely expressed in contrast to results obtained in the adult mice, where the Akt3 protein expression is quite restricted. Our findings provide important insights and help better understand the developmental defects in Akt1/Akt3 compound mutant mice and Akt1/Akt2 Akt1/Akt3 DKO mice. In the single knockouts of Akt isoforms, Akt2 and Akt3 null mice have no neonatal survival disadvantages (7, 16, 25, 27). Nevertheless, we and others have observed that 40% of Akt1 null mice fail to survive past the neonatal period (8). This raises the intriguing possibility that the Akt1 gene is the most critical isoform of the three for early mouse development. Nearly all Akt1−/− Akt3+/− mice died at an early age, while Akt1 +/− Akt3−/− mice survived normally. This suggests that the Akt1 gene is more essential than Akt3 for mouse survival. In a recent report, Akt1/Akt2 double knockout mice developed to term but died shortly after birth with severe developmental defects, including dwarfism, skeletal deformity, and skin abnormalities (22). We found that Akt1−/− Akt3+/− mice have severe growth deficiencies, and nearly all died young with multiple organ and tissue pathologies. Mice lacking both Akt1 and Akt3 genes died at approximately E11 to E12 and displayed multiple fatal developmental defects. Akt1/Akt2 double knockout mice with only Akt3 developed to term (22), but Akt1/Akt3 DKO mice (i.e., only Akt2 was expressed) did not develop beyond the midterm (E12). This indicates a more critical role for Akt3 than Akt2 in early animal development and survival. Thus, the order of apparent importance of the three Akt genes in mouse development and survival is Akt1 > Akt3 > Akt2. The phenotypes observed with Akt1/Akt3 DKO and Akt1/Akt2 DKO mice are largely due to loss of Akt1 function. Taken together, these results indicate that the three Akt genes have isoform-specific and dosage-dependent effects on mouse survival and development.
In this study, we found that Akt1 and Akt3 have essential roles in cardiovascular development and function. The probable cause of sudden death of Akt1−/− Akt3+/− mice is heart failure, possibly as a result of defective heart development and cardiomyocyte function (6). Akt1/Akt3 DKO mice embryonic lethality could also be attributed to cardiovascular anomalies. These embryos died at around E12, with significant vascular reduction in embryo and placenta; large vessels in the branchial arches and dorsal aorta were also abnormal. As these large branchial arch and dorsal aorta arteries are converted to aortic artery and other large arteries, the developmental defects of these vessels at E12 inhibits further development of mice and enhances morbidity (9, 20, 21).
Our studies of Akt1/Akt3 DKO mice also indicate that Akt1 and Akt3 are critical for nervous system development. Previously, we found that Akt3 protein levels are the highest in the brain, and deletion of this isoform substantially impaired brain development (13, 25). In Akt1/Akt3 DKO embryos, many apoptotic cells were found in the neuroepithelium and neurons of ganglia. Therefore, Akt1 and Akt3 are indispensable for nervous system development in the embryo through their maintenance of cell survival (12, 29, 30).
Another interesting finding is the Hassall's corpuscle-like structures in, Akt1−/− Akt3+/− thymus, as this structure is well developed in the thymus of humans and guinea pigs but poorly developed in the thymus of mice and rats (26). In humans, the Hassall's corpuscles have been proposed to remove apoptotic thymocytes (26). Previously, Hay's group found increased apoptotic thymocytes in Akt1 null mice (5). The formation and function of these structures in Akt1−/− Akt3+/− thymus need tobe studied further.
In conclusion, our results indicate that Akt1 and Akt3 genes apparently regulate multiple developmental processes in mice. Akt1 and Akt3 genes share some redundant functions required to maintain mouse development and survival. However, these two proteins seem to play distinct roles based on the phenotypes of the individual knockouts. To rigorously understand the causes of the different phenotypes, it will be necessary to identify the specific Akt substrates involved in the development of the different organs.
Acknowledgments
We thank D. Hynx (FMI) for management of the mouse colonies and J. Günthard and T. Dieterle at the Children's Hospital and Canton Hospital, Basel, Switzerland, for consultation on Akt1−/− Akt3+/− heart phenotype. We are grateful to B. Sücürü and E. Fayard for antibody preparation. The Friedrich Miescher Institute for Biomedical Research is a part of the Novartis Research Foundation.
B.D. is supported by the Swiss Cancer League (KFS 1002-02-2000).
REFERENCES
- 1.Alessi, D., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Altomare, D. A., G. E. Lyons, Y. Mitsuuchi, J. Q. Cheng, and J. R. Testa. 1998. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene 16:2407-2411. [DOI] [PubMed] [Google Scholar]
- 3.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 4.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W. S., P.-Z. Xu, K. Gottlob, M.-L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien, K. R., and E. N. Olson. 2002. Converging pathways and principles in heart development and disease: CV@CSH. Cell 110:153-162. [DOI] [PubMed] [Google Scholar]
- 7.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 8.Cho, H., J. L. Thorvaldsen, Q. Chu, F. Feng, and M. J. Birnbaum. 2001. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349-38352. [DOI] [PubMed] [Google Scholar]
- 9.Conway, S. J., A. Kruzynska-Frejtag, P. L. Kneer, M. Machnicki, and S. V. Koushik. 2003. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis 35:1-21. [DOI] [PubMed] [Google Scholar]
- 10.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 11.Di-Poi, N., C. Y. Ng, N. S. Tan, Z. Yang, B. A. Hemmings, B. Desvergne, L. Michalik, and W. Wahli. 2005. Epithelium-mesenchyme interactions control the activity of peroxisome proliferator-activated receptor β/δ during hair follicle development. Mol. Cell. Biol. 25:1696-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudek, H., S. R. Datta, T. F. Franke, M. J. Birnbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan, and M. E. Greenberg. 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661-665. [DOI] [PubMed] [Google Scholar]
- 13.Easton, R. M., H. Cho, K. Roovers, D. W. Shineman, M. Mizrahi, M. S. Forman, V. M.-Y. Lee, M. Szabolcs, R. de Jong, T. Oltersdorf, T. Ludwig, A. Efstratiadis, and M. J. Birnbaum. 2005. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol. Cell. Biol. 25:1869-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, J., R. Tamaskovic, Z. Yang, D. P. Brazil, A. Merlo, D. Hess, and B. A. Hemmings. 2004. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J. Biol. Chem. 279:35510-35517. [DOI] [PubMed] [Google Scholar]
- 15.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 16.Garofalo, R. S., S. J. Orena, K. Rafidi, A. J. Torchia, J. L. Stock, A. L. Hildebrandt, T. Coskran, S. C. Black, D. J. Brees, J. R. Wicks, J. D. McNeish, and K. G. Coleman. 2003. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J. Clin. Investig. 112:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanada, M., J. Feng, and B. A. Hemmings. 2004. Structure, regulation and function of PKB/AKT-a major therapeutic target. Biochimica et Biophysica Acta Proteins Proteom. 1697:3-16. [DOI] [PubMed] [Google Scholar]
- 18.Jones, P., T. Jakubowicz, F. Pitossi, F. Maurer, and B. Hemmings. 1991. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. USA 88:4171-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 20.Liu, C., W. Liu, J. Palie, M. F. Lu, N. A. Brown, and J. F. Martin. 2002. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development 129:5081-5091. [DOI] [PubMed] [Google Scholar]
- 21.Papaioannou, V. E., and R. R. Behringer. 2005. Mouse phenotypes, a handbook of mutation analysis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Peng, X.-d., P.-Z. Xu, M.-L. Chen, A. Hahn-Windgassen, J. Skeen, J. Jacobs, D. Sundararajan, W. S. Chen, S. E. Crawford, K. G. Coleman, and N. Hay. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17:1352-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheid, M. P., and J. R. Woodgett. 2001. PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell Biol. 2:760-768. [DOI] [PubMed] [Google Scholar]
- 24.Stainier, D. Y., R. K. Lee, and M. C. Fishman. 1994. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development 120:2539-2553. [DOI] [PubMed] [Google Scholar]
- 25.Tschopp, O., Z.-Z. Yang, D. Brodbeck, B. Duemmler, M. Hemmings-Mieszczak, T. Watanabe, T. Michaelis, J. Frahm, and B. A. Hemmings. 2005. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development, but not for glucose homeostasis. Development 132:2943-2954. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe, N., Y. H. Wang, H. K. Lee, T. Ito, Y. H. Wang, W. Cao, and Y. J. Liu. 2005. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436:1181-1185. [DOI] [PubMed] [Google Scholar]
- 27.Yang, Z.-Z., O. Tschopp, A. Baudry, B. Duemmler, D. Hynx, and B. A. Hemmmngs. 2004. Physiological function of PKB/Akt. Biochem. Soc. Trans. 32:350-354. [DOI] [PubMed] [Google Scholar]
- 28.Yang, Z.-Z., O. Tschopp, M. Hemmings-Mieszczak, J. Feng, D. Brodbeck, E. Perentes, and B. A. Hemmings. 2003. Protein kinase Bα/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278:32124-32131. [DOI] [PubMed] [Google Scholar]
- 29.Yuan, J., M. Lipinski, and A. Degterev. 2003. Diversity in the mechanisms of neuronal cell death. Neuron 40:301-413. [DOI] [PubMed] [Google Scholar]
- 30.Yuan, J., and B. A. Yankner. 2000. Apoptosis in the nervous system. Nature 407:802-809. [DOI] [PubMed] [Google Scholar]