Abstract
Regulation of Src family kinase (SFK) activity is indispensable for a functional immune system and embryogenesis. The activity of SFKs is inhibited by the presence of the carboxy-terminal Src kinase (Csk) at the cell membrane. Thus, recruitment of cytosolic Csk to the membrane-associated SFKs is crucial for its regulatory function. Previous studies utilizing in vitro and transgenic models suggested that the Csk-binding protein (Cbp), also known as phosphoprotein associated with glycosphingolipid microdomains (PAG), is the membrane adaptor for Csk. However, loss-of-function genetic evidence to support this notion was lacking. Herein, we demonstrate that the targeted disruption of the cbp gene in mice has no effect on embryogenesis, thymic development, or T-cell functions in vivo. Moreover, recruitment of Csk to the specialized membrane compartment of “lipid rafts” is not impaired by Cbp deficiency. Our results indicate that Cbp is dispensable for the recruitment of Csk to the membrane and that another Csk adaptor, yet to be discovered, compensates for the loss of Cbp.
The Src family protein tyrosine kinases (SFKs) Lck and Fyn play an essential role in T-cell immunity (27). Activation of these kinases, which follows engagement of the T-cell antigen receptor (TCR), coreceptors, and adhesion molecules, initiates multiple signaling processes that define the fate of developing and mature T cells. In the absence of Lck and Fyn, the surface-expressed pre-T-cell receptor fails to support survival and proliferation of early T-cell progenitors and their differentiation into double-positive (DP) thymocytes expressing CD4 and CD8 (2, 14, 24, 37). Like developing thymocytes, peripheral T cells require Lck and Fyn for their TCR-dependent survival in vivo and proliferation in response to antigenic stimulation in vitro (32, 33). Conversely, constitutive up-regulation of SFK expression or activity leads to abnormal TCR-independent T-cell development, to autoimmunity, or to tumorigenesis (1, 9).
Our earlier findings showed that maintenance of T-cell development under TCR governance requires the activity of the C-terminal Src kinase (Csk) (30, 31). Csk inhibits SFKs by phosphorylating a conserved C-terminal tyrosine residue on SFKs, thereby inducing an intramolecular interaction between the C-terminal phosphotyrosine and the SH2 domain (17, 25). This interaction renders the catalytic domain of SFKs inaccessible to substrates. To inhibit membrane-associated SFKs in resting T cells and to achieve signal attenuation, a fraction of the cytosolic Csk protein pool (∼5%) is brought to the membrane into close proximity with the SFKs (39, 41). Artificial tethering of large amounts of Csk to the plasma membrane leads to virtually complete ablation of TCR signaling. The inhibitory activity of Csk is counteracted by the tyrosine phosphatase CD45, which “unlocks” SFKs by dephosphorylation of the C-terminal phosphotyrosine residue (35). This balance between inhibitory Csk and activating CD45 is essential for the proper regulation of the resting state and for TCR-induced activation of T cells.
In a search for the mechanism controlling the membrane association of Csk to enable the negative regulation of SFK, we and others identified a transmembrane Csk-binding protein, Cbp/PAG (hereafter referred to as Cbp) (7, 20). Several features of Cbp suggested its potential role in Csk-mediated SFK inhibition and T-cell activation. First, Cbp is localized exclusively within the SFK-enriched and functionally distinct signaling “lipid raft” membrane domain. Second, phospho-Cbp binds and activates Csk in vitro (38), suggesting a mechanism that potentiates the activity of lipid raft-associated Csk in vivo. It was also demonstrated that Fyn phosphorylates Cbp on the Csk-binding tyrosine residue (34, 44), indicating that the Csk-binding and -activating capacities of Cbp are likely to increase in proportion to Fyn activity. This, in turn, may provide a feedback control mechanism restraining the activity of lipid raft-associated SFKs by Csk.
Despite the relative wealth of biochemical data supporting an important role of Cbp in regulation of SFKs, the physiological significance of the Cbp-Csk interaction in T cells is not understood. High levels of Cbp phosphorylation in resting T cells and the rapid dephosphorylation of Cbp in the course of T-cell activation and rephosphorylation during signal attenuation (7, 18, 39) led to the hypothesis that Cbp controls the level of SFK activity in T cells during the resting state and following antigen-induced immune responses. The Csk-regulatory function of Cbp also suggested that Cbp might regulate antigen-dependent T-cell selection that was found to be controlled by Csk (30, 31).
To test these hypotheses as well as to reveal potentially unknown functions of Cbp, we have generated Cbp-deficient mice and analyzed T-cell development, TCR signaling features, and immune responses in these mice.
MATERIALS AND METHODS
Inactivation of the cbp gene.
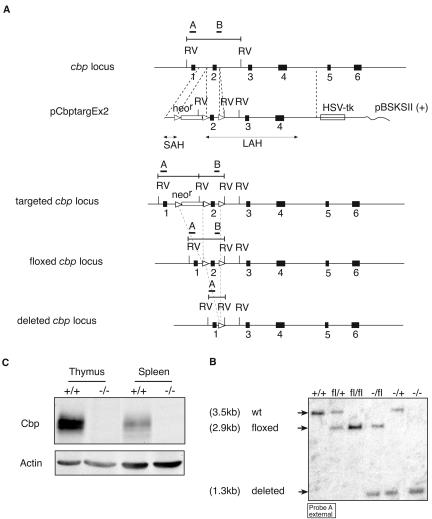
The design of the cbp-targeting vector was based on the analysis of the splicing of the Cbp pre-mRNA. Using the splice site score calculation program (http://rulai.cshl.edu/new_alt_exon_db2/HTML/score.html), we found that Cre-mediated deletion of exon 2 will result in a frameshift mutation when splicing from exon 1 to exon 3 or to downstream exons utilizing real or predicted splice acceptor sides and should lead to nonsense-mediated decay of the targeted RNA (5). Therefore deletion of exon 2 of the cbp gene will preclude generation of either truncated or full-length Cbp protein. Accordingly, we have generated a cbp-targeting vector that, upon recombination with the endogenous cbp allele, will introduce loxP sites around exon 2. The cbp gene was cloned from a C57BL/6 genomic DNA library by screening with a full-length cbp cDNA as probe. To create the targeting vector pCbptargEx2, the XbaI-AflII cbp gene fragment upstream of exon 2 was blunted and cloned into the blunted BamHI site of pEasyFlox (M. Alimzanov, unpublished data) upstream of the Neor selection marker cassette flanked by loxP sites. The cbp gene fragment containing exon 2 and flanking regions (AflII-XhoI) was cloned into the SalI site of pEasyFlox, between loxP sites. To generate a long arm of homology, the cbp gene fragment containing exons 3 and 4 (XhoI-FspI) was cloned into the HindIII site of pEasyFlox (Fig. 1). The DNA of the resulting targeting vector was transfected into Bruce4 embryonic stem (ES) cells, transfected ES cells were selected as described previously (40), and cells carrying a modified cbp gene locus were identified by Southern blot analysis (EcoRV digest, probe A and probe B). The Neor cassette was removed from targeted ES cells by transient transfection with a Cre expression vector as described previously (40). Cells carrying the cbp allele with exon 2 flanked with loxP sequences (Cbp-loxP) were identified by Southern blotting and used to generate mice carrying the cbp-loxP allele by standard techniques (40).
FIG. 1.
Disruption of the cbp gene in mice by conditional gene targeting. (A) cbp gene organization, targeting construct, and schematic of targeting strategy. The exon structure of the mouse cbp gene and a depiction of the targeting construct are shown. The positions of the DNA fragments used as probes for Southern blotting are shown (probe A, external; probe B, internal). Probe A hybridizes to a 3.5-kb EcoRV fragment in wild-type mice, to a 3-kb EcoRV fragment in targeted cells, to a 2.9-kb EcoRV fragment in floxed mice (with deletions of Neo), and to a 1.3-kb EcoRV fragment in mice with the deletion of this allele. Filled rectangles depict exons, open rectangles depict selection markers, and open triangles depict the loxP sites and their orientation. Abbreviations: RV, EcoRV; SAH, short arm of homology; LAH, long arm of homology. (B) Targeting of the Cbp allele. Southern blot depicting wild-type (+), floxed (fl)/+, fl/fl, deleted (−)/fl, −/+, and −/− targeted alleles. Genomic DNA from mouse tails was digested with EcoRV and analyzed by Southern blotting using external probe A. (C) Deletion of the Cbp protein. Immunoblots were prepared from total thymus and total spleen. The presence of Cbp was detected by immunoblotting with a polyclonal rabbit anti-Cbp antibody (top panel). Equal protein loading was confirmed by reprobing the membrane with an antiactin antibody (bottom panel).
Mice.
Cbp-deficient mice were on a pure C57BL/6 background and used with age- and sex-matched controls, or, where the mutant Cbp allele was crossed onto mixed genetic backgrounds, littermates were used as controls. For the analysis of the superantigen-mediated negative selection of T cells, the Cbp-deficient mice were backcrossed to BALB/c mice for six generations. All mice were bred and maintained under specific-pathogen-free conditions, and all mouse protocols were approved by the Rockefeller University Institutional Animal Care and Use Committees.
T-cell isolation.
Peripheral CD4 T cells were isolated by magnetic cell sorting depletion of single-cell suspensions from spleen or lymph nodes according to the manufacturer's protocol (Miltenyi Biotech). In short, cells were incubated with a mixture of biotinylated antibodies (anti-CD8, anti-CD19, anti-CD11b, anti-CD49b, anti-NK1.1, anti-TCR-γδ, anti-GR1, and anti-TER 119), washed, and incubated with streptavidin-coated beads. Streptavidin-labeled non-CD4 T cells were then depleted using magnetic cell sorting. The resulting CD4 T cells were more than 98% viable and more than 95% pure.
Cell stimulation.
Purified T cells were incubated on ice for 20 min in complete RPMI 1640 (Gibco BRL) (10% fetal calf serum) containing biotinylated or purified antibodies. Cells were washed once in complete RPMI medium, resuspended in prewarmed complete medium containing 20 μg/ml recombinant streptavidin or anti-species-specific antibodies (goat anti-hamster antibodies), and incubated at 37°C for the indicated time periods. After the incubation, cells were washed in plain medium and lysed in octyl-d-glucoside (ODG) lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 20 mM NaF, 1 mM EDTA [pH 8], 1 mM EGTA [pH 8], 2% octyl-d-glucoside [wt/vol], 10% glycerol [vol/vol]).
Antibodies.
The following antibodies were used for Western blot analysis of protein expression and phosphorylation: antiactin (Ab-1) (CP01; Oncogene Research Products), anti-Csk (C-20) (sc-286; Santa Cruz), anti-Fyn (Fyn3) (sc-16; Santa Cruz), LAT (06-807; Upstate), anti-phospho-LAT (Tyr171) (3581; Cell Signaling), anti-phospho-LAT (Tyr191) (3584; Cell Signaling), anti-Lck (2102)-G (sc-13; Santa Cruz), anti-p-Tyr (PY99) (sc-7020; Santa Cruz), anti-p-Tyr (4G10) (16-103; Upstate), anti-phospho-Src (Tyr418) (44-660; Biosource), anti-PLCγ1 (B-6-4) (05-366; Upstate Biotechnology), phospho-PLCγ1 (Tyr783) (2821; Cell Signaling), anti-α-tubulin (DM 1A) (T9026; Sigma), anti-Vav1 (C-14) (sc-132; Santa Cruz), anti-phospho-Vav1 (Tyr174)-R (sc-16408-R; Santa Cruz), anti-ZAP-70 (29) (610239; BD Transduction Laboratories), and anti-phospho-ZAP-70 (Tyr319) (2701; Cell Signaling). All secondary horseradish peroxidase-coupled antibodies were obtained from Amersham. Cbp was detected by Western blotting using a rabbit polyclonal antibody raised by us against a peptide corresponding to the last 14 amino acid residues of the C terminus of the Cbp protein.
Western blotting.
Cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 8 to 15% acrylamide gels and blotted onto polyvinylidene difluoride membranes according to the manufacturer's instructions (Millipore). Membranes were then blocked in Superblock (Pierce) or in TB buffer (Tris-buffered saline, 0.2% Tween 20, 5% bovine serum albumin) for 1 h at room temperature. Membranes were incubated in primary antibody for 1 h at room temperature or overnight at 4°C with constant agitation and in secondary horseradish peroxidase-conjugated antibody for 45 min at room temperature and washed three times in between for 10 min. All incubations and washes were performed with Tris-buffered saline containing 0.2% Tween. An ECL detection kit (Amersham) was used for protein visualization.
Flow cytometric analysis.
Mice were sacrificed and lymphoid organs removed. Single-cell suspensions were prepared by gentle tearing of spleens, lymph nodes, or thymi in complete RPMI 1640. When necessary, erythrocytes were lysed by incubating cells in red blood cell lysis buffer (0.75% NH4Cl, 100 mM Tris-HCl [pH 7.65]) for 2 to 4 min at room temperature. Lysis was stopped by adding 10 ml of complete RPMI. Cells were incubated with chromophore-labeled antibodies as described previously (12). In brief, 1 × 106 to 3 × 106 cells were incubated with chosen antibodies for 20 min at 4°C in phosphate-buffered saline-1% bovine serum albumin-0.01% sodium azide (PBA), and frequencies of the antibody-bound cells were measured by four-color analysis using a FACSCalibur flow cytometer (Becton Dickinson). The data were analyzed with FlowJo software (Treestar). For the exclusion of dead cells during analysis, TO-PRO-3 (1 nM; Molecular Probes) was added to the cells before acquisition. The following antibodies were purchased from BD Pharmingen: B220 (RA3-6B2), CD3 (145-2C11), CD4 (RM4-5), CD5 (53-7.3), CD8α (53-6.7), CD11b (M1/70), CD19 (1D3), CD24/HSA (M1/69), CD25 (7D4), CD49b (DX5), CD69 (H1.2F3), CD90/Thy1.2 (53-2.1), GR1 (RB6-8C5), NK1.1 (PK136), TCRβ (H57-597), TCRγδ (GL3), TCR-Vβ3 (KJ25), TCR-Vβ5.1, 5.2 (MR9-4), TCR-Vβ8 (F23.1), and TER119 (Ly76). Streptavidin-Cy7-phycoerythrin was purchased from BD Pharmingen.
Calcium flux analysis.
For calcium flux analysis, cells were washed with plain medium (RPMI 1640) and loaded for 30 min with 2 μM Indo 1 (Molecular Probes) at 37°C. For stimulation, different amounts of biotinylated anti-CD3 antibody were added and cross-linked with two times the concentration of recombinant streptavidin. The data were acquired on a BD LSR 1 system (Becton Dickinson) and analyzed with FlowJo software (Treestar).
Lipid raft isolation.
Thymocytes (2 × 107) were lysed in 200 μl ice-cold TNNE buffer (10 mM Tris [pH 7.5], 150 mM NaCl, 5 mM EDTA, 20 mM NaF) containing 0.1% Triton X-100, protease inhibitors (P8340; Sigma), and 2 mM Na3VO4. The lysate was transferred to precooled UltraCone polyallomer centrifuge tubes (Seton Scientific), and lipid rafts were isolated using an iodixanol gradient (Optiprep; Axis-Shield). Samples were layered on the bottom at a final concentration of 40% iodixanol. Iodixanol (30%) and TNNE buffer were separately layered into the tube to provide a gradient. The samples were spun at 60,000 rpm in a Sorvall TH-660 rotor for 3 h. Five to 8 equivalent volume fractions were collected from each tube, with the top fractions containing the lipid rafts.
Induction of oral tolerance.
Cbp−/− Rag1−/− DO11.10 TCR transgenic mice and age-matched controls were given water containing 20 mg/ml ovalbumin (OVA; Sigma) for 5 days. With an average consumption of 5.5 ml/day, this equaled around 100 mg/day. Mice were sacrificed on the sixth day, and proliferation in response to OVA peptide of CD4 T cells from the mesenteric lymph nodes was assessed in vitro. Briefly, 2 × 104 CD4 T cells were cultured with 1 × 105 irradiated syngeneic antigen-presenting cells and either 30 nM, 300 nM, or 3 mM OVA323-339 peptide per triplicate well in a 96-well round-bottom plate. Cells were cultured for 72 h at 37°C, and 1 μCi/well of [3H]thymidine was added for the final 8 h of culture. Incorporated [3H]thymidine was measured using a scintillation counter.
RESULTS
Cbp is dispensable for embryonic development and postnatal growth.
Deficiency in Csk in developing embryos leads to their death (25). Given the important role of Cbp in Csk regulation, we assumed that, similar to Csk deficiency, the lack of Cbp would lead to the death of mouse embryos. Therefore, our initial plan was to generate mice carrying the cbp loxP/loxP allele and to use these mice for inducible or cell-lineage-specific cbp inactivation. To inactivate Cbp in αβ T cells, cbp loxP/loxP mice (CbploxP/loxP) were bred to CD4-cre transgenic mice (Fig. 1A).
While generating the mice with αβ-T-cell-specific deficiency in Cbp, we also generated mice heterozygous for the cbp-null allele (Cbp+/− mice) by crossing CbploxP/loxP mice to EIIa-cre mice, which leads to Cre-mediated deletion of the floxed allele in all tissues of the zygote (21). The Cbp+/− mice were interbred for homozygosity to address the role of Cbp in embryonic development. Against our expectations, Cbp−/− mice were born in Mendelian ratios, appeared grossly normal, were fertile, and developed no obvious abnormalities later in their life. Southern blot analysis of DNA isolated from wild-type and Cbp-deficient mice confirmed the complete deletion of the loxP-flanked exon 2 (Fig. 1B). Western blot analysis of Cbp expression in thymocytes and splenocytes of Cbp−/− mice did not show the presence of the full-length or truncated Cbp protein (Fig. 1C). Therefore mice homozygous for the deletion of exon 2 of the cbp gene are in fact Cbp deficient. Thus, unlike Csk, Cbp is not required for normal embryonic development and is dispensable for all vital functions in the adult mouse.
Cbp is not required for the antigen-dependent control of T-cell development.
The Csk-recruiting function of Cbp suggested that Cbp might regulate SFK functions in T cells in a manner similar to that of Csk (7, 20, 39). Loss of Csk at early stages of T-cell development is associated with TCR- and major histocompatibility complex (MHC)-independent T-cell development, resulting in TCR-deficient T cells accumulating in the peripheral lymphoid organs. On the other hand, TCR-expressing Csk-deficient T cells express lower levels of αβTCR, CD4, or CD8 coreceptors (30, 31). None of these features were observed in Cbp-deficient thymocytes or peripheral T cells. Deficiency in Cbp affects neither the frequency nor the number of T cells developing in the various subpopulations in the thymus. Based on the representation of DN (CD4− CD8−), DP (CD4+ CD8+), and CD4+ SP or CD8+ SP cells in the thymus, T-cell development is normal in Cbp-deficient mice. Also, a further analysis of the DN stage for expression of CD25 and CD44 revealed no difference between wild-type and Cbp-deficient mice (Fig. 2). Deficiency in Cbp also does not influence the expression pattern and levels of expression of the surface proteins CD3, CD4, and CD8. The markers CD5 and CD69, which become up-regulated in the course of TCR-dependent positive selection of thymocytes, follow the wild-type pattern of expression in Cbp-deficient mice (Fig. 2) (3, 6). Furthermore the T-cell-to-B-cell ratio, the ratio of CD4 to CD8 cells, and the percentage of regulatory T cells, as defined by the simultaneous expression of CD4 and CD25, are not affected in spleens or lymph nodes of Cbp-deficient mice.
FIG. 2.
Wild-type percentages of thymic and peripheral lymphocyte populations in Cbp-deficient mice. Total thymocytes, splenocytes, and lymph node cells were stained with the indicated antibodies and analyzed by flow cytometry. Numbers indicate percentages of gated cells. The dotted line in each histogram represents double-positive cells, the gray area represents CD8 single-positive cells, and the black area represents CD4 single-positive (SP) cells. Abbreviations: Thy, thymus; DN, gated on CD4− and CD8− (double-negative) cells; Spl, spleen; LN, lymph nodes, CD4+, gated on CD4+ cells; +/+, Cbp+/+; −/−, Cbp−/−.
Cbp does not control the resting state of peripheral T cells.
TCR-mediated activation of ex vivo-isolated T cells is associated with rapid but transient dephosphorylation of Cbp at the tyrosine residue responsible for the Cbp-Csk interaction (7, 18, 39). This observation led to the hypothesis that Cbp plays a positive role in maintenance of T cells in the resting state, such that the absence of Cbp would result in spontaneous T-cell activation. In mice, spontaneously or antigenically activated T cells are characterized by increased cell size and up-regulation of the cell surface proteins CD25, CD44, and CD69 but decreased levels of CD62L (L-selectin). The expression levels of these activation markers and cell sizes were similar for Cbp-deficient and control T cells analyzed ex vivo (Fig. 2 and data not shown). Thus, deficiency in Cbp does not lead to changes that would be consistent with spontaneous T-cell activation. Furthermore, Cbp-deficient peripheral T cells did not proliferate spontaneously in vitro and required antigenic stimulation for their activation (see below).
As the above-mentioned analyses were performed in Cbp-null mice, it is possible that the loss of Cbp expression during mouse embryonic and early postnatal development led to the selection of T cells that use other means of maintaining their resting state. To test for such a possibility, we investigated the effect of Cbp deficiency induced at the DP stage of T-cell development on further development of Cbp-deficient T cells and their phenotype. To inactivate Cbp in DP cells, the CbploxP/loxP mice were bred to CD4-cre transgenic mice to generate CbploxP/loxP CD4-cre mice. Expression of the CD4-driven Cre leads to deletion of the loxP-flanked exon 2 in the DP stage and to subsequent Cbp deficiency in SP thymocytes and peripheral T cells, based on the absence of Cbp protein and loxP-flanked exon 2 determined by Western blotting and PCR, respectively (data not shown).
As defined by the analysis of surface-expressed differentiation and activation markers, such as CD3, CD4, CD8, CD5, CD25, CD44, CD62L, and CD69, conditional deficiency in Cbp had no effect on T-cell development or the state of T-cell activation in peripheral lymphoid organs (data not shown).
In conclusion, our data show that Cbp is not required for T-cell development and maintenance at the resting state.
Cbp does not control TCR-mediated protein phosphorylation in peripheral T cells.
The ability of Cbp to recruit Csk to the plasma membrane suggested a negative role for Cbp in SFK activation in T cells. To address this possibility, the kinetics and levels of TCR-induced protein tyrosine phosphorylation in CD4+ Cbp-deficient T cells were measured. Purified control and Cbp-deficient CD4 T cells were stimulated in vitro with anti-CD3 antibody and analyzed for total protein tyrosine phosphorylation as well as for the phosphorylation of specific proteins in the TCR signaling pathway. The patterns, levels, and kinetics of total protein tyrosine phosphorylation were similar in Cbp-deficient and wild-type T cells (Fig. 3A). The ∼90-kDa phosphotyrosine band in wild-type cells, missing in Cbp−/− cells, migrates in the area normally occupied by Cbp. Like what is seen with total tyrosine phosphorylation, the phosphorylation patterns of individual proteins before and after TCR stimulation were not affected by Cbp deficiency (Fig. 3B).
FIG. 3.
Unaltered proximal signaling events in Cbp−/− CD4 T cells. (A) Unaltered total tyrosine phosphorylation in Cbp−/− CD4 T cells. Purified CD4 T cells were incubated on ice with 10 μg/ml anti-CD3 antibody and stimulated for the indicated times by cross-linking with goat anti-hamster antibodies at 37°C. Cell lysates were prepared, blotted, and probed with a phosphotyrosine-specific antibody, 4G10 (top panel). Equal protein loading was assessed by blotting for actin (bottom panel). (B) Purified CD4 T cells were incubated on ice with 10 μg/ml anti-CD3 or 10 μg/ml anti-CD3 plus 10 μg/ml anti-CD4 antibody and stimulated by cross-linking with goat anti-hamster antibodies at 37°C. Control cells were incubated only with goat anti-hamster antibodies. Cell lysates were prepared, blotted, probed with protein-specific phosphotyrosine antibodies and reprobed with protein-specific antibodies to confirm equal loading.
Therefore, Cbp is not essential for the TCR-induced phosphorylation of key signaling proteins. These data suggest indirectly that Cbp is dispensable for SFK activation.
Cbp does not control sensitivity of T cells to TCR activation.
Recent studies by Davidson and colleagues (10) showed that transgenic expression of a mutant form of Cbp that does not bind Csk increases sensitivity of T cells to antigenic stimulation. This observation is consistent with the signaling model that postulates a negative role of Cbp in TCR signaling. However, our data argue against a significant role for Cbp in the regulation of TCR signal strength. For example, the TCR dose-dependent effects on intracellular calcium release were similar in Cbp-deficient and control cells (Fig. 4A). The lack of evident Cbp contribution to T-cell activation was further underscored by unaltered Cbp-deficient T-cell activation in response to TCR cross-linking in vitro. Incubation of Cbp- deficient cells with plate-bound anti-CD3 antibody led to wild-type-like changes in the expression levels of CD25, CD44, CD69, and CD62L (Fig. 4B).
FIG. 4.
Cbp deficiency does not control T-cell sensitivity to TCR-mediated signals in vitro. (A) Cbp deficiency does not alter TCR-induced calcium flux. Calcium flux of CD4 SP, CD8 SP, and DP thymocytes and of splenic CD4 T cells is depicted. Wild-type (gray area) or Cbp-deficient (black line) cells were stimulated with titrated amounts of anti-CD3 antibody (in micrograms), and an overlay of the histograms of their calcium fluxes is shown. Arrowheads indicate the addition of anti-CD3 and cross-linking antibodies. (B) Unaltered regulation of activation markers in the absence of Cbp. CD4 T cells isolated from spleen and lymph nodes were incubated for 12 h either in medium alone or with plate-bound anti-CD3 (black lines) and analyzed by flow cytometry for the expression of CD25, CD44, CD62L, and CD69. Gray areas represent uninduced controls, and black lines represent stimulated cells. (C) Unaltered TCR-induced proliferation in Cbp−/− T cells. CD4 T cells isolated from spleen and lymph nodes were incubated for 3 days either in medium alone or with plate-bound anti-CD3 (in micrograms) with or without plate-bound anti-CD28 (in micrograms). Proliferation was assessed by carboxyfluorescein succinimidyl ester (CFSE) dilution and analyzed by flow cytometry. Gray areas represent uninduced controls, and black lines represent stimulated cells. Stimulation with phorbol myristate acetate (P+I; 2.5 ng/ml) and the ionophore A23187 (100 ng/ml) was used to control for the proliferative potentials of wild-type and Cbp−/− cells. (D) Unaltered kinetics of TCR-induced proliferation in Cbp−/− T cells. CD4 T cells were purified from spleen and lymph nodes from two mice per genotype, wild type (open symbols) and Cbp−/− (closed symbols). The cells were incubated for 12 h, 24 h, or 36 h on titrated amounts of anti-CD3 antibody, transferred into uncoated 96-well plates, and further incubated for a total of 72 h. Proliferation was scored by [3H]thymidine incorporation for the last 8 h of the culture. (E) Normal Th1/Th2 differentiation in the absence of Cbp. CD4 T cells were differentiated in vitro under Th1-polarizing and Th2-polarizing conditions. Numbers show percentages of live gated cells.
To address a possible impact of Cbp on T-cell proliferation in vitro, wild-type and Cbp-deficient CD4 T cells were incubated with different amounts of anti-CD3 antibody with or without anti-CD28 (Fig. 4C). There was no difference between the sensitivity of Cbp-deficient T cells to TCR-mediated proliferation and that of wild-type T cells. The strength of the TCR-induced proliferative signal depends not only on the amount of the TCR agonist but also on the length of time the TCR is engaged (15). Therefore, we measured the amplitudes of T-cell responses to titrated amounts of anti-CD3 antibody that stimulated cells for various time periods (12 to 36 h). Following incubation with anti-CD3 antibodies for defined periods of time, T cells were moved to agonist-free fresh medium, and their proliferation was measured by [3H]thymidine incorporation (Fig. 4D). No differences were detected in the proliferative responses to titrated antibody concentrations or to variable activation times.
Perturbation of TCR function by mutating signaling proteins frequently affects terminal T-cell differentiation into cytokine-producing Th1 or Th2 cells (4, 16, 29). To test the ability of Cbp to control Th1/Th2 differentiation, purified Cbp-deficient and control CD4 T cells were stimulated with anti-CD3 and anti-CD28 in the presence of interleukin-12 and interleukin-4, respectively (Fig. 4E). Wild-type and Cbp-deficient cells displayed equal abilities to differentiate into Th1 or Th2 cells.
Collectively, the results of these in vitro T-cell activation experiments rule out a significant role for Cbp in TCR-mediated T-cell activation, if it has any.
Cbp deficiency does not influence negative selection or induction of T-cell anergy in vivo.
The wild-type pattern of Cbp-deficient T-cell activation in vitro suggested that Cbp-deficient T-cell function in vivo could also be unaltered. Nonetheless, we tested whether Cbp controls T-cell function in vivo, where effects that were undetectable in the in vitro analysis could become apparent. To measure in vivo T-cell responses to antigenic stimulation, we used two experimental models to test T-cell development in response to self-antigens. Engagement of TCR by self-antigens leads either to clonal deletion or functional inactivation (anergy) of these thymocytes (11, 26, 36, 43). We reasoned that potential subtle changes in TCR signaling in the absence of Cbp might affect self-antigen-mediated selection.
For the analysis of thymic selection, the HY TCR transgenic system was chosen since it is well established and allows the analysis of both positive and negative selection. The HY TCR reacts with the male-specific antigen HY, causing negative selection of HY-specific T cells in males but positive selection in females, which lack the male self-peptide(s) (42). Overall, the deficiency in Cbp has no effect on positive or negative selection in this model system. Male wild-type and Cbp-deficient HY transgenic mice lacked CD8hi T cells that stained positive for the HY TCR-specific antibody (T3.70) in the periphery but had T3.70 CD8lo transgenic cells, which have been described before (42). In female wild-type and Cbp-deficient mice, the expected large numbers of T3.70 CD8hi cells could be found in the periphery (Fig. 5A).
FIG. 5.
Cbp-deficient mice show no change in T-cell function in vivo. (A) Thymocytes and lymph node cells from HY Cbp+/+ (+/+ HY) and HY Cbp−/− (−/− HY) male mice (top two rows) and from HY Cbp+/+ and HY Cbp−/− female mice (bottom two rows) were analyzed by flow cytometry. CD4-versus-CD8 dot plots are shown for either ungated or gated T3.70hi cells. Numbers indicate the total thymic cellularities. (B) Clonal deletion of T cells by superantigens. CD4 T cells were stained for their Vβ expression and analyzed by flow cytometry. Percentages of Vβ3-, 5-, 8-, and 11-expressing cells are depicted. White bars represent mice on the nonselecting background (H-2b), and gray bars represent mice on the selecting background (H-2d). Five to 20 mice were analyzed in each group. (C) Cbp-deficient mice are able to undergo oral tolerization. Cbp−/− Rag1−/− DO11.10 transgenic mice (open symbols) and age-matched controls (filled symbols) were administered water (angular symbols) or 100 mg OVA (circles) as described in Materials and Methods. CD4 T cells were isolated from mesenteric lymph nodes after 5 days of feeding and incubated with congenic irradiated splenic cells from BALB/c mice and titrated amounts of OVA323-339 peptide. Cells were incubated for 3 days, and proliferation was determined by [3H]thymidine incorporation.
Negative selection of T cells carrying TCR recognized by superantigens shapes the peripheral T-cell repertoire. Mice carry well-defined mouse mammary tumor virus-encoded superantigens (19, 23), which direct clonal deletion of CD4 T cells expressing TCR with certain Vβ gene segments (Vβ 3, 5, 11, and 12) when bound by MHC class II molecules of the H-2d haplotype. To evaluate the influence of Cbp deficiency on T-cell reactivity toward endogenous superantigens, Cbp-deficient mice (nonselecting C57BL/6 [H-2b background]) had to be backcrossed onto the selecting BALB/c (H-2d background). The percentages of T cells expressing various Vβ proteins were examined in mice on both backgrounds. As shown in Fig. 5B, T-cell clones expressing TCR Vβ 3, 5, and 11 were efficiently eliminated in wild-type and Cbp-deficient mice of the H-2d haplotype. The frequencies of T cells expressing Vβ 8, which does not lead to negative selection, were also similar in wild-type and Cbp-deficient mice.
To determine whether the loss of Cbp has any influence on T-cell anergy induction in vivo, we set up a model of oral tolerance in which high doses of ingested antigen induce systemic, antigen-specific T-cell tolerance (13). Cbp−/− Rag1−/− DO11.10 TCR transgenic and age-matched control mice were fed OVA for 5 days, after which the proliferative response of CD4 T cells to congenic antigen-presenting cells and OVA323-339 peptide was measured. As expected, T cells from OVA-fed mice showed proliferation that was profoundly diminished compared to that of T cells from control transgenic mice that were not given OVA (Fig. 5C). No significant differences in anergy induction levels were seen between wild-type and Cbp-deficient mice.
Cbp is dispensable for raft localization of Csk in T cells.
The systematic analysis of the signaling function in Cbp-deficient T cells did not reveal a role for Cbp in the regulation of TCR signaling and T-cell activation. These observations suggested a possible redundancy of Cbp in the regulation of Csk localization at the plasma membrane in general and at lipid rafts in particular. Indeed, we found that Cbp is not required for the association of Csk with lipid rafts in T-lineage cells. This was demonstrated through the quantification of Csk associated with lipid rafts in thymocytes, which was unaltered in the absence of Cbp (Fig. 6B). Moreover, the phosphorylation levels of Fyn and Lck in lipid rafts were not affected by the absence of Cbp (Fig. 6A, bottom row).
FIG. 6.
Csk localizes to rafts in the absence of Cbp. (A) Lipid rafts were isolated from thymocytes by high-speed centrifugation. Fractionations were collected and controlled by Western analysis of Cbp, LAT, and cholera toxin B subunit (CTX-B). The activities of Fyn and Lck were controlled by a Src phosphotyrosine-specific antibody. pFyn and pLck indicate the positions of phospho-Fyn and phospho-Lck, respectively. (B) The top three fractions containing rafts were probed for Csk.
Thus, Cbp is required neither for Csk localization nor for Csk-mediated SFK phosphorylation in lipid rafts.
DISCUSSION
The control of SFKs through the kinase Csk is essential for a functional immune system. Recruitment of Csk to the plasma membrane is an integral part of the control of SFKs, and previous data implicate Cbp as the membrane adaptor for Csk. In this study, we attempted to prove the role of Cbp in the regulation of Csk utilizing genetic inactivation of the cbp gene in mice.
Our results exclude Cbp as an essential gatekeeper of the T-cell resting state. In the absence of stimulation, Cbp-deficient T cells show no sign of altered protein tyrosine phosphorylation, calcium mobilization, or spontaneous up-regulation of activation-specific protein markers. Similarly to wild-type T cells, Cbp-deficient cells do not proliferate spontaneously and undergo rapid apoptosis in the absence of antibody-induced TCR engagement in vitro.
Our data also show that Cbp has no important dampening role in TCR signaling via the recruitment of Csk to the plasma membrane. In vitro induction of T cells with various concentrations of anti-CD3 antibody over different time periods indicated no influence of Cbp on the signal strength downstream of the TCR and led to T-cell activation and proliferation at levels similar to those of control cells.
The findings from the Cbp-deficient mice are in striking contrast to those from Csk-deficient mice, both in the whole animal and in the immune system in particular, which argues against Cbp being a major regulator of Csk function. During embryogenesis, Csk is essential for neural tube closure and for the development of the embryo (17, 25), while Cbp, on the other hand, is dispensable. During thymic development, Csk robustly regulates SFK function, and Csk deficiency leads to TCR- and MHC-independent development (30, 31), while Cbp plays no important role. Furthermore, Cbp is not essential for antigen-specific negative or positive selection, showing that Cbp plays no role in the modulation of weak or strong signals received through the TCR.
The benign phenotype of Cbp-deficient cells could be explained by a potential redundancy in the Csk-recruiting function of Cbp. Indeed, the amount of lipid raft-associated Csk in the thymus is not affected by the absence of Cbp. Moreover, the unaltered levels of phosphorylation of the Csk-dependent tyrosine residues within the carboxyl termini of Lck and Fyn in the Cbp-deficient thymocytes excludes an important role for Cbp in negative regulation of the membrane-associated Src family kinases. It is likely that previously reported associations of Csk with other plasma membrane-localized adaptors, such as Dok-3, SIT, and/or LIME, can compensate for the lack of Cbp (8, 22, 28).
In conclusion, our data show that Cbp is not essential in embryonic development, the regulation of T-cell immunity, or Csk compartmentalization. Our preliminary data show no significant changes in Cbp-deficient B cells and neurons. Hence, Cbp is likely to be generally redundant with other membrane adaptor proteins as a regulator of Csk function in various cell types.
Acknowledgments
We thank Ingrid Mecklenbräuker, Laura Donlin, Eva Besmer, and Sukhvinder Sahota for critical reading of the manuscript.
This work was supported by The Irene Diamond Fund/Professorship Program (A.T.) and by National Institutes of Health grants 5-RO1-AI050867 and 5-RO1-AI053545 (A.T.). This work was supported in part by The Cancer Research Institute Predocotoral Emphasis Pathway in Tumor Immunology (M.-W.D.).
REFERENCES
- 1.Abraham, K. M., S. D. Levin, J. D. Marth, K. A. Forbush, and R. M. Perlmutter. 1991. Thymic tumorigenesis induced by overexpression of p56lck. Proc. Natl. Acad. Sci. USA 88:3977-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleby, M. W., J. A. Gross, M. P. Cooke, S. D. Levin, X. Qian, and R. M. Perlmutter. 1992. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell 70:751-763. [DOI] [PubMed] [Google Scholar]
- 3.Azzam, H. S., A. Grinberg, K. Lui, H. Shen, E. W. Shores, and P. E. Love. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188:2301-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badou, A., M. Savignac, M. Moreau, C. Leclerc, G. Foucras, G. Cassar, P. Paulet, D. Lagrange, P. Druet, J. C. Guery, and L. Pelletier. 2001. Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-gamma production. Eur. J. Immunol. 31:2487-2496. [DOI] [PubMed] [Google Scholar]
- 5.Baker, K. E., and R. Parker. 2004. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr. Opin. Cell Biol. 16:293-299. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac, A., P. Matzinger, R. A. Seder, W. E. Paul, and R. H. Schwartz. 1992. Activation events during thymic selection. J. Exp. Med. 175:731-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191:1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brdickova, N., T. Brdicka, P. Angelisova, O. Horvath, J. Spicka, I. Hilgert, J. Paces, L. Simeoni, S. Kliche, C. Merten, B. Schraven, and V. Horejsi. 2003. LIME: a new membrane raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J. Exp. Med. 198:1453-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke, M. P., K. M. Abraham, K. A. Forbush, and R. M. Perlmutter. 1991. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell 65:281-291. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, D., M. Bakinowski, M. L. Thomas, V. Horejsi, and A. Veillette. 2003. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol. Cell. Biol. 23:2017-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fink, P. J., and M. J. Bevan. 1995. Positive selection of thymocytes. Adv. Immunol. 59:99-133. [DOI] [PubMed] [Google Scholar]
- 12.Forster, I., and K. Rajewsky. 1987. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 17:521-528. [DOI] [PubMed] [Google Scholar]
- 13.Garside, P., and A. M. Mowat. 2001. Oral tolerance. Semin. Immunol. 13:177-185. [DOI] [PubMed] [Google Scholar]
- 14.Groves, T., P. Smiley, M. P. Cooke, K. Forbush, R. M. Perlmutter, and C. J. Guidos. 1996. Fyn can partially substitute for Lck in T lymphocyte development. Immunity 5:417-428. [DOI] [PubMed] [Google Scholar]
- 15.Iezzi, G., K. Karjalainen, and A. Lanzavecchia. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8:89-95. [DOI] [PubMed] [Google Scholar]
- 16.Iezzi, G., E. Scotet, D. Scheidegger, and A. Lanzavecchia. 1999. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur. J. Immunol. 29:4092-4101. [DOI] [PubMed] [Google Scholar]
- 17.Imamoto, A., and P. Soriano. 1993. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell 73:1117-1124. [DOI] [PubMed] [Google Scholar]
- 18.Itoh, K., M. Sakakibara, S. Yamasaki, A. Takeuchi, H. Arase, M. Miyazaki, N. Nakajima, M. Okada, and T. Saito. 2002. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol. 168:541-544. [DOI] [PubMed] [Google Scholar]
- 19.Kappler, J. W., U. Staerz, J. White, and P. C. Marrack. 1988. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature 332:35-40. [DOI] [PubMed] [Google Scholar]
- 20.Kawabuchi, M., Y. Satomi, T. Takao, Y. Shimonishi, S. Nada, K. Nagai, A. Tarakhovsky, and M. Okada. 2000. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404:999-1003. [DOI] [PubMed] [Google Scholar]
- 21.Lakso, M., J. G. Pichel, J. R. Gorman, B. Sauer, Y. Okamoto, E. Lee, F. W. Alt, and H. Westphal. 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93:5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemay, S., D. Davidson, S. Latour, and A. Veillette. 2000. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell. Biol. 20:2743-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald, H. R., R. Schneider, R. K. Lees, R. C. Howe, H. Acha-Orbea, H. Festenstein, R. M. Zinkernagel, and H. Hengartner. 1988. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature 332:40-45. [DOI] [PubMed] [Google Scholar]
- 24.Molina, T. J., K. Kishihara, D. P. Siderovski, W. van Ewijk, A. Narendran, E. Timms, A. Wakeham, C. J. Paige, K. U. Hartmann, A. Veillette, et al. 1992. Profound block in thymocyte development in mice lacking p56lck. Nature 357:161-164. [DOI] [PubMed] [Google Scholar]
- 25.Nada, S., T. Yagi, H. Takeda, T. Tokunaga, H. Nakagawa, Y. Ikawa, M. Okada, and S. Aizawa. 1993. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell 73:1125-1135. [DOI] [PubMed] [Google Scholar]
- 26.Nossal, G. J. 1994. Negative selection of lymphocytes. Cell 76:229-239. [DOI] [PubMed] [Google Scholar]
- 27.Palacios, E. H., and A. Weiss. 2004. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23:7990-8000. [DOI] [PubMed] [Google Scholar]
- 28.Pfrepper, K. I., A. Marie-Cardine, L. Simeoni, Y. Kuramitsu, A. Leo, J. Spicka, I. Hilgert, J. Scherer, and B. Schraven. 2001. Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein). Eur. J. Immunol. 31:1825-1836. [DOI] [PubMed] [Google Scholar]
- 29.Rogers, P. R., and M. Croft. 1999. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J. Immunol. 163:1205-1213. [PubMed] [Google Scholar]
- 30.Schmedt, C., K. Saijo, T. Niidome, R. Kuhn, S. Aizawa, and A. Tarakhovsky. 1998. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature 394:901-904. [DOI] [PubMed] [Google Scholar]
- 31.Schmedt, C., and A. Tarakhovsky. 2001. Autonomous maturation of alpha/beta T lineage cells in the absence of COOH-terminal Src kinase (Csk). J. Exp. Med. 193:815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seddon, B., G. Legname, P. Tomlinson, and R. Zamoyska. 2000. Long-term survival but impaired homeostatic proliferation of naive T cells in the absence of p56lck. Science 290:127-131. [DOI] [PubMed] [Google Scholar]
- 33.Seddon, B., and R. Zamoyska. 2002. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J. Immunol. 169:2997-3005. [DOI] [PubMed] [Google Scholar]
- 34.Shima, T., S. Nada, and M. Okada. 2003. Transmembrane phosphoprotein Cbp senses cell adhesion signaling mediated by Src family kinase in lipid rafts. Proc. Natl. Acad. Sci. USA 100:14897-14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieh, M., J. B. Bolen, and A. Weiss. 1993. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 12:315-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starr, T. K., S. C. Jameson, and K. A. Hogquist. 2003. Positive and negative selection of T cells. Annu. Rev. Immunol. 21:139-176. [DOI] [PubMed] [Google Scholar]
- 37.Stein, P. L., H. M. Lee, S. Rich, and P. Soriano. 1992. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 70:741-750. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi, S., Y. Takayama, A. Ogawa, K. Tamura, and M. Okada. 2000. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J. Biol. Chem. 275:29183-29186. [DOI] [PubMed] [Google Scholar]
- 39.Torgersen, K. M., T. Vang, H. Abrahamsen, S. Yaqub, V. Horejsi, B. Schraven, B. Rolstad, T. Mustelin, and K. Tasken. 2001. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 276:29313-29318. [DOI] [PubMed] [Google Scholar]
- 40.Torres, R., and R. Kuhn. 1997. Laboratory protocols for conditional gene targeting. Oxford University Press, Oxford, United Kingdom.
- 41.Vang, T., K. M. Torgersen, V. Sundvold, M. Saxena, F. O. Levy, B. S. Skalhegg, V. Hansson, T. Mustelin, and K. Tasken. 2001. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J. Exp. Med. 193:497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Boehmer, H. 1990. Developmental biology of T cells in T cell-receptor transgenic mice. Annu. Rev. Immunol. 8:531-556. [DOI] [PubMed] [Google Scholar]
- 43.von Boehmer, H. 1994. Positive selection of lymphocytes. Cell 76:219-228. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda, K., M. Nagafuku, T. Shima, M. Okada, T. Yagi, T. Yamada, Y. Minaki, A. Kato, S. Tani-Ichi, T. Hamaoka, and A. Kosugi. 2002. Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 169:2813-2817. [DOI] [PubMed] [Google Scholar]