Abstract
The Vibrio cholerae ToxT regulon includes the genes encoding cholera toxin (CT) and the toxin-coregulated pilus (TCP), which are the major virulence factors required for causing cholera disease and colonizing the upper small intestine of the host, respectively. The genes encoding CT, ctxAB, and the genes encoding the components of the TCP, tcpA to tcpJ, are organized within operons, upstream of which are DNA binding sites for the transcriptional activator ToxT. ToxT is a member of the large AraC/XylS family of transcriptional regulators and also activates transcription of five other genes whose roles in V. cholerae pathogenesis, if any, are poorly understood. acfA and acfD are divergently transcribed genes required for efficient colonization of the intestine. Transcriptional activation of acfA and acfD requires a pair of central ToxT binding sites in an inverted-repeat configuration for ToxT-directed transcription of both genes. tcpI has an unknown role in pathogenesis. aldA and tagA are divergently transcribed genes that also have unknown roles in pathogenesis. In this study, we map the aldA and tagA promoters and identify the ToxT binding sites upstream of each gene. Our results suggest that two ToxT binding sites in an inverted-repeat configuration are required for ToxT-directed transcription of tagA and that a single ToxT binding site is required for ToxT-directed transcription of aldA. Furthermore, to direct transcription of tagA and aldA, ToxT uses independent binding regions upstream of each gene, in contrast to what we previously found for the divergently transcribed acfA and acfD genes, which share ToxT binding sites between the two genes.
The gram-negative, comma-shaped bacterium Vibrio cholerae is the causative agent of the severe diarrheal disease cholera. Cholera continues to be a significant problem in the developing world, where morbidity and mortality levels remain high. There have been seven cholera pandemics since 1817, and the seventh, current pandemic began in 1961 in Indonesia and later spread throughout Africa, South America, and parts of Europe. In 2003 alone, approximately 20,000 people are estimated to have died of cholera worldwide (47).
The two major virulence factors produced by V. cholerae are the cholera toxin (CT) and the toxin-coregulated pilus (TCP). Cholera disease is induced by the activity of the cholera toxin, which has an AB5 stoichiometry (9, 36). The five CT-B subunits are responsible for binding of CT to the host ganglioside-GM1 receptor in the upper small intestine, whereas the single CT-A subunit confers CT activity (18). The CT-A subunit catalyzes ADP-ribosylation of a Gα protein, resulting in elevated levels of cyclic AMP. This then results in secretion of chloride, followed by a massive outpouring of water into the intestinal lumen to produce the voluminous watery diarrhea, also known as rice water stool, characteristic of cholera (7). The genes encoding CT, ctxAB, are located within the genome of a lysogenic filamentous phage, CTXΦ (58). The toxin-coregulated pilus, so named because it was originally found to be produced under the same conditions as CT (46, 53, 54), is required for V. cholerae to colonize the upper small intestine. TCP is a type IV pilus, and the genes required for TCP biogenesis, tcpA to tcpJ, are located within a region of V. cholerae chromosome I having some phage-like properties, the vibrio pathogenicity island (VPI) (24, 25, 41, 46). TCP is also the receptor for CTXΦ (58).
Expression of V. cholerae virulence genes is subject to regulation by a cascade of transcription factors. The direct activator of transcription of the majority of V. cholerae virulence genes is the ToxT protein (5), which is a member of the large AraC/XylS family of transcriptional regulators (17). Expression of ToxT is extensively regulated. The membrane-localized transcriptional activators ToxR and TcpP, together with their respective cofactors, ToxS and TcpH, are required to activate transcription of the toxT gene by binding to a region upstream of the toxT promoter (14, 15, 32, 33). Because toxT is also located within the large tcpA operon (3, 16), once ToxT protein has been produced it is able to activate its own expression through a positive-feedback loop (61). Expression of the tcpPH operon is also subject to regulation by a pair of transcriptional activators, AphA and AphB (26-29, 52). The cyclic AMP receptor protein, CRP, and another protein, PepA, negatively regulate expression of tcpPH (2, 29). Finally, in some strains of V. cholerae expression of AphA is regulated by the HapR protein, which is controlled by the V. cholerae quorum-sensing system (23, 26, 30, 40, 63).
The ToxT protein is 276 amino acids in length, with the 100-amino-acid AraC/XylS family domain at its C-terminal end (17). The AraC/XylS domain contains two helix-turn-helix motifs and confers both DNA binding and transcriptional-activation properties (8, 38, 55). The function of the remaining 176 amino acids of ToxT, which presumably form a second domain, is unknown, and this domain has no homology to any other proteins when a BLAST search is performed with it alone. Possible roles for this second ToxT domain include multimerization of the protein and/or binding to effector molecules. There are no known effectors that are required for transcriptional activation by ToxT, although there is some evidence that bile may act as a negative effector (51).
In addition to its role as the direct activator of ctx and tcpA transcription, ToxT is known to activate transcription of five other genes whose roles in pathogenesis are poorly understood: acfA, acfD, tcpI, aldA, and tagA (43-46). All five of these genes are located within the VPI. The acfA and acfD genes, which are divergently transcribed, encode components of the accessory colonization factor (ACF), which is required for efficient intestinal colonization by V. cholerae in the infant mouse model system (46). How these genes function in colonization is unknown; acfA encodes a putative outer membrane protein and acfD encodes a putative lipoprotein (44, 45). ToxT binds to a pair of binding sites in an inverted-repeat configuration between acfA and acfD and activates transcription of both genes from this central location (59). tcpI encodes a putative methyl-accepting chemotaxis protein (MCP) (13). tcpI is divergently transcribed from the tcpPH operon, although these operons are relatively distant from one another and are controlled independently. The role of TcpI in pathogenesis, if any, is unknown. aldA and tagA are also divergently transcribed (43). aldA encodes an aldehyde dehydrogenase and tagA encodes a putative lipoprotein (12, 43, 45). Again, the roles of AldA and TagA in V. cholerae pathogenesis, if any, are unknown.
Previously identified ToxT binding sites upstream of tcpA, ctx, acfA, and acfD are consistent with two ToxT monomers binding upstream of each gene (21, 59, 62; J. H. Withey and V. J. DiRita, submitted for publication). AraC/XylS family members are able to bind DNA and activate transcription as both monomers (SoxS, Rob, and MarA) (11, 37) and dimers (AraC and RhaS) (6, 49, 50). The results of experiments on the ToxT binding sites between acfA and acfD, in which alteration of the spacing between the two sites by the insertion of 5 or 10 bp did not affect the ability of ToxT to footprint both binding sites, strongly suggest that ToxT monomers bind independently to the two binding sites (59).
The sequences of the ToxT binding sites that have been found upstream of tcpA, ctx, acfA, and acfD are quite degenerate, which made their identification not a trivial exercise. The major common element in all ToxT binding sites is a tract of four or more T nucleotides on one strand near the 5′ end of the binding site. Mutations to this T tract result in a site to which ToxT may still bind but from which it is unable to activate transcription (59). The 3′ portions of the binding sites have very little sequence conservation but exhibit a preference for A and T nucleotides. Mutations to these 3′ regions generally decrease but do not abrogate activation of transcription by ToxT. In addition to having a degenerate consensus binding sequence, ToxT also uses binding sites oriented in both direct- and inverted-repeat configurations to activate transcription. Upstream of tcpA, ToxT binds to a pair of binding sites in a direct-repeat conformation (J. H. Withey and V. J. DiRita, unpublished data); ToxT most likely binds to sites in a direct-repeat orientation upstream of ctx as well. However, between acfA and acfD, ToxT binds to a pair of sites in an inverted-repeat conformation (59). Both of these sites are required for activation by ToxT of both acfA and acfD transcription. Because the consensus ToxT binding site is so degenerate, it is impossible to identify other ToxT binding sites by a simple sequence search for the consensus. Such a search of only the VPI region of the large V. cholerae chromosome yields hundreds of potential ToxT binding sites, the vast majority of which are not used by ToxT.
This study focused on the identification of binding sites from which ToxT activates tagA and aldA. We located the start sites of transcription and identified the DNA binding sites from which ToxT activates transcription of each gene. Using a combination of nested lacZ fusions, directed mutagenesis, and copper-phenanthroline footprinting, we identified ToxT binding sites upstream of both tagA and aldA from which transcription is activated. Activation of tagA transcription by ToxT requires a pair of ToxT binding sites in an inverted-repeat conformation upstream of the core promoter elements. Activation of aldA transcription by ToxT requires only a single ToxT binding site upstream of the core promoter elements, an unexpected result given our knowledge of other ToxT-dependent promoters. Although tagA and aldA are divergently transcribed genes, the minimal DNA sequences from which ToxT activates transcription of tagA and aldA do not overlap, suggesting that these genes are controlled independently by ToxT.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains used in this study are listed in Table 1. Strains were grown at 37°C in Luria broth (LB) for overnight cultures and at 30°C in LB adjusted to a starting pH of 6.5 (inducing conditions) for use in β-galactosidase assays. Strains were maintained at −70°C in LB plus 20% glycerol. Antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1, and streptomycin, 100 μg ml−1. Plasmids were introduced into V. cholerae strains by electroporation using a Bio-Rad Escherichia coli pulser.
TABLE 1.
Strains used in this study
| Strain | Plasmid | Relevant genotype | Parent strain | Source or reference |
|---|---|---|---|---|
| O395 | Strr | Laboratory collection | ||
| VJ740 | ΔtoxT | O395 | 3a | |
| JW21 | pTL61T | Ampr | O395 | 59 |
| JW22 | pTL61T | Ampr | VJ740 | 59 |
| JW32 | pJW61 | tagA1::lacZ | O395 | This work |
| JW33 | pJW62 | tagA2::lacZ | O395 | This work |
| JW38 | pJW61 | tagA1::lacZ ΔtoxT | VJ740 | This work |
| JW39 | pJW62 | tagA2::lacZ ΔtoxT | VJ740 | This work |
| JW42 | pJW65 | aldA1::lacZ | O395 | This work |
| JW43 | pJW66 | aldA2::lacZ | O395 | This work |
| JW46 | pJW65 | aldA1::lacZ ΔtoxT | VJ740 | This work |
| JW47 | pJW66 | aldA2::lacZ ΔtoxT | VJ740 | This work |
| JW51 | pJW70 | tagA3::lacZ | O395 | This work |
| JW55 | pJW70 | tagA3::lacZ ΔtoxT | VJ740 | This work |
| JW65 | pJW76 | aldA3::lacZ | O395 | This work |
| JW67 | pJW76 | aldA3::lacZ ΔtoxT | VJ740 | This work |
| JW81 | pJW80 | tagA4::lacZ | O395 | This work |
| JW84 | pJW80 | tagA4::lacZ ΔtoxT | VJ740 | This work |
| JW87 | pJW82 | aldA4::lacZ | O395 | This work |
| JW92 | pJW82 | aldA4::lacZ ΔtoxT | VJ740 | This work |
| JW97 | pJW89 | tagA5::lacZ | O395 | This work |
| JW98 | pJW90 | tagA11::lacZ | O395 | This work |
| JW100 | pJW92 | aldA11::lacZ | O395 | This work |
| JW101 | pJW93 | aldA12::lacZ | O395 | This work |
| JW102 | pJW94 | aldA13::lacZ | O395 | This work |
| JW103 | pJW95 | aldA14::lacZ | O395 | This work |
| JW105 | pJW89 | tagA5::lacZ ΔtoxT | VJ740 | This work |
| JW106 | pJW90 | tagA11::lacZ ΔtoxT | VJ740 | This work |
| JW108 | pJW92 | aldA11::lacZ ΔtoxT | VJ740 | This work |
| JW109 | pJW93 | aldD12::lacZ ΔtoxT | VJ740 | This work |
| JW110 | pJW94 | aldD13::lacZ ΔtoxT | VJ740 | This work |
| JW114 | pJW99 | tagA13::lacZ | O395 | This work |
| JW120 | pJW99 | tagA13::lacZ ΔtoxT | VJ740 | This work |
| JW139 | pJW106 | aldA14::lacZ | O395 | This work |
| JW140 | pJW107 | tagA14::lacZ | O395 | This work |
| JW145 | pJW106 | aldA14::lacZ ΔtoxT | VJ740 | This work |
| JW146 | pJW107 | tagA14::lacZ ΔtoxT | VJ740 | This work |
| JW155 | pJW114 | tagA12::lacZ | O395 | This work |
| JW160 | pJW114 | tagA12::lacZ ΔtoxT | VJ740 | This work |
| JW234 | pJW131 | tagA15::lacZ | O395 | This work |
| JW243 | pJW131 | tagA15::lacZ ΔtoxT | VJ740 | This work |
DNA manipulations.
Plasmids were purified using QIAGEN Spin Miniprep or Plasmid Midi kits. PCR was performed using Taq DNA polymerase from Roche as specified by the manufacturer. Restriction enzymes were purchased from New England Biolabs and used as specified by the manufacturer.
Plasmid construction.
The aldA and tagA nested lacZ fusions were constructed by PCR of the appropriate region, using fresh O395 colonies as the template. PCR products were cloned between the XbaI and HindIII sites of pTL61T (34). Site-directed mutations were created using the splicing by overlap extension technique (19, 20), after which inserts having the desired mutations were cloned between the XbaI and HindIII sites of pTL61T. The nucleotide sequences of all plasmid constructs were confirmed by DNA sequencing at the University of Michigan Sequencing Core.
Primer extensions.
Transcription start sites were mapped by primer extension as previously described (48). Primers were designed to hybridize approximately 50 bp downstream of the translational start site. Whole-cell RNA was purified using TRIzol reagent (Invitrogen) according to the manufacturer's directions. Bacteria were grown at 30°C for 5 h in LB at pH 6.5 prior to RNA purification. RNA was purified from four strains: for mapping aldA, strains JW42 (wild-type [WT] toxT, plasmid aldA::lacZ) and JW46 (ΔtoxT, plasmid aldA::lacZ) were used, and for mapping tagA, strains JW33 (WT toxT, plasmid tagA::lacZ) and JW46 (ΔtoxT, plasmid tagA::lacZ) were used (see Fig. 1). Superscript II reverse transcriptase (Invitrogen) was used to elongate primers end labeled with 32P.
FIG. 1.
Primer extensions to determine the start sites of transcription for tagA and aldA. Primers were designed to hybridize approximately 50 bp downstream of the translational start site. Experiments used total bacterial RNA from V. cholerae grown under ToxT-inducing conditions. Arrows indicate the band at the position from which transcription is initiated. The DNA sequencing ladders on the left sides of the figures were produced using the same primers with DNA from wild-type V. cholerae colonies as the template. (A) Primer extension of aldA. (B) Primer extension of tagA. The plus sign indicates that RNA was purified from V. cholerae having wild-type toxT, and the minus sign indicates that RNA was purified from V. cholerae having a toxT deletion. The dashed arrow and bracket indicate the bands produced by transcriptional stuttering as described in the text.
β-Galactosidase assays.
For β-galactosidase assays, V. cholerae strains were grown overnight at 37°C, subcultured at a 1:40 dilution into fresh LB, pH 6.5, and grown for 3 h at 30°C. Bacteria were then placed on ice, and chloramphenicol was added to 0.5 mg ml−1. Assays were performed according to the method of Miller (39).
Copper-phenanthroline footprinting.
An electrophoretic mobility shift assay (EMSA) was performed as previously described (59). The amount of H6-ToxT (a purified form of ToxT having six histidines added to its N terminus) used was determined empirically to be the amount required to shift approximately 50% of the labeled DNA. Plasmids used in the β-galactosidase assays were used as PCR templates as indicated in the text and Table 1. After EMSA, the procedure used was that of Papavassiliou (42), as previously described (59). Briefly, the gel was soaked in 200 ml 10 mM Tris-HCl, pH 8, in a glass tray. One milliliter 40 mM 1,10-phenanthroline (Sigma) was mixed with 1 ml 9 mM CuSO4 (Sigma) for 1 min and then diluted with 18 ml distilled H2O. This was added to the gel tray and mixed by shaking. A 1:200 dilution of 3-mercaptopropionic acid (20 ml; Sigma) was then added to the gel tray and briefly mixed. The reaction continued for 7 min and was stopped by addition of 20 ml 28 mM neocuproine (Sigma), followed by shaking for 2 min. The gel was rinsed with 1,000 ml distilled H2O and placed on X-ray film for 3 h. After the film was developed, bands corresponding to free DNA and H6-ToxT/DNA complexes were excised from the gel based on their locations in the film. The gel slices were crushed, and the DNA was eluted overnight in 0.5 ml 0.5 M ammonium acetate, pH 7.5, 1 mM EDTA, 0.1% sodium dodecyl sulfate, 10 mM MgCl2. Gel pieces were pelleted by centrifugation, and the supernatant was passed through a 0.2-μm syringe filter and ethanol precipitated. The pellets were resuspended in a 1:1 mixture of Tris-EDTA:sequencing stop solution (USB), and radioactivity was measured with a Geiger counter. Approximately equal amounts of labeled DNA from the free DNA and H6-ToxT/DNA complex bands were loaded on the subsequent sequencing gel. The sequencing ladder was produced with a Thermo Sequenase radiolabeled terminator cycle sequencing kit (USB) as specified by the manufacturer, and the sequencing gel was prepared and run as specified by the sequencing-kit manual. The same plasmid template and the primer that was end labeled in the EMSA/footprinting experiment were used in the sequencing reactions. Autoradiography was performed with the resulting gel, and typical exposure times were 5 to 10 days.
RESULTS
Determination of the start sites of tagA and aldA transcription.
We began our efforts to map the regulatory regions of tagA and aldA by determining the nucleotides at which transcription is initiated. This was done using primer extension experiments. Oligonucleotide primers were designed to hybridize approximately 50 nucleotides (nt) downstream of the translational start sites of tagA and aldA. The primer extensions were performed on total cell RNA preparations isolated from bacteria grown under conditions known to induce expression of genes in the ToxT regulon. RNA was isolated from both strains O395 and VJ740 carrying no plasmids and strains JW33 and JW39 (for tagA) and JW42 and JW46 (for aldA), which carry fusions of the respective gene to lacZ on a plasmid, as discussed below. Identical primer extension results were observed from mRNAs produced from either the chromosomal copy (data not shown) or the plasmid copy of either gene, although the bands produced using the plasmid-based genes were significantly more intense (Fig. 1). A single band was observed using primers for aldA (Fig. 1A). However, multiple bands were observed using primers for tagA (Fig. 1B). This may be due to RNA polymerase (RNAP) slippage or stuttering at a tract of T nucleotides on the template strand 4 nt downstream from the site we propose as the primary tagA transcriptional start (Fig. 2). Transcriptional slippage would lead to insertion of a random number of additional A nucleotides before RNAP resumes normal elongation, resulting in the ladder of bands observed just above the band corresponding to the start site in Fig. 1B. Stuttering or transcriptional slippage, which leads to incorporation of nontemplated nucleotide tracts within the mRNA, has been observed in several other instances when there is a mononucleotide tract in the template strand at or near the start site of transcription (1, 4, 10, 22, 35, 56, 57, 60). Because the added nucleotides in this case would be upstream of the tagA translational start site, stuttering would affect neither translation nor the reading frame of tagA. Our assignment of the true tagA start site is based primarily on the observation that a near-consensus −10 element is present at the appropriate position relative only to this nucleotide and on the fact that the band in Fig. 1B corresponding to this nucleotide is the highest in the gel aside from the stuttering ladder above it. Our goal with the series of experiments described here was to map the ToxT binding sites responsible for activating tagA, so the apparent transcriptional slippage phenomenon we observed was not examined further.
FIG. 2.
Sequence of the tagA-aldA intergenic region. Both strands of the DNA are shown, with landmarks for tagA on the top strand and landmarks for aldA on the bottom strand. The start sites of transcription are shown by an arrow on the top strand for tagA and an arrow on the bottom strand for aldA. The putative core −35 and −10 elements for each promoter are indicated by boxes. The putative ToxT binding sites upstream of each gene are indicated by arrows between the top- and bottom-strand sequences. The end points of the tagA3 and aldA3 constructs are also shown. The positions of mutations are indicated by arrows pointing from the mutant designation to the mutated base pairs. Each of the mutations changes an AT base pair to a GC base pair or vice versa. The bracket below and to the right of the tagA start site indicates the poly-T tract on which transcriptional slippage likely occurs. The dots below the sequence indicate 10-bp distances from the start site of aldA transcription, and the dots above the sequence indicate 10-bp distances from the start site of tagA transcription.
The putative core promoter −10 and −35 elements were identified based on the locations of the start sites of transcription for aldA and as noted above for tagA (Fig. 2). There is a near-consensus −10 box (TATAAT) found upstream of both genes. However, both genes have a degenerate putative −35 box (TTGACA) at the appropriate spacing relative to the −10 element. We previously identified degenerate −35 boxes upstream of acfA, acfD, and tcpA (59, 62), suggesting that this may be common among genes in the ToxT regulon.
Identification of the minimal tagA promoter region.
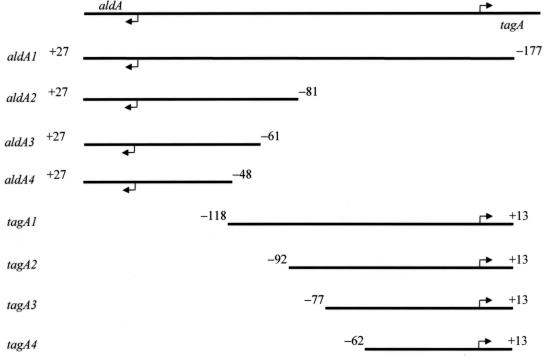
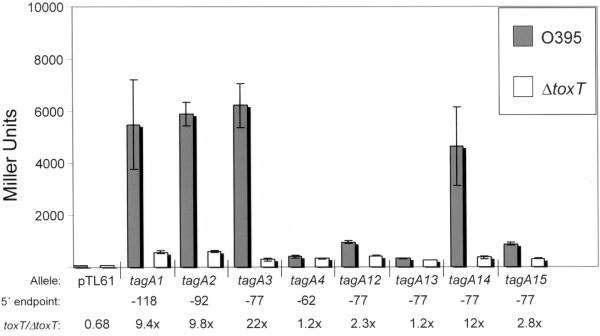
To determine the length of DNA upstream of the core promoter elements of tagA that is required for ToxT-directed transcription, we constructed nested tagA::lacZ fusions in which the 5′ endpoint of DNA upstream from tagA varies from −118 to −62 relative to the start site of transcription (Fig. 3). These fusions were constructed in plasmid pTL61T, which carries a promoterless lacZ gene downstream from multiple restriction sites. The ability of ToxT to activate transcription of these tagA::lacZ constructs was assessed by measuring β-galactosidase activity in V. cholerae strains having either wild-type toxT or a toxT deletion. Constructs extending to −118, −92, and −77 exhibited similar levels of ToxT-directed transcription (Fig. 4). However, a construct extending to −62 did not exhibit significant ToxT-directed transcription. This suggests that the sequence between −77 and −62 contains sequence elements that are essential for activation of tagA transcription by ToxT.
FIG. 3.
Map of the nested lacZ fusion constructs. The black line at the top of the figure represents the entire aldA-tagA intergenic region. Arrows indicate transcriptional start sites. The fusion construct names are listed on the left side, and the size of each construct is represented by the length of the black line. The numbers on either side of the black line indicate the length of DNA present in the construct relative to the transcriptional start site.
FIG. 4.
Results of β-galactosidase assays with strains carrying tagA::lacZ fusions. The results of experiments done with V. cholerae strains having wild-type ToxT (O395) are represented as gray bars, and the results of experiments done with V. cholerae strains having a ToxT deletion (VJ740) are represented as white bars. “Allele” indicates which tagA::lacZ fusion construct was used in the experiment, except for pTL61T, in which the empty vector was present in the indicated strains; “5′ endpoint” indicates the length of DNA upstream of the transcriptional start site that is present in the construct; and “toxT/ΔtoxT” indicates the difference (n-fold) between the mean β-galactosidase values for that construct measured with O395 and measured with VJ740. β-Galactosidase assays were performed a minimum of three times with each strain, and the values shown are the means ± standard deviations.
Mutational analysis of the tagA promoter region.
Previous work suggested that ToxT binds to a 13-bp DNA sequence having a tract of 4 or more consecutive T nucleotides on one strand, followed by a sequence rich in A and T nucleotides (59). The region upstream of tagA between the −35 box and −77, the 5′ endpoint of the minimal ToxT-activated tagA3 construct, contains two T tracts that would be consistent with this ToxT consensus sequence. We targeted these tracts for mutagenesis, along with some other A/T-rich sequences in this region, to examine the DNA sequence requirements for ToxT-directed transcription of tagA. Site-directed mutations were created in the tagA3 construct, which extends to −77 relative to the start of transcription. Each of the mutations changes an AT base pair to a GC base pair.
Mutations to the base pairs at −49 and −51 (tagA13), in the midst of a tract of 7 T nucleotides (Fig. 2, bottom strand), caused the largest defect in ToxT-directed transcription of tagA (Fig. 4). The tagA13 construct showed no significant difference in β-galactosidase activity between strains having wild-type toxT and strains having a toxT deletion. Mutations to the base pairs at −75 and −76 (tagA12), in the midst of a tract of 4 T nucleotides (Fig. 2, top strand), also caused a large defect in ToxT-directed transcription, reducing the difference in β-galactosidase activity between toxT and ΔtoxT strains to 2.3-fold, versus the 22-fold difference seen with the parent tagA3 construct (Fig. 4). These results are consistent with a requirement for two ToxT binding sites upstream of tagA for transcriptional activation. The observation that the T tracts in these two putative binding sites are on opposite strands of the DNA suggests that these sites are arranged in an inverted configuration, similar to what we found previously between acfA and acfD (59). Mutations in the 3′ portion of the promoter-distal ToxT activation site upstream of tagA, at −69 and −70 (tagA15), caused a large reduction in the ability of ToxT to activate transcription. However, mutations located between the two putative ToxT binding sites (Fig. 2), at −63 and −64 (tagA14), had no significant effect on ToxT-directed transcription of tagA.
Identification of the minimal aldA promoter region.
We used a method similar to that described above for tagA to determine the length of DNA upstream of the core promoter elements of aldA that is required for ToxT-directed transcription. Nested lacZ fusions were constructed in pTL61T, and β-galactosidase levels for strains carrying these fusions and having either wild-type toxT or a deletion in toxT were assayed. This series extended from −177 bp upstream to −48 bp upstream of the transcription start site (Fig. 3). Compared to the levels of induction of other ToxT-activated genes, those conferred by ToxT to the aldA::lacZ fusions were relatively low. Constructs extending to −177, −81, and −61 all had similar levels of expression in the wild-type toxT background, with values in the range of 1,200 to 1,600 Miller units (Fig. 5). For other ToxT-activated genes, the range is from 5,000 to over 20,000 Miller units. Significantly, however, the aldA::lacZ construct extending to −48 expressed β-galactosidase at levels markedly lower than those expressed in the longer constructs and exhibited little to no ToxT-directed transcription.
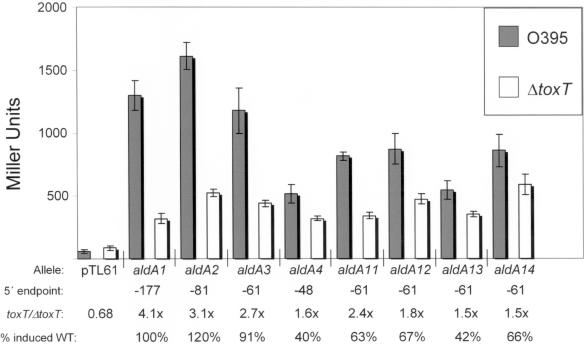
FIG. 5.
Results of β-galactosidase assays with strains carrying aldA::lacZ fusions. The results of experiments done with V. cholerae strains having wild-type ToxT (O395) are represented as gray bars, and the results of experiments done with V. cholerae strains having a ToxT deletion (VJ740) are represented as white bars. “Allele,” “5′ endpoint,” and “toxT/ΔtoxT” are defined in the legend for Fig. 4. “% induced WT” was calculated by dividing the mean β-galactosidase level for the indicated construct by the mean β-galactosidase level from the aldA1 construct which contains the entire tagA-aldA intergenic region. β-Galactosidase assays were performed a minimum of three times with each strain, and the values shown are the means ± standard deviations.
Comparison of these nested constructs in terms of the level of induction (n-fold) by ToxT, as we have done previously, is complicated by the observation that the basal levels of expression in the ΔtoxT background varied between different aldA::lacZ fusion constructs. While this is commonly observed for other lacZ fusion constructs as well, including those we describe here for tagA, because the relative levels of expression of the aldA::lacZ constructs in wild-type toxT strains are lower than those of tagA::lacZ constructs, small differences in measurements of basal activity of the aldA::lacZ constructs in the ΔtoxT strains have a large effect on the calculation of induction due to ToxT activity. For example, the −61 construct (aldA3) produced 91% of the β-galactosidase activity of the longest (−177) construct (aldA1) in strains having wild-type toxT. However, the differences (n-fold) between the wild-type toxT and ΔtoxT strains carrying these constructs were 2.7-fold and 4.1-fold, respectively. Therefore, we believe it is most useful to assess these constructs as percentages of the ToxT-induced expression observed for the full-length, −177 construct (aldA1) (Fig. 5). Using this assessment, it is clear that the −48 construct, aldA4, has a defect in ToxT-directed transcription, whereas the −177, −81, and −61 constructs have similar levels of expression. This suggests that the DNA sequence between −48 and −61 is important for ToxT-directed transcription of aldA.
Mutational analysis of the aldA promoter region.
We targeted A/T-rich sequences upstream of aldA for mutagenesis to determine whether any of these sequences are important for ToxT-directed transcription of aldA. Mutations were constructed in the minimal ToxT-directed aldA::lacZ construct, aldA3, which extends to −61 relative to the start of transcription. Each of the mutations changes an AT base pair to a GC base pair or vice versa.
The sequence between the −35 box and −61 upstream of aldA has three tracts of 3 or more consecutive T nucleotides, and we mutated each of these. Mutations at −52 and −54 (aldA13) had the strongest negative effect, resulting in a ToxT-directed transcription level of only 42% of the wild-type level. This is very similar to the level seen for the truncated construct extending to −48 (aldA4). The other mutations each resulted in a minimal decrease in ToxT-directed transcription to around 65% of that observed with the wild-type −177 construct. The observations that (i) the length of the minimal aldA::lacZ fusion exhibiting ToxT-directed transcription is much shorter than that which we have observed for other genes in the ToxT regulon and (ii) that mutations to only one T tract caused a drastic decrease in the ability of ToxT to activate transcription of aldA suggest that a single ToxT binding site may be located upstream of aldA, in contrast to the other ToxT-activated operons we have studied.
Copper-phenanthroline footprinting of the aldA-tagA intergenic region.
The genetic experiments described above allowed us to determine regions between tagA and aldA that are required for ToxT-directed transcription. To more precisely identify the DNA sequence bound by ToxT, we used DNA footprinting. The copper-1,10-phenanthroline footprinting procedure was used because of its superior resolution and relative lack of sequence and structural cleavage specificity relative to DNase I (42, 59). We used a purified form of ToxT having six histidines added to its N terminus (H6-ToxT) for these experiments; previous work showed that H6-ToxT is functional both in vivo and in vitro (59, 62).
Three regions of protection were conferred by H6-ToxT within the tagA-aldA intergenic region. The first and second of these protected regions were proximal to tagA (Fig. 6A); DNA segments between −45 and −57 and between −79 and −74 relative to the start of tagA transcription were protected by H6-ToxT. These footprints encompass both of the T tracts to which directed mutagenesis produced the strongest negative effect on ToxT-directed transcription of tagA. The third protected region encompassed the T tract between −50 and −56 relative to the start of aldA transcription (Fig. 6B). Again, this was the region to which directed mutagenesis produced the strongest negative effect on ToxT-directed transcription of aldA. The sequence between the putative tagA and aldA ToxT binding sites was not significantly protected by H6-ToxT (not shown).
FIG. 6.
Copper-phenanthroline footprint of H6-ToxT in the tagA-aldD intergenic region. −, DNA cleaved by copper-phenanthroline in the absence of H6-ToxT; +, H6-ToxT/DNA complexes cleaved by copper-phenanthroline. The numbers to the right of the autoradiographs indicate the distances upstream of the transcriptional start sites of tagA and aldD. The −35 core promoter elements are represented by empty boxes. (A) Footprinting of H6-ToxT on the minimal tagA3 construct. The vertical arrows indicate the positions of the putative binding sites, and the heavy black lines indicate the regions of protection. (B) Footprinting of H6-ToxT proximal to aldA alone. The vertical arrow indicates the position of the binding site, and the heavy black line indicates the region of protection.
Our assignment of the binding-site sequences (indicated by the arrows in Fig. 2 and 6) is based on both the genetic data described in this report and our studies of ToxT binding sites upstream of acfA, acfD, and tcpA that indicate ToxT binds to a 13-bp sequence with a T tract near the 5′ end (59; J. H. Withey and V. J. DiRita, submitted). The 5′ regions of both tagA binding sites, containing the T tracts, exhibited the highest levels of protection. However, there is a significant disparity in the degrees of protection conferred by H6-ToxT to the tagA-proximal and tagA-distal binding sites, particularly in the 3′ portions of the binding sites; the tagA-proximal binding site is strongly protected along its length, whereas the tagA-distal binding site is more weakly protected within the T tract, and little to no protection is observed for the 3′ portion of this binding site. This result is consistent with our observation that mutations to the T tract in the tagA-proximal binding site at −55 to −49 resulted in a complete loss of activation by ToxT (tagA13 mutant [Fig. 4]), whereas mutations to the T tract in the tagA-distal binding site at −76 to −79 resulted in a large decrease, but not a complete loss, of activation by ToxT (tagA12 mutant [Fig. 4]). Significant protection by H6-ToxT was not observed for the sequence between the two tagA ToxT binding sites in this experiment.
DISCUSSION
The experiments in this report were designed to identify the DNA binding sites from which ToxT activates transcription of the divergent tagA and aldA genes, which were previously shown to be within the ToxT regulon (5, 12, 43, 45, 46) but which have unknown roles, if any, in V. cholerae pathogenesis. Earlier work by Parsot and Mekalanos (43) had roughly mapped the regulatory region for tagA to a region within about 400 bp upstream of the translational start site. Using nested lacZ fusions, we identified the minimal DNA sequences upstream of both tagA and aldA required for ToxT-directed transcription of the respective genes. Unlike what we previously found for the similarly divergent ToxT-activated genes acfA and acfD, the minimal ToxT-directed tagA and aldA constructs do not overlap (Fig. 3); there are 24 bp between the endpoints of tagA3 and aldA3, the minimal constructs for each gene that permit ToxT-directed transcription (Fig. 2). This finding suggests that tagA and aldA have independent ToxT binding regions from which transcription is activated.
Mutational analysis of the A/T-rich sequences upstream of tagA gave us further information about the DNA sequence requirements for its ToxT-directed transcription. Two T tracts upstream of tagA are required for ToxT-directed transcription of tagA. Mutations in the promoter-proximal T tract (tagA13) completely abrogated transcriptional activation by ToxT, whereas mutations in the promoter-distal T tract (tagA12) drastically reduced transcriptional activation by ToxT. The observation that these two T tracts are on opposite DNA strands suggests that there are two putative ToxT binding sites upstream of tagA arranged as an inverted repeat. This is similar to what we observed for the ToxT sites between acfA and acfD (59). A notable difference between the tagA ToxT sites and the acfA to acfD ToxT sites is their spacing relative to each other; there are 2 bp between the two acfA to acfD ToxT sites and 9 bp between the two tagA ToxT sites (Fig. 2). One likely explanation for this difference is that the two ToxT sites located between acfA and acfD are used to activate transcription of both genes (59), whereas the two ToxT sites upstream of tagA are used only to activate tagA transcription. Therefore, there are likely different requirements for optimal spacing of the sites for each individual site and relative to the core promoter elements in these different situations.
Copper-phenanthroline footprinting of H6-ToxT in the tagA region further localized the ToxT binding sites upstream of tagA. The two T tracts we identified as most critical for transcriptional activation by ToxT were in the regions of greatest protection by H6-ToxT. However, greater protection was conferred by H6-ToxT to the tagA-proximal binding site than to the tagA-distal binding site (Fig. 6A). This finding is consistent with the results of the lacZ fusion experiments with mutated binding sites, which indicated that mutagenesis to the tagA-proximal binding site had a larger negative effect on ToxT-directed transcription of tagA than did mutagenesis to the tagA-distal binding site. Perhaps binding by ToxT to the tagA-distal binding site is more dependent on nonspecific interactions with the phosphate DNA backbone or the likely interaction between ToxT and the α-C-terminal domain of RNAP provides more binding energy at this position.
Mutational analysis of the A/T-rich sequences upstream of aldA identified a 7-nt T tract as important for ToxT-directed transcription of aldA. The results of the aldA experiments are significantly more difficult to interpret due to the relatively low level of induction conferred by ToxT even from a construct carrying the entire tagA-aldA intergenic region. This level of induction is low only compared to other ToxT-activated promoters, however; over 1,200 Miller units of β-galactosidase is considerable activity. Because the levels of expression of the truncated aldA4 construct, which presumably lacks a ToxT binding site, and the mutated aldA13 construct, which has a pair of mutations in the 7-nt T tract, are very similar, it is safe to say that the aldA13 mutations cause as large a defect in ToxT-directed transcription as we are likely to see using this promoter construct. The other aldA::lacZ mutant constructs decreased but did not abolish ToxT-directed transcription of aldA. This is similar to what we have observed for mutations in other ToxT binding sites outside of the conserved T tract. We also observed protection conferred by H6-ToxT to the 7-nt aldA T tract in footprinting experiments (Fig. 6B), confirming the position of ToxT in the aldA promoter region, but did not observe significant protection outside of the T tract.
The most notable difference between the region upstream of aldA that we found to be required for ToxT-directed transcription and the regions upstream of other operons in the ToxT regulon that we have studied is the relative shortness of the minimal aldA DNA sequence from which ToxT-directed transcription occurs (aldA3 [Fig. 2 and 3]). Based on our observations of the requirements for ToxT-directed transcription of acfA, acfD (59), tagA, tcpA (J. H. Withey and V. J. DiRita, submitted), and tcpI (J. H. Withey and V. J. DiRita, submitted), there is not sufficient DNA sequence between the −61 endpoint of the ald3 construct and the −35 box in which to fit two ToxT binding sites. Furthermore, there is only a single T tract at an appropriate position in this region to which mutagenesis produces a dramatic decrease in ToxT-directed transcription. Therefore, we propose that there is a single ToxT binding site upstream of aldA. This would be the first ToxT-activated gene that has been found to use only one binding site; this also suggests that ToxT acts as a monomer at aldA. We have previously observed that ToxT binds to DNA as monomers to both of the binding sites between acfA and acfD (59) and to both of the binding sites upstream of tcpA (J. H. Withey and V. J. DiRita, submitted). The putative single ToxT site at aldA is however different in its orientation relative to the promoter from other promoter-proximal ToxT binding sites we have identified. The promoter-proximal binding sites at the acfA, acfD, tagA, tcpA, and tcpI promoters are all oriented so that the conserved T tract is on the template strand, and thus the binding site “points away” from the promoter (Fig. 2). The aldA ToxT site has the conserved T tract on the nontemplate DNA strand, and so the binding site “points toward” the promoter (Fig. 2). However, the aldA ToxT site is located at a position similar to the promoter-proximal ToxT sites of the other genes, and the sequence of the aldA ToxT site fits with the consensus ToxT binding sequence we have proposed based on ToxT sites upstream of other genes (Fig. 7). The difference in orientation could potentially explain why the level of aldA induction conferred by ToxT is low relative to the level of induction conferred by ToxT at other promoters.
FIG. 7.
Alignment of ToxT binding sites. “Gene” indicates the locus upstream of which the ToxT binding sites were identified. “Sequence” indicates the sequence of the ToxT binding site. “Repeat” indicates the orientation of the binding sites relative to each other except for the aldA binding site. “Spacing” indicates the number of base pairs between the two ToxT binding sites, except for aldA, for which “NA” indicates not applicable. “Promoter proximity” indicates the promoter-proximal end of the nearest ToxT binding site relative to the start site of transcription. “W” in the consensus sequence represents either an A or a T nucleotide.
Our observation that the divergent aldA and tagA genes are controlled independently by ToxT adds to the question of what the roles these genes have as members of the ToxT regulon. There are several operons within the VPI that are oriented divergently, including acfA-acfD and tcpI-tcpPH. As we have shown previously, transcription of acfA and acfD is activated by ToxT from a central location between the genes (59), and presumably acfA and acfD expression levels are coregulated. Because both of these genes encode components of ACF, it is logical that their expression would be coordinated. tcpI and tcpPH, on the other hand, have separate control regions and a relatively large intergenic distance, and their transcription levels are controlled by different transcriptional regulators (2, 26-29, 31, 52). Because TcpP and TcpH are required for expression of toxT and ToxT activates expression of tcpI, these genes would have no obvious reason to be coordinately regulated. This suggests that the independent regulation by ToxT of aldA and tagA may be due to TagA and AldA proteins having different roles in either cell physiology or pathogenesis and that their expression levels may differ under different ToxT levels. Because the ToxT-directed expression of aldA is relatively low and aldA uses only a single ToxT binding site, high concentrations of ToxT may be required for full induction of aldA. Conversely, because tagA is activated to a relatively high degree by ToxT and uses two ToxT binding sites, lower concentrations of ToxT may allow full induction of tagA. Further work on the roles of these gene products in V. cholerae biology should address whether this hypothesis is sound.
In summary, we have identified the ToxT binding sites upstream of tagA and aldA from which ToxT activates transcription of these genes. tagA utilizes two ToxT binding sites in an inverted-repeat conformation, whereas aldA utilizes a single ToxT binding site. The sequences of these newly identified binding sites are in accordance with the ToxT consensus binding sequence we have previously proposed (59). The minimal constructs from which ToxT is able to activate tagA and aldA transcription do not overlap, suggesting that ToxT controls expression of tagA and aldA independently.
Acknowledgments
This work was supported by grant AI31645 (to V.J.D.) from the National Institutes of Health (NIH). J.H.W. was supported by a Kirschstein National Research Service Award (1 F32 AI51074) from NIH.
REFERENCES
- 1.Baranov, P. V., A. W. Hammer, J. Zhou, R. F. Gesteland, and J. F. Atkins. 2005. Transcriptional slippage in bacteria: distribution in sequenced genomes and utilization in IS element gene expression. Genome Biol. 6:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behari, J., L. Stagon, and S. B. Calderwood. 2001. pepA, a gene mediating pH regulation of virulence genes in Vibrio cholerae. J. Bacteriol. 183:178-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, R. C., and R. K. Taylor. 1995. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol. Microbiol. 16:425-439. [DOI] [PubMed] [Google Scholar]
- 3a.Champion, G. A., M. N. Neely, M. A. Breenan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, Y., S. M. Dylla, and C. L. Turnbough, Jr. 2001. A long T · A tract in the upp initially transcribed region is required for regulation of upp expression by UTP-dependent reiterative transcription in Escherichia coli. J. Bacteriol. 183:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan, S. M., and R. F. Schleif. 1994. DNA-dependent renaturation of an insoluble DNA binding protein. Identification of the RhaS binding site at rhaBAD. J. Mol. Biol. 243:821-829. [DOI] [PubMed] [Google Scholar]
- 7.Field, M., D. Fromm, Q. al-Awqati, and W. B. Greenough III. 1972. Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J. Clin. Investig. 51:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallegos, M. T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. Arac/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill, D. M. 1976. The arrangement of subunits in cholera toxin. Biochemistry 15:1242-1248. [DOI] [PubMed] [Google Scholar]
- 10.Gott, J. M., and R. B. Emeson. 2000. Functions and mechanisms of RNA editing. Annu. Rev. Genet. 34:499-531. [DOI] [PubMed] [Google Scholar]
- 11.Griffith, K. L., and R. E. Wolf, Jr. 2001. Systematic mutagenesis of the DNA binding sites for SoxS in the Escherichia coli zwf and fpr promoters: identifying nucleotides required for DNA binding and transcription activation. Mol. Microbiol. 40:1141-1154. [DOI] [PubMed] [Google Scholar]
- 12.Harkey, C. W., K. D. Everiss, and K. M. Peterson. 1995. Isolation and characterization of a Vibrio cholerae gene (tagA) that encodes a ToxR-regulated lipoprotein. Gene 153:81-84. [DOI] [PubMed] [Google Scholar]
- 13.Harkey, C. W., K. D. Everiss, and K. M. Peterson. 1994. The Vibrio cholerae toxin-coregulated-pilus gene tcpI encodes a homolog of methyl-accepting chemotaxis proteins. Infect. Immun. 62:2669-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häse, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins, D. E., and V. J. DiRita. 1996. Genetic analysis of the interaction between Vibrio cholerae transcription activator ToxR and toxT promoter DNA. J. Bacteriol. 178:1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins, D. E., and V. J. DiRita. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol. Microbiol. 14:17-29. [DOI] [PubMed] [Google Scholar]
- 17.Higgins, D. E., E. Nazareno, and V. J. DiRita. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174:6974-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmgren, J., I. Lonnroth, and L. Svennerholm. 1973. Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand. J. Infect. Dis. 5:77-78. [DOI] [PubMed] [Google Scholar]
- 19.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 20.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 21.Hulbert, R. R., and R. K. Taylor. 2002. Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J. Bacteriol. 184:5533-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, D. J. 1994. Slippage synthesis at the galP2 promoter of Escherichia coli and its regulation by UTP concentration and cAMP · cAMP receptor protein. J. Biol. Chem. 269:17221-17227. [PubMed] [Google Scholar]
- 23.Jobling, M. G., and R. K. Holmes. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023-1034. [DOI] [PubMed] [Google Scholar]
- 24.Karaolis, D. K., J. A. Johnson, C. C. Bailey, E. C. Boedeker, J. B. Kaper, and P. R. Reeves. 1998. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc. Natl. Acad. Sci. USA 95:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman, M. R., C. E. Shaw, I. D. Jones, and R. K. Taylor. 1993. Biogenesis and regulation of the Vibrio cholerae toxin-coregulated pilus: analogies to other virulence factor secretory systems. Gene 126:43-49. [DOI] [PubMed] [Google Scholar]
- 26.Kovacikova, G., W. Lin, and K. Skorupski. 2003. The virulence activator AphA links quorum sensing to pathogenesis and physiology in Vibrio cholerae by repressing the expression of a penicillin amidase gene on the small chromosome. J. Bacteriol. 185:4825-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacikova, G., and K. Skorupski. 2002. Binding site requirements of the virulence gene regulator AphB: differential affinities for the Vibrio cholerae classical and El Tor tcpPH promoters. Mol. Microbiol. 44:533-547. [DOI] [PubMed] [Google Scholar]
- 28.Kovacikova, G., and K. Skorupski. 2000. Differential activation of the tcpPH promoter by AphB determines biotype specificity of virulence gene expression in Vibrio cholerae. J. Bacteriol. 182:3228-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacikova, G., and K. Skorupski. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41:393-407. [DOI] [PubMed] [Google Scholar]
- 30.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1147. [DOI] [PubMed] [Google Scholar]
- 31.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krukonis, E. S., and V. J. DiRita. 2003. DNA binding and ToxR responsiveness by the wing domain of TcpP, an activator of virulence gene expression in Vibrio cholerae. Mol. Cell 12:157-165. [DOI] [PubMed] [Google Scholar]
- 33.Krukonis, E. S., R. R. Yu, and V. J. Dirita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67-84. [DOI] [PubMed] [Google Scholar]
- 34.Linn, T., and R. St. Pierre. 1990. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J. Bacteriol. 172:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, C., L. S. Heath, and C. L. Turnbough, Jr. 1994. Regulation of pyrBI operon expression in Escherichia coli by UTP-sensitive reiterative RNA synthesis during transcriptional initiation. Genes Dev. 8:2904-2912. [DOI] [PubMed] [Google Scholar]
- 36.Lonnroth, I., and J. Holmgren. 1973. Subunit structure of cholera toxin. J. Gen. Microbiol. 76:417-427. [DOI] [PubMed] [Google Scholar]
- 37.Martin, R. G., W. K. Gillette, S. Rhee, and J. L. Rosner. 1999. Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol. Microbiol. 34:431-441. [DOI] [PubMed] [Google Scholar]
- 38.Martin, R. G., and J. L. Rosner. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132-137. [DOI] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 41.Ogierman, M. A., S. Zabihi, L. Mourtzios, and P. A. Manning. 1993. Genetic organization and sequence of the promoter-distal region of the tcp gene cluster of Vibrio cholerae. Gene 126:51-60. [DOI] [PubMed] [Google Scholar]
- 42.Papavassiliou, A. G. 1994. 1,10-Phenanthroline-copper ion nuclease footprinting of DNA-protein complexes in situ following mobility-shift electrophoresis assays, p. 43-78. In G. G. Kneale (ed.), Methods in molecular biology, vol. 5. Humana Press, Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 43.Parsot, C., and J. J. Mekalanos. 1991. Expression of the Vibrio cholerae gene encoding aldehyde dehydrogenase is under control of ToxR, the cholera toxin transcriptional activator. J. Bacteriol. 173:2842-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsot, C., and J. J. Mekalanos. 1992. Structural analysis of the acfA and acfD genes of Vibrio cholerae: effects of DNA topology and transcriptional activators on expression. J. Bacteriol. 174:5211-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsot, C., E. Taxman, and J. J. Mekalanos. 1991. ToxR regulates the production of lipoproteins and the expression of serum resistance in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:1641-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson, K. M., and J. J. Mekalanos. 1988. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect. Immun. 56:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sack, D. A., R. B. Sack, G. B. Nair, and A. K. Siddique. 2004. Cholera. Lancet 363:223-233. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schleif, R. 2003. AraC protein: a love-hate relationship. Bioessays 25:274-282. [DOI] [PubMed] [Google Scholar]
- 50.Schleif, R. 2000. Regulation of the l-arabinose operon of Escherichia coli. Trends Genet. 16:559-565. [DOI] [PubMed] [Google Scholar]
- 51.Schuhmacher, D. A., and K. E. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skorupski, K., and R. K. Taylor. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763-771. [DOI] [PubMed] [Google Scholar]
- 53.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1986. Identification of a pilus colonization factor that is coordinately regulated with cholera toxin. Ann. Sclavo Collana Monogr. 3:51-61. [PubMed] [Google Scholar]
- 54.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tobes, R., and J. L. Ramos. 2002. AraC-XylS database: a family of positive transcriptional regulators in bacteria. Nucleic Acids Res. 30:318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vijesurier, R. M., L. Carlock, R. M. Blumenthal, and J. C. Dunbar. 2000. Role and mechanism of action of C · PvuII, a regulatory protein conserved among restriction-modification systems. J. Bacteriol. 182:477-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner, L. A., R. B. Weiss, R. Driscoll, D. S. Dunn, and R. F. Gesteland. 1990. Transcriptional slippage occurs during elongation at runs of adenine or thymine in Escherichia coli. Nucleic Acids Res. 18:3529-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 59.Withey, J. H., and V. J. DiRita. 2005. Activation of both acfA and acfD transcription by Vibrio cholerae ToxT requires binding to two centrally located DNA sites in an inverted repeat conformation. Mol. Microbiol. 56:1062-1077. [DOI] [PubMed] [Google Scholar]
- 60.Xiong, X. F., and W. S. Reznikoff. 1993. Transcriptional slippage during the transcription initiation process at a mutant lac promoter in vivo. J. Mol. Biol. 231:569-580. [DOI] [PubMed] [Google Scholar]
- 61.Yu, R. R., and V. J. DiRita. 1999. Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. J. Bacteriol. 181:2584-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, R. R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]
- 63.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]