Abstract
We have shown that Staphylococcus aureus biofilm production is induced in iron-restricted conditions and is repressed by iron via a Fur-independent mechanism, while Fur has both positive and negative regulatory roles in low iron. Furthermore, there is no significant increase in polymeric N-acetylglucosamine polysaccharide expression to account for induction of biofilms in low iron.
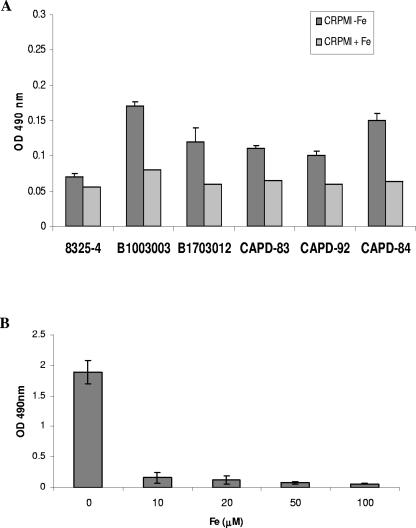
Staphylococcus aureus is one of the most frequently isolated pathogens associated with nosocomial sepsis. Patients particularly at risk of developing staphylococcal infections are those with indwelling medical devices, which S. aureus colonizes as a biofilm (11). Several environmental factors have been shown to influence S. aureus biofilm formation, including anaerobic growth (5), osmotic stress (15, 20), and glucose availability (15). A major environmental stress encountered by bacteria in vivo is severe iron restriction; to investigate the role of iron in the regulation of biofilm formation, S. aureus strains Newman (7) and 8325-4 (18) and clinical strains CAPD-83, CAPD-84, and CAPD-92 (University Hospitals of Leicester) and B1003003 and B1703012 (Queen's Medical Centre, Nottingham, United Kingdom) were grown in CRPMI medium in 5% CO2 in air (18) for 24 h, diluted to an optical density at 595 nm (OD595) of 0.1 in fresh CRPMI with and without the addition of 50 μM Fe2(SO4)3, and aliquoted into quadruplicate wells of 96-well flat-bottomed tissue culture plates (Nunc). The plates were incubated for 24 h, and biofilm formation was assessed as described previously (6). All strains showed enhanced biofilm formation under low-iron conditions, whereas addition of iron resulted in a significant decrease in biofilm formation (Fig. 1A and B). All strains besides Newman produced low levels of biofilm when iron was depleted, although the enhancement of biofilm formation in comparison to that in iron-replete medium was significant. Slight differences in growth of the different strains in CRPMI were observed, but these were not significant. The effect of iron on biofilm formation is concentration dependent (Fig. 1B); it occurs in other iron-restricted media and is not due to changes in pH resulting from addition of ferric sulfate to the medium, nor is it due to growth in 5% CO2 in air compared to growth in air (data not shown). Moreover, inhibition of biofilm formation is specific for iron; addition of other metal ions such as magnesium, manganese, copper, and calcium did not consistently affect biofilm formation to any significant degree (data not shown). Thus biofilm formation, an important virulence factor of S. aureus, is induced in low-iron growth conditions and repressed by iron.
FIG. 1.
S. aureus biofilm formation is regulated by iron. S. aureus strains 8325-4, B1003003, B1703012, CAPD-84, CAPD-83, and CAPD-92 (A) and Newman (B) were grown in CRPMI overnight, diluted to an OD595 of 0.1 in CRPMI supplemented with Fe2(SO4)3 either at 50 μM (A) or at the concentrations indicated (B), and inoculated into quadruplicate wells of a polystyrene microtiter plate. After 24 h, the wells were washed with phosphate-buffered saline, adherent bacteria were stained with 1% (wt/vol) safranin, and biofilm formation was assessed by OD490 measurements. Results represent the averages of at least three independent experiments and the standard error of the mean.
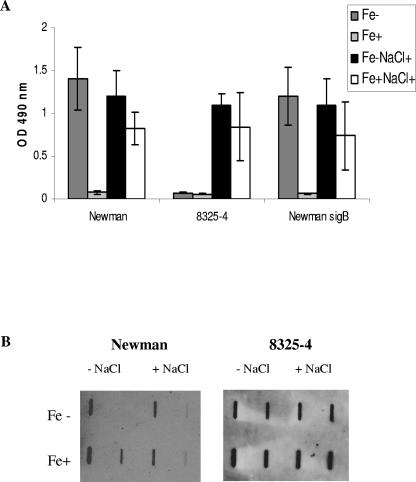
Previous studies have shown that osmotic stress induces S. aureus biofilm formation and that the alternative transcription factor σB is involved in this induction (20). S. aureus Newman and 8325-4, representing extremes of biofilm-forming ability in low-iron CRPMI, and the isogenic Newman ΔsigB::erm mutant (10) were used to investigate the combined effect of low iron and osmotic stress on the induction of biofilms and the role of σB in these conditions. Strains were grown in iron-restricted and iron-replete CRPMI in the presence and absence of 3% NaCl for 24 h. As shown in Fig. 2A, low iron and high osmotic stress resulted in equally high levels of biofilm formation in Newman and 8325-4, which was comparable to the amount of biofilm produced by Newman in low iron and low osmotic stress. Interestingly, the presence of iron did not completely repress biofilm formation in high osmotic conditions as it does in low osmotic conditions, suggesting that different factors are induced in response to low iron and osmotic stress and that some of these are not repressed by iron. In addition, there were no significant differences in the patterns of biofilm formation between Newman and the Newman ΔsigB::erm mutant (Fig. 2A) or between 8325-4, which is σB deficient, and the σB-positive isogenic strain SH1000 (13; data not shown), suggesting that σB does not have a significant role in the regulation of biofilm formation in low-iron conditions.
FIG. 2.
Biofilm assays and slot blot analysis of PNAG extracts indicate that PNAG levels do not correspond with maximal biofilm levels in S. aureus. (A) Biofilm formation by S. aureus Newman, 8325-4, and the Newman ΔsigB::erm mutant. (B) PNAG production by strains Newman and 8325-4. Bacteria were grown in CRPMI overnight and diluted to an OD595 of 0.1 in CRPMI (Fe−) or CRPMI plus 50 μM Fe2(SO4)3 (Fe+) with (NaCl+) or without the addition of 3% (vol/vol) NaCl and inoculated in quadruplicate into wells of a polystyrene microtiter plate. After 24 h the wells were washed with phosphate-buffered saline, adherent bacteria were stained with safranin, and biofilm formation was assessed by OD490 measurements. Results represent the averages of at least eight independent experiments and the standard error of the mean. For measurement of PNAG production, cells were harvested by centrifugation from 24-h static cultures. PNAG was extracted by boiling cell suspensions in EDTA; cell debris was removed by centrifugation, and 50-μl samples of serial dilutions (1:10 and 1:100) were applied to nitrocellulose membrane for probing with PNAG-specific rabbit antiserum. Each experiment was repeated at least three times using PNAG extracts obtained from cultures grown on different days with similar results in each case.
The polymeric N-acetylglucosamine polysaccharide (PNAG) (16) encoded by the ica locus is proposed to be critical for biofilm formation (4). To determine the effect of low iron and osmotic stress on PNAG production, PNAG levels in cell extracts and culture supernatants were measured directly using a polysaccharide slot blot assay (1) with polyclonal rabbit antiserum specific for PNAG (16). Surprisingly, there was no reproducible significant increase in the level of PNAG extracted from strain Newman grown in low-iron conditions compared to cells grown in iron-replete conditions (as measured by densitometry of repeat blots from individual experiments) to account for the significant difference between Newman biofilm formation in iron-restricted and iron-replete growth conditions (Fig. 2B). Furthermore, 8325-4 produced a level of PNAG similar to that produced by Newman, and yet 8325-4, in contrast to Newman, produces only a very low level of biofilm in CRPMI (Fig. 2B), and there was no significant increase in PNAG levels in either strain to account for the observed increase in biofilm under osmotic stress conditions. Additionally, although some PNAG was detected in the supernatants, there was again no difference in the relative levels compared to the cell surface extracts that would account for the differences in biofilm levels (data not shown). Thus, these results suggest that biofilm factors other than PNAG are induced in response to low iron and osmotic stress in vitro. These are relevant environmental conditions in vivo, and therefore this observation may explain why a number of studies have shown that ica mutants are not attenuated in experimental device-related infection models (8, 9, 14). Therefore, factors other than PNAG may be critical for biofilm formation in vivo.
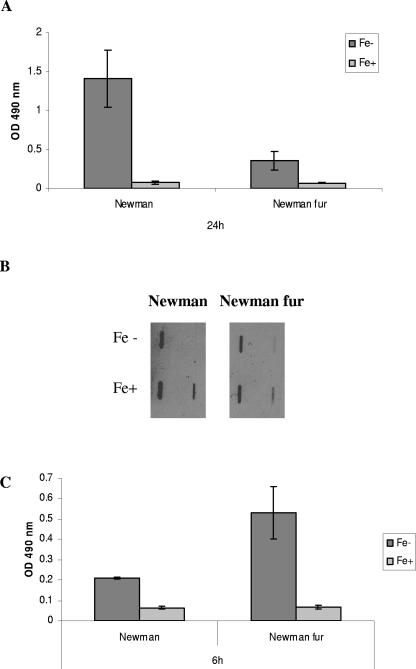
Iron-responsive gene regulation in S. aureus is mediated by the global regulator Fur (12, 21). To determine the role of Fur in iron regulation of biofilm formation, S. aureus Newman and the isogenic Newman Δfur::tet mutant, constructed by transducing the fur::tet mutation from 8325-4 (12) with phage φ11 (3), were assayed for biofilm formation in iron-restricted and iron-replete CRPMI. If Fur were involved in iron-dependent repression of biofilm production, we would expect to see equal levels of biofilm regardless of iron status. However, biofilm levels were still significantly repressed in the presence of iron in the Newman fur mutant (P = 0.046), suggesting that Fur is not responsible for the negative iron regulation of S. aureus biofilm formation (Fig. 3A). Nevertheless, Fur does appear to have a significant role in biofilm regulation in Newman; the fourfold decrease (P = 0.039) in the level of biofilm produced by the fur mutant strain compared to that of wild-type Newman suggests a positive regulatory role in low-iron conditions. This observed decrease was not due to any significant difference in growth between the wild-type and fur mutant strains (data not shown). PNAG levels of the S. aureus Newman Δfur::tet mutant were also measured, but no significant difference was observed in any of the growth conditions or in comparison to the wild type, indicating that Fur does not regulate expression of PNAG (Fig. 3B).
FIG. 3.
Effect of fur mutation on S. aureus biofilm formation. Strain Newman and the isogenic Newman Δfur::tet mutant were grown in CRPMI overnight, diluted to an OD595 of 0.1 in CRPMI (Fe−) or CRPMI plus 50 μM Fe2(SO4)3 (Fe+), and inoculated in quadruplicate into wells of a polystyrene microtiter plate. After (C) 6 h or (A) 24 h, the wells were washed with phosphate-buffered saline, adherent bacteria were stained with safranin, and OD490 measurements were obtained. Results represent the averages of at least eight independent experiments and the standard error of the mean. For measurement of PNAG production (B), cells were harvested by centrifugation from 24-h static cultures. Fifty-microliter samples of serial dilutions of PNAG extracts (1:10 and 1:100) were applied to nitrocellulose membrane for probing with PNAG-specific rabbit antiserum. Each experiment was repeated at least three times using PNAG extracts obtained from cultures grown on different days with similar results in each case.
The observation that Fur does not repress S. aureus biofilm formation was surprising, as our previous studies indicated that Fur may have a negative regulatory role in initial adhesion (19). Therefore, we investigated the effect of Fur and iron on the earlier stages of S. aureus biofilm formation. At 6 h, as at 24 h, biofilm formation by wild-type S. aureus Newman showed Fur-independent negative regulation by iron (Fig. 3C). In contrast to positive regulation by Fur at the later stages, however, it appears that Fur negatively regulates the early stages of S. aureus biofilm formation in low-iron conditions, as there is a significant increase in biofilm formation at 6 h in the fur mutant (P = 0.008) (Fig. 3C). There were no significant differences in the growth of the wild-type and mutant strains at 6 h. Thus, Fur both positively and negatively regulates different stages of biofilm formation in low iron. To our knowledge, there have been no reports of Fur acting as an activator protein in low iron in any other bacteria. The mechanisms of regulation in S. aureus are unknown: Fur may act indirectly on another regulatory mechanism such as a small RNA (17), or it may repress gene expression by directly binding promoters in low iron as observed in Helicobacter pylori (2).
In summary, we have demonstrated that iron-mediated repression of biofilm production is Fur independent and that Fur has both positive and negative regulatory roles in low iron. We have also demonstrated that factors other than PNAG are critical for biofilms induced in low-iron growth conditions; there is significant strain variation in the expression of these factors, suggesting that regulation of S. aureus biofilm formation is extremely complex.
Acknowledgments
This work was supported by project grant 24681 from the Royal Society.
We thank Simon Foster, University of Sheffield, for providing SH1000 and the 8325-4 fur mutant; Gerry Pier, Harvard Medical School, Boston, Mass., for providing the PNAG antiserum; and M. Bischoff, University of Zurich, for providing Newman ΔrsbUVW-sigB::erm.
REFERENCES
- 1.Beenken, K. E., P. M. Dunman, F. McAleese, D. Macapagal, E. Murphy, S. J. Projan, J. S. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bereswill, S., S. Greiner, A. H. M. Van Vliet, B. Waidner, F. Fassbinder, E. Schiltz, J. G. Kusters, and M. Kist. 2000. Regulation of ferritin-mediated cytoplasmic iron storage by the ferric uptake regulator homolog (Fur) of Helicobacter pylori. J. Bacteriol. 182:5948-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung, A. L., J. M. Koomey, C. A. Butler, S. J. Projan, and V. A. Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 89:6462-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramton, S. E., M. Ulrich, F. Götz, and G. Döring. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:4079-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deighton, M. A., J. Capstick, E. Domalewski, and T. van Nguyen. 2001. Methods for studying biofilms produced by Staphylococcus epidermidis. Methods Enzymol. 336:177-204. [DOI] [PubMed] [Google Scholar]
- 7.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 8.Fluckiger, U., M. Ulrich, A. Steinhuber, G. Döring, D. Mack, R. Landmann, C. Goerke, and C. Wolz. 2005. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 73:1811-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francois, P., P. H. Tu Quoc, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, S. E. Cramton, F. Gotz, and P. Vaudaux. 2003. Lack of biofilm contribution to bacterial colonisation in an experimental model of foreign body infection by Staphylococcus aureus and Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 35:135-140. [DOI] [PubMed] [Google Scholar]
- 10.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 12.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristian, S. A., T. Golda, F. Ferracin, S. E. Cramton, B. Neumeister, A. Peschel, F. Gotz, and R. Landmann. 2004. The ability of biofilm formation does not influence virulence of Staphylococcus aureus and host response in a mouse tissue cage infection model. Microb. Pathog. 36:237-245. [DOI] [PubMed] [Google Scholar]
- 15.Lim, Y., M. Jana, T. T. Luong, and C. Y. Lee. 2004. Control of glucose- and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J. Bacteriol. 186:722-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maira-Litrán, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrissey, J. A., A. Cockayne, P. J. Hill, and P. Williams. 2000. Molecular cloning and analysis of a putative siderophore ABC transporter from Staphylococcus aureus. Infect. Immun. 68:6281-6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrissey, J. A., A. Cockayne, J. Hammacott, K. Bishop, A. Denman-Johnson, P. J. Hill, and P. Williams. 2002. Conservation, surface exposure, and in vivo expression of the Frp family of iron-regulated cell wall proteins in Staphylococcus aureus. Infect. Immun. 70:2399-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong, A., V. K. Singh, G. Cabrera, and R. K. Jayaswal. 2000. Molecular characterisation of the ferric regulator, Fur, from Staphylococcus aureus. Microbiology 146:659-668. [DOI] [PubMed] [Google Scholar]