Abstract
Phenotypic and genetic evidence supporting the notion of biofilm formation as a developmental process is growing. In the present work, we provide additional support for this hypothesis by identifying the onset of accumulation of biofilm-stage specific proteins during Pseudomonas aeruginosa biofilm maturation and by tracking the abundance of these proteins in planktonic and three biofilm developmental stages. The onset of protein production was found to correlate with the progression of biofilms in developmental stages. Protein identification revealed that proteins with similar function grouped within similar protein abundance patterns. Metabolic and housekeeping proteins were found to group within a pattern separate from virulence, antibiotic resistance, and quorum-sensing-related proteins. The latter were produced in a progressive manner, indicating that attendant features that are characteristic of biofilms such as antibiotic resistance and virulence may be part of the biofilm developmental process. Mutations in genes for selected proteins from several protein production patterns were made, and the impact of these mutations on biofilm development was evaluated. The proteins cytochrome c oxidase, a probable chemotaxis transducer, a two-component response regulator, and MexH were produced only in mature and late-stage biofilms. Mutations in the genes encoding these proteins did not confer defects in growth, initial attachment, early biofilm formation, or twitching motility but were observed to arrest biofilm development at the stage of cell cluster formation we call the maturation-1 stage. The results indicated that expression of theses genes was required for the progression of biofilms into three-dimensional structures on abiotic surfaces and the completion of the biofilm developmental cycle. Reverse transcription-PCR analysis confirmed the detectable change in expression of the respective genes ccoO, PA4101, and PA4208. We propose a possible mechanism for the role of these biofilm-specific proteins in biofilm formation.
Biofilms are complex, organized communities of bacteria that grow in association with a wide array of biotic and abiotic surfaces (73). They can be found at almost any solid-liquid interface in industrial and clinical settings (12, 23, 31-32). It is now widely accepted that microorganisms undergo profound changes during their transition from planktonic (free-swimming) organisms to cells that are part of a complex, surface-attached community. These changes are reflected in the new phenotypic characteristics developed by biofilm bacteria. Biofilms are inherently resistant to antimicrobial agents and are often the root cause of persistent implant- and non-implant-related bacterial infections and diseases such as cystic fibrosis, otitis media, and periodontitis (11, 22, 37, 58). One of the most medically important biofilm-forming species is Pseudomonas aeruginosa, which is frequently associated with nosocomial infections of the urogenital tract and skin (21) and the lungs of patients suffering from cystic fibrosis (6, 9, 58).
Thus, it is not surprising that the formation of biofilms has been proposed to be a developmental process (16, 18, 52). The formation of biofilms has been shown in many species to progress through multiple developmental stages (18, 52, 61). In Pseudomonas aeruginosa, five stages can be distinguished (52). Biofilm formation is believed to begin when bacteria sense certain environmental parameters that trigger the transition from planktonic growth to life on a surface (14, 16). Biofilm development begins with the reversible and irreversible attachment to a surface. For optimal attachment to glass or plastic substrata, flagella and type IV pili are required (42, 52-53). Attachment is followed by immigration and division of bacteria to form small clusters exceeding 10 μm in width and height (maturation-1 stage) (16, 19, 52) and finally maturation of microcolonies (maturation-2 stage) into an exopolysaccharide-encased mature biofilms (3, 52). The biofilm developmental cycle comes full circle when biofilms disperse (52). Biofilm dispersion has been demonstrated to be influenced by environmental factors such as the availability of organic nutrients (15, 29, 39, 54-55) and iron and oxygen (5, 8, 59, 62, 74), all of which can have profound impacts on biofilm development. Mechanistically, biofilm dispersion may involve the differential regulation of the adhesiveness of the bacteria, potentially through the activation of lytic phages (69) or through cyclic-di-GMP signaling (24) in a protein phosphorylation-mediated event (54).
Global analyses of gene expression of bacterial biofilms using P. aeruginosa, Escherichia coli, Bacillus subtilis, Pseudomonas putida, and Shewanella oneidensis (56, 60, 62, 68, 72) revealed that gene expression in biofilms compared to that in planktonic bacteria varies from 1% to 38% of the total genome (50, 56, 60, 62, 72) and up to 50% of the total proteome, for instance, in P. aeruginosa (40, 52). The studies listed above have assisted in the identification of transcription factors that affect the formation of biofilms and responses to biofilm formation, including the repression of flagellum genes and hyperexpression of genes for adhesion and ribosomal protein. For example, biofilm formation in E. coli was shown to involve the differential expression of 230 genes, including those encoding proteins associated with adhesion and autoaggregation, outer membrane proteins (OmpC, OmpF, OmpT, Slp), and proteins involved in lipid A biosynthesis (56). A number of these genes have recently been associated with the initial steps of E. coli biofilm formation on abiotic surfaces (43, 50). Biofilm formation in Bacillus subtilis involves the differential expression of 519 genes, including those associated with phage-related functions, membrane bioenergetics, glycolysis, and the tricarboxylic acid cycle, as well as a large number of genes involved in motility and chemotaxis (60).
The majority of the gene expression analysis studies investigated only one or two biofilm growth stages. Thus, information is lacking whether multiple biofilm developmental stages and the attendant features that are characteristic of biofilms are accompanied by large-scale changes in temporal and spatial gene expression. However, recent studies have hinted at the involvement of regulatory pathways in the progression of biofilm development, thus indicating that biofilm development may be accompanied by stage-specific temporal and spatial gene expression patterns. Kuchma et al. have demonstrated that the development to mature biofilms in P. aeruginosa is associated with a three-component regulator system (sadARS) by showing that a deletion in any of the three sadARS genes resulted in a failure in progression of biofilm maturation (36). Furthermore, several other regulatory systems have been reported to influence the early stages of biofilm formation, including the global virulence factor regulator GacA (45), the catabolite repression control protein Crc (42), and the response regulator proteins AlgR (71). Bacteria in which these functions have been blocked by mutation have shown a decrease in biofilm formation and a failure to form microcolonies. Quorum sensing has been observed to be important for later stages of biofilm formation. Studies by Davies and colleagues (17) demonstrated that P. aeruginosa strains lacking a functional las quorum-sensing system initiated biofilms normally but were unable to establish the typical architecture of a mature biofilm; the las mutant strain producing a biofilm comprised only a relatively thin layer of cells (17, 33). The rhl quorum-sensing system of P. aeruginosa, which is active in early stages of biofilm formation (52), has been reported to influence biofilm formation, probably via RpoN, an alternative sigma factor for RNA polymerase, which regulates expression of rhlR and rhlI (25). Both quorum-sensing systems have been shown to influence 616 and 315 genes, respectively (57, 67). In addition, RpoN controls expression of a diverse set of genes, including those for flagella and pili and gacA (25, 27, 30, 66), which affect early stages of biofilm formation. Furthermore, the starvation-activated transcription factor sigma-H and the transcriptional regulator Spo0A were shown to be required for biofilm formation in Bacillus subtilis (60). Overall, these studies indicate that biofilm development is a regulated process and that each biofilm developmental stage may be accompanied by large-scale changes in temporal and spatial gene expression. This finding indicates that it may be possible to arrest biofilm development by interfering with protein production or gene expression for critical biofilm specific proteins and regulators.
To determine whether the onset of biofilm-stage specific protein production may be used to arrest the progression of biofilm development, we tracked biofilm proteins and protein abundance in planktonic cells and three distinct biofilm developmental stages. We present evidence that the development of biofilms by P. aeruginosa requires the production of proteins in a progressive manner. Biofilm stage-specific proteins were discernible throughout the biofilm developmental life cycle. Deletion of genes encoding biofilm specific proteins resulted in arresting the progression of biofilm maturation. Together, our findings indicate that biofilm formation is a progressive process and that knowledge of temporal expression of biofilm-specific genes can be used to control biofilm formation or to arrest biofilm formation at an early biofilm developmental stage.
MATERIALS AND METHODS
Bacterial strains and media.
The parental strain for all studies is P. aeruginosa PAO1, unless otherwise stated. All planktonic strains were grown aerobically in minimal medium (2.56 g Na2HPO4, 2.08 g KH2PO4, 1.0 g NH4Cl, 0.04 g CaCl2 · 2 H2O, 0.5 g MgSO4 · 7H2O, 0.1 mg CuSO4 · 5H2O, 0.1 mg ZnSO4 · H2O, 0.1 mg FeSO4 · 7H2O, and 0.004 mg MnCl2 · 4H2O per liter, pH 7.2) at 22°C in shake flasks at 220 rpm. Biofilms were grown as described below at 22°C in minimal medium. Glutamate (130 mg/liter) was used as the sole carbon source. All strains used in this study are listed in Table 1.
TABLE 1.
Bacterial strains used in this study
| Strain or mutant of P. aeruginosa | Relevant genotype | Protein name | Source or reference |
|---|---|---|---|
| PAO1 | Wild type | B. H. Holloway | |
| MPAO1 | Wild type | 28 | |
| 29990 | ccoO::ISphoA/hah (PA1553::ISphoA/hah) | Probable cytochrome c oxidase subunit | 28 |
| 32275 | PA0180::ISphoA/hah | Probable chemotaxis transducer protein | 28 |
| 40994 | PA4101::ISphoA/hah | Probable two-component response regulator | 28 |
| 8945 | mexH::ISlacZ/hah (PA4206::ISlacZ/hah) | Probable outer-membrane efflux protein MexH | 28 |
| 42631 | PA0720::ISphoA/hah | Probable helix-destabilizing protein of bacteriophage Pf1 | 28 |
| 10143 | PA2677::ISlacZ/hah | Probable type II secretion protein | 28 |
| 1122 | PA1596::ISlacZ/hah | Heat shock protein | 28 |
| 13987 | PA2627::ISlacZ/hah | Conserved hypothetical protein | 28 |
| 7993 | PA4844::ISlacZ/hah | Probable chemotaxis transducer | 28 |
Biofilm formation: (i) continuous flow tube reactor.
Biofilms were grown as described previously (52). Briefly, the interior surfaces of silicone tubing of a once-through continuous flow tube reactor system were used to cultivate biofilms at 22°C. Biofilms were harvested after 3 days (maturation-1 stage), 6 days (maturation-2 stage), and 9 days (dispersion stage) of growth under flowing conditions. Biofilm cells were harvested from the interior surface by pinching the tube along its entire length, resulting in extrusion of the cell material from the lumen. The resulting cell paste was collected on ice. Prior to sampling, the bulk liquid was purged from the tubing to prevent interference from detached, planktonic cells.
(ii) Ninety-six-well microtiter dish assay.
Initial biofilm formation was measured by using the microtiter dish assay system, as described previously (41). Microtiter wells were inoculated from overnight LB-grown cultures diluted 1:50 in minimal medium or LB broth. The cells were grown for 8 and 24 h before they were stained with crystal violet (CV) and quantified. Biofilm formation was quantified by solubilization of the CV staining in 95% ethanol. The absorbance of the resulting solution was measured at 570 nm.
(iii) Flow cells.
Biofilm architecture was studied by using flow cells, as described previously (52). To exclude the effects of the media, biofilms were grown in both 1:10-diluted LB broth and minimal medium.
Preparation of crude protein extract and protein determination.
Batch- and biofilm-grown P. aeruginosa cells were immediately washed after being sampled by centrifugation at 12,000 × g for 10 min at 4°C and resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0), containing 0.3 mg phenylmethanesulfonyl fluoride (Boehringer Mannheim, Indianapolis, IN)/ml. All cell samples were lysed by sonication on ice by six 10-s bursts at 4 W (Cole Parmer Instrument Co., Vernon Hills, IL) and stored at −20°C until used for protein determination. Cell debris and unbroken cells were removed from all samples by centrifugation at 30,600 × g for 30 min at 4°C. The total protein concentration was determined by the modified method of Lowry (47) with reagents from Sigma. Bovine serum albumin was used as the standard. Experiments for each time point were repeated at least three times.
Two-dimensional gel electrophoresis and image analysis.
The protein production patterns of total cell extracts of planktonic and biofilm cells were analyzed by two-dimensional polyacrylamide gel electrophoresis (2D-gel) as described previously (52-53) with a Multiphor II from GE Healthcare (Piscataway, NJ) and a 2D-gel system from Genomic Solutions Inc. (Ann Arbor, MI). 2D-gels were stained with silver nitrate (7). 2D-gel analyses were performed in triplicate for each growth condition to confirm the reproducibility of the protein pattern under planktonic and attached growth conditions. Only differences in protein spots that were reproduced three times are described here. 2D-gels were scanned using a calibrated image scanner (GE Healthcare) to insure even spot detection and higher accuracy for the subsequent image analysis. Computational image analysis was carried out with Image Master 2D Platinum software (GE Healthcare).
Correlation of protein concentration and CFU over the course of Pseudomonas aeruginosa biofilm development.
The population size of planktonic and biofilm cells was determined by the number of CFU by using serial dilution plate counts. To do so, biofilms were harvested from the interior surface as described above, and the resulting cell pastes were resuspended in saline (total volume = 1 ml) and homogenized for 30 s to disrupt cell clusters. The protein concentration was determined in parallel by the modified method of Lowry (47). Planktonic cells were harvested in logarithmic phase. Experiments for each time point were repeated at least three times.
Protein identification by MS.
Protein spots of interest were excised from the gel and digested in situ with trypsin using a ProGest workstation (Genomics Solutions Inc., Michigan). After digestion for 6 h at 37°C, tryptic peptides were extracted and desalted if necessary with ZipTips (Millipore), and an aliquot of the supernatant was taken for analysis by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using an Ettan MALDI-TOF Pro (GE Healthcare, Piscataway, NJ). Data were obtained using the following parameters: reflective mode; 20 kV accelerating voltage; 95% grid voltage; and 0.2-ns delay. Trypsin peptides were used as internal standards for every peptide sample to ensure high mass accuracy. Database searches were performed using MASCOT and ProFound. All proteins were identified with significant certainty (Est-Z score of >0.3 or a probability score of <0.02). Proteins were identified with 3 to 15 matched peptides and a minimum of 5% sequence coverage. The correlation between apparent and theoretical molecular masses of the identified proteins was found to be very similar (y = 0.9553x − 0.2294), with an R2 of 0.9736.
Twitching motility assay.
Twitching motility was determined as previously described (4). Briefly, overnight cultures of P. aeruginosa strains grown in LB medium were stabbed through a twitching motility plate (2% LB medium, 1% agar). The plates were incubated at 37°C for 48 h, at which time the agar was removed, the bacteria attached to the plate were stained with CV, and the diameter of the zone of twitching was measured.
Microscopy and image acquisition.
Images of biofilms grown in once-through flow cells were viewed by transmitted light with an Olympus BX60 microscope (Olympus, Melville, NY) and a ×100 magnification A100PL objective lens. Images were captured using a Magnafire cooled three-chip charge-coupled device camera (Optronics Inc., Galena, CA) and a 30-ms exposure. In addition, confocal scanning laser microscopy was performed with an LSM 510 Meta inverted microscope (Zeiss, Heidelberg, Germany). Images were obtained with a LD-Apochrome 40×/0.6 lens and with the LSM 510 Meta software (Zeiss).
RNA isolation and reverse transcription-PCR (RT-PCR).
Biofilm cells were harvested after 4 and 6 days of growth (maturation-1 and -2 stages), collected in RNA Protect, and spun down at 4°C. Planktonic cells were harvested similarly. Total RNA was isolated by using the RNeasy kit from QIAGEN (Valencia, CA). Residual DNA was removed by treatment with DNase I. The purity of the RNA was determined by gel electrophoresis, and the concentration was determined by spectrophotometry.
RT was performed using 1 μg of total RNA, random hexamers, and the first-strand synthesis kit for RT-PCR, RETROscript, from Ambion (Austin, TX) as indicated by the manufacturer. PCR was carried out with either 2 or 4 μl of first-strand cDNA in a total volume of 50 μl. The transcripts were amplified using a cycle as follows: 94°C, 5 min; 30 cycles, each consisting of 94°C for 25 s, 50°C for 30 s, and 72°C for 50 s, followed by a final extension step at 72°C for 5 min. The following primer sets were used to quantify expression of individual genes: ccoO (PA0180, 5′-CAGATCGTTCCGCTG and 5′-GTCGTCGGAGTAACG), PA4101 (5′-GAAGCCAACACGGTG and 5′-CTGGTGTCCAGTTGC), mexH (PA4208, 5′-CCTGATGTTCGTCGC and 5′-CGATGTGTTCGAGGG), and crc (PA5332, 5′-GAGCTACCCGAACAG and 5′-GCGCCAGTTCTTCAC). PCR products were analyzed using 2% agarose gels. To ascertain that no residual DNA was present in the RNA preparations, a PCR was performed with the same primers and overall conditions, except that no RT was added.
RESULTS
The formation of biofilms has been proposed to be a developmental process wherein bacterial populations adapt to life at a surface. Furthermore, recent studies have indicated the involvement of regulatory pathways in the progression of biofilm maturation, thus indicating that the biofilm developmental stages may be accompanied with stage-specific expression patterns. However, while previous results from our laboratory suggested that biofilms display multiple phenotypes (52, 54), detailed information is lacking on whether biofilm developmental stages are accompanied by large-scale changes at the protein and/or gene level.
Biofilm developmental stages coincide with the onset and the abundance of distinct groups of proteins.
Here, we characterized biofilm proteins and their protein abundance at the planktonic growth stage and at three distinct biofilm developmental stages, at the maturation-1 and -2 biofilm developmental stages, and at the dispersion stage. To do so, 2D-gel images of all three biofilm developmental stages and the planktonic growth stage were compared and analyzed with the image analysis program Image Master 2D Platinum (GE Healthcare). Then, proteins that appeared to be produced in a biofilm-specific manner were tracked within all four biofilm developmental stages, and onset and protein abundance was recorded. By thus comparing 2D-gel images of the four growth stages, eight distinct populations of biofilm proteins were identified, comprising >700 proteins. Each protein population consisted of sets of unique proteins; no single protein was assigned to more than one population. The locations of proteins displaying distinct patterns are indicated in the 2D-gel images provided in the supplemental material. We did not analyze proteins that were more abundant at the planktonic cell stage.
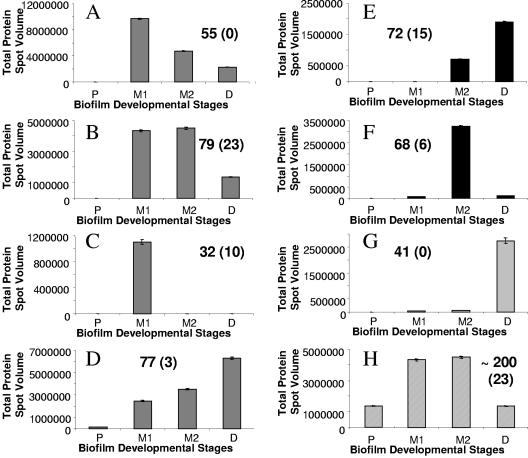
Four distinct trends in protein patterns were discernible, based on the onset of detection of protein abundance. Proteins showing trend I behavior were first detected at the maturation-1 stage and comprise protein abundance patterns A to D. However, while all proteins belonging to patterns A to D were first detectable or produced at maturation-1 stage, their maximal abundance differed depending on the biofilm developmental stage. Protein abundance pattern A comprised 155 proteins that were maximally abundant during the maturation-1 stage of biofilm development and then displayed a continuous decrease in abundance through to late-stage biofilm development. Protein abundance pattern B (79 proteins) showed proteins that were only maximally abundant during maturation-1 and maturation-2 stages of biofilm development, while the 77 proteins belonging to protein abundance pattern D displayed a continuous increase in abundance through to late-stage biofilm development. Proteins comprising pattern C represented maturation-1 stage-specific proteins (32 proteins). Proteins showing trend II behavior comprised patterns E and F (Fig. 1E and F). Proteins displaying this trend II behavior were found to be first detectable at the maturation-2 stage. Pattern E comprised 72 proteins that were only present during maturation-2 and the late or dispersion stage of biofilm development. Proteins comprising pattern F represent maturation-1 stage-specific proteins (68 proteins). Trends III and IV comprised only one protein abundance pattern each (Fig. 1G and H). Proteins belonging to pattern G (trend III) were produced when P. aeruginosa biofilms entered the dispersion stage. The 41 proteins comprising this pattern were found to be dispersion-stage specific. Protein production pattern H (trend IV) comprised ∼200 proteins that were present at all four biofilm developmental stages. However, the proteins were maximally abundant during the maturation-I or maturation-II stage of biofilm development. Proteins belonging to pattern H were not considered biofilm specific. Overall, our findings indicate that the onset of protein production correlated with distinct biofilm developmental stages.
FIG. 1.
Protein production patterns A to H over the course of biofilm development. The production pattern analysis was carried out from 2D images that were scanned using a calibrated image scanner (GE Healthcare). Image analysis was done using Image Master 2D Platinum software (GE Healthcare). Computational analysis was based on 2D protein spot volumes. Four biofilm developmental stages previously described by Sauer et al. (52) were thus analyzed: P, planktonic cell stage; M1, maturation-1 stage (3-day-old biofilms); M2, maturation-2 stage (6-day-old biofilms); and D, early dispersion stage (9-day old biofilms). The data shown here represent average spot volumes for all proteins belonging to distinct protein production patterns. Experiments were carried out in triplicate for each growth stage.
Biofilm stage-specific trends in protein abundance and the progression of protein production are not a result of various protein loads.
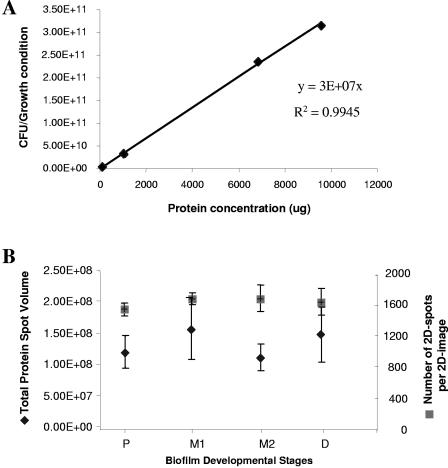
While a consistent amount of protein was separated per 2D-gel for all four developmental stages, we nevertheless ensured that the protein production patterns shown in Fig. 1 were a result of bacteria adapting to life at a surface and not a result of differences in cell numbers over the course of biofilm development and thus the result of various protein loads per 2D image. To do so, the number of viable cells (CFU) was correlated to the total protein concentration obtained from planktonic cells used as inoculum and for biofilm cells obtained after 3, 6, and 9 days of biofilm growth. The results are shown in Fig. 2A. The ratio of viable cells to the protein yield over the course of biofilm development was observed to be constant with 3.6 × 107 cells yielding 1 μg of protein (Fig. 2A). This finding indicated that by loading a constant amount of protein per 2D-gel, a constant number of viable cells were analyzed.
FIG. 2.
Protein yield, total protein spot volume, and the number of detectable 2D spots per 2D image are constant over the course of biofilm development. (A) The correlation between CFU and protein yield. CFU and protein concentration were obtained from four biofilm developmental stages. P, planktonic cell stage; M1, maturation-1 stage (3-day-old biofilms); M2, maturation-2 stage (6-day-old biofilms); and D, early dispersion stage (9-day old biofilms). The average viable cell count (CFU) corresponding to 1 μg of protein was 3.6 × 107 (standard deviation, 6.3 × 106). (B) Average total protein spot volume and average number of spots per 2D image. 2D images obtained from the planktonic stage and biofilms in the maturation-1 stage (M1, 3-day-old biofilms), maturation-2 stage (M2, 6-day-old biofilms), and early dispersion stage (D, 9 day-old biofilms) were analyzed with Image Master 2D Platinum software. Analyses of variance indicated that the spot volume (P = 0.425) and the numbers of 2D spots (P = 0.584) remained constant over the course of biofilm formation. Experiments were carried out in triplicate for each growth stage. CFU, viable cell counts.
An average of 1,600 protein spots were detected per 2D-gel. The total number of spots per 2D image and the resulting total protein spot volumes detected per 2D image showed no significant difference over the course of biofilm development (Fig. 2B). Analysis of variance confirmed that the number of 2D spots remained constant (F = 0.69; P = 0.584). Several proteins were selected that remained constant in all four stages tested and used as controls for equal protein loading (not shown). The spot volume of these proteins correlated with the constant spot volumes given in Fig. 2B, as detected by Image Master 2D Platinum software, indicating equal protein concentrations per gel (F = 1.04; P = 0.425). Our data indicated that the number of proteins and the overall detectable protein spot volume remained constant over the course of biofilm development. Overall, our data indicate that the protein production patterns shown in Fig. 1 were not a result of unequal protein loading (Fig. 2) but a result of bacteria adapting to life at a surface and of biofilm development.
Proteins with similar functions group within the same protein abundance patterns.
Several proteins following the production patterns shown in Fig. 1 were identified by MALDI-TOF MS using peptide mass fingerprinting (Table 2). Proteins that were first produced in maturation-1 stage biofilms (Fig. 3A to D) were identified by MALDI-TOF MS to be involved in adhesion (pilus assembly chaperone CupA2), regulation (probable serine/threonine-protein kinase PA1782, two-component sensor PA1336, and probable transcriptional regulator PA3420), antibiotic resistance (components of multidrug efflux pumps MexF, OprN homologue PA1238, and probable transcriptional regulator PA4878), in adaptation and stress (superoxide dismutase, a probable glutathione peroxidase, heat shock protein HtpG, sigma factor PvdS, PvdQ, and and l-ornithine N5-oxygenase PvdA), virulence (secreted hemolysin-coregulated protein Hcp), and denitrification (c-type cytochrome NirN). Proteins that were produced at maturation-2 stage (patterns E and F) were also involved in antibiotic resistance (components of MexCD and MexGHI efflux pumps) and pyoverdine synthesis (PvdD, -J, and -L) but also included proteins involved in quorum sensing (LasR, a MexGHI efflux pump involved in acyl-homoserine lactone [HSL] homeostasis by actively exporting 3-oxo-C12-HSL), as well as regulation and chemotaxis (PA4101, PA3420, PA0180, and PA5072). To date, no protein for pattern G has been identified.
TABLE 2.
List of identified proteins that were found to be produced in a progressive manner over the course of biofilm developmenta
| Pattern or protein identified | Gene |
|---|---|
| Trend I: onset of protein production at maturation-1 stage | |
| Pattern A | None identified |
| Pattern B | |
| Conserved hypothetical protein | PA0344 |
| Conserved hypothetical protein | PA0423 |
| Conserved hypothetical protein | PA1122 |
| Hypothetical protein | PA4788 |
| 3-Demethylubiquinone-9 3-methyltransferase UbiG | PA3171 |
| Thioredoxin reductase, TrxB1 | PA2616 |
| PvdS, sigma factor | PA2426 |
| PvdQ, probable acylase | PA2385 |
| PvdA, l-ornithine N5-oxygenase | PA2386 |
| Superoxide dismutase | PA4468 |
| Probable glutathione peroxidase | PA2826 |
| Probable c-type cytochrome NirN, part of nirSMCFDLGHJEN gene cluster | PA0509 |
| Mismatch repair system component of mutS | PA3620 |
| Non-hemolytic phospholipase C precursor | PA3319 |
| Probable pilus assembly chaperone CupA2 | PA2129 |
| Probable transcriptional regulator | PA4878 |
| Probable chemotaxis transducer | PA4844 |
| Probable two-component sensor | PA1336 |
| Probable acid phosphatase | PA0190 |
| Probable outer membrane component of multidrug efflux pump (OprN homologue) | PA1238 |
| RND multidrug efflux transporter MexF | PA2494 |
| Probable ribosomal protein L25 | PA4671 |
| Pattern C | None identified |
| Pattern D | |
| Heat shock protein HtpG (chaperone hsp90) | PA1596 |
| Probable serine/threonine protein kinase | PA1782 |
| Secreted hemolysin-coregulated protein Hcp | PA0263 |
| Trend II: onset of protein production at maturation-2 stage | |
| Pattern E | |
| Hypothetical protein | PA3068 |
| Hypothetical protein | PA1893 |
| Probable two-component response regulator | PA4101 |
| Probable transcriptional regulator | PA3420 |
| Probable chemotaxis transducer | PA0180 |
| Probable chemotaxis transducer | PA5072 |
| LasR (fragment) | PA1430 |
| Probable cytochrome c oxidase subunit | PA1553 |
| Probable electron transfer flavoprotein alpha subunit energy | PA5400 |
| Phosphopantetheine adenylyltransferase; CoaD | PA0363 |
| Probable outer membrane efflux protein precursor OpmD, part of mexGHI-opmD operon | PA4208 |
| RND multidrug efflux membrane fusion protein MexC precursor, part of mexCD-oprJ operon | PA4599 |
| Probable bacteriophage protein | PA0641 |
| PvdD, pyoverdine synthase D | PA2399 |
| PvdJ | PA2400 |
| PvdL | PA2424 |
| Pattern F | |
| Conserved hypothetical protein | PA4939 |
| Hypothetical protein | PA0588 |
| Hypothetical protein | PA2363 |
| Probable chemotaxis transducer | PA1608 |
| Probable transcriptional regulator | PA4145 |
| Exopolyphosphatase (fragment) | PA5241 |
| Trend III: onset of protein production at dispersion stage | |
| Pattern G | None identified |
| Pattern or protein identified | Gene |
| Trend IV: Proteins present at all stages | |
| Pattern H | |
| Probable molybdopterin-guanine dinucleotide biosynthesis protein MobA | PA3030 |
| Glyceraldehyde-3-phosphate dehydrogenase | PA3001 |
| Succinate semialdehyde dehydrogenase | PA0265 |
| Aconitate hydratase 2; AcnB | PA1787 |
| Arginine deiminase; ArcA | PA5171 |
| Ornithine carbamoyltransferase; ArcB | PA5172 |
| Carbamate kinase; ArcC | PA5173 |
| Dihydrolipoamide dehydrogenase 3 | PA4829 |
| NADH dehydrogenase I chain B; NuoB | PA2638 |
| Probable electron transfer flavoprotein alpha subunit | PA5400 |
| Alkyl hydroxyperoxide reductase subunit C | PA0139 |
| β-hydroxydecanoyl-acyl carrier protein dehydrase, FabA | PA1610 |
| Biotin carboxylase, AccC | PA4848 |
| Probable ClpA/ClpB-type chaperone | PA0090 |
| Probable heme utilization protein precursor HxuC | PA1302 |
| Sulfate-binding protein precursor | PA0283 |
| Nucleotide sugar epimerase/dehydratase WbpM | PA3141 |
| Probable chemotaxis sensor/effector fusion protein | PA3704 |
| Conserved hypothetical protein | PA2627 |
| Sigma factor RpoD (C-terminal portion) | PA0576 |
| DNA polymerase alpha chain | PA0669 |
| DNA polymerase II | PA1886 |
| Probable type II secretion protein | PA2677 |
| Alternate pattern (see reference 52) | |
| Probable helix-destabilizing protein of bacteriophage Pf1 | PA0720 |
The protein production patterns correlated with the patterns shown in Fig. 1. Proteins were identified by tryptic in-gel digest, followed by peptide mass fingerprinting using the ProFound and Mascot databases. All proteins listed here were identified with significant certainty (Est-Z score of >1 or probability score of <0.02). Proteins were identified with 3 to 15 matched peptides and a minimum of 5% sequence coverage. The correlation between apparent and theoretical molecular masses of the proteins identified was found to be very similar (y = 0.9553x − 0.2294) with R2 = 0.9736.
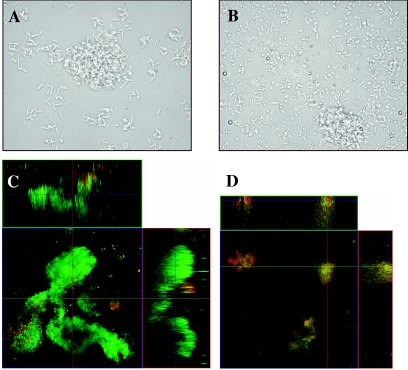
FIG. 3.
Biofilm formation under continuous flow. Biofilm formation after 3 days (A and B) and 6 days (C and D) of growth is shown for minimal medium-grown bacteria in a flow cell. Phase-contrast micrographs at a magnification of ×1,000 obtained after 3 days of growth (maturation-1 stage) are shown for P. aeruginosa PAO1 (A) and a representative mutant, ΔPA1553::Tetr (B). Confocal scanning laser microscopy images of maturation-2 stage biofilms at a magnification of ×400 of the P. aeruginosa PAO1 (C) and the ΔPA1553::Tetr mutant (D) after 6 days of growth (maturation-2 stage), showing the xy and xz planes. Flow cell experiments were performed in triplicate as described in Materials and Methods.
While all of the identified proteins listed in Table 2 were found to be more abundant under biofilm growth conditions, we nevertheless noticed that proteins with similar functions clustered within certain protein production patterns. The majority of proteins belonging to pattern H (trend IV) were identified as being either housekeeping or metabolic proteins such as the arginine deiminase system ArcABC, DNA polymerase II (PA1886), glyceraldehyde-3-phosphate dehydrogenase (PA3001), and succinate semialdehyde dehydrogenase (PA0265) (Table 2). Furthermore, proteins involved in pyoverdine synthesis (PvdAQ, PvdDJ, PvdL, and PvdS) were found to belong to protein production patterns B and E. Proteins identified as components of efflux pumps such as OpmD, MexC, and the probable outer membrane component of multidrug efflux pump PA1238 were found to cluster within the same protein production patterns that were maximally abundant during the maturation-2 or dispersion stage of biofilm development (Fig. 1; Table 2). A similar correlation was found for regulatory proteins such as transcriptional regulators, two-component regulatory proteins, and proteins involved in chemotaxis, which clustered in patterns B, D, and E, comprising proteins that were mainly maximally abundant during the later stages of biofilm development (Table 2). Interestingly, virulence factors and proteins involved in adaptation and protection such as nonhemolytic phospholipase C precursor PA3319, LasR PA1430, probable type II secretion system protein PA2672, superoxide dismutase PA4468, and secreted hemolysin-coregulated protein Hcp PA0263 were found to cluster within the same protein production patterns (patterns B, D, and E) (Table 2).
Mutants in biofilm-specific proteins are affected in the progression of biofilm development.
Since protein production appeared to be progressive and stage specific over the course of biofilm development, we were interested whether distinct, biofilm-specific proteins produced in mature biofilms might have important implications for biofilm development and whether deletion of these proteins would arrest biofilm development. We therefore tested nine P. aeruginosa mutant strains with mutations in proteins belonging to the abundance patterns B, D, E, and H (and alternate patterns) for biofilm formation (Tables 1 and 2; Fig. 1). No mutation was generated for trend III proteins, since we were interested in arresting biofilm development at an early biofilm developmental stage. Since several studies indicated that bacterial twitching motility is critical for early attachment events in P. aeruginosa biofilm formation, we tested whether the mutants were capable of this behavior. None of the tested mutants were observed to be impaired in twitching motility (not shown). In addition, none of the mutants showed a reduction in initial attachment when tested in 96-well microtiter plates for 8 h (not shown). Since no apparent difference in initial attachment was detected, we tested all 9 mutants for mature stage biofilm formation under flowing conditions.
In continuous culture, initial attachment of mutant cells after 1 day of growth (irreversible attachment stage) was indistinguishable from the wild-type strain. Cells were fixed to the substratum and formed nascent cell clusters (not shown). By day 3 (maturation-1 stage), both the wild-type and mutant cells began to form small cell clusters (Fig. 3A and B). We therefore concluded that none of the tested mutants had any apparent defects in attachment or cluster formation in the flow cell at day 3, also known as maturation-1 stage (52 and data not shown). However, by day 6, we observed obvious differences in the pattern of biofilm formation in the ΔPA1553, ΔPA4101, ΔPA4208, and ΔPA0180 mutant strains relative to the wild-type strains P. aeruginosa MPAO1 and PAO1, respectively (Fig. 3C and D). The gene PA1553 encodes a probable cytochrome c oxidase which is part of a cbb3-type heme-copper oxidase complex; PA4101 encodes a probable two-component response regulator; PA4208 encodes the probable resistance-nodulation-cell division efflux membrane fusion protein precursor MexH, which is part of the mexGHI-OpmD operon; and PA0180 encodes a probable chemotaxis transducer protein (www.pseudomonas.com) (Table 2).
After 6 days of growth under flowing conditions, the mutant biofilms appeared to have failed in the progression of biofilm development to reach maturation-2 stage, lacking large, distinct microcolonies and pronounced water channels (Fig. 3C). As shown in Fig. 3B and D, biofilms formed by the mutant ΔPA1553 after 6 days of growth consisted of small clusters that were only several cells thick and approximately 10 μm in width and height, while the wild type had formed biofilms with pronounced three-dimensional architecture (Fig. 3C). Biofilms formed by the mutants ΔPA4101, ΔPA4208, and ΔPA0180 appeared similar to the biofilms formed by the mutant ΔPA1553 under the same growth conditions (not shown). The architecture observed in the mutants appeared similar to the maturation-1 stage recently described for the P. aeruginosa wild type to occur after 3 days of growth under flowing conditions under the same conditions (52). Interestingly, mutations affecting proteins belonging to pattern H (probable type II secretion protein PA2677 and a conserved hypothetical protein PA2627), pattern B (probable chemotaxis transducer PA4844), pattern D (heat shock protein PA1596), and the alternate pattern (probable helix-destabilizing protein of bacteriophage Pf1 PA0720) had no apparent defect in biofilm formation (not shown).
Overall, disruption mutations in genes encoding proteins belonging to the abundance patterns B, D, and H (and alternate patterns) affected neither initial attachment nor biofilm formation. In contrast, mutants defective in proteins belonging to abundance pattern E (Fig. 1E) showed no defect in initial attachment and early biofilm developmental stages but were affected in the progression of biofilm development into pronounced three-dimensional architecture (Fig. 3). Overall, the mutant biofilms appeared to be unable to establish the typical architecture of a mature biofilm (Fig. 3C) and only formed a thin layer of cells at the surface with dispersed small cell clusters (Fig. 3D). Taken together, the visual observations suggested that biofilms formed by the mutants failed in the progression of biofilm development, appearing arrested between the stages of maturation-1 and maturation-2. These results support the observation that P. aeruginosa displays multiple phenotypes during biofilm development and that knowledge of biofilm-specific protein production and stage-specific physiology may be important in detecting and controlling biofilm growth.
Quantitative analysis of the mutant biofilm structure.
Visual inspection of the biofilm formed by the ΔPA0180, ΔPA1553, ΔPA4101, and ΔPA4208 mutants relative to that formed by the P. aeruginosa wild type indicated that the mutants are defective in developing mature biofilm architecture. To confirm these observations, we utilized the COMSTAT image analysis program to perform a quantitative analysis of biofilm architecture at the 6-day time point (26). As shown in Table 3, four variables were used to evaluate biofilm architecture. The mutant strains differed from the P. aeruginosa wild-type strains with respect to mean and maximum thicknesses, as well as total biomass. The decreased total biomass in the biofilms formed by the mutant strains was consistent with the appearance of small clusters and a sparsely covered substratum (Fig. 3D). The lack of mature biofilm architecture in the mutant biofilms was consistent with the appearance of the biofilms in the flow cells (Fig. 3C and D) and the decrease in biofilm thickness (Table 3). Taken together, these quantitative analyses support our visual observations that the mutants were unable to establish the typical architecture of a mature biofilm.
TABLE 3.
COMSTAT: Quantitative analysis of biofilm structurea
| Strain or strain identifier | Mean thickness (μm) | Maximum thickness (μm) | Total biomass (μm3/μm2) | Roughness coefficient |
|---|---|---|---|---|
| WT | 29.2 (8.3) | 84.1 (20.6) | 18.2 (2.6) | 0.8 (0.1) |
| ΔPA0180::Tetr | 8.5 (6.5) | 13.7 (2.3) | 8.8 (8.0) | 0.3 (0.1) |
| ΔPA1553::Tetr | 2.1 (0.3) | 30.7 (9.5) | 2.3 (1.9) | 1.9 (0.08) |
| ΔPA4101::Tetr | 4.4 (1.3) | 18.7 (3.3) | 3.5 (0.6) | 1.5 (0.3) |
| ΔPA4206::Tetr | 2.0 (0.6) | 18.9 (10.8) | 1.4 (0.2) | 1.7 (0.2) |
Values are means of data from six z-series image stacks for each strain taken at day 6 (maturation-2 stage). Numbers in parentheses indicates standard deviations.
Confirmation of biofilm-specific gene expression.
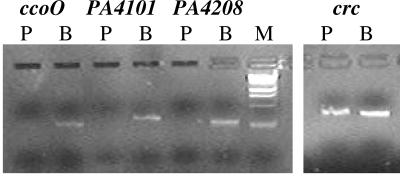
RT-PCR was used to examine the relative steady-state levels of the ccoO, PA4101, and PA4208 transcripts, using primer pairs specific for each transcript. ccoO encodes a probable cytochrome c oxidase, PA4208 encodes the efflux protein MexH, and PA4101 encodes a probable two-component response regulator. Overall, we found differential expression for the ccoO, PA4101, and PA4208 transcripts in biofilm cells, compared to planktonic cells (Fig. 4). The ccoO transcript was expressed as early as 4 days of biofilm growth (transition between maturation-1 and -2 stages) while the PA4101 and PA4208 transcripts were detectable after 6 days of biofilm growth (maturation-2 stage) (Fig. 4). The PA4101 and PA4208 transcripts were not detectable in 4-day-old biofilms (not shown). No transcripts were detectable under planktonic growth conditions (Fig. 4). Parallel experiments were performed using crc as a positive control. crc has been shown in P. putida to be produced relatively equally under planktonic growth conditions in minimal medium (51) and to be equally expressed in P. aeruginosa under mature biofilm and dispersing conditions (54). As shown in Fig. 4, the crc transcript was detectable in both planktonic and biofilm bacteria. Overall, these findings indicate a correlation of protein production and gene expression for these genes over the course of biofilm formation (Fig. 1 and 4; Table 2).
FIG. 4.
RT-PCR analysis of abundance of transcripts in P. aeruginosa PAO1 grown planktonically and as a biofilm. RT-PCR analysis of ccoO (PA1553), PA4101, mexH (PA4206), and crc expression in P. aeruginosa PAO1 grown planktonically (P) and as a biofilm (B). Amplification of the ccoO transcript resulted in a 270-bp PCR product and amplification of the PA4101 and PA4208 transcripts in 320- and 280-bp PCR products, respectively. The PCR product for the crc transcript was 330 bp. For the determination of ccoO transcript abundance in biofilms, cDNA from 4-day-old biofilms was used; for PA4101 and PA4208 transcript abundance in biofilms, cDNA obtained from 6-day-old biofilms (maturation-2 stage) was used. The oligonucleotide pairs used for the RT-PCR analysis are listed in Material and Methods. M, DNA marker.
DISCUSSION
In this study, we identified the onset of protein production and the protein abundance patterns of biofilm-specific proteins. Based on the onset of protein production, three different trends in protein abundance were observed, indicating that the transition between biofilm developmental stages was associated with the synthesis of unique sets of proteins. The transition from the planktonic mode of growth to the maturation-1 stage of biofilm formation was associated with the abundance of proteins belonging to the patterns A through D (Fig. 1; Table 2). Transition to maturation-2 stage correlated with the abundance of proteins belonging to the patterns E and F, while the transition to the dispersion stage required proteins belonging to pattern D (Fig. 1; Table 2). In contrast, proteins in pattern H (trend IV) were more abundant at mature biofilm stages but were detectable at all four biofilm developmental stages; their temporal production or abundance did not coincide with transitional episodes of biofilm development (Fig. 1).
Protein identification revealed that proteins with similar function showed similar abundance patterns. Metabolic or housekeeping proteins were found to cluster within protein production pattern H (Fig. 1C; Table 2). Within this group were proteins involved in cofactor biosynthesis, such as biotin carboxylase, and enzymes involved in the tricarboxylic acid and citrullin cycles and other biosynthetic pathways (glyceraldehyde-3-phosphate dehydrogenase, succinate semialdehyde dehydrogenase, aconitate hydratase 2, the arginine deiminase system [ArcABC], β-hydroxydecanoyl-acyl carrier protein dehydrase, and dihydrolipoamide dehydrogenase 3), energy generation (NADH dehydrogenase I chain B and probable electron transfer flavoprotein alpha subunit), and proteins involved in nucleotide synthesis (DNA polymerase alpha chain and DNA polymerase II) (Table 2). The differences in protein abundance of this group of proteins may indicate that biofilms exhibit different rates of growth over the course of biofilm development (63-64, 70). The finding of increased production of the arginine deiminase system ArcABC may indicate not only changes in the growth or metabolic rate but also a response to changes in the pH of the environment. The enzyme system catalyzes the conversion of arginine to ornithine, ammonia, and CO2 (13) and thus protects biofilms from lethal acidification through the production of ammonia and subsequent increases in local pH (38). The arginine deiminase system has been shown to be one of two major ammonia-generating pathways in the oral cavity (20).
Interestingly, our findings showed that metabolic and housekeeping proteins grouped within a pattern separate from virulence, antibiotic resistance, and quorum-sensing-related proteins. Virulence factors were first detected at the maturation-1 stage of biofilm development. These include proteins involved in pyoverdine synthesis (PvdA, PvdQ, and the sigma factor PvdS), as well as the secreted hemolysin-coregulated protein Hcp, superoxide dismutase, and glutathione peroxidase. At the maturation-2 stage, additional proteins involved in pyoverdine synthesis (PvdDJ and PvdL) were detected. The finding that the number of virulence proteins, including those involved in pyoverdine synthesis, appears to increase over the course of biofilm formation may indicate that virulence factors are produced in a progressive manner and thus that pathogenesis develops as biofilm development takes place. Furthermore, the findings may indicate that protein production within the biofilm is influenced by environmental factors such as the availability of iron and oxygen (as indicative by the finding of c-type cytochrome NirN being produced at the onset of the maturation-1 stage; nirN is part of the nirSMCFDLGHJEN gene cluster involved in denitrification). The availability of both iron and oxygen has been shown to profoundly impact biofilm formation by P. aeruginosa and other biofilm-forming bacteria (5, 8, 59, 62, 74). Furthermore, protein production in response to external conditions was apparent by the number of detected two-component response regulators and chemotaxis transducer proteins that were produced in a biofilm-specific manner. The proteins clustered in patterns B, D, and E and were maximally abundant during the later stages of biofilm development (Fig. 1; Table 2).
Proteins involved in antibiotic resistance were found to have a similar abundance pattern to proteins involved in pyoverdine synthesis and were maximally abundant during the maturation-2 or dispersion stage of biofilm development (Fig. 1B and E). Several components of resistance-nodulation-cell division multidrug efflux pumps, as well as the probable transcriptional regulator PA4878, were identified. The probable transcriptional regulator PA4878 belongs to the MerR family of helix-turn-helix transcriptional activators (www.pseudomonas.com). Interestingly, the N-terminal helix-turn-helix DNA binding motif of is 47% similar to a region of multidrug efflux transporter regulator TipA of Streptomyces lividans (www.pseudomonas.com). The MexGHI-OpmD pump has recently been described by Aendekerk et al. to facilitate cell-to-cell communication and to promote virulence, and growth in P. aeruginosa via 4-quinolone-dependent cell-to-cell communication (2). Furthermore, the MexGHI-OpmD system has been shown to confer resistance to vanadium (1). Taken together, the progressive production of proteins related to antibiotic resistance and virulence, attendant features that are characteristic of biofilms, may indicate that biofilms become more resistant and virulent in a progressive manner as part of the developmental process of biofilm formation. This is supported by the findings that the vast majority of these proteins were not detectable under planktonic growth conditions (Fig. 1; Table 2).
In this study, we describe the identification and initial characterization of four proteins, cytochrome c oxidase, two-component response regulator PA4101, a chemotaxis transducer protein PA0180, and MexH, required for biofilm formation. The proteins were initially selected to determine whether the onset of protein production at distinct biofilm developmental stages may be used to arrest biofilm development. The four particular proteins belonging to pattern E (Fig. 1E) were first detected during the maturation-2 stage. All four proteins appeared to play a critical role in biofilm maturation (Fig. 3), in particular, in the transition from the stage of cluster formation to biofilm maturation, which is usually indicated by the formation of large microcolonies and fluid-filled channels observed with some mature P. aeruginosa biofilms (34-35, 52). Since the proper production of these proteins was shown to be critical for normal biofilm development in P. aeruginosa, they can be considered to be true biofilm-specific proteins.
The mechanisms by which these four proteins affect biofilm maturation may be as diverse as the proteins themselves. For instance, the effect of MexH on biofilm formation observed in our study may be considered an indirect effect via the N-acyl homoserine lactone 3-oxo-C12-HSL. Recent findings indicate that the MexGHI-OpmD (www.pseudomonas.com) (1) and potentially the MexAB-OprM (46) efflux pumps are involved in acyl-homoserine lactone homeostasis by actively exporting 3-oxo-C12-HSL. In P. aeruginosa, N-acyl homoserine lactone signals control the expression of extracellular virulence factors and the type II secretion apparatus. Furthermore, homoserine lactone signals were shown to control biofilm differentiation. P. aeruginosa biofilms defective in 3-oxo-C12-HSL synthesis were shown to produce a flat and relatively unstructured biofilm (17, 33).
The probable cytochrome c oxidase PA1553 is part of a cbb3-type heme-copper oxidase complex. Cytochrome cbb3 oxidases are found almost exclusively in Proteobacteria and represent a distinctive class of proton-pumping respiratory heme-copper oxidases; genes encoding a cytochrome cbb3 oxidase were initially identified in Bradyrhizobium japonicum and designated fixNOQP (ccoNOQP) (49). Recently, homologues have been identified in other Proteobacteria, including three human pathogens (Campylobacter jejuni, Helicobacter pylori, and Neisseria meningitidis), in which cytochrome cbb3 is the only respiratory oxidase encoded by the genome (44, 48, 65). According to sequence analyses (www.pseudomonas.com), P. aeruginosa contains two operons (PA1557-PA1555 and PA1554- PA1552) with homology to fixNOP and with both of the P. aeruginosa operons missing fixQ. Recently, it has been suggested that the expression of cytochrome oxidases is required for the successful colonization of anoxic tissues and may be an important determinant of pathogenicity (48). We observed that the cytochrome c oxidase CcoO mutant was unable to form mature biofilms containing microcolonies. Microcolonies are typically characterized by gradients in the concentration of oxygen. This observation, therefore, supports the link between colonization, energy generation under anoxic conditions, and the action of cytochrome c oxidases.
The two-component response regulator PA4101 can be added to a growing list of regulatory factors that have been shown to be involved in the regulation of biofilm formation (10, 17, 36, 45). For example, the three-component system SadARS has been observed to play a role in P. aeruginosa biofilm maturation, in particular, the formation of large microcolonies and fluid-filled channels (36). The biofilm phenotype observed under flowing conditions in a flow cell was distinct from those previously described, suggesting that the sadARS system affected a step in biofilm maturation distinct from that affected by other regulatory loci (36). A comparison of gene expression between the wild type and the sadARS mutant biofilms showed ∼200 genes to be differentially expressed. Interestingly, mutations within the two-component response regulator PA4101 described here affected biofilm formation in a manner similar to that described for SadARS, with initial attachment, early biofilm formation, and twitching motility being unaffected while biofilm maturation was impaired. PA4101 mutant biofilms failed in the progression of development past the stage of maturation-1 and maturation-2 as indicated by visual observations (Fig. 3) and COMSTAT analysis (Table 3). Maturation-1 is considered the third stage over the course of biofilm development and refers to the point in time where cell clusters become progressively layered, exceeding 10 μm in width and height, while maturation-2 refers to the biofilm developmental stage at which cell clusters attain their maximum average thickness of ∼100 μm (52). However, while the sadARS system was constitutively expressed at low levels in the P. aeruginosa wild type at both planktonic and biofilm modes of growth (36), the two-component response regulator PA4101 identified in this study was produced in a biofilm stage-specific manner during the maturation-2 stage (Fig. 1E). Maturation-2 stage-specific expression of the PA4101 transcript was confirmed by RT-PCR (Fig. 4). The inhibition of progression of biofilm development at the transition from maturation-1 to maturation-2 in the PA4101 mutant strain was, therefore, consistent with the expression of this protein during the maturation-2 stage. The same was true for the PA0180 mutant biofilm. The probable chemotaxis transducer protein PA0180 contains a methyl-accepting chemotaxis protein domain; proteins belonging to this family are known to be involved in cell motility, secretion, and signal transduction mechanisms and are believed to be involved in reversible methylation in response to attractants or repellants during bacterial chemotaxis (www.pseudomonas.com). The mechanism by which this protein impairs biofilm maturation is unclear.
Together, our findings indicate that biofilm formation by P. aeruginosa is a progressive developmental process characterized by distinct phenotypic stages identifiable by unique cellular protein patterns. The biofilm developmental process was found to be accompanied by a progressive increase in the production of proteins related to antibiotic resistance and virulence, indicating that biofilm characteristics may develop over the course of biofilm development. We have identified a number of key biofilm-specific proteins which control the transition to late stage biofilm development. Knowledge of the temporal production of biofilm-specific proteins may be used in the future to arrest biofilm formation during early biofilm developmental stages and to reduce biofilm-associated damage. Furthermore, knowledge of stage-specific physiology may be important in detecting the degree of development of a biofilm and in leading to targeted antibacterial treatments.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL073835-01) and the National Science Foundation (DBI-0321046 and -0311307).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aendekerk, S., B. Ghysels, P. Cornelis, and C. Baysse. 2002. Characterization of a new efflux pump, MexGHI-OpmD, from Pseudomonas aeruginosa that confers resistance to vanadium. Microbiology 148:2371-2381. [DOI] [PubMed] [Google Scholar]
- 2.Aendekerk, S., S. P. Diggle, Z. Song, N. Hoiby, P. Cornelis, P. Williams, and M. Camara. 2005. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology 151:1113-1125. [DOI] [PubMed] [Google Scholar]
- 3.Allison, D. G. 2003. The biofilm matrix. Biofouling 19:139-150. [DOI] [PubMed] [Google Scholar]
- 4.Alm, R. A., and J. S. Mattick. 1997. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene 192:89-98. [DOI] [PubMed] [Google Scholar]
- 5.Applegate, D. H., and J. D. Bryers. 1991. Effects on carbon and oxygen limitations and calcium concentrations on biofilm removal processes. Biotechnol. Bioeng. 37:17-25. [DOI] [PubMed] [Google Scholar]
- 6.Bagge, N., M. Schuster, M. Hentzer, O. Ciofu, M. Givskov, E. P. Greenberg, and N. Hoiby. 2004. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production. Antimicrob. Agents Chemother. 48:1175-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 8.Bollinger, N., D. J. Hassett, B. H. Iglewski, J. W. Costerton, and T. R. McDermott. 2001. Gene expression in Pseudomonas aeruginosa: evidence of iron override effects on quorum sensing and biofilm-specific gene regulation. J. Bacteriol. 183:1990-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciofu, O., V. Fussing, N. Bagge, C. Koch, and N. Hoiby. 2001. Characterization of paired mucoid/non-mucoid Pseudomonas aeruginosa isolates from Danish cystic fibrosis patients: antibiotic resistance, beta-lactamase activity and RiboPrinting. J. Antimicrob. Chemother. 48:391-396. [DOI] [PubMed] [Google Scholar]
- 10.Cochran, W. L., S. J. Suh, G. A. McFeters, and P. S. Stewart. 2000. Role of RpoS and AlgT in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide and monochloramine. J. Appl. Microbiol. 88:546-553. [DOI] [PubMed] [Google Scholar]
- 11.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:318-322. [DOI] [PubMed] [Google Scholar]
- 12.Costerton, J. W., Z. Lewandowski, D. DeBeer, D. Caldwell, D. Korber, and G. James. 1994. Biofilms, the customized microniche. J. Bacteriol. 176:2137-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagastino, L., A. E. Goodman, and K. C. Marshall. 1991. Physiological responses induced in bacteria adhering to surfaces. Biofouling 4:113-119. [Google Scholar]
- 15.Dalaquis, P. J., D. E. Caldwell, J. R. Lawrence, and A. R. WcCurdy. 1989. Detachment of Pseudomonas fluorescens from biofilms on glass surfaces in response to nutrient stress. Microb. Ecol. 18:199-210. [DOI] [PubMed] [Google Scholar]
- 16.Davies, D. G., and G. G. Geesey. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 18.Davey, M. E., and G. A. O'Toole. 2000. Microbioal biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doggett, R. G. 1969. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl. Microbiol. 18:936-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong, Y., Y. Y. Chen, J. A. Snyder, and R. A. Burne. 2002. Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Appl. Environ. Microbiol. 68:5549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drenkard, E. 2003. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 5:1213-1219. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich, G. D., R. Veeh, X. Wang, J. W. Costerton, J. D. Hayes, F. Z. Hu, B. J. Daigle, M. D. Ehrlich, and J. C. Post. 2002. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287:1710-1715. [DOI] [PubMed] [Google Scholar]
- 23.Geesey, G. G., W. T. Richardson, H. G. Yeomans, R. T. Irvin, and J. W. Costerton. 1977. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can. J. Microbiol. 23:1733-1736. [DOI] [PubMed] [Google Scholar]
- 24.Gjermansen, M., P. Ragas, C. Sternberg, S. Molin, and T. Tolker-Nielsen. 2005. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms. Environ. Microbiol. 7:894-906. [DOI] [PubMed] [Google Scholar]
- 25.Heurlier, K., V. Denervaud, G. Pessi, C. Reimmann, and D. Haas. 2003. Negative control of quorum sensing by RpoN (σ54) in Pseudomonas aeruginosa PAO1. J. Bacteriol. 185:2227-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heydorn, A., B. K. Ersboll, M. Hentzer, M. R. Parsek, M. Givskov, and S. Molin. 2000. Experimental reproducibility in flow-chamber biofilms. Microbiology 146:2409-2415. [DOI] [PubMed] [Google Scholar]
- 27.Ishimoto, K. S., and S. Lory. 1989. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc. Natl. Acad. Sci. USA 86:1954-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, S. Ernst, O. Will, R. Kaul, C. Raymond, R. Levy, L. Chun-Rong, D. Guenthner, D. Bovee, M. V. Olson, and C. Manoil. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 100:14339-14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James, G. A., D. R. Korber, D. E. Caldwell, and J. W. Costerton. 1995. Digital image analysis of growth and starvation responses of a surface-colonizing Acinetobacter sp. J. Bacteriol. 177:907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jyot, J., N. Dasgupta, and R. Ramphal. 2002. FleQ, the major flagellar gene regulator in Pseudomonas aeruginosa, binds to enhancer sites located either upstream or atypically downstream of the RpoN binding site. J. Bacteriol. 184:5251-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr, C. J., K. S. Osborn, A. H. Rickard, G. D. Robson, and P. S. Handley. 2003. Biofilms in water distribution systems, p. 757-776. In M. Duncan and N. J. Horan (ed.), Water and wastewater engineering. Academic Press, London, United Kingdom.
- 32.Khoury, A. E., K. Lam, B. Ellis, and J. W. Costerton 1992. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 38:M174-M178. [DOI] [PubMed] [Google Scholar]
- 33.Kjelleberg, S., and S. Molin. 2002. Is there a role for quorum sensing signals in bacterial biofilms? Curr. Opin. Microbiol. 5:254-258. [DOI] [PubMed] [Google Scholar]
- 34.Klausen, M., A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50: 61-68. [DOI] [PubMed] [Google Scholar]
- 35.Klausen, M., A. Heydorn, P. Ragas, L. Lambertsen, A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511-1524. [DOI] [PubMed] [Google Scholar]
- 36.Kuchma, S. L., J. P. Connolly, and G. A. O'Toole. 2005. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 187:1441-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marquis, R. E., G. R. Bender, D. R. Murray, and A. Wong. 1987. Arginine deiminase system and bacterial adaptation to acid environments. Appl. Environ. Microbiol. 53:198-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall, J. C. 1988. Adhesion and growth of bacteria at surfaces in oligotrophic habitats. Can. J. Microbiol. 34:503-506. [Google Scholar]
- 40.Oosthuizen, M. C., B. Steyn, J. Theron, P. Cosette, D. Lindsay, A. Von Holy, and V. S. Brozel. 2002. Proteomic analysis reveals differential protein expression by Bacillus cereus during biofilm formation. Appl. Environ. Microbiol. 68:2770-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 42.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otto, K., J. Norbeck, T. Larsson, K. A. Karlsson, and M. Hermansson. 2001. Adhesion of type 1-fimbriated Escherichia coli to abiotic surfaces leads to altered composition of outer membrane proteins. J. Bacteriol. 183:2445-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 45.Parkins, M. D., H. Ceri, and D. G. Storey. 2001. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 40:1215-1226. [DOI] [PubMed] [Google Scholar]
- 46.Pearson, J. P., C. Van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson, G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83:346-356. [DOI] [PubMed] [Google Scholar]
- 48.Pitcher, R. S., and N. J. Watmough. 2004. The bacterial cytochrome cbb3 oxidases. Biochim. Biophys. Acta 1655:388-399. [DOI] [PubMed] [Google Scholar]
- 49.Preisig, O., D. Anthamatten, and H. Hennecke. 1993. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc. Natl. Acad. Sci. USA 90:3309-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prigent-Combaret, C., O. Vidal, C. Dorel, and P. Lejeune. 1999. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 181:5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiz-Manzano, A., L. Yuste, and F. Rojo. 2005. Levels and activity of the Pseudomonas putida global regulatory protein Crc vary according to growth conditions. J. Bacteriol. 187:3678-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauer, K., and A. K. Camper. 2001. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 183:6579-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sauer, K., M. C. Cullen, A. H. Rickard, L. A. Zeef, D. G. Davies, and P. Gilbert. 2004. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 186:7312-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawyer, L. K., and S. W. Hermanowicz. 1998. Detachment of biofilm bacteria due to variations in nutrient supply. Water Sci. Technol. 37:211-214. [Google Scholar]
- 56.Schembri, M. A., K. Kjaergaard, and P. Klemm. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253-627. [DOI] [PubMed] [Google Scholar]
- 57.Schuster, M., C. P. Lostroch, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 59.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Welsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552-555. [DOI] [PubMed] [Google Scholar]
- 60.Stanley, N. R., R. A. Britton, A. D. Grossman, and R. A. Lazazzera. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 185:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 62.Thormann, K. M., R. M. Saville, S. Shukla, and A. M. Spormann. 2005. Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 187:1014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tolker-Nielsen, T., and S. Molin. 2000. Spatial organization of microbial biofilm communities. Microb. Ecol. 40:75-84. [DOI] [PubMed] [Google Scholar]
- 64.Tolker-Nielsen, T., U. C. Brinch, P. C. Ragas, J. B. Andersen, C. S. Jacobsen, and S. Molin. 2000. Development and dynamics of Pseudomonas sp. biofilms. J. Bacteriol. 182:6482-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomb. J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 66.Totten, P. A., J. C. Lara, and S. Lory. 1990. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J. Bacteriol. 172:389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environments. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waite. R. D., A. Papakonstantinopoulon, E. Litter, and M. A. Curtis. 2005. Transcriptome analysis of Pseudomonas aeruginosa growth: comparison of gene expression in planktonic cultures and developing and mature biofilms. J. Bacteriol. 187:6571-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Webb, J. S., M. Lau, and S. Kjelleberg. 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:8066-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Werner, E., F. Roe, A. Bugnicourt, M. J. Franklin, A. Heydorn, S. Molin, B. Pitts, and P. S. Stewart. 2004. Stratified growth in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 70:6188-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitchurch, C. B., T. E. Erova, J. A. Emery, J. L. Sargent, J. M. Harris, A. B. Semmler, M. D. Young, J. S. Mattick, and D. J. Wozniak. 2002. Phosphorylation of the Pseudomonas aeruginosa response regulator AlgR is essential for type IV fimbria-mediated twitching motility. J. Bacteriol. 184:4544-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 73.Wimpenny, J. W. T. 2000. An overview of biofilms as functional communities, p. 1-24. In D. G. Allison, P. Gilbert, H. M. Lappin-Scott, and M. Wilson (ed.), Community structure and co-operation in biofilms. Cambridge University Press, Cambridge, United Kingdom.
- 74.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms. Relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.