Abstract
In bacteria, thiamine pyrophosphate (TPP) is an essential cofactor that is synthesized de novo. Thiamine, however, is not an intermediate in the biosynthetic pathway but is salvaged from the environment and phosphorylated to TPP. We have isolated and characterized new mutants of Bacillus subtilis that deregulate thiamine biosynthesis and affect the export of thiamine products from the cell. Deletion of the ydiA gene, which shows significant similarity to the thiamine monophosphate kinase gene of Escherichia coli (thiL), did not generate the expected thiamine auxotroph but instead generated a thiamine bradytroph that grew to near-wild-type levels on minimal medium. From this ΔthiL deletion mutant, two additional ethyl methanesulfonate-induced mutants that derepressed the expression of a thiC-lacZ transcriptional reporter were isolated. One mutant, Tx1, contained a nonsense mutation within the B. subtilis yloS (thiN) gene that encodes a thiamine pyrophosphokinase, a result which confirmed that B. subtilis contains a single-step, yeast-like thiamine-to-TPP pathway in addition to the bacterial TPP de novo pathway. A second mutant, strain Tx26, was shown to contain two lesions. Genetic mapping and DNA sequencing indicated that the first mutation affected yuaJ, which encodes a thiamine permease. The second mutation was located within the ykoD cistron of the ykoFEDC operon, which putatively encodes the ATPase component of a unique thiamine-related ABC transporter. Genetic and microarray studies indicated that both the mutant yuaJ and ykoD genes were required for the derepression of thiamine-regulated genes. Moreover, the combination of the four mutations (the ΔthiL, thiN, yuaJ, and ykoD mutations) into a single strain significantly increased the production and excretion of thiamine products into the culture medium. These results are consistent with the proposed “riboswitch” mechanism of thiamine gene regulation (W. C. Winkler, A. Nahvi, and R. R. Breaker, Nature 419:952-956, 2002).
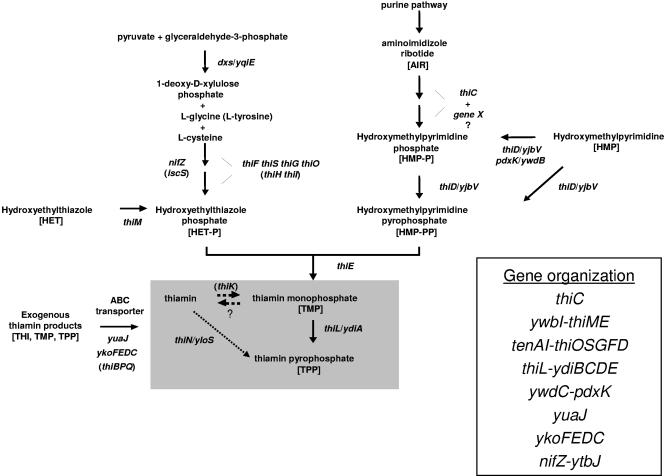
Vitamin B1 (thiamine pyrophosphate), which can be synthesized by microorganisms, plants, and fungi but not by mammals, is a cofactor of a number of important enzymes in carbohydrate and amino acid metabolisms (30). Unlike other vitamin biosynthesis pathways in bacteria (e.g., for riboflavin and biotin), thiamine is not part of the de novo pathway but is actually part of the salvage pathway. In Bacillus subtilis, thiamine pyrophosphate biosynthesis occurs by a complex multistep pathway (1). The pyrimidine moiety, 4-amino-2-methyl-5-hydroxymethylpyrimidine pyrophosphate (HMP-PP), is derived from an intermediate in the de novo purine biosynthetic pathway, 5-aminoimidazole ribotide, in a conversion catalyzed by the thiC gene product (1). HMP-P is then phosphorylated to HMP-PP by the product of the thiD (yjbV) gene prior to coupling with the thiazole unit (25). The thiazole moiety, 5-(2-hydroxyethyl)-4-methylthiazole phosphate (HET-P), is derived from 1-deoxy-d-xylulose 5-phosphate, glycine, and cysteine in a complex oxidative condensation reaction requiring the products of at least five different genes, thiF, thiS, thiO, thiG, and a nifS-like gene (26). The coupling of HMP-PP and HET-P is catalyzed by thiamine phosphate pyrophosphorylase encoded by thiE, resulting in thiamine monophosphate (TMP) (1). TMP is then phosphorylated to form thiamine pyrophosphate (TPP) by the action of thiamine monophosphate kinase, encoded by thiL. B. subtilis contains another potential way to produce TPP, through a thiamine one-step salvage pathway formerly discovered in Saccharomyces cerevisiae (23). This pathway, initially thought to exist only in lower eukaryotes, is catalyzed by the product of the thiN (yloS) gene (19). Two other salvage kinases, one specific for hydroxyethylthiazole and the other with a broad range of substrate specificities, including vitamin B6 compounds and HMP, have been identified in B. subtilis and are encoded by the genes thiM and pdxK (ywdB), respectively (25). However, an ortholog to the E. coli gene thiK (ycfN) encoding thiamine kinase, a salvage enzyme that converts thiamine to TMP, has not been identified in B. subtilis (19). The B. subtilis thiamine biosynthesis pathway showing all known and proposed genes and intermediates is shown in Fig. 1. (By recent convention, B. subtilis thi gene nomenclature has been changed to the E. coli nomenclature; some literature cited here may contain the original B. subtilis thi gene designations.)
FIG. 1.
Thiamine biosynthesis and salvage pathway of Bacillus subtilis. B. subtilis thi genes were previously identified by genetic, biochemical, and/or in silico evidence (1, 19, 25, 26, 29); in some cases, their original y gene designation is listed. E. coli intermediates or genes not present in B. subtilis but with similar functions are given in parentheses (1). Abbreviations are in brackets. The thiamine-TMP-TPP salvage pathway is shown in a shaded box. See the text for details. Question marks indicate possible unknown genes involved in the thiamine biosynthesis and salvage pathway (15, 19). The organization of Bacillus thiamine biosynthesis genes is shown on the right.
Interestingly, a thiamine regulatory gene has not been identified so far in bacteria (9, 14, 38). Instead, it has been proposed that thiamine pyrophosphate directly regulates the expression of the thiamine biosynthesis genes by a novel mechanism called riboswitch, in which TPP interacts with the nascent mRNA message at a cis-acting site within the 5′ leader region (called the thi box) to form a secondary structure that allows for the formation of a transcription terminator (17, 21, 22, 39). In addition to TPP, which was shown by transcription profiling to regulate the expression of the thiC gene and the tenA-tenI-thiOSGFD operon (16), thiazole (HET) was also described to repress the ywbI-thiME operon in B. subtilis (40, 41).
In silico screening of the B. subtilis genome with the thi box sequence has resulted in the identification of several new putative thiamine-regulated genes (29): the yuaJ gene and the ykoF-ykoE-ykoD-ykoC operon. yuaJ could encode an ATP-independent thiamine transporter containing multiple trans-membrane segments. YkoE and YkoC are likely two trans-membrane components, while YkoD is an ATPase component and YkoF acts as a novel HMP/thiamine-binding protein (6). However, no genetic experiments have confirmed the role of these genes in thiamine transport, and their products show no similarity to the known thiamine ABC transporter genes of Salmonella enterica serovar Typhimurium (thiBPQ) (36).
In this study, we used mutagenesis and transcriptomics to identify and characterize mutants of B. subtilis that deregulate thiamine gene expression. As predicted by the riboswitch model of gene regulation, none of these mutations were located in genes predicted to encode a regulatory protein. Instead, these mutations mapped to several genes previously identified as encoding a thiamine pyrophosphokinase activity (thiN) or encoding thiamine-related transport proteins (ykoD and yuaJ). Moreover, we also show that these mutations must be combined to deregulate thiamine gene expression, resulting in bacterial strains that are altered in the production and export of thiamine. These results provide genetic support that intracellular TPP levels control thiamine biosynthesis gene expression.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Plasmids and strains used in this study are listed in Table 1. Antibiotic resistance genes that confer resistance to neomycin (neo), tetracycline (tet), and chloramphenicol (cat) were obtained from plasmids pBEST501, pDG1514, and pC194, respectively (Bacillus Genetic Stock Center, Ohio State University [BGSC]). B. subtilis was grown on minimal medium (MM; 1× Spizizen salts, 0.04% sodium glutamate, and 0.5% glucose) or veal infusion-yeast extract complete medium or grown on agar plates consisting of tryptose blood agar base (Difco, Maryland) or MM. E. coli was grown on MM or Luria-Bertani medium without glucose. The compositions of these media are described elsewhere (11). For testing thiamine production in liquid test tube cultures, a thiamine-free medium was used (7). Thiamine supplements (thiamine, TMP, and TPP) required for auxotrophy were added at 1 μM. Resistance to the thiamine antimetabolite pyrithiamine was tested at 0.1 or 10 μM.
TABLE 1.
List of plasmids and strains
| Strain or plasmid | Relevant characteristic or genotype | Reference |
|---|---|---|
| Plasmids | ||
| pBR322 | E. coli plasmid cloning vector | 2 |
| pMUTIN2 | B. subtilis integration plasmid for gene inactivation | 33 |
| pDG1728 | B. subtilis plasmid for ectopic integration of lacZ transcriptional fusions at amyE | 10 |
| pEpUCΔ1 | E. coli-B. subtilis shuttle vector to generate in-frame mutations | 31 |
| pTH1 | Internal 348-bp fragment of thiL (ydiA) cloned into pMUTIN2 | This work |
| pTH12 | 712-bp thiC promoter fragment cloned into pDG1728 | This work |
| pTH30 | In-frame ΔthiL (ydiA) deletion (G79-G202) cloned into pEpUCΔ1 | This work |
| pTH31 | Internal 353-bp yuaJ fragment cloned into pMUTIN2 | This work |
| Strains | ||
| B. subtilis | ||
| PY79 | SPβc (cured) prototroph | BGSC (1A747) |
| TH12 | SPβc ΔthiL::cat4 | This work |
| TH21 | SPβc ΩamyE::thiC-lacZ | This work |
| TH22 | SPβc ΔthiL::cat4 ΩamyE::thiC-lacZ | This work |
| TH48 | ΔthiL::cat4 yuaJ (the first lesion in Tx26) ykoD (the second lesion in Tx26) | This work |
| TH76 | ΩyuaJ::pMUTIN2 | This work |
| TH83 | ΔthiL yuaJ ykoD | This work |
| TH95 | ΔthiL thiN (tx1) yuaJ ykoD ΩyloA::Tn917 | This work |
| TH105 | ΔthiL::cat4 | This work |
| TH106 | ΔthiL::cat4Ω thiL::pMUTIN2 | This work |
| TH112 | ΔthiL::cat4 ΩamyE::thiC-lacZ ykoD ΩyufR::Tn917 | This work |
| TH128 | ΔispA::neo | This work |
| TH137 | ΩyuaJ::pMUTIN2 ΔykoD::cat4 | This work |
| TH138 | ΔykoD::cat4 | This work |
| 1A603 | (SPβc2) trpC2 ΩthiC84::Tn917 | 34 |
| 1A631 | (SPβc2) trpC2 ΩmotA::Tn917 | 34 |
| 1A633 | (SPβc2) trpC2 ΩyloA::Tn917 | 34 |
| 1A642 | (SPβc2) trpC2 ΩyufR::Tn917 | 34 |
| Tx1 | ΔthiL::cat4 ΩamyE::thiC-lacZ thiN (tx1) | This work |
| Tx26 | ΔthiL::cat4 ΩamyE::thiC-lacZ yuaJ ykoD | This work |
| RL2066 | ΔspoVM::spec | 5 |
| E. coli | ||
| PT-R1 | thiO-35 | E. coli Genetic Stock Center; 13 |
| K12 | Wild type | E. coli Genetic Stock Center |
| S. enterica serovar Typhimurium | ||
| DM456 | ΩthiD906::MudJ(Km) | 37 |
| DM1856 | ΩthiL934::Tn10d(Tc) | 28 |
Thiamine biological assays.
Total thiamine compounds were assayed using indicator strains derived from Salmonella enterica serovar Typhimurium by known methods (7). Strain DM456 (ΩthiD906::mudJ) responds to thiamine, TMP, and TPP in MM, whereas strain DM1856 (ΩthiL934::Tn10d) responds to only TPP (28, 37). The responses of DM456 to known concentrations of thiamine, TMP, and TPP ranging from 0.0256 to 100 μg/liter were similar. In addition, DM456 was found to be more sensitive to TPP than DM1856. To assay B. subtilis cultures, supernatants were filter sterilized before the preparation of dilutions. Intracellular thiamine levels were measured from dilutions of 1-ml filter-sterilized cellular extracts that were obtained by French press breaking of the cells and centrifugation at 10,000 × g for 10 min. Indicator strains were grown overnight at 37°C in thiamine assay medium. Turbidity readings were made at 600 nm and compared to a range of standard solutions.
Thiamine thiochrome/HPLC assay.
Individual thiamine compounds, thiamine, TMP, and TPP were measured using a modified thiochrome/high-performance liquid chromatography (HPLC) assay procedure described previously (3). Briefly, 10 μl of culture supernatant or intracellular extracts is added to 200 μl of 4 M potassium acetate. The sample is then oxidized by the addition of 100 μl fresh 3.8 mM potassium ferricyanide in 7 M NaOH. The mixture is vigorously mixed and then quenched by the addition of 100 μl fresh 0.06% H2O2 in saturated KH2PO4. Samples are transferred to HPLC vials and injected onto a Supelcosil LC-18-T column (15 cm by 4.6 mm, 3 μm) (Supelco reference no. 58970-U). Elution is made by a 10% to 35% methanol (H2O, 50% to 25%) gradient in the presence of 40% 0.1 M K2HPO4 (pH 6.6) and 4 mM tetrabutyl ammonium hydrogen sulfate. Fluorescence is measured at 444 nm after excitation at 365 nm. The chronological order of elution from the column is thiamine, TMP, and TPP. This procedure was utilized to monitor both internal and external thiamine production during fermentation. In our HPLC/diode array detector assay used to directly measure thiamine and the intermediates HMP and HET in the fermentation broth, chromatography of samples was performed with a Phenomenex Luna C18 column, using an Agilent 1100 HPLC system equipped with an autosampler plus thermostat and a diode array detector. The column dimensions are 150 by 4.6 mm, with a particle size of 5 μm. The column temperature was kept constant at 20°C. The mobile phase is a mixture of 0.4 g pentane sulfonate in water, pH 2 (component A), and methanol (component B). Gradient elution, ranging from 2% (3 min) to 20% (20 min) component A, is applied. The flow rate is 1 ml/min. The detection method is based on UV absorption at 254 nm. The selectivity of the method was verified by injecting 10-μl standard solutions of the relevant reference compounds, thiamine, HMP, and HET, each at 100 μg/ml. The target compounds were completely separated without special sample preparation.
Molecular and genetic techniques.
Standard genetic and molecular biology techniques used in this study have been previously described by Maniatis et al. (18). DNA transformation, PBS1 generalized transduction, and other standard B. subtilis genetic techniques have been described previously (4).
Construction of thiL (ydiA) mutant.
Comparison of the E. coli ThiL protein sequence to the SubtiList protein database detected significant similarity to only one protein, YdiA [P(N) = 8.1e−15, where P is the probability of an alignment occurring with the score N or better]. The gene encoding this protein, ydiA, is 975 base pairs in length and is the first gene of a five-gene operon located at 55° on the B. subtilis chromosome. The thiL deletion mutant was generated by inserting a chloramphenicol acetyltransferase cassette (cat4) lacking the endogenous rho-independent transcription termination site between nucleotides 267 and 272 of thiL. Removal of the terminator in cat4 results in nonpolar insertional mutations. To do this, PCR primer pair 1-2 (Table 2) was used to first generate the DNA cassette cat4 gene. This fragment was then individually ligated to two DNA fragments containing either the 3′ or 5′ ends of thiL, which were also generated by PCR using primer pairs 3-4 and 5-6 (Table 2). The PCR fragment containing the ΔthiL::cat4 cassette was then inserted directly into the chromosomal thiL gene of strain PY79 by DNA transformation, selecting for colonies resistant to 5 μg/ml chloramphenicol. Strain TH12 containing ΔthiL::cat4 was recovered.
TABLE 2.
List of PCR primers
| Primer no. | Sequence |
|---|---|
| 1 | 5′-GGGGGTACCGAAAATTGGATAAAGTGGG-3′ |
| 2 | 5′-GGGACGCGTAAGTACAGTCGGCATTATCTCATA-3′ |
| 3 | 5′-GGGGAATTCTACCAGTTGTTCTGCCAAGGGCAT-3′ |
| 4 | 5′-GGGGGTACCGCAAGTGATACCAGATAAAACTTAGG-3′ |
| 5 | 5′-GGGACGCGTCTTCAAAATGGACTGAATCTGAAA-3′ |
| 6 | 5′-GGGGGATCCTTCAACGAGACAGACACCTTGTCCG-3′ |
| 7 | 5′-ATGCGGATCCCGTCCGGACCGCC-3′ |
| 8 | 5′-CGATCCCGGGGCCTCCCATCGCGGC-3′ |
| 9 | 5′-ATGCCCCGGGATTTGCCTAAGCTTCATCCTAAC-3′ |
| 10 | 5′-CGATGAATTCAGCCCTTCTGCAAAACCTT-3′ |
| 11 | 5′-AGCTAAGCTTGGCAGCCGTTATTTTAGACATTG-3′ |
| 12 | 5′-TGCAGGATCCATAAAAACTGCGCTGACCACTGA-3′ |
Plasmid pEpUCΔ1 (31) was used to construct an in-frame, markerless deletion mutation in B. subtilis. This E. coli vector contains a selectable erythromycin resistance (erm) cassette and the pE194 temperature-sensitive origin of replication that does not function over 51°C. To do this, a DNA fragment containing an in-frame gene deletion was prepared using standard PCR methods and inserted between the BamHI and EcoRI sites of pEpUCΔ1. Competent cells of the recipient strain were transformed at 51°C with the in-frame mutation-containing plasmid pEpUCΔ1, selecting for erythromycin resistance at 5 μg/ml erythromycin. Emr colonies that are also Cmr were recovered and grown overnight at 28°C in the absence of antibiotic selection for 72 h. Bacteria were then plated onto tryptose blood agar plates, and the plates were incubated overnight at 37°C. Approximately 25% of the colonies were found to be sensitive to both erythromycin and chloramphenicol. PCR analysis of chromosomal DNA from several Ems Cms colonies was used to confirm the presence of the in-frame mutation.
Isolation of EMS-induced thiamine-deregulated mutants.
The strategy to isolate thiamine-deregulated mutants of B. subtilis was to mutagenize bacteria that contained a thiC-lacZ fusion and then screen for colonies that were Lac+ in the presence of TPP or thiamine. A 732-bp-long DNA fragment containing 417 bp of the 5′ promoter region of thiC was prepared by PCR using standard methods and cloned unidirectionally in front of the promoterless lacZ gene of the pDG1728 vector (10), resulting in plasmid pTH12. This vector is designed to introduce ectopic transcriptional lacZ fusions into the nonessential amyE locus of B. subtilis. Plasmid pTH12 was linearized by restriction enzyme digestion and transformed into B. subtilis PY79, selecting for colonies that were resistant to 100 μg/ml spectinomycin (TH21). The same transcriptional fusion was also introduced into TH12 to generate TH22 (ΔthiL::cat4 ΩamyE::thiC-lacZ). Based on these results, thiC-lacZ reporter strain TH22 (ΔthiL::cat4) was used to screen for deregulated mutants under repressing growth conditions. Two methods were used to isolate such mutants. In the first method, MM agar plates that contained 1 μM thiamine and 25 μg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) were prepared. After a uniform dilution of logarithmic-growth-phase TH22 cells was applied, a paper disk containing 3 drops of ethyl methanesulfonate (EMS; d = 1.17 g/ml solution) was placed in the center of the plate. Lac+ colonies appeared over a period of 7 days of incubation at 37°C. In the second method, banks of EMS-mutagenized cells were prepared and screened. Accordingly, logarithmic-stage TH22 cells were treated with 9.4 mM EMS for 90 min, and aliquots were frozen in 10% glycerol at −90°C. Cells from the frozen stocks were diluted in veal infusion-yeast extract complete medium, incubated at room temperature for 30 min, and then plated onto MM containing 1 μM thiamine and 25 μg/ml X-Gal. Screening of Lac+ colonies led to mutants Tx1 and Tx26.
Combination of ΔthiL and the lesions in the mutant strains Tx1 and Tx26.
As a first step, the mutations in Tx26 were transferred into TH12 (ΔthiL::cat4) by DNA transformation, followed by selection for resistance to 0.1 μM pyrithiamine (P-0256; Sigma, St. Louis, MO). One pyrithiamine-resistant (Pyrr) colony that was also Lac+ in the presence of TPP was recovered and designated TH48. Each strain was grown in MM supplemented with micronutrients and 2.5% Difco nutrient broth for 18 h at 37°C. The cat-interrupted thiL gene was next replaced by an in-frame deletion using pEpUCΔ1 (method described above). Using standard PCR methods, an in-frame deletion of thiL (removing amino acid residues Gly79 through Gly202) was first constructed using primer pairs 7-8 and 9-10 (Table 2) and inserted between the BamHI and EcoRI sites of pEpUCΔ1, creating pTH30. This plasmid was then used to replace the ΔthiL::cat4 mutation in TH48 with the in-frame thiL mutation (ΔthiL), resulting in strain TH83. The mutation in Tx1 (thiN) was next introduced into TH83 by PBS1 transduction by standard procedures using linkage to a silent Tn917 insertion, ΩyloA::Tn917 (strain 1A633 of the Bacillus Genetic Stock Center, also called CU4153 [Ωzdi-82::Tn917]). The resulting strain was called TH95.
Mapping experiments and constructions of mapping strains.
Genetic mapping studies using PBS1 generalized transduction were performed under standard conditions (4) using donor strains from the collection of phenotypically silent Tn917 mapping insertions (34) and strains listed in Table 1. In order to inactivate the yuaJ gene, a 324-bp internal fragment of yuaJ starting at position 353 was PCR amplified and inserted between the BamHI and HindIII sites of pMUTIN2 using primers 14 and 15 (Table 2), creating plasmid pTH31. To determine if the mutation in Tx1 is allelic to thiN (yloS), a 448-bp deletion mutation, starting at base 124 of the thiN gene (ΔthiN::cat4), was constructed using the cat4 gene and cloned into pBR322 (2). Long-flanking-homology PCR was used to construct strain PY79 derivatives TH128 and TH138, which contain cat4-mediated deletions of the ispA and ykoD genes, respectively (35).
Microarray profiling.
TH22, Tx1, Tx26, and TH48 were grown in shake flask cultures containing 50 ml Spizizen MM with added thiamine pyrophosphate (0.34 μg/ml). Overnight cultures were diluted to 10 Klett units in fresh medium and grown to exponential growth phase (100 Klett units). Cells from half of the culture were collected by centrifugation, and the total RNA was immediately extracted as previously described (16). The remaining culture was grown to early stationary phase before RNA extraction. Early stationary phase was judged to be 30 min after glucose exhaustion; glucose content in the medium was measured by a glucose analyzer 2 (Beckman, Fullerton, CA) using standard procedures. The preparation of RNA, labeled cDNA targets, microarray hybridization and staining procedures, and data analysis used were described by Lee et al. (16).
RESULTS
Bacterial thiamine levels.
As a first step in isolating B. subtilis thiamine deregulatory mutants, the intra- and extracellular levels of thiamine products from wild-type and engineered B. subtilis strains were determined as described in Materials and Methods. As a control, the thiamine-deregulated E. coli PT-R1 mutant was similarly tested; this mutant is reported to produce only intracellular thiamine (no excretion of thiamine products into the medium), mostly in the form of TPP (>90%). Bioassay results indicated that thiamine products were readily detected from extracts of sonicated cells but not from the culture medium (data not shown). The intracellular level of thiamine products in logarithmic- or stationary-phase wild-type B. subtilis was calculated to be approximately 100 to 200 μg/liter (Table 3). This thiamine level was lower than that for E. coli PT-R1, which reached approximately 1.6 mg/liter in stationary-phase cells. Determination of the individual thiamine forms using thiochrome/HPLC showed only trace amounts of TMP and TPP in the supernatant of PY79 cultures (<0.05 μg/ml). However, intracellular thiamine products were 80% TPP and 20% TMP, in good agreement with the bioassay results. Intra- or extracellular levels of free thiamine were not observed.
TABLE 3.
Thiamine production and expression of thiA-lacZ transcriptional fusions in wild-type and thiamine-deregulated mutants of B. subtilis
| Strain | Genotype | Accumulation (μg/liter)
|
Regulation (β-galactosidase sp act [Miller units])c
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Extracellulara | Intracellularb | Log
|
Stationary
|
||||||
| None | HMPd | TPPd | None | HMP | TPP | ||||
| TH21 | thiC+ ΩamyE::thiC-lacZ | <0.1 | 190 | 110 | 6 | 0.5 | 31 | 3 | 1.2 |
| TH22 | thiC+ ΩamyE::thiC-lacZ ΔthiL::cat4 | 0.9 | 530 | 140 | 26 | 1.3 | 75 | 9 | 1.8 |
| Tx1 | thiC+ ΩamyE::thiC-lacZ ΔthiL::cat4 thiN | 5 | 500 | NG | 135 | 8 | NG | 177 | 5 |
| Tx26 | thiC+ ΩamyE::thiC-lacZ ΔthiL::cat4 yuaJ ykoD | 15 | 500 | 280 | 35 | 130 | 120 | 20 | 36 |
Thiamine concentration in MM (30 ml) after 24 hours of growth at 37°C.
Thiamine concentration of 1-ml supernatant of sonicated cells collected from a 30-ml MM culture after 24 hours of growth at 37°C.
Calculated according to Miller (20). NG, no growth.
Final concentrations of TPP and HMP were 1 μM each.
Construction and isolation of thiamine-deregulated mutants.
The strategy to isolate and characterize thiamine-deregulated mutants was based on monitoring the activity of a thiC-lacZ transcriptional fusion in the presence of TPP or thiamine. As described in Materials and Methods, TH21 (ΩamyE::thiC-lacZ) is a derivative of PY79, in which a thiC-lacZ transcriptional fusion was integrated in single copy into the amyE gene. In control studies, TH21 displayed thiamine-regulated expression of the lacZ fusion. On MM agar plates containing the indicator X-Gal, colonies of TH21 were Lac+ (i.e., blue) but were Lac− if the medium was supplemented with thiamine or TPP (1 μM). Moreover, when colonies were grown to early logarithmic phase (optical density at 600 nm of 0.8 to 0.9) in shake flask cultures containing 1 μM TPP, the β-galactosidase activity was repressed approximately 200-fold compared to cells grown in MM without TPP (Table 3). In stationary growth phase, the difference caused by TPP repression was less pronounced (30-fold repression). HMP (1 μM) also repressed expression of the fusion but to a lesser extent. Both thiazole and adenosine (1 μM each) did not show any thiamine-repressing activity (data not shown).
The thiC-lacZ transcriptional reporter fusion was introduced into a ΔthiL::cat4 mutant (TH22) to screen for Lac+ (i.e., blue) colonies under thiamine- or TPP-repressing growth conditions. In E. coli, inactivation of the thiL gene produces a thiamine-deregulated strain which is a strict thiamine auxotroph (37). However, the B. subtilis ΔthiL mutant was not a thiamine or TPP auxotroph; instead, it appeared to be a thiamine bradytroph (data not shown) and was slightly deregulated for thiamine gene expression (Table 3). We chose this strain to generate thiamine-deregulated mutants in order to exclude thiL mutants from our screen. Out of 50,000 EMS-mutagenized colonies screened, 26 Lac+ mutants were recovered. Among these, two (Tx1 and Tx26) were selected for on the basis of their unique phenotypes. Tx1 appeared to be a thiamine bradytroph based on its slow-growth profile on MM without TPP. Conversely, Tx26 was a prototroph but conveyed a much stronger Lac+ phenotype than Tx1. In addition, Tx26 was found to be resistant to pyrithiamine (Pyrr), a toxic thiamine analog, at concentrations up to 10 μM.
Thiamine-deregulated mutations are allelic to genes that are involved in thiamine salvage and transport.
PBS1 generalized transduction and DNA transformation were used to determine the genetic map positions of the lesions in Tx1 and Tx26. In most cases, the Tn917 mapping strains were used as donors; since these mapping strains carry the wild-type alleles, we reasoned that replacement of the mutation in Tx1 or Tx26 with the wild-type allele would result in a concomitant change in Lac phenotype (i.e., Lac+ to Lac−). In addition, the conversion of Pyrr to Pyrs could also be used to map the lesion(s) in the Tx26 mutant.
Initial mapping studies showed that the mutation in Tx1 was not linked to either the ΔthiL::cat4 mutation or the ΩamyE::thiC-lacZ fusion. However, the ΩspoVM::Tn917 mutation (strain RL2066) showed the highest transduction (>99%) and transformation linkages (72%) to the mutation in Tx1, indicating that this lesion was within or adjacent to spoVM. Inspection of this region located a possible candidate, thiN (yloS), a gene adjacent to spoVM, which has been previously shown to have thiamine pyrophosphokinase activity (thiamine-to-TPP activity) (19). To determine if the mutation in Tx1 is allelic to thiN (yloS), the DNA sequence of thiN in the Tx1 mutant was determined. The results revealed a single base mutation that caused a leucine-to-phenylalanine substitution at amino acid residue 116 (Leu116 [CTT] > Phe116 [TTT]).
Initial DNA transformation studies of Tx26 indicated that the transformation frequency of the pyrithiamine resistance marker to sensitive bacteria was nonlinear with respect to DNA concentration (data not shown). This result suggested that Pyrr in Tx26 was caused by more than one mutation. Transduction experiments confirmed that the Tx26 mutant contained two mutations located at different regions of the chromosome. One mutation showed 70% linkage to ΩyufR::Tn917, and the other mutation showed 59% linkage to ΩmotA::Tn917. Further transformation mapping studies showed strong linkage of the first lesion in Tx26 to yuaJ, a thiamine-regulated gene proposed to encode a thiamine permease (29). To determine if this lesion is allelic to yuaJ, a knockout mutation of yuaJ was first constructed using pMUTIN. As expected, the introduction of ΩyuaJ::pMUTIN into wild-type strains (e.g., PY79) or thiC mutants did not produce a phenotype. However, the introduction of ΩyuaJ::pMUTIN into strain TH112 (with only the second Tx26 lesion and ΔthiL) resulted in Emr colonies that were resistant to 0.1 μM pyrithiamine. Finally, DNA sequence analysis of yuaJ from Tx26 confirmed that the first lesion was an allele of yuaJ. YuaJ is a predicted membrane-bound thiamine permease. Sequencing detected a single base mutation that resulted in the change of a glutamine residue at amino acid position 35 to an ochre stop codon (Gln35 [CAA] > Stop [TAA]). The introduction of the first Tx26 mutation is predicted to prematurely terminate translation, resulting in an inactive, 35-amino-acid truncated protein. In similar mapping studies, the second Tx26 allele showed strong linkage (65%) to the ispA locus (117°). This genetic map position corresponded to a cluster of genes, ykoFEDC, previously predicted to encode HMP transport proteins (29). DNA sequencing of PCR fragments containing this operon and the promoter region detected a single missense mutation in the ykoD gene that resulted in an Asp180 (GAC)-to-Asn180 (AAC) substitution.
Mutant yloS (thiN) has decreased pyrophosphokinase activity.
Growth of Tx1 was found to be slower on MM lacking thiamine or TPP, taking more than 3 days to form colonies at 37°C. The addition of TPP or thiamine restored full growth within 1 day, suggesting that Tx1 was a strong thiamine bradytroph. In addition, Tx1 was strongly Lac+ on MM containing thiamine, HMP, or TMP but showed no Lac activity in the presence of TPP. These phenotypes strongly suggested that the lesion in the pyrophosphokinase gene decreases the conversion of thiamine to TPP whether thiamine is added externally or is formed by the dephosphorylation of TMP. Reconstruction of the Tx1 mutant confirmed this suspension. To do this, a double mutant (TH106) that consisted of a stable thiN deletion mutation (ΔthiN::cat4) and a thiL disruption mutation (ΩthiL::pMUTIN2) was constructed. Growth on minimal or complex medium of this double mutant was similar to that displayed by the original Tx1 mutant; TH106 could grow on MM in the presence of TPP only, with the addition of thiamine or TMP failing to restore growth. The ΔthiN::cat4 mutation alone in PY79 (TH105) produced no discernible phenotype in terms of growth on MM.
Simultaneous inactivation of YkoD and YuaJ is required for thiamine deregulation.
Unlike Tx1, Tx26 containing mutant ykoD and yuaJ genes was a thiamine prototroph and grew like the parental strain on both minimal and complex media. Reconstruction experiments confirmed that the inactivation of both yuaJ and ykoD was required for resistance to pyrithiamine. To do this, a double mutant (TH137) that consisted of a stable ykoD deletion mutation (ΔykoD::cat4) and a yuaJ disruption mutation (ΩyuaJ::pMUTIN2) was constructed. This mutant was found to be resistant to pyrithiamine (Fig. 2). However, the yuaJ and ykoD single mutants were pyrithiamine sensitive. To further investigate thiamine deregulation in Tx26, β-galactosidase levels of the thiC-lacZ reporter were measured when Tx26 was grown under thiamine-repressing and -derepressing conditions. As shown in Table 3, β-galactosidase activity was induced more than 200-fold relative to that of the wild-type control when cells were grown to logarithmic stage in MM containing TPP. The level of deregulation was reduced to about 30-fold in stationary phase. In addition, β-galactosidase levels were only twofold higher in cells grown to logarithmic stage in MM without TPP than in cells grown with TPP (1 μM); this difference in β-galactosidase levels was less pronounced in stationary growth phase. This variability in derepression of the thiC-lacZ fusion probably reflects fluctuation in TPP intracellular levels during growth. Interestingly, expression of the fusion was still partially repressed in the presence of HMP, which was likely caused by the conversion of HMP to TPP via the salvage pathway.
FIG. 2.
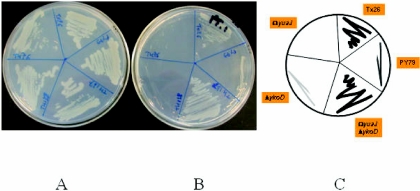
Reconstitution of the Tx26 pyrithiamine resistance phenotype. The strains with the indicated mutations were streaked for single colonies on MM (A) or MM containing 0.1 μM pyrithiamine and incubated for 3 days at 37°C (B). Panel C is a cartoon rendition of the results in panel B.
To determine if the expression of other thiamine biosynthesis genes was deregulated in Tx26, transcription profiling was carried out using a custom Bacillus subtilis oligonucletotide microarray (16). A reconstructed, backcrossed thiamine-deregulated mutant, TH48, was used in these first transcription profile experiments (see Materials and Methods) because the mutation(s) causing thiamine deregulation (i.e., pyrithiamine resistance) could be separated away from the thiC-lacZ fusion and any other unrelated EMS-induced mutation from the original Tx26 mutant (see below). Previous profiling results of wild-type B. subtilis PY79 demonstrated a 30- to 90-fold thiamine-specific repression of the two major thiamine biosynthesis operons, thiC and tenAI-thiOSGFD, but not of a third operon, ywbI-thiME (16). As shown in Table 4, high-level, deregulated expression of both thiC and tenAI-thiOSGFD was observed in TH48. Transcript levels of thiC increased 105-fold in TH48 compared to those in PY79 when both strains were grown in MM containing repressing levels of TPP. Transcription levels of tenAI-thiOSGFD increased 30- to 100-fold depending on the gene. The transcription of a third biosynthesis operon containing thiM and thiE and other known thiamine biosynthesis or salvage genes, pdxK, dxs (yqiE), thiI (ytbJ), and thiN (yloS), remained unchanged. A similar result was obtained by comparing transcription profiles of the original Tx26 mutant with those of the parental TH21 strain (data not shown). The ΔthiL::cat4 mutation in both TH48 and Tx26 had little effect on the transcription profile of thiamine biosynthesis genes other than thiC (a two- to threefold increase compared to PY79 when strains were grown to logarithmic stage in the presence of TPP) (data not shown). More interestingly, the transcript levels of genes recently shown to contain thi box regulatory sequences (29) also exhibited thiamine-specific derepression. For example, transcription of the ykoFEDC gene cluster was significantly increased in TH48 relative to PY79: ykoF, ykoD, and ykoC transcripts increased 15- to 23-fold, whereas ykoE increased 32-fold. Other examples include the yuaJ gene and ylmB encoding a protein similar to acetylornithine deacetylase. The average levels of yuaJ and ylmB transcripts were 8- and 11-fold higher, respectively, in TH48 than in PY79. Similar patterns of regulated gene expression of ykoFEDC, yuaJ, and ylmB were also observed in microarray analyses of wild-type cells grown in the presence or absence of thiamine. Finally, in HMP-grown TH48, the expression of only thiC, thiOSGFD, and ykoFEDC was repressed relative to cells grown in the absence of HMP (data not shown).
TABLE 4.
Change in transcript levels of genes in B. subtilis in response to TPP
| Genea | thi Box elemente | Enzyme/functiona | Ratiob
|
|
|---|---|---|---|---|
| WT/WT+c | Dereg+/WT+d | |||
| thiC | + | Biosynthesis of hydroxymethylpyrimidine phosphate | 58 | 105 |
| ywbI | − | Similar to LysR family of transcriptional regulators | NC | NC |
| thiM | − | Hydroxyethylthiazole kinase | NC | NC |
| thiE | − | Thiamine phosphate synthase | NC | NC |
| tenA | + | Thiaminasef | 68 | 62 |
| tenI | + | Possible antagonist of TenA | 25 | 27 |
| thiO (goxB) | + | Glycine oxidase | 67 | 56 |
| thiS (yjbS) | + | Sulfur carrier protein | 87 | 86 |
| thiG (yjbT) | + | Thiazole synthase | 82 | 61 |
| thiF (yjbU) | + | ThiS adenylyltransferase | 87 | 96 |
| thiD (yjbV) | + | Phosphomethylpyrimidine kinase | 27 | 34 |
| pdxK (thiD) | − | Pyridoxal/pyridoxal/pyridoxamine kinase | NC | NC |
| thiL (ydiA) | − | TMP kinase | NC | NC |
| thiN (yloS) | − | Thiamine pyrophosphorylase | NC | NC |
| ytbJ | − | Possible thiI orthologs (sulfur transferase) | NC | NC |
| dxs (yqiE) | − | 1-Deoxy-d-xylulose synthase | NC | NC |
| ykoC | + | Possible transmembrane component of thiamine-related ABC transporter | 10 | 15 |
| ykoD | + | Possible ATPase component of thiamine-related ABC transporter | 11 | 16 |
| ykoE | + | Possible transmembrane component of thiamine-related ABC transporter | 22 | 32 |
| ykoF | + | Possible ligand-binding protein of thiamine-related ABC transporter | 17 | 23 |
| yuaJ | + | Possible thiamine permease | 6.6 | 8.4 |
| ylmB | + | Similar to acetylornithine deacetylase | 15 | 11 |
Gene names and functions were described by Perkins and Pero (27) and in SubtiList (http://genolist.pasteur.fr/SubtiList).
Transcript ratios were calculated by dividing the average difference values (after normalization) from hybridization experiments of wild-type cells grown to exponential phase in MM without TPP treatment by those with TPP treatment (WT−/WT+) or from hybridization experiments of deregulated mutant cells grown to exponential phase in MM with TPP treatment by those of wild-type cells grown under the same condition (Dereg+/WT+). For some genes (in bold), average difference values were obtained from duplicate probe sets per hybridization experiment: NC, no change in average difference values. Some data were taken from Table 1 in a report by Lee et al. (16).
r value of 0.95 (TPP-treated versus untreated data sets).
r value of 0.85 (TPP-treated TH48 versus TPP-treated PY79 data sets).
Under thi box element control according to report by Rodionov et al. (29).
See reference 32.
Strains with mutant yloS, yuaJ, and ykoD genes overproduce and excrete thiamine products.
As shown in Table 3, there appeared to be a direct correlation between the degree of thiamine deregulation (i.e., expression level of the thiC-lacZ fusion in the presence of TPP) and the excretion of thiamine products. Strain TH22 with mutant thiL alone was only weakly thiamine deregulated and excreted the least amount of thiamine products relative to the TH21 wild-type control strain (ninefold higher). Tx1 with mutant thiL and thiN genes, which showed partial thiamine deregulation, produced a 50-fold-higher level of excreted thiamine products (5 μg/liter). Lastly, Tx26, containing mutant thiL, yuaJ, and ykoD genes, which showed the highest level of thiamine deregulation, produced the highest level of excreted thiamine production (15 μg/liter). This level of production was 100 to 150 times higher than that excreted by the control strain, and slightly over 50% of that excreted by Tx26 was in the form of TPP (data not shown). The level of intracellular thiamine production of all mutants was approximately 500 μg/liter, or about threefold higher than that of the wild-type strain.
The four thiamine mutations, two in Tx26, thiL, and that in Tx1, were next combined into a single strain (as described in Materials and Methods) to determine if higher levels of thiamine overproduction and excretion could be obtained. Although Tx26 is a prototroph, the resulting strain (TH95) grew very poorly on MM unless TPP or nutrient broth (reported as containing significant levels of thiamine precursors HMP and thiazole [8]) was added to the medium. In this respect, TH95 was a stronger bradytroph than Tx1 (i.e., TH95 colonies took longer to appear on MM than Tx1 colonies). Despite this growth defect, TH95 produced threefold more excreted thiamine products in shake flask cultures than Tx26 (Table 5). Moreover, a shift in the excretion profile from predominantly TPP in Tx26 to a combination of thiamine and TMP in TH95 was observed. This shift in the thiamine product profile was confirmed by thiochrome/HPLC (data not shown).
TABLE 5.
Thiamine production of TH95
| Straina | OD600b | Concn produced (μg/liter)
|
|||
|---|---|---|---|---|---|
| Extracellular
|
Intracellular
|
||||
| Thiamine + TMP + TPP | TPP | Thiamine + TMP + TPP | TPP | ||
| Tx26 (ΔthiL::cat4 yuaJ ykoD amyE::thiC-lacZ) | 9.2 | 380 | 390 | 830 (25)c | 370 |
| TH95 (ΔthiL thiN yuaJ ykoD ΩyloA::Tn917) | 10.4 | 1270 | 120 | 1150 (40)c | 270 |
Bacteria were grown in MM supplemented with 2.5% nutrient broth for 18 hours at 37°C. Culture media and cell-free extracts were measured for total thiamine products (thiamin + TMP + TPP) and TPP by a biological assay using S. enterica serovar Typhimurium indicators DM456 (thiD906::MudJ) and DMI856 (thiL934::Tn10), respectively.
OD600, optical density at 600 nm.
Values in parentheses are the estimated extracellular concentrations of thiamine product if all are excreted into the culture medium in μg/liter; see Table 1.
DISCUSSION
It is now well accepted that “thi box” genes are regulated by a mechanism referred to as “riboswitch.” Using mainly in vitro methods, Winkler et al. (39) convincingly showed that TPP alone acts to regulate thiamine gene expression by directly interacting with the nascent RNA transcript at the thi box sequence to block either transcription or translation. The work presented here describes mutagenesis studies that identified and characterized new thiamine-deregulated mutations of Bacillus subtilis that are located within the coding regions of three genes: thiN, yuaJ, and ykoD. The encoded proteins have been lately suggested by in silico analysis to be involved in thiamine salvage and transport. We demonstrate here that indeed an important phenotype of these deregulatory mutants is the ability to excrete significant quantities of thiamine products and, therefore, that an important role of these proteins seems to be the control of TPP levels. It is important to note that the identification of deregulatory mutations within thiamine transport genes is strikingly similar to the recent results of Johansen et al. (12) and Nygaard and Saxild (24), which showed that mutations that increase the activity of the pbuE-encoded purine-specific pump deregulate expression of the G-box regulon by increasing the efflux of hypoxanthine, a metabolite that regulates purine gene expression by a riboswitch mechanism.
Melnick et al. (19) first demonstrated that B. subtilis contained a thiamine pyrophosphorylation activity normally found in yeast. This activity was encoded by yloS, subsequently renamed thiN. We present here experimental evidence confirming the function of this enzyme in thiamine biosynthesis. Indirect genetic evidence for this gene came from mutagenesis studies of the B. subtilis thiL gene, which resulted in mutants that were thiamine bradytrophs rather than strict auxotrophs, as shown for thiL mutants of S. enterica serovar Typhimurium and E. coli. This shows that B. subtilis has two pathways to produce TPP. Direct evidence was obtained by showing that the mutation in Tx1 mapped to thiN and that reconstitution experiments indicated that inactivation of both thiN and thiL together resulted in a synthetic TPP auxotrophy in B. subtilis. In addition, the differential Lac activity displayed by Tx1 in the presence of thiamine (Lac+) and TPP (Lac−) is consistent with the presence of a direct thiamine-to-TPP route (Table 3). By blocking the ThiL reaction (TMP to TPP) and reducing the ThiN (YloS) reaction (THI to TPP) by the Tx1 mutation, the expected intracellular TPP level would be too low to repress thi gene expression. What is not clear, however, is whether these two pathways are linked (Fig. 1). Preliminary genetic studies suggest that B. subtilis contains both thiamine kinase and TMP phosphatase activities (data not shown). However, because the B. subtilis genome does not contain genes that display significant similarity to known thiamine kinase (e.g., E. coli thiK [ycfN]) or TMP phosphatase (yeast PHO3) genes, it will be interesting to determine if these putative enzymes are specific to thiamine biosynthesis or represent activities from housekeeping or other biosynthesis pathways.
The mutations in the Tx26 mutant were found to be allelic to two putative thiamine transport genes, yuaJ (the first lesion) and ykoD (the second lesion). The yuaJ gene is predicted to encode a thiamine permease (passive transport) that contains six predicted trans-membrane domains (29). The ykoD gene is predicted to encode an ATPase activity, which together with the ykoC and ykoE gene products is predicted to form an ABC transporter (29). The Tx26 mutations were found to generate three phenotypes: deregulation of thiamine gene expression, an increase in pyrithiamine resistance, and an increase in extracellular levels of thiamine products. The fact that inactivation of both yuaJ and ykoD was required to cause all three phenotypes suggested that these transporters control the influx of TPP and other thiamine products into the cell (mutations that inactivate efflux transporters would not be consistent with our observation of higher extracellular levels of thiamine product with Tx26). Based on these results, we recommend that yuaJ be renamed thiT and that the ykoFEDC operon be renamed thiUVWX. Although in silico analysis does not indicate a function for ykoF, we have tentatively designated it a thi gene because it is cotranscribed with the thiVWX genes and because the crystal structure of its encoded protein indicates that it binds thiamine molecules (6).
Both lacZ reporter fusion (Table 3) and microarray experiments indicated that the expression of all known thi box-containing genes (e.g., thiC, thiOSGFD, ykoFEDC, and yuaJ) was highly derepressed in the Tx26 mutant. Since a key phenotype of this mutant is the efflux of thiamine products from the cell, gene deregulation is most likely caused by changes in TPP intracellular levels. Interestingly, the addition of exogenous HMP appeared to partially regulate thiamine gene expression (Table 3) in Tx26. We do not believe HMP is functioning as a repressor (or corepressor) independent of TPP because lacZ reporter fusion experiments showed that HMP did not repress thiC expression in the Tx1 mutant. It seems more likely that HMP enters the cell by a different transport route and is converted to the TPP effector via the salvage pathway (HMP to HMP phosphate to TMP to TPP), which then interacts with thi box sequences of the nascent mRNA to repress thi gene expression via the riboswitch mechanism.
Finally, an important application of our study was that these thiamine-deregulated mutants could be further manipulated to generate new strains that excrete more thiamine products. The combination of four mutations in thiL, thiN, thiT (yuaJ), and thiW (ykoD) has led to a strain that under 10-liter-scale fermentation conditions excreted thiamine products in the mg/liter range (data not shown). Genetic engineering of the thiamine biosynthesis and precursor genes should lead to strains with a further increase in thiamine production.
Acknowledgments
We gratefully acknowledge Werner Bretzel for analytical support, Markus Goese for fermentation support, and Jian-ming Lee for microarray analysis of thiamine-deregulated mutants. We thank Hans-Peter Hohmann for helpful discussion and critical reading of the manuscript. We also thank Tadhg Begley and Diana Downs for providing data before publication and for their expertise and support during the course of this work.
REFERENCES
- 1.Begley, T. P., D. M. Downs, S. E. Ealick, F. W. McLafferty, A. P. Van Loon, S. Taylor, N. Campobasso, H. J. Chiu, C. Kinsland, J. J. Reddick, and J. Xi. 1999. Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 171:293-300. [DOI] [PubMed] [Google Scholar]
- 2.Bolivar, F., R. L. Rodriguez, M. C. Betlach, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. I. Amplicillin-resistant derivatives of the plasmid pMB9. Gene 2:75-91. [DOI] [PubMed] [Google Scholar]
- 3.Chiu, H. J., J. J. Reddick, T. P. Begley, and S. E. Ealick. 1999. Crystal structure of thiamin phosphate synthase from Bacillus subtilis at 1.25 Å resolution. Biochemistry 38:6460-6470. [DOI] [PubMed] [Google Scholar]
- 4.Cutting, S., and P. B. Vander Horn. 1990. Genetic analysis, p. 74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, New York, N.Y.
- 5.Cutting, S., M. Anderson, E. Lysenko, A. Page, T. Tomoyasu, K. Tatematsu, T. Tatsuta, L. Kroos, and T. Ogura. 1997. SpoVM, a small protein essential to development in Bacillus subtilis, interacts with the ATP-dependent protease FtsH. J. Bacteriol. 179:5534-5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devedjiev, Y., Y. Surendranath, U. Derewenda, A. Gabrys, D. R. Cooper, R. Zhang, L. Lezondra, A. Joachimiak, and Z. S. Derewenda. 2004. The structure and ligand binding properties of the B. subtilis YkoF gene product, a member of a novel family of thiamin/HMP-binding proteins. J. Mol. Biol. 343:395-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Difco Laboratories. 1998. Difco manual, 11th ed. Difco Laboratories, Sparks, Md.
- 8.Downs, D. Personal communication.
- 9.Downs, D. M., and L. Petersen. 1994. apbA, a new genetic locus involved in thiamine biosynthesis in Salmonella typhimurium. J. Bacteriol. 176:4858-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guérout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57-61. [DOI] [PubMed] [Google Scholar]
- 11.Harwood, C. R., and A. R. Archibald. 1990. Growth, maintenance and general techniques, p. 1-26. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, New York, N.Y.
- 12.Johansen, L. E., P. Nygaard, C. Lassen, Y. Agersø, and H. H. Saxild. 2003. Definition of a second Bacillus subtilis pur regulon comprising the pur and xpt-pbuX operons plus pbuG, nupG (yxjA), and pbuE (ydhL). J. Bacteriol. 185:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawasaki, T., and Y. Nose. 1969. Thiamine regulatory mutants in Escherichia coli. J. Biochem. (Tokyo) 65:414-425. [DOI] [PubMed] [Google Scholar]
- 14.Lawhorn, B. G., S. Y. Gerdes, and T. P. Begley. 2004. A genetic screen for the identification of thiamin metabolic genes. J. Biol. Chem. 279:43555-43559. [DOI] [PubMed] [Google Scholar]
- 15.Lawhorn, B. G., R. A. Mehl, and T. P. Begley. 2004. Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction. Org. Biomol. Chem. 2:2538-2546. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J.-M., S. Zhang, S. Saha, S. Santa Anna, C. Jiang, and J. Perkins. 2001. RNA expression analysis using an antisense Bacillus subtilis genome array. J. Bacteriol. 183:7371-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandal, M., B. Boese, J. E. Barrik, W. C. Winkler, and R. R. Breaker. 2003. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113:577-586. [DOI] [PubMed] [Google Scholar]
- 18.Maniatis, T., E. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Melnick, J., E. Lis, J.-H. Park, C. Kinsland, H. Mori, T. Baba, J. Perkins, G. Schyns, O. Vassieva, A. Osterman, and T. P. Begley. 2004. Identification of the two missing bacterial genes involved in thiamine salvage: thiamine pyrophosphokinase and thiamine kinase. J. Bacteriol. 186:3660-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Miranda-Rios, J., M. Navarro, and M. Soberson. 2001. A conserved RNA structure (thi box) is involved in regulation of thiamin biosynthesis gene expression in bacteria. Proc. Natl. Acad. Sci. USA 98:9736-9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mironov, A. S., I. Gusarov, R. Rafikov, L. E. Lopez, K. Shatalin, R. A. Kreneva, D. A. Perumov, and E. Nudler. 2002. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. Cell 111:747-756. [DOI] [PubMed] [Google Scholar]
- 23.Nosaka, K., Y. Kaneko, H. Nishimura, and A. Iwashima. 1993. Isolation and characterization of a thiamin pyrophoshokinase gene, THI80, from Saccharomyces cerevisiae. J. Biol. Chem. 268:17440-17447. [PubMed] [Google Scholar]
- 24.Nygaard, P., and H. H. Saxild. 2005. The purine efflux pump PbuE in Bacillus subtilis modulates expression of the PurR and G-box (XptR) regulons by adjusting the purine base pool size. J. Bacteriol. 187:791-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, J.-H., K. Burns, C. Kinsland, and T. P. Begley. 2004. Characterization of two kinases involved in thiamine pyrophosphate and pyridoxal phosphate biosynthesis in Bacillus subtilis: 4-amino-5-hydroxymethyl-2-methylpyrimidine kinase and pyridoxal kinase. J. Bacteriol. 186:1571-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, J. H., P. C. Dorrestein, H. Zhai, C. Kinsland, F. W. McLafferty, and T. P. Begley. 2003. Biosynthesis of the thiazole moiety of thiamin pyrophosphate (vitamin B1). Biochemistry 42:12430-12438. [DOI] [PubMed] [Google Scholar]
- 27.Perkins, J. B., and J. Pero. 2001. Vitamin biosynthesis, p. 271-286. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 28.Petersen, L. A., and D. M. Downs. 1997. Identification and characterization of an operon in Salmonella typhimurium involved in thiamine biosynthesis. J. Bacteriol. 179:4894-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodionov, D. A., A. G. Viteschak, A. A. Mironov, and M. S. Gelfand. 2002. Comparative genomics of thiamin biosynthesis in prokaryotes. J. Biol. Chem. 277:48949-48959. [DOI] [PubMed] [Google Scholar]
- 30.Schowen, R. 1998. Thiamin-dependent enzymes, p. ••-••. In L. Sinnott (ed.), Comprehensive catalysis. Academic Press, San Diego. Calif.
- 31.Seror, S. Personal communication.
- 32.Toms, A. V., A. L. Haas, J. H. Park, T. P. Begley, and S. E. Ealick. 2005. Structural characterization of the regulatory proteins TenA and TenI from Bacillus subtilis and identification of TenA as a thiaminase II. Biochemistry 44:2319-2329. [DOI] [PubMed] [Google Scholar]
- 33.Vagner, V., E. Dervyn, and S. D. Erhlick. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 34.Vandeyar, M. A., and S. A. Zahler. 1986. Chromosomal insertions of Tn917 in Bacillus subtilis. J. Bacteriol. 167:530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259-265. [DOI] [PubMed] [Google Scholar]
- 36.Webb, E., K. Claas, and D. M. Downs. 1998. thiBPQ encodes an ABC transporter required for transport of thiamine and thiamine pyrophosphate in Salmonella typhimurium. J. Biol. Chem. 273:8946-8950. [DOI] [PubMed] [Google Scholar]
- 37.Webb, E., and D. M. Downs. 1997. Characterization of thiL, encoding thiamin-monophosphate kinase, in Salmonella typhimurium. J. Biol. Chem. 272:15702-15707. [DOI] [PubMed] [Google Scholar]
- 38.Webb, E., F. Febres, and D. M. Downs. 1996. Thiamine pyrophosphate (TPP) negatively regulates transcription of some thi genes in Salmonella typhimurium. J. Bacteriol. 178:2533-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler, W. C., A. Nahvi, and R. R. Breaker. 2002. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419:952-956. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Y., and T. P. Begley. 1997. Cloning, sequencing, and regulation of thiA, a thiamin biosynthesis gene from Bacillus subtilis. Gene 198: 73-82. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, Y., S. V. Taylor, H.-J. Chiu, and T. P. Begley. 1997. Characterization of the Bacillus subtilis thiC operon involved in thiamine biosynthesis. J. Bacteriol. 179:3030-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]