Abstract
Bacterial histidine kinases have been proposed as targets for the discovery of new antibiotics, yet few specific inhibitors of bacterial histidine kinases have been reported. We report here a novel thienopyridine (TEP) compound that inhibits bacterial histidine kinases competitively with respect to ATP but does not comparably inhibit mammalian serine/threonine kinases. Although it partitions into membranes and does not inhibit the growth of bacterial or mammalian cells, TEP could serve as a starting compound for a new class of histidine kinase inhibitors with antibacterial activity.
Two-component regulatory systems (TCSs) are found in all eubacterial species except mycoplasmas (18, 22). TCSs are one of the main signal transduction systems that allow bacteria to adapt to changing environmental conditions (reviewed in references 3, 19, 34, 42, 45, and 46). Consequently, each bacterial species contains multiple TCSs that are required for a wide range of responses that affect growth, survival, and virulence (reviewed in references 15, 18, 21, 22, and 43). In several different bacteria, at least one TCS is essential for growth in laboratory media through its regulation of essential genes or processes (5, 9, 10, 16, 20, 23, 26, 29, 31, 37, 43, 44). A typical TCS consists of a histidine kinase (HK) and a cognate response regulator (RR). HKs autophosphorylate at conserved histidine residues in response to environmental or metabolic signals (reviewed in references 7, 18, 19, 22, 34, 42, 45, and 46). Phosphoryl groups on the histidine residues of HKs are then transferred to conserved aspartate residues in the receiver domains of cognate RRs. Phosphorylation of an RR alters its conformation and its interactions with other components of the signal transduction pathway, often resulting in a change in the ability of the RR to bind to DNA and influence transcription (7, 18, 19, 22, 34, 42, 45, 46).
Because of their central roles in bacterial physiology and their essentiality in some cases, TCSs have frequently been proposed as targets for new antibiotics (1, 21, 27, 30, 35, 39, 40). A general inhibitor of multiple TCSs would be highly desirable, because it is unlikely that antibiotic resistance would develop as quickly to multiple targets. However, the quest for small molecules that specifically inhibit TCS function has proven to be particularly challenging. The most common inhibitors reported to date are hydrophobic compounds that inhibit HK autokinase activity noncompetitively with respect to ATP (see references 17, 39, and 40). However, these compounds generally inhibit by binding to the four-helix bundle that contains the conserved histidine residues, thereby causing aggregation of the HKs (40, 41). This kind of inhibitor usually prevents bacterial growth by nonspecific mechanisms independent of HK inhibition (17, 39, 41). Several recently described hydrophobic inhibitors of HKs may fall into this class (13, 24).
In contrast, competitive ATP inhibitors of HKs have not generally been reported, except for standard nucleotide analogues, such as AMP-PNP, which are not drug candidates (33, 40, 41). As discussed recently by Stephenson and Hoch (39), competitive inhibitors of HKs present a considerable specificity challenge. HKs per se have not been found in mammals; however, the unusual Bergerat ATP binding fold in HKs is shared with several proteins, such as mammalian homologues of the MutL mismatch repair protein, the Hsp90 chaperone, and certain mitochondrial kinases (6, 39, 48). Indeed, the antifungal compound radicicol is a competitive ATP inhibitor of HKs and of mammalian α-keto acid dehydrogenase, which also contains a Bergerat fold (2). On the other hand, significant advances have been made in discovering specific competitive ATP inhibitors of other classes of mammalian kinases, despite strong conservation of the ATP binding site (reviewed in 4, 11, 32, and 36). These advances have relied on a combination of screening, combinatorial chemistry, and structure-based design, often starting with a compound that lacks specificity or other desirable medicinal chemistry properties. Thus, it is important to find other competitive ATP inhibitors of HKs which might serve as a starting point for maximizing specificity.
We report here a new class of competitive ATP inhibitors of bacterial HKs, 3,6-diamino-5-cyano-4-phenyl-thieno[2,3-b]pyridine-2-carboxylic acid (4-bromo-phenyl)-amide (TEP) (Fig. 1). TEP (CAS [Chemical Abstracts] registry number 332175-01-6) was obtained from AsInEx Company (Moscow, Russia) or ChemBridge Corporation (San Diego, CA). The structure of the compound used in this study was confirmed by physical chemical methods and by resynthesis. We identified TEP as an inhibitor of TCS function in a high-throughput screen of compound libraries. In this screen, we used a coupled assay containing the HpkA HK and DrrA RR of Thermotoga maritima that allows multiple turnovers of HpkA, linear formation of phosphorylated DrrA, and Michaelis-Menten analysis of inhibitors of HpkA autokinase activity or phosphoryl group transfer from HpkA to DrrA (12). The coupled assay measures the formation of phosphorylated DrrA (Drr-P) in reaction mixtures containing catalytic amounts of HpkA and excess amounts of ATP and DrrA, which are treated as substrates in the kinetic analyses (Fig. 2 and 3) (12). In the high-throughput screens, we used a filter format of the coupled assay, as described previously (12), which employs 96-well Immobilon P filter plates (Millipore, Inc.).
FIG. 1.
Chemical structure of TEP {3,6-diamino-5-cyano-4-phenyl-thieno[2,3-b]pyridine-2-carboxylic acid (4-bromo-phenyl)-amide}.
FIG. 2.
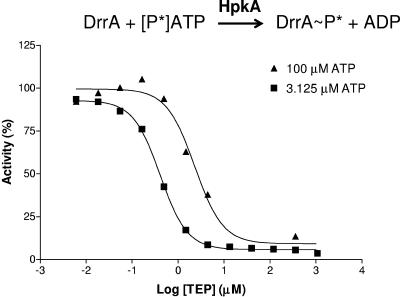
Dependence of the IC50 of TEP on ATP concentration in the coupled HpkA-DrrA reaction. IC50s were determined as previously described (12) in reaction mixtures containing 10% (vol/vol) dimethyl sulfoxide. The IC50s were 0.41 μM and 2.30 μM at ATP concentrations of 3.13 μM (▪) and 100 μM (▴), respectively. Standard errors were less than 20%.
FIG. 3.
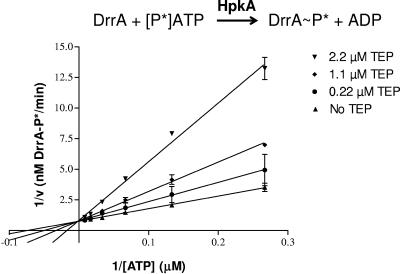
Lineweaver-Burk analysis demonstrating competitive ATP inhibition of the coupled HpkA-DrrA reaction by TEP. Initial rates of the coupled reaction were determined at various concentrations of ATP as previously described (12) in the presence of the indicated concentrations of TEP and 5% (vol/vol) dimethyl sulfoxide. Lines were drawn based on linear regression analysis, and standard errors are indicated. Intersection on the ordinate indicates competitive ATP inhibition. The experiment was repeated independently numerous times, with an average Ki of TEP of 0.62 ± 0.11 μM.
Fifty-percent inhibitory concentration (IC50) determinations performed by methods described previously (12) showed that TEP competes with ATP in the coupled assay (Fig. 2). When the ATP concentration in the reaction mixtures was dropped from 100 μM to 3.1 μM, the IC50 dropped from 2.30 μM to 0.41 μM, indicating stronger inhibition at the lower ATP concentration (Fig. 2). In contrast, the formation of DrrA-P in the coupled assay was insensitive to variations of the DrrA amount at fixed concentrations of ATP (data not shown). Inhibition analysis on Lineweaver-Burk plots demonstrated that TEP was indeed a competitive inhibitor of the coupled reaction, with a Ki of 0.62 μM (Fig. 3). By comparison, TEP was a much stronger competitive inhibitor of the coupled reaction than the nonhydrolyzable ATP analogue AMP-PNP was (12, 41). The Ki for AMP-PNP in the coupled reaction was 100 μM, and the IC50 of AMP-PNP was 67.3 μM in reaction mixtures containing 1.0 μM ATP (12).
The only step in the coupled assay that uses ATP directly is HpkA autophosphorylation. We confirmed that TEP was competing with ATP by performing autokinase assays as described previously (12) for purified, soluble, carboxy-terminal domains of the following histidine kinases, where the number in parentheses refers to the amino acids removed from amino termini and the reference cited reports the same or a very similar truncated construct: His10-HpkA (Δ77) from T. maritima (12), glutathione S-transferase-VicK (YycG) (Δ36) from Streptococcus pneumoniae (9), maltose-binding protein-VanS (Δ94) from Enterococcus faecium (47), and His6-EnvZ (Δ222) from Escherichia coli (8). TEP inhibited the autokinase activities of all four of these HKs with the indicated IC50s in reaction mixtures containing 10 μM ATP and 10% (vol/vol) dimethyl sulfoxide: HpkA, 5.5 μM (standard errors, +3.2 or −2.0 μM; n = 4); VicK, 13.2 μM (+4.8/−3.5 μM; n = 5); VanS, 103.8 μM (+16.5/−14.2 μM; n = 5); and EnvZ, 26.8 μM (+7.7/−6.0 μM; n = 1). Thus, the inhibition of autokinase activity was relatively strong for two different bacterial histidine kinases, intermediate for one, and weak for another. Moreover, TEP was not a general kinase inhibitor. TEP did not strongly inhibit (IC50 > 20 μM) any of 10 common mammalian serine/threonine kinases (protein kinase B [PKB, AKT], transforming growth factor β-R1 [TGFβ-R1], TGFβ-R2, Ca2+/calmodulin-dependent kinase II [CAMKII], cyclin-dependent kinase 2 [CDK2], CDK4, glycogen synthase kinase 3β [GSK3β], protein kinase C [PKC], mixed lineage kinase 7 [MLK7], and p38 mitogen-activated protein kinase [p38 MAPK]) (data not shown).
TEP is a hydrophobic compound that starts to become insoluble in aqueous solutions lacking dimethyl sulfoxide at concentrations above 20 μg per ml (∼44 μM). Stephenson et al. were the first to show that many hydrophobic noncompetitive ATP inhibitors of bacterial HKs act by a nonspecific mechanism that leads to aggregation (41). In contrast, competitive ATP inhibitors, such as AMP-PNP, do not lead to aggregation (12, 41). We tested whether TEP led to an aggregation of the HpkA HK by comparing autokinase inhibition with the disappearance of HpkA dimers upon glutaraldehyde cross-linking as described previously (12). Inhibition by TEP is not correlated with the loss of an HpkA dimer (Fig. 4); therefore, TEP does not inhibit HpkA by causing extensive aggregation.
FIG. 4.
Inhibition of autophosphorylation and lack of aggregation of His-HpkA77 in the presence of various concentrations of TEP. The inhibition of His-HpkA77 autophosphorylation and percentage of His-HpkA dimer remaining following cross-linking with glutaraldehyde were determined as previously described (12) in reaction mixtures containing 2 μM His-HpkA77 and 20 μM ATP that were incubated at 42°C for 30 min. Reaction mixtures were then divided into two aliquots, one of which was quenched while the other was cross-linked with glutaraldehyde for 30 min longer before being quenched (12). Samples lacking TEP were defined as 100% autokinase activity or dimer amount. Standard errors are indicated, and the experiment was performed independently twice. At 20 μM, the IC50 of TEP was 2.35 μM (+0.08/−0.09; n = 2) for the autokinase reaction, whereas the DC50 ([TEP] at which 50% dimer was present) was >1,700 μM for loss of the His-HpkA77 dimer.
Finally, we tested whether TEP inhibited the growth of bacterial cells or was toxic to mammalian cells. TEP did not inhibit (MIC, >256 μg per ml) a panel of gram-positive and -negative bacteria, including Streptococcus pneumoniae R6, Enterococcus faecium SP180, Enterococcus faecalis SP409, Haemophilus influenzae ATCC 49247, Escherichia coli K-12 MG1655, E. coli MG1655 envA1, and E. coli MG1655 tolC::kan (25), and was not toxic to rat R6 myoblasts in standard assays (38). In addition, TEP did not affect the function of the VanRS TCS of Enterococcus faecium in a reporter assay reported previously (14). We found that TEP exhibited fluorescence in aqueous solution (excitation wavelength, 348 nm; emission wavelength, 702 nm), but not in 100% dimethyl sulfoxide. Because of this fluorescence, we examined Staphylococcus aureus cells treated with TEP by epifluorescence microscopy (Fig. 5B). For a control, we stained cells with DAPI (4′,6′-diamidino-2-phenylindole), which binds to nucleic acids (Fig. 5A). DAPI stained compact nucleoids that had already separated in cells that were about to divide (Fig. 5A). In contrast, TEP localized to the periphery of cells and to the septa of predivisional cells. This pattern suggests that either the cell membrane or peptidoglycan was stained by TEP. Consistent with staining of the cell membrane, an L-form strain of S. aureus, which lacks a cell wall (28), showed peripheral staining with TEP, as did other bacteria, such as S. pneumoniae R6, and mammalian tissue culture cells (data not shown). In other experiments, partition of TEP into the membranes of Streptococcus pneumoniae R6 did not result in a detectable MIC but did increase the transcript amounts of several heat shock and stress response proteins in microarray analyses (data not shown; see reference 31).
FIG. 5.
TEP stains the peripheries of bacterial cells. Staphylococcus aureus strain ATCC 12598 was grown in brain heart infusion broth and stained with (A) 0.2 μg per ml DAPI (Sigma) or (B) 8.0 μg per ml TEP in 1% dimethyl sulfoxide. Suspensions were observed immediately by phase-contrast microscopy (lower panels) and by epifluorescence microscopy (upper panels) using a 100× oil emersion lens with excitation at 330 to 380 nm and emission above 420 nm. DAPI is a control for staining bacterial nucleoids. Peripheral staining with TEP was also observed for L-form cells of S. aureus, which lack a cell wall, and for mammalian tissue culture cells (data not shown).
In summary, TEP is a new class of competitive ATP inhibitors of bacterial HK autophosphorylation that does not cause extensive protein aggregation. TEP contains a core ring structure (Fig. 1) that resembles that of purines, but the structural basis for inhibition of HKs by TEP is yet to be determined. On the other hand, TEP does not strongly inhibit mammalian serine/threonine kinases and thus is not a general inhibitor of protein kinases. TEP is hydrophobic and seems to partition into the membranes of cells, which probably prevents inhibition of the growth of bacterial cells. TEP could serve as the starting compound for new inhibitors that specifically inhibit bacterial HKs. Such inhibitors could be useful in structure-function studies of the mechanism of autophosphorylation by bacterial HKs. In addition, a structure-activity relationship study starting with TEP could determine whether inhibition of HK autokinase activity can be maintained in derivatives that are imported into the cytoplasm of bacterial cells.
Acknowledgments
We thank Jana Chain, Wen Luo, Debbie Mullen, Bill Vassiliou, and Jingyong Zhao for helpful discussions and Krystyna Kazmierczak for reading the manuscript.
This work was supported by resources provided by Lilly Research Laboratories and Indiana University at Bloomington.
REFERENCES
- 1.Barrett, J. F., and J. A. Hoch. 1998. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42:1529-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besant, P. G., M. V. Lasker, C. D. Bui, and C. W. Turck. 2002. Inhibition of branched-chain alpha-keto acid dehydrogenase kinase and Sln1 yeast histidine kinase by the antifungal antibiotic radicicol. Mol. Pharmacol. 62:289-296. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma, J. J., and E. A. Groisman. 2003. Making informed decisions: regulatory interactions between two-component systems. Trends Microbiol. 11:359-366. [DOI] [PubMed] [Google Scholar]
- 4.Cherry, M., and D. H. Williams. 2004. Recent kinase and kinase inhibitor X-ray structures: mechanisms of inhibition and selectivity insights. Curr. Med. Chem. 11:663-673. [DOI] [PubMed] [Google Scholar]
- 5.Dubrac, S., and T. Msadek. 2004. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J. Bacteriol. 186:1175-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta, R., and M. Inouye. 2000. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 25:24-28. [DOI] [PubMed] [Google Scholar]
- 7.Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. [DOI] [PubMed] [Google Scholar]
- 8.Dutta, R., T. Yoshida, and M. Inouye. 2000. The critical role of the conserved Thr247 residue in the functioning of the osmosensor EnvZ, a histidine kinase/phosphatase, in Escherichia coli. J. Biol. Chem. 275:38645-38653. [DOI] [PubMed] [Google Scholar]
- 9.Echenique, J. R., and M. C. Trombe. 2001. Competence repression under oxygen limitation through the two-component MicAB signal-transducing system in Streptococcus pneumoniae and involvement of the PAS domain of MicB. J. Bacteriol. 183:4599-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabret, C., and J. A. Hoch. 1998. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J. Bacteriol. 180:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer, P. M. 2004. The design of drug candidate molecules as selective inhibitors of therapeutically relevant protein kinases. Curr. Med. Chem. 11:1563-1583. [DOI] [PubMed] [Google Scholar]
- 12.Foster, J. E., Q. Sheng, J. R. McClain, M. Bures, T. I. Nicas, K. Henry, M. E. Winkler, and R. Gilmour. 2004. Kinetic and mechanistic analyses of new classes of inhibitors of two-component signal transduction systems using a coupled assay containing HpkA-DrrA from Thermotoga maritima. Microbiology 150:885-896. [DOI] [PubMed] [Google Scholar]
- 13.Furuta, E., K. Yamamoto, D. Tatebe, K. Watabe, T. Kitayama, and R. Utsumi. 2005. Targeting protein homodimerization: a novel drug discovery system. FEBS Lett. 579:2065-2070. [DOI] [PubMed] [Google Scholar]
- 14.Grissom-Arnold, J., W. E. Alborn, Jr., T. I. Nicas, and S. R. Jaskunas. 1997. Induction of VanA vancomycin resistance genes in Enterococcus faecalis: use of a promoter fusion to evaluate glycopeptide and nonglycopeptide induction signals. Microb. Drug Resist. 3:53-64. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, L., and M. Perego. 2002. Two-component signal transduction in Enterococcus faecalis. J. Bacteriol. 184:5819-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, L. E., and M. Perego. 2004. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J. Bacteriol. 186:7951-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilliard, J. J., R. M. Goldschmidt, L. Licata, E. Z. Baum, and K. Bush. 1999. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob. Agents Chemother. 43:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoch, J. A., and T. J. Silhavy (ed.). 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 19.Hoch, J. A., and K. I. Varughese. 2001. Keeping signals straight in phosphorelay signal transduction. J. Bacteriol. 183:4941-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell, A., S. Dubrac, K. K. Andersen, D. Noone, J. Fert, T. Msadek, and K. Devine. 2003. Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol. Microbiol. 49:1639-1655. [DOI] [PubMed] [Google Scholar]
- 21.Hubbard, J., M. K. Burnham, and J. P. Throup. 2003. Pathogenicity and histidine kinases: approaches toward the development of a new generation of antibiotics, p. 459-481. In M. Inouye and R. Dutta (ed.), Histidine kinases in signal transduction. Academic Press, San Diego, Calif.
- 22.Inouye, M., and R. Dutta (ed.). 2003. Histidine kinases in signal transduction. Academic Press, San Diego, Calif.
- 23.Kallipolitis, B. H., and H. Ingmer. 2001. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 204:111-115. [DOI] [PubMed] [Google Scholar]
- 24.Kitayama, T., R. Iwabuchi, S. Minagawa, F. Shiomi, J. Cappiello, S. Sawada, R. Utsumi, and T. Okamoto. 2004. Unprecedented olefin-dependent histidine-kinase inhibitory of zerumbone ring-opening material. Bioorg. Med. Chem. Lett. 14:5943-5946. [DOI] [PubMed] [Google Scholar]
- 25.Kulanthaivel, P., A. J. Kreuzman, M. A. Strege, M. D. Belvo, T. A. Smitka, M. Clemens, J. R. Swartling, K. L. Minton, F. Zheng, E. L. Angleton, D. Mullen, L. N. Jungheim, V. J. Klimkowski, T. I. Nicas, R. C. Thompson, and S. B. Peng. 2004. Novel lipoglycopeptides as inhibitors of bacterial signal peptidase I. J. Biol. Chem. 279:36250-36258. [DOI] [PubMed] [Google Scholar]
- 26.Le Breton, Y., G. Boel, A. Benachour, H. Prevost, Y. Auffray, and A. Rince. 2003. Molecular characterization of Enterococcus faecalis two-component signal transduction pathways related to environmental stresses. Environ. Microbiol. 5:329-337. [DOI] [PubMed] [Google Scholar]
- 27.Lyon, G. J., P. Mayville, T. W. Muir, and R. P. Novick. 2000. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc. Natl. Acad. Sci. USA 97:13330-13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madoff, S. (ed.). 1986. The bacterial L-forms. Marcel Dekker, Inc., New York, N.Y.
- 29.Martin, P. K., T. Li, D. Sun, D. P. Biek, and M. B. Schmid. 1999. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J. Bacteriol. 181:3666-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita, M., and K. D. Janda. 2002. Histidine kinases as targets for new antimicrobial agents. Bioorg. Med. Chem. 10:855-867. [DOI] [PubMed] [Google Scholar]
- 31.Ng, W. L., G. T. Robertson, K. M. Kazmierczak, J. Zhao, R. Gilmour, and M. E. Winkler. 2003. Constitutive expression of PcsB suppresses the requirement for the essential VicR (YycF) response regulator in Streptococcus pneumoniae R6. Mol. Microbiol. 50:1647-1663. [DOI] [PubMed] [Google Scholar]
- 32.Pratt, D. J., J. A. Endicott, and M. E. Noble. 2004. The role of structure in kinase-targeted inhibitor design. Curr. Opin. Drug Discov. Dev. 7:428-436. [PubMed] [Google Scholar]
- 33.Ramstrom, H., M. Bourotte, C. Philippe, M. Schmitt, J. Haiech, and J. J. Bourguignon. 2004. Heterocyclic bis-cations as starting hits for design of inhibitors of the bifunctional enzyme histidine-containing protein kinase/phosphatase from Bacillus subtilis. J. Med. Chem. 47:2264-2275. [DOI] [PubMed] [Google Scholar]
- 34.Robinson, V. L., D. R. Buckler, and A. M. Stock. 2000. A tale of two components: a novel kinase and a regulatory switch. Nat. Struct. Biol. 7:626-633. [DOI] [PubMed] [Google Scholar]
- 35.Roychoudhury, S., N. A. Zielinski, A. J. Ninfa, N. E. Allen, L. N. Jungheim, T. I. Nicas, and A. M. Chakrabarty. 1993. Inhibitors of two-component signal transduction systems: inhibition of alginate gene activation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 90:965-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawyers, C. L. 2003. Opportunities and challenges in the development of kinase inhibitor therapy for cancer. Genes Dev. 17:2998-3010. [DOI] [PubMed] [Google Scholar]
- 37.Schär, J., A. Sickmann, and D. Beier. 2005. Phosphorylation-independent activity of atypical response regulators of Helicobacter pylori. J. Bacteriol. 187:3100-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scudiero, D. A., R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff, and M. R. Boyd. 1988. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 48:4827-4833. [PubMed] [Google Scholar]
- 39.Stephenson, K., and J. A. Hoch. 2004. Developing inhibitors to selectively target two-component and phosphorelay signal transduction systems of pathogenic microorganisms. Curr. Med. Chem. 11:765-773. [DOI] [PubMed] [Google Scholar]
- 40.Stephenson, K., and J. A. Hoch. 2002. Two-component and phosphorelay signal-transduction systems as therapeutic targets. Curr. Opin. Pharmacol. 2:507-512. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson, K., Y. Yamaguchi, and J. A. Hoch. 2000. The mechanism of action of inhibitors of bacterial two-component signal transduction systems. J. Biol. Chem. 275:38900-38904. [DOI] [PubMed] [Google Scholar]
- 42.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 43.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35:566-576. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe, T., Y. Hashimoto, Y. Umemoto, D. Tatebe, E. Furuta, T. Fukamizo, K. Yamamoto, and R. Utsumi. 2003. Molecular characterization of the essential response regulator protein YycF in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 6:155-163. [DOI] [PubMed] [Google Scholar]
- 45.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 46.Wolanin, P. M., P. A. Thomason, and J. B. Stock. 2002. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol. 3:REVIEWS3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright, G. D., T. R. Holman, and C. T. Walsh. 1993. Purification and characterization of VanR and the cytosolic domain of VanS: a two-component regulatory system required for vancomycin resistance in Enterococcus faecium BM4147. Biochemistry 32:5057-5063. [DOI] [PubMed] [Google Scholar]
- 48.Yang, W. 2003. Histidine kinases: extended relationship with GHL ATPases, p. 219-236. In M. Inouye and R. Dutta (ed.), Histidine kinases in signal transduction. Academic Press, San Diego, Calif.