Abstract
The alternative sigma factor σB of Staphylococcus aureus controls the expression of a variety of genes, including virulence determinants and global regulators. Genetic manipulations and transcriptional start point (TSP) analyses showed that the sigB operon is transcribed from at least two differentially controlled promoters: a putative σA-dependent promoter, termed sigBp1, giving rise to a 3.6-kb transcript covering sa2059-sa2058-rsbU-rsbV-rsbW-sigB, and a σB-dependent promoter, sigBp3, initiating a 1.6-kb transcript covering rsbV-rsbW-sigB. TSP and promoter-reporter gene fusion experiments indicated that a third promoter, tentatively termed sigBp2 and proposed to lead to a 2.5-kb transcript, including rsbU-rsbV-rsbW-sigB, might govern the expression of the sigB operon. Environmental stresses, such as heat shock and salt stress, induced a rapid response within minutes from promoters sigBp1 and sigBp3. In vitro, the sigBp1 promoter was active in the early growth stages, while the sigBp2 and sigBp3 promoters produced transcripts throughout the growth cycle, with sigBp3 peaking around the transition state between exponential growth and stationary phase. The amount of sigB transcripts, however, did not reflect the concentration of σB measured in cell extracts, which remained constant over the entire growth cycle. In a guinea pig cage model of infection, sigB transcripts were as abundant 2 and 8 days postinoculation as values found in vitro, demonstrating that sigB is indeed transcribed during the course of infection. Physical interactions between staphylococcal RsbU-RsbV, RsbV-RsbW, and RsbW-σB were inferred from a yeast (Saccharomyces cerevisiae) two-hybrid approach, indicating the presence of a partner-switching mechanism in the σB activation cascade similar to that of Bacillus subtilis. The finding that overexpression of RsbU was sufficient to trigger an immediate and strong activation of σB, however, signals a relevant difference in the regulation of σB activation between B. subtilis and S. aureus in the cascade upstream of RsbU.
Staphylococcus aureus is one of the leading causes for nosocomial- and community-acquired infections (11, 46). Its capacity to cause a wide spectrum of diseases and to survive in unfavorable conditions is due to a network of global regulatory elements enabling it to rapidly sense changes and to respond appropriately. These elements comprise two-component regulatory systems, including the agr locus, the SarA protein family, and alternative σ factors (reviewed in reference 16 and references within).
Computational analysis of the published staphylococcal genomes suggests that S. aureus harbors only two alternative sigma factors, σB and σH (45). σB of S. aureus was demonstrated to influence the expression of a variety of genes (6, 26, 33, 43, 78, 79), including virulence factors (23, 27, 34, 38, 43, 50, 51, 52, 62, 78, 79) and regulatory elements (5, 6, 21, 26, 34, 47, 60, 66). Moreover, it affects methicillin and glycopeptide resistance (4, 56, 65, 74), biofilm production (58), and internalization into endothelial cells (51) and is involved in the general stress response (12, 25, 27, 34, 42, 43). Although σB interferes with the expression of virulence determinants, its effect in animal models seemed not apparent (12, 23, 34, 52) except in a murine model of septic arthritis (35).
The genetic organization of the staphylococcal sigB operon closely resembles that of the distal part of the homologous Bacillus subtilis operon (reviewed in references 32 and 57), and its corresponding products were shown to fulfil similar functions: the staphylococcal RsbU positively regulates σB activity (27, 34, 54), RsbW is an anti-σ factor (50), and SigB operates as an alternative σ factor (21, 50). The drastic decrease in σB activity upon deletion of rsbV in S. aureus furthermore supports the role of RsbV as an anti-anti-σ factor (54). However, apart from physical interaction between RsbW and σB (50), very little experimental evidence exists about the modes of interaction and activation of the elements regulating σB activity in S. aureus. It remains to be determined whether additional factors are needed to induce the postulated RsbV-specific phosphatase activity of RsbU and whether RsbU responds solely to environmental stresses or to energy limitation as well. In addition, the regulation of the sigB operon has not been fully elucidated yet. No experimental confirmation about the predicted promoters has been provided (42, 54, 74), which is required for the interpretation of the multiple sigB-containing transcripts observed, ranging from 3.6 to 1.2 kb in size (25, 27, 42).
In this study, we demonstrate that the sigB operon of S. aureus is controlled by three distinct promoters, with two of them being induced by environmental stresses. We confirm, by yeast two-hybrid technology, protein-protein interactions between the factors encoded by the sigB operon, and we show that, unlike in B. subtilis, an increase in RsbU was sufficient to induce σB activity.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and relevant phenotypes are listed in Table 1. Bacteria were routinely grown in Luria-Bertani medium (Difco Laboratories, Detroit, Mich.) with aeration (200 rpm) at 37°C. Where indicated, media were supplemented with either 100 μg of ampicillin, 10 μg of erythromycin, or 10 μg of tetracycline per ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or phenotypea | Reference or source |
|---|---|---|
| Strains | ||
| S. aureus | ||
| BB255 | NCTC8325 derivative, rsbU | 3 |
| Newman | Clinical isolate (ATCC 25904), rsbU+ | 22 |
| COL | mec, high-Mcr clinical isolate; Mcr Tcr | 40 |
| RN4220 | NCTC8325-4 r− m+rsbU | 41 |
| GP266 | RN4220 rsbU+sigB1(Am), Tcr | 4 |
| GP267 | RN4220 (rsbU-V-W-sigB)+-tet(L), Tcr | 27 |
| GP268 | BB255 (rsbU-V-W-sigB)+-tet(L), Tcr | 27 |
| MB25 | RN4220 asp23+ (asp23p::luc+)-pEC-ermB, Emr | 27 |
| MB32 | Newman asp23+ (asp23p::luc+)-pEC-ermB Emr | 27 |
| MB55 | RN4220 (rsbU-V-W-sigB-luc+)+-erm(B), Emr | This study |
| MB58 | Newman (rsbU-V-W-sigB-luc+)+-erm(B), Emr | This study |
| MB89 | MB25 (rsbU-V-W-sigB)+-tet(L), Tcr, Emr | This study |
| MS64 | Newman sigB1(Am), Tcr | This study |
| E. coli | ||
| XL1Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15Tn10 (Tcr)] | Stratagene |
| Yeast | ||
| YRG-2 | MATaura3-52 his3-200 ade2-101 lys2-801 trp1-901 leu2-3 112 gal4-542 gal80-538 LYS::UASGAL1-TATAGAL1- HIS3 URA3::UASGAL4 17mers(×3)-TATACYC1lacZ | Stratagene |
| Plasmids | ||
| pAD-GAL4-2.1 | Yeast two-hybrid target plasmid, harboring the GAL4 activation domain, Apr | Stratagene |
| pBD-GAL4 Cam | Yeast two-hybrid bait plasmid, harboring the GAL4 DNA-binding domain, Cmr | Stratagene |
| pADrsbU | pAD-GAL4-2.1 derivative, harboring the rsbU ORF of COL cloned into the MCS in frame with the GAL4 DNA-binding domain, Apr | This study |
| pADrsbV | pAD-GAL4-2.1 derivative, harboring the rsbV ORF of COL cloned into the MCS in frame with the GAL4 DNA-binding domain, Apr | This study |
| pADrsbW | pAD-GAL4-2.1 derivative, harboring the rsbW ORF of COL cloned into the MCS in frame with the GAL4 DNA-binding domain, Apr | This study |
| pADsigB | pAD-GAL4-2.1 derivative, harboring the sigB ORF of COL cloned into the MCS in frame with the GAL4 DNA-binding domain, Apr | This study |
| pAW8 | S. aureus-E. coli shuttle vector harboring tet(L), Tcr | 27 |
| pBDrsbU | pBD-GAL4 Cam derivative, harboring the rsbU ORF of COL cloned into the MCS in frame with the GAL4 activation domain, Cmr | This study |
| pBDrsbV | pBD-GAL4 Cam derivative, harboring the rsbV ORF of COL cloned into the MCS in frame with the GAL4 activation domain, Cmr | This study |
| pBDrsbW | pBD-GAL4 Cam derivative, harboring the rsbW ORF of COL cloned into the MCS in frame with the GAL4 activation domain, Cmr | This study |
| pBDsigB | pBD-GAL4 Cam derivative, harboring the sigB ORF of COL cloned into the MCS in frame with the GAL4 activation domain, Cmr | This study |
| pBus1 | pAW8 with multicloning site from pBluescript II SK (Stratagene) and the rrnT14 terminator sequence from pLL2443, Tcr | 61 |
| pBus1-luc+ | pBus1 with a 1.7-kb Kpnl/EcoRI fragment of pSP-luc+, Tcr | This study |
| pBus1-sigBp2-luc+ | pBus1 with a 363-bp PCR fragment of the upstream region of rsbU fused to the reporter gene luc+, Tcr | This study |
| pCX15 | Xylose-inducible expression vector for staphylococci, Cmr | 71 |
| pEC4 | pBluescript KS+ (Stratagene) containing the 1.45-kb Clal erm(B) fragment of Tn551, Apr Emr | 10 |
| pIK7 | pUC18 with 6.6-kb Pstl/EcoRI S. aureus sigB fragment, Apr | 42 |
| pPG4 | plK7 with 1.45-kb Clal erm(B) fragment of pEC4 into blunted Bsp119I site downstream of sigB; Apr Emr | This study |
| pSP-luc+ | Firefly luciferase cassette vector, Apr | Promega |
| pSPslt | pSP-luc+ with rsbW and sigB upstream of luc+ and the sigB-sa2053 intergenic region containing the sigB terminator and erm(B) downstream of luc+; Apr Emr | This study |
| pXyl | pAW8 with 1.7-kb HindIII-BamHI fragment of pCX15 containing the xylR and the xylA promoter-operator region from S. xylosus, Tcr | This study |
| pXylrsbU | pXyl derivative containing rsbU from COL under the control of the xylose-inducible promoter Pxyl, Tcr | This study |
Apr, ampicillin resistant; Cmr, chloramphenicol resistant; Emr, erythromycin resistant; Mcr, methicillin resistant; MCS, multiple cloning site; Tcr, tetracycline resistant.
Yeast two-hybrid analyses.
rsbU, rsbV, rsbW, or sigB was PCR-amplified from S. aureus COL chromosomal DNA using the respective 2Hyb primer pairs shown in Table 2, introducing an EcoRI site upstream of the translation initiation codon and a PstI site downstream of the stop codon. The PCR fragments were digested and ligated into the respective sites of plasmids pAD-GAL4-2.1 or pBD-GAL4 Cam and subsequently transformed into Escherichia coli XL1Blue to obtain target plasmids pADrsbU, pADrsbV, pADrsbW, and pADsigB as well as bait plasmids pBDrsbU, pBDrsbV, pBDrsbW, and pBDsigB. The nucleotide sequences of the cloned PCR fragments were confirmed to be identical to the sequences denoted under GenBank accession no. NC_002951. All combinations of pAD- and pBD-based derivatives were cotransformed into yeast strain YRG-2 (Stratagene), a dual reporter strain of Saccharomyces cerevisiae carrying the auxotrophic markers histidine (his3), leucine (leu2), and tryptophan (trp1). The handling of YRG-2 and its derivatives was performed as described in the manufacturer's manual. YRG-2 transformants were selected on synthetic dextrose (SD) minimal agar plates lacking the amino acids leucine (Leu) and tryptophan (Trp) to ensure the presence of both plasmids. The yeast transformants obtained were plated on SD agar plates without histidine (His), Leu, and Trp to screen for protein-protein interactions.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′-3′)a | Location (accession no. NC_002951) or reference |
|---|---|---|
| asp23probe+ | ATGACTGTAGATAACAATAAAGC | 27 |
| asp23probe− | TTGTAAACCTTGTCTTTCTTGG | 27 |
| clfAprobe+ | CGTGGCTTCAGTGCTTGTAG | 880693-880712 |
| clfAprobe− | GAGTTGTTGCCGGTGTATTAGC | 881015-881036 |
| gyr864 | GTACGATTTAATACCGCCCTCATA | 29 |
| gyr297 | TTAGTGTGGGAAATTGTCGATAAT | 5199-5222 |
| gyr570 | AGTCTTGTGACAATGCGTTTACA | 5450-5472 |
| gyrFL1 | ATTTTAACTGTTTTACATGCTGGTGGTAA-FL | 72 |
| gyrLC1 | LCred640-TTTGGCGGTGGCGGATACA-ph | 72 |
| 2Hyb-rsbU+ | atcgaattcaTGGAAGAATTTAAGCAAC | 2123847-2123864 |
| 2Hyb-rsbU− | atctctgcagTTAATTTACTCTTTTTATAATC | 2122864-2122885 |
| 2Hyb-rsbV+ | atcgaattcATGAATCTTAATATAGAAACAACC | 2122721-2122744 |
| 2Hyb-rsbV− | atctctgcagTTATTCGACCTCCGTTCCTTC | 2122418-2122438 |
| 2Hyb-rsbW+ | atcgaattcATGCAATCTAAAGAAGATTTTATCG | 2122392-2122416 |
| 2Hyb-rsbW− | atctctgcagTTAGCTGATTTCGACTCTTTCG | 2121937-2121958 |
| 2Hyb-sigB+ | atcgaattcaTGGCGAAAGAGTCGAAATCAGC | 2121940-2121961 |
| 2Hyb-sigB− | atctctgcagCTATTGATGTGCTGCTTCTTG | 2121192-2121212 |
| rsbUpe+ | GCTGAATGCAGTAGCTCACC | 2124226-2124245 |
| rsbUpe− | CGTTAAACTTTCATCAATTAAACCC | 2123815-2123839 |
| rsbUprobe+ | GACGTTAAACTTAACGCGTG | 2123685-2123704 |
| rsbUprobe− | GAACAATATCTTGTGGGTGC | 2122950-2122969 |
| rsbU-RBSBamHI+ | gcggatccGAAGTGAGGAGGCAACTAATCaTGGAAGAATTTAAGC | 2123857-2123886 |
| rsbVpe− | CGTAAAATTTATCTTGAGTGG | 2122702-2122722 |
| rsbVpe+ | CGGATGGTGTGACTGAAGC | 2123039-2123057 |
| rsbWKpnl+ | gaaggtaccGAGGTCGAATAACATGC | 2122413-2122429 |
| rsbWprobe+ | CGAAATGCGCGTGCCAGC | 2122376-2122393 |
| rsbWprobe− | GTCATACTGATTGTCACACC | 2121995-2122014 |
| sigBFL2 | GGACAAAGTTATAATGCGTTAAGTGT-FL | 2121469-2121494 |
| sigBHindlll− | tatcataagcttcctACAAATTCTATTGATGTGCTGC | 2121185-2121206 |
| sigBLC2 | LCred640-GATCATTCCATTGAAGCTGATAAA-ph | 2121444-2121467 |
| sigBp2Kpnl+ | cggatccGTTTAGGGCTGAATGCAGTAGC | 2124231-2124252 |
| sigBp2Ncol− | cttccatggTTAGTTGCCTCCTCACTTCCC | 2123868-2123888 |
| sigBprobe+ | CAGCTAATGAAGTTTCACCTG | 2121923-2121943 |
| sigBprobe− | CTCTGAAGTCGTGATACATGC | 2121232-2121252 |
| sigB3368 | TAAACCGATACGCTCACCTGT | 2121261-2121281 |
| sigB2922 | TTGAACGGAAGTTTGAAGC | 2121709-2121727 |
| sigB3232 | CATCTTGTTGCCCCATAATA | 2121398-2121417 |
| sa2053EcoRI− | gcGAATTCAAACTTTGCAGAT | 2120042-2120060 |
| sa2058probe+ | CTGGTAGGATTAATAAAGCG | 2124419-2124438 |
| sa2058probe− | GTGAGCTACTGCATTCAGCC | 2124227-2124246 |
| sa2059pe− | GCTTCGTTCGCTAGGGAGAG | 2124644-2124663 |
| sa2059pe+ | CCTATTGGATATGCAGATGGC | 2125117-2125137 |
| TermXbaI+ | ggcaatctAGAAATTACAAGAAGCAGC | 2121202-2121220 |
| T7-gyr | taatacgactcactatagggagATTATGGTGCTGGGCAAATACA | 5098-5119 |
| T7-sigB2747 | taatacgactcactataAAAGAACACCAAGAAAATAAG | 2121882-2121902 |
Lowercase letters represent nucleotide additions. FL, fluorescein; LCred640, LightCycler-Red 640-N-hydroxysuccinimide ester; ph, 3′-phosphate.
Construction of plasmid pSPslt.
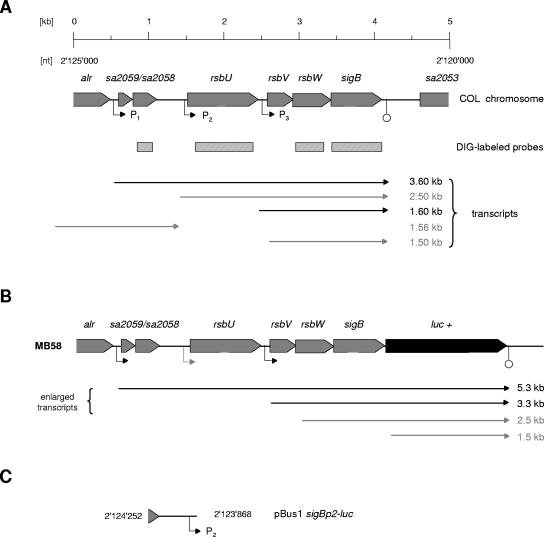
To obtain a chromosomal sigB-luciferase fusion, the following strategy was used: a DNA fragment covering rsbW and sigB of S. aureus BB255 was amplified by PCR using primers RsbWKpnI+ and SigBHindIII−. The resulting PCR product was digested with KpnI and HindIII and cloned into plasmid pSP-luc+ (Promega), yielding plasmid pSPsl with the reporter gene luc+ downstream of rsbW and sigB. In a second step, the 1.45-kb erm(B) cassette of plasmid pEC4 (10) was excised by ClaI digestion, blunted by Klenow treatment, and subsequently cloned into the blunted Bsp119I site of plasmid pIK7 (42), which is located downstream of the putative terminator sequence in the intergenic region between sigB and sa2053 (Fig. 1A). The plasmid obtained, pPG4, was then used as a template to amplify a 2.6-kb DNA fragment covering the proposed terminator sequence of the sigB operon (42, 74), the intergenic region between sigB and sa2053, including the erm(B) cassette, and the 5′-end of sa2053 using primer pair TermXbaI+ and SA2053EcoRI−. The resulting PCR product was digested with XbaI and EcoRI and cloned into plasmid pSPsl to obtain pSPslt, which was electroporated into S. aureus GP267 (27), an rsbU+ derivative of RN4220 that harbors a tet(L) cassette downstream of the sigB operon. A screen for double-crossover transformants that were sensitive to tetracycline and resistant to erythromycin was then carried out. In the final step, the engineered chromosomal region of a positive transformant, MB55, was transduced into strain Newman to obtain MB58 (Fig. 1B) (53).
FIG. 1.
Genetic organization of the S. aureus sigB operon. Schematic representations of the sigB operons of S. aureus COL (A) and MB58 (B) and of the rsbU promoter-luciferase reporter gene plasmid pBus1sigBp2-luc (C). Open reading frames, termination signals (open circles), promoters (P; closed triangles), and sigB operon-specific probes used for Northern blot analyses are indicated. sigB-containing transcripts identified in this study to originate from specific promoters are indicated in black, while those of unclear nature are indicated in gray. Open reading frame notations and nucleotide (nt) numbers given correspond to those of the respective genomic regions of strain COL (accession no. NC_002951).
Construction of plasmid pBus1-sigBp2-luc+.
A DNA fragment covering the putative sigBp2 promoter of S. aureus COL was amplified by PCR using primer pair sigBp2KpnI+-sigBp2NcoI− (Table 2). The resulting PCR product was KpnI/NcoI-digested and cloned into pSP-luc+ (Promega) in front of the reporter gene luc+. A plasmid harboring the promoter fragment was used to excise the promoter-reporter gene fragment by digestion with KpnI/EcoRI, which was subsequently cloned into the multiple cloning site of the E. coli-S. aureus shuttle vector pBus1 (61) to obtain plasmid pBus1-sigBp2-luc+ (Fig. 1C). The nucleotide sequence of the cloned PCR fragment was confirmed to be identical with the sequence deposited under GenBank accession no. NC_002951. As a control, a HindIII/EcoRI fragment of pSP-luc+, including luc+ and its ribosomal binding site, was cloned into pBus1. pBus1-sigBp2-luc+ and the control plasmid pBus1-luc+ were finally used for electroporation of RN4220, from which they were transduced into strain Newman by phage transduction (53).
Luciferase assays.
Bacterial cells of Newman derivatives harboring plasmids pBus1sigBp2-luc+ and pBus1-luc+ were grown in LB supplemented with 10 μg ml−1 tetracycline at 37°C and 200 rpm and sampled after 3 h of growth. Cell harvesting and downstream applications were carried out as described previously (27).
Northern blot and microarray analyses.
For in vitro growth studies, overnight cultures of S. aureus were diluted 1:100 into fresh prewarmed LB medium and grown at 37°C and 200 rpm. Samples were removed from the culture after 1, 3, 5, and 8 h of growth and centrifuged at 7,000 × g and 4°C for 5 min, the culture supernatants were discarded, and the cell sediments were snap-frozen in liquid nitrogen. For stress experiments, cells were grown in LB at 37°C to an optical density at 600 nm of 1. At this growth stage, heat shock was applied by transferring the culture to a 48°C water bath shaker, and salt stress was applied by adding solid NaCl to a final concentration of 2 M. Cells were sampled 0, 5, and 10 min after the stresses were applied and centrifuged at 16,000 × g at room temperature for 1 min, the culture supernatants were discarded, and the cell sediments were snap-frozen in liquid nitrogen. For the Northern analyses, total RNAs were isolated according to Cheung et al. (14). Blotting, hybridization, and labeling were performed as previously described (27). For the in vitro growth studies, primer pairs sa2058probe+-sa2058probe−, rsbUprobe+-rsbUprobe−, rsbWprobe+-rsbWprobe−, and sigBprobe+-sigBprobe− (Table 2) were used to generate digoxigenin-labeled sigB operon probes by PCR labeling (Fig. 1A). For the stress studies, sigB-, asp23-, and clfA-specific probes were used. For the microarray analyses, RNA isolation and transcriptional profiling were performed as described earlier (6).
Primer extension experiments.
Total RNA (50 μg) was dissolved in 60 μl of hybridization buffer {40 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] [pH 6.4], 1 mM EDTA, 0.4 M NaCl, 80% [vol/vol] formamide} at 65°C, denatured together with 0.3 pmol 32P-labeled oligonucleotide primer (sa2059pe−, rsbUpe−, or rsbVpe−) for 5 min at 95°C, and annealed for 4 h at 45°C. Samples were ethanol precipitated and dissolved in 8.5 μl of water and the following components were added: 0.75 μl RNasin (Promega), 3 μl 5× avian myeloblastosis virus-reverse transcriptase (RT) buffer (Promega), 0.75 μl dATP, dGTP, dTTP, dCTP (5 mM each), and 0.75 μl actinomycin (4 mg ml−1). This mixture was incubated for 2 min at 42°C. Primer extension was initiated by adding 2 μl of avian myeloblastosis virus-RT (18 U; Promega). The reaction was terminated after incubation for 2 h at 42°C with 25 μl RNase mix (100 μg ml−1 DNase-free RNase A, 30 μg ml−1 sonicated salmon sperm DNA, 10 mM Tris-EDTA, [pH 8]) and by incubating for 30 min at 37°C. After the addition of 20 μl 1 M NaCl, the mixture was extracted by the alkaline phenol-chloroform method and DNA was precipitated with ethanol. The pellet was dissolved in 5 μl loading buffer (80% [vol/vol] formamide, 10 mM NaOH, 1 mM EDTA, 0.05% xylene cyanol, 0.05% bromphenol blue) and heated for 2 min at 95°C, and an aliquot was separated on a 6% denaturing gel together with G+A and T+C sequencing ladders (48) derived from the corresponding end-labeled DNA fragments. DNA fragments were PCR amplified using plasmid pIK7 as a template. The probes sa2059-pe (493 bp), rsbU-pe (430 bp), and rsbV-pe (355 bp) were prepared using the forward primers sa2059pe+, rsbUpe+, and rsbVpe+, respectively, together with the 5′-end-labeled reverse primers sa2059pe−, rsbUpe−, and rsbVpe−, respectively, all lying within the corresponding coding regions. Oligonucleotides were 5′-end-labeled with [γ-32P]ATP (ICN; 4,500 Ci mmol−1) and T4 polynucleotide kinase (Biolabs).
Real-time PCR of in vivo-expressed sigB.
The guinea pig model of implant infection was used as described previously (80). Four perforated Teflon tubes were inserted subcutaneously in the flanks of guinea pigs (63). Two weeks after the implantation, the interstitial fluid inside the tissue cages was checked for sterility and 105 CFU of strain Newman isolates was injected into each of the devices. The exudates were aspirated with a syringe at days 2 and 8 after infection and transferred immediately to liquid nitrogen until RNA preparation. For RNA preparation from exudates, the frozen samples were thawed rapidly and 200 μl aliquots were used. RNA was isolated and purified as described previously (30). To remove residual DNA, a DNaseI digest step was performed as described elsewhere (29). For transcript analysis in vitro, cells were grown and sampled as depicted above. RNA preparation from the in vitro samples was performed as described previously (25). Sequence-specific RNA standards for LightCycler RT-PCR were engineered using the following protocol: PCR was performed using a gene-specific primer with a 5′ extension encompassing the T7 phage promoter sequence, thus generating transcription-competent amplicons. For construction of the standards, the primer pairs T7-gyr-gyr864 and T7-sigB2747-sigB3368 were used (Table 2). T7-driven in vitro transcription was performed using a transcription assay with T7-MEGAShortscript (Ambion, Huntingdon, United Kingdom). The reaction mixtures were subjected to DNaseI treatment (Roche Biochemicals), and the RNAs were recovered with the MEGAclear kit (Ambion). The transcripts were quantified by spectrophotometric analysis and by ethidium bromide staining on agarose gels. LightCycler RT-PCR was carried out using the LightCycler RNA amplification kit for hybridization probes (Roche Biochemicals). Master mixes were prepared following the manufacturer's instructions using the following primer pairs: gyr297-gyr574 for gyrB and sigB2922-sigB3232 for sigB. The hybridization probes were selected for specific binding to an internal part of the respective RNA standard using the end-labeled oligonucleotides gyrFL1 and gyrLC1 for gyrB and sigBFL2/sigBLC2 for sigB. Standard curves were generated using 10-fold serial dilutions (104 to 108 copies/μl) of the specific RNA standards. The number of copies of each sample transcript was then determined with the LightCycler software. At least two independent RT-PCR runs were performed for each sample. The specificity of the PCR was verified by ethidium bromide staining on 3% agarose gels. Specific sigB transcripts were quantified in reference to the transcription of gyrase (in copies per copy of gyrB). Values from two separate RNA isolations and two independent RT-PCRs each were used to calculate the mean expression (±standard errors of the mean).
RsbU and σB content.
Cytoplasmic protein fractions (10 μg/lane) obtained from S. aureus Newman grown in LB at 37°C and sampled on an hourly basis were separated using sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, blotted onto nitrocellulose, and subjected to Western blot analysis using antigen-purified anti-RsbU or anti-σB antibodies (27).
Overexpression of rsbU.
A 1.7-kb HindIII-BamHI fragment of plasmid pCX15 (71), harboring xylR and the xylA promoter-operator region from S. xylosus, was cloned into the E. coli-S. aureus shuttle vector pAW8 (27) to obtain plasmid pXyl. A DNA fragment covering the open reading frame and the native ribosomal binding site of rsbU of S. aureus COL was amplified by PCR using the primers RsbURBSBamHI+ and RsbUEcoRI−. The resulting PCR product was digested with BamHI and EcoRI and cloned into plasmid pXyl downstream of the xylA promoter-operator region to create pXylrsbU, which was transformed into E. coli, and subsequently transduced into RN4220 derivative MB25 and Newman. For overexpression of RsbU, pXylrsbU harboring derivatives were grown in LB to an optical density at 600 nm of 1, where overexpression of RsbU was induced by adding 0.5% xylose (final volume). σB activities were determined by measuring the luciferase activities of Luc+, the product of the luc+ reporter gene fused to the σB-dependent promoters of asp23 as described previously (27). RsbU and Asp23 production was determined by Western blot analyses using antigen-purified anti-RsbU and anti-Asp23 antibodies (27).
RESULTS
Yeast two-hybrid experiments.
We used the yeast two-hybrid technology (reference 24, reviewed in reference 28) in order to identify protein-protein interactions between the gene products encoded by the sigB operon of S. aureus. For this purpose, proteins linked to either the activator domain or the DNA-binding domain of the yeast transcriptional activator GAL4 were coexpressed in the yeast reporter strain YRG-2 (his3 trp1 leu2). Target (pAD) and bait (pBD) plasmids carried LEU2 or TRP1 markers, respectively. Only in cases of protein-protein interaction were subdomains brought into sufficiently close proximity as to allow functional complementation of GAL4. For read-out of interaction, a third auxotrophic marker was used, HIS3, which in YRG-2 is under the control of GAL4. Growth of YRG-2 on SD agar plates lacking the amino acids His, Leu, and Trp was observable only when plasmids containing either rsbU and rsbV, rsbV and rsbW, or rsbW and sigB were present at the same time (Fig. 2). These combinations complemented YRG-2 irrespective of which interacting partner was cloned into the bait or target vector. Growth of YRG-2 was also observed in the presence of rsbW-containing bait and target vectors (data not shown). All other plasmid combinations failed to complement the auxotrophic yeast strain, indicating that protein-protein interactions occurred only between RsbV and either RsbU or RsbW as well as between RsbW and either RsbV, RsbW, or SigB.
FIG. 2.
Yeast two-hybrid analysis of protein-protein interactions among sigB operon gene products.
Transcriptional control of σB.
The multiple, differently sized mRNA transcripts shown to hybridize with a sigB probe (25, 27, 42) have never been attributed to specific promoters, nor have any of the postulated transcriptional starts of the sigB operon been mapped. Moreover, the 1.5-kb transcript, migrating approximately at the 16S rRNA position, is thought to represent degraded sigB-containing transcripts stabilized in the bulk of ribosomal RNAs (25). To discern specific sigB transcripts, we fused the 1.7-kb promoterless luc+ gene between the 3′ end of sigB and the putative sigB terminator sequence, thereby elongating any sigB transcript by 1.7 kb (Fig. 1B). While sigB Northern blot experiments with strain Newman yielded the previously reported four bands of 3.6, 2.5, 1.6, and 1.5 kb, we observed signals at 5.3, 3.3, 2.5 and 1.5 kb in MB58 (Fig. 3). The latter signal pattern was additionally observed for MB58 when a probe specific for luc+ was used (data not shown). The substitution of the 3.6- and 1.6-kb bands with the 5.3- and 3.3-kb bands, respectively, in MB58 indicated that the wild-type transcripts were shifted by the expected 1.7 kb, signaling their origin from specific promoters. The presence of the 2.5- and 1.5-kb bands in both Newman and MB58, on the other hand, suggests that these transcripts might be fragments captured within rRNA, since they did not appear in the ΔrsbUVWsigB mutant IK184 (data not shown).
FIG. 3.
Heat shock induction of sigB transcription of the S. aureus strains Newman and MB58. Sizes of relevant bands are indicated on the right.
TSP determinations.
Primer extension analyses suggested a transcriptional start point (TSP) for the 3.6-kb transcript at position 2,124,790 of the S. aureus COL genome (accession no. NC_002951), 46 bp upstream of the putative start codon of sa2059 (Fig. 4). It was preceded by a sequence (TGGTCA-17-TATCAT) highly similar to that of the E. coli σA consensus promoter (TTGACA-16/18-TATAAT) (31), which is thought to be similar to that of S. aureus σA (20, 59). σA-dependence of this promoter, termed sigBp1, was further supported by the finding that the signals for the 3.6-kb TSP of strains GP268 (rsbU+) and BB255 (rsbU) were almost identical (Fig. 4A), whereas those for the σB-dependent 1.6-kb transcripts were significantly enhanced in the rsbU-positive GP268 relative to the rsbU-negative BB255. The TSP of the 1.6-kb transcripts mapped at position 2,122,819, 75 bp upstream of rsbV, and in close proximity to a σB consensus sequence (GATTAG-13-GGGTAT) predicted to give rise to the 1.6-kb transcript (42, 74). Since various groups suggested the 2.5-kb transcript to originate from a σA-dependent promoter located immediately upstream of rsbU (15, 25, 42, 54, 74), TSP analyses were extended in order to determine the TSP for this transcript. Unlike as suggested by the gene-shift assay (Fig. 3), primer extension experiments indeed allowed the identification of a third TSP, mapping at position 2,124,009, 144 bp upstream of rsbU. The intensities of the signals obtained from GP268 and BB255, being virtually identical, suggested that this transcript was unaffected by σB activity. Screening the nucleotide sequence upstream of the TSP, however, did not succeed in the identification of a potential σA promoter, even when allowing two mismatches per hexamer.
FIG. 4.
TSPs of the S. aureus promoters directing expression of the sigB operon. Primer extension products obtained with primers sa2059-pe (A), rsbU-pe (B), and rsbV-pe (C) were analyzed on DNA-sequencing gels together with G+A (lane A)- and T+C (lane T)-sequencing ladders (48). Horizontal arrows indicate the positions of the extension products. (D) Nucleotide sequence of the putative S. aureus sigB operon promoters. The TSPs are indicated by vertical arrows corresponding to the extension products designated in panel A (plain), panel B (diamond), or panel C (circle). Boxes (−10 and −35) of putative promoters are shown in bold characters and underlined.
sigBp2 promoter-reporter gene fusion experiments.
We fused the genomic region preceding rsbU to the reporter gene luc+ (Fig. 1C) in order to confirm the presence of the putative sigBp2 promoter suggested by the primer extension analysis. Newman derivatives transformed with pBussigBp2-luc+ produced significant amounts of luciferase activity (34.9 ± 1.3 relative light units), in contrast to the Newman derivatives that harbored the control plasmid pBus1luc+ (0.17 ± 0.04 relative light units), supporting the presence of a functional sigBp2 promoter.
Growth phase dependence of the sigB transcripts.
Production of the transcripts of the sigB locus during in vitro growth was monitored by Northern blot analyses using sa2058-, rsbU-, rsbW-, and sigB-specific probes (Fig. 5). The 3.6-kb transcript was detectable predominantly during the early exponential growth phase but not at later growth stages. All probes used allowed the identification of this transcript, signaling that it covered sa2059-sa2058-rsbU-rsbV-rsbW-sigB (Fig. 5B). The 1.6-kb transcript was detectable with the rsbW- and sigB-specific probes, but not with probes specific for sa2058 and rsbU, in agreement with the prediction that it includes the genetic information for RsbV, RsbW, and SigB (42, 74). The 1.6-kb transcript appeared to be the most prominent transcript originating from the sigB operon and was visible throughout growth (Fig. 5B), although its amount seemed to vary somewhat, being highest during the transition from late exponential phase to stationary phase (i.e., 5 h). The 2.5-kb transcript was detectable with rsbU-, rsbW-, and sigB-specific probes at all time points analyzed, although its expression was most intense early in growth (i.e., 1 h). An approximately 1.56-kb band showed up with the sa2058-specific probe, indicating the presence of a second sa2058-encoding transcript.
FIG. 5.
Expression patterns of genes in the region of sigB during in vitro growth. (A) Growth curve of S. aureus Newman in LB at 37°C. Arrows indicate time points of sampling. OD600, optical density at 600 nm. (B) Northern blot analyses of sigB operon transcripts using sa2058-, rsbU-, rsbW-, and sigB-specific probes. RNAs were obtained from cells sampled at the time points indicated. Relevant transcripts are indicated on the right. (C) Microarray expression patterns of genes within the sigB operon. Transcript levels for Newman cells sampled at different time points of growth (x axes). Data points were plotted as relative intensity values (y axes). (D) Quantitative sigB-transcript analysis of S. aureus strain Newman by LightCycler RT-PCR in vitro and in vivo. In vivo expression of sigB was determined in exudates from infected tissue cages in guinea pigs 2 days (d) and 8 days after inoculation.
Transcriptional profiling of the sigB operon.
We made use of a recently described microarray strategy (6) to further analyze the transcriptional profiles of the sigB operon, containing genes sa2058, rsbU, rsbW, and sigB (Fig. 5C). Based on the transcripts identified by Northern analyses, we expected the microarray-derived signal intensities for sa2058 to be representative for the 3.6-kb transcript, the rsbU microarray values to represent the 3.6- and 2.5-kb transcripts, and the rsbW and sigB data to include all three transcripts. In line with the Northern analyses, rsbW and sigB expression were found to be highest during later growth stages and to be significantly stronger than sa2058 and rsbU. The latter two genes appeared in the microarray analyses to be transcribed on a rather constant level throughout growth, in contrast to the Northern blots, which showed higher intensities in early exponential growth phase.
In vivo expression of sigB.
Transcription of sigB was also followed in the guinea pig cage model of infection (80). Expression of sigB was determined by real-time PCR by using RNAs that were obtained from cells isolated either 2 days or 8 days after inoculation (in vivo) and, as comparison, from cells grown in LB (in vitro) (Fig. 5D). The in vitro sigB transcription profile observed by real-time PCR was highest during the later growth stages and corresponded well with the Northern results presented before. Interestingly, sigB transcripts were as abundant in vivo as from in vitro cultures, demonstrating that sigB was transcribed at a high rate at these time points of infection (Fig. 5D).
Stress inducibility of the sigB operon and σB activity.
Heat shock is known to induce σB activity (25, 27). Shifting growing S. aureus cells from 37°C to 48°C yields a rapid but short increase in σB activity within 3 to 5 min that declines when the heat stress is present for more than 10 min (25, 27). Additionally, heat shock was shown to increase the expression of the 3.6-, 2.5-, and 1.6-kb transcripts (25). Our Northern blot analyses confirmed the heat shock induction of the 3.6- and 1.6-kb transcripts but failed to identify the increase in expression of the 2.5-kb transcript (Fig. 6). The temperature shift very rapidly induced the expression of the σB-dependent 1.6-kb transcript, with a peak shortly after the stress was applied, followed by an immediate decline, in agreement with previous findings (25, 27). Interestingly, heat induction of the 3.6-kb transcript resulted in a different kinetic, increasing steadily over the time monitored. While the 1.6-kb transcript was missing in the sigB mutant MS64, as expected, the 3.6-kb transcript remained heat inducible, suggesting an additional σB-independent stimulus for the heat shock response. Similarly, salt stress with 2 M NaCl induced expression of the 3.6- and 1.6-kb transcripts (Fig. 6), although the stress induction occurred slower than by heat shock, possibly requiring prior solubilization of the solid NaCl to become effective.
FIG. 6.
Stress inducibility of the sigB operon transcripts and of σB activity. Effect of heat shock (48°C) and salt stress (2 M NaCl) on the expression of sigB operon transcripts, asp23, and clfA in Newman and its sigB-defective derivative, MS64. Relevant transcript signals are indicated on the right of each panel.
The σB activity in response to these environmental stresses was followed by analyzing the expression of the σB-dependent genes asp23 and clfA. Expression of both genes was significantly increased in S. aureus wild-type cells subjected for 5 min to heat shock but diminished upon longer heat exposure. No such transcripts were detectable in the sigB-negative MS64. Similarly, salt stress significantly induced σB activity, again with a time delay compared with the σB activity induced by heat stress.
RsbU and σB levels during in vitro growth.
Western blot analyses demonstrated that RsbU amounts were basically constant throughout growth (Fig. 7), with a slight increase in later growth stages (i.e., hours 5 to 8). Surprisingly, the σB protein content seemed to be constant throughout growth as well, although the transcriptional studies hinted to a clear growth phase-dependent expression of sigB transcripts.
FIG. 7.
Western blot analyses of RsbU (top) and σB (bottom) in cytoplasmic protein fractions of S. aureus Newman cells grown in vitro. Samples were taken at the time points indicated. Relevant protein signals are indicated.
RsbU-dependent induction of σB activity.
To measure the effect of RsbU on σB activation, the rsbU mutant MB25 was complemented in trans with a xylose-inducible rsbU wild-type allele. The induction of rsbU resulted in an immediate increase of the otherwise negligible σB activity (Fig. 8) and was independent of the growth stage (data not shown). Interestingly, RsbU overproduction was able to further induce the already considerable σB activity in the rsbU+ strain Newman (Fig. 8B), suggesting a positive correlation between amounts of RsbU and σB activity. Xylose-induced RsbU production and its effect on σB activity were additionally monitored by Western blot analyses of the trans-complemented MB25 (Fig. 8C). A faint RsbU band was detectable in MB25 complemented with pXylrsbU, while in control strain MB25 trans-complemented with pXyl, RsbU was absent. Accordingly, increasing amounts of the σB-dependent Asp23 were detectable only in MB25 pXylrsbU, not in MB25 harboring the control plasmid (Fig. 8C).
FIG. 8.
Effect of RsbU overexpression on derivatives of strains MB25 (RN4220 asp23p::luc+) (A) and MB32 (Newman asp23p::luc+) (B), harboring either plasmid pXylrsbU (•) or control plasmid pXyl (▪) on σB activity (determined by luciferase activity). (C) Western blot analyses of RsbU and Asp23 in MB25 cells harboring plasmid pXylrsbU or pXyl after xylose induction. Samples were taken at the time points indicated. Relevant protein signals are indicated.
DISCUSSION
Much of what has been hypothesized so far about the regulation of S. aureus σB was adopted from the well-characterized homologous operon of B. subtilis (Fig. 9). However, key regulatory elements of B. subtilis σB activity, such as RsbU and RsbV, are influenced by factors that are absent in S. aureus, strongly suggesting that the activation of σB may significantly differ among these two bacterial species. This hypothesis is also supported by the observation that σB activity profiles under in vitro conditions clearly differ. B. subtilis σB is known to be a stationary phase σ factor, which is activated only during exponential growth in response to environmental stresses (2, 7, 8, 69). In contrast, σB activity of S. aureus was shown to be clearly detectable during exponential phase with a peak at late exponential phase and a significant decrease thereafter (27).
FIG. 9.
Comparison of the σB activation models in B. subtilis (A) and S. aureus (B). Factors thought to be present in only B. subtilis or S. aureus are indicated in gray, and factors thought to fulfill similar functions in both organisms are indicated by open symbols. (A) Model of the σB signal transduction network in B. subtilis (adapted from references 39, 44, and references within). σB activity is thought to be controlled by two independent signaling cascades converging on the anti-anti-σ factor RsbV and the anti-σ factor RsbW, the primary regulators of σB. In unstressed cells, σB is held inactive in a complex with RsbW as long as RsbV is inactivated due to an RsbW-catalyzed phosporylation (RsbV-P). Two stress-specific PP2C serine phosphatases, RsbU (induced by nutritional stress) and RsbP (induced by physical stress), can dephosphorylate and thus reactivate RsbV-P, allowing it to displace σB from the RsbW-σB complex. Nutritional stress triggers the RsbV phosphatase RsbP and a predicted hydrolyase (RsbQ) to dephosphorylate RsbV-P. Although the metabolic inducer(s) of RsbP/Q is still unknown, a drop in ATP/GTP levels and the presence of RelA, a (p)ppGpp synthetase, seem to be of importance. Physical stress leads to the activation of the RsbV phosphatase RsbU by a complex mechanism requiring protein-protein interaction between RsbU and its activator RsbT. Under unstressed conditions, RsbT is thought to be trapped by its negative regulator RsbS in a large complex comprising RsbRA and its paralogues RsbR (B-D). Physical stress activates the kinase activity of RsbT, allowing it to phosporylate and inactivate RsbRA and RsbS, thereby freeing itself from the complex to bind and activate RsbU. The stress-derived signal(s) activating RsbT is unknown but seems to involve the ribosome protein L11 and the ribosome-associated GTP-binding protein Obg. A third phosphatase, RsbX, returns the system to prestress conditions in the presence of high σB levels by dephosphorylating and reactivating RsbS and RsbRA, allowing them to sequester RsbT again. (B) Proposed model for the regulation of σB in S. aureus. Based on the known functions of the RsbU, RsbV, and RsbW homologues from B. subtilis, it is assumed that the anti-σB protein RsbW from S. aureus can form mutually exclusive complexes with either σB or its antagonist, RsbV. RsbV is normally inactive (RsbV-P) due to phosphorylation by RsbW and is thus unable to complex with RsbW, leaving the latter free to interact with σB. When bound to RsbW, σB is unable to aggregate with the RNA polymerase core enzyme to form an active holoenzyme. Upon stress, the RsbV-P-specific phosphatase activity of RsbU, a positive activator of σB, becomes activated and thus reactivates RsbV. Unphosphorylated RsbV interacts and complexes highly specifically with RsbW, thereby releasing σB. RsbV-complexed RsbW is unable to bind to σB, leaving the latter free to form an active σB-holoenzyme. Even though the exact mode of activation of RsbU in S. aureus remains unclear, there is evidence that RsbU is required for σB activation in response to nutritional and physical stress signals and that its activation differs substantially from that of the RsbU homologue in B. subtilis. The strong decrease of σB activity after mid-exponential growth phase observed in orange-pigmented teicoplanin-selected first-step mutants with affected rsbW loci suggests that a further, as-yet-unidentified negative regulator of σB is present in S. aureus. The interaction of this factor, tentatively named RsbW2, with σB is different from that of RsbW (4). Analyses of S. aureus rsbU and rsbV mutants indicate that RsbV might be controlled by an additional, as-yet-unidentified factor (X), which acts independently from RsbU (54). Recent results signal that a subset of σB-dependent genes seems to require the action of an additional still unknown factor (Y) for full expression (64).
Previous Northern analyses suggested a complex regulation of this transcription factor by identifying sigB transcripts of various sizes, some of which were heat shock inducible (25, 27, 42). However, it remained unclear whether all transcripts reported so far represented products from distinct promoters, or if some of them were degradation products, as speculated by Gertz et al. (25). Our data suggest that the sigB operon of S. aureus is controlled by at least two distinct promoters which give rise to a 3.6-kb transcript covering sa2059, sa2058, rsbU, rsbV, rsbW, and sigB, and a 1.6-kb transcript, covering rsbV, rsbW, and sigB. Expression from the sigBp1 and sigBp3 promoters was quickly induced by different environmental stresses, by either σB-dependent (1.6 kb) or σB-independent mechanisms (3.6 kb), indicating a complex regulatory circuit to control the expression of this operon. The presence of sigBp1 and sigBp3 was verifiable by various approaches, such as Northern analyses, TSP determination, and shift assay. However, this was not the case with sigBp2, a putative third promoter of the sigB operon, which is expected to give rise to the 2.5-kb transcript detected here (Fig. 3 and 5) and elsewhere (25, 27, 42), that is supposed to cover rsbU, rsbV, rsbW, and sigB. The findings that primer extension experiments identified a clear signal immediately upstream of rsbU and that luciferase activity was measurable with a sigBp2-luc+ fusion strongly support the hypothesis that rsbU is preceded by a distinct promoter. However, the lack of an apparent promoter consensus upstream of the TSP determined with rsbU-pe (Fig. 4), the fact that both the 2.5- and 1.5-kb transcripts but no 4.2- and 3.2-kb transcripts were detectable in MB58 harboring the 1.7-kb luc+ gene within its sigB operon, and the finding that the 2.5-kb transcript also appeared in MB58 when a luc+ specific probe was used are arguing against the presence of such a promoter. Instead, the latter findings somehow suggest the 2.5- and 1.5-kb bands to represent products of sigB-containing transcripts which might comigrate with the bulk of ribosomal RNAs, as has been suggested by Gertz et al. (25). Alternatively, the 2.5- and 1.5-kb bands might represent specifically cleaved products of larger sigB operon transcripts and additional work is clearly needed to elucidate the true nature of these two bands.
Monitoring the in vitro transcription of sigB over time revealed the expression of this σ factor to be growth-phase dependent (Fig. 5), which is in agreement with previous findings (5, 27). Western blot analysis of σB, however, showed that the alternate σ factor was present in more or less similar quantities throughout the growth cycle. This suggests that in addition to the transcriptional control, there are posttranslational mechanisms active.
A fundamental difference between the B. subtilis and S. aureus σB-activating cascades (Fig. 9) seems to exist for the control of the phosphorylation state of RsbV. In B. subtilis, reactivation of the inactivated anti-anti-σ factor, RsbV-P, is mediated by either of two stress-specific PP2C serine phosphatases, RsbU or RsbP, which can dephosphorylate RsbV-P, allowing it to displace σB from the RsbW-σB complex. Upon nutritional energy stress, reactivation of RsbV-P is thought to be triggered by activation of RsbP, together with additional factors (9, 67, 77). Environmental stress, on the other hand, leads to the activation of RsbU by a complex mechanism requiring protein-protein interaction between RsbU and its activator RsbT (1, 18, 19, 36, 39, 44, 73, 75). The resetting of σB activity to prestress levels after the imposition of environmental stress involves a third phosphatase, RsbX, that is part of the B. subtilis sigB operon (13, 68, 75). Since none of the six currently available genome sequences revealed the existence of either RsbR(A-D), RsbP, RsbQ, RsbT, or RsbX homologues in S. aureus, other regulatory mechanisms controlling RsbU and RsbV are likely to be present in this pathogen. Several experimental observations support this theory. In B. subtilis, RsbU activity requires RsbT and the overexpression of RsbU has no effect on σB activity (U. Völker, personal communication). We could demonstrate that in S. aureus, the overexpression of RsbU resulted in an almost immediate activation of σB. This suggests either that RsbU is active without an additional factor or the abundant presence of an RsbU-activator allowing immediate activation of the newly synthesized RsbU. However, the observation that RsbU levels seemed to be quite constant throughout the growth cycle, combined with previous findings showing σB activity to vary during in vitro growth (6, 27) favors the theory that RsbU activity is being regulated. This regulation could be due to an activator responding to stress sensed by the cell. Alternatively, the regulation of RsbU activity may be controlled by an inhibitor inactivated upon stress. In B. subtilis, RsbU is not involved in the activation of σB under energy-limiting conditions (69). S. aureus rsbU mutants, however, failed to activate σB throughout the growth cycle, indicating that RsbU might be of importance for response to both environmental and nutritional stresses (6, 27).
While the activation of RsbU differs significantly between S. aureus and B. subtilis, the partner-switching mechanism between RsbV, RsbW, and σB, which builds up the end part of the σB activation cascade, is likely to be the same in both bacterial species, as shown by the yeast two-hybrid system. We identified protein-protein interactions between RsbV and RsbU as well as between RsbW and RsbV, σB, and RsbW itself. All protein interactions observed are in agreement with what is known for B. subtilis (9, 18, 70) and what was shown for RsbW and σB in S. aureus (50). Thus, the major players of σB activity control, the anti-anti-σ factor RsbV and the anti-σ factor RsbW, are likely to function in a similar fashion in both organisms.
Still, further regulatory elements are likely to be present in the control of σB activity in S. aureus (4, 54). Many of the factors regulating σB activity in B. subtilis are encoded in the sigB operon. Our transcriptional studies demonstrate that the S. aureus sigB operon contains the ORFs sa2059 and sa2058 in addition to rsbU, rsbV, rsbW, and sigB. It is currently unknown what functions SA2059 and SA2058 may have in S. aureus, why they are cotranscribed with sigB and its regulatory elements, and whether they serve as regulators of σB activity. Interestingly, the genetic organization with sa2059 and sa2058 homologues located upstream of sigB is not restricted to staphylococci but is also found in other gram-positive bacteria. The S. aureus ORFs were previously recognized to share some homology with antitoxin/toxin systems, such as pemI-K or mazE-F of E. coli (reviewed in references 49 and 76), initially thought to be involved in programmed cell death. More recently, MazE-F function was reassigned from cell killing to inducing a bacteriostatic condition from which the cells could be resuscitated (17). In the latter study, MazF was reported to inhibit translation by inducing cleavage of mRNAs on translating ribosomes in a fashion similar to that shown for the E. coli RelB-E system (55). Noteworthy, the RelB-E system was suggested to play a regulatory role in bacteria during the adaptation to poor growth conditions (55). Overlapping of the termination codon of sa2059 with the putative initiation codon of sa2058 suggests a translational coupling of these two ORFs. Cotranslation is further suggested by the lack of a significant ribosomal binding site in the vicinity of the proposed sa2058 start codon, indicating that both gene products may act as a module. It is feasible that the S. aureus SA2059-SA2058 module serves as a regulatory element inhibiting translation. However, no experiments supporting such a function have been published so far and additional work is clearly needed in order to elucidate their roles.
Several animal model studies demonstrated that mutations in sigB had no apparent effect on pathogenicity of S. aureus (12, 23, 34, 52), calling into question whether and how this σ factor is produced under in vivo conditions. Our data clearly demonstrate that sigB is indeed expressed in vivo, arguing against the possibility that the missing effect of sigB mutations on pathogenicity might be due to a lack of sigB expression. Assuming that gyrB was expressed in comparable amounts under both conditions, transcription of sigB seemed to be high at both in vivo time points. Although interesting, the significance of this finding remains open for several reasons. First, our in vitro studies indicate that sigB transcript levels do not necessarily reflect the σB content within a cell. Second, the σB content is not a direct measure for σB activity, as it depends upon posttranslational processes involving the regulatory elements RsbU, -V, and -W. Third, the interpretation of the in vivo observations is further complicated by the fact that sigB expression is driven by σB-dependent and -independent mechanisms. The attribution of the high sigB transcript content observed under in vivo conditions to a high level of σB activity is thus uncertain. Whether the effect of σB on the ability of S. aureus to invade its host and to cause an infection is positive or negative remains unclear to date. Its proven influence on the expression of a variety of virulence determinants somehow suggests the σ factor to be beneficial for S. aureus infectivity (23, 27, 34, 37, 38, 43, 50, 51, 52, 62, 78, 79). The recent findings of Kanth (37), showing that mutations within the sigB operon resulting in low levels of σB activity were more common than previously expected, however, signal that this might not be the case for all kinds of infections this pathogen is able to cause. Further studies are therefore clearly required in order to better define the importance of σB in the pathogenicity of S. aureus.
Acknowledgments
This study was supported by Swiss National Science Foundation grants 31-105390 and 3100A0-100234 and the European Community grant EU-LSH-CT2003-50335 (BBW 03.0098). J.K. was supported by grant 2/3010/23 from the Slovak Academy of Sciences.
We thank C. Goerke, C. Wolz, and M. Bayer for their support in establishing the protocol for the LightCycler RT-PCR. We are also grateful to S. Burger for her excellent technical support and to S. Projan, P. Dunman, and the Wyeth Antimicrobial Research Department for providing us with the necessary materials for the GeneChip experiments.
REFERENCES
- 1.Akbar, S., T. A. Gaidenko, C. M. Kang, M. O'Reilly, K. M. Devine, and C. W. Price. 2001. New family of regulators in the environmental signaling pathway which activates the general stress transcription factor σB of Bacillus subtilis. J. Bacteriol. 183:1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson, A. K., and W. G. Haldenwang. 1993. The σB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger-Bächi, B. 1983. Increase in transduction efficiency of Tn551 mediated by the methicillin resistance marker. J. Bacteriol. 154:533-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff, M., and B. Berger-Bächi. 2001. Teicoplanin stress-selected mutations increasing σB activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff, M., P. Dunman, J. Kormanec, D. Macapagal, E. Murphy, W. Mounts, B. Berger-Bächi, and S. Projan 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 186:4085-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boylan, S. A., A. Rutherford, S. M. Thomas, and C. W. Price. 1992. Activation of Bacillus subtilis transcription factor σB by a regulatory pathway responsive to stationary-phase signals. J. Bacteriol. 174:3695-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody, M. S., K. Vijay, and C. W. Price. 2001. Catalytic function of an α/β hydrolase is required for energy stress activation of the σB transcription factor in Bacillus subtilis. J. Bacteriol. 183:6422-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2001. National Nosocomial Infections Surveillance (NNIS) report, data summary from January 1992-June 2001. Am. J. Infect. Control 29:404-421. [DOI] [PubMed] [Google Scholar]
- 12.Chan, P. F., S. J. Foster, E. Ingham, and M. O. Clements. 1998. The Staphylococcus aureus alternative sigma factor σB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J. Bacteriol. 180:6082-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, C. C., M. D. Yudkin, and O. Delumeau. 2004. Phosphorylation and RsbX-dependent dephosphorylation of RsbR in the RsbR-RsbS complex of Bacillus subtilis. J. Bacteriol. 186:6830-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung, A. L., K. J. Eberhardt, and V. A. Fischetti. 1994. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal. Biochem. 222:511-514. [DOI] [PubMed] [Google Scholar]
- 15.Cheung, A. L., Y. T. Chien, and A. S. Bayer. 1999. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect. Immun. 67:1331-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Christensen, S. K., K. Pedersen, F. G. Hansen, and K. Gerdes. 2003. Toxin-antitoxin loci as stress-response-elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. J. Mol. Biol. 332:809-819. [DOI] [PubMed] [Google Scholar]
- 18.Delumeau, O., R. J. Lewis, and M. D. Yudkin. 2002. Protein-protein interactions that regulate the energy stress activation of σB in Bacillus subtilis. J. Bacteriol. 184:5583-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delumeau, O., S. Dutta, M. Brigulla, G. Kuhnke, S. W. Hardwick, U. Volker, M. D. Yudkin, and R. J. Lewis. 2004. Functional and structural characterization of RsbU, a stress signaling protein phosphatase 2C. J. Biol. Chem. 279:40927-40937. [DOI] [PubMed] [Google Scholar]
- 20.Deora, R., and T. K. Misra. 1996. Characterization of the primary σ factor of Staphylococcus aureus. J. Biol. Chem. 271:21828-21834. [DOI] [PubMed] [Google Scholar]
- 21.Deora, R., T. Tseng, and T. K. Misra. 1997. Alternative transcription factor σSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J. Bacteriol. 179:6355-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 23.Entenza, J., P. Moreillon, M. M. Senn, J. Kormanec, P. Dunman, B. Berger-Bächi, S. Projan, and M. Bischoff. 2004. Role of σB in the expression of Staphylococcus aureus cell-wall adhesins ClfA and FnbA and contribution to infectivity in a rat model of experimental endocarditis. Infect. Immun. 73:990-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 25.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 2000. Regulation of σB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261:558-566. [DOI] [PubMed] [Google Scholar]
- 26.Gertz, S., S. Engelmann, R. Schmid, A.-K. Ziebandt, K. Tischer, C. Scharf, J. Hacker, and M. Hecker. 2000. Characterization of the σB regulon in Staphylococcus aureus. J. Bacteriol. 182:6983-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gietz, I. D. 2005. Yeast two-hybrid system screening. Methods Mol. Biol. 313:345-372. [DOI] [PubMed] [Google Scholar]
- 29.Goerke, C., S. Campana, M. G. Bayer, G. Döring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goerke, C., M. G. Bayer, and C. Wolz. 2001. Quantification of bacterial transcripts during infection using competitive RT-PCR and LightCycler RT-PCR. Clin. Diagn. Lab. Immunol. 8:279-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hecker, M., and U. Volker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35-91. [DOI] [PubMed] [Google Scholar]
- 33.Homerova, D., M. Bischoff, A. Dumoulin, and J. Kormanec. 2004. Optimization of a two-plasmid system for the identification of promoters recognized by RNA polymerase containing Staphylococcus aureus alternative sigma factor σB. FEMS Microbiol. Lett. 232:173-179. [DOI] [PubMed] [Google Scholar]
- 34.Horsburgh, M. J., J. L. Aish, I. L. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonsson, I. M., S. Arvidson, S. Foster, and A. Tarkowski. 2004. Sigma factor B and RsbU are required for virulence in Staphylococcus aureus-induced arthritis and sepsis. Infect. Immun. 72:6106-6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang, C. M., K. Vijay, and C. W. Price. 1998. Serine kinase activity of a Bacillus subtilis switch protein is required to transduce environmental stress signals but not to activate its target PP2C phosphatase. Mol. Microbiol. 30:189-196. [DOI] [PubMed] [Google Scholar]
- 37.Kanth, A. 2003. Studies on global regulators involved in virulence gene expression in Staphylococcus aureus. Inaugural thesis. Karolinska Institute, Stockholm, Sweden.
- 38.Karlsson, A., and S. Arvidson. 2002. Variation in extracellular protease production among clinical isolates of Staphylococcus aureus due to different levels of expression of the protease repressor sarA. Infect. Immun. 70:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, T. J., T. A. Gaidenko, and C. W. Price. 2004. In vivo phosphorylation of partner switching regulators correlates with stress transmission in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 186:6124-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornblum, J., B. J. Hartmann, R. P. Novick, and A. Tomasz. 1986. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur. J. Clin. Microbiol. 5:714-718. [DOI] [PubMed] [Google Scholar]
- 41.Kreiswirth, B. N., S. Löfdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 42.Kullik, I., and P. Giachino. 1997. The alternative sigma factor sigB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch. Microbiol. 167:151-159. [DOI] [PubMed] [Google Scholar]
- 43.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo, S., S. Zhang, R. L. Woodbury, and W. G. Haldenwang. 2005. Associations between Bacillus subtilis σB regulators in cell extracts. Microbiology 150:4125-4136. [DOI] [PubMed] [Google Scholar]
- 45.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, et. al. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 46.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 47.Manna, A. C., M. G. Bayer, and A. L. Cheung. 1998. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J. Bacteriol. 180:3828-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maxam, A. M., and W. Gilbert. 1980. Sequencing end-labeled DNA with base specific chemical cleavages. Methods Enzymol. 65:449-560. [DOI] [PubMed] [Google Scholar]
- 49.Mittenhuber, G. 1999. Occurrence of mazEF-like antitoxin/toxin systems in bacteria. J. Mol. Microbiol. Biotechnol. 1:295-302. [PubMed] [Google Scholar]
- 50.Miyazaki, E., J. M. Chen, C. Ko, and W. R. Bishai. 1999. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J. Bacteriol. 181:2846-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair, S. P., M. Bischoff, M. M. Senn, and B. Berger-Bächi. 2003. The σB regulon influences internalization of Staphylococcus aureus by osteoblasts. Infect. Immun. 71:4167-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholas, R. O., T. Li, D. McDevitt, A. Marra, S. Sucoloski, P. L. Demarsh, and D. R. Gentry. 1999. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect. Immun. 67:3667-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novick, R. P. 1991. Genetic systems in staphylococci. Methods Enzymol. 204:587-636. [DOI] [PubMed] [Google Scholar]
- 54.Palma, M., and A. L. Cheung. 2001. σB activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect. Immun. 69:7858-7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen, K., A. V. Zavialov, M. Y. Pavlov, J. Elf, K. Gerdes, and M. Ehrenberg. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112:131-140. [DOI] [PubMed] [Google Scholar]
- 56.Price, C. T., V. K. Singh, R. K. Jayaswal, B. J. Wilkinson, and J. E. Gustafson. 2004. Pine oil cleaner-resistant Staphylococcus aureus: reduced susceptibility to vancomycin and oxacillin and involvement of SigB. Appl. Environ. Microbiol. 68:5417-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price, C. W. 2000. Protective function and regulation of the general stress response in Bacillus subtilis and related gram-positive bacteria, p. 179-197. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 58.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao, L., R. K. Karls, and M. J. Betley. 1995. In vitro transcription of pathogenesis-related genes by purified RNA polymerase from Staphylococcus aureus. J. Bacteriol. 177:2609-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rice, K. C., T. Patton, S.-J. Yang, A. Dumoulin, M. Bischoff, and K. W. Bayles. 2004. Transcription of the Staphylococcus aureus cid and lrg murein hydrolase regulators is affected by sigma factor B. J. Bacteriol. 186:3029-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossi, J., M. Bischoff, A. Wada, and B. Berger-Bächi. 2003. MsrR, a putative cell envelope-associated element involved in Staphylococcus aureus sarA attenuation. Antimicrob. Agents Chemother. 47:2558-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt, K. A., N. P. Donegan, W. A. Kwan Jr., and A. Cheung. 2004. Influences of σB and agr on expression of staphylococcal enterotoxin B (seb) in Staphylococcus aureus. Can. J. Microbiol. 50:351-360. [DOI] [PubMed] [Google Scholar]
- 63.Schwank, S., Z. Rajacic, W. Zimmerli, and J. Blaser. 1998. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob. Agents Chemother. 42:895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Senn, M. M., M. Bischoff, C. von Eiff, and B. Berger-Bächi. 2005. σB activity in a Staphylococcus aureus hemB mutant. J. Bacteriol. 87:7397-7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh, V. K., J. L. Schmidt, R. K. Jayaswal, and B. J. Wilkinson. 2003. Impact of sigB mutation on Staphylococcus aureus oxacillin and vancomycin resistance varies with parental background and method of assessment. Int. J. Antimicrob. Agents. 21:256-261. [DOI] [PubMed] [Google Scholar]
- 66.Tegmark, K., A. Karlsson, and S. Arvidson. 2000. Identification and characterization of SarH1, a new global regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 37:398-409. [DOI] [PubMed] [Google Scholar]
- 67.Vijay, K., M. S. Brody, E. Fredlund, and C. W. Price. 2000. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the σB transcription factor of Bacillus subtilis. Mol. Microbiol. 35:180-188. [DOI] [PubMed] [Google Scholar]
- 68.Völker, U., A. Dufour, and W. G. Haldenwang. 1995. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of σB. J. Bacteriol. 177:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Völker, U., A. Vökler, B. Maul, M. Hecker, A. Dufour, and W. G. Haldenwang. 1995. Separate mechanisms activate σB of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 177:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Völker, U., A. Völker, and W. G. Haldenwang. 1996. The yeast two-hybrid system detects interactions between Bacillus subtilis σB regulators. J. Bacteriol. 178:7020-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wieland, K. P., B. Wieland, and F. Götz. 1995. A promoter-screening plasmid and xylose-inducible, glucose-repressible expression vectors for Staphylococcus carnosus. Gene 158:91-96. [DOI] [PubMed] [Google Scholar]
- 72.Wolz, C., C. Goerke, R. Landmann, W. Zimmerli, and U. Flückiger. 2002. Transcription of clumping factor A in attached and unattached Staphylococcus aureus in vitro and during device-related infection. Infect. Immun. 70:2758-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodbury, R. L., T. Luo, L. Grant, and W. G. Haldenwang. 2004. Mutational analysis of RsbT, an activator of the Bacillus subtilis stress response transcription factor, σB. J. Bacteriol. 186:2789-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 178:6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang, X., C. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265-2275. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, J., Y. Zhang, and M. Inouye. 2003. Characterization of the interactions within the mazEF addiction module of Escherichia coli. J. Biol. Chem. 278:32300-32306. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, S., and W. G. Haldenwang. 2003. RelA is a component of the nutritional stress activation pathway of the Bacillus subtilis transcription factor σB. J. Bacteriol. 185:5714-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ziebandt, A. K., H. Weber, J. Rudolph, R. Schmid, D. Hoper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and σB. Proteomics 1:480-493. [DOI] [PubMed] [Google Scholar]
- 79.Ziebandt, A. K., D. Becher, K. Ohlsen, J. Hacker, M. Hecker, and S. Engelmann. 2004. The influence of agr and σB in growth phase dependent regulation of virulence factors in Staphylococcus aureus. Proteomics 4:3034-3047. [DOI] [PubMed] [Google Scholar]
- 80.Zimmerli, W., F. A. Waldvogel, P. Vaudaux, and U. E. Nydegger. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J. Infect. Dis. 146:487-497. [DOI] [PubMed] [Google Scholar]