Abstract
Motility and chemotaxis are believed to be important in the pathogenesis of Lyme disease caused by the spirochete Borrelia burgdorferi. Controlling the phosphorylation state of CheY, a response regulator protein, is essential for regulating bacterial chemotaxis and motility. Rapid dephosphorylation of phosphorylated CheY (CheY-P) is crucial for cells to respond to environmental changes. CheY-P dephosphorylation is accomplished by one or more phosphatases in different species, including CheZ, CheC, CheX, FliY, and/or FliY/N. Only a cheX phosphatase homolog has been identified in the B. burgdorferi genome. However, a role for cheX in chemotaxis has not been established in any bacterial species. Inactivating B. burgdorferi cheX by inserting a flgB-kan cassette resulted in cells (cheX mutant cells) with a distinct motility phenotype. While wild-type cells ran, paused (stopped or flexed), and reversed, the cheX mutant cells continuously flexed and were not able to run or reverse. Furthermore, swarm plate and capillary tube chemotaxis assays demonstrated that cheX mutant cells were deficient in chemotaxis. Wild-type chemotaxis and motility were restored when cheX mutant cells were complemented with a shuttle vector expressing CheX. Furthermore, CheX dephosphorylated CheY3-P in vitro and eluted as a homodimer in gel filtration chromatography. These findings demonstrated that B. burgdorferi CheX is a CheY-P phosphatase that is essential for chemotaxis and motility, which is consistent with CheX being the only CheY-P phosphatase in the B. burgdorferi chemotaxis signal transduction pathway.
Bacteria move toward or away from environments that are favorable or unfavorable, respectively, to enhance their survival (reviewed in references 5, 63, and 65). When this movement is in response to chemicals, the process is termed chemotaxis. Flagella or periplasmic flagella, depending upon their location in a cell, are responsible for locomotion in many species of bacteria. Regulation of flagellar rotation and chemotaxis has been studied most extensively in Escherichia coli and Salmonella enterica serovar Typhimurium, and phosphorylation of the response regulator CheY plays an important role in regulating the swimming pattern of cells (reviewed in references 5, 8, 12, 60, 63, and 65). The concentration of phosphorylated CheY (CheY-P) determines whether a cell runs or tumbles. In the absence of attractants, the concentration of CheY-P is relatively high, and CheY-P diffuses to and binds the flagellar switch protein FliM, switching flagellar rotation from a default counterclockwise (CCW) state to a clockwise (CW) rotation. CW rotation of one or more flagella disrupts flagellar bundles, causing cells to tumble and reorient direction during the next run (37, 64). Although CheY-P autodephosphorylates, E. coli CheZ is required for efficient CheY-P dephosphorylation, allowing rapid responses to the environment (53). Thus, functionally reducing CheY-P in null mutants of cheA (encoding the protein that transfers phosphate to CheY) or cheY results in cells with constant running phenotypes. In contrast, functionally elevating CheY-P in cheZ mutants results in cells that constantly tumble; all of these mutants are also nonchemotactic (1, 6, 43).
Borrelia burgdorferi is the flat-wave, motile spirochete that causes Lyme disease. Chemotaxis and motility are believed to be important in the pathogenesis induced by B. burgdorferi (10, 13, 36, 45). Motility of this bacterium is attributed to 7 to 11 periplasmic flagella that are attached near each end of the cell cylinder and overlap in the center of the cell (3, 21, 27, 39, 51). These flagella reside in the periplasmic space between the outer membrane sheath and cell cylinder and are involved in both cell morphology and motility. Inactivating the gene encoding the major periplasmic flagellar protein, FlaB, results in cells that are rod-shaped and nonmotile (39, 51).
Relatively little is known about spirochete chemotaxis. Several defined compounds, as well as undefined substances such as serum, are chemoattractants for Spirochaeta aurantia, B. burgdorferi, and Treponema denticola (2, 26, 29, 34, 57). A membrane potential appears to play a role in the chemotaxis signal transduction pathway of S. aurantia (24). Transcriptional and genomic analysis of several spirochete species reveals motility and chemotaxis gene clusters/operons. Many of these genes show sequence similarity to E. coli and S. enterica serovar Typhimurium motility and chemotaxis genes (15, 16, 44, 54). The B. burgdorferi genome contains multiple homologs of several motility and chemotaxis genes, e.g., there are two cheA, three cheY, three cheW, and two cheB genes. B. burgdorferi lacks a cheZ homolog (15), which appears to be restricted to β- and γ-proteobacteria (63). Some bacterial species that lack cheZ contain multiple copies of CheY, and one or more CheY protein(s) may function as a phosphate sink to sequester phosphate from a CheY that binds flagellar motors (25, 55, 56, 59).
In bacteria lacking cheZ, other chemotaxis genes, such as cheC, cheX, and fliY or fliY/N, have recently been shown to encode proteins which catalyze dephosphorylation of CheY-P and therefore may have a functional role analogous to that of cheZ in E. coli (31, 42, 62, 63). Except for fliY, which is exclusively found in gram-positive bacteria, these genes are present in Thermotoga maritima as well as the vast majority of chemotactic bacteria whose genomes have been sequenced to date. Many of these species are human pathogens (42, 61, 62). B. burgdorferi CheX shares approximately 25% amino acid sequence identity with Bacillus subtilis CheC, as well as with T. maritima CheC and CheX (31). In B. subtilis, a cheC mutant has decreased flagellar switching frequency (i.e., longer CCW and CW rotations), but the flagellar rotational bias (CCW versus CW) is unaltered (52). In addition, CheC possesses an enzymatic activity that weakly dephosphorylates CheY-P and increases significantly in the presence of CheD (62). Recently, Park et al. (42) determined the structure and biochemical activity of CheC and CheX of T. maritima. Both of these proteins possess phosphatase activity towards CheY-P, with CheX having a higher specific activity. The homodimeric state of CheX was postulated to increase its phosphatase activity (42).
Although T. maritima CheX has been shown to dephosphorylate CheY-P, the importance of this protein in chemotaxis or motility has not yet been determined in any bacterial species. To investigate the function of CheX in B. burgdorferi, we inactivated cheX by targeted mutagenesis, and the phenotypes of the mutant, as well as the complemented strains (cheX+), were analyzed. cheX mutant cells exhibited a unique motility phenotype, i.e., they constantly flexed and were unable to translate. In addition, cheX mutant cells were nonchemotactic. Biochemical analyses confirmed that B. burgdorferi CheX is indeed a phosphatase that efficiently dephosphorylates CheY-P in vitro. Taken together, these results indicate that cheX is an essential CheY-P phosphatase in the B. burgdorferi chemosensory system.
MATERIALS AND METHODS
Bacterial strains and growth conditions and cheX inactivation and complementation.
High-passage, avirulent B. burgdorferi strain B31A, the nonmotile flaB mutant, and their growth conditions have been described previously (39). Targeted inactivation of cheX (486 base pairs; Bb0671) was achieved using an flgB-kan cassette (7, 39). PCR amplification of cheX, construction of the inactivation plasmids, and electroporation of linear cheX-kan DNA into competent cells were carried out as described previously (39, 49). Briefly, cheX DNA was PCR amplified using primers (5′-3′) CheX-F (GGGGAGCTGATTGTTTGGAAG) and CheX-R (CCTTTGCCCTATCTAATGGT) and then ligated into the pGEM-T Easy vector (Promega), yielding pCheX-Easy. flgB-kan was similarly PCR amplified as reported previously (39), except the AgeI restriction sequences were replaced with XmaI. The XmaI-restricted flgB-kan DNA was then inserted into a unique XmaI site within cheX (located 92 base pairs from the cheX ATG start codon) of the pCheX-Easy vector, yielding pCheX-kan. Restriction mapping indicated that the direction of transcription of kan was the same as that of cheX. Approximately 2.8 kb of cheX-kan DNA was PCR amplified for electroporation. To complement cheX::kan, the coding sequence of cheX was PCR amplified with primers (5′-3′) CheX/com/F (CATATGAGAATAGATTATATAGAG) and CheX/com/R (AAGCTTCAAACCCTCTCTCTTATTG) and ligated into a plasmid (pGEM-T Easy) containing the B. burgdorferi flgB promoter, using NdeI and HindIII restriction sites. The flgB-cheX DNA was then inserted into shuttle vector pKFSS1 (14) using HindIII restriction digestion, yielding pKFCheX. This shuttle vector and the backbone of pKFSS1 vector, pBSV2, have been successfully used to complement targeted genes in this and other laboratories (14, 67; M. Motaleb, C. Li, and N. Charon, unpublished). Twenty micrograms of the resultant plasmid, pKFCheX, was electroporated into the cheX::kan mutant cells. Growth medium contained 350 μg/ml kanamycin and 80 μg/ml streptomycin.
Dark-field microscopy.
Video sequences were taken with a Basler A600fm digital camera at a resolution of 640 by 480 pixels and a speed of 50 frames per second. Dark-field images at a magnification of 100× on the camera sensor were produced with Zeiss optics and a modified Chadwick-Helmuth stroboscope, as previously described (22). Individual frames were selected with MoviePlayer and edited in Photoshop.
Protein preparation and antibody production.
To express CheX, the gene encoding the protein sequence was PCR amplified (without the ATG codon) using primers (5′-3′) F RCheX (GGATCCAGAATAGATTATATAGAGC) and CheX/com/R (AAGCTTCAAACCCTCTCTCTTATTG); the amplified DNA was then cloned into the BamHI-HindIII-restricted pQE30 vector (QIAGEN Inc.) and overexpressed with a six-His tag in E. coli M15(pREP4) by using 0.1 M isopropyl-β-d-thiogalactoside. The His-tagged protein was purified as recommended by the manufacturer (QIAGEN Inc.) and dialyzed against 25 mM NaCl, 50 mM Tris-HCl, pH 8.5 (enzyme studies) or pH 7.0 (antibody production). Approximately 400 μg of purified His-CheX was used to immunize rats to produce CheX-specific antiserum (Strategic Biosolutions, Newark, DE). Amino-terminal His-tagged CheY3 and CheA2 were prepared using similar procedures. Rat or rabbit polyclonal antisera against recombinant CheY3 or CheA2, respectively, were produced in a similar manner and are described elsewhere (M. Motaleb, R. Bakker, C. Li, and N. Charon, unpublished data). Antibody specificities were confirmed by Western blotting using their respective targeted mutants constructed in our laboratory (32, 40).
Gel electrophoresis and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with an enhanced chemiluminescent detection method (Amersham Pharmacia) were carried out as reported previously (19). The concentration of proteins in cell lysates was determined by a Bio-Rad protein assay kit. Unless otherwise noted, 10 μg of lysate protein was subjected to SDS-PAGE and Western blotting using specific antibodies. Monoclonal antibodies kindly provided by other investigators included the following: anti-FlaB (H9724) by A. Barbour (University of California, Irvine, CA), anti-FlaA by B. Johnson (Centers for Disease Control and Prevention, Atlanta, GA), and anti-DnaK by J. Benach (SUNY, Stony Brook, NY). Specific reactivity to B. burgdorferi FlaA, FlaB, and DnaK has been reported previously (4, 11, 20, 39, 40).
Swarm plate and capillary tube chemotaxis assays.
Swarm plate chemotaxis assays were performed as described previously (32, 39). Approximately 106 washed cells in a 5-μl volume were spotted onto 0.35% (wt/vol) agarose plates containing Barbour-Stoenner-Kelly II (BSK-II) medium diluted 1:10 in Dulbecco's phosphate-buffered saline. Capillary tube assays were carried out as reported previously (32), with some modifications (2). Briefly, cells were centrifuged and resuspended in a motility buffer (57) containing 1% (wt/vol) bovine serum albumin and 1% (wt/vol) methylcellulose (400 mesh). Capillary tubes filled with attractant (0.1 M glucosamine) or motility buffer controls were sealed and inserted into microcentrifuge tubes containing resuspended cells. Tubes were incubated for 2 h at 33°C in a humidified chamber, after which solutions were expelled from the capillary tubes. To determine the concentration of B. burgdorferi cells in capillary tubes, aliquots of expelled cells were mixed with 10 mM HEPES, pH 7.4, containing cell-permeant nucleic acid fluorescent dye Syto61 (Molecular Probes) and analyzed by flow cytometry. Data were acquired and analyzed with a FACSCalibur using CellQuest Pro software (Becton Dickinson).
CheY-P dephosphorylation assays.
Phosphotransfer and dephosphorylation experiments were performed as reported previously (6). Briefly, CheA2 (200 pmol) was autophosphorylated by incubation with TKM buffer (50 mM Tris-HCl, pH 8.5, 50 mM KCl, 5 mM MgCl2), and 0.3 mM [γ-32P]ATP (5,000 Ci/mmol, 10 mCi/ml; from MP Biomedicals) in a total volume of 0.1 ml at room temperature for 30 min. Unincorporated [γ-32P]ATP was removed using Micro Bio-spin 6 chromatography columns (Bio-Rad). Twenty picomoles of autophosphorylated [32P]CheA2-P was then added to premixed TKM buffer containing 150 pmol of CheY3, with or without CheX (0 to 20 pmol), for 1 min. The reactions were stopped with 4× SDS-PAGE sample buffer (50 mM Tris-HCl, pH 6.8, 100 mM dithiothreitol, 2% [wt/vol] SDS, 0.1% [wt/vol] bromophenol blue, 10% [vol/vol] glycerol, and 5% [vol/vol] 2-mercaptoethanol), and proteins were separated by SDS-PAGE (15% acrylamide). The gels were dried and subjected to analysis on a PhosphorImager system (Storm 860; Molecular Dynamics).
Gel filtration chromatography.
Purified His-CheX (225 μg in 200 μl 150 mM NaCl, 50 mM Tris, pH 7.4) or protein standards (bovine serum albumin [66 kDa], carbonic anhydrase [29 kDa], and cytochrome c [12.4 kDa]; Sigma molecular weight marker kit for gel chromatography) were applied to a Superdex 75 HR 10/30 gel filtration column and eluted at 0.3 ml/min with 150 mM NaCl, 50 mM Tris, pH 7.4, using Pharmacia LCC 501 Plus Controller and P500 pump. Eluted proteins were detected by monitoring absorbance at 280 nm.
RESULTS
Construction and complementation of cheX strains.
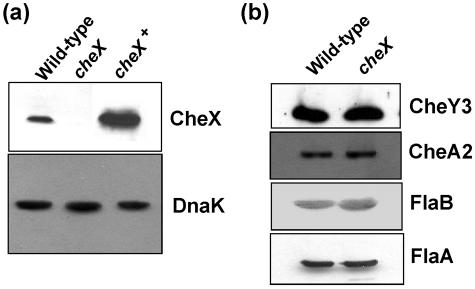
B. burgdorferi cheX is located in a motility/chemotaxis operon consisting of flaA, cheA2, cheW3, cheX, and cheY3 genes that is driven by a σ70 promoter (17-19, 32, 33). Western blot analysis (see below) indicates that all of the genes in this operon are expressed in growing cells (32, 40). To investigate the function of cheX, cheX was inactivated by targeted mutagenesis using a kan cassette as described in Materials and Methods and reference 39. Western blot analysis confirmed cheX inactivation, using antiserum from rats immunized with purified CheX. Figure 1a shows that B. burgdorferi CheX antiserum reacted with an 18-kDa protein in wild-type cells, as expected, and that this 18-kDa protein is absent in the cheX mutant. PCR analysis confirmed the insertion of kan into the targeted gene (data not shown). Together, these results indicate that CheX synthesis is inhibited by inserting kan into cheX. Since cheX is located within an operon, kan insertion within cheX might have caused an alteration of downstream gene expression, thus complicating interpretation of any phenotype associated with cheX mutant cells. However, several lines of evidence indicate that a polar effect due to the inactivation of cheX is unlikely. First, Western blot analysis demonstrated that the expression of CheY3, which is immediately downstream of cheX, was essentially identical in cheX mutant and wild-type cells (Fig. 1b). Second, targeted mutation of cheY3 results in cells that constantly run (M. Motaleb, R. Bakker, C. Li, and N. Charon, unpublished data), which is markedly different from the swimming behavior of cheX mutant cells (see below). Finally, complementing cheX mutant cells (cheX+) with a shuttle vector expressing CheX restored wild-type swimming (see below), indicating that the phenotype of cheX mutant cells was not due to an unexpected secondary alteration. Western blot analysis demonstrated that CheX is expressed in the cheX+ strain (Fig. 1a).
FIG. 1.
(a) Western blot analysis of CheX expression in wild-type, cheX mutant, and cheX+ cells. Cell lysates from wild-type, cheX mutant, and cheX+ cells were analyzed by SDS-PAGE and Western blotting with anti-CheX antiserum (top panel). The overexpression of CheX in the cheX+ strain was likely due to the strong flgB promoter and multicopy nature of the shuttle vector used. As an internal control, 2 μg of each cell lysate was probed with anti-DnaK antibodies (bottom panel). (b) Western blot analysis of CheY3, CheA2, FlaB, and FlaA expression in wild-type and cheX mutant cells. Cell lysates (2 μg for FlaB) from wild-type and cheX mutant cells were probed with antibodies specific for CheY3, CheA2, FlaB, and FlaA.
Altered swimming in cheX mutant cells.
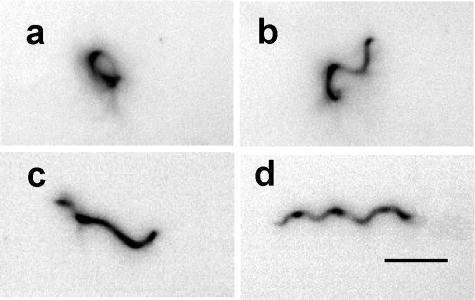
Because CheX shares amino acid sequence similarity with the recently characterized CheY-P phosphatases CheC of B. subtilis and CheX of T. maritima (31, 42, 62), it was important to characterize the phenotypes associated with B. burgdorferi cheX mutant cells. Video analysis of swimming cells demonstrated that wild-type B. burgdorferi ran, paused (stopped or flexed), and reversed; they had a regular wave-like morphology during the interval when they translated in either BSK-II or BSK-II supplemented with 1% methylcellulose (Fig. 2d). The latter compound, which causes the medium to be gel-like, has been shown to promote enhanced translation of B. burgdorferi and other spirochetes (10, 23, 30). For B. burgdorferi, a “flex” is considered to be when cells bend in the middle or appear distorted in BSK-II medium (23). In contrast to wild-type cells, cheX mutant cells had a distorted morphology (Fig. 2a to c). Such cells appeared to be locked into the flexing mode. Although cheX mutant cells were motile, as the ends of the cells gyrated, they did not translate in BSK-II with or without methylcellulose. As expected, the wild-type swimming behavior and morphology were restored in cheX+ cells (data not shown). The possibility that the cheX mutation caused altered expression of motility genes, resulting in a constant flexing phenotype, was investigated. Previous studies in this laboratory demonstrated that periplasmic flagella are crucial for B. burgdorferi morphology and motility (39). The levels of expression of major and minor flagellins, FlaB and FlaA, were similar in wild-type and cheX mutant cells, as shown by Western blotting (Fig. 1b). Furthermore, microscopic examination of cheX mutant cells demonstrated that both ends of the cell gyrate at a given time. This observation indicates that both bundles of the periplasmic flagella are rotating (data not shown). Therefore, the constant flexing phenotype in cheX mutant cells can be attributed solely to inactivation of cheX.
FIG. 2.
Dark-field images with contrast reversed of cheX mutant and wild-type B. burgdorferi cells. (a to c) cheX mutant cells illustrating their distorted morphological forms. Cells change their shape from one form to another. (d) Morphological form of wild-type cell during translational motility interval. This form is rarely seen in cheX mutant cells. The bar represents 5 μm.
Altered chemotaxis in cheX mutant cells.
CheC and especially CheX are considered to be functional analogues of E. coli CheZ, based on their biochemical properties as CheY-P phosphatases in B. subtilis and T. maritima (42, 62, 63). If cheX is an essential B. burgdorferi CheY-P phosphatase, a null mutant in cheX should result in cells with a nonchemotactic behavior, such as E. coli cheZ mutants (6, 43). To test this hypothesis, swarm plate and capillary tube chemotaxis assays were performed with the wild-type, cheX mutant, and cheX+ cells. The swarm diameter of cheX mutant cells was significantly reduced compared to wild-type cells (Fig. 3a). Furthermore, the swarm diameter of cheX+ cells was similar to that of the wild type, indicating that deficient chemotaxis of cheX mutant cells was due to cheX inactivation and not an unknown mutation elsewhere. Noticeable growth defects were not observed in cheX mutant cells, indicating the decreased swarming phenotype was not due to a growth defect (data not shown). The swarm diameter likely depends on the precise cell run/pause/reverse frequency (50, 66). Therefore, excess CheX synthesized in the cheX+ cells (Fig. 1a) would likely alter the run/pause/reverse frequency and modestly reduce swarm size, as seen in Fig. 3a. In addition, capillary tube chemotaxis assays were performed using glucosamine as an attractant. Glucosamine is a chemoattractant for S. aurantia (26) and B. burgdorferi (2). Whereas the wild-type and cheX+ strains were chemotactic toward glucosamine, the cheX mutant cells failed to respond to this compound (Fig. 3b). These capillary tube results support those from the swarm plate assays and further indicate that cheX mutant cells are nonchemotactic.
FIG. 3.
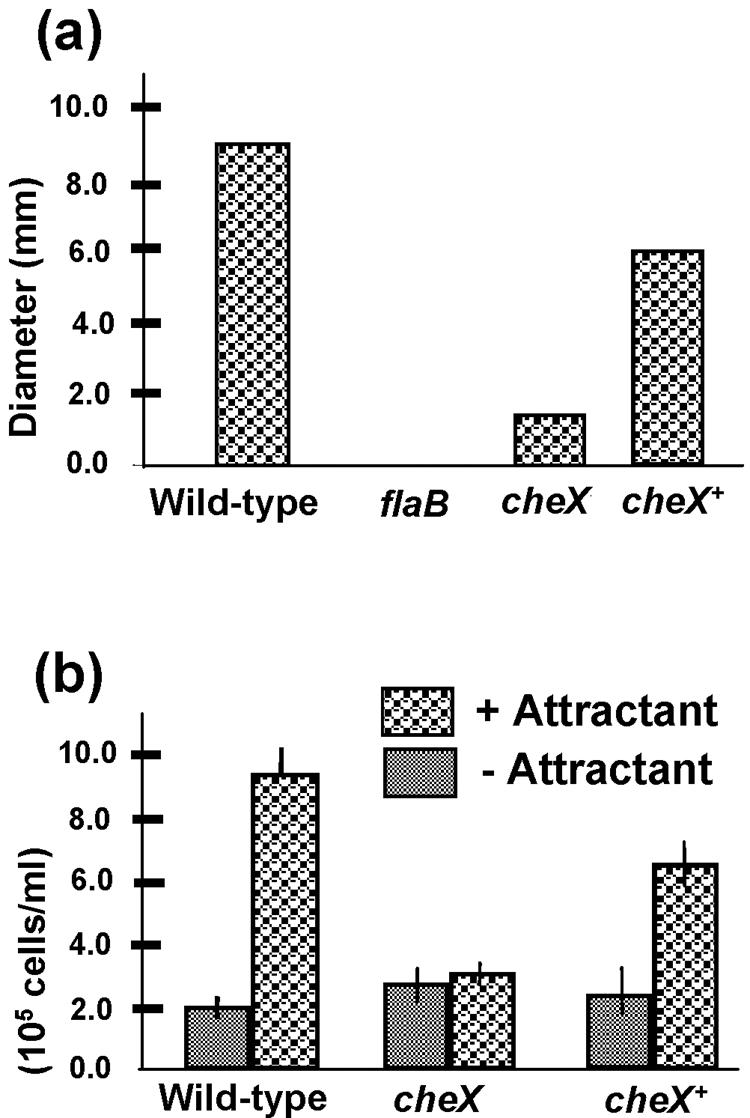
Chemotaxis assays. (a) Swarm plate assays were performed using BSK-II medium diluted 1:10 with phosphate-buffered saline. Wild-type, cheX mutant, and cheX+ cells were spotted onto agarose plates and incubated for 3 days. The results are expressed as swarm diameter after subtracting the diameter of a nonmotile flaB mutant. (b) Capillary tube chemotaxis assay coupled with flow cytometry. Cells from each strain shown were incubated with capillary tubes filled with attractant or no attractant as described in Materials and Methods. After 2 h of incubation, the concentration of cells in each tube was determined using flow cytometry. At least three independent experiments were performed with each strain. Results are expressed as the average number of cells/ml ± standard deviation.
CheX is a CheY-P phosphatase.
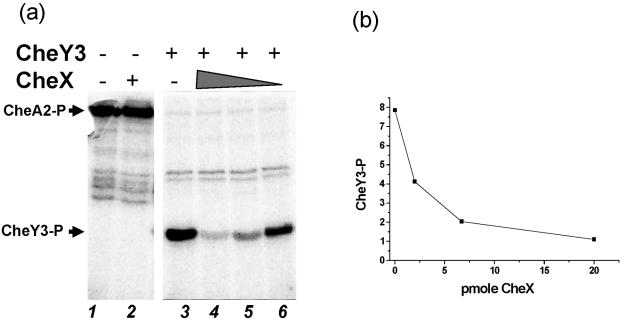
Recently, CheC and CheX of B. subtilis and T. maritima have been shown to dephosphorylate CheY-P in vitro (42, 62, 63). Amino acid sequence alignment indicates that the functional residues of CheC and CheX of T. maritima are conserved in B. burgdorferi CheX (references 31 and 42 and data not shown). Therefore, the effect of B. burgdorferi CheX on CheY-P was determined in vitro. Although the three CheYs of B. burgdorferi each possess all of the conserved functional residues of a response regulator (data not shown), initial studies focused on the CheA2-CheY3 pathway for the following reasons: cheA2 and cheY3 are located in the same operon as cheX, and cheA2 and cheY3 are the only sensor kinase and response regulator genes, respectively, that exhibit detectable nonchemotactic phenotypes when inactivated (32; M. Motaleb, R. Bakker, C. Li, and N. Charon, unpublished data). After overexpression and purification of B. burgdorferi CheA2 and CheY3, optimal conditions (pH, time course, and KCl concentration) for CheA2 autophosphorylation with [γ-32P]ATP were determined. As shown in Fig. 4a, CheA2 autophosphorylated (lane 1) and effectively transferred its phosphoryl group to CheY3 (lane 3), confirming that CheA2 is a phosphodonor for CheY3. CheY3 was not phosphorylated by ATP itself (not shown). CheX dephosphorylated [32P]CheY3-P, in a concentration-dependent manner, when added to a reaction mixture of [32P]CheA2-P and CheY3 (Fig. 4a, lanes 4 to 6, and b). However, the stability of [32P]CheA2-P was unaffected by even a sevenfold molar excess of CheX (Fig. 4a, lane 2, and data not shown). These results indicate that CheX of B. burgdorferi is a functional CheY-P phosphatase in the B. burgdorferi chemotaxis signal transduction pathway.
FIG. 4.
CheY3-P dephosphorylation assay. (a) Twenty picomoles of autophosphorylated [32P]CheA2-P was incubated alone (lane 1), with 20 pmol CheX for 15 min (lane 2), or with 150 pmol CheY3 for 1 min with no CheX (lane 3), 20 pmol CheX (lane 4), 6.3 pmol CheX (lane 5), or 2.1 pmol CheX (lane 6). [32P]CheY3-P did not significantly autodephosphorylate during this time period. Arrows indicate the positions of [32P]CheA2-P and [32P]CheY3-P. (b) The intensity of [32P]CheY3-P bands as a function of CheX concentration is depicted as relative phosphorimage units.
CheX is a dimer.
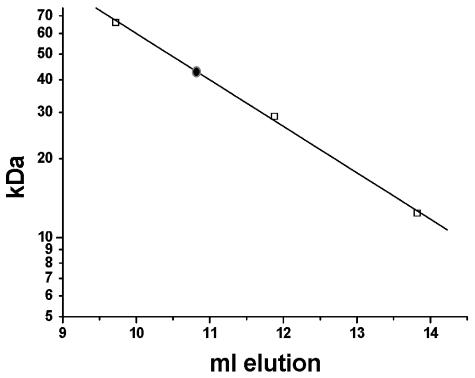
CheX and CheC share some sequence similarity; however, a major difference between these two proteins is that T. maritima CheC is a monomer whereas CheX is a dimer (42). Furthermore, the dimeric state of T. maritima CheX was proposed to be important for the stronger phosphatase activity exhibited by CheX (42). The monomer/dimer status of B. burgdorferi CheX was investigated by gel filtration chromatography to further validate that this protein is properly classified as a CheX rather than a CheC. The calculated mass of purified CheX is ∼19 kDa, including the six-His tag; however, all of the CheX applied to a Superdex column eluted as an ∼40-kDa protein (Fig. 5), indicating that B. burgdorferi CheX is a homodimer, consistent with classification as a CheX protein.
FIG. 5.
CheX chromatography on Superdex 75. The elution positions of protein standards bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.4 kDa) from a Superdex 75 column are depicted as open squares; the position of CheX elution is indicated by a filled circle.
DISCUSSION
The motility and chemotaxis system of the Lyme disease spirochete B. burgdorferi is unique and complex. Motility and chemotaxis are important for many pathogenic organisms to colonize and/or cause disease, including Vibrio cholerae, S. enterica serovar Typhimurium, Helicobacter pylori, Brachyspira hyodysenteriae, and possibly T. denticola (9, 28, 38, 41, 46). In T. denticola, mutation of chemotaxis genes cheA, dmcA, or dmcB produced cells that were unable to penetrate eukaryotic cell monolayers (35, 36). Several studies suggest that motility and chemotaxis are also important for B. burgdorferi to penetrate mammalian hosts (36, 47, 48). In addition, during B. burgdorferi's life cycle, it shuttles between mammalian and tick hosts, and it swims in viscous gel-like media where most other bacteria fail to swim (10, 30). Multiple variants of chemotaxis and motility proteins may be necessary for these spirochetes to transit to the different hosts and adapt to these very different environments (10, 15, 32). The goal of the present investigation was to determine the extent to which cheX is important for B. burgdorferi chemotaxis and motility and to begin to characterize the properties of this protein.
CheX represents a recently identified family of protein phosphatases involved in catalyzing the dephosphorylation of CheY-P (42, 62, 63), a reaction essential for rapid chemotactic responses (53). While CheZ is a CheY-P phosphatase in E. coli and other β- and γ-proteobacteria (63), in other species CheY-P dephosphorylation is mediated by CheC, CheD, FliY, and/or CheX (42, 62, 63). B. burgdorferi CheX shares amino acid sequence similarity to CheX, CheC, and FliY, which have recently been reported to be CheY-P phosphatases of B. subtilis and T. maritima (42, 62). However, fliY and cheC were not identified in the B. burgdorferi genome (see below). While other spirochetes, e.g., T. denticola, Treponema pallidum, and Leptospira interrogans, contain homologs of fliY in addition to cheX or cheC (16, 44, 54), B. burgdorferi is the only spirochete whose genome sequence has been published that does not contain a fliY homolog (15). In fact, the only known potential CheY-P phosphatase in the B. burgdorferi genome is CheX, suggesting that this protein plays a critical role in B. burgdorferi chemotaxis.
Inactivating cheX (Fig. 1a) produced B. burgdorferi cells that constantly flexed (Fig. 2a to c) and were nonchemotactic (Fig. 3), demonstrating an essential role of CheX in B. burgdorferi's chemosensory pathway. Neither the constantly flexing phenotype nor the loss of chemotaxis in cheX mutant cells appeared to be attributed to polar effects or secondary mutations: first, no appreciable alteration in levels of expression of the motility proteins FlaB or FlaA or the chemotaxis proteins CheY3 or CheA2 was observed (Fig. 1) (cheY3 is immediately downstream of cheX, and flaA and cheA2 are upstream of cheX); second, both flagellar bundles appear to be rotating; and finally, the wild-type swimming behavior and chemotactic ability were restored in cheX+ complemented cells. In B. burgdorferi, cheX inactivation likely removes the only CheY-P phosphatase, resulting in highly elevated CheY-P and disruption of the chemosensory signal transduction pathway. Most bacteria that do not contain a cheZ gene have multiple presumptive CheY-P phosphatases, and inactivating one of those phosphatases may have less dramatic effects than those observed in B. burgdorferi cheX mutant cells. For example, in B. subtilis, inactivating either of the two CheY-P phosphatase genes, fliY or cheC, altered flagellar rotation, indicating increased levels of CheY-P. The fliY mutant exhibited a stronger phenotype than the cheC mutant, and a cheC fliY double mutant had the strongest phenotype, consistent with the highest levels of CheY-P (62). Recently, Sim et al. (58) reported that T. denticola CheX interacted with itself and CheA in a yeast two-hybrid system. They also postulated that cheX is likely to be a phosphatase in T. denticola (58); however, the effects of inactivating cheX in T. denticola have not yet been reported. All these observations are consistent with the hypothesis that B. burgdorferi relies solely on CheX for CheY-P dephosphorylation. Furthermore, a double mutant of cheY3 and cheX would be expected to have a constantly running phenotype (as a single cheY3 mutant) instead of a constantly flexing phenotype (such as the cheX mutant cells). Consistent with this prediction, a double mutation that inactivated both cheX and cheY3 did exhibit a constantly running phenotype (M. Motaleb and N. Charon, unpublished).
In vitro phosphorylation studies confirmed that CheX stimulates dephosphorylation of B. burgdorferi CheY3-P (Fig. 4), and gel filtration studies indicate B. burgdorferi CheX is a homodimer (Fig. 5). Park et al. (42) proposed to differentiate CheX and CheC, based in part on CheX being smaller than CheC and on the presence of a conserved Gly residue in CheX that is involved in forming a β-sheet important for CheX dimerization. Based on these criteria, B. burgdorferi CheX is correctly classified as a CheX rather than a CheC. While results of the CheA2-CheY3-CheX pathway have been presented herein (Fig. 4), additional studies suggest that CheX also dephosphorylates CheY1-P in the CheA1-CheY1-CheX pathway (M. Motaleb, C. Li, N. Charon, and M. Miller, unpublished data). Recent reports indicate that the mechanism of T. maritima CheX-mediated dephosphorylation of CheY-P is different from that of E. coli CheZ (42, 68). B. burgdorferi and T. maritima CheX share ∼25% amino acid sequence identity but no sequence similarity to CheZ. The ability of T. maritima CheX to form homodimers was proposed to be one factor in the higher phosphatase activity of CheX relative to CheC (42). B. burgdorferi CheX is also a homodimer. Taken together, this information suggests B. burgdorferi and T. maritima CheX may dephosphorylate CheY-P by similar mechanisms. Determining the structure of a B. burgdorferi CheX/CheY3-P complex should provide more definitive information about the mechanism of B. burgdorferi CheX-mediated CheY-P dephosphorylation.
Acknowledgments
We thank A. Barbour, J. Benach, B. Johnson, and D. S. Samuels for sharing antibodies and plasmid. We also thank David Yelton for comments on the manuscript and Pang Jia for technical assistance.
This research was supported by U.S. Public Health Service grants AI29743 to N.W.C., GM 050860 to R.B.B., and AR050656-01 to C.L.; NSF grant DMS 0201063 to S.F.G.; U.S. Public Health Service grant RR16440 to The West Virginia Flow Cytometric Core Facility; American Heart Association grant 0365225B to M.A.M.; and West Virginia University Health Science Center Internal Grants, Office of Research and Graduate Education, to M.A.M. and M.R.M.
REFERENCES
- 1.Alon, U., L. Camarena, M. G. Surette, B. A. Y. Arcas, Y. Liu, S. Leibler, and J. B. Stock. 1998. Response regulator output in bacterial chemotaxis. EMBO J. 17:4238-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker, R. G., and N. W. Charon. 2004. Chemically defined attractants for Borrelia burgdorferi the Lyme disease spirochete: a FACS based approach, abstr. I-142, p. 347. Abstr. 104th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, D.C.
- 3.Barbour, A. G., and S. F. Hayes. 1986. Biology of Borrelia species. Microbiol. Rev. 50:381-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour, A. G., S. F. Hayes, R. A. Heiland, and M. E. Schrumpf. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect. Immun. 52:549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19-54. [DOI] [PubMed] [Google Scholar]
- 6.Boesch, K. C., R. E. Silversmith, and R. B. Bourret. 2000. Isolation and characterization of nonchemotactic CheZ mutants of Escherichia coli. J. Bacteriol. 182:3544-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bono, J. L., A. F. Elias, J. J. Kupko III, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 182:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourret, R. B., and A. M. Stock. 2002. Molecular information processing: lessons from bacterial chemotaxis. J. Biol. Chem. 277:9625-9628. [DOI] [PubMed] [Google Scholar]
- 9.Butler, S. M., and A. Camilli. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 101:5018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charon, N. W., and S. F. Goldstein. 2002. Genetics of motility and chemotaxis of a fascinating group of bacteria: the Spirochetes. Annu. Rev. Genet. 36:47-73. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, J. L., and J. L. Benach. 1992. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J. Infect. Dis. 165:658-666. [DOI] [PubMed] [Google Scholar]
- 12.Falke, J. J., and G. L. Hazelbauer. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank, K. L., S. F. Bundle, M. E. Kresge, C. H. Eggers, and D. S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, and J. Gocayne. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, C. M., S. J. Norris, C. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, and M. D. Cotton. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 17.Ge, Y., and N. W. Charon. 1997. An unexpected flaA homolog is present and expressed in Borrelia burgdorferi. J. Bacteriol. 179:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge, Y., and N. W. Charon. 1997. Molecular characterization of a flagellar/chemotaxis operon in the spirochete Borrelia burgdorferi. FEMS Microbiol. Lett. 153:425-431. [DOI] [PubMed] [Google Scholar]
- 19.Ge, Y., C. Li, L. Corum, C. A. Slaughter, and N. W. Charon. 1998. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi. J. Bacteriol. 180:2418-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmore, R. D., Jr., R. L. Murphree, A. M. James, S. A. Sullivan, and B. J. Johnson. 1999. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is the flaA gene product. J. Clin. Microbiol. 37:548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein, S. F., K. F. Buttle, and N. W. Charon. 1996. Structural analysis of Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J. Bacteriol. 178:6539-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein, S. F., and N. W. Charon. 1990. Multiple exposure photographic analysis of a motile spirochete. Proc. Natl. Acad. Sci. USA 87:4895-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein, S. F., N. W. Charon, and J. A. Kreiling. 1994. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc. Natl. Acad. Sci. USA 91:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulbourne, E. A., Jr., and E. P. Greenberg. 1981. Chemotaxis of Spirochaeta aurantia: involvement of membrane potential in chemosensory signal transduction. J. Bacteriol. 148:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greck, M., J. Platzer, V. Sourjik, and R. Schmitt. 1995. Analysis of a chemotaxis operon in Rhizobium meliloti. Mol. Microbiol. 15:989-1000. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg, E. P., and E. Canale-Parola. 1977. Chemotaxis in Spirochaeta aurantia. J. Bacteriol. 130:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hovind-Hougen, K. 1984. Ultrastructure of spirochetes isolated from Ixodes ricinus and Ixodes dammini. Yale J. Biol. Med. 57:543-548. [PMC free article] [PubMed] [Google Scholar]
- 28.Josenhans, C., and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605-614. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka, M., H. Li, S. Arakawa, and H. Kuramitsu. 1997. Characterization of a methyl-accepting chemotaxis protein gene, dmcA, from the oral spirochete Treponema denticola. Infect. Immun. 65:4011-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimsey, R. B., and A. Spielman. 1990. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J. Infect. Dis. 162:1205-1208. [DOI] [PubMed] [Google Scholar]
- 31.Kirby, J. R., C. J. Kristich, M. M. Saulmon, M. A. Zimmer, L. F. Garrity, I. B. Zhulin, and G. W. Ordal. 2001. CheC is related to the family of flagellar switch proteins and acts independently from CheD to control chemotaxis in Bacillus subtilis. Mol. Microbiol. 42:573-585. [DOI] [PubMed] [Google Scholar]
- 32.Li, C., R. G. Bakker, M. A. Motaleb, M. L. Sartakova, F. C. Cabello, and N. W. Charon. 2002. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc. Natl. Acad. Sci. USA 99:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, C., M. A. Motaleb, M. Sal, S. F. Goldstein, and N. W. Charon. 2000. Spirochete periplasmic flagella and motility. J. Mol. Microbiol. Biotechnol. 2:345-354. [PubMed] [Google Scholar]
- 34.Li, H., S. Arakawa, Q. D. Deng, and H. Kuramitsu. 1999. Characterization of a novel methyl-accepting chemotaxis gene, dmcB, from the oral spirochete Treponema denticola. Infect. Immun. 67:694-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lux, R., J. N. Miller, N. H. Park, and W. Shi. 2001. Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola. Infect. Immun. 69:6276-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lux, R., A. Moter, and W. Shi. 2000. Chemotaxis in pathogenic spirochetes: directed movement toward targeting tissues? J. Mol. Microbiol. Biotechnol. 2:355-364. [PubMed] [Google Scholar]
- 37.Macnab, R. M., and M. K. Ornston. 1977. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J. Mol. Biol. 112:1-30. [DOI] [PubMed] [Google Scholar]
- 38.Merrell, D. S., S. M. Butler, F. Qadri, N. A. Dolganov, A. Alam, M. B. Cohen, S. B. Calderwood, G. K. Schoolnik, and A. Camilli. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motaleb, M. A., L. Corum, J. L. Bono, A. F. Elias, P. Rosa, D. S. Samuels, and N. W. Charon. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA 97:10899-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motaleb, M. A., M. S. Sal, and N. W. Charon. 2004. The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. J. Bacteriol. 186:3703-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 42.Park, S. Y., X. Chao, G. Gonzalez-Bonet, B. D. Beel, A. M. Bilwes, and B. R. Crane. 2004. Structure and function of an unusual family of protein phosphatases: the bacterial chemotaxis proteins CheC and CheX. Mol. Cell 16:563-574. [DOI] [PubMed] [Google Scholar]
- 43.Parkinson, J. S. 1978. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J. Bacteriol. 135:45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren, S. X., G. Fu, X. G. Jiang, R. Zeng, Y. G. Miao, H. Xu, Y. X. Zhang, H. Xiong, G. Lu, L. F. Lu, H. Q. Jiang, J. Jia, Y. F. Tu, J. X. Jiang, W. Y. Gu, Y. Q. Zhang, Z. Cai, H. H. Sheng, H. F. Yin, Y. Zhang, G. F. Zhu, M. Wan, H. L. Huang, Z. Qian, S. Y. Wang, W. Ma, Z. J. Yao, Y. Shen, B. Q. Qiang, Q. C. Xia, X. K. Guo, A. Danchin, I. Saint Girons, R. L. Somerville, Y. M. Wen, M. H. Shi, Z. Chen, J. G. Xu, and G. P. Zhao. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422:888-893. [DOI] [PubMed] [Google Scholar]
- 45.Rosa, P. A., K. Tilly, and P. E. Stewart. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 3:129-143. [DOI] [PubMed] [Google Scholar]
- 46.Rosey, E. L., M. J. Kennedy, and R. J. Yancey, Jr. 1996. Dual flaA1 flaB1 mutant of Serpulina hyodysenteriae expressing periplasmic flagella is severely attenuated in a murine model of swine dysentery. Infect. Immun. 64:4154-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saba, S., B. A. VanderBrink, G. Perides, L. J. Glickstein, M. S. Link, M. K. Homoud, R. T. Bronson, M. Estes III, and P. J. Wang. 2001. Cardiac conduction abnormalities in a mouse model of Lyme borreliosis. J. Interv. Card. Electrophysiol. 5:137-143. [DOI] [PubMed] [Google Scholar]
- 48.Sadziene, A., D. D. Thomas, V. G. Bundoc, S. C. Holt, and A. G. Barbour. 1991. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J. Clin. Investig. 88:82-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuels, D. S., K. E. Mach, and C. F. Garon. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 176:6045-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanna, M. G., and M. I. Simon. 1996. In vivo and in vitro characterization of Escherichia coli protein CheZ gain- and loss-of-function mutants. J. Bacteriol. 178:6275-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sartakova, M. L., E. Y. Dobrikova, M. A. Motaleb, H. P. Godfrey, N. W. Charon, and F. C. Cabello. 2001. Complementation of a nonmotile flaB mutant of Borrelia burgdorferi by chromosomal integration of a plasmid containing a wild-type flaB allele. J. Bacteriol. 183:6558-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saulmon, M. M., E. Karatan, and G. W. Ordal. 2004. Effect of loss of CheC and other adaptational proteins on chemotactic behaviour in Bacillus subtilis. Microbiology 150:581-589. [DOI] [PubMed] [Google Scholar]
- 53.Segall, J. E., M. D. Manson, and H. C. Berg. 1982. Signal processing times in bacterial chemotaxis. Nature (London) 296:1-3. [DOI] [PubMed] [Google Scholar]
- 54.Seshadri, R., G. S. Myers, H. Tettelin, J. A. Eisen, J. F. Heidelberg, R. J. Dodson, T. M. Davidsen, R. T. DeBoy, D. E. Fouts, D. H. Haft, J. Selengut, Q. Ren, L. M. Brinkac, R. Madupu, J. Kolonay, S. A. Durkin, S. C. Daugherty, J. Shetty, A. Shvartsbeyn, E. Gebregeorgis, K. Geer, G. Tsegaye, J. Malek, B. Ayodeji, S. Shatsman, M. P. McLeod, D. Smajs, J. K. Howell, S. Pal, A. Amin, P. Vashisth, T. Z. McNeill, Q. Xiang, E. Sodergren, E. Baca, G. M. Weinstock, S. J. Norris, C. M. Fraser, and I. T. Paulsen. 2004. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc. Natl. Acad. Sci. USA 101:5646-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah, D. S., S. L. Porter, D. C. Harris, G. H. Wadhams, P. A. Hamblin, and J. P. Armitage. 2000. Identification of a fourth cheY gene in Rhodobacter sphaeroides and interspecies interaction within the bacterial chemotaxis signal transduction pathway. Mol. Microbiol. 35:101-112. [DOI] [PubMed] [Google Scholar]
- 56.Shah, D. S., S. L. Porter, A. C. Martin, P. A. Hamblin, and J. P. Armitage. 2000. Fine tuning bacterial chemotaxis: analysis of Rhodobacter sphaeroides behaviour under aerobic and anaerobic conditions by mutation of the major chemotaxis operons and cheY genes. EMBO J. 19:4601-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi, W., Z. M. Yang, Y. Geng, L. E. Wolinsky, and M. A. Lovett. 1998. Chemotaxis in Borrelia burgdorferi. J. Bacteriol. 180:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim, J. H., W. Shi, and R. Lux. 2005. Protein-protein interactions in the chemotaxis signalling pathway of Treponema denticola. Microbiology 151:1801-1807. [DOI] [PubMed] [Google Scholar]
- 59.Sourjik, V., and R. Schmitt. 1998. Phosphotransfer between CheA, CheY1, and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry 37:2327-2335. [DOI] [PubMed] [Google Scholar]
- 60.Sourjik, V. 2004. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 12:569-576. [DOI] [PubMed] [Google Scholar]
- 61.Szurmant, H., M. W. Bunn, V. J. Cannistraro, and G. W. Ordal. 2003. Bacillus subtilis hydrolyzes CheY-P at the location of its action, the flagellar switch. J. Biol. Chem. 278:48611-48616. [DOI] [PubMed] [Google Scholar]
- 62.Szurmant, H., T. J. Muff, and G. W. Ordal. 2004. Bacillus subtilis CheC and FliY are members of a novel class of CheY-P-hydrolyzing proteins in the chemotactic signal transduction cascade. J. Biol. Chem. 279:21787-21792. [DOI] [PubMed] [Google Scholar]
- 63.Szurmant, H., and G. W. Ordal. 2004. Diversity in chemotaxis mechanisms among the bacteria and archaea. Microbiol. Mol. Biol. Rev. 68:301-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner, L., W. S. Ryu, and H. C. Berg. 2000. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 182:2793-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wadhams, G. H., and J. P. Armitage. 2004. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5:1024-1037. [DOI] [PubMed] [Google Scholar]
- 66.Wolfe, A. J., and H. C. Berg. 1989. Migration of bacteria in semisolid agar. Proc. Natl. Acad. Sci. USA 86:6973-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao, R., E. J. Collins, R. B. Bourret, and R. E. Silversmith. 2002. Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nat. Struct. Biol. 9:570-575. [DOI] [PubMed] [Google Scholar]