Abstract
Isotope-coded affinity tag analysis and two-dimensional gel electrophoresis followed by tandem mass spectrometry were used to identify Pseudomonas aeruginosa proteins expressed during anaerobic growth. Out of the 617 proteins identified, 158 were changed in abundance during anaerobic growth compared to during aerobic growth, including proteins whose increased expression was expected based on their role in anaerobic metabolism. These results form the basis for future analyses of alterations in bacterial protein content during growth in various environments, including the cystic fibrosis airway.
Pseudomonas aeruginosa is a ubiquitous environmental gram-negative bacterium found in soil and water. It is also an opportunistic pathogen that causes infections in individuals with innate immune defects, including cystic fibrosis (CF) patients (8). P. aeruginosa encounters low-oxygen environments in soil and water. Evidence indicates that in humans with CF, bacteria may, at least in part, be in a low-oxygen environment within mucopurulent masses or biofilms within the respiratory tracts (19). P. aeruginosa is able to grow anaerobically in the presence of terminal electron acceptors, such as nitrate (NO3−), nitrite (NO2−), and nitrous oxide (N2O), or when l-arginine is a substrate for growth (21). The CF airway mucus is sufficiently rich in NO3− and NO2− to support the anaerobic growth of P. aeruginosa (7, 19). In this study, a comparison of the P. aeruginosa proteome during growth in the presence and absence of oxygen was performed.
P. aeruginosa strain PAO1 obtained from Steve Lory (Harvard Medical School, Boston, MA) was grown in 125-ml flasks in Luria broth (LB) supplemented with 1% KNO3 with shaking at 200 rpm at 37°C for aerobic growth. Anaerobic growth was completed as previously described (9) in 80 ml of medium in 100-ml Wheaton serum bottles (Fisher Scientific) with rubber stoppers. The medium was deprived of oxygen by being subjected to bubbling with N2 gas for 1 h. For both aerobic and anaerobic conditions, bacteria were harvested at the late logarithmic phase of growth, at which point the cell density (optical density at 600 nm) of the anaerobic culture was 44% of the density of the aerobic culture. There was no significant difference between the pHs of the harvested cultures (pH 7.6 for the anaerobic culture and pH 7.4 for the aerobic culture). Equal amounts of denatured and reduced whole-cell protein (2.0 mg from each growth state) were labeled with either light (12C) or heavy (13C) cleavable isotope-coded affinity tag (ICAT) reagent (Applied Biosystems, Foster City, CA), processed, and analyzed as previously described (3). The reported data are the averages of at least two independent experiments.
Six hundred ten P. aeruginosa proteins were identified and quantified using ICAT (for a complete list of proteins, see Table S1 in the supplemental material). Among 151 proteins whose abundances changed during anaerobic growth, 76 were higher in abundance (Table 1) and 75 were lower in abundance (Table 2). As expected, 13 proteins that participate in anaerobic growth and denitrification (including products of nir, nos, and nar genes) were expressed at higher levels during anaerobic growth (Table 1). These results suggest that the observed changes in protein content include those resulting specifically from growth at different oxygen levels.
TABLE 1.
P. aeruginosa proteins with increased abundance during anaerobic growth
| Genea | Protein | Gene name | nb | Ratioc | SD |
|---|---|---|---|---|---|
| PA0025* | Shikimate dehydrogenase | aroE | 3 | 1.79 | 0.04 |
| PA0130 | Probable aldehyde dehydrogenase | 10 | 2.28 | 0.22 | |
| PA0132 | Beta-alanine-pyruvate transaminase | 10 | 1.64 | 0.31 | |
| PA0286 | Probable fatty acid desaturase | 5 | 4.61 | 0.42 | |
| PA0300 | Polyamine transport protein | spuD | 7 | 1.65 | 0.17 |
| PA0321 | Probable acetylpolyamine aminohydrolase | 1 | 1.91 | NAd | |
| PA0336 | Nudix hydrolase YgdP | ygdP | 13 | 1.54 | 0.40 |
| PA0396 | Twitching motility protein PilU | pilU | 8 | 1.88 | 0.25 |
| PA0408 | Twitching motility protein PilG | pilG | 2 | 1.63 | 0.10 |
| PA0413 | Component of signal transduction system | chpA | 12 | 2.10 | 0.35 |
| PA0520 | Regulatory protein NirQ | nirQ | 59 | 2.21 | 0.33 |
| PA0655 | Hypothetical protein | 34 | 2.63 | 0.41 | |
| PA0658 | Probable short-chain dehydrogenase | 1 | 1.96 | NA | |
| PA0844 | Hemolytic phospholipase C precursor | plcH | 1 | 1.72 | NA |
| PA0867 | Hypothetical protein | 4 | 2.33 | 0.12 | |
| PA0934 | GTP pyrophosphokinase | relA | 6 | 1.70 | 0.08 |
| PA0936 | LPS biosynthetic protein LpxO2 | lpxO2 | 14 | 2.17 | 0.35 |
| PA1155 | Ribonucleoside reductase, small chain | nrdB | 3 | 12.15 | 5.64 |
| PA1156 | Ribonucleoside reductase, large chain | nrdA | 4 | 3.57 | 1.37 |
| PA1398 | Hypothetical protein | 1 | 1.56 | NA | |
| PA1566 | Conserved hypothetical protein | 3 | 3.12 | 0.58 | |
| PA1681 | Chorismate synthase | aroC | 5 | 1.65 | 0.14 |
| PA1766 | Hypothetical protein | 3 | 1.60 | 0.13 | |
| PA1847 | Conserved hypothetical protein | 1 | 1.88 | NA | |
| PA1919 | Probable radical-activating enzyme | 5 | 7.34 | 0.98 | |
| PA1920 | Conserved hypothetical protein | 15 | 10.80 | 5.21 | |
| PA2119 | Alcohol dehydrogenase (Zn dependent) | 25 | 1.84 | 0.22 | |
| PA2127 | Conserved hypothetical protein | 6 | 2.46 | 0.12 | |
| PA2323 | Probable glyceraldehyde-3-phosphate dehydrogenase | 2 | 1.95 | NA | |
| PA2567 | Hypothetical protein | 1 | 1.54 | NA | |
| PA2945 | Conserved hypothetical protein | 2 | 2.36 | 0.30 | |
| PA2991 | Soluble pyridine nucleotide transhydrogenase | sth | 20 | 1.93 | 0.37 |
| PA2994 | NA+-translocating NADH:quinone oxidoreductase | nqrF | 15 | 1.60 | 0.27 |
| PA2999* | NA+-translocating NADH:ubiquinone oxidoreductase | nqrA | 5 | 1.74 | 0.11 |
| PA3002 | Transcription-repair coupling protein Mfd | mfd | 2 | 1.52 | 0.06 |
| PA3150 | LPS biosynthesis protein WbpG | wbpG | 1 | 3.72 | NA |
| PA3185 | Hypothetical protein | 4 | 1.82 | 0.08 | |
| PA3391 | Regulatory protein NosR | nosR | 5 | 7.75 | 0.98 |
| PA3392 | Nitrous oxide reductase precursor | nosZ | 69 | 3.65 | 0.72 |
| PA3394 | NosF protein | nosF | 9 | 4.09 | 0.46 |
| PA3438 | GTP cyclohydrolase I precursor | folEI | 1 | 5.58 | NA |
| PA3515 | Hypothetical protein | 1 | 4.21 | NA | |
| PA3562* | Probable phosphotransferase system enzyme I | 3 | 2.91 | 0.17 | |
| PA3694 | Hypothetical protein | 4 | 1.92 | 0.07 | |
| PA3871 | Probable peptidyl-prolyl cis-trans isomerase, PpiC type | 3 | 2.50 | 0.64 | |
| PA3873 | Respiratory nitrate reductase delta chain | narJ | 1 | 3.20 | NA |
| PA3874 | Respiratory nitrate reductase beta chain | narH | 67 | 7.89 | 2.83 |
| PA3875 | Respiratory nitrate reductase alpha chain | narG | 35 | 7.70 | 3.23 |
| PA3880 | Conserved hypothetical protein | 8 | 3.88 | 0.98 | |
| PA3886 | Hypothetical protein | 1 | 7.25 | NA | |
| PA3895 | Probable transcriptional regulator | 2 | 1.49 | 0.00 | |
| PA3913 | Probable protease | 1 | 5.30 | NA | |
| PA3914* | Molybdenum cofactor biosynthetic protein A1 | moeA1 | 21 | 3.41 | 0.63 |
| PA3915* | Molybdopterin biosynthetic protein B1 | moaB1 | 5 | 4.40 | 0.72 |
| PA3918* | Molybdopterin biosynthetic protein C | moaC | 23 | 1.88 | 0.41 |
| PA3958 | Hypothetical protein | 1 | 2.29 | NA | |
| PA4180 | Probable acetolactate synthase large subunit | 2 | 2.16 | 0.53 | |
| PA4811 | Nitrate-inducible formate dehydrogenase, beta subunit | fdnH | 3 | 5.85 | 1.85 |
| PA4812 | Formate dehydrogenase-O, major subunit | fdnG | 4 | 3.46 | 0.64 |
| PA4868 | Urease alpha subunit | ureC | 1 | 1.51 | NA |
| PA4922 | Azurin precursor | azu | 4 | 2.96 | 0.81 |
| PA5005 | Probable carbamoyl transferase | 42 | 1.59 | 0.24 | |
| PA5011 | Heptosyltransferase I | waaC | 4 | 1.49 | 0.17 |
| PA5012 | Heptosyltransferase II | waaF | 6 | 1.45 | 0.11 |
| PA5015 | Pyruvate dehydrogenase | aceE | 111 | 1.98 | 0.42 |
| PA5064 | Hypothetical protein | 1 | 1.93 | NA | |
| PA5223 | UbiH protein | ubiH | 3 | 1.67 | 0.10 |
| PA5296 | ATP-dependent DNA helicase Rep | rep | 2 | 1.77 | 0.00 |
| PA5300 | Cytochrome c5 | cycB | 13 | 1.91 | 0.21 |
| PA5332 | Catabolite repression control protein | crc | 3 | 1.90 | 0.21 |
| PA5440 | Probable peptidase | 1 | 18.54 | NA | |
| PA5496* | Hypothetical protein | 8 | 6.46 | 2.07 | |
| PA5497* | Hypothetical protein | 10 | 11.28 | 3.17 | |
| PA5508 | Probable glutamine synthetase | 11 | 2.73 | 0.26 | |
| PA5564 | Glucose inhibited division protein B | gidB | 2 | 1.53 | 0.02 |
Genes marked with an asterisk (*) were identified as up-regulated during anaerobic growth (1).
Number of peptides identified and quantified for each protein.
Values represent relative protein abundance, or the ratio of protein expression in cells grown anaerobically to protein expression in cells grown aerobically.
NA, not applicable.
TABLE 2.
P. aeruginosa proteins with decreased abundance during anaerobic growth
| Genea | Proteine | Gene name | nb | Ratioc | SD |
|---|---|---|---|---|---|
| PA0085 | Conserved hypothetical protein | 3 | 2.15 | 0.26 | |
| PA0100 | Hypothetical protein | 1 | 1.53 | NAd | |
| PA0128 | Conserved hypothetical protein | 9 | 2.10 | 0.35 | |
| PA0139 | Alkyl hydroperoxide reductase subunit C | ahpC | 655 | 2.50 | 1.29 |
| PA0195 | Still frameshift pyridine nucleotide transhydrogenase | pntA | 10 | 2.21 | 0.55 |
| PA0399 | Cystathionine beta-synthase | 6 | 3.39 | 0.52 | |
| PA0447* | Glutaryl-CoA dehydrogenase | gcdH | 24 | 5.30 | 1.04 |
| PA0534 | Conserved hypothetical protein | 4 | 5.46 | 1.51 | |
| PA0588 | Conserved hypothetical protein | 78 | 5.56 | 2.52 | |
| PA0746 | Probable acyl-CoA dehydrogenase | 2 | 2.52 | 0.51 | |
| PA0853 | Probable oxidoreductase | 16 | 2.19 | 0.30 | |
| PA0854 | Fumarate hydratase | fumC2 | 9 | 2.36 | 0.34 |
| PA0870 | Aromatic amino acid aminotransferase | phhC | 24 | 1.74 | 0.22 |
| PA0871 | Pterin-4-alpha-carbinolamine dehydratase | phhB | 27 | 2.37 | 0.57 |
| PA0872 | Phenylalanine-4-hydroxylase | phhA | 60 | 2.11 | 0.65 |
| PA0916 | Conserved hypothetical protein | 6 | 1.93 | 0.28 | |
| PA0997* | Quinolone signal biosynthesis protein | pqsB | 3 | 15.54 | 6.73 |
| PA0998* | Quinolone signal biosynthesis protein | pqsC | 5 | 9.11 | 3.39 |
| PA0999* | 3-Oxoacyl-[acyl-carrier-protein] synthase III | pqsD | 12 | 5.62 | 1.50 |
| PA1002* | Anthranilate synthase component II | phnB | 1 | 2.30 | NA |
| PA1228 | Hypothetical protein | 13 | 2.55 | 0.52 | |
| PA1529 | DNA ligase | lig | 21 | 2.50 | 0.33 |
| PA1574 | Conserved hypothetical protein | 1 | 2.25 | NA | |
| PA1662 | Probable ClpA/B-type protease | 2 | 2.65 | 0.27 | |
| PA1756 | 3′-Phosphoadenosine-5′-phosphosulfate reductase | cysH | 3 | 2.89 | 0.13 |
| PA1772 | Probable methyltransferase | 4 | 2.34 | 0.43 | |
| PA1894 | Hypothetical protein | 9 | 2.48 | 0.94 | |
| PA1964 | Probable ATP-binding component of ABC transporter | 1 | 1.00 | NA | |
| PA2001 | Acetyl-CoA acetyltransferase | atoB | 149 | 1.74 | 1.11 |
| PA2007 | Maleylacetoacetate isomerase | maiA | 10 | 2.45 | 0.47 |
| PA2008 | Fumarylacetoacetase | fahA | 47 | 11.02 | 4.75 |
| PA2009 | Homogentisate 1,2-dioxygenase | hmgA | 4 | 20.39 | 11.50 |
| PA2012* | Probable acyl-CoA carboxylase alpha chain | 7 | 2.24 | 0.19 | |
| PA2014* | Probable acyl-CoA carboxyltransferase beta chain | 69 | 2.14 | 0.44 | |
| PA2044 | Hypothetical protein | 4 | 3.49 | 0.24 | |
| PA2069 | Probable carbamoyl transferase | 10 | 4.11 | 1.13 | |
| PA2081 | Hypothetical protein | 4 | 2.25 | 0.12 | |
| PA2112* | Conserved hypothetical protein | 28 | 3.94 | 0.90 | |
| PA2116 | Conserved hypothetical protein | 35 | 3.67 | 0.97 | |
| PA2194 | Hydrogen cyanide synthase HcnB | hcnB | 9 | 3.26 | 0.50 |
| PA2195 | Hydrogen cyanide synthase HcnC | hcnC | 3 | 4.11 | 0.15 |
| PA2247 | 2-Oxoisovalerate dehydrogenase (alpha subunit) | bkdA1 | 7 | 3.54 | 1.00 |
| PA2248 | 2-Oxoisovalerate dehydrogenase (beta subunit) | bkdA2 | 59 | 2.80 | 1.07 |
| PA2250 | Lipoamide dehydrogenase Val | lpdV | 18 | 2.79 | 0.59 |
| PA2366* | Conserved hypothetical protein | 1 | 2.70 | NA | |
| PA2552* | Probable acyl-CoA dehydrogenase | 13 | 1.99 | 0.70 | |
| PA2553* | Probable acyl-CoA thiolase | 48 | 2.17 | 0.50 | |
| PA2555* | Probable AMP-binding enzyme | 10 | 2.22 | 0.56 | |
| PA2850 | Organic hydroperoxide resistance protein | ohr | 6 | 2.26 | 0.37 |
| PA2939 | Probable aminopeptidase | 3 | 2.67 | 0.80 | |
| PA2981 | Tetraacyldisaccharide 4′-kinase | lpxK | 1 | 13.49 | NA |
| PA3049 | Ribosome modulation factor | rmf | 15 | 3.84 | 0.92 |
| PA3195 | Glyceraldehyde 3-phosphate dehydrogenase | gapA | 1 | 2.76 | NA |
| PA3327 | Probable nonribosomal peptide synthetase | 1 | 2.16 | NA | |
| PA3328 | Probable FAD-dependent monooxygenase | 6 | 4.58 | 1.28 | |
| PA3329* | Hypothetical protein | 1 | 2.08 | NA | |
| PA3331 | Cytochrome P450 | 17 | 5.10 | 2.00 | |
| PA3347 | Hypothetical protein | 4 | 1.96 | 0.20 | |
| PA3365 | Probable chaperone | 1 | 2.35 | NA | |
| PA3366 | Aliphatic amidase | amiE | 1 | 2.00 | NA |
| PA3481 | Conserved hypothetical protein | 1 | 1.54 | NA | |
| PA3537 | Ornithine carbamoyltransferase, anabolic | argF | 1 | 5.57 | NA |
| PA3569 | 3-Hydroxyisobutyrate dehydrogenase | mmsB | 25 | 3.67 | 0.94 |
| PA3570 | Methylmalonate-semialdehyde dehydrogenase | mmsA | 1 | 3.17 | NA |
| PA3842 | Probable chaperone | 8 | 3.17 | 1.44 | |
| PA3919* | Conserved hypothetical protein | 7 | 2.19 | 0.36 | |
| PA4015 | Conserved hypothetical protein | 11 | 2.11 | 0.67 | |
| PA4129* | Hypothetical protein | 3 | 3.75 | 1.17 | |
| PA4132 | Conserved hypothetical protein | 6 | 2.36 | 1.11 | |
| PA4217 | Flavin-containing monooxygenase | phzS | 5 | 5.09 | 1.26 |
| PA4362 | Hypothetical protein | 2 | 2.17 | 0.29 | |
| PA4412* | MurG protein | murG | 1 | 3.28 | NA |
| PA4498 | Probable metallopeptidase | 4 | 2.00 | 0.06 | |
| PA5100 | Urocanase | hutU | 10 | 4.50 | 1.23 |
| PA5410 | Probable ring hydroxylating dioxygenase, alpha subunit | 1 | 2.76 | NA |
Genes marked with an asterisk (*) were identified as down-regulated during anaerobic growth (1).
Number of peptides identified and quantified for each protein.
Values represent relative protein abundance, or the ratio of protein expression in cells grown aerobically to protein expression in cells grown anaerobically.
NA, not applicable.
CoA, coenzyme A; FAD, flavin adenine dinucleotide.
The changes in the detected proteome could also reflect differences in all density-dependent regulation in addition to effects of oxygen tension, given the lower relative cell density of the harvested anaerobic culture. Indeed, 29 proteins detected in lower abundance in anaerobically grown cells are encoded by genes previously shown to be quorum sensing induced (5, 16, 17). These include the hydrogen cyanide synthase subunits HcnB and HcnC; the Pseudomonas quinolone signal biosynthetic enzymes PqsB, PqsC, and PqsD; and PhnB (Table 2). Consistent with our results, hcn and pqs genes were also found to be transcriptionally repressed during anaerobic growth by a recent DNA microarray analysis using aerobic and anaerobic cultures harvested at the same cell density (1) (Table 2).
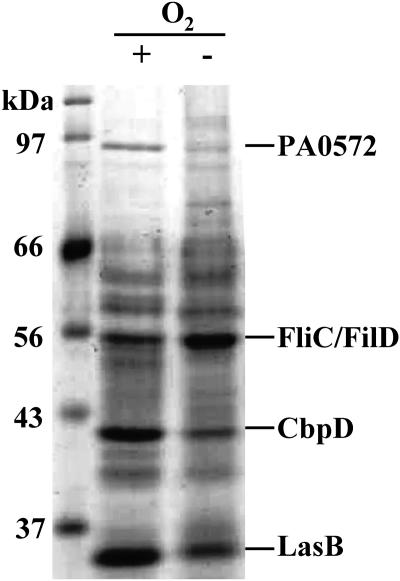
To identify secreted P. aeruginosa proteins with altered levels during anaerobic growth, culture supernatant proteins were concentrated (11) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1). Four Coomassie-stained protein bands, corresponding to differentially expressed proteins, were identified and analyzed as in a previous study (4) (Fig. 1). The abundances of three secreted proteins appeared to decrease during anaerobic growth: the CbpD chitin-binding protein, LasB elastase, and a protein of unknown function encoded by PA0572. Previous proteomic studies found that all three of these proteins are quorum sensing induced (11). One protein appeared to be increased in abundance during anaerobic growth and was identified as either the flagellar filament protein FliC or the flagellar capping protein FliD (due to the overlap in characteristics of these two proteins).
FIG. 1.
P. aeruginosa secreted proteins expressed during anaerobic growth. P. aeruginosa culture supernatant proteins were separated by 12% SDS-PAGE and detected by staining with Coomassie. Proteins that changed in abundance during anaerobic growth (relative to aerobic growth) are labeled. −O2, anaerobic growth; +O2, aerobic growth.
Most P. aeruginosa outer membrane proteins do not contain cysteine residues and hence cannot be analyzed by ICAT (4). Therefore, two-dimensional (2D) PAGE was used as a complementary method (4). Several outer membrane proteins (Fig. 2) were excised from the 2D gel and identified (4). OprE appeared to increase in abundance during anaerobic growth, while OprF and OprH appeared to decrease in abundance (Fig. 2). All three proteins migrated as multiple species during isoelectric focusing (Fig. 2). Decreased abundance of OprF during anaerobic growth was confirmed by immunoblotting of outer membrane proteins (data not shown), using a polyclonal anti-OprF antiserum (a gift from Robert Hancock, University of British Columbia at Vancouver, Canada).
FIG. 2.
P. aeruginosa outer membrane proteins expressed during anaerobic growth. Outer membrane proteins were separated by 12% 2D PAGE and detected by staining with Coomassie. Proteins were separated in the first dimension by isoelectric focusing (IEF) at pI ranges of 4 to 7 (A) and 6 to 11 (B). Proteins that changed in abundance during anaerobic growth (relative to aerobic growth) are labeled with arrows. −O2, anaerobic growth; +O2, aerobic growth.
Among the P. aeruginosa proteins that showed increased abundance during anaerobic growth (Table 1; Fig. 1), several contribute to functions involved in the formation and development of biofilms. These proteins include the catabolite repression control protein Crc and the twitching motility proteins PilU, PilG, and ChpA (12, 13, 18). Consistent with an increased level of Crc in anaerobically grown cells (Table 1), known targets of Crc repression were decreased in abundance (Table 2), including the hmgA and bkd gene products (6, 10). ChpA and PilG are components of a complex regulatory system controlling twitching motility (18). Taken together, these results suggest that expression or function of cell surface appendages that affect biofilm formation is altered during anaerobic growth. Such changes may contribute to the increased biofilm formation observed for P. aeruginosa growing anaerobically (20).
In addition to the changes in outer membrane proteins observed during anaerobic growth, ICAT analysis showed that several enzymes involved in the biosynthesis of P. aeruginosa lipopolysaccharide (LPS) were expressed at higher levels during anaerobic growth (Table 1). These included a homologue of beta-hydroxylase LpxO2, which hydroxylates lipid A fatty acids (14); LPS core heptosyltransferases WaaC and WaaF (2, 15); and WbpG, which is encoded by a gene cluster that participates in the synthesis of a long B-band O antigen. These results suggest that LPS content could be altered as a consequence of anaerobiosis.
In summary, the P. aeruginosa proteome changes significantly during anaerobic growth. We identified 617 proteins in total: 610 by ICAT analysis, 4 by SDS-PAGE analysis, and 3 by 2D PAGE analysis. Of the 617 identified proteins, the abundances of 158 varied between anaerobically grown and aerobically grown cells. Because P. aeruginosa reached a lower cell density under our anaerobic growth conditions than under aerobic growth conditions, density-dependent changes in protein expression may have contributed to the proteome that we detected during anaerobic growth. Nevertheless, bacterial cell density is likely to be similarly limited in many environmental niches where multiple nutrients (including oxygen) are scarce. Therefore, the changes in protein levels that we have detected contribute to an understanding of how the proteome and metabolic state of bacteria vary in response to different environments. Direct analysis of bacterial protein content is a robust technology to observe the adaptation of bacteria to specific environmental niches, including the CF airway.
Supplementary Material
Acknowledgments
We thank David D'Argenio and Robert Ernst for helpful discussions, Xiaojun Li and Biaoyang Lin for help with the data analysis, and Susan Farmer and Robert Hancock for providing the anti-OprF antibodies.
This work was supported by NIH grant DK 064954 to S. I. Miller and by Cystic Fibrosis Foundation grant R565-CR02 to T. Guina.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Filiatrault, M. J., V. E. Wagner, D. Bushnell, C. G. Haidaris, B. H. Iglewski, and L. Passador. 2005. Effect of anaerobiosis and nitrate on gene expression in Pseudomonas aeruginosa. Infect. Immun. 73:3764-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gronow, S., C. Oertelt, E. Ervela, A. Zamyatina, P. Kosma, M. Skurnik, and O. Holst. 2001. Characterization of the physiological substrate for lipopolysaccharide heptosyltransferases I and II. J. Endotoxin Res. 7:263-270. [PubMed] [Google Scholar]
- 3.Guina, T., S. O. Purvine, E. C. Yi, J. Eng, D. R. Goodlett, R. Aebersold, and S. I. Miller. 2003. Quantitative proteomic analysis indicates increased synthesis of a quinolone by Pseudomonas aeruginosa isolates from cystic fibrosis airways. Proc. Natl. Acad. Sci. USA 100:2771-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guina, T., M. Wu, S. I. Miller, S. O. Purvine, E. C. Yi, J. Eng, D. R. Goodlett, R. Aebersold, R. K. Ernst, and K. A. Lee. 2003. Proteomic analysis of Pseudomonas aeruginosa grown under magnesium limitation. J. Am. Soc. Mass Spectrom. 14:742-751. [DOI] [PubMed] [Google Scholar]
- 5.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Høiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hester, K. L., K. T. Madhusudhan, and J. R. Sokatch. 2000. Catabolite repression control by Crc in 2xYT medium is mediated by posttranscriptional regulation of bkdR expression in Pseudomonas putida. J. Bacteriol. 182:1150-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, R. N., M. A. Croco, K. C. Kugler, M. A. Pfaller, and M. L. Beach. 2000. Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). Diagn. Microbiol. Infect. Dis. 37:115-125. [DOI] [PubMed] [Google Scholar]
- 8.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 9.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales, G., J. F. Linares, A. Beloso, J. P. Albar, J. L. Martínez, and F. Rojo. 2004. The Pseudomonas putida Crc global regulator controls the expression of genes from several chromosomal catabolic pathways for aromatic compounds. J. Bacteriol. 186:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nouwens, A. S., S. A. Beatson, C. B. Whitchurch, B. J. Walsh, H. P. Schweizer, J. S. Mattick, and S. J. Cordwell. 2003. Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology 149:1311-1322. [DOI] [PubMed] [Google Scholar]
- 12.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 14.Raetz, C. R. 2001. Regulated covalent modifications of lipid A. J. Endotoxin Res. 7:73-78. [PubMed] [Google Scholar]
- 15.Raetz, C. R., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitchurch, C. B., A. J. Leech, M. D. Young, D. Kennedy, J. L. Sargent, J. J. Bertrand, A. B. Semmler, A. S. Mellick, P. R. Martin, R. A. Alm, M. Hobbs, S. A. Beatson, B. Huang, L. Nguyen, J. C. Commolli, J. N. Engel, A. Darzins, and J. S. Mattick. 2004. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol. Microbiol. 52:873-893. [DOI] [PubMed] [Google Scholar]
- 19.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Döring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]
- 21.Zannoni, D. 1989. The respiratory chains of pathogenic pseudomonads. Biochim. Biophys. Acta 975:299-316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.