Abstract
Since the first report of clonidine, an α2-adrenoceptor agonist, the indications for this class of drugs have continued to expand. In December 1999, dexmedetomidine was approved as the most recent agent in this group and was introduced into clinical practice as a short-term sedative (<24 hours). α2-Adrenoceptor agonists have several beneficial actions during the perioperative period. They decrease sympathetic tone, with attenuation of the neuroendocrine and hemodynamic responses to anesthesia and surgery; reduce anesthetic and opioid requirements; and cause sedation and analgesia. They allow psychomotoric function to be preserved while letting the patient rest comfortably. With this combination of effects, α2-adrenoceptor agonists may offer benefits in the prophylaxis and adjuvant treatment of perioperative myocardial ischemia. Furthermore, their role in pain management and regional anesthesia is expanding. Side effects consist of mild to moderate cardiovascular depression, with slight decreases in blood pressure and heart rate. The development of new, more selective α2-adrenoceptor agonists with improved side effect profiles may provide a new concept for the administration of perioperative anesthesia and analgesia. This review aims to give background information to improve understanding of the properties and applications of the novel α2-adrenoceptor agonist, dexmedetomidine.
The first α2-adrenoceptor agonist was synthesized in the early 1960s to be used as a nasal decongestant. Early application of the new substance, now known as clonidine, showed unexpected side effects, with sedation for 24 hours and symptoms of severe cardiovascular depression. Subsequent testing led to the introduction of clonidine as an antihypertensive drug in 1966. Over the years, clonidine gained acceptance as a powerful therapy not only for high blood pressure but also for the management of alcohol and drug withdrawal, for adjunctive medication in myocardial ischemia, and for pain and intrathecal anesthesia (1).
The use of α2-adrenoceptor agonists as anesthetics is not new. Veterinarians employed xylazine and detomidine for a long time to induce analgesia and sedation in animals, and much of our knowledge was gained from this application (2). It has recently become evident that complete anesthesia is possible by employing new, more potent α2 agonists, such as medetomidine and its stereoisomer, dexmedetomidine.
Dexmedetomidine was approved by the Food and Drug Administration at the end of 1999 for use in humans as a short-term medication (<24 hours) for analgesia and sedation in the intensive care unit (ICU). Its unique properties render it suitable for sedation and analgesia during the whole perioperative period. Its applications as a premedication, as an anesthetic adjunct for general and regional anesthesia, and as a postoperative sedative and analgesic are similar to those of the benzodiazepines, but a closer look reveals that the α2-adrenoceptor agonist has more beneficial side effects.
Dexmedetomidine became available at Baylor University Medical Center in August 2000. Between that time and mid October 2000, the drug was used in about 25 patients, most commonly as a supplement to anesthesia in patients undergoing cardiac procedures. In this patient population, dexmedetomidine serves as a sedative and analgesic agent in fast-tracking anesthesia regimens. When dexmedetomidine is started at the end of the case, patients are sedated but remain arousable and are able to cooperate when stimulated upon entry to the ICU.
This review attempts to provide an understanding of the current role of α2-adrenoceptor agonists in anesthesiologic practice and their potential as prospective drugs for sedation and analgesia. Rather than focusing on the use of α2-adrenoceptor agonists in the ICU, this article describes the physiologic and pharmacologic bases of this group of agents, with special reference to the perioperative applications of the most recently introduced compound, dexmedetomidine.
PHYSIOLOGY OF THE α2 RECEPTOR
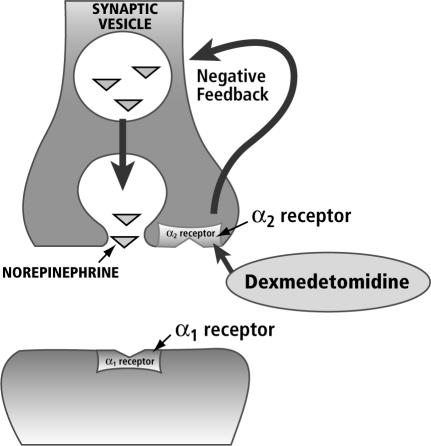
Adrenergic receptors were originally differentiated into α and β receptors on the basis of the rank order of potency of various natural and synthetic catecholamines in different physiologic preparations. It was believed that activation of either α- or β-adrenergic receptors produced excitatory effects in some tissues and inhibitory effects in others (3, 4). Later, a subclass of α adrenoceptors was discovered that regulates the release of neurotransmitters. From this, it was inferred that the receptor is located at the presynaptic site (4). However, classification of the receptors on the basis of anatomic location alone is problematic, because α2receptors have also been found at postsynaptic and extrasynaptic sites (5). Presynaptic α2 receptors may be of the greatest clinical import, because they regulate the release of norepinephrine and adenosine triphosphate through a negative feedback mechanism (Figure 1).
Figure 1.
Physiology of the α2-adrenoceptor agonists receptor. Reprinted from dexmedetomidine.com with permission of DocMD.com.
At least 3 different α2 isoreceptors have been defined both by pharmacologic studies (affinity for different α2 antagonists) and by biological probes. Receptors for α2 are found in the peripheral and central nervous systems, platelets, and a variety of organs, including the liver, pancreas, kidney, and eye. Physiologic responses mediated by α2 adrenoreceptors vary with location.
The α2-adrenergic receptor mediates its effects by activating guanine-nucleotide regulatory binding proteins (G proteins). Activated G proteins modulate cellular activity by signaling a second messenger system or by modulating ion channel activity.
The second messenger system, when activated, leads to the inhibition of adenylate cyclase, which, in turn, results in decreased formation of 3,5-cyclic adenosine monophosphate (cAMP). Specific cAMP-dependent kinases modify the activity of target proteins by controlling their phosphorylation status (6). Modulation of ion channel activity leads to hyperpolarization of the cell membrane. Efflux of potassium through an activated channel hyperpolarizes the excitable membrane and provides an effective means of suppressing neuronal firing. Stimulation of the α2 adrenoceptor also suppresses calcium entry into the nerve terminal, which may be responsible for its inhibitory effect on secretion of neurotransmitters. From an anesthesiologic viewpoint, neuronal hyperpolarization is a key element in the mechanism of action of α2-adrenoceptor agonists (7).
Mechanisms of action
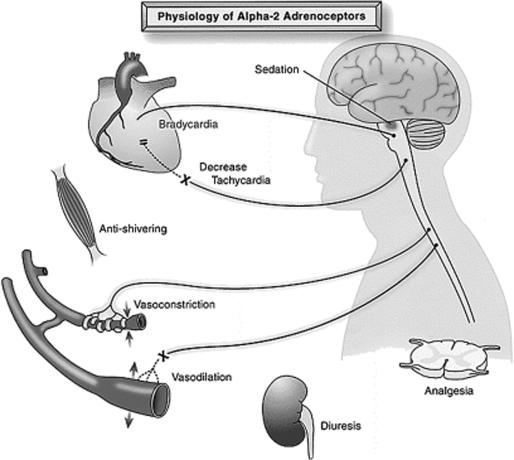
Dexmedetomidine, an imidazole compound, is the pharmacologically active dextroisomer of medetomidine that displays specific and selective α2-adrenoceptor agonism. The mechanism of action is unique and differs from those of currently used sedative agents, including clonidine. Activation of the receptors in the brain and spinal cord inhibits neuronal firing, causing hypotension, bradycardia, sedation, and analgesia. The responses to activation of the receptors in other areas include decreased salivation, decreased secretion, and decreased bowel motility in the gastrointestinal tract; contraction of vascular and other smooth muscle; inhibition of renin release, increased glomerular filtration, and increased secretion of sodium and water in the kidney; decreased intraocular pressure; and decreased insulin release from the pancreas (8) (Figure 2).
Figure 2.
Responses that can be mediated by α2-adrenergic receptors. Reprinted from Kamibayashi T, Maze M. Clinical uses of alpha 2-adrenergic agonists. Anesthesiology 2000;93:1345–1349 with the permission of the American Society of Anesthesiologists.
In general, presynaptic activation of the α2adrenoceptor inhibits the release of norepinephrine, terminating the propagation of pain signals. Postsynaptic activation of α2 adrenoceptors in the central nervous system (CNS) inhibits sympathetic activity and thus can decrease blood pressure and heart rate. Combined, these effects can produce analgesia, sedation, and anxiolysis. Dexmedetomidine combines all these effects, thus avoiding some of the side effects of multiagent therapies.
The mechanisms of the analgesic actions of α2 agonists have not been fully elucidated. A number of sites, both supraspinal and spinal, modulate the transmission of nociceptive signals in the CNS. Even peripheral α2 adrenoceptors may mediate antinociception (9). Drugs may act at any of these sites to reduce nociceptive transmission, leading to analgesia. The activation of inwardly rectifying G1-protein-gated potassium channels results in membrane hyperpolarization, decreasing the firing rate of excitable cells in the CNS. This is considered a significant mechanism of the inhibitory neuronal actions of α2-adrenoceptor agonists (7). Another prominent physiologic action ascribed to α2 adrenoceptors is their reduction of calcium conductance into cells, thus inhibiting neurotransmitter release. This effect involves direct regulation of calcium entry through N-type voltage-gated calcium channels and is independent of cAMP and protein phosphorylation. It is mediated by G0 proteins. These 2 mechanisms represent 2 very different ways of effecting analgesia: in the first, the nerve is prevented from ever firing, and in the second, it cannot propagate its signal to its neighbor.
One of the highest densities of α2 receptors has been detected in the locus coeruleus, the predominant noradrenergic nucleus in the brain and an important modulator of vigilance. The hypnotic and sedative effects of α2-adrenoceptor activation have been attributed to this site in the CNS. The locus coeruleus is also the site of origin for the descending medullospinal noradrenergic pathway, known to be an important modulator of nociceptive neurotransmission. In this region of the brain, α2-adrenergic and opioidergic systems have common effector mechanisms, indicating that dexmedetomidine has a supraspinal site of action.
These findings lead to the conclusion that the major sedative and antinociceptive effects of dexmedetomidine are attributable to its stimulation of the α2 adrenoceptors in the locus coeruleus. Furthermore, studies in transgenic mice have demonstrated that the α2A-adrenoceptor subtype is responsible for relaying the sedative and analgesic properties of dexmedetomidine (10). The improved specificity of dexmedetomidine for the α2 receptor, especially for the 2A subtype of this receptor, causes it to be a much more effective sedative and analgesic agent than clonidine. Studies have shown that dexmedetomidine is 8 times more specific for α2 adrenoceptors than clonidine (ratios of α2:α1 activity, 1620:1 for dexmedetomidine, 220:1 for clonidine).
In addition to dexmedetomidine's action in the locus coeruleus of the brain stem, it has been shown to stimulate α2 receptors directly in the spinal cord, thus inhibiting the firing of nociceptive neurons. The substantia gelatinosa of the dorsal horn of the spinal cord contains receptors which, when stimulated, inhibit the firing of nociceptive neurons stimulated by peripheral Aδ and C fibers and also inhibit the release of the nociceptive neurotransmitter substance P (11). This spinal mechanism is most likely why anesthesiologists have found success in using clonidine as an epidurally administered agent in addition to its primary use as an intravenous drug (1).
PHARMACODYNAMICS AND PHARMACOKINETICS
Dexmedetomidine is an α-adrenoceptor agonist with dosedependent α2-adrenoceptor selectivity (12). In animals that receive low to medium doses at slow rates of infusion (10 to 300 μg/kg), high levels of α2-adrenoceptor selectivity are observed, placing dexmedetomidine in the same therapeutic category as clonidine but with more affinity for the α2 adrenoceptor (12). At higher doses (>1000 μg/kg) or in rapid infusions of lower doses, both α1- and α2-adrenoceptor activities are observed. The majority of patients receiving dexmedetomidine as a primary therapy experienced clinically effective sedation yet were still easily arousable, a unique feature not observed with other clinically available sedatives (13). Clinical trials indicate that patients treated with dexmedetomidine required either no additional sedative medication or only small doses of add-on medications. This was significantly different from the add-on medication requirements of patients who did not receive dexmedetomidine.
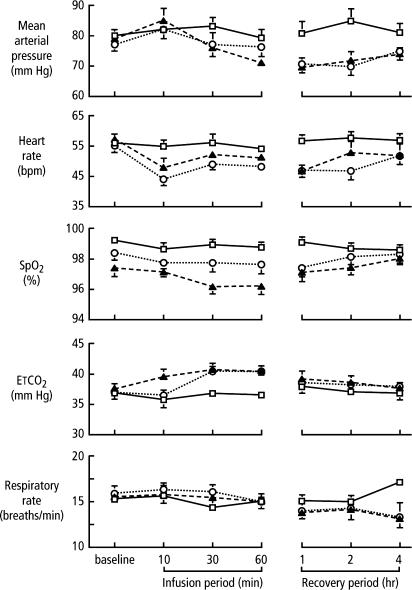
Dexmedetomidine does not appear to have any direct effects on the heart (14). A biphasic cardiovascular response has been described after the application of dexmedetomidine (15–17) (Figure 3). The administration of a bolus of 1 μg/kg dexmedetomidine initially results in a transient increase of the blood pressure and a reflex decrease in heart rate, especially in younger, healthy patients (16). The initial reaction can be explained by the peripheral α2B-adrenoceptor stimulation of vascular smooth muscle and can be attenuated by a slow infusion over 10 or more minutes. Even at slower infusion rates, however, the increase in mean arterial pressure over the first 10 minutes was shown to be in the range of 7%, with a decrease in heart rate between 16% and 18% (17). The initial response lasts for 5 to 10 minutes and is followed by a decrease in blood pressure of approximately 10% to 20% below baseline and a stabilization of the heart rate, also below baseline values; both of these effects are caused by the inhibition of the central sympathetic outflow overriding the direct stimulating effects (18). Another possible explanation for the subsequent heart rate decrease is the stimulation of the presynaptic α2-adrenoceptor, leading to a decreased norepinephrine release(19). The application of a single high dose of dexmedetomidine reduced norepinephrine release by as much as 92% in young healthy volunteers (20). The release of epinephrine is also reduced by the same amount (21). This seems to be more important than either central α2-adrenoceptor agonism or non-α adrenaline imidazole-preferring receptors in effecting the change (18).
Figure 3.
Cardiorespiratory variables before, during, and after infusion of dexmedetomidine of 0.2 (▴) or 0.6 (○) μg·kg-1/hr1 or placebo (□). SpO2 indicates arterial oxyhemoglobin saturation; ETCO2, end-tidal carbon dioxide. Adapted with permission from Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg 2000;90:699–705.
The baroceptor reflex is well preserved in patients who receive dexmedetomidine, and the reflex heart rate response to a pressor stimulus is augmented (20). These results illustrate that the cardiovascular response is evoked mainly by decreases in central sympathetic outflow.
Dexmedetomidine could result in cardiovascular depression, i.e., bradycardia and hypotension. The incidence of postoperative bradycardia has been reported as high as 40% in healthy surgical patients who received dexmedetomidine, especially high doses (22). Usually, these temporary effects were successfully treated with atropine or ephedrine and volume infusions (23). There are, of course, clinical situations in which the sympatholytic or bradycardic actions of α2-adrenoceptor agonists may be deleterious (e.g., in hypovolemic patients or patients with fixed stroke volume).
At clinically effective doses, dexmedetomidine has been shown to cause much less respiratory depression than other sedatives (24). However, coadministration of dexmedetomidine with anesthetic agents, sedatives, hypnotics, or opioids is likely to cause additive effects (19).
Although dexmedetomidine has no significant effect on adrenocorticotropic hormone (ACTH) secretion at therapeutic doses, cortisol's response to ACTH may be reduced after prolonged use or high doses of dexmedetomidine (25). The prolonged infusion of dexmedetomidine in dogs for 1 week diminished the response to ACTH by 40% (P < 0.05 vs control). Receptor binding of dexamethasone was not inhibited. Similar suppression of steroido-genesis has been reported after the administration of etomidate, another imidazole compound. Imidazole agents are able to inhibit mitochondrial cytochrome P450 enzymes; 11 β hydroxylase at low concentrations; and, at higher concentrations, cholesterol side-chain cleavage enzyme activity. The ratio of levels of inhibition caused by etomidate and dexmedetomidine was shown to be on the order of 100:1, suggesting that the biologic effects of the inhibitory activities of dexmedetomidine in patients probably are not clinically important (25).
Juxtaglomerular cells in the kidneys participate in the control and release of renin. Renin release is stimulated by β-adrenoceptor mechanisms, whereas α2-adrenoceptor agonists directly inhibit renin release (26).
Stimulation of α2 adrenoceptors on islet cells directly inhibits the release of insulin; this effect has unproven clinical importance, because hyperglycemia has never been reported to be significant in patients receiving clonidine (8).
Pharmocokinetics
Dexmedetomidine undergoes almost complete biotransformation through direct glucuronidation and cytochrome P450 metabolism (hydroxylation, mediated by CYP2A6), all hepatic processes, with very little excretion of unchanged molecules in the urine or feces. Although dexmedetomidine is dosed to effect, it may be necessary to decrease the typical dose in patients with hepatic failure, since they will have lower rates of metabolism of the active drug. Metabolites of biotransformation are excreted in the urine (about 95%) and in the feces (4%). It is unknown whether they possess intrinsic activity. The elimination half-life is approximately 2 hours.
Dexmedetomidine exhibits linear kinetics when infused in the recommended dose range of 0.2 to 0.7 μg/kg/hr for no more than 24 hours. The steady-state volume of distribution is 118 L, and the distribution phase is rapid, with a half-life of distribu tion of approximately 6 minutes (27).
The average protein binding of dexmedetomidine is 94%, with negligible protein binding displacement by fentanyl, ketorolac, theophylline, digoxin, and lidocaine, all drugs commonly used during anesthesia and in the ICU. There have been no significant sex- or age-based differences in the pharmocokinetic profile, even in elderly patients, and pharmacokinetics of the active dexmedetomidine molecule do not change in patients with renal failure. There is, however, a theoretical possibility of accumulation of metabolites of biotransformation, which has not yet been studied. This possibility is suspected because of the high degree of renal clearance of these metabolites.
TOXICOLOGY
The teratogenic effects of dexmedetomidine have not been adequately studied at this time, but the drug does cross the placenta and should be used during pregnancy only if the benefits justify the risk to the fetus. No studies have been performed in children.
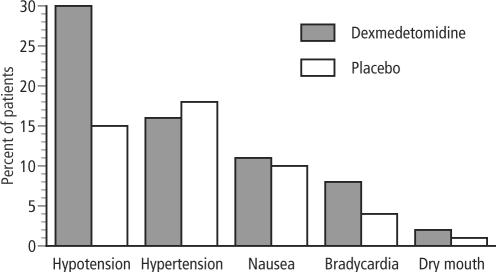
The adverse effects of dexmedetomidine include hypotension, hypertension, nausea, bradycardia, atrial fibrillation, and hypoxia (22, 28) (Figure 4). Overdose may cause first-degree or second-degree atrioventricular block. Most of the adverse events associated with dexmedetomidine use occur during or briefly after loading of the drug. By omitting or reducing the loading dose, adverse effects can be reduced.
Figure 4.
Side effects of dexmedetomidine. Adapted from Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs 2000;59:263–268.
No study has described the long-term use of dexmedetomidine, but adaptive changes and withdrawal syndrome like those seen with the use of clonidine can be expected from dexmedetomidine.
PREOPERATIVE EFFECTS
Because dexmedetomidine possesses anxiolytic, sedative, analgesic, and sympatholytic properties (13, 22, 28–31), it might be a useful adjunct for premedication, especially for patients susceptible to preoperative and perioperative stress. Clonidine has been used for a long time to attenuate sympathetic activation during induction of anesthesia and to provide a more stable hemodynamic profile. Dexmedetomidine seems to offer the same beneficial properties. Both were able to decrease oxygen consumption in the intraoperative period (up to 8%) and in the postoperative period (up to 17%) (32). The maximum heart rate decrease was 18% more in both treatment groups than in the placebo group.
Dexmedetomidine and clonidine potentiate the anesthetic effects of all intraoperative anesthetics, regardless of method of administration (intravenous, volatile, or even regional block). Intravenous or intramuscular administration of dexmedetomidine reduced induction requirements of thiopentone by 17% in a group that received low doses and by up to 30% in a group that received high doses (33).
INTRAOPERATIVE EFFECTS
α2-Adrenoceptor agonists have been shown to attenuate stress-induced sympathoadrenal responses. Protecting the patient from noxious sympathetic stimulation and hemodynamic changes during surgery is one of the goals of anesthesia. Dexmedetomidine exerted anesthetic-sparing effects, increased hemodynamic stability, and reduced unwarranted responses to endotracheal intubation.
There is evidence that dexmedetomidine alters the pharma-cokinetics of intravenous anesthetic agents by decreasing cardiac output (34) and by inhibiting alfentanil microsome metabolism in the liver (35) but not the pharmacokinetics of inhaled agents such as isoflurane. The first report of reduced isoflurane requirements in humans with dexmedetomidine was published in 1991 (36). Aho et al showed 25% reductions of maintenance concentrations of isoflurane in patients who received dexmedetomidine. Khan et al found 35% to 50% reductions of isoflurane requirements in patients treated with either low or high doses of dexmedetomidine and isoflurane without premedication (37). In a recent study in elderly patients undergoing elective surgery, administration of dexmedetomidine was associated with a 17% decrease of sevoflurane requirements for the maintenance of anesthesia (38).
In animals, the profound reduction of anesthetic requirements raised the possibility that α2-adrenoceptor agonists may be considered anesthetic agents when administered alone (39). It was subsequently shown that a central α2-adrenergic C4 isoreceptor is the probable receptor that mediated the anesthetic response (40). Possible anesthetic effects also have been suggested in humans (28).
Endotracheal intubation is associated with significant increases of arterial pressure, heart rate, and plasma catecholamine concentrations. Dexmedetomidine attenuated the sympathoadrenal stimulation during tracheal intubation effectively but did not completely abolish the cardiovascular response (41).
Analgesic properties have been demonstrated in studies that used dexmedetomidine as the sole analgesic after minor surgery (42). Used without any adjuncts, dexmedetomidine provided analgesia with a ceiling effect at doses >0.5 μg/kg. Thus, the effect was not dose dependent (29).
Opioid requirements in the intraoperative period and in the postanesthesia care unit (PACU) are reduced by dexmedetomidine (41) and clonidine (43). Nakagawa et al suggested that α2-adrenergic mechanisms are involved in the modulation of nociception at the level of spinal noradrenergic systems (44). There is clear evidence that α2-adrenoceptors are located on the dorsal horn neurons of the spinal cord and might release endogenous opiate compounds (45). Thus, the α2-adrenoceptor agonists may offer interesting new possibilities in the treatment of pain and may help to reduce intraoperative opioid requirements, as clonidine does (1, 41, 42, 46). Overall, giving dexmedetomidine in patients allowed lower doses of anesthetics to be used, resulting in more rapid recovery from anesthesia and a reduced need for pain medication in the PACU, thereby reducing the length of stay.
Only 1 study to date investigated the muscle relaxant effects of dexmedetomidine on the neuromuscular blockade (47). Using a steady-state infusion with rocuronium, the authors showed that increasing plasma concentrations of dexmedetomidine resulted in further decreased muscle force using mechanomyog-raphy. Although these changes were statistically significant, the investigators concluded that they were not clinically relevant.
When used in combination with isoflurane or halothane, dexmedetomidine decreased cerebral blood flow in dogs by 30% to 45% without evidence of ischemia (48). The cerebral metabolic rate was not affected, nor were intracranial pressures; α2-adrenoceptor agonists even seem to be neuroprotective in an animal model of brain ischemia (49).
In humans, cerebral blood flow was decreased up to 25% in a group of patients who received high-dose dexmedetomidine (goal: plasma level, 1.1 ng/mL). In this study by Zornow et al, the decrease appeared even in the presence of an increase of arterial carbon dioxide pressure from 39 mm Hg to 45 mm Hg, a change in value usually associated with cerebral vasodilation (48). Within 2 hours of cessation of dexmedetomidine, the values returned to baseline. Consequently, dexmedetomidine should not be used in patients with intracranial pathologies until further studies have proven its safety in this group.
Like clonidine, dexmedetomidine is associated with a lower rate of shivering. Intravenous infusion of dexmedetomidine reduced the vasoconstriction threshold and the shivering threshold. Dexmedetomidine did not change the sweating threshold and decreased the concentration-response curves for vasocon-striction and shivering in a linear fashion (50). Therefore, with dexmedetomidine, thermoregulatory responses were inhibited within a wider range of temperatures.
POSTOPERATIVE EFFECTS
Recovering from anesthesia often results in pain, elevating catecholamine concentrations. At the same time, anesthesia residuals compromise breathing. Therefore, α2-adrenoceptor agonists may prove beneficial in the postoperative period because of their sympatholytic and analgesic effects without respiratory depression.
All effects of dexmedetomidine could be antagonized easily by administering the α2-adrenoceptor antagonist atipamezole (51), which, like dexmedetomidine, reverses sedation and sympatholysis and has a half-life of 1.5 to 2 hours (52). The combination of dexmedetomidine and atipamezole might be the basis for a reversible intravenous anesthetic technique that could provide timely independent recovery from anesthesia and sedation in the future (53).
With dexmedetomidine, patients are able to return to their baseline level of consciousness when stimulated. This feature of dexmedetomidine was shown by Hall and colleagues, who used the Bispectral Index System and psychometric tests such as the Visual Analog Scale for sedation, Observer's Assessment of Alertness/Sedation scale, Digit Symbol Substitution Scale, and specific memory tests. All values were reduced by dexmedetomidine but had returned to baseline 4 hours after treatment (17). A more objective sign was the return of the Bispectral Index System, a processed electroencephalogram signal analysis, from 60 to 65 before stimulus back to normal baseline values when encouraged. A larger European phase III trial underlined these findings, stating that even “complex tasks, such as communication by pen and paper, are possible” (13).
Dexmedetomidine also provides intense analgesia during the postoperative period. Postoperative analgesic requirements were reduced by 50% in cardiac patients, and the need for rescue midazolam for sedation was diminished by 80% (13). However, dexmedetomidine may lack amnestic properties; a small number of patients who received the drug were able to recall their ICU stay and found the experience very stressful.
Dexmedetomidine seems to have few respiratory side effects (24). Indeed, receptor-binding studies suggest that its effects on respiration should be minor. Belleville et al reported episodes of obstructive apnea in a group of patients who received high doses of the drug (24). The effects were seen more commonly with doses of 1 or 2 μg/kg given over 2 minutes, doses that provide rapid sedation. The obstructive respiration pattern and irregular breathing seen with such doses are probably related more to deep sedation and anatomical features of the patient. Our own experience suggests that this could be easily overcome by insertion of an oral airway.
The danger of respiratory depression with sedative agents often necessitates their discontinuation during the extubation period, whereas a dexmedetomidine infusion can be continued safely in the extubated, spontaneously breathing patient. Whether this is true when the patient has also received opioids needs to be proven for dexmedetomidine; it has been shown to be true for clonidine (54). In rats, the addition of dexmedetomidine did not worsen alfentanil-induced respiratory depression (55).
In human volunteers, dexmedetomidine showed some depression and rightward shift of the carbon dioxide response curve in human volunteers (24). In a recent report about respiratory effects, respiratory rates and arterial blood gas values of postsurgical patients were reported. This study showed no differences in the respiratory parameters. Respiratory rates were lower in treated patients and respiration was more economic, with preserved minute ventilations, which yielded better oxygenation (56). Many agents used in the ICU have been shown to modify immune response. Midazolam, a frequently used sedative agent, has been shown to reduce phagocytic effects and decrease the interleukin-8 release in response to lipopolysaccharide, an effect not seen with opioids. On the other hand, dexmedetomidine at clinically relevant concentrations did not influence chemotaxis, phagocytosis, or O2– free radical production by neutrophils. Also, α2-adrenoceptor agonists failed to scavenge the O2– generated by the cell-free system (57). Overall, there seems to be little evidence for any clinically relevant immunomodulation by dexmedetomidine.
The postoperative hemodynamic effects of dexmedetomidine were comparable to its intraoperative effects. These postoperative changes in heart rate and blood pressure may be important factors in outcome in high-risk patients, e.g., those who have had vascular surgery or coronary artery bypass graft surgery (58). Therefore, they are discussed in detail below.
EFFECTS OF DEXMEDETOMIDINE IN CARDIOVASCULAR PATIENTS
Surgical stimulation and postoperative stress evoke a general sympathetic stimulation evinced by increased levels of epinephrine and norepinephrine, increased blood pressure and heart rate, a state of hypercoagulopathy, and thermal instability. All these are associated with an increased myocardial oxygen demand and an increased incidence of postoperative complications. The hyperdynamic changes predispose the myocardium to ischemia, especially in patients with coronary artery disease and a decreased reserve for coronary blood flow. Perioperative ischemia is associated with a 9-fold increase in the risk of having postoperative cardiac death, nonfatal myocardial infarction (MI), or unstable angina while in the hospital (59). The long-term risk for adverse cardiac events increases 2-fold in patients who have perioperative ischemia alone and 14-fold to 20-fold in patients who have perioperative MI or unstable angina (60). α2-Adrenoceptor agonists blunt hemodynamic variability during surgery and recovery, may exert anti-ischemic effects in the perioperative setting, and may also be effective in reducing these high rates of early postoperative ischemic events.
The first trial investigating the cardioprotective effects of α2-adrenoceptor agonists was the Multicenter Study of Perioperative Ischemia (McSPI) Europe trial (61). The application of mivazerol, an α2-adrenoceptor agonist, resulted in a 50% reduction of perioperative ischemic events in the group that received high doses. These results were not confirmed by the European Mivazerol Trial (EMIT), probably because that trial lacked the power of the McSPI-Europe study (62). In the EMIT study, mivazerol did not alter the rates of MI or cardiac death in 2854 patients who had known coronary artery disease or were at high cardiac risk undergoing noncardiac surgery. However, mivazerol did protect a subgroup of patients who were undergoing vascular surgery from further coronary events (62–64).
High-risk patients who received dexmedetomidine from 1 hour before until 48 hours after vascular surgery experienced significantly fewer ischemic episodes than did patients in the placebo group (63, 64). The incidence was 8% in the dexmedetomidine group and 29% in the placebo group. During emergence from anesthesia, norepinephrine levels in the placebo group were 2 to 3 times higher than those in the dexmedetomidine group (63). All ischemic events were associated with significant increases (>40%) in heart rate and systolic pressure and lasted for 1 to 5 minutes (64). The reduction of the rate-pressure product, such as that seen during the intraoperative period, might lead to fewer ischemic events because of reduced oxygen demand (32, 63). The decreased blood pressure in dexmedetomidine-treated patients did not result in any adverse effects.
The fluid volume needed during the intraoperative period to avoid hypotension was significantly higher in the dexmede-tomidine group, a side effect that may be unfavorable in volume-sensitive patients with reduced left ventricular function. This effect might be outweighed, however, by the diuretic effects of α2-adrenoceptor agonists, whose mechanisms may include attenuation of the secretion or effect of antidiuretic hormone, inhibition of renin, or release of natriuretic peptide (18). In the study in coronary artery bypass graft patients, despite the fact that the volume of fluid challenge was higher in the dexmedetomidine group, the overall volume administered was similar between placebo and treatment groups (23).
Although α2-adrenoceptor agonists appear to be beneficial in terms of ischemic adverse events, there is some controversy about the vasoconstrictive effects of α2 agonism. α2-Adrenoceptor agonists may cause peripheral and coronary vasoconstriction by stimulation of postjunctional α2-adrenergic receptors (65). In a study on dogs, dexmedetomidine reduced the myocardial oxygen deficiency by preserving the blood flow to the ischemic inner layers of the heart. This was related to its hemodynamic effects, the reduction in heart rate, and the reduction of myocardial wall tension (66). Local metabolic effects stimulated by the nitric-oxide–mediated release of activated α2A receptors are probably able to counteract the sympathetic stimulation in the heart (65). This conclusion is an extrapolation from an animal model to humans, but clinical results with clonidine seem to confirm these results (67, 68).
The decrease of cardiac output and the increase in systemic vascular resistance seen in response to dexmedetomidine do not seem to be related to a decreased contractility, relaxation, or intracellular calcium-channel block (14). Instead, the hemodynamic changes can be attributed to the dexmedetomidine-induced bradycardia, α2-adrenergic stimulation, and a decrease in oxygen requirements (32).
In conclusion, dexmedetomidine might be a useful adjunct to cardiovascular anesthesia, providing a protective pharmacologic profile with only moderate sympathetic depression (58). However, patients who depend on a high level of sympathetic tone or have reduced myocardial function might not tolerate the decrease in sympathetic tone. The possibility of ongoing sedation and sympathetic block during the administration of dexmedetomidine could be beneficial for high-risk patients undergoing noncardiac surgery as well as cardiac patients with good to moderately decreased left ventricular function.
SUMMARY
We are only beginning to understand the molecular pharmacology of many agents we use on a daily basis as sedatives or anesthetics; dexmedetomidine appears to be unique in that our insight into its mechanism of action was far advanced before its introduction into human clinical practice.
α2-Adrenoceptor agonists may provide an attractive alternative to anesthetic adjunctive agents now in use because of their anesthetic-sparing and hemodynamic-stabilizing effects. Dexmedetomidine provides better perioperative hemodynamic stability than many agents now in use and may offer protection from ischemia due to the attenuated neuroendocrine response, but the incidence of hypotension and bradycardia requiring intervention during clinical studies was higher in the dexmedetomidine groups than in the placebo groups. Dexmedetomidine-treated patients were more sedated at the time of arrival in the PACU, emerged more rapidly from anesthesia, required less volatile anesthetic to achieve hemodynamic endpoints, and had greater overall stability in the perioperative period with fewer episodes of tachycardia requiring intervention.
α2-Adrenoceptor agonists do not affect the synthesis, storage, or metabolism of neurotransmitters and do not block the receptors, thus providing the possibility of reversing the hemodynamic effects with vasoactive drugs or the α2-agonist effects with a specific α2-adrenoceptor antagonist (22). Therefore, they may have a role in anesthesia for patients who are at high risk of myocardial ischemia while undergoing major surgery.
Appropriate patient selection is crucial, as a patient's hemodynamic properties may increase his or her risk of serious adverse effects. For example, ICU patients who are hypovolemic or severely vasoconstricted should not receive dexmedetomidine. The drug should not be administered as a bolus, and the association of higher doses with systemic and pulmonary hypertension limits its use as a single anesthetic or sedative agent.
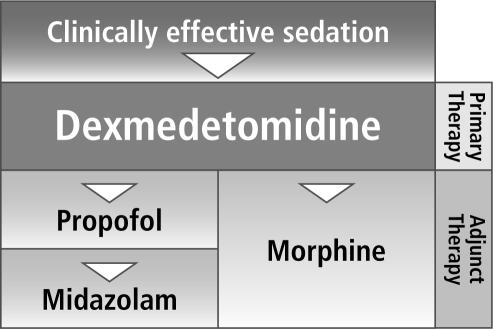
Finally, the possibility afforded by dexmedetomidine of continuing sedation throughout the extubation process without significant respiratory impairment and with lower analgesic requirements may shorten the ICU stay (Figure 5). It has not yet been determined if this will have any cost implications.
Figure 5.
Potential role of dexmedetomidine for sedation in the intensive care unit. Reprinted from dexmedetomidine.com with permission of DocMD.com.
References
- 1.Tamsen A, Gordh T. Epidural clonidine produces analgesia. Lancet. 1984;2:231–232. doi: 10.1016/s0140-6736(84)90523-3. [DOI] [PubMed] [Google Scholar]
- 2.Clarke KW, Hall LW. “Xylazine”—a new sedative for horses and cattle. Vet Rec. 1969;85:512–517. doi: 10.1136/vr.85.19.512. [DOI] [PubMed] [Google Scholar]
- 3.Alquist RP. A study of adrenergic receptors. Am J Physiol. 1948;153:586–589. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 4.Langer SZ. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974;23:1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- 5.Drew GM, Whiting SB. Evidence for two distinct types of postsynaptic alpha-adrenoceptor in vascular smooth muscle in vivo. Br J Pharmacol. 1979;67:207–215. doi: 10.1111/j.1476-5381.1979.tb08668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotecchia S, Kobilka BK, Daniel KW, Nolan RD, Lapetina EY, Caron MG, Lefkowitz RJ, Regan JW. Multiple second messenger pathways of alphaadrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem. 1990;265:63–69. [PubMed] [Google Scholar]
- 7.Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- 8.Metz SA, Halter JB, Robertson RP. Induction of defective insulin secretion and impaired glucose tolerance by clonidine. Selective stimulation of metabolic alpha-adrenergic pathways. Diabetes. 1978;27:554–562. doi: 10.2337/diab.27.5.554. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M, Ferreira SH. Peripheral analgesic action of clonidine: mediation by release of endogenous enkephalin-like substances. Eur J Pharmacol. 1988;146:223–228. doi: 10.1016/0014-2999(88)90296-8. [DOI] [PubMed] [Google Scholar]
- 10.Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, Secchi R, Sutton J, Eglen RM. Assessment of the role of alpha 2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 13.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Vedio A, Singer M, Feneck R, Treacher D, Willatts SM, Grounds RM. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 14.Housmans PR. Effects of dexmedetomidine on contractility, relaxation, and intracellular calcium transients of isolated ventricular myocardium. Anesthesiology. 1990;73:919–922. doi: 10.1097/00000542-199011000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:813–820. doi: 10.1097/00000542-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Aibiki M, Seki K, Ogura S, Ogli K. Effects of dexmedetomidine, an alpha 2-adrenoceptor agonist, on renal sympathetic nerve activity, blood pressure, heart rate and central venous pressure in urethane-anesthetized rabbits. J Auton Nerv Syst. 1998;71:48–54. doi: 10.1016/s0165-1838(98)00061-7. [DOI] [PubMed] [Google Scholar]
- 19.Aantaa R, Kanto J, Scheinin M, Kallio A, Scheinin H. Dexmedetomidine, an alpha 2-adrenoceptor agonist, reduces anesthetic requirements for patients undergoing minor gynecologic surgery. Anesthesiology. 1990;73:230–235. doi: 10.1097/00000542-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kallio A, Scheinin M, Koulu M, Ponkilainen R, Ruskoaho H, Viinamaki O, Scheinin H. Effects of dexmedetomidine, a selective alpha 2-adrenoceptor agonist, on hemodynamic control mechanisms. Clin Pharmacol Ther. 1989;46:33–42. doi: 10.1038/clpt.1989.103. [DOI] [PubMed] [Google Scholar]
- 21.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and perioperative fentanyl. Br J Anaesth. 1992;68:126–131. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 22.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Comparison of dexmedetomidine and midazolam sedation and antagonism of dexmedetomidine with atipamezole. J Clin Anesth. 1993;5:194–203. doi: 10.1016/0952-8180(93)90014-6. [DOI] [PubMed] [Google Scholar]
- 23.Jalonen J, Hynynen M, Kuitunen A, Heikkila H, Perttila J, Salmenpera M, Valtonen M, Aantaa R, Kallio A. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997;86:331–345. doi: 10.1097/00000542-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–1133. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Maze M, Virtanen R, Daunt D, Banks SJ, Stover EP, Feldman D. Effects of dexmedetomidine, a novel imidazole sedative-anesthetic agent, on adrenal steroidogenesis: in vivo and in vitro studies. Anesth Analg. 1991;73:204–208. doi: 10.1213/00000539-199108000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Pettinger WA, Umemura S, Smyth DD, Jeffries WB. Renal alpha 2-adrenoceptors and the adenylate cyclase-cAMP system: biochemical and physiological interactions. Am J Physiol. 1987;252(2 Pt 2):F199–F208. doi: 10.1152/ajprenal.1987.252.2.F199. [DOI] [PubMed] [Google Scholar]
- 27.Abbott Labaratories. Precedex. Dexmedetomidine hydrochloride injection prescribing information. Abbott Labaratories, USA, 2000.
- 28.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine—a novel alpha 2-adrenoceptor agonist—in healthy volunteers. Pain. 1991;46:281–285. doi: 10.1016/0304-3959(91)90111-A. [DOI] [PubMed] [Google Scholar]
- 30.Aantaa R, Kanto J, Scheinin M. Intramuscular dexmedetomidine, a novel alpha 2-adrenoceptor agonist, as premedication for minor gynaecological surgery. Acta Anaesthesiol Scand. 1991;35:283–288. doi: 10.1111/j.1399-6576.1991.tb03290.x. [DOI] [PubMed] [Google Scholar]
- 31.Aantaa RE, Kanto JH, Scheinin M, Kallio AM, Scheinin H. Dexmedetomidine premedication for minor gynecologic surgery. Anesth Analg. 1990;70:407–413. doi: 10.1213/00000539-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Taittonen MT, Kirvela OA, Aantaa R, Kanto JH. Effect of clonidine and dexmedetomidine premedication on perioperative oxygen consumption and haemodynamic state. Br J Anaesth. 1997;78:400–406. doi: 10.1093/bja/78.4.400. [DOI] [PubMed] [Google Scholar]
- 33.Aantaa R, Jaakola ML, Kallio A, Kanto J. Reduction of the minimum alveolar concentration of isoflurane by dexmedetomidine. Anesthesiology. 1997;86:1055–1060. doi: 10.1097/00000542-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Bührer M, Mappes A, Lauber R, Stanski DR, Maitre PO. Dexmedetomidine decreases thiopental dose requirement and alters distribution pharmacokinetics. Anesthesiology. 1994;80:1216–1227. doi: 10.1097/00000542-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Kharasch ED, Hill HF, Eddy AC. Influence of dexmedetomidine and clonidine on human liver microsomal alfentanil metabolism. Anesthesiology. 1991;75:520–524. doi: 10.1097/00000542-199109000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74:997–1002. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 1: Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83:372–380. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 38.Fragen RJ, Fitzgerald PC. Effect of dexmedetomidine on the minimum alveolar concentration (MAC) of sevoflurane in adults age 55 to 70 years. J Clin Anesth. 1999;11:466–470. doi: 10.1016/s0952-8180(99)00081-1. [DOI] [PubMed] [Google Scholar]
- 39.Doze VA, Chen BX, Maze M. Dexmedetomidine produces a hypnoticanesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology. 1989;71:75–79. doi: 10.1097/00000542-198907000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Regan JW, Doze VA, Daniel K, Maze M. Is dexmedetomidine's anesthetic activity dependent on isoreceptor selectivity? Anesthesiology. 1989;71:A579. [Google Scholar]
- 41.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and perioperative fentanyl. Br J Anaesth. 1992;68:126–131. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 42.Aho MS, Erkola OA, Scheinin H, Lehtinen AM, Korttila KT. Effect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligation. Anesth Analg. 1991;73:112–118. doi: 10.1213/00000539-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Ghignone M, Quintin L, Duke PC, Kehler CH, Calvillo O. Effects of clonidine on narcotic requirements and hemodynamic response during induction of fentanyl anesthesia and endotracheal intubation. Anesthesiology. 1986;64:36–42. doi: 10.1097/00000542-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa I, Omote K, Kitahata LM, Collins JG, Murata K. Serotonergic mediation of spinal analgesia and its interaction with noradrenergic systems. Anesthesiology. 1990;73:474–478. doi: 10.1097/00000542-199009000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Fleetwood-Walker SM, Mitchell R, Hope PJ, Molony V, Iggo A. An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurons. Brain Res. 1985;334:243–254. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- 46.Xu M, Kontinen VK, Kalso E. Effects of radolmidine, a novel alpha 2-adrenergic agonist compared with dexmedetomidine in different pain models in the rat. Anesthesiology. 2000;93:473–481. doi: 10.1097/00000542-200008000-00027. [DOI] [PubMed] [Google Scholar]
- 47.Talke PO, Caldwell JE, Richardson CA, Kirkegaard-Nielsen H, Stafford M. The effects of dexmedetomidine on neuromuscular blockade in human volunteers. Anesth Analg. 1999;88:633–639. doi: 10.1097/00000539-199903000-00031. [DOI] [PubMed] [Google Scholar]
- 48.Zornow MH, Maze M, Dyck JB, Shafer SL. Dexmedetomidine decreases cerebral blood flow velocity in humans. J Cereb Blood Flow Metab. 1993;13:350–353. doi: 10.1038/jcbfm.1993.45. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–332. doi: 10.1097/00000542-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87:835–841. doi: 10.1097/00000542-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Jones JG, Taylor PM. Receptor-specific reversible sedation: dangers of vascular effects. Anesthesiology. 1999;90:1489–1490. doi: 10.1097/00000542-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 52.Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha 2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998;89:574–584. doi: 10.1097/00000542-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Talke P. Receptor-specific reversible sedation: beginning of new era of anesthesia? Anesthesiology. 1998;89:560–561. doi: 10.1097/00000542-199809000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Jarvis DA, Duncan SR, Segal IS, Maze M. Ventilatory effects of clonidine alone and in the presence of alfentanil, in human volunteers. Anesthesiology. 1992;76:899–905. doi: 10.1097/00000542-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Furst SR, Weinger MB. Dexmedetomidine, a selective alpha 2-agonist, does not potentiate the cardiorespiratory depression of alfentanil in the rat. Anesthesiology. 1990;72:882–888. doi: 10.1097/00000542-199005000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Joseph AA, Cassell C, Gargia-Rodriguez CR, El-Moalem HE, Sum-Ping ST. Effects of dexmedetomidine on respiration. ASA Meeting Abstracts 2000:A483.
- 57.Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, Niwa Y. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesth Analg. 1999;88:452–458. doi: 10.1097/00000539-199902000-00042. [DOI] [PubMed] [Google Scholar]
- 58.Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–1142. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 59.Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–1788. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 60.Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992;268:233–239. doi: 10.1001/jama.268.2.233. [DOI] [PubMed] [Google Scholar]
- 61.Perioperative sympatholysis, Beneficial effects of the alpha 2-adrenoceptor agonist mivazerol on hemodynamic stability and myocardial ischemia, McSPI—Europe Research Group Anesthesiology. 1997;86:346–363. [PubMed] [Google Scholar]
- 62.Oliver MF, Goldman L, Julian DG, Holme I. Effect of mivazerol on perioperative cardiac complications during non-cardiac surgery in patients with coronary heart disease: the European Mivazerol Trial (EMIT) Anesthesiology. 1999;91:951–961. doi: 10.1097/00000542-199910000-00014. [DOI] [PubMed] [Google Scholar]
- 63.Talke P, Li J, Jain U, Leung J, Drasner K, Hollenberg M, Mangano DT. Effects of perioperative dexmedetomidine infusion in patients undergoing vascular surgery. The Study of Perioperative Ischemia Research Group. Anesthesiology. 1995;82:620–633. doi: 10.1097/00000542-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, Heard S, Cheung A, Son SL, Kallio A. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–839. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Coughlan MG, Lee JG, Bosnjak ZJ, Schmeling WT, Kampine JP, Warltier DC. Direct coronary and cerebral vascular responses to dexmedetomidine. Significance of endogenous nitric oxide synthesis. Anesthesiology. 1992;77:998–1006. doi: 10.1097/00000542-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 66.Pagel PS, Hettrick DA, Kersten JR, Warltier DC. Dexmedetomidine produces similar alterations in the determinants of left ventricular afterload in conscious dogs before and after the development of pacing-induced cardiomyopathy. Anesthesiology. 1998;89:741–748. doi: 10.1097/00000542-199809000-00026. [DOI] [PubMed] [Google Scholar]
- 67.Heusch G, Schipke J, Thamer V. Clonidine prevents the sympathetic initiation and aggravation of poststenotic myocardial ischemia. J Cardiovasc Pharmacol. 1985;7:1176–1182. doi: 10.1097/00005344-198511000-00026. [DOI] [PubMed] [Google Scholar]
- 68.Roekaerts PM, Prinzen FW, De Lange S. Beneficial effects of dexmedetomidine on ischaemic myocardium of anaesthetized dogs. Br J Anaesth. 1996;77:427–429. doi: 10.1093/bja/77.3.427. [DOI] [PubMed] [Google Scholar]