Abstract
Background
It is becoming apparent that perhaps as much as half of the genome of the human blood fluke Schistosoma mansoni is constituted of mobile genetic element-related sequences. Non-long terminal repeat (LTR) retrotransposons, related to the LINE elements of mammals, comprise much of this repetitive component of the schistosome genome. Of more than 12 recognized clades of non-LTR retrotransposons, only members of the CR1, RTE, and R2 clades have been reported from the schistosome genome.
Results
Inspection of the nucleotide sequence of bacterial artificial chromosome number 49_J_14 from chromosome 1 of the genome of Schistosoma mansoni (GenBank AC093105) revealed the likely presence of several RTE-like retrotransposons. Among these, a new non-LTR retrotransposon designated SR3 was identified and is characterized here. Analysis of gene structure and phylogenetic analysis of both the reverse transcriptase and endonuclease domains of the mobile element indicated that SR3 represented a new family of RTE-like non-LTR retrotransposons. Remarkably, two full-length copies of SR3-like elements were present in BAC 49-J-14, and one of 3,211 bp in length appeared to be intact, indicating SR3 to be an active non-LTR retrotransposon. Both were flanked by target site duplications of 10–12 bp. Southern hybridization and bioinformatics analyses indicated the presence of numerous copies (probably >1,000) of SR3 interspersed throughout the genome of S. mansoni. Bioinformatics analyses also revealed SR3 to be transcribed in both larval and adult developmental stages of S. mansoni and to be also present in the genomes of the other major schistosome parasites of humans, Schistosoma haematobium and S. japonicum.
Conclusion
Numerous copies of SR3, a novel non-LTR retrotransposon of the RTE clade are present in the genome of S. mansoni. Non-LTR retrotransposons of the RTE clade including SR3 appear to have been remarkably successful in colonizing, and proliferation within the schistosome genome.
Background
Schistosomiasis is considered among the most important of the tropical diseases in terms of morbidity and mortality, ranking only behind malaria [1]. International efforts are underway to sequence the entire genomes of two of the three major schistosome species, S. mansoni and S. japonicum [2]. It is anticipated that an enhanced understanding of the schistosome genome will aid in the control of this disease, including the development of vaccines and new anti-parasite medications [3]. Up to half of the schistosome genome may be composed of repetitive sequences, including LTR and non-LTR retrotransposons, mobile genetic elements that transpose through an RNA intermediate (reviewed by Brindley et al. [4]). Mobile genetic elements are drivers of genome evolution [5,6]. In addition to this role, from a practical perspective mobile genetic elements offer potential as transgenesis vectors [7]. Problematically, however, their interspersed, repetitive nature can impede progress during genome sequencing using shotgun sequencing approaches through the confounding effects of their repetitions on sequence assembly algorithms [8,9]. For these and other reasons, we and others have been characterizing the retrotransposons of the schistosome genome [10-15]. Here we report a novel non-LTR retrotransposon termed SR3, a member of the RTE clade of non-LTR retrotransposons, from the genome of S. mansoni. Based on the multi-copy, interspersed nature of SR3, and the presence of other RTE elements characterized previously from the genomes of schistosomes, it appears that members of the RTE clade may be the most common and successful of the non-LTR retrotransposons to have colonized the genomes of these metazoan parasites.
Results and Discussion
New retrotransposons identified in bacterial artificial chromosome 49_J_14 from the genome of S. mansoni
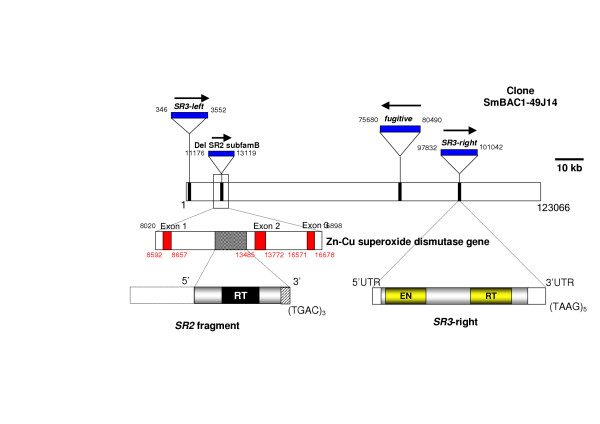
BLASTn searches revealed the presence of reverse transcriptase (RT)-encoding sequences in the S. mansoni bacterial artificial chromosome (BAC) number 49_J_14 [16], the entire sequence of which has been deposited in GenBank with accession number AC093105 by El Sayed and co-workers [3]. Annotation provided with GenBank AC093105 indicated that the sequence included in BAC 49_J_14 is from chromosome 1 of the genome of S. mansoni. Inspection of the nucleotide sequence of BAC 49_J_14, of ~123 kb in length, indicated the presence of a number of discrete retrotransposons. One of these encodes a novel long terminal repeat (LTR) retrotransposon, which we have described in a recent report [11] (Fig. 1). In addition, at least three non-LTR retrotransposons appeared to be located in BAC 49_J_14. One of these appeared to be a degenerate copy of an SR2 element. SR2 elements are non-LTR retrotransposons of the RTE clade [17] which are present in high copy (estimated at up to 10,000 copies) in the genome of S. mansoni [10,18]. This fragment of SR2 was located between nucleotide residues numbers 11,176 and 13,119 of BAC 49_J_14 and, more specifically appeared to be located within intron number 1 of the gene encoding cytosolic Zn/Cu superoxide dismutase [19]. As illustrated in Fig. 1, the Cu/Zn superoxide dismutase gene is present in BAC 49_J_14 between residues 8,020 and 16,898 of BAC 49_J_14. The copy of SR2 in the intron of the Cu/Zn superoxide dismutase gene is ~1,830 nucleotides (nt) in length, and included regions encoding the retrotransposon RT domain (Fig. 1). Over the putative RT-encoding region, the sequence was 47% identical to the RT sequence of SR2. At only ~1.8 kb in length, and since full-length copies of SR2 are ~3.9. kb in length [18], this appears to be a truncated copy of SR2 that is unlikely to be autonomously mobile. In like fashion to the location of this truncated copy of SR2, copies of other SR2 elements (and indeed other retrotransposons) have been identified previously in introns of other S. mansoni protein encoding genes [20,21].
Figure 1.
Schematic diagram of the location, size and structure of copies of the SR3 non-LTR retrotransposon in bacterial artificial chromosome number 49_J_14 from chromosome 1 of the genome of Schistosoma mansoni. The location of copies of the SR2 and fugitive retrotransposons is also presented. The arrows indicate the direction of transcription of the mobile elements. The numbers on each bar indicate the nucleotide position of the elements within the bacterial artificial chromosome. A degenerate copy of the SR2 element was evident within intron 1 of Zn-Cu superoxide dismutase (SOD) gene, in particular within intron 1 of the gene: three exons of the SOD are indicated in red, with the position and structure of the degenerate copy of SR2 indicated within intron 1. On the bottom right, a schematic of the length and domain structure of the SR3-right copy of the SR3 retrotransposon is presented. RT, reverse transcriptase; EN, endonuclease.
SR3 represents a new family of non-LTR retrotransposon from the genome of S. mansoni
In addition to the fugitive LTR retrotransposon [11], and the truncated copy of SR2, at least two other retrotransposons were readily identifiable in BAC 49_J_14. The first of these was located between nt 346 and 3,552 (i.e., 3,207 bp in length), and the second between nt 97,832 and 101,042 (3,211 bp in length). Comparison of the sequences of these two prospective retrotransposons revealed that they were closely related to one another and appeared to represent discrete copies of a novel family of retrotransposons. We have termed the new retrotransposon SR3, whose phylogenetic analysis indicated a new family of the RTE clade of non-LTR retrotransposons (see below). (SR3 stands for Schistosome Retrotransposon 3 because two other non-LTR retrotransposons described previously from S. mansoni are termed SR1 and SR2 [18,22]). (A recent article, published after this present report was submitted for publication, identified a SR3-like element in the S. mansoni transcriptome, termed Perere-3, and also identified several other novel retrotransposons [15].) For convenience of description, we refer here to the copy of SR3 resident between nt 346 and 3,552 of BAC 49_J_14 as SR3-left and the other copy between nt 97,842 and 101,042 as SR3-right, because they are located on the left and right sides of the BAC as in Figure 1. The full-length SR3-left and SR3-right elements were comprised of a single, read through open reading frame (ORF) encoding two functional domains similar to apurinic-apyrimidic (AP) endonuclease (EN) and RT, in that order. The element terminated with a short repeat sequence, (TAAG)4 or (TAAG)5 (Fig. 1). The nucleotide and deduced amino acid sequences of the SR3-left and SR3-right copies are provided in Additional files 1 and 2, respectively.
The sequence of 3,211 bp long SR3-right element translated into a single, deduced open reading frame (ORF) of 922 amino acid residues that did not include any apparent frameshift or stop codon mutations (Additional file 2). By contrast, the deduced ORF of SR3-left was interrupted by stop codons at amino acid positions 719 and 913 of the ORF (Additional files 1, 3). SR3-right has a longer terminal repeat unit than SR3-left, (TAAG)5 compared with (TAAG)4, which accounts for the difference in total lengths of the two copies (3,207 and 3,211 bp). (By contrast, comparison of the ORFs of Perere-3 (Accession CAJ00236.1) and the SjR2 retrotransposon (AY027869) of S. japonicum, with the deduced ORFs of both SR3-left and SR3-right revealed that the similarity extends well beyond the predicted ORF of 922 deduced amino acids of SR3-right [not shown]. Whereas this suggests the possibility of premature stop codon in the SR3 copies presented here, it may also simply reflect phylogenetic relatedness in the carboxy-terminal encoding regions and 3'UTRs of these elements.) Nonetheless, SR3-left and SR3-right are very similar to each other in sequence, with the ORFs region exhibiting 94 % identity and 97 % similarity over the predicted ORF of 922 residues (Additional file 3). Together, these findings suggest that both SR3-left and SR3-right are full-length copies and, moreover, that SR3-right is an intact, putatively functional and active copy, capable of autonomous retrotransposition activity. It was remarkable not only that two copies (SR3-left and SR3-right) of this retrotransposon reside in close proximity to each other in the region of the S. mansoni genome represented by BAC 49_J_14, but also that both copies are full-length and intact or close to intact. Most copies of non-LTR retrotransposons are 5'-truncated, due to deficits in their elongation processes, and generally include deletions or insertions (indels), and are thereby rendered inactive [6,23,24].
Four other non-LTR retrotransposons have been reported from the genome of S. mansoni. These are SR1 and Perere, discrete members of the CR1 clade, and SR2 and Perere-3, members of the RTE-1 clade [14,15,18,22]. SR3 was dissimilar to these non-LTR retrotransposons reported previously from the genome of S. mansoni: when compared with the deduced amino acid sequence of the ORF of SR3, SR1 shared 23 %/ 38 % amino acid sequence identity/similarity with SR3, Perere shared 22 %/35 % identity/similarity, SR2 shared 39 %/55 % identity/similarity and Perere-3 shared 78 %/88 % amino acid sequence identity/similarity with SR3 (not shown). Together, these differences indicated that SR3 was a novel element distinct from these other schistosome non-LTR retrotransposons.
SR3 represents a new member of a family of the RTE-1 non-LTR retrotransposons
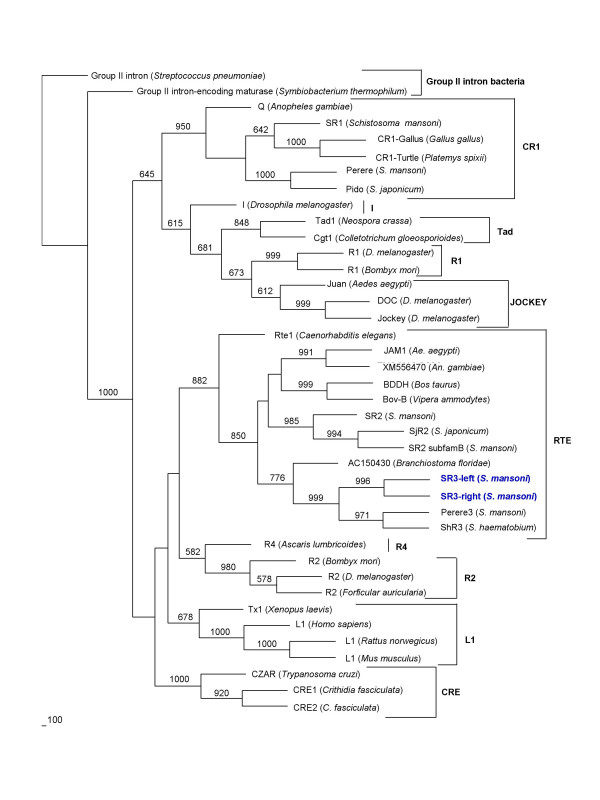
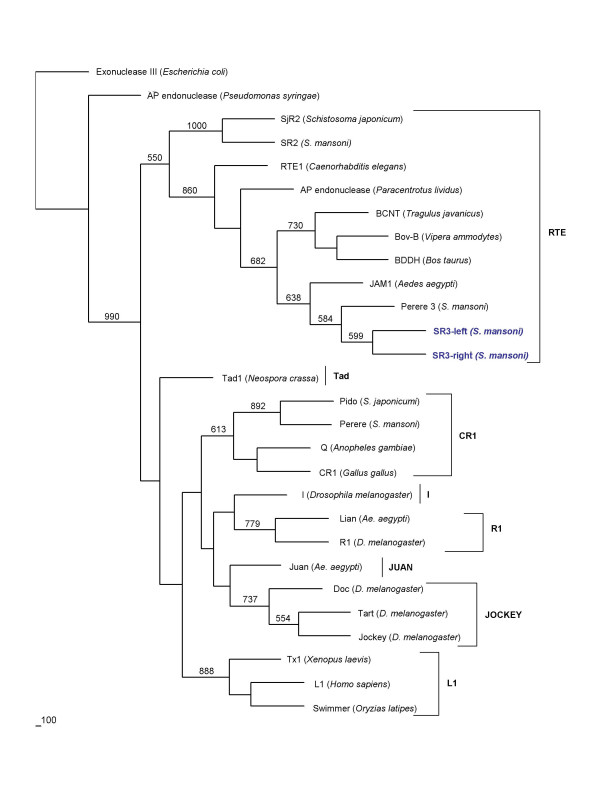
The predicted RT domain of SR3 was aligned with orthologous domains of numerous other non-LTR retrotransposons including representatives from 11 clades of non-LTR retrotransposons, as defined by Eickbush and colleagues [25,26]. Phylogenetic comparison of the RT domains of these diverse elements revealed that the closest relatives of SR3 were ShR3 from S. haematobium and Perere-3 from S. mansoni, with close identity also to AC150430 element from Branchiostoma floridae, SR2 from S. mansoni, SjR2 from S. japonicum and also to RTE-1 from Caenorhabditis elegans (Figure 2; and Additional file 4), placing SR3 in the RTE-1 clade of non-LTR retrotransposons. In like fashion, a phylogenetic tree was constructed based on the EN domain of eight clades of non-LTR retrotransposons. The topography of the EN tree, and the position of SR3 within the RTE clade, was similar to the topography represented on the RT-based tree, confirming both the inclusion of SR3 as an RTE clade element and that SR3 and SR2 were discrete families of RTE-like retrotransposons (Figure 3; and Additional file 5). Indeed, in the EN tree, SR3 was more closely related to RTE-1 of C. elegans than to SR2 of S. mansoni (Figs. 2, 3).
Figure 2.
Phylogram constructed using the neighbor-joining method to compare the relationships among reverse transcriptases of SR3 and of representative elements belonging to the major clades of non-LTR retrotransposons [25] from a range of host genomes. Representatives of 11 clades of non-LTR retrotransposons including the RTE, CR1, L1, R1 and Jockey clades were included in the analysis. Bootstrap values, where 500 or greater from a maximum of 1,000 replicates, are presented at the nodes.
Figure 3.
Phylogram constructed using the neighbor-joining method to compare the relationships among endonucleases of SR3 and of representative elements belonging to the major clades of non-LTR retrotransposons [25] from a range of host genomes. Representatives of eight major clades of non-LTR retrotransposons including the RTE, L1, CR1, Jockey, and I clades were included in the analysis. Bootstrap values of 500 or greater from 1,000 replicates are presented at the nodes.
Structure of SR3
Youngman et al. [27] provided the first report of a RTE retrotransposon, from the genome of C. elegans. RTE clade elements display a broad host range, having been described from numerous invertebrate and vertebrate taxa, and from algae and flowering plants [14,15,17,18]. RTE-1 encodes a 1,066-amino-acid ORF containing both apurinic-apyrimidic endonuclease and reverse-transcriptase domains. A possible first ORF of only 43 amino acids overlaps with the larger ORF and may be the site of translation initiation. Members of the RTE clade are characterized by unusually short 3' untranslated regions that are predominantly composed of AT-rich trimer, tetramer, and/or pentamer repeats [17]. RTE-derived SINE elements are also found in mollusc and flatworm genomes.
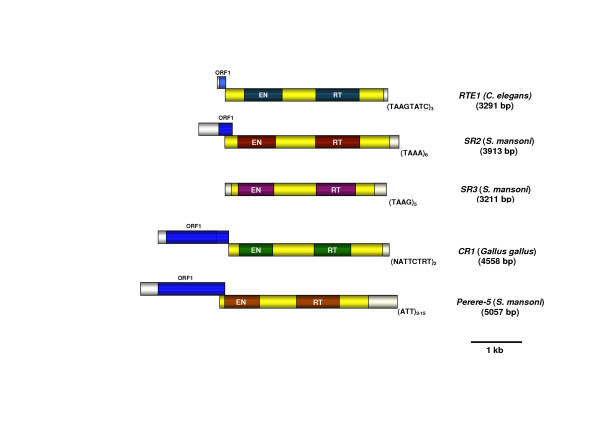
In addition to the demonstration by phylogenetic analyses targeting both the RT and EN domains that SR3 is an RTE like element, we compared the structural motifs and domains of SR3 with RTE-1 of C. elegans and SR2 of S. mansoni in order to confirm the identity of SR3 as an RTE clade non-LTR retrotransposon. First, the three elements were of generally similar length; 3,291 bp for RTE-1 of C. elegans [17], 3,913 bp for SR2 [18], and 3,211 kb for SR3-right. Second, the length of the ORF was somewhat similar; 1066, 1016, and 922 amino acids for RTE-1, SR2, and SR3 respectively. The RTE-1 and SR2 elements may also contain a short ORF upstream of the major ORF, although this has not been confirmed by functional analysis [17,18,25]. Third, the 3'-UTRs of RTE clade elements are usually short in length and terminate in several tetrameric or pentameric, A-rich repeats. SR3 conformed to RTE-1 in this regard, with SR3 exhibiting a short 3'-UTR of 177 bp in length and terminating with several copies of the tetramer, TAAG (Fig. 1; Additional files 1, 2).
A schematic comparison of the structures of RTE-1, SR2, SR3, CR1, and an SR1-like element, Perere-5 [15,22], is presented in Figure 4. In summary, the SR3 elements of S. mansoni conform in all respects to the generalized structure of the RTE clade of non-LTR retrotransposons. Moreover, as with other RTE elements, SINE-like elements reported from schistosomes may be derived from SR3-like elements [4].
Figure 4.
Schematic representation of the structure of non-LTR retrotransposons of the RTE and CR1 clades, including RTE-1 from Caenorhabditis elegans, SR2 and SR3 from Schistosoma mansoni, CR1 from Gallus gallus, and Perere-5, and SR1-like retrotransposon from S. mansoni. (The structure of a full-length copy of SR1 has not been reported [15, 22].) The sequence motifs of the 3'-termini are shown, along with positions of enzymatic domains, EN (endonuclease) and RT (reverse transcriptase). The RTE-1 element (3291 bp) illustrated here includes the 3'-UTR so that it is longer than the 3258 bp, described in Malik and Eickbush [17] (which included only the region between the 5'-end and the termination codon).
SR3 is present in genomes of other schistosome species
Investigation of SR3 sequences in the genomes of other human schistosomes by BLAST search analysis revealed many sequences similar to SR3 in the transcriptomes of S. japonicum (e.g., GenBank AY810372, AY915175, AY813885 and AY915893). In addition, when the nucleotide sequence of SR3-right was employed as the query in BLASTx analysis against the GenBank non-redundant database, SR3-like sequences were identified within introns 1 and 6 of the gene encoding S. haematobium acetylcholinesterase (AChE) (GenBank AY167025) [28]. The two copies are similar in sequence (~70% identical), both copies are 5' truncated, and both include regions encoding the RT domain of the retrotransposon (not shown). The fragment within intron 1 was located between nt 1,023–2,474, and the fragment in intron 6 was located between nt 18,742–20,658. The predicted RT domain of the SR3 like element from S. haematobium (termed ShR3) was included in the phylogenetic tree presented in Fig. 2 and was found to be phylogenetically similar to SR3 from S. mansoni. The presence of SR3 elements in other schistosome species can be explained by vertical transmission from a progenitor schistosome species [29], given that vertical transmission is the expected route of transmission of non-LTR retrotransposons [24].
Numerous copies of SR3 are interspersed throughout the genome of S. mansoni
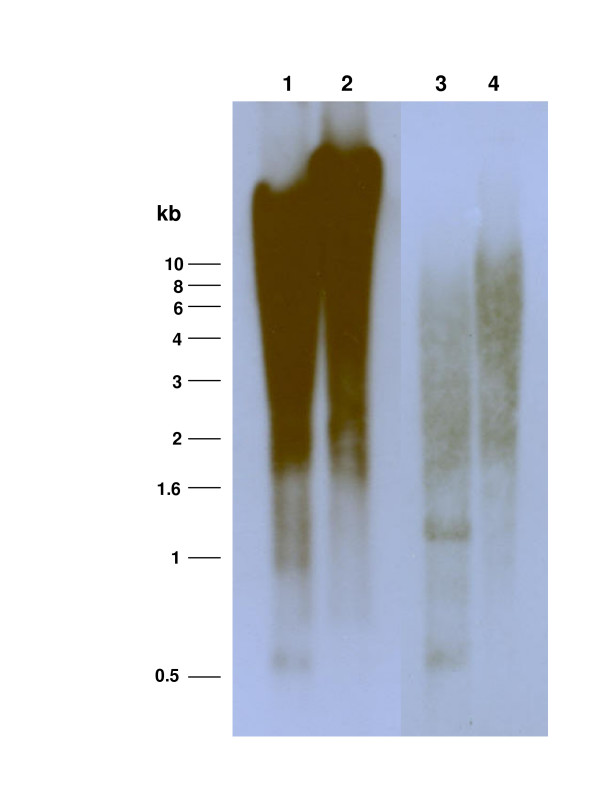
Southern hybridization analysis revealed that multiple bands of digested genomic DNA of S. mansoni hybridized to the SR3 specific probe, indicating the presence of numerous copies of SR3 in the S. mansoni genome (Fig. 5, lanes 1 and 2). Hybridization to the gDNA fragments released by double enzyme digestions revealed an even more smeared pattern (Fig. 5, lanes 3, 4), clearly suggesting that SR3 elements have interspersed throughout the genome of S. mansoni. In addition, a bioinformatics analysis using the approach of Copeland et al. [13] was used to estimate copy number of SR3 by comparisons with reference copy number estimates of other mobile genetic elements and genes reported previously. BLASTn searches were undertaken using the nucleotide sequences of these reference genes and the complete nucleotide sequence of SR3-right. Because the construction of the S. mansoni BAC library (from which BAC 49_J_14 was isolated) involved partial digestion of the genomic DNA with Hind III [16], genes without Hind III sites will be underrepresented in the BAC end sequences. Accordingly, since sequenced BAC ends from this library constitute a large proportion of the genomic S. mansoni sequences in the public domain, we used only genes containing Hind III sites as reference sequences. As shown in Table 1, the number of hits for SR3, 110, was higher than the number of hits for the single-copy cathepsin D gene (0 hits) and for three high copy number retrotransposons Boudicca (100 hits, 1,000–10,000 reported copies), SR2 (102 hits, 1,000–10,000 copies), and SR1 (104 hits, 200–2,000 reported copies) but lower than that for the multiple-copy 28S ribosomal RNA gene (157 hits) (100–200 copies). Although it is difficult with these available data to obtain a good estimate of the number of copies, however a comparison with the other 3 retrotransposons would give a tentative copy number for SR3 of between 1,000 and 10,000.
Figure 5.
Southern hybridization analysis of genomic DNA of S. mansoni probed with a SR3 retrotransposon specific probe. Genomic DNA was cleaved with endonucleases Hind III (lane 1), BamH I (lane 2), EcoR I plus Xba I (lane 3) and Hind III plus Xho I (lane 4). Molecular size standards in kilobase pairs (kb) are shown at the left. (Lanes 1 and 2 were exposed to film longer than lanes 3 and 4.)
Table 1.
Estimation by bioinformatics approaches of gene copy number of the SR3 non-LTR retrotransposon in the genome of Schistosoma mansoni.
| Gene | GenBank Accession | Query Length (bp) | Number of hits (Expect 0.000001) | Copy number | Key references |
| Cathepsin D, Intron 4 | AY309267 | 1636 | 0 | 1 | [20] |
| 28S rRNA | Z46503 | 1694 | 157 | 100–200 | [46] |
| Sinbad | AY506538 | 6288 | 38 | 50 | [13] |
| Boudicca | AY662653 | 5858 | 100 | 1,000–10,000 | [12] |
| SR3 | 3211 | 110 | >1,000 | This study | |
| SR2 | AF025672 | 3913 | 102 | 1,000–10,000 | [18] |
| SR1 | U66331 | 2337 | 104 | 200–2,000 | [22] |
| Saci-2 | BK004069 | 4946 | 107 | 85–850* | [14] |
| Saci-1 | BK004068 | 5980 | 133 | 70–700* | [14] |
SR3 is transcribed in all developmental stages of S. mansoni
The nucleotide sequences of the full length of SR3-left and SR3-right elements were employed as query sequences for BLAST searches of the GenBank EST database of S. mansoni sequences. The database includes more than 160,000 EST sequences from six developmental stages of S. mansoni – egg, miracidium, cercaria, germball (= sporocyst), schistosomulum, and mixed sex adults [30]. Significant hits were found to ESTs from all six of these stages (not shown). Representative accession numbers of the positive matches are presented in Additional files 6 and 7, along with brief details of the regions where the matches were located and statistical significance of the matches. In brief, positive ESTs spanning all of the 5'UTR, 3'UTR, EN and RT were located in most of these developmental stages. Based on these findings, it appeared that SR3 was expressed in developmental stages throughout the life cycle of S. mansoni.
SR3 integration sites
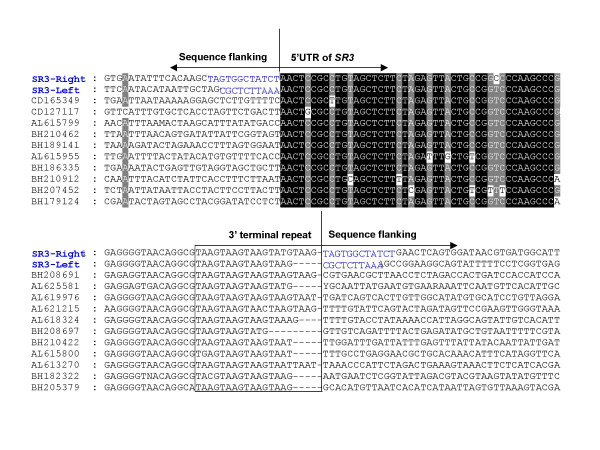
In order to investigate the nature of integration sites or target sequences of the new retrotransposon within the schistosome genome, five kilobases of nucleotide sequences flanking the 5'- and 3'-UTRs of both SR3-left and SR3-right were employed as queries to search the GenBank non-redundant nucleotide and protein databases, and the GSS and EST entries for S. mansoni. These searches revealed no significant matches to any sequences encoding genes of Schistosoma species (not shown). However, they did reveal that SR3 elements appear to target AT-rich sites, as indicated in Figure 6, a similar preference to L1 retrotransposons within the human genome [31,32]. More specifically, the average AT content of the integration sites of the 21 copies of SR3 shown in Figure 6 was 68 % AT. Whereas target site specificity does not appear to be stringent for SR3, it can be expected to reflect the recognition sequence of the SR3 endonuclease. For example, L1 elements apparently integrate at numerous sites in the genome because the endonuclease of L1 preferably cleaves DNA at the short consensus sequence, 5'-TTTT/A-3', where/designates the cleavage site [31,33].
Figure 6.
Multiple sequence alignment of the nucleotide sequences flanking the insertion sites of copies of the SR3 non-LTR retrotransposon within the genome of Schistosoma mansoni. Sequences flanking the 5'UTR of SR3-left and SR3-right are aligned in the top panel, while those flanking the 3'-terminus of SR3-left and SR3-right are presented in the bottom panel. Target site duplications are evident at the sites of SR3-left and SR3-right integration, and are indicated with bold font. Conservation of residues is indicated by the shading of boxes. Target sequences were identified among entries in the GSS database of S. mansoni sequences at GenBank or the Sanger Institute [39, 40].
To propagate, non-LTR retrotransposons employ their EN and RT enzymes respectively to nick a genomic target site and reverse transcribe the retrotransposon, integrating the element into a new genomic locus [33-35]. This process is termed target-site-primed reverse transcription. For the L1 elements in the human genome, a new L1 insertion is usually flanked by short direct repeats derived from the target DNA locus upon L1 integration [32,36]. These repeats are called target site duplications (TSDs), and can range from several to several hundred nucleotides in length [32,37]. Interestingly, both SR3-left and SR3-right are flanked by TSDs of 10 and 12 bp, respectively; TAGTGGCTAATCT for SR3-right and CGCTCTTAAA for SR3-left (Fig. 6). The presence of these TSDs provides further indication, along with their intact structure, of recent activity of these two copies of SR3 localized in BAC 49_J_14 [see [32]]. Apparently unlike SR3, and certainly unlike L1, some other clades of non-LTR retrotransposons exhibit extreme target site specificity, the well-known examples being the R2 and R4 elements which are found exclusively in the ribosomal RNA genes of insects (e.g., Bombyx mori, Drosophila melanogaster) and nematodes (e.g., Ascaris lumbricoides) or in simple repeats (e.g., the Dong element from B. mori) [25].
Nonetheless, as noted above, we have detected the presence of SR3 of S. haematobium within introns of the AChE gene [28], and in addition, other RTE elements have been reported from gene-rich sites of the schistosome genome. The degenerate copy of a non-LTR retrotransposon, SR2 [18] in BAC clone BAC 49_J_14 has integrated into intron 1 of the Zn-Cu superoxide dismutase (SOD) gene of S. mansoni (Figure 1). SR2 from schistosomes has been recorded from several other target genes including 28 kDa glutathione S transferase [18], cathepsin D [20] and the UTR of heat shock protein 70 [10]. Furthermore, the RTE-1 retrotransposon of C. elegans was found inserted in the intron of pim related kinase-1 (prk-1) gene [27]. Thus, although SR3 and other RTE clade retrotransposons do not exhibit tight target site specificity, they seem to prefer to integrate into AT-rich sites and, in addition, are frequently found in introns and other-non coding areas of protein encoding gene loci.
Conclusion
A new non-LTR retrotransposon, SR3, is reported from the genome of the human blood fluke Schistosoma mansoni. Numerous copies of SR3 are interspersed throughout the S. mansoni genome, and given the apparently intact sequence of the SR3-right copy of SR3 located in BAC 49_J_14 and the presence of transcripts from at least six developmental stages of S. mansoni, SR3 appears to be an active or recently active retrotransposon. This element is also present in the related human schistosomes, S. haematobium and S. japonicum. Based on phylogenetic comparisons of both the reverse transcriptase and endonuclease domains, SR3 represents a distinct family of RTE elements, discrete from the SR2 family described previously from schistosomes [18]. While there are numerous non-LTR retrotransposons in the schistosome genome, most elements so far described belong either to the RTE clade or CR1 clades [4], both of which are considered to be more advanced clades of non-LTR retrotransposons with progressive features including lack of target site specificity and an ORF encoding endonuclease and reverse transcriptase, respectively [25]. The presence of these and the apparent absence of some other clades of non-LTR retrotransposons should be informative in understanding the influence of mobile genetic elements in shaping the schistosome genome and its evolution and in studies of the phylogeny of schistosomes and related taxa. Finally, for studies with transgenesis of schistosomes, it may be possible to adapt an active copy of SR3 – such as SR3-right – for the introduction of transgenes into the schistosome genome in similar fashion to the adaptation of L1 elements of humans for studies on the movement of LINE elements in cultured human cell lines [23,37,38].
Methods
Bioinformatics approaches for detection of mobile sequences in the schistosome genome
The keyword phrase <Reverse Transcriptase> was used as the query to search the EST_others and GSS databases at GenBank for novel schistosome sequences associated with mobile genetic elements. Schistosome RT-like sequences that were retrieved were employed subsequently to search for matches in the GenBank non-redundant sequence database using BLASTn, BLASTx and/or tBLASTn [39]. Sequences of the previously characterized schistosome retrotransposons including Gulliver, pido, SjR2 of S. japonicum [4] and Boudicca [12] also were employed as queries. In addition, retrotransposon integration sites were investigated by interrogation of the S. mansoni genome survey sequences (GSS) at the Sanger Institute, Hinxton, U.K [40].
Parasites and parasite DNA
The life cycle of Schistosoma mansoni (NMRI strain, of Puerto Rican origin) was maintained at the Queensland Institute of Medical Research, Brisbane, Australia using experimentally infected mice and albino Biomphalaria glabrata snails. Genomic DNAs (gDNAs) of adult mixed sex parasites perfused from mice and cercariae (shed from snails) of S. mansoni were extracted using Qiagen's Genomic Tip-100 system according to the manufacturer's instructions.
Southern hybridization
Thirty micrograms of S. mansoni gDNA was cleaved with restriction enzymes Hind III, EcoR I, BamH I and Xho I. Digested gDNA was fractionated through 0.8% agarose gel and then was transferred to nylon membrane (Hybond-N+, Amersham Biosciences) by capillary action [41]. Southern hybridization analysis was performed using a horseradish peroxidase labelled probe and the ECL detection system (Amersham Biosciences). The membrane was incubated in hybridization medium (provided with kit) supplemented with the labeled probe overnight at 42°C, after which the membrane was washed in 0.4% SDS, 0.5× SSC at 42°C (two washes, 20 min. each) and subsequently in 2× SSC at room temperature (two washes, 5 min. each). The retrotransposon-like gene probe was amplified by polymerase chain reaction (PCR) with specific primers using S. mansoni gDNA as a template. Specific primers targeting the amplification of the RT domain of SR3 were SR3-forward, 5'-GAAGATTTGGGAAGAGGAACA and SR3-reverse, 5'-AACGATGCTCCCCAGATAAT (spanning nucleotides 1,809–2,622, Additional file 1). The SR3-right gene probe was amplified using specific primer SR3 forward (same as for the SR3-left probe) and SR3-right reverse 5'-CAACGATGCTCCCCAGGTACTTG (nt 1,809–2,622). Probes were sized in gels, isolated and purified before use. These probe sequences have been assigned GenBank accession numbers DQ008120 and DQ008121 for the SR3-left- and SR3-right-based probes, respectively.
Sequence analysis and phylogenetic analysis of new retrotransposons
The amino acid sequences of the functional domains of both RT and EN of both copies of the new non-LTR retrotransposon were aligned to other non-LTR retrotransposons by the ClustalW method [42] using BioEdit software [43] and optimized gaps and errors were referenced to conserved domains defined by Malik et al. [25]. Edited sequence alignments of the RT and EN domains were analyzed for phylogenetic relationships using the PHYLIP package [44]. Phylograms were generated and assessed for bootstrap values of 1,000 replicates using the neighbor-joining method with assistance from SEQBOOT and NEIGHBOR in the PHYLIP software suite [44]. Trees were displayed by TreeView [45]. Sequences used in the phylogenetic analyses were obtained from the GenBank, EMBL and PIR databases. They included family representatives from 11 major clades of non-LTR retrotransposons [25]. RT sequences of Group II introns from bacteria and EN sequences from bacteria were used as outgroups for the RT and EN trees, respectively. The names and accession numbers of the aligned sequences were: SR1 (U66331), SR2 (AF025672), Perere (BK004067) and Perere 3 (BN000794) from S. mansoni, SjR2 (AY027869) and pido (AY034003) from S. japonicum, ShR3 (AY167025) from S. haematobium, RTE1 from C. elegans (AF054983), JAM1 (Z86117) and Lian (U87543) from Ae. aegypti, Bov-B LINE from Vipera ammodytes (AF332697), Branchiostoma floridae clone CH302-99K22 (AC150430), BDDH from Bos taurus (AC150753), BCNT from Tragulus javanicus (AB191483), ENSANGP00000028171 from Anopheles gambiae strain PEST (XM556470), Tx1 from Xenopus laevis (M26915), Swimmer from the medaka fish, Oryzias latipes (AF055640), L1 from the rat (U83119), L1 from the mouse (AF081114), L1 from the human (U93574), R4 from Ascaris lumbricoides (U29445), R2 from Bombyx mori (M16558), R2 from the earwig, Forficular auricularia (AF015819), R2 from Drosophila melanogaster (X51967), CZAR from Trypanosoma cruzi (M62862), CRE2 from Crithidia fasciculata (U19151), CRE1 from C. fasciculata (M33009), CR1 from the turtle Platymys spixii (AB005891), CR1 from the chicken (U88211), Q from Anopheles gambiae (U03849), Tad1 from Neurospora crassa (L25662), CgT1 from the fungal phytopathogen, Colletotrichum gloeosporioides (L76169), R1 from B. mori (M19755), R1 from D. melanogaster (X51968), Tart from D. melanogaster (U14101), Juan from Ae. aegypti (M95171), Jockey (M22874), Doc (X17551), and I (M14954) from D. melanogaster, Group II intron-encoding maturase from Symbiobacterium thermophilum (BAD41717), Group II intron protein from Streptococcus pneumoniae (CAI33690), AP1 endonuclease from Paracentrotus lividus (AAY37515), AP endonuclease from Pseudomonas syringae (AAY37515), and exonuclease III from Escherichia coli (NP288182).
Copy number estimation
Estimates of the copy number of the SR3 retrotransposon were established by a comparative bioinformatics approach [12-14] wherein BLAST analysis of the BAC-end database of S. mansoni genomic sequences targeted more well-characterized retrotransposable elements from S. mansoni, and some other reference genes, for which copy numbers have been reported. These included the Boudicca and Sinbad LTR retrotransposons [12,13], the non-LTR retrotransposons SR1 and SR2 [18,22], the 18S ribosomal RNA genes, a middle repetitive element [46], and cathepsin D, a single copy gene [20]. The NCBI database was searched by BLAST using the sequences of these mobile genetic elements and some other genes of S. mansoni, all of which included at least one Hind III site. Specifically, the Advanced BLAST function was used, set to search only the S. mansoni sequences in the GSS database (Limit by Entrez Query: <Schistosoma mansoni [organism]>), and with the E (Expect) value at 0.000001. This stringent cutoff value was used to minimize the chance of counting other RTE-1-like elements in the total copy number of SR3. Since the formula for E is based not only on the bit scores of the local alignment of each pair of sequences, but also on the lengths of the subject and query [47], no additional correction was made for the length of the query sequence. Only hits with a Blast score of ≥100 were counted.
Investigation of integration sites
Five kilobases of the sequence flanking both 5'- and 3'-termini of SR3-left and SR3-right were employed as queries in BLAST searches of the non-redundant and dbEST GenBank databases limited by the organism [Schistosoma mansoni]. Sequences flanking additional copies of SR3 identified in other GenBank entries were also used as queries in BLAST searches to investigate the target site of SR3 integration. Multiple sequence alignments of integration sites were assembled and examined for target site preferences.
List of abbreviations
LTR, long terminal repeat; RT, reverse transcriptase; EN, endonuclease; UTR, untranslated region; SR3, schistosome retrotransposon 3; ORF, open reading frame; BAC, bacterial artificial chromosome; AChE, acetylcholineesterase; GSS, genome survey sequence; TSD, target site duplication.
Authors' contributions
TL carried out the sequence analysis, multiple sequence alignments, phylogenetic trees and drafted the manuscript. NK performed the Southern hybridization and assisted with the bioinformatics analyses. AL contributed to the experimental designs, sequence alignments, bioinformatics, and with drafting the manuscript. PJB oversaw the project, carried out copy number and other bioinformatics analyses, and drafted the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Nucleotide and deduced amino acid sequence of the SR3-left retrotransposon.
Nucleotide and deduced amino acid sequence of the SR3-right retrotransposon.
Sequence alignment of deduced open reading frames of SR3-left and SR3-right.
Multiple sequence alignment of the reverse transcriptase domain of SR3 and related non-LTR retrotransposons.
Multiple sequence alignment of the endonuclease domain of SR3 and related non-LTR retrotransposons.
Table of representative GenBank accessions to show the presence in the Schistosoma mansoni transcriptomes of messenger RNAs encoding SR3 expressed in six developmental stages of the parasite.
Table of representative GenBank accessions to show the presence in the Schistosoma mansoni transcriptomes of messenger RNAs encoding SR3 expressed in six developmental stages of the parasite.
Acknowledgments
Acknowledgements
We thank Mary Duke for maintenance of the Schistosoma mansoni life cycle, and the anonymous reviewers for helpful suggestions. Additional S. mansoni parasites were supplied by Dr. Fred Lewis through NIAID-NIH supply contract NO1-A1-55270. This investigation received financial support from the UNICEP/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (ID A20723). PJB is a recipient of a Burroughs Wellcome Fund scholar award in Molecular Parasitology and AL is a recipient of an R. Douglas Wright Biomedical Career Development Award from the National Health and Medical Research Council of Australia.
Contributor Information
Thewarach Laha, Email: thewa_la@kku.ac.th.
Nonglack Kewgrai, Email: nong261@hotmail.com.
Alex Loukas, Email: alex.loukas@qimr.edu.au.
Paul J Brindley, Email: paul.brindley@tulane.edu.
References
- Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol. 2004;2:12–13. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- Hu W, Brindley PJ, McManus DP, Feng Z, Han ZG. Schistosome transcriptomes: new insights into the parasite and schistosomiasis. Trends Mol Med. 2004;10:217–225. doi: 10.1016/j.molmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- El-Sayed NM, Bartholomeu D, Ivens A, Johnston DA, LoVerde PT. Advances in schistosome genomics. Trends Parasitol. 2004;20:154–157. doi: 10.1016/j.pt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Brindley PJ, Laha T, McManus DP, Loukas A. Mobile genetic elements colonizing the genomes of metazoan parasites. Trends Parasitol. 2003;19:79–87. doi: 10.1016/S1471-4922(02)00061-2. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Sniegowski P, Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994;371:215–220. doi: 10.1038/371215a0. [DOI] [PubMed] [Google Scholar]
- Kazazian HH., Jr Mobile elements: drivers of genome evolution. Science. 2004;303:1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- Plasterk RH, Izsvak Z, Ivics Z. Resident aliens: the Tc1/mariner superfamily of transposable elements. Trends Genet. 1999;15:326–332. doi: 10.1016/S0168-9525(99)01777-1. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Fischer C, Bouneau L, Coutanceau JP, Weissenbach J, Volff JN, Ozouf-Costaz C. Global heterochromatic colocalization of transposable elements with minisatellites in the compact genome of the pufferfish Tetraodon nigroviridis. Gene. 2004;336:175–183. doi: 10.1016/j.gene.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Laha T, Brindley PJ, Smout MJ, Verity CK, McManus DP, Loukas A. Reverse transcriptase activity and untranslated region sharing of a new RTE-like, non-long terminal repeat retrotransposon from the human blood fluke, Schistosoma japonicum . Int J Parasitol. 2002;32:1163–1174. doi: 10.1016/S0020-7519(02)00063-2. [DOI] [PubMed] [Google Scholar]
- Laha T, Loukas A, Smyth DJ, Copeland CS, Brindley PJ. The fugitive LTR retrotransposon from the genome of the human blood fluke, Schistosoma mansoni. Int J Parasitol. 2004;34:1365–1375. doi: 10.1016/j.ijpara.2004.08.007. Corrigendum, Int J Parasitol 2005, 35: 461. [DOI] [PubMed] [Google Scholar]
- Copeland CS, Brindley PJ, Heyers O, Michael SF, Johnston DA, Williams DL, Ivens AC, Kalinna BH. Boudicca, a retrovirus-like long terminal repeat retrotransposon from the genome of the human blood fluke Schistosoma mansoni. J Virol. 2003;77:6153–6166. doi: 10.1128/JVI.77.11.6153-6166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Mann VH, Morales ME, Kalinna BH, Brindley PJ. The Sinbad retrotransposon from the genome of the human blood fluke, Schistosoma mansoni, and the distribution of related Pao-like elements. BMC Evol Biol. 2005;5:20. doi: 10.1186/1471-2148-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco R, Kowaltowski AT, Machado AA, Soares MB, Gargioni C, Kawano T, Rodrigues V, Madeira AM, Wilson RA, Menck CF, Setubal JC, Dias-Neto E, Leite LC, Verjovski-Almeida S. Saci-1, -2, and -3 and Perere, four novel retrotransposons with high transcriptional activities from the human parasite Schistosoma mansoni. J Virol. 2004;78:2967–2978. doi: 10.1128/JVI.78.6.2967-2978.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco R, Machado AA, Bisson-Filho AW, Verjovski-Almeida S. Identification of 18 new transcribed retrotransposons in Schistosoma mansoni. Biochem Biophys Res Commun. 2005;333:230–240. doi: 10.1016/j.bbrc.2005.05.080. [DOI] [PubMed] [Google Scholar]
- Le Paslier MC, Pierce RJ, Merlin F, Hirai H, Wu W, Williams DL, Johnston D, LoVerde PT, Le Paslier D. Construction and characterization of a Schistosoma mansoni bacterial artificial chromosome library. Genomics. 2000;65:87–94. doi: 10.1006/geno.2000.6147. [DOI] [PubMed] [Google Scholar]
- Malik HS, Eickbush TH. The RTE class of non-LTR retrotransposons is widely distributed in animals and is the origin of many SINEs. Mol Biol Evol. 1998;15:1123–1134. doi: 10.1093/oxfordjournals.molbev.a026020. [DOI] [PubMed] [Google Scholar]
- Drew AC, Minchella DJ, King LT, Rollinson D, Brindley PJ. SR2 elements, non-long terminal repeat retrotransposons of the RTE-1 lineage from the human blood fluke Schistosoma mansoni. Mol Biol Evol. 1999;16:1256–1269. doi: 10.1093/oxfordjournals.molbev.a026216. [DOI] [PubMed] [Google Scholar]
- Mei H, Hirai H, Tanaka M, Hong Z, Rekosh D, LoVerde PT. Schistosoma mansoni: cloning and characterization of a gene encoding cytosolic Cu/Zn superoxide dismutase. Exp Parasitol. 1995;80:250–259. doi: 10.1006/expr.1995.1031. [DOI] [PubMed] [Google Scholar]
- Morales ME, Kalinna BH, Heyers O, Schulmeister A, Mann VH, Copeland CS, Loukas A, Brindley PJ. Genomic organization of the Schistosoma mansoni aspartic protease gene, a platyhelminth orthologue of mammalian lysosomal cathepsin D. Gene. 2004;338:99–109. doi: 10.1016/j.gene.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Foulk BW, Pappas G, Hirai Y, Hirai H, Williams DL. Adenylosuccinate lyase of Schistosoma mansoni: gene structure, mRNA expression, and analysis of the predicted peptide structure of a potential chemotherapeutic target. Int J Parasitol. 2002;32:1487–1495. doi: 10.1016/S0020-7519(02)00161-3. [DOI] [PubMed] [Google Scholar]
- Drew AC, Brindley PJ. A retrotransposon of the non-long terminal repeat class from the human blood fluke Schistosoma mansoni. Similarities to the chicken-repeat-1-like elements of vertebrates. Mol Biol Evol. 1997;14:602–610. doi: 10.1093/oxfordjournals.molbev.a025799. [DOI] [PubMed] [Google Scholar]
- Han JS, Boeke JD. A highly active synthetic mammalian retrotransposon. Nature. 2004;429:314–318. doi: 10.1038/nature02535. [DOI] [PubMed] [Google Scholar]
- Laha T, Brindley PJ, Verity CK, McManus DP, Loukas A. pido, a non-long terminal repeat retrotransposon of the chicken repeat 1 family from the genome of the Oriental blood fluke, Schistosoma japonicum. Gene. 2002;284:149–159. doi: 10.1016/S0378-1119(02)00381-5. [DOI] [PubMed] [Google Scholar]
- Malik HS, Burke WD, Eickbush TH. The age and evolution of non-LTR retrotransposable elements. Mol Biol Evol. 1999;16:793–805. doi: 10.1093/oxfordjournals.molbev.a026164. [DOI] [PubMed] [Google Scholar]
- Malik HS, Eickbush TH. NeSL-1, an ancient lineage of site-specific non-LTR retrotransposons from Caenorhabditis elegans. Genetics. 2000;154:193–203. doi: 10.1093/genetics/154.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman S, van Luenen HG, Plasterk RH. Rte-1, a retrotransposon-like element in Caenorhabditis elegans. FEBS Lett. 1996;380:1–7. doi: 10.1016/0014-5793(95)01525-6. [DOI] [PubMed] [Google Scholar]
- Bentley GN, Jones AK, Agnew A. Mapping and sequencing of acetylcholinesterase genes from the platyhelminth blood fluke Schistosoma. Gene. 2003;314:103–112. doi: 10.1016/S0378-1119(03)00709-1. [DOI] [PubMed] [Google Scholar]
- Hirai H, Taguchi T, Saitoh Y, Kawanaka M, Sugiyama H, Habe S, Okamoto M, Hirata M, Shimada M, Tiu WU, Lai K, Upatham ES, Agatsuma T. Chromosomal differentiation of the Schistosoma japonicum complex. Int J Parasitol. 2000;30:441–452. doi: 10.1016/S0020-7519(99)00186-1. [DOI] [PubMed] [Google Scholar]
- Verjovski-Almeida S, DeMarco R, Martins EA, Guimaraes PE, Ojopi EP, Paquola AC, Piazza JP, Nishiyama MY, Jr, Kitajima JP, Adamson RE, Ashton PD, Bonaldo MF, Coulson PS, Dillon GP, Farias LP, Gregorio SP, Ho PL, Leite RA, Malaquias LC, Marques RC, Miyasato PA, Nascimento AL, Ohlweiler FP, Reis EM, Ribeiro MA, Sa RG, Stukart GC, Soares MB, Gargioni C, Kawano T, Rodrigues V, Madeira AM, Wilson RA, Menck CF, Setubal JC, Leite LC, Dias-Neto E. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet. 2003;35:148–157. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- Jurka J. Sequence patterns indicate an enzymatic involvement in integration of mammalian retroposons. Proc Natl Acad Sci USA. 1997;94:1872–1827. doi: 10.1073/pnas.94.5.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szak TS, Pickeral OK, Makalowski W, Boguski MS, Landsman D, Boeke JD. Molecular archeology of L1 insertions in the human genome. Genome Biol. 2002;3:10. doi: 10.1186/gb-2002-3-10-research0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Moran JV, Kazazian HH, Jr, Boeke JD. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell. 1996;87:905–916. doi: 10.1016/S0092-8674(00)81997-2. [DOI] [PubMed] [Google Scholar]
- Luan DD, Korman MH, Jakubczak JL, Eickbush TH. Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell. 1993;72:595–605. doi: 10.1016/0092-8674(93)90078-5. [DOI] [PubMed] [Google Scholar]
- Feng Q, Schumann G, Boeke JD. Retrotransposon R1Bm endonuclease cleaves the target sequence. Proc Natl Acad Sci U S A. 1998;95:2083–2088. doi: 10.1073/pnas.95.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning TG, Singer MF. a mammalian transposable element. Biochim Biophys Acta. 1987;910:203–212. doi: 10.1016/0167-4781(87)90112-6. [DOI] [PubMed] [Google Scholar]
- Moran JV, Holmes SE, Naas TP, DeBerardinis RJ, Boeke JD, Kazazian HH., Jr High frequency retrotransposition in cultured mammalian cells. Cell. 1996;87:917–927. doi: 10.1016/S0092-8674(00)81998-4. [DOI] [PubMed] [Google Scholar]
- Perepelitsa-Belancio V, Deininger P. RNA truncation by premature polyadenylation attenuates human mobile element activity. Nat Genet. 2003;35:363–366. doi: 10.1038/ng1269. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1006/jmbi.1990.9999. [DOI] [PubMed] [Google Scholar]
- The Sanger Institute: S. mansoni blast server http://www.sanger.ac.uk/cgi-bin/blast/submitblast/s_mansoni
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Felsenstein J. Distributed by the author. Department of Genetics, University of Washington, Seattle; 1993. PHYLIP (Phylogeny Inference Package) version 3.5c. [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Littlewood DT, Johnston DA. Molecular phylogenetics of the four Schistosoma species groups determined with partial 28S ribosomal RNA gene sequences. Parasitology. 1995;111:167–175. doi: 10.1017/s003118200006491x. [DOI] [PubMed] [Google Scholar]
- The statistics of sequence similarity scores http://www.ncbi.nlm.nih.gov/BLAST/tutorial/Altschul-1.html#head2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nucleotide and deduced amino acid sequence of the SR3-left retrotransposon.
Nucleotide and deduced amino acid sequence of the SR3-right retrotransposon.
Sequence alignment of deduced open reading frames of SR3-left and SR3-right.
Multiple sequence alignment of the reverse transcriptase domain of SR3 and related non-LTR retrotransposons.
Multiple sequence alignment of the endonuclease domain of SR3 and related non-LTR retrotransposons.
Table of representative GenBank accessions to show the presence in the Schistosoma mansoni transcriptomes of messenger RNAs encoding SR3 expressed in six developmental stages of the parasite.
Table of representative GenBank accessions to show the presence in the Schistosoma mansoni transcriptomes of messenger RNAs encoding SR3 expressed in six developmental stages of the parasite.