Abstract
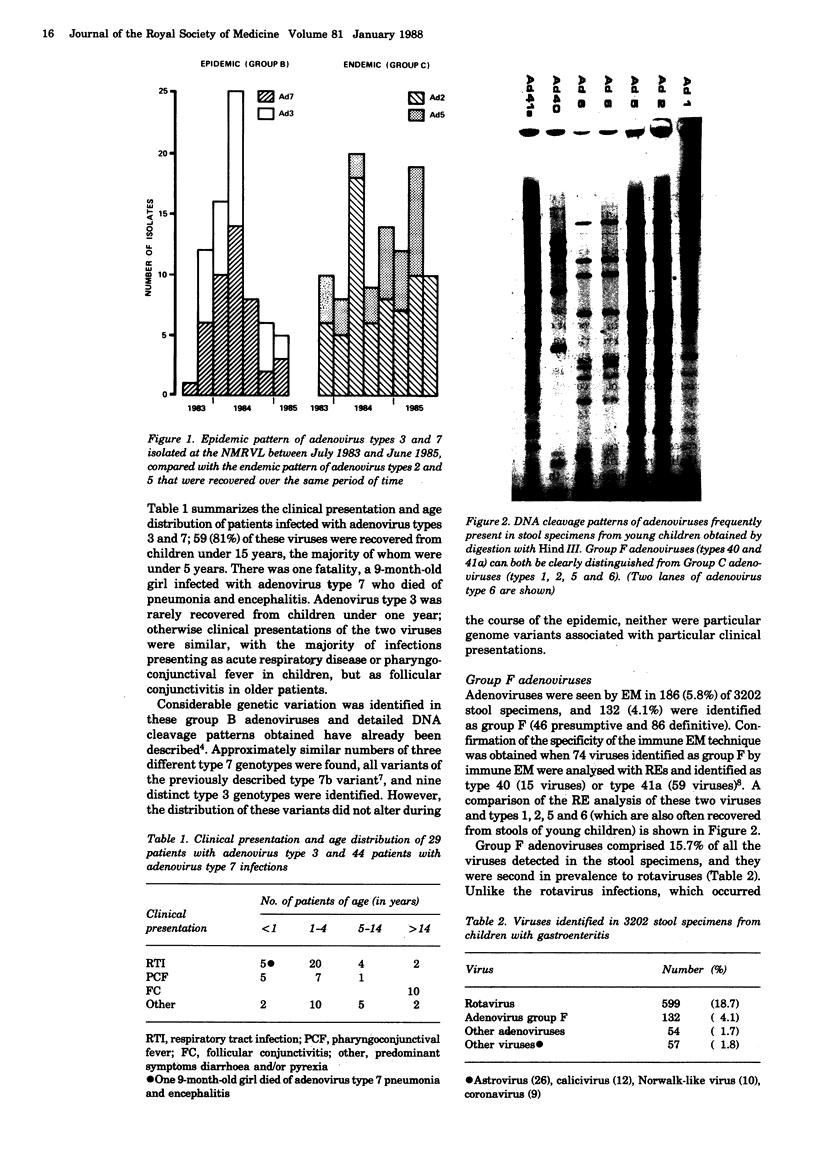
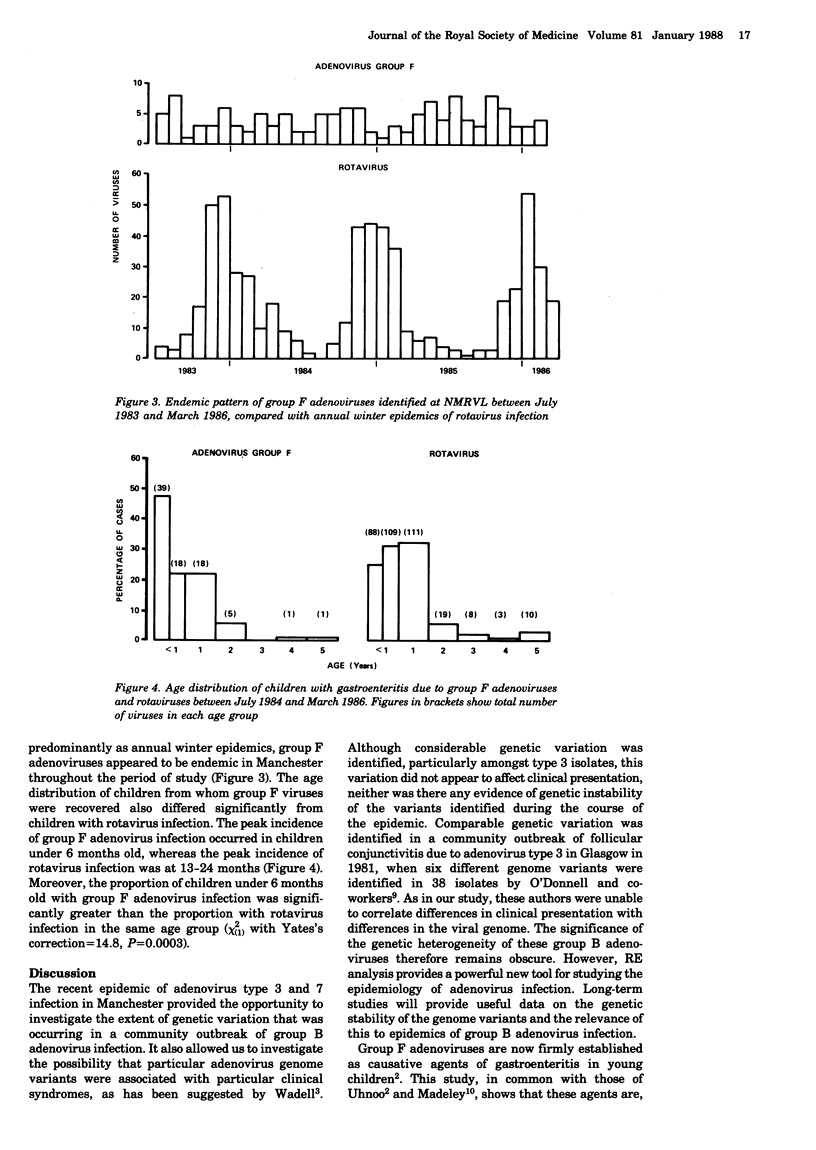
Seventy-three group B adenoviruses (29 type 3 and 44 type 7) identified in a recent community outbreak were analysed with restriction endonucleases. Considerable genetic heterogeneity was identified, particularly amongst the type 3 isolates, but this genome variation could not be correlated with either clinical or epidemiological findings. Group F adenoviruses were found in 132 (4.1%) of 3202 stool specimens from children with gastroenteritis and, after rotaviruses, they were the most common viruses identified. Unlike rotaviruses, these enteric adenoviruses were endemic throughout the 3-year study period and the greatest proportion of infections (47.6%) were found in babies under 6 months old.
Full text
PDF

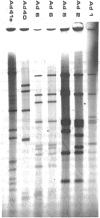
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. S., Richmond S. J. Genetic heterogeneity of recent isolates of adenovirus types 3, 4, and 7. J Clin Microbiol. 1986 Jul;24(1):30–35. doi: 10.1128/jcm.24.1.30-35.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Gardner M. K., Parrott R. H. Adenoviruses and pediatric gastroenteritis. J Infect Dis. 1985 Mar;151(3):437–443. doi: 10.1093/infdis/151.3.437. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Nogrady M. B., Spence L., Wiglesworth F. W. An outbreak of adenovirus type 7 infection in children in Montreal. Can Med Assoc J. 1973 Feb 17;108(4):434–439. [PMC free article] [PubMed] [Google Scholar]
- Champsaur H., Questiaux E., Prevot J., Henry-Amar M., Goldszmidt D., Bourjouane M., Bach C. Rotavirus carriage, asymptomatic infection, and disease in the first two years of life. I. Virus shedding. J Infect Dis. 1984 May;149(5):667–674. doi: 10.1093/infdis/149.5.667. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Kidd A. H. Genome variants of adenovirus 41 (subgroup G) from children with diarrhoea in South Africa. J Med Virol. 1984;14(1):49–59. doi: 10.1002/jmv.1890140108. [DOI] [PubMed] [Google Scholar]
- O'Donnell B., Bell E., Payne S. B., Mautner V., Desselberger U. Genome analysis of species 3 adenoviruses isolated during summer outbreaks of conjunctivitis and pharyngoconjunctival fever in the Glasgow and London areas in 1981. J Med Virol. 1986 Mar;18(3):213–227. doi: 10.1002/jmv.1890180303. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., Hammarskjöld M. L., Winberg G., Varsanyi T. M., Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
- Wadell G. Molecular epidemiology of human adenoviruses. Curr Top Microbiol Immunol. 1984;110:191–220. doi: 10.1007/978-3-642-46494-2_7. [DOI] [PubMed] [Google Scholar]
- Wood D. J., Bailey A. S. Detection of adenovirus types 40 and 41 in stool specimens by immune electron microscopy. J Med Virol. 1987 Feb;21(2):191–199. doi: 10.1002/jmv.1890210211. [DOI] [PubMed] [Google Scholar]