Abstract
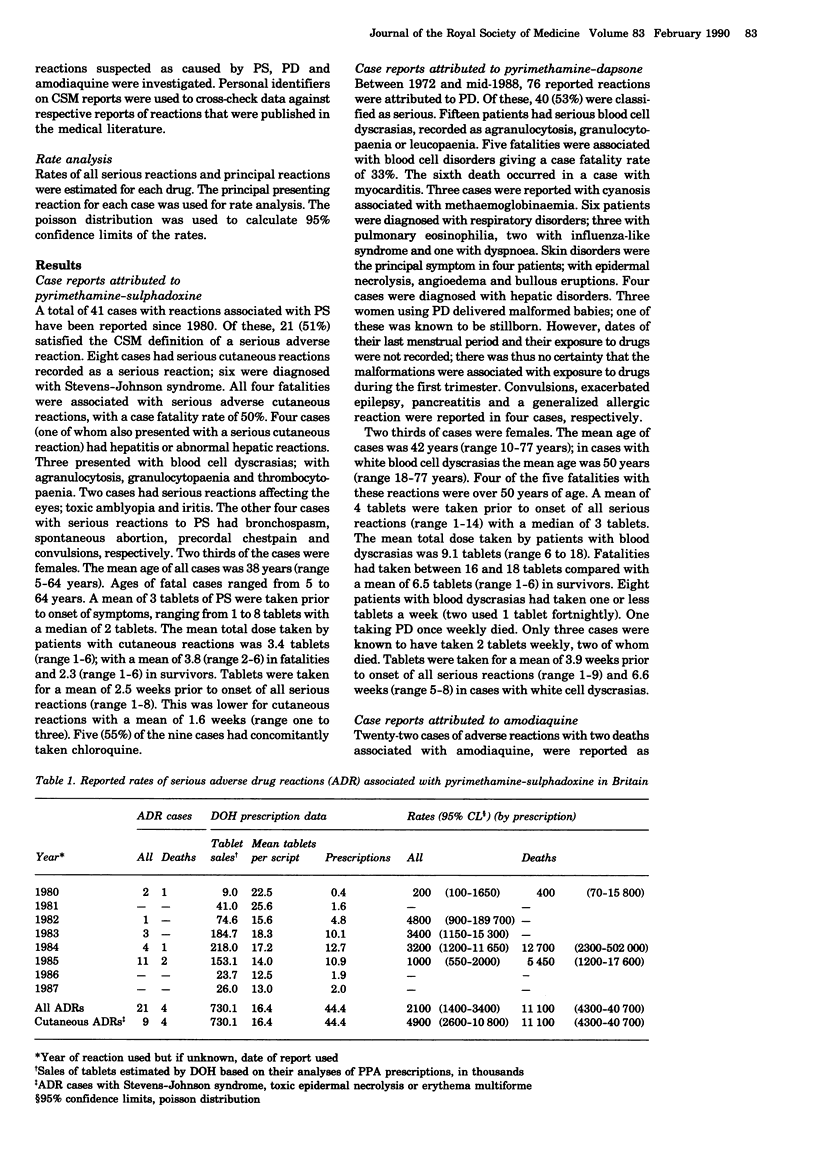
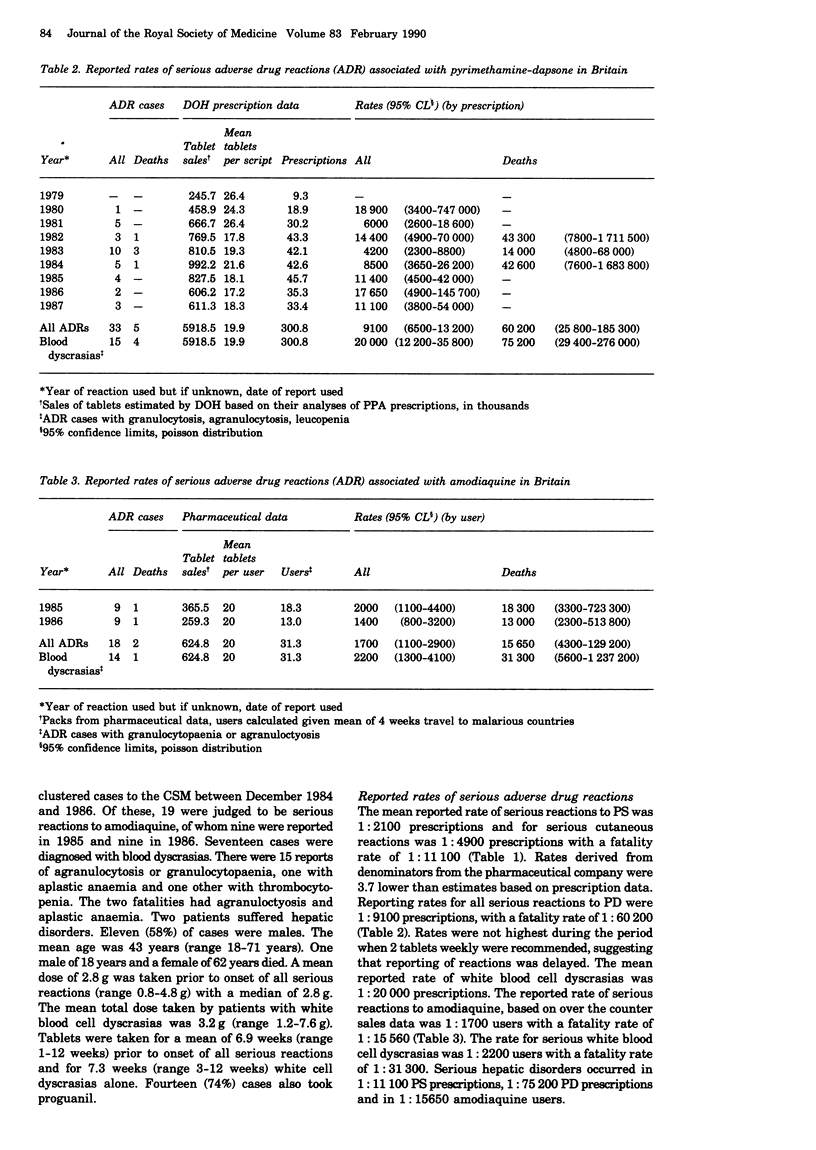
All reports of adverse reactions with pyrimethamine-sulphadoxine (Fansidar), pyrimethamine-dapsone (Maloprim), and amodiaquine spontaneously reported through the UK national post-marketing system were reviewed. Retrospective reporting rates of serious reactions associated with these drugs were analysed using prescription data from the Department of Health, derived from the Prescription Pricing Authority, and relevant pharmaceutical companies. Whilst interpretation of these data requires caution, they allowed comparison with reporting rates from other studies. The reported rate for all serious reactions to pyrimethamine-sulphadoxine was 1:2100 prescriptions, and for cutaneous reactions was 1:4900 prescriptions, with a fatality rate of 1:11,100. The reported rate for serious reactions to pyrimethamine-dapsone was 1:9100 prescriptions, and for blood dyscrasias was 1:20,000 prescriptions, with a fatality rate of 1:75,000. The reported rate of blood dyscrasias associated with amodiaquine was 1:2100 users with a fatality rate of 1:31,000. Serious hepatic disorders occurred in 1:11 1000 pyrimethamine-sulphadoxine prescriptions, 1:75,200 pyrimethamine-dapsone prescriptions, and in 1:15,650 amodiaquine users. 35% of cases received these drugs needlessly as they were not exposed to drug resistant strains of Plasmodium falciparum. Since few serious reactions have been reported to chloroquine plus proguanil, these data support guidelines which restrict the use of reviewed drugs for those at greatest risk of infection. Dosage data indicated that fatalities had taken higher doses and continued prophylaxis after onset of symptoms. Two thirds of serious reactions to the compound antimalarials were reported in females.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friman G., Nyström-Rosander C., Jonsell G., Björkman A., Lekås G., Svendsrup B. Agranulocytosis associated with malaria prophylaxis with Maloprim. Br Med J (Clin Res Ed) 1983 Apr 16;286(6373):1244–1245. doi: 10.1136/bmj.286.6373.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton C. S., Peto T. E., Bunch C., Pasvol G., Russell S. J., Singer C. R., Edwards G., Winstanley P. Frequency of severe neutropenia associated with amodiaquine prophylaxis against malaria. Lancet. 1986 Feb 22;1(8478):411–414. doi: 10.1016/s0140-6736(86)92371-8. [DOI] [PubMed] [Google Scholar]
- Hellgren U., Rombo L., Berg B., Carlson J., Wiholm B. E. Adverse reactions to sulphadoxine-pyrimethamine in Swedish travellers: implications for prophylaxis. Br Med J (Clin Res Ed) 1987 Aug 8;295(6594):365–366. doi: 10.1136/bmj.295.6594.365-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. D., Lobel H. O., Satriale R. F., Kuritsky J. N., Stern R., Campbell C. C. Severe cutaneous reactions among American travelers using pyrimethamine-sulfadoxine (Fansidar) for malaria prophylaxis. Am J Trop Med Hyg. 1986 May;35(3):451–458. doi: 10.4269/ajtmh.1986.35.451. [DOI] [PubMed] [Google Scholar]
- Peto T. E., Gilks C. F. Strategies for the prevention of malaria in travellers: comparison of drug regimens by means of risk-benefit analysis. Lancet. 1986 May 31;1(8492):1256–1261. doi: 10.1016/s0140-6736(86)91395-4. [DOI] [PubMed] [Google Scholar]
- Steffen R., Somaini B. Severe cutaneous adverse reactions to sulfadoxine-pyrimethamine in Switzerland. Lancet. 1986 Mar 15;1(8481):610–610. doi: 10.1016/s0140-6736(86)92825-4. [DOI] [PubMed] [Google Scholar]


