Abstract
The plant heat stress protein, Hsp101, and the yeast ortholog, Hsp104, are required to confer thermotolerance in plants and yeast (Saccharomyces cerevisiae), respectively. In addition to its function during stress, Hsp101 is developmentally regulated in plants although its function during development is not known. To determine how the expression of Hsp101 is regulated in cereals, we investigated the Hsp101 expression profile in developing maize (Zea mays). Hsp101 protein was most abundant in the developing tassel, ear, silks, endosperm, and embryo. It was less abundant in the vegetative and floral meristematic regions and was present at only a low level in the anthers and tassel at anthesis, mature pollen, roots, and leaves. As expected, heat treatment resulted in an increase in the level of Hsp101 protein in several organs. In expanding foliar leaves, husk leaves, the tassel at the premeiosis stage of development, or pre-anthesis anthers, however, the heat-mediated increase in protein was not accompanied by an equivalent increase in mRNA. In contrast, the level of Hsp101 transcript increased in the tassel at anthesis following a heat stress without an increase in Hsp101 protein. In other organs such as the vegetative and floral meristematic regions, fully expanded foliar leaves, the young ear, and roots, the heat-induced increase in Hsp101 protein was accompanied by a corresponding increase in Hsp101 transcript level. However, anthers at anthesis, mature pollen, developing endosperm, and embryos largely failed to mount a heat stress response at the level of Hsp101 protein or mRNA, indicating that Hsp101 expression is not heat inducible in these organs. In situ RNA localization analysis revealed that Hsp101 mRNA accumulated in the subaleurone and aleurone of developing kernels and was highest in the root cap meristem and quiescent center of heat-stressed roots. These data suggest an organ-specific control of Hsp101 expression during development and following a heat stress through mechanisms that may include posttranscriptional regulation.
Aspects of the response to heat stress have been highly conserved from bacteria to humans, including the induction of heat stress protein (Hsp) synthesis. Heat stress results in the production of mis-folded proteins during their synthesis and the denaturation of existing proteins. Prevention of denaturation or refolding of already denatured proteins appears to be the principle function of the Hsps. Several classes of Hsps have been described in plants including Hsp100, Hsp90, Hsp70, Hsp60, and the small Hsps (for review, see Vierling, 1991; Winter and Sinibaldi, 1991; Miernyk, 1999). The chaperone function of some Hsps, such as Hsp100, has been reported to promote protein disaggregation following a thermal stress (Parsell et al., 1994; Glover and Lindquist, 1998) whereas that of others, such as Hsp70, promotes refolding of denatured proteins once released from the protein aggregates (for review, see Parsell and Lindquist, 1993; Miernyk, 1999).
In addition to their function during heat stress, Hsps can serve important functions under non-stress conditions. For example, members of the Hsp70 family bind to nascent peptide chains, facilitate protein assembly in the endoplasmic reticulum, and promote protein transport across membranes during protein import into mitochondria, the endoplasmic reticulum, or chloroplasts (for review, see Rothman, 1989; Parsell and Lindquist, 1993; Becker and Craig, 1994; Mihara and Omura, 1996; Caliebe and Soill, 1999). Hsp90 interacts with and is important for the folding of protein kinases and steroid hormone receptors in mammalian cells (for review, see Caplan, 1999). Members of the Hsp100 family in plants are targeted to the chloroplast intermembrane space and stroma to facilitate protein import of nuclear-encoded proteins (Nielsen et al., 1997).
In addition to their induction following thermal stress, the expression of many Hsps is developmentally regulated (for review, see Vierling 1991; Winter and Sinibaldi, 1991). The most prominent developmental stage in plants in which Hsps are expressed is during embryogenesis. The function of Hsps in the developing embryo is not well understood but their expression is a conserved feature of animal oogenesis and embryogenesis (for review, see Heikkila et al., 1997; Krone et al., 1997; Michaud et al., 1997; Giudice et al., 1999; Luft and Dix, 1999). Developmental regulation of Hsps is observed even in lower eukaryotes such as yeast (Saccharomyces cerevisiae), in which expression of Hsp104, the yeast ortholog of the plant Hsp101, is induced once cells have entered stationary phase of growth (Sanchez and Lindquist, 1990). However, the developmental regulation of Hsps has been less well studied than its heat induction.
The Hsp100 family is unusual in that its primary role is thought to be to confer thermotolerance, i.e. the ability to survive exposure to what would otherwise be a lethal temperature (Sanchez and Lindquist, 1990; Parsell et al., 1991) rather than the initial heat stress that is required for its induction. Moreover, Hsp100 is conserved in bacteria, yeast, and plants (also known as ClpB, Hsp104, and Hsp101, respectively) and has been shown to be essential for thermotolerance in yeast and plants (Sanchez and Lindquist, 1990; Hong and Vierling, 2000; Queitsch et al., 2000). cDNAs encoding Hsp101 have been isolated from several plant species, including soybean (Glycine max; Lee et al., 1994), Arabidopsis (Schirmer et al., 1994), tobacco (Nicotiana tabacum), and wheat (Triticum aestivum; Wells et al., 1998). The Hsp101 encoded from these plant species can complement a thermotolerance defect in yeast caused by the deletion of the Hsp104 gene (Lee et al., 1994; Schirmer et al., 1994; Wells et al., 1998), suggesting conservation of the molecular chaperone activity required for the acquisition of thermotolerance. In addition to its thermotolerance, Hsp101 functions to mediate a high rate of translation from those mRNAs that are able to efficiently recruit the protein (Wells et al., 1998). The 5′-leaders of tobacco mosaic viral mRNA and the nuclear-encoded Ferredoxin 1 mRNA are two examples of leader sequences that function as translational enhancers that employ the Hsp101-mediated mechanism of translational enhancement (Wells et al., 1998; Ling et al., 2000).
In contrast to most Hsps, the regulation of Hsp101 expression in plants has not been well studied, particularly for cereals. A protein of 110 kD that cross-reacted with yeast anti-Hsp104 antibodies was detected in rice (Oryza sativa) and limited peptide sequencing of a rice 104-kD protein revealed similarity with plant Hsp101 proteins. Because these two proteins are distinct immunologically, in size, and their expression differs in response to different stresses (Pareek et al., 1995; Singla et al., 1997), it is not clear whether either are true members of the Hsp101 family. In maize (Zea mays), a genomic clone and partial cDNA sequence encoding Hsp101 have been reported (Nieto-Sotelo et al., 1999).
As a first step in the analysis of the regulation of Hsp101 in cereals, we have examined the expression of Hsp101 during the development of maize and wheat and following heat stress. Under non-stress conditions, Hsp101 protein was most abundant in the premeiosis stage of tassel development, the ear, silks, endosperm, and embryo and was present at only a low level in foliar leaves and roots. A heat-induced increase in Hsp101 protein was accompanied by a corresponding increase in Hsp101 transcript level in the vegetative and floral meristematic regions, fully expanded foliar leaves, the young ear, and roots. However, Hsp101 transcript abundance increased in the heat-stressed tassel at anthesis without a similar increase in Hsp101 protein. Moreover, in heat-stressed, expanding foliar leaves, husk leaves, and at the premeiosis stage of tassel development, the level of Hsp101 protein increased without an equivalent increase in Hsp101 mRNA. Little or no increase in the level of Hsp101 protein or mRNA was observed in heat-stressed anthers at anthesis, mature pollen, developing endosperm or embryos, suggesting that these tissues are not capable of mounting a heat stress response. These observations suggest that Hsp101 expression is regulated in an organ-specific manner during development and following a heat stress and that its expression is regulated at the RNA level as well as posttranscriptionally.
RESULTS
Maize Hsp101 Confers Thermotolerance to Thermosensitive Yeast
As a first step toward investigating the developmental regulation of Hsp101 in cereals, a full-length Hsp101 cDNA was isolated from maize and rice cDNA libraries. The amino acid sequence predicted from this maize Hsp101 cDNA (Fig. 1) is identical to that recently reported for a genomic Hsp101 clone (Nieto-Sotelo et al., 1999). Maize and rice Hsp101 exhibit a high degree of conservation with each other (96.4% identity) and two wheat Hsp101 cDNAs (Table I). The previously reported amino acid sequence obtained from peptides of a rice 104-kD protein (Singla et al., 1997) were similar but not identical with rice Hsp101. Maize and rice Hsp101 are also conserved with Hsp101 from dicots such as tobacco, soybean, and Arabidopsis but to a lower extent than that observed among monocots (Table I).
Figure 1.
Alignment of Hsp101 from monocot and dicot species. The complete sequence for maize Hsp101 (accession no. AF133840) is shown and only those positions within OsHsp101 (rice; accession no. AF332981), TaHsp101-1 and TaHsp101-2 (wheat; accession nos. AF083344 and AF097363, respectively), NtHsp101 (tobacco; accession no. AF083343), GmHsp101 (soybean; accession no. L35272), and AtHsp101 (Arabidopsis; accession no. U13949) that differ from the maize ortholog are indicated. Identity with maize Hsp101 is indicated by dots, whereas gaps introduced to maintain alignment are indicated by dashes. The start of each of the five domains is indicated by an arrow. Conserved signature sequences (Schirmer et al., 1996) within each domain are indicated above the maize amino acid sequence where non-conserved or hydrophobic amino acids are indicated with an “x” or “h,” respectively, and the roman numeral or letter designations by which they are known (Schirmer et al., 1996) appears above each conserved signature sequence.
Table I.
Conservation of HSP101 in dicot and monocot speciesa
| Species | Maize | Rice | Wheat-1 | Wheat-2 | Tobacco | Soybean | Arabidopsis |
|---|---|---|---|---|---|---|---|
| Maize | – | 97.1 | 96.0 | 96.4 | 88.9 | 89.2 | 89.6 |
| Rice | 96.4 | – | 96.2 | 96.6 | 88.3 | 88.9 | 89.0 |
| Wheat-1 | 93.5 | 94.3 | – | 99.3 | 88.4 | 88.6 | 88.0 |
| Wheat-2 | 94.1 | 95.0 | 98.9 | – | 88.5 | 89.0 | 88.4 |
| Tobacco | 82.8 | 82.8 | 81.3 | 81.8 | – | 91.9 | 90.1 |
| Soybean | 84.4 | 84.5 | 82.9 | 83.5 | 86.9 | – | 91.5 |
| Arabidopsis | 85.2 | 84.5 | 82.8 | 83.7 | 85.5 | 87.4 | – |
Percent similarity and identity are indicated above and below the diagonal of dashes, respectively. Values were calculated using BESTFIT (Blosum62) of the GCG Wisconsin Package Version 10.
Maize and rice Hsp101 are structurally conserved with members of the Hsp101/Hsp104/ClpB family in that each contains two ATP-binding domains highly conserved with the other members, which are separated by a middle domain and bounded by N-terminal and C-terminal domains. Hsp101 present in monocots has diverged from dicot Hsp101 in the N-terminal, middle, and C-terminal domains but most of the sequence differences are conserved among the monocot Hsp101 proteins (Fig. 1). Maize and rice Hsp101 also contain the conserved signature sequences within each domain of the B-type Hsp100 proteins (Fig. 1), which are distinguished from other subclasses (e.g. A and C) by the length of their middle domain (Schirmer et al., 1996).
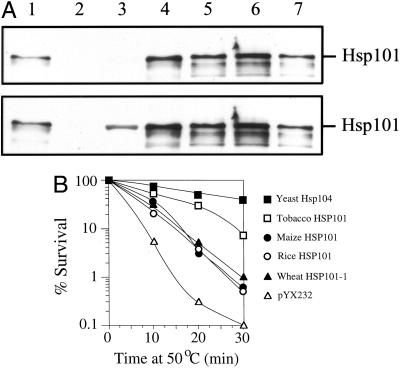
In the absence of Hsp104, yeast survives poorly when treated at 50°C. To assay the thermotolerance function of monocot Hsp101, the cDNAs encoding the maize, rice, and wheat Hsp101 as well as tobacco Hsp101 and the yeast Hsp104 were introduced into a yeast expression vector under the control of the constitutive triose phosphate isomerase (TPI) promoter and each construct was introduced into the hsp104 yeast strain, SL304A. Expression from each construct was confirmed by western analysis (Fig. 2A) and the ability of each plant Hsp101 to complement the thermotolerance defect of SL304A was assayed by exposing the yeast to a potentially lethal heat treatment. Maize, rice, and wheat Hsp101 increased the thermotolerance of the yeast confirming their thermotolerance function (Fig. 2B). Yeast Hsp104 conferred the greatest degree of thermotolerance followed by tobacco Hsp101. These data demonstrate that the maize cDNA isolated encodes a functional Hsp101 that confers a level of thermotolerance typical for monocot Hsp101 proteins. The maize cDNA was used in the northern analysis of Hsp101 expression during development and following a heat stress (see below).
Figure 2.
Analysis of tobacco, maize, rice, and wheat Hsp101 expression and thermotolerance function in yeast. Each Hsp101 cDNA was introduced into a yeast expression vector under the control of the TPI promoter and the constructs introduced into the hsp104 yeast mutant, SL304A. A, Yeast extract from 6 × 106 exponentially growing cells was resolved on a 10% (w/v) SDS-PAGE gel, transferred to nitrocellulose membrane, probed using anti-wheat Hsp101 antibodies (top), and then probed with anti-yeast Hsp104 antibodies (bottom). Detection in each case used peroxidase-linked secondary antibody and chemiluminescence. Lane 1, Purified wheat Hsp101; lane 2, yeast containing pYX232; lane 3, yeast expressing Hsp104; lane 4, yeast expressing tobacco Hsp101; lane 5, yeast expressing wheat Hsp101-1; lane 6, yeast expressing maize Hsp101; and lane 7, yeast expressing rice Hsp101. The bands below the full-length Hsp101 represent degradation products. B, SL304A, containing the tobacco, maize, rice, and wheat Hsp101-1 cDNAs or yeast Hsp104 under the control of the TPI promoter, was grown to an early exponential stage in synthetic dextrose medium prior to assaying for thermotolerance. The expression vector, pYX232, was used as a negative control. The percentage of survival at 50oC was plotted as a function of the length of the heat treatment. The results shown are representative for these constructs under the conditions employed.
The Level of Hsp101 Is Developmentally Regulated during Germination
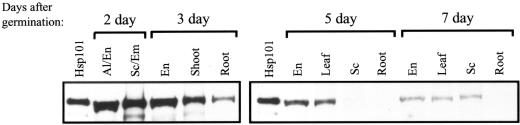
Mature wheat embryos had been used previously to purify Hsp101 (Tanguay and Gallie, 1996), suggesting that Hsp101 is expressed during embryo development. To examine whether Hsp101 is also present during growth of the seedling, and if so, in what tissues, maize and wheat seed were germinated, the seedlings dissected, and the presence of Hsp101 determined through western analysis. In wheat, the level of Hsp101 was highest during early germination and progressively decreased (Fig. 3). In 2-d-old seedlings, Hsp101 was present at approximately equal levels in the embryo and endosperm (composed of the living aleurone and the dead starchy endosperm). Hsp101 was also detected in the emerging coleoptile and roots of 3-d seedlings. In 5- to 7-d-old seedlings, Hsp101 was no longer detectable in whole roots and its amount decreased progressively in the endosperm, scutellum, and leaves. Previous work that probed extract prepared from whole rice seedlings with antiserum raised to a 104-kD, heat-inducible protein noted a similar decline within the first 4 d of growth (Singla et al., 1998).
Figure 3.
Presence of Hsp101 in wheat seedling tissues. Wheat seedlings were grown for the times indicated above the panels and the seedlings dissected into endosperm (En), aleurone (Al), embryo (Em), scutellum (Sc), shoot, and root tissue. Five micrograms of soluble protein extracted from each tissue was resolved using SDS-PAGE, transferred to nitrocellulose membrane, and incubated with anti-Hsp101 antibodies. Detection used peroxidase-linked secondary antibody and chemiluminescence. Purified wheat Hsp101 was included as a control in the first lane of each panel.
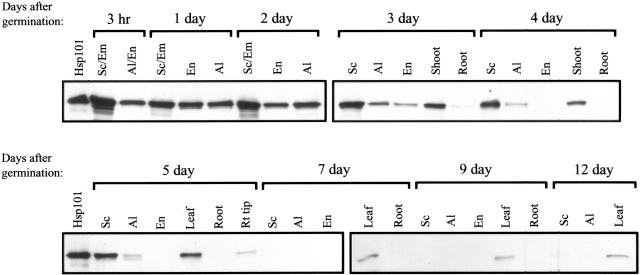
A similar pattern was observed following germination of maize. Most of the Hsp101 in mature maize kernels was present in the scutellum/embryo (see Sc/Em, 3-h germinated kernels, Fig. 4) although a lower level of Hsp101 was also detected in the aleurone/endosperm (see Al/En, 3 h germinated kernels, Fig. 4). Approximately equal amounts of Hsp101 were present in scutellum, endosperm, and aleurone in kernels germinated for 1 d but by 2 d of germination, the level of Hsp101 in the endosperm and aleurone had declined relative to the scutellum/embryo. As in wheat seed, the decrease in the level of Hsp101 in the starchy endosperm is presumably a result of its degradation as part of the mobilization of protein that occurs during the germination program. The 104-kD, heat-inducible protein in rice was not detected in the endosperm of 5-d-old seedlings (Singla et al., 1998), supporting the conclusion that Hsp101 is rapidly lost from this tissue during seedling growth. In 3-d-old seedlings, Hsp101 was detected in the emerging shoot and root and the level of Hsp101 in the endosperm and aleurone declined further (Fig. 4). By 4 d, Hsp101 became undetectable in the endosperm and root although it remained at a detectable level in root tips of 5-d-old seedlings. During subsequent growth, the level of Hsp101 decreased in all tissues, and by 12 d, it was present at just a low level in leaves (Fig. 4). The low level of Hsp101 present in root tips and in leaves most probably represents new synthesis of the protein as Hsp101 mRNA is detected in leaves and root tips (see below).
Figure 4.
Presence of Hsp101 in germinating maize seedling tissues. Maize seedlings were grown for the times indicated above the panels and the seedlings dissected into endosperm (En), aleurone (Al), embryo (Em), scutellum (Sc), shoot, leaf, and root or root tip tissue. Note that the scutellum tissue includes the embryonic axis for the 3-h, 1- and 2-d germinated kernels (Sc/Em), whereas the endosperm tissue includes the aleurone layer for the 3-h germinated kernels (Al/En). Five micrograms of soluble protein extracted from each tissue was resolved using SDS-PAGE, transferred to nitrocellulose membrane, and incubated with anti-Hsp101 antibodies. Purified wheat Hsp101 was included as a control in the first lane of each left panel.
Control of Hsp101 Expression Is Regulated in an Organ-Specific Manner
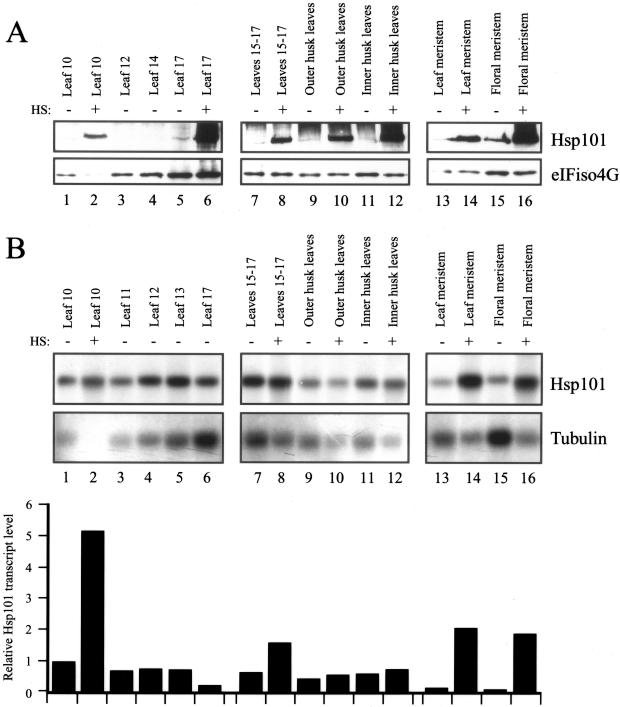
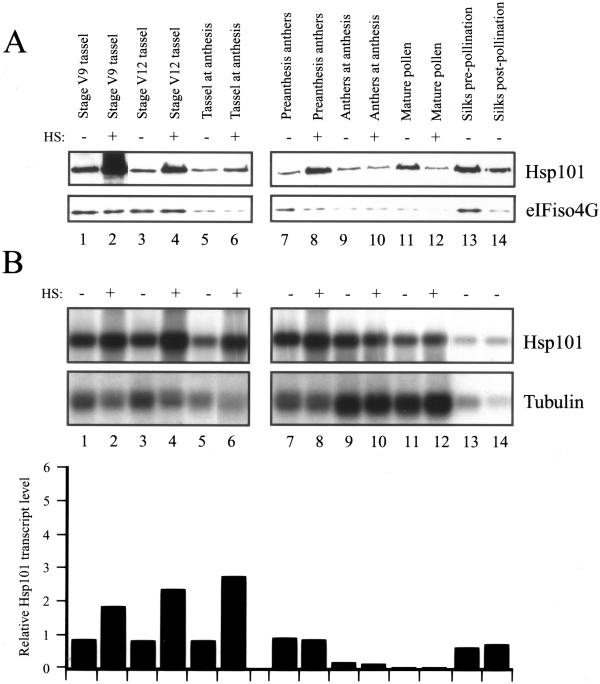
The developmental regulation of Hsp101 was examined in multiple organs of maize grown under non-stress conditions. Moreover, each organ was examined for its heat responsiveness by examining whether the amount of Hsp101 increased following a heat stress at 41°C for 1 h. Poly(A+) RNA was isolated from stressed and non-stressed tissues and assayed for Hsp101 RNA using the maize Hsp101 cDNA isolated above as the probe in northern analysis. Probing the same membrane for maize tubulin RNA served as an internal control against which the level of Hsp101 could be normalized. Western analysis of total protein from each tissue was also performed. Because antiserum against maize tubulin was not available, antiserum to the eukaryotic initiation factor (eIF) iso4G was used to serve as an internal control for Hsp101 protein levels in stressed and non-stressed tissues.
Hsp101 protein was present at a very low level in non-stressed, adult foliar leaves (lanes 1, 3–5, and 7, Fig. 5A) whereas Hsp101 mRNA was detected in all foliar leaves examined (lanes 1 and 3–7, Fig. 5B). With the exception of the youngest foliar leaf, the level of Hsp101 mRNA was relatively constant during the expansion of leaves (lanes 1 and 3–7 and the relative transcript data in Fig. 5B). Heat stress of a fully expanded foliar leaf (i.e. leaf 10) resulted in a substantial increase in transcript level (lanes 1 and 2 and the relative transcript data in Fig. 5B) with an increase in the level of Hsp101 protein from just detectable to prominent (lanes 1 and 2, respectively, Fig. 5A). The induction of Hsp101 transcript level in heat-stressed, expanding leaves was lower than in a fully expanded leaf (compare heat induction of leaves 15–17 with that of leaf 10, Fig. 5B). Nevertheless, a substantial increase in Hsp101 protein was observed in expanding leaves following a heat stress (lanes 7 and 8, Fig. 5A). Similar results were observed in husk leaves: Hsp101 transcript was readily detected in non-stressed, outer and inner husk leaves but did not increase significantly following a heat stress (lanes 9–12 and the relative transcript data in Fig. 5B). Despite the lack of a heat-mediated increase in Hsp101 transcript levels, a substantial increase in Hsp101 protein was observed in heat-stressed husk leaves, particularly so in inner husk leaves (lanes 9–12, Fig. 5A). These observations demonstrate that Hsp101 transcript is constitutively accumulated in foliar and husk leaves but its level is not substantially heat inducible in husk leaves. Despite this, the level of Hsp101 protein is highly heat inducible in all leaves examined, suggesting that posttranscriptional control of Hsp101 expression may occur in expanding foliar and husk leaves.
Figure 5.
Developmental and heat-regulated expression of Hsp101 in maize foliar and husk leaves. A, The level of maize Hsp101 protein was determined by western analysis in the organs indicated above each lane. Total protein was isolated, transferred to nitrocellulose membrane following resolution using SDS-PAGE, and probed for Hsp101 or eIFiso4G. Whether an organ was heat stressed (HS) at 41°C for 1 h or maintained at 21°C is indicated above each lane. B, The level of maize Hsp101 mRNA was determined by northern analysis in the organs indicated above each lane. Poly(A+) mRNA was isolated, transferred to nylon membrane following resolution on a 7% (w/v) formaldehyde/MOPS [3-(N-morpholino)-propanesulfonic acid] gel, and probed with maize Hsp101 or maize tubulin antisense RNA. The level of Hsp101 mRNA was quantitated by densitometry, normalized to the ribosomal RNA and tubulin mRNA, and expressed in the histograms relative to the level of Hsp101 mRNA in the fully expanded, foliar leaf 10 that was set at a value of 1. Numbering of maize leaves begins with the first leaf that emerges from the kernel. All foliar leaves were collected at the same time from six plants to eliminate plant-to-plant variation. The husk leaves were collected at silking stage.
A higher level of Hsp101 protein was present in the vegetative and male floral meristematic regions, which for this study included leaf and tassel inflorescence primordia, respectively, and a significant increase in the level of Hsp101 protein was observed following a heat stress, particularly for the male floral meristematic region (lanes 13–16, Fig. 5A). In a similar manner, only a low level of Hsp101 transcript was observed in the non-stressed, meristematic regions and a substantial increase in the amount of RNA was observed following a heat stress (lanes 13–16 and the relative transcript data in Fig. 5B).
The level of Hsp101 protein was high in developing, premeiotic stage tassels and was highly heat inducible (lanes 1 and 2, Fig. 6A). However, the degree of induction of Hsp101 protein following exposure to heat decreased with the age of the tassel so that by anthesis, little increase in Hsp101 protein was observed following a heat stress (lanes 5 and 6, Fig. 6A). In the developing tassel, the level of Hsp101 mRNA increased moderately following a heat stress (lanes 1–4, Fig. 6B) and despite the lack of a substantial heat-mediated increase in Hsp101 protein during late development of the tassel, a significant increase in Hsp101 transcript levels was observed in heat-stressed tassels at anthesis (lanes 5 and 6, Fig. 6B).
Figure 6.
Developmental and heat-regulated expression of Hsp101 in maize tassel, anthers, pollen, and silks. A, The level of maize Hsp101 protein was determined by western analysis in the organs indicated above each lane. The western analysis was performed as described in Figure 5 and in the same experiment. Whether an organ was heat stressed (HS) at 41°C for 1 h or maintained at 21°C is indicated above each lane. B, The level of maize Hsp101 mRNA was determined by northern analysis in the organs indicated above each lane. The northern analysis was performed as described in Figure 5 and in the same experiment. The level of Hsp101 mRNA was quantitated by densitometry, normalized to the ribosomal RNA and tubulin mRNA, and expressed in the histograms relative to the level of Hsp101 mRNA in the fully expanded, foliar leaf 10 (as shown in Fig. 5) that was set at a value of 1. The preanthesis anthers were taken 1 week prior to anthesis. The post-pollination silks were taken 1 week following pollination. The organs were collected from six plants to eliminate plant-to-plant variation.
Hsp101 protein was observed in pre-anthesis anthers and only a moderate induction in the level of protein was observed following a heat stress (lanes 7 and 8, Fig. 6A). No increase in Hsp101 protein was observed in heat-stressed anthers at anthesis or in mature pollen (lanes 9–12, Fig. 6A). Hsp101 transcript was present at a moderate level in developing, non-stressed anthers, decreased in anthers at anthesis, and was present at only a low level in mature pollen (lanes 7, 9, and 11, respectively, and the relative transcript data in Fig. 6B). Little to no increase in the amount of Hsp101 RNA was observed in heat-stressed, developing anthers, anthers at anthesis, or mature pollen (lanes 7–12, Fig. 6B), observations correlating with the lack of a heat-mediated increase in Hsp101 protein level (lanes 7–12, Fig. 6A). These results suggest that, with respect to Hsp101, pollen may be only slightly heat responsive during its development and this response is lost upon its maturation. Hsp101 protein and RNA were also detected in silks prior to pollination (lane 13, Fig. 6, A and B, respectively) and remained in silks even 1 week following pollination (lane 14, Fig. 6, A and B, respectively).
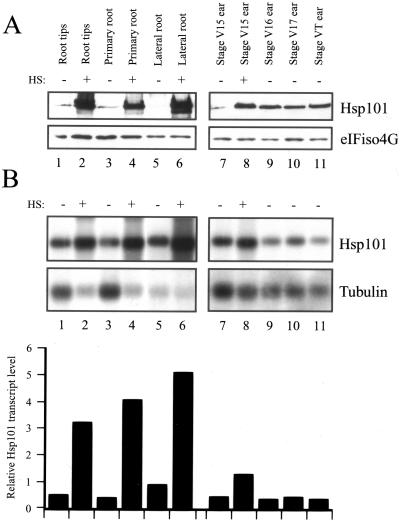
Hsp101 protein was present at a low level during early ear development and was also heat inducible (lanes 7 and 8, Fig. 7A) as were Hsp101 transcript levels (lanes 7 and 8 and the relative transcript data in Fig. 7B). The level of Hsp101 protein increased during later ear development (compare lanes 9–11 with lane 7, Fig. 7A) despite a lack of a corresponding increase in transcript levels (compare lanes 9–11 with lane 7, Fig. 7B).
Figure 7.
Developmental and heat-regulated expression of Hsp101 in maize roots and ear. A, The level of maize Hsp101 protein was determined by western analysis in the organs indicated above each lane. The western analysis was performed as described in Figure 5 and in the same experiment. Whether an organ was heat-stressed (HS) at 41°C for 1 h or maintained at 21°C is indicated above each lane. B, The level of maize Hsp101 mRNA was determined by northern analysis in the organs indicated above each lane. The northern analysis was performed as described in Figure 5 and in the same experiment. The level of Hsp101 mRNA was quantitated by densitometry, normalized to the ribosomal RNA and tubulin mRNA, and expressed in the histograms relative to the level of Hsp101 mRNA in the fully expanded, foliar leaf 10 (as shown in Fig. 5) that was set at a value of 1. The roots used for this analysis were taken from a 4-week-old plant and the terminal 5 mm was collected. Root tips represented the terminal 2 mm of root tissue. The organs were collected from six plants to eliminate plant-to-plant variation.
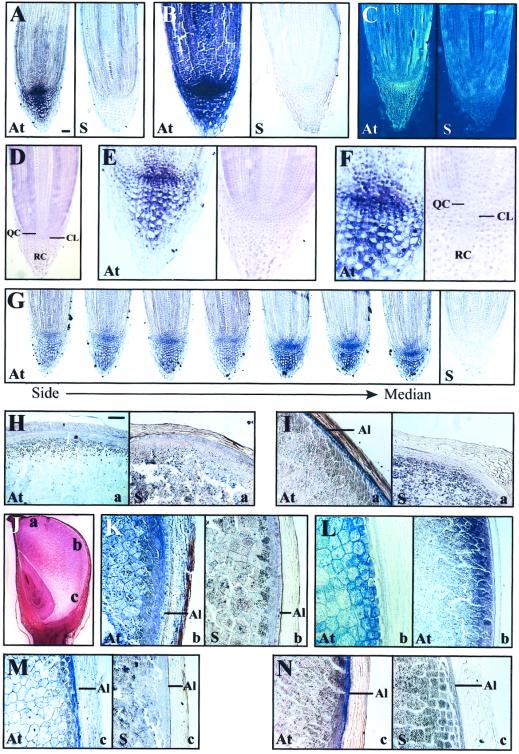
Hsp101 protein was present at a low level in the primary/nodal roots and lateral roots and was strongly heat inducible (lanes 1–6, Fig. 7A). Similar results were observed at the transcript level: Hsp101 mRNA was present at a moderate level under non-stressed conditions and was highly heat inducible (lanes 1–6 and the relative transcript data in Fig. 7B). In situ RNA localization of Hsp101 mRNA within heat-stressed, primary roots revealed that the RNA accumulated to the highest level in the root cap (RC) and quiescent center (QC) of stressed roots (Fig. 8A). Within the RC, Hsp101 mRNA was highest in the calyptrogen layer (CL), i.e. the RC meristem (see Fig. 8F). The level of Hsp101 mRNA decreased as a function of cell age in the RC so that the lowest level of Hsp101 mRNA was observed in the oldest cells, i.e. outermost layers (Fig. 8E). Serial sectioning through a single root tip (from one side to the median section, Fig. 8G) revealed that the Hsp101 mRNA detected in the CL and QC was concentrated most in the apical regions of each. Although Hsp101 mRNA was most abundant in the CL and QC, Hsp101 mRNA could be detected throughout the root when the sections were developed for a longer period (Fig. 8B). No signal was detected when heat-stressed roots were probed with Hsp101 sense RNA (see right, Fig. 8, A, B, and G). In non-stressed roots, Hsp101 mRNA was detectable at a low level in the apical region of the CL that was not seen in non-stressed roots when probed with Hsp101 sense RNA (Fig. 8C). These results suggest that although the amount of Hsp101 transcript increases in all root tip cell types following a heat stress, it is the CL and QC that exhibit the greatest response to the stress.
Figure 8.
In situ localization of Hsp101 mRNA during maize root and kernel development. Sections of root (A–G) and kernels (H–N) were hybridized with riboprobes containing digoxygenin. Hybridization was detected as a blue precipitate by staining with nitroblue tetrazolium. A, Median section of a 10-d-old, heat-stressed (all heat-treatments performed at 41oC for 1 h) root probed with a low concentration of antisense (At) Hsp101 RNA to detect Hsp101 mRNA or sense (S) RNA to serve as a control. B, Median section of a 10-d-old, heat-stressed root probed with a high concentration of antisense or sense RNA. C, Median section of a non-stressed root in which the positive signal (At) is represented by yellow staining region in this inverted image. D, Median section of 10-d-old root stained with safranin O to show the QC, CL, and RC. E, Median section of 10-d-old, heat-stressed root tip probed with antisense Hsp101 RNA (left) or stained with safranin O (right). F, Median section at high magnification of 10-d-old, heat-stressed root tip probed with antisense Hsp101 RNA (left) or stained with safranin O (right). G, Serial sections from the side to the median section of a 12-d-old, heat-stressed root probed with antisense RNA (first seven images) to detect Hsp101 mRNA or sense RNA (right) to serve as a control. H, Median section of the crown region of a 26-d after pollination (DAP) developing kernel probed with antisense Hsp101 (At) RNA to detect Hsp101 mRNA or sense (S) RNA to serve as a control. I, Median section of the crown region of an 18-DAP developing kernel probed with antisense Hsp101 (At) or sense (S) RNA. J, Median section of an 18-DAP kernel stained with safranin O to illustrates the regions (labeled a, b, or c) analyzed in H through N. K, Median section (region b) of a 26-DAP kernel probed with antisense Hsp101 (At) or sense (S) RNA. L, Median section (region b) of an 18-DAP kernel probed with antisense α-zein RNA at a high (left) or low (right) magnification. M, Median section (region c) of a 26-DAP kernel probed with antisense Hsp101 (At) or sense (S) RNA. N, Median section (region c) of an 18-DAP kernel probed with antisense Hsp101 (At) or sense (S) RNA. The bar in A represents 100 mm and in H represents 350 mm.
Hsp101 Expression Is Subject to Developmental Control But Is Not Stress Regulated in Kernels
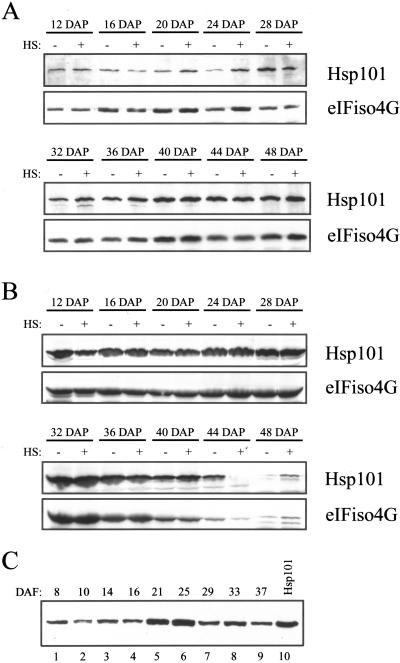
The developmental expression of Hsps during embryogenesis is characteristic of plant and animals (Lindquist, 1986; Winter and Sinibaldi 1991; Wehmeyer et al., 1996; Heikkila et al., 1997; Krone et al., 1997; Michaud et al., 1997; Giudice et al., 1999; Luft and Dix, 1999) although their function during embryogenesis remains unknown. To examine the expression characteristics of Hsp101 during kernel development in maize, kernels were collected at stages during their development and the level of Hsp101 transcript and protein examined. The heat responsiveness of developing kernels was also examined by subjecting the kernels to a 41°C heat stress for 1 h. Poly(A+) RNA and protein from the embryo and endosperm were analyzed using northern and western analysis, respectively. Hsp101 protein was expressed in the endosperm at 12 DAP when zein storage protein is being actively synthesized, and remained constant up to 32 DAP (at which stage the kernel is competent for germination) after which point its amount declined (Fig. 9). A similar decline was observed for eIFiso4G (Fig. 9), data supporting the observation that many soluble proteins disappear during late endosperm development (Gallie et al., 1998). The decline in Hsp101 protein level was accompanied by a corresponding decline in Hsp101 (and tubulin) transcript levels (data not shown). Hsp101 protein was present throughout embryo development, accumulating slightly up to maturity (Fig. 9). In contrast to the endosperm, no decline in the level of Hsp101 protein was observed during the late development of the embryo. A similar protein expression profile was observed during the development of whole (i.e. endosperm and embryo) wheat seed in which Hsp101 accumulated during early seed development up to 21 DAF, which represents the period of greatest deposition of storage reserves, remained constant to 25 DAF, and declined somewhat by 37 DAF (Fig. 9C), which represents late development of the seed. In situ RNA localization analysis of Hsp101 transcript levels in developing maize kernels at 26 DAP revealed that the mRNA was most abundant in the subaleurone region of the endosperm (Fig. 8, K and M), similar to the accumulation of α-zein mRNA (Fig. 8L). Hsp101 transcript level in the subaleurone region was higher in the basal half of the kernel (Fig. 8, K and M) than in the crown region (see Fig. 8H), where little signal was observed. At this developmental stage, dessication of the crown region, including the aleurone and subaleurone, had already begun as part of the maturation program of kernel development. At 18 DAP, Hsp101 mRNA could be detected in the aleurone of the crown (Fig. 8I) as well as in the basal half of the kernel (Fig. 8N), data suggesting that Hsp101 accumulates in the aleurone layer at an earlier stage of development than it does in the subaleurone. No signal was detected in the endosperm when probed with Hsp101 sense RNA (see right, Fig. 8, H, I, K, M, and N). In addition, no signal was detected in the embryo (data not shown) despite detection of the mRNA using northern analysis and Hsp101 protein using western analysis (Fig. 9B), suggesting that Hsp101 transcript may be uniformly present in the embryo.
Figure 9.
The level of Hsp101 protein is developmentally regulated during seed development in maize and wheat. Hsp101 protein in maize embryo (A) or endosperm (B) from kernels collected at DAP or from whole wheat seed (C) collected at selected days after flowering (DAF) was measured by western analysis. Total protein was resolved using SDS-PAGE, transferred to nitrocellulose membrane, and incubated with anti-Hsp101 antibodies or reprobed with anti-eIFiso4G antibodies. A and B, The heat-stress treatment (HS) is indicated above the lanes. The 12-DAP samples represent whole kernels. C, Purified wheat Hsp101 (lane 10) was included as a control.
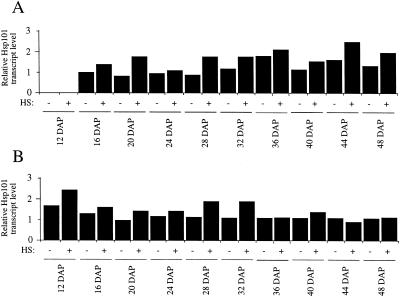
No significant increase in Hsp101 protein levels was observed in heat-stressed maize endosperm or embryo at any developmental stage examined (Fig. 9), whereas only a slight increase in the level of Hsp101 transcript was observed in these tissues following a heat stress and only during their early development (Fig. 10). Hsp101 protein was present at a similar level in shrunken2 (sh2) maize kernels in which starch biosynthesis is blocked, resulting in sugar accumulation and osmotic stress (data not shown), suggesting that osmotic stress does not influence the amount of Hsp101 protein in either developing endosperm or embryos. These observations suggest that kernels are not significantly heat responsive with regard to Hsp101 nor does the amount of Hsp101 increase in response to osmotic stress. However, Hsp101 transcript and protein are present during the normal development of the endosperm and embryo. It is interesting that the level of Hsp101 protein declines during late endosperm development but remains at a high level during late embryo development.
Figure 10.
Hsp101 transcript level is not significantly induced in developing maize endosperm and embryo following a heat stress. Hsp101 transcript abundance in maize embryo (A) or endosperm (B) from kernels collected at time points following pollination was measured by northern analysis and normalized to maize tubulin RNA. The level of Hsp101 RNA is indicated relative to that in non-stressed 16-DAP embryos, which is given a value of 1. DAP and the heat stress treatment (HS) are indicated below the histograms. The 12-DAP samples represent whole kernels.
DISCUSSION
Expression of heat shock proteins at specific developmental stages has been observed in plants for several of the known Hsps (for review, see Vierling, 1991; Winter and Sinibaldi, 1991), suggesting that the chaperone function associated with many of these proteins is required even under non-stress conditions. That expression of many Hsps is induced during specific stages of development, e.g. during embryogenesis, indicates that the requirement for Hsps is organ specific and perhaps stage specific. In this study, we have examined how Hsp101 expression in maize is regulated during development and following a heat stress.
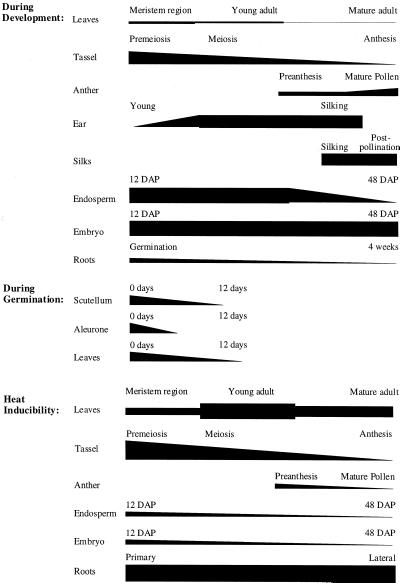
Our results can be integrated into a whole-plant model of Hsp101 expression during development and following a heat stress (Fig. 11). The initial high levels of Hsp101 present in the embryonic shoot decrease within days of the emergence of the shoot. Under non-stress conditions, Hsp101 protein is present in the vegetative meristematic region at a low to moderate level and declines during subsequent leaf development. The heat inducibility of Hsp101 expression also declines somewhat during leaf expansion. Hsp101 protein is abundant in the young male inflorescence and is highly heat inducible, whereas its level and heat inducibility decline with further development of the tassel until it is not heat responsive at all by anthesis (Fig. 11). The level of Hsp101 in the developing ear increases during its early development and is present in silks to a similar level, both prior to and up to 1 week following fertilization. Hsp101 is present at a relatively constant level during the growth and development of the embryo, whereas in the endosperm, Hsp101 is concentrated in the aleurone and subaleurone regions as suggested by in situ RNA localization analysis. Although the level of Hsp101 increases in the young ear following a heat stress, expression of Hsp101 in the endosperm and embryo is not heat responsive. A similar lack in the heat induction of Hsps, including Hsp70, Hsp40, and small Mr (SMW) Hsps, has been observed in developing wheat embryos (Helm and Abernethy, 1990).
Figure 11.
Summary of the relative expression of Hsp101 protein in maize during development, germination, and following a heat stress. The change in the level of Hsp101 protein during the development of those organs analyzed in Figures 4 through 7 and 9 are illustrated where changes in the thickness of a bar represents an estimate of the degree of change in the level of Hsp101.
Hsp101 protein is present at a low but detectable level in non-stressed primary roots and at a higher level in lateral roots, mostly in the root tip, which also exhibited a strong heat response. In situ RNA localization analysis revealed that Hsp101 mRNA is detectable in the CL of non-stressed roots and, following a heat stress, accumulates to a higher level in this tissue (and the QC) than observed for any other cell type in the root. It is interesting to note that the meristematic cells comprising the CL are known to have the highest rate of cell division in the root (Esau, 1977), suggesting that heat stress may be most detrimental to rapidly dividing cells.
Assuming that the level of Hsp101 in a tissue reflects the need for the protein, these observations suggest those organs involved in supporting growth of the germ cells (tassel/anthers and ear), those of the kernel (endosperm and embryo), and those that generate new aerial organs (the shoot and floral meristematic regions) require the highest level of Hsp101, whereas other organs involved in the general growth of the plant, e.g. leaves and roots, do not require a high level of Hsp101 under non-stressed conditions. Given the abundance of Hsp101 in the developing tassel, the amount of Hsp101 in mature pollen is surprisingly low and is not heat inducible. The level of Hsp101 protein in mature pollen appears to decrease following a heat stress, an observation that has also been made for some SMW Hsps under similar conditions in maize pollen (Magnard et al., 1996). The inability to maintain Hsp101 protein levels following a heat stress cannot be a consequence of a reduction in Hsp101 mRNA because this remains unaltered by the stress treatment.
Our observation that Hsp101 protein is present at a moderate to low level in anthers and mature pollen is consistent with the finding that several other Hsps, such as Hsp90, Hsp70, Hsp60, and some SMW Hsps, are expressed during the early stages of pollen development (Marrs et al., 1993; Magnard et al., 1996). Moreover, the failure of mature and germinating maize pollen to mount a heat stress response has been observed for other Hsps (Cooper et al., 1984; Hopf et al., 1992; Magnard et al., 1996) and correlates with its pronounced loss of viability when exposed to elevated temperatures (Herrero and Johnson, 1980; Schoper et al., 1986; Mitchell and Petolino, 1988; Dupuis and Dumas, 1990). The observation that maize pollen progressively loses its ability to induce Hsp synthesis during its development (Frova et al., 1989) is consistent with the progressive loss of heat induction of Hsp101, suggesting that the regulation of Hsp101 expression in pollen is similar to that for Hsps in general. These observations suggest that the developmental expression of Hsp101 and other Hsps in mature pollen is insufficient to meet the demand following a heat stress. In contrast, the higher level of expression of Hsp101 protein in silks prior to and following fertilization and in the developing ear correlates with the high degree of thermotolerance reported for these tissues (Schoper et al., 1986; Mitchell and Petolino, 1988).
In several organs, alterations in the amount of Hsp101 mRNA did not correlate with changes in the level of Hsp101 protein. First, no heat-mediated increase in the level of Hsp101 mRNA was observed in husk leaves despite a substantial increase in Hsp101 protein observed following a heat stress. Second, the substantial heat-mediated increase in Hsp101 protein observed in the tassel at the premeiosis stage of development or in expanding foliar leaves was not accompanied by a similar increase in transcript abundance. Third, although no increase in Hsp101 protein was observed following a heat stress in the tassel at anthesis, the level of Hsp101 transcript did increase. Fourth, the level of Hsp101 transcript is higher in lateral roots than in primary roots, whereas the level of Hsp101 protein is similar in both. These discrepancies between mRNA and protein levels may indicate developmental or stress-related changes in the translational efficiency of Hsp101 mRNA such that the mRNA is more efficiently translated in husk leaves, expanding foliar leaves, or in young tassels when heat-stressed, which would facilitate rapid synthesis of Hsp101 protein and minimize any delay associated with transcriptional induction. Preferential translation of Hsp mRNAs has been suggested to account for an increase in Hsp protein without a corresponding increase in Hsp mRNA in heat-treated, imbibed wheat embryos (Helm and Abernethy, 1990). However, discrepancies between Hsp101 mRNA and protein levels could also be a result of changes in protein stability if, for example, Hsp101 protein is rapidly stabilized in leaves following a heat stress. Given that the heat treatment in the present study was only for 1 h, this would require that the half-life of Hsp101 protein be substantially less than 1 h in non-stressed tissues to explain the large increase in the steady-state level of protein following the heat stress. Regardless of the basis for the observed discrepancies between mRNA and protein expression, they indicate that measuring protein amounts is essential for an accurate determination of Hsp101 in an organ.
MATERIALS AND METHODS
cDNA Library Screening
A cDNA library made from developing maize (Zea mays) endosperm was provided by Dr. Brian Larkins (University of Arizona, Tucson) and a full-length maize Hsp101 cDNA obtained by screening the library using the previously isolated wheat (Triticum aestivum) Hsp101-1 cDNA (Wells et al., 1998). The full-length rice (Oryza sativa) HSP101 cDNA was obtained following screening the available rice expressed sequence tag database using the sequence of wheat HSP101. These materials will be made available upon request.
Plasmid Construction and Thermotolerance Assay
The tobacco (Nicotiana tabacum), wheat, maize, and rice Hsp101 cDNAs or yeast (Saccharomyces cerevisiae) Hsp104 were introduced into the yeast expression vectors, pYX222 or pYX232 (Novagen, Madison, WI), in which HSP101 expression is controlled by the triose phosphate isomerase (TPI) promoter. Each construct was introduced into SL304A (leu2-3, 112 trp1-1 ura3-1 ade2-1 his3-11, 15 lys2Δ can1-100 hsp104::LEU2; Schirmer et al., 1994), a Δhsp104 yeast mutant (generously provided by Dr. Susan Lindquist, University of Chicago) using the polyethylene glycol/LiCl method as described (Hill et al., 1991; Gietz et al., 1992) and grown in synthetic dextrose medium containing the appropriate supplements. The yeast were diluted to an optical density of 0.6 and complementation of Hsp104 function in SL304A was determined in a thermotolerance assay as described (Sanchez and Lindquist, 1990; Parsell et al., 1991).
Western Analysis
Inbred B73 was grown in the greenhouse during the winter to avoid heat stress that may occur in field-grown maize. For heat treatments, isolated plant organs were incubated in a 41oC water bath for 1 h and frozen in liquid nitrogen prior to storage at −80°C. Kernels received similar treatment except they were intact during the heat treatment and then dissected into endosperm and embryo. For the seedling studies, kernels were germinated in vermiculite, the organs dissected at the indicated times, and stored at −80°C. For seedlings, the tissues were ground in liquid nitrogen re-suspended in aqueous buffer {50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.5, 120 mm KOAc, 5 mm MgOAc, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 1 μg mL−1 leupeptin, 1 μg mL−1 pepstatin, and 1 mm EDTA}, the cell debris pelleted, and the protein concentration determined as described (Bradford, 1976). Five micrograms of soluble protein was resolved on a 10% (w/v) SDS-PAGE gel and transferred to nitrocellulose membrane. The other tissues were ground in liquid nitrogen, extracted with trichloroacetic acid/acetone, the precipitated protein re-suspended in urea, and 20 μg of soluble protein was resolved on an 8% (w/v) SDS-PAGE gel and transferred to nitrocellulose membrane.
Anti-Hsp101 antiserum that was raised against wheat Hsp101 protein was described previously (Tanguay and Gallie, 1996) as was that for wheat eIFiso4G (Browning et al., 1987). The antiserum used to detect yeast Hsp104 was raised against the recombinant protein in rabbits. For western analysis, a membrane containing the protein of interest was blocked for 30 min in Tween 20/phosphate-buffered saline (TPBS) (0.1% [w/v] Tween 20, 13.7 mm NaCl, 0.27 mm KCl, 1 mm Na2HPO4, and 0.14 mm KH2PO4) with 5% (w/v) reconstituted dry milk and incubated with anti-wheat HSP101, anti-yeast Hsp104, or anti-eIFiso4G antiserum diluted (1:500 to 1:2,000) in TPBS with 1% (w/v) milk for 1.5 h. The blots were then washed with TPBS, incubated with goat anti-rabbit-horseradish peroxidase antibody (Southern Biotechnology, Birmingham, AL) diluted 1:5,000 (v/v) to 1:10,000 (v/v) for 1 h, and HSP101 detected using chemilumensence (Amersham Corporation, Piscataway, NY). Yeast extracts were prepared by boiling 6 × 106 cells in 2× SDS-loading buffer, the protein resolved on a 10% (w/v) SDS-PAGE gel, and Hsp101 measured by western analysis as described above.
Northern Analysis
Total RNA was extracted from the same tissues used for the western analysis as described (Chomczynski and Sacchi, 1987) and poly(A+) mRNA isolated using binding to oligo dT resin. The RNA was resolved on a denaturing formaldehyde-agarose gel, followed by northern transfer to nylon membrane, and probed with in vitro-synthesized, radiolabeled maize anti-Hsp101 or maize anti-tubulin RNA. The antisense strand of maize Hsp101 or anti-tubulin probes were synthesized in vitro as previously described (Yisraeli and Melton, 1989) using 40 mm Tris-HCl, pH 7.5, 6 mm MgCl2, 100 μg mL−1 bovine serum albumin, 0.5 mm each of ATP, UTP, GTP, and 70 μC 32P-CTP, 10 mm dithiothreitol, 0.3 units μL−1 RNasin (Promega, Madison, WI), and 0.5 units μL−1 T7 RNA polymerase.
RNA in Situ Localization
RNA in situ localization was carried out as described previously (Langdale, 1994) with modifications. Control or heat-stressed (41oC for 1 h) roots (10–20 mm long including the root tip) or developing kernels were placed into formalin, acetic acid, and alcohol (50% [v/v] ethanol, 10% [v/v] formalin, and 5% [v/v] acetic acid), vacuum infiltrated, and stored for 2 d at 4oC. The fixative was replaced with 70% (v/v) ethanol and the samples dehydrated through an ethanol series (85%, 95%, and 100% [v/v]) at 1-d intervals at 4oC. Ethanol was replaced with Hemo-De through a graded series (2 h 50% [v/v] ethanol: 50% [v/v] Hemo-De [Fisher, Pittsburgh] and three treatments in 100% [v/v] Hemo-De for 2 h). Samples were then infiltrated in increasing concentrations of Paraplast Plus, embedded in 100% (v/v) Paraplast Plus, sectioned on a rotary microtome (15 μm thick), and fixed on Probe-On-Plus slides (Fisher). Sections are treated as described by Jackson (1991) with modifications. Sections were deparaffinized in 100% (v/v) Hemo-De, rehydrated through an ethanol series, equilibrated in phosphate-buffered saline, deproteinized with pronase, treated with Gly, and washed twice in phosphate-buffered saline. Sections were post-fixed with 4% (w/v) paraformaldehyde, acetylated with acetic anhydride, washed, and finally dehydrated through an ethanol series. For RNA in situ hybridization, sense or antisense maize Hsp101 RNA was denatured at 80oC, added to hybridization solution (0.3 m NaCl, 10 mm Tris-HCl, pH 6.8, 10 mm NaHPO4, 5 mm EDTA, 50% [w/v] formamide, 10% [w/v] dextran sulfate, 1× Denhardts, and 1 mg mL−1 tRNA), and applied to the slide for overnight hybridization at 55oC. The sections were washed, treated with RNase, blocked (using 1.0% [w/v] Boehringer Block, Boehringer, Indianapolis), and incubated with anti-digoxigenin antibody. The sections were washed, covered with an nitroblue tetrazolium substrate solution, and developed in the dark for 1 to 3 d until a signal was visible.
ACKNOWLEDGMENTS
The authors thank Dr. Susan Lindquist for SL304A and the yeast Hsp104 gene, Dr. Brian Larkins for the maize cDNA library, Dr. Caroline Siflow for the tubulin cDNA, Dr. Karen Browning for the eIFiso4G antiserum, and Dr. Patricia Springer for the generous use of the microtome and microscope for the RNA localization studies.
Footnotes
This work was supported by the U.S. Department of Agriculture (grant no. NRICGP 00–35301–9086) and by the National Science Foundation (grant no. MCB–9816657).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010160.
LITERATURE CITED
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browning KS, Lax SR, Ravel JM. Identification of two messenger RNA cap binding proteins in wheat germ: evidence that the 28-kDa subunit of eIF-4B and the 26-kDa subunit of eIF-4F are antigenically distinct polypeptides. J Biol Chem. 1987;262:11228–11232. [PubMed] [Google Scholar]
- Caliebe A, Soill J. News in chloroplast protein import. Plant Mol Biol. 1999;39:641–645. doi: 10.1023/a:1006170321840. [DOI] [PubMed] [Google Scholar]
- Caplan AJ. Hsp90's secrets unfold: new insights from structural and functional studies. Trends Cell Biol. 1999;9:262–268. doi: 10.1016/s0962-8924(99)01580-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper P, Ho THD, Hauptmann RM. Tissue specificity of the heat shock response in maize. Plant Physiol. 1984;75:431–441. doi: 10.1104/pp.75.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis I, Dumas C. Influence of temperature stress on in vitro fertilization and heat shock protein synthesis in maize (Zea mays L.) reproductive tissues. Plant Physiol. 1990;94:665–670. doi: 10.1104/pp.94.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. Anatomy of Seed Plants. New York: John Wiley and Sons; 1977. p. 231. [Google Scholar]
- Frova C, Taramino G, Binelli G. Heat-shock proteins during pollen development in maize. Dev Genet. 1989;10:324–332. [Google Scholar]
- Gallie DR, Le H, Tanguay RL, Browning KS. Translation initiation factors are differentially regulated in cereals during development and following heat shock. Plant J. 1998;14:715–722. [Google Scholar]
- Gietz D, St Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice G, Sconzo G, Roccheri MC. Studies on heat shock proteins in sea urchin development. Dev Growth Differ. 1999;41:375–380. doi: 10.1046/j.1440-169x.1999.00450.x. [DOI] [PubMed] [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ, Ohan N, Tam Y, Ali A. Heat shock protein gene expression during Xenopus development. Cell Mol Life Sci. 1997;53:114–121. doi: 10.1007/PL00000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KW, Abernethy RH. Heat shock proteins and their mRNAs in dry and early imbibing embryos of wheat. Plant Physiol. 1990;93:1626–1633. doi: 10.1104/pp.93.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MP, Johnson RR. High temperature stress and pollen viability of maize. Crop Sci. 1980;20:796–800. [Google Scholar]
- Hill J, Donald KA, Griffiths DE. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991;19:5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA. 2000;97:4392–4397. doi: 10.1073/pnas.97.8.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf N, Plesofsky-Vig N, Brambl R. The heat shock response of pollen and other tissues of maize. Plant Mol Biol. 1992;19:623–630. doi: 10.1007/BF00026788. [DOI] [PubMed] [Google Scholar]
- Jackson D. In situ hybridization in plants. In: Bowles DJ, Gurr SJ, McPherson M, editors. Molecular Plant Pathology: A Practical Approach. Vol. 1. Oxford: IRC Press; 1991. pp. 163–174. [Google Scholar]
- Krone PH, Sass JB, Lele Z. Heat shock protein gene expression during embryonic development of the zebrafish. Cell Mol Life Sci. 1997;53:22–129. doi: 10.1007/PL00000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA. In situ hybridization. In: Freeling M, Walbot V, editors. The Maize Handbook. New York: Springer-Verlag; 1994. pp. 165–180. [Google Scholar]
- Lee Y-RJ, Nagao RT, Key JL. A soybean 101-kD heat shock protein complements a yeast HSP104 deletion mutant in acquiring thermotolerance. Plant Cell. 1994;6:1889–1897. doi: 10.1105/tpc.6.12.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Ling J, Wells DR, Tanguay RL, Dickey LF, Thompson WF, Gallie DR. Heat shock protein HSP101 binds to the Fed-1 internal light regulatory element and mediates its high translational activity. Plant Cell. 2000;12:1213–1228. doi: 10.1105/tpc.12.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft JC, Dix DJ. Hsp70 expression and function during embryogenesis. Cell Stress Chaperones. 1999;4:162–170. doi: 10.1379/1466-1268(1999)004<0162:heafde>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnard J-L, Vergne P, Dumas C. Complexity and genetic variability of heat-shock protein expression in isolated maize microspores. Plant Physiol. 1996;111:1085–1096. doi: 10.1104/pp.111.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA, Casey ES, Capitant SA, Bouchard RA, Dietrich PS, Mettler IJ, Sinibaldi RM. Characterization of two maize HSP90 heat shock protein genes: expression during heat shock, embryogenesis, and pollen development. Dev Genet. 1993;14:27–41. doi: 10.1002/dvg.1020140105. [DOI] [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay RM. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53:104–113. doi: 10.1007/PL00000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk JA. Protein folding in the plant cell. Plant Physiol. 1999;121:695–703. doi: 10.1104/pp.121.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Omura T. Cytoplasmic chaperones in precursor targeting to mitochondria: the role of MSF and hsp70. Trends Cell Biol. 1996;6:104–108. doi: 10.1016/0962-8924(96)81000-2. [DOI] [PubMed] [Google Scholar]
- Mitchell JC, Petolino JF. Heat stress effects on isolated reproductive organs of maize. J Plant Physiol. 1988;133:625–628. [Google Scholar]
- Nielsen E, Akita M, Davila-Aponte J, Keegstra K. Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J. 1997;16:935–946. doi: 10.1093/emboj/16.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Sotelo J, Kannan KB, Martinez LM, Segal C. Characterization of a maize heat-shock protein 101 gene, HSP101, encoding a ClpB/Hsp100 protein homologue. Gene. 1999;230:187–195. doi: 10.1016/s0378-1119(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Pareek A, Singla SL, Grover A. Immunological evidence for accumulation of two high-molecular-weight (104 and 90 kDa) HSPs in response to different stresses in rice and in response to high temperature stress in diverse plant genera. Plant Mol Biol. 1995;29:293–301. doi: 10.1007/BF00043653. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Kowal AS, Singer MA, Lindquist S. Protein disaggregation mediated by heat-chock protein Hsp104. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Ann Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Sanchez Y, Stitzel JD, Lindquist S. Hsp104 is a highly conserved protein with two essential nucleotide-binding sites. Nature. 1991;353:270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell. 2000;12:479–92. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989;59:591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Lindquist S. HSP104 required for induced thermotolerance. Science. 1990;248:1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Glover JR, Singer MA, Lindquist S. HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci. 1996;21:289–296. [PubMed] [Google Scholar]
- Schirmer EC, Lindquist S, Vierling E. An Arabidopsis heat shock protein complements a thermotolerance defect in yeast. Plant Cell. 1994;6:1899–1909. doi: 10.1105/tpc.6.12.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoper JB, Lambert RJ, Vasilas BL. Maize pollen viability and ear receptivity under water and high temperature stress. Crop Sci. 1986;26:1029–1033. [Google Scholar]
- Singla SL, Pareek A, Grover A. Yeast HSP 104 homologue rice HSP 110 is developmentally- and stress-regulated. Plant Sci. 1997;125:211–219. [Google Scholar]
- Singla SL, Pareek A, Kush AK, Grover A. Distribution patterns of 104 kDa stress-associated protein in rice. Plant Mol Biol. 1998;37:911–919. doi: 10.1023/a:1006099715375. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Gallie DR. Isolation and characterization of the 102-kilodalton RNA-binding protein that binds to the 5′ and 3′ translational enhancers of tobacco mosaic virus RNA. J Biol Chem. 1996;271:14316–14322. doi: 10.1074/jbc.271.24.14316. [DOI] [PubMed] [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E. Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol. 1996;112:747–757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DR, Tanguay RL, Le H, Gallie DR. HSP101 functions as a specific translational regulatory protein whose activity is regulated by nutrient status. Genes Dev. 1998;12:3236–3251. doi: 10.1101/gad.12.20.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Sinibaldi R. The expression of heat shock protein and cognate genes during plant development. In: Nover L, editor. Results and Problems in Cell Differentiation: Heat Shock and Development. Vol. 17. Berlin: Hightower, Springer-Verlag; 1991. pp. 85–105. [DOI] [PubMed] [Google Scholar]
- Vierling E. The heat shock response in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Yisraeli JK, Melton DA. Synthesis of long, capped transcripts in vitro by SP6 and T7 RNA polymerases. Methods Enzymol. 1989;180:42–50. doi: 10.1016/0076-6879(89)80090-4. [DOI] [PubMed] [Google Scholar]