Abstract
Methionine (Met) and threonine (Thr) are members of the aspartate family of amino acids. In plants, their biosynthetic pathways diverge at the level of O-phosphohomo-serine (Ser). The enzymes cystathionine gamma-synthase and Thr synthase (TS) compete for the common substrate O-phosphohomo-Ser with the notable feature that plant TS is activated through S-adenosyl-Met, a metabolite derived from Met. To investigate the regulation of this branch point, we engineered TS antisense potato (Solanum tuberosum cv Désirée) plants using the constitutive cauliflower mosaic virus 35S promoter. In leaf tissues, these transgenics exhibit a reduction of TS activity down to 6% of wild-type levels. Thr levels are reduced to 45% wild-type controls, whereas Met levels increase up to 239-fold depending on the transgenic line and environmental conditions. Increased levels of homo-Ser and homo-cysteine indicate increased carbon allocation into the aspartate pathway. In contrast to findings in Arabidopsis, increased Met content has no detectable effect on mRNA or protein levels or on the enzymatic activity of cystathionine gamma-synthase in potato. Tubers of TS antisense potato plants contain a Met level increased by a factor of 30 and no reduction in Thr. These plants offer a major biotechnological advance toward the development of crop plants with improved nutritional quality.
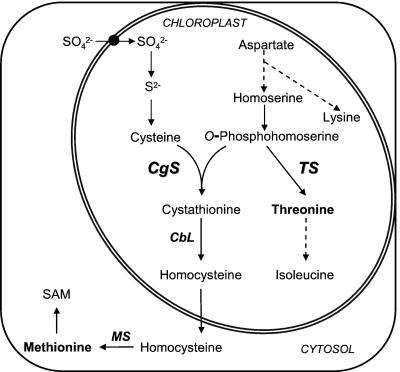
Thr, Lys, Met, and iso-Leu, as members of the Asp family of amino acids, are synthesized via a branched pathway (Fig. 1) with complex regulatory control circuits (Bryan, 1980; Giovanelli et al., 1980; Azevedo et al., 1997; Matthews, 1999). Control of synthesis is exerted through feedback inhibition of specific isoforms of Asp kinase early in the pathway. These isoforms are sensitive to changes in Thr, Lys, or Lys in conjunction with changes in S-adenosyl-met (SAM) levels (Galili, 1995). The diverting branches of Lys and Ile biosynthesis are controlled additionally through feedback inhibition of the first enzyme of each branch, dihydrodipicolinate synthase and Thr deaminase, respectively (Bryan, 1980). In plants, the branch point intermediate of Thr and Met synthesis is O-phosphohomo-Ser (OPHS), which represents the common substrate for both Thr synthase (TS) and cystathionine-gamma synthase (CgS; Fig. 1). OPHS is either directly converted to Thr by TS, or, in a three-step mechanism, to Met through condensation of Cys and OPHS to cystathionine, which is subsequently converted to homo-Cys and then Met (Anderson, 1990; Hell, 1997; Ravanel et al., 1998; Matthews, 1999). The central position of OPHS in plants is different from other organisms able to synthesize Met and Thr, such as bacteria and yeasts, in which homo-Ser, the immediate precursor of OPHS, is the last common substrate (Bryan, 1980; Giovanelli et al., 1980). Thr and Met are either incorporated into proteins or serve as precursors for Ile or SAM biosynthesis, respectively. SAM is one of the central metabolites in plants involved in methylation reactions and polyamine, ethylene, and biotine biosynthesis (Ravanel et al., 1998).
Figure 1.
Biosynthetic pathway of the Asp family of amino acids and of the sulfur assimilation and Cys biosynthetic pathway in plants. Met biosynthesis comprises two biosynthetic domains: sulfur assimilation reduction, and Cys biosynthesis and one branch of the Asp amino acid family biosynthetic pathway. A dashed line represents parts of the pathway in which detailed descriptions of the enzymatic steps have been omitted. Most of the pathway is localized in the chloroplast, as indicated, but the final step of Met biosynthesis takes place in the cytosol. Met serves as a precursor for protein and SAM biosynthesis.
The fact that plant TS and CgS are branch point enzymes competing for the same substrate demands the effective regulation of the respective enzymatic activities. There is no evidence suggesting the occurrence of feedback inhibition of CgS activity by either Met or SAM (Ravanel et al., 1998). However, in Lemna paucicostata and Arabidopsis, the enzymatic activity or the stability of the CgS mRNA, respectively, seems to be regulated by Met levels. Feeding studies with Met in L. paucicostata lead to decreased enzymatic activity (Thompson et al., 1982; Giovanelli et al., 1985). The identification of an Arabidopsis mutant, mto1, in which a mutation of the CgS gene increases the stability of the cognate mRNA in the presence of increased levels of Met, further supported the occurrence of posttranscriptional CgS regulation (Inaba et al., 1994; Chiba et al., 1999). This mutation leads to up to 40-fold increases in Met content in the mto1 mutant during certain developmental stages.
Differences in TS mRNA levels were observed among several different potato (Solanum tuberosum cv Désirée) plant organs; abundance in flowers, leaves, and roots and scarcity in stems and tubers (Casazza et al., 2000). Neither precursors (Suc, oxalacetate, homo-Ser, and OPHS), nor reaction products (phosphate and Thr), nor nitrogenous compounds (Gln and Asn) have any effect on expression when fed to detached leaves, essentially excluding metabolic regulation of TS transcription (Casazza et al., 2000). Whereas fungal and bacterial TSs are not activated by SAM levels (Bryan, 1980), the enzymatic activity of plant TS is activated by low concentrations of SAM, the product of the competing pathway, and is inhibited by Cys (Thoen et al., 1978; Giovanelli et al., 1984, 1985; Curien et al., 1996). Thus, increasing levels of Met and, hence, SAM increase TS activity. Under these conditions, the Km values of TS for OPHS have been shown to be 250- to 500-fold lower as compared with the competing enzyme, CgS, thus favoring carbon flow into Thr biosynthesis in preference to Met synthesis (Madison and Thompson, 1976; Curien et al., 1996, 1998; Laber et al., 1999). Transgenic tobacco (Nicotiana tabacum) plants expressing an SAM-insensitive Escherichia coli TS yielded a 5-fold increase of Thr (Muhitch, 1997), whereas the Arabidopsis mutant mto2, which displays a reduced TS activity, exhibits a 16-fold decrease in Thr content (Bartlem et al., 2000).
To determine in vivo the physiological relevance of TS and its role in controlling the competing biosynthetic pathways of Thr and Met, we reduced TS mRNA availability via the antisense inhibition of a previously cloned endogenous potato TS (Casazza et al., 2000). The data garnered from potatoes expressing this construct demonstrate that TS is a major control point for Met biosynthesis in potato. Furthermore, these findings allow the development of a strategy to increase Met content and thus improve the nutritional quality of plants.
RESULTS
Engineering Plants Inhibited in TS Expression
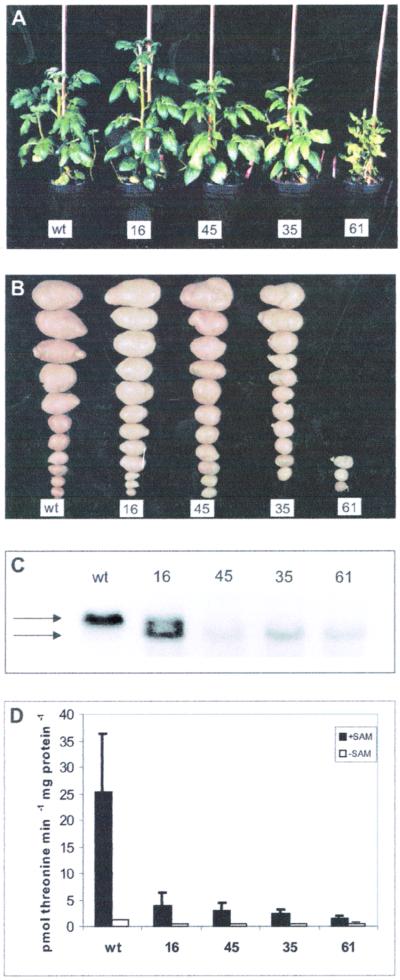
Transgenic potato plants were generated containing an antisense RNA coding construct under the control of a constitutive promoter directed against the endogenous TS (Casazza et al., 2000). Sixty independent transgenic plant lines were regenerated and selected based on reduced TS steady-state mRNA levels (data not shown). Four lines were chosen for detailed analysis based on their reduced transcript levels and differences in phenotypes. These lines are representative examples of weak (16), medium (45 and 35), and strong (61) inhibition. All transgenic plants (lines 16, 45, 35, and 61) expressed the truncated antisense transcript, which is 300 nucleotides shorter than the sense transcript (Fig. 2C). The mRNA steady-state transcript for TS is clearly detectable in wild-type plant leaf tissue but not in three (45, 35, and 61) of the four transgenic lines that show only the antisense RNA signal. In line 16, a weak hybridization signal from the intrinsic TS mRNA is detectable together with the antisense RNA signal.
Figure 2.
Phenotype, RNA-blot analysis, and TS activity of TS antisense compared with wild-type potato plants. A, Phenotype of transgenic lines (16, 45, 35, and 61) and control plants (wt). Plant 16 displays essentially a wild-type appearance, whereas lines 45 and 35 display weak symptoms including slight chlorosis along leaf nervature, whereas line 61 shows growth retardation, leaf chlorosis, and alterations in leaf morphology. B, TS antisense inhibition affects tuber development parallel with increasing phenotypical alteration of the green matter. A reduction in tuber yield due to decreases in size and number of tubers was observed. C, RNA-blot analysis of leaf RNA of transgenic and wild-type plants for the TS transcript. The size of the sense-transcript is 1.7 kb (upper arrow); the antisense-transcript has a length of 1.4 kb (lower arrow). Wild-type plants (wt) only show the presence of the sense-RNA of TS. In transgenic plants 45, 35, and 61, the presence of the antisense RNA is detected, whereas the sense messenger is absent. In plant 16, both the antisense and the sense RNA are visible. D, Determination of TS activity. 14C-Labeled OPHS was used as substrate to determine the enzyme activity of TS in wild-type and transgenic plants in the presence of the inductor SAM (white bars) and in its absence (black bars). sds of three samples per determination are indicated as error bars.
Greenhouse-grown plant material was evaluated and scored based on macroscopic phenotypic alterations (Fig. 2A). Transgenic line 16 was phenotypically indistinguishable from wild-type plants, lines 45 and 35 exhibited only marginal alterations such as slight growth retardation and mild chlorosis along leaf nervature, and line 61, the most strongly inhibited line, shows a more drastic phenotype characterized by severe growth retardation, strong chlorosis, and an acute reduction in tuber yield (Fig. 2B).
Determination of Potato TS Enzyme Activity
TS activity was determined in the transgenic lines 16, 45, 35, and 61 and compared with controls based on desalted plant leaf extracts using radioactively labeled [14C]OPHS as substrate and monitoring the formation of Thr both in the absence and presence of SAM, the activator of TS. In the presence of SAM, wild-type extracts revealed a TS activity of 25.3 pmol per minute and mg protein. For the antisense lines, the activities were 16% (line 16), 12% (line 45), 10% (line 35), and 6% (line 61; Fig. 2D). When SAM was omitted from the reaction mixture, TS activity in wild-type potato plants was reduced about 20-fold to 4.8% as compared with the activity measured in the presence of SAM. TS activity in the transgenic lines was 2.0% (line 16), 1.7% (line 45), 1.9% (line 35), and 2.3% (line 61) of TS activity in wild-type plants in the presence of SAM. The relative decrease of TS in the transgenic lines as compared with wild type is clearly smaller in the absence of SAM than it is in the presence of SAM. This observation might indicate the presence of a higher degree of basal activity in the transgenic lines. Perhaps this increase compensates in part for the loss due to reduced expression.
Effect of TS Antisense Inhibition on Metabolite Levels in Source Leaves
It is believed that the leaf is the main organ of amino acid biosynthesis in plants (Wallsgrove et al., 1983; Ravanel et al., 1998). To address the question whether or not the down-regulation of TS through antisense inhibition results in changes in amino acid content, source leaf extracts from TS antisense plants were analyzed using gas chromatography (GC)/mass spectrometry-based technology (Roessner et al., 2000, 2001). The amount of free Thr in lines 16, 45, 35, and 61 was reduced to 69%, 56%, 46%, and 45%, respectively, of the wild-type level, which is in agreement with the block in the Thr pathway occurring at the level of the TS. To score the effect of TS inhibition on the competing Met pathway, three successive sets of plants (set I–III) were grown in the greenhouse and analyzed. The level of free Met was increased by between about 2- and 240-fold in all four transgenic lines (Table I). Although variations in the Met levels of individual plants within the same experiment and among the three sets of experiments were observed, it is important to note that the significant increases in Met in lines 16, 45, and 35 were not accompanied by severe phenotypic changes under greenhouse conditions.
Table I.
Relative Met content of TS antisense plants compared with wild type
| Experiment | Wild Type | 16 | 45 | 35 | 61 |
|---|---|---|---|---|---|
| Set I | 1 | 91 | 192 | 222 | 181 |
| Set II | 1 | 2 | 13 | 53 | 239 |
| Set III | 1 | 2 | 10 | 10 | 16 |
Potato plants were grown in three successive sets of experiments. Relative responses of methionine in leaf extracts of 8-week-old plants were determined in comparison with an internal ribitol standard using GC/mass spectrometry measurements. Due to that fact that absolute Met concentrations were only determined for the third set of plants, the relative response per gram fresh weight has been taken to compare the different plant sets. For each plant set, the Met level in respective wild-type extracts was set as 1 and for extracts of transgenic TS antisense plant lines (16, 45, 35, and 61) the ratio to the wild-type level is presented.
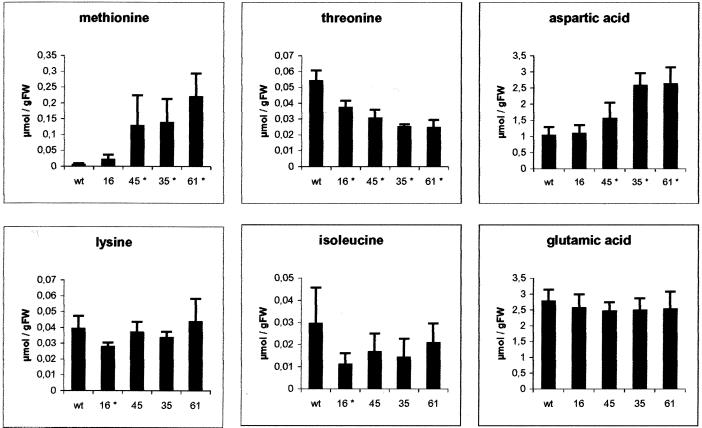
For experimental set III, we performed a detailed metabolite analysis to determine actual metabolite concentrations. The GC/mass spectrometry measurements were calibrated with externally supplied standards (Fig. 3). Met content was determined to be 24, 128, 137, and 220 nmol Met g fresh weight−1 in lines 16, 45, 35, and 61, respectively, which is in vast excess when compared with the low levels of free Met present in wild-type plants (5 nmol g fresh weight−1).
Figure 3.
Determination of leaf metabolite compositions in TS antisense plants. Polar metabolites were extracted from source leaves of 8-week-old control plants (wt) and the TS antisense transgenic lines (five samples each). Metabolites were determined using GC/mass spectrometry. Actual concentrations of Met, Thr, Asp, Ile, Lys, and Glu were determined using external standards for calibration. Statistically significant changes (P < 0.05) are identified with an asterisk.
The GC/mass spectrometry-based analysis of further amino acids and intermediates of Met biosynthesis in source leaves gives credence to the idea that increased Met levels result from the redirection of carbon flow from the Thr to the Met branch (Fig. 3). The small but significant increase observed for Asp indicates the importance of TS for carbon allocation into the Asp pathway. Most other amino acids, including Lys, Ile, Ala, Val, and Glu, did not change significantly in the transgenic lines as compared with wild-type plants. The same holds true for Cys (the second substrate for cystathionine biosynthesis and the source of reduced sulfur) except for line 61 (data not shown). This line shows in general a rather perturbed amino acid profile reflecting major metabolic problems resulting from Thr deficiency. However, the transgenic lines did have elevated concentrations of the pathway intermediates homo-Ser (max of 175-fold) and homo-Cys (max of 46-fold), the direct precursor of Met that is hardly detectable in wild-type plants.
Effect of TS Antisense Inhibition on Metabolite Levels in Tubers
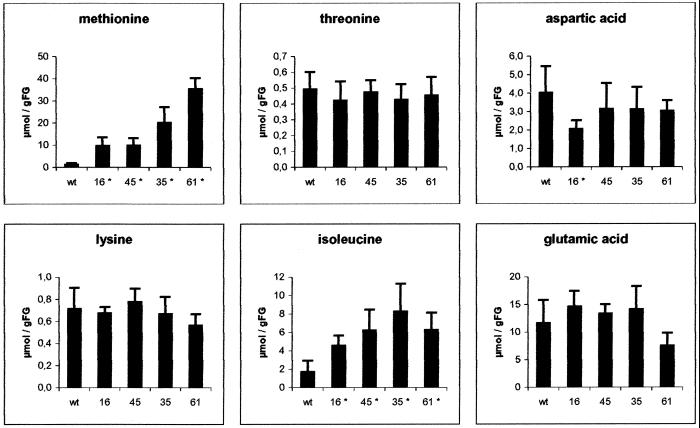
Tubers are potato's major sink tissue. We analyzed the parenchyma tissue of tubers in experimental set III for their amino acid composition using GC/mass spectrometry (Roessner et al., 2000, 2001). In wild-type plants, the amount of free Met is about one order of magnitude higher in tuber tissue (1.2 μmol g fresh weight−1) as compared with leaf tissue of the corresponding plants (0.005 μmol g fresh weight−1). In contrast to the results obtained for leaves, we did not observe a reduction of Thr content in TS antisense tubers; instead, we saw a constant level of free Thr in these plants equivalent to that seen in wild type—about 0.5 μg g fresh weight−1 (Fig. 4). Yet, Met content was increased up to 30-fold above wild-type levels in TS antisense tubers (Fig. 4). Lines 16, 45, 35, and 61 contained 9.7, 9.8, 20.2, and 35.4 μmol Met g fresh weight−1, respectively. Similar to the situation in leaves, Lys contents were not altered, yet Ile levels were increased (e.g. in line 35 by a factor of 5–8.3 μmol g fresh weight−1). Val, Ala (data not shown), and Glu (Fig. 4), amino acids not related to the Asp family, were not altered in these tissues. No increase in Asp was seen in TS antisense tubers, which is consistent with the lack of reduction in Thr levels. It remains to be determined if the observed increases are due to effective import processes or to in situ amino acid biosynthesis.
Figure 4.
Determination of tuber metabolite compositions in TS antisense plants. Polar metabolites were extracted from sink tuber parenchyma tissue of 8-week-old control plants (wt) and the TS antisense transgenic lines (five samples each). Metabolites were determined using GC/mass spectrometry. Actual concentrations of Met, Thr, Asp, Lys, Ile, and Glu were determined using external standards for calibration. Statistically significant changes (P < 0.05) are identified with an asterisk.
Analysis of Met Pathway-Related Genes in TS Antisense Plants
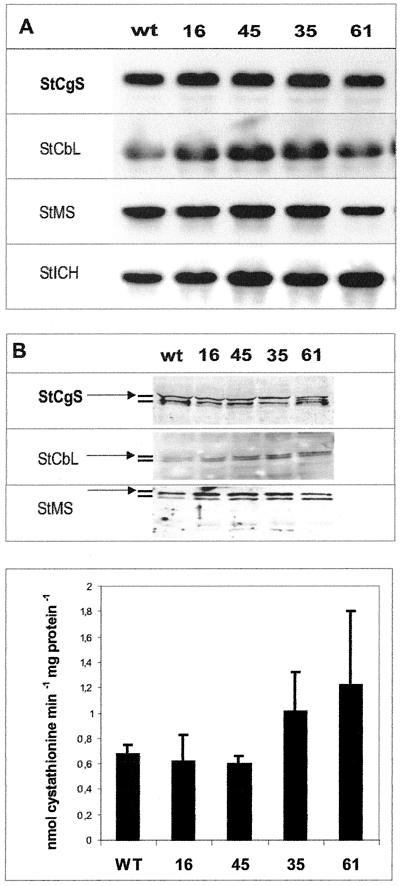
In Arabidopsis and L. paucicostata, elevated levels of Met result in reduced steady-state levels of CgS mRNA or CgS protein, respectively (Thompson et al., 1982; Inaba et al., 1994; Chiba et al., 1999). Because we had TS antisense lines with increased Met levels at hand, we decided to test whether or not the expression of Met biosynthetic genes, i.e. CgS, cystathionine beta-lyase (CbL) and Met synthase (MS), is altered in these lines. RNA-blot analysis using CgS, CbL, and MS as probes did not reveal significant differences in the steady-state RNA levels in leaves of transgenic potato plants when compared with wild-type plants (Fig. 5A). An isocitrate dehydrogenase (ICDH) cDNA clone from potato known to be constitutively expressed in leaf tissue (Fieuw et al., 1995) was used to prove equal loading of the RNA samples (Fig. 5A). Protein-blot analysis of CgS, CbL, and MS using polyclonal antibodies (Maimann et al., 2000) provided no evidence of changes in the respective protein amounts either (Fig. 5B).
Figure 5.
Analysis of Met pathway related genes in TS antisense plants. A, Leaf RNA of 8-week-old control plants (wt) and transgenic plant lines 16, 45, 35, and 61 was extracted and in an RNA-blot experiment hybridized to cDNA probes of potato CgS (StCgS), CbL (StCbL), and MS (StMS). ICDH of potato (ICDH) was used as positive control of expected constitutive expression. B, Protein extracts of plant samples similar to those described in A were subjected to a protein-blot analysis using polyclonal antibodies generated against CgS (StCgS), CbL (StCbL), and Met synthase (StMS). The arrow indicates the position of each of these bands relative to the positions determined in pre-experiments. C, The activity of CgS of leaf extracts of wild type plants (wt) and transgenic lines was determined by supplying OPHS and Cys as substrates and quantifying the product, cystathionine, by HPLC. Five samples per measurement were used and standard deviations are indicated as error bars.
Determination of CgS Enzyme Activity in TS Antisense Plants
Given the fact that the activity of CgS, the enzyme competing with TS for the common substrate OPHS, is of interest with respect to understanding the allocation of metabolites, we determined the activity of CgS in desalted plant extracts. The enzymatic activity of wild-type leaf extracts was determined to be 0.68 nmol cystathionine per minute per mg total protein. The corresponding activities determined for the transgenic lines (16, 45, 35, and 61) are 0.62, 0.60, 1.0, and 1.2 nmol min−1 mg−1, respectively (Fig. 5C). Although this might indicate a slight increase of CgS activity in parallel to decreasing TS activity, the increase was statistically insignificant.
DISCUSSION
OPHS represents the common substrate for both TS and CgS leading to either Thr or Met biosynthesis. In wild-type plants, the ratio of CgS to TS changes transiently and rapidly because of either increasing cellular concentrations of SAM or Cys (Thompson et al., 1982; Ravanel et al., 1998; Bartlem et al., 2000). Furthermore, high levels of orthophosphate and AMP inhibit the activity of L. paucicostata TS (Giovanelli et al., 1986). Therefore, increasing Cys content inhibits TS activity, whereas SAM leads to a higher affinity of TS toward OPHS. Under these conditions, the Km values of TS for OPHS have been shown to be a factor 250 to 500 lower as compared with the competing enzyme, CgS, thus favoring the flow of metabolites into Thr biosynthesis over Met synthesis.
Starting from these models, we postulated that TS, or rather the ratio of TS to CgS, controls Met and Thr synthesis. To test this assumption, transgenic potato plants were constructed that, due to antisense RNA-mediated inhibition, display gradually decreased TS expression at both the transcript and the enzymatic activity level of TS. Analysis of these plants clearly revealed a significant increase in Met in leaves, the factor varying between 2- and 239-fold. This is even more impressive when considering that the increased Met content most likely results in increased SAM concentrations as observed in the mto1 and mto2 mutants of Arabidopsis, which are mutated in the CgS and TS genes, respectively (Inaba et al., 1994; Chiba et al., 1999; Bartlem et al., 2000). Increases in SAM lead, as described above, to the up-regulation of the remaining TS activity. Due to this property of TS, it is likely that, in transgenic potato plants, in situ TS activity is actually higher then the activity measured using in vitro assays with equally added amounts of SAM.
The data described here argue for the central role of the CgS and TS with respect to the flow of carbon skeletons into the Thr and Met paths, respectively, and support the outcome of several previous investigations. Chief among these were the overexpression of an E. coli TS in tobacco plants that yielded a 5-fold increase in Thr (Muhitch, 1997) and the impairment of TS activity in an Arabidopsis mutant, mto2, that resulted in a 16-fold decrease in Thr content, accompanied by 22-fold elevated Met levels (Bartlem et al., 2000).
Metabolite contents of the TS antisense potato plants were analyzed to score for consequences beyond the immediate effects on Met and Thr. The analysis of the amino acid composition of the TS antisense plants correlates to predictions inherent to generally accepted models (Bryan, 1980; Galili; 1995; Azevedo et al., 1997; Matthews, 1999). In leaves, Lys and Ile levels were essentially unaltered, both being effectively controlled by feedback inhibition at the level of the enzymes dihydrodipicolinate synthase and Thr deaminase, respectively, despite the reduction of Thr, the immediate precursor of Ile biosynthesis. Asp and pathway intermediates such as homo-Ser and homo-Cys accumulate in the transgenics, as befits a model in which Thr is reduced to a level insufficient for the feedback inhibition of Thr-sensitive aspartate kinase (AK) and homo-Ser dehydrogenase early in the pathway. Such a situation results in an increased allocation of carbon backbones to Asp amino acid biosynthesis (Karchi et al., 1993; Galili, 1995). Accumulation of the intermediates probably indicates the insufficient activity of homo-Ser kinase, CgS, and MS and therefore the inability to cope with increased substrate supply. In particular, homo-Ser accumulation indicates that CgS may have a lower affinity for OPHS than TS does. It is likely that in wild-type plants, most of the common substrate is channeled toward Thr synthesis, leaving the level of OPHS below the maximal substrate concentration for CgS, whereas in the transgenic plants, CgS activity is pushed to its limits. If this is true, it might also explain why CgS overproduction leads to increases in Met content even in the presence of TS (Chiba et al., 1999). Furthermore, our data correspond to findings indicating that the expression of feedback-insensitive AKs in planta lead to Thr accumulation (2- to 9-fold) and slight increases in Lys and iso-Leu, but not to changes in Met levels in transgenic tobacco leaves (Shaul and Galili, 1992). In a similar manner, Thr accumulation and Lys and Ile increases were observed in Arabidopsis when a desensitized AK was expressed (Heremans and Jacobs, 1995). However, in this case Met accumulated about 2-fold, indicating a different metabolite flow control between Arabidopsis and solanaceous plants.
Furthermore, a potential new relationship between Thr and Asp levels was observed in this study: In leaves, reduced Thr levels corresponded with increased Asp levels, whereas in tubers, constant levels of Thr were concomitant with unaltered Asp concentrations. This result might indicate that a demand for carbon can be conveyed upstream of the main regulatory enzyme, AK, and might hint at a yet unknown regulatory control mechanism for Asp family amino acid biosynthesis.
Although Met accumulates, Cys, the second substrate of CgS (Anderson, 1990; Azevedo et al., 1997; Ravanel et al., 1998; Matthews, 1999), seems to remain constant in TS anitisense plants (data not shown). This could indicate that the sulfur assimilation and reduction pathway, as well as the biosynthesis of Cys (Hell, 1997; Saito, 1999), is flexible enough to cope with the strongly increased demand for reduced sulfur in TS antisense plants. Finally, with respect to Thr, the observation that the transgenic potato plants display a 2 to 7-fold decreased Thr level in their leaves meets expectations, demonstrating nicely that the block set by the antisense RNA has worked. Although this finding is straightforward, the constant Thr levels observed in tubers are more difficult to reconcile. One possible explanation for this result is that, whereas leaves represent the primary location of amino acid and thus Thr biosynthesis, they directly reflect changes in TS activity, tubers, which probably import a large proportion of their amino acids from leaves through phloem transport, may have other means to compensate for this loss (compare with below).
Another interesting observation made in TS antisense plants concerns the influence of Met on CgS. CgS mRNA and CgS activity are reduced in the presence of excess Met in Arabidopsis and L. paucicostata, respectively (Thompson et al., 1982; Inaba et al., 1994; Ravanel et al., 1998; Chiba et al., 1999). Our findings, in contrast, indicate that Met accumulation does not affect expression and protein content of the corresponding biosynthetic genes (CgS, CbL, and MS) of the Met branch in potato. Moreover, the enzyme activity of CgS is not affected at all in transgenic potato plants with increased Met levels and decreased TS activity. The marked difference in CgS response indicates a different mode of regulation in potato as compared with Arabidopsis and L. paucicostata.
Though the observed increase in Met content and some other intermediates of this pathway were consistently observed and proved statistically significant, our data also show that the actual amounts of the respective amino acids show large variations among various leaf samples. This could be due to slight variation in developmental stage among plants of different sets (even among plants of the same age) or microclimatic variation among plant positions within the greenhouse. It should be emphasized, however, that temporal and spatial variations in Met content have also been observed in the Arabidopsis mutants, mto1 and mto2 (Inaba et al., 1994; Bartlem et al., 2000).
The harvested crop and major sink tissue of potato plants is the tuber. The amount of free Met is about one order of magnitude higher in tubers than it is in leaf tissues of wild-type plants. The constitutive antisense mediated down-regulation of TS in potato plants resulted in a further, significant 30-fold increase in tuber Met. It is most interesting that Thr levels in tubers were not reduced. The possibility that TS activity is higher in tubers then in leaves of antisense plants cannot be excluded, but DNA- and RNA-blot analysis of tubers from wild-type potato plants indicate that such activity would be unlikely to result from the expression of a second TS gene (Casazza et al., 2000). Even though antisense inhibition under the control of the cauliflower mosaic virus 35S promoter should be constitutive, differences between transgene expression in tubers and leaves have been previously observed (Höfgen and Willmitzer, 1992; Holtorf et al., 1995). Such differences may contribute to the disparate Thr levels observed in leaves and tubers. The degrees to which these levels are affected by sink tissue import processes and by biosynthesis within the tuber itself remain to be determined. Even so, the concomitant increases in pathway intermediates such as homo-Ser and homo-Cys and the fact that Ile does not accumulate indicate the substantial involvement of in situ biosynthetic processes, at least in leaves.
From an applied perspective, this result is a major achievement for plant breeding (Sun et al., 1992; Tabe and Higgins, 1998; Hesse et al., 2001). Not only is the essential amino acid Met increased in tubers, this increase is not accompanied by the reduction of another essential amino acid, i.e. Thr. And, from a basic research perspective, our findings have led us to propose a model for Met biosynthesis in which the ratio of TS to CgS activity acts as a switch, diverting carbon backbones into Thr synthesis as soon as sufficient Met or Met-derived products, such as SAM, are available. CgS itself acts as a shunt pathway—at least in potato—because neither CgS expression nor activity is affected by Met accumulation.
Therefore, the data presented here provide a general and in all probability, widely applicable biotechnological tool to manipulate crop plants with respect to increase Met content and, hence, nutritional quality. This increase in Met abundance will be best taken advantage of by concomitantly increasing the synthesis of nutritionally valuable sulfur-rich proteins (Tabe and Higgins, 1998; Chakraborty et al., 2000).
MATERIALS AND METHODS
Generation of Transgenic Potato (Solanum tuberosum cv Désirée) Lines
Potato (Saatzucht Lange AG, Bad Schwartau, Germany) TS (Casazza et al., 2000) was cut from pBluescript SK− as a truncated Asp718/XbaI fragment and cloned in its reverse orientation to the promoter into the vector pBinAR-Kan (Höfgen and Willmitzer, 1990) previously cut with Asp718/XbaI to generate an antisense construct for plant transformation. The transformation of potato by Agrobacterium tumefaciens (Rocha-Sosa et al., 1989) using the strain C58C1/pGV2260 (Deblaere et al., 1985) has been carried out as described (Dietze et al., 1995). Transgenic plants were selected on medium containing kanamycin (10 mg L−1) and supplemented with Thr (35 mg L−1) and casein hydrolysate (200 mg L−1). The resulting transgenic plants were planted in soil and grown in the greenhouse under a 16-h-light, 8-h-dark regime at 20°C. Leaf material was screened for reduced expression of TS by RNA-blot analysis. Standard techniques were essentially executed as described in Sambrook et al. (1989).
Plant Cultivation and Metabolite Analysis
Three successive sets of transgenic TS antisense plants and controls, 4 weeks apart, were propagated in tissue culture and transferred to soil. These were termed sets I, II, and III, respectively. The rooted shoots were planted in small pots and cultivated at the phytotron with a light regime of 200 to 250 μmol s−1 m−1, 16 h/8 h under a hood to retain high air humidity. After 2 weeks, the plants were transferred into pots with a diameter of 20 cm and thereafter cultivated in the greenhouse. Sets I and III were grown under a constant light regime of 200 to 250 μmol s−1 m−1 with a light/dark rhythm of 16 h/8 h, whereas set II was grown using moving lights providing variation in light intensity plus additional natural sunlight. Leaf material (about 250 mg per plant) was harvested after approximately 8 weeks at the onset of flowering. Leaf discs were excised from tissues of similar developmental stage. Tuber samples (about 120 mg) were taken from tubers of 5-month-old plants. Sampling took place between 10 and 12 am and plant material was immediately frozen in liquid nitrogen before storage at −80°C. All metabolites were determined using GC/mass spectrometry-based technology. Leaf tissues were ground to a fine powder in liquid nitrogen in a bead mill. Methanol (1,400 μL), ribitol (50 μL; 0.2 mg mL−1 as an internal standard), and distilled, deionized water (50 μL) were added to each sample and successive methanol and chloroform (750 μL) extractions were performed (Maimann et al., 2000). Tuber analysis was performed according to previously published protocols (Roessner et al., 2000, 2001). For the quantification of amino acids, external standards were used and recoveries were determined before analysis.
RNA- and Protein-Blot Analysis
Total potato leaf RNA was prepared according to Logemann et al. (1987). Forty micrograms of total RNA was loaded per lane on denaturing agarose gels (1.2%, w/v) containing 15% (v/v) formaldehyde. Gels were blotted to nylon membranes, hybridized under stringent conditions with specific radioactively-labeled cDNA probes (StTS, full-length cDNA fragment [Casazza et al., 2000]; StCgS, 1.3-kb internal EcoRI fragment [Riedel et al., 1999]; StCbL, 1.3-kB BamHI/SacI fragment, a strand-specific cRNA probe [Riboprobe, Promega GmbH, Mannheim, Germany]; and StMS, full-length cDNA [accession no. AF082893]), and exposed to x-ray film. To compare gene expression of different genes of interest, the membrane was hybridized with a constitutively expressed gene (ICDH) to control gel loading. The level of gene expression was estimated from x-ray films. Protein-blot analysis was performed as described by Maimann et al. (2000) using the polyclonal antibodies described there.
Preparation of [U-14C]OPHS
l-[U-14C]homo-Ser (specific activity 463 MBq mmol−1; Amersham, Braunschweig, Germany) was purified by preparative thin-layer chromatography (methanol:acetic acid:water = 6:3:1, v/v) and converted with Escherichia coli homo-Ser kinase to [U-14C]OPHS according to the protocol of Rognes (1990). Phosphorylation of l-homo-Ser was performed at 37°C, for 4 h in 500 μL of 5 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.5, 1.5 mm ATP, 1.5 mm MgCl, and 1 mm l-[U-14C]homo-Ser. The reaction was started by the addition of 5 units of homo-Ser kinase. The reaction was terminated by addition of 15 μL 1 n HCl and the solution was applied immediately to a cation exchange column (AG 50W, Bio-Rad, Munich). [U-14C]OPHS was eluted with water.
TS Assay
TS assay was adapted from Giovanelli et al. (1984). Total protein was extracted from source leaves: three 8-week-old plants per line, 1 sample each plant. Leaves were immediately frozen in liquid nitrogen and stored at −80°C. Frozen leaf tissue was homogenized at 4°C with a micropestle in 500 μL extraction buffer containing 50 mm HEPES (pH 7.8), 10% (v/v) glycerol, and 20 μm pyridoxal 5′-phosphate (PLP). After centrifugation at 4°C (15,000g, 20 min), the supernatant was desalted via pre-equilibrated NAP-5 columns. Protein concentration was determined according to Bradford (1976). The TS activities of 25 μg desalted protein extracts were determined in radioactive assays in which Thr formation was monitored in a scintillation counter. The enzyme was assayed in 100 mm HEPES (pH 7.8), 5% (v/v) glycerol, 250 μm PLP, 200 μm SAM, 1 μm Na2WO4, and 0.2 μm OPHS in a final volume of 100 μL. Incubation was for 60 min at 30°C. The reaction was terminated by addition of 5 μL 1 n NaOH and 495 μL water. To separate radioactive Thr from radioactive OPHS, the mixture was incubated for 5 min with anion-exchange resin (Bio-Rad AG 1). After two centrifugation steps (15,000g, 2 min), 400 μL of the Thr containing supernatant was measured in 2 mL scintillation cocktail. Controls had either protein omitted or were incubated with heat-denatured protein. Assays were performed with or without SAM to determine the activity with and without induction. To ensure that the radioactivity measured in the mixture after anion-exchange chromatography resulted from Thr and not from homo-Ser, some of the assays were analyzed by thin-layer chromatography. Four hundred microliters of dried assay reactions were diluted in a solution of unlabeled amino acids (20 mm OPHS, 20 mm homo-Ser, and 20 mm Thr). Radioactive and non-radioactive components of the solution were separated (butanol:acetone:diethylamine:water, 10:10:2:5) and amino acids on the thin-layer chromatography plate were stained with ninhydrin before exposure to x-ray film.
CgS Assay
CgS activity was measured as described by Ravanel et al. (1995). Leaf tissues (100 mg) from source leaves of five replicas of 8-week-old plants, one sample each, were collected and immediately frozen in liquid nitrogen. The samples were then kept at −80°C until the assay was performed. Frozen leaf tissue was ground using a micro pestle in 500 μL ice-cold extraction buffer containing 20 mm MOPS [3-(N-morpholino)-propanesulfonic acid]-NaOH (pH 7.5), 2 mm dithiothreitol, 100 μm PLP, 0.1% (v/v) Triton 100, 1 mm EDTA, and 0.2% (w/v) phenylmethylsulfonylfluoride. After two centrifugation steps (14,000g, 15 min, 4°C) the supernatant was desalted using pre-equilibrated NAP-5 columns (Pharmacia, Erlangen, Germany). CgS activity was measured in a 100-μL volume containing 20 mm MOPS-NaOH (pH 7.5), 2 mm dithiothreitol, 0.1 mm PLP, 2 mm l-Cys, 5 mm O-phospho-l-homo-Ser, and 0.2 mm l-α-(aminoethoxyvinyl) Gly. l-α-(aminoethoxyvinyl) Gly is known to act as a specific inhibitor for CbL (Droux et al., 1995), the enzyme catalyzing the subsequent step in Met biosynthesis in plants, and was added to prevent enzymatically formed l-cystathionine from being further converted to l-homo-Cys. Assays were initiated by adding the desalted protein extract (100 μg). After incubating the mixture for 60 min at 30°C, the reaction was stopped by boiling for 5 min and l-cystathionine formation was analyzed by HPLC after derivatization with O-phthaldialdehyde.
ACKNOWLEDGMENTS
We wish to thank the “Greenteam” (Max-Planck-Institute, Golm, Germany), especially Helga Kulka and Katrin Lepa for greenhouse work, Romy Ackermann and Astrid Basner for tissue culture work, and Josef Bergstein for photographical support. We are also grateful to Dr. Karin Köhl for supporting the statistical calculations. We would like to thank Megan McKenzie for editing the manuscript and Stefanie Maimann for providing the idea for Figure 1 and the antibodies for CbL. Dr. Bernd Laber (Aventis, Frankfurt) provided technical advice for setting up the TS assay and provided radioactive substrates and homo-Ser kinase.
Footnotes
This project was partially supported by the European Framework Programme 4 (project grant no. Bio–4CT–97–2182) and by the Max-Planck-Society.
LITERATURE CITED
- Anderson JW. Sulfur Metabolism in Plants. In: Miflin BJ, Lea PJ, editors. The Biochemistry of Plants. Vol. 16. San Diego: Academic Press; 1990. pp. 327–3381. [Google Scholar]
- Azevedo RA, Arruda P, Turner WL, Lea PJ. The biosynthesis and metabolism of the aspartate derived amino acids in higher plants. Phytochem. 1997;46:395–419. doi: 10.1016/s0031-9422(97)00319-1. [DOI] [PubMed] [Google Scholar]
- Bartlem D, Lambein I, Okamoto T, Itaya A, Uda Y, Kijima F, Tamaki Y, Nambara E, Naito S. Mutation in the threonine synthase gene results in an over-accumulation of soluble methionine in Arabidopsis. Plant Physiol. 2000;123:101–110. doi: 10.1104/pp.123.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryan JK. Synthesis of the aspartate family and branched-chain amino acids. In: Miflin BJ, editor. The Biochemistry of Plants. Vol. 5. New York: Academic Press; 1980. pp. 403–452. [Google Scholar]
- Casazza AP, Basner A, Höfgen R, Hesse H. Expression of threonine synthase from Solanum tuberosum L. is not metabolically regulated by photosynthesis-related signals or by nitrogenous compounds. Plant Sci. 2000;157:43–50. doi: 10.1016/s0168-9452(00)00265-x. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Chakraborty N, Datta A. Increased nutritive value of transgenic potato by expressing a nonallergenic seed albumin gene from Amaranthus hypochondriacus. Proc Natl Acad Sci USA. 2000;97:3724–3729. doi: 10.1073/pnas.050012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Ishikawa M, Kijima F, Tyson RH, Kim J, Yamamoto A, Mambara E, Leustek T, Wallsgrove RM, Naito S. Evidence for autoregulation of cystathionine γ-synthase mRNA stability in Arabidopsis. Science. 1999;286:1371–1374. doi: 10.1126/science.286.5443.1371. [DOI] [PubMed] [Google Scholar]
- Curien G, Dumas R, Ravanel S, Douce R. Characterization of an Arabidopsis thaliana cDNA encoding an S-adenosylmethionine-sensitive threonine synthase. FEBS Lett. 1996;390:85–90. doi: 10.1016/0014-5793(96)00633-3. [DOI] [PubMed] [Google Scholar]
- Curien G, Job D, Douce R, Dumas R. Allosteric activation of Arabidopsis threonine synthase by S-adenosylmethionine. Biochemistry. 1998;31:13212–13221. doi: 10.1021/bi980068f. [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, de Greve H, Debroek F, Schell J, van Montagu M, Leemanns J. Efficient octopine Ti plasmid-derived vectors of Agrobacterium mediated gene transfer to plants. Nucleic Acid Res. 1985;13:4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze J, Blau A, Willmitzer L. Agrobacterium-mediated transformation of potato (Solanum tuberosum) In: Potrykus I, Spangenberg G, editors. Gene Transfer to Plants XXII. Berlin: Springer-Verlag; 1995. pp. 24–29. [Google Scholar]
- Droux M, Ravanel S, Douce R. Methionine biosynthesis in higher plants: II. Purification and characterization of cystathionine β-lyase from spinach chloroplasts. Arch Biochem Biophys. 1995;316:585–595. doi: 10.1006/abbi.1995.1078. [DOI] [PubMed] [Google Scholar]
- Fieuw S, Müller-Röber B, Gálvez S, Willmitzer L. Cloning and expression analysis of the cytosolic NADP+-dependent isocitrate dehydrogenase from potato. Plant Physiol. 1995;107:905–913. doi: 10.1104/pp.107.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili G. Regulation of lysine and threonine synthesis. Plant Cell. 1995;7:899–906. doi: 10.1105/tpc.7.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J, Mudd SH, Datko AH. Sulfur amino acids in plants. In: Miflin BJ, editor. The Biochemistry of plants: A comprehensive Treatise. Vol. 5. New York: Academic Press; 1980. pp. 453–505. [Google Scholar]
- Giovanelli J, Mudd SH, Datko AH. In vivo regulation of de novo methionine biosynthesis in a higher plant (Lemna) Plant Physiol. 1985;77:450–455. doi: 10.1104/pp.77.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J, Mudd SH, Datko AH, Thompson GA. Effects of orthophosphate and adenosine 5′-phosphate on threonine synthase and cystathionine g-Synthase of Lemna paucicostata Hegelm.6746. Plant Physiol. 1986;81:577–583. doi: 10.1104/pp.81.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J, Veluthambi K, Thompson GA, Mudd SH, Datko AH. Threonine synthase of Lemna paucicostata Hegelm. 6746. Plant Physiol. 1984;76:285–292. doi: 10.1104/pp.76.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R. Molecular physiology of plant sulfur metabolism. Planta. 1997;202:138–148. doi: 10.1007/s004250050112. [DOI] [PubMed] [Google Scholar]
- Heremans B, Jacobs M. Threonine accumulation in a mutant of Arabidopsis thaliana (L.) Heynh. with an altered aspartate kinase. J Plant Physiol. 1995;146:249–257. [Google Scholar]
- Hesse H, Kreft O, Maimann S, Zeh M, Willmitzer L, Höfgen R. Approaches towards understanding methionine biosynthesis in higher plants. Amino Acids. 2001;20:281–289. doi: 10.1007/s007260170044. [DOI] [PubMed] [Google Scholar]
- Höfgen R, Willmitzer L. Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum) Plant Sci. 1990;66:221–230. [Google Scholar]
- Höfgen R, Willmitzer L. Transgenic potato plants depleted for the major tuber protein patatin via expression of antisense RNA. Plant Sci. 1992;87:45–54. [Google Scholar]
- Holtorf S, Apel K, Bohlmann H. Comparison of different constitutive and inducible promoters for the overexpression of transgenes in Arabidopsis thaliana. Plant Mol Biol. 1995;29:637–646. doi: 10.1007/BF00041155. [DOI] [PubMed] [Google Scholar]
- Inaba K, Fujiwara T, Chino M, Komeda Y, Naito S. Isolation of an Arabidopsis thaliana mutant, mto1, that overacumulates soluble methionine. Plant Physiol. 1994;104:881–887. doi: 10.1104/pp.104.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchi H, Shaul O, Galili G. Seed specific expression of a bacterial desensitized aspartate kinase increases the production of seed threonine and methionine in transgenic tobacco. Plant J. 1993;3:721–727. [Google Scholar]
- Laber B, Maurer W, Hanke C, Gräfe S, Ehlert S, Messerschmidt A, Clausen T. Characterization of recombinant Arabidopsis thaliana threonine synthase. Eur J Biochem. 1999;263:212–221. doi: 10.1046/j.1432-1327.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Madison JT, Thompson JF. Threonine synthetase from higher plants: stimulation by S-adenosylmethionine and inhibition by cysteine. Biochem Biophys Res Commun. 1976;71:684–691. doi: 10.1016/0006-291x(76)90842-1. [DOI] [PubMed] [Google Scholar]
- Maimann S, Wagner C, Kreft O, Zeh M, Willmitzer L, Höfgen R, Hesse H. Transgenic potato plants reveal the indispensable role of cystathionine beta-lyase in plant growth and development. Plant J. 2000;23:747–758. doi: 10.1046/j.1365-313x.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- Matthews BF. Lysine, threonine and methionine biosynthesis. In: Singh BK, editor. Plant Amino Acids: Biochemistry and Biotechnology. New York: Dekker; 1999. pp. 205–225. [Google Scholar]
- Muhitch MJ. Effects of expressing E. coli threonine synthase in tobacco (Nicotiana tabacum) suspension culture cells on free amino acid levels, aspartate pathway enzyme activities and uptake of aspartate into the cells. J Plant Physiol. 1997;150:16–22. [Google Scholar]
- Ravanel S, Droux M, Douce R. Methionine biosynthesis in higher plants: I. Purification and characterization of cystathionine γ-synthase from spinach chloroplasts. Arch Biochem Biophys. 1995;316:572–584. doi: 10.1006/abbi.1995.1077. [DOI] [PubMed] [Google Scholar]
- Ravanel S, Gakière B, Job D, Douce R. The specific features of methionine biosynthesis and metabolism in plants. Proc Nat Acad Sci USA. 1998;95.:7805–7812. doi: 10.1073/pnas.95.13.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel K, Mangelsdorf C, Streber W, Willmitzer L, Höfgen R, Hesse H. Isolation and characterization of a cDNA encoding cystathionine gamma-synthase from potato. Plant Biol. 1999;1:638–644. [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L. Both developmental and metabolic signals activate the promoter of the class I patatin gene. EMBO J. 1989;8:23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Willmitzer L, Fernie AR. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell. 2001;13:11–29. doi: 10.1105/tpc.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000;23:1–12. doi: 10.1046/j.1365-313x.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- Rognes SE. Threonine biosynthesis. In: Dey PM, Harbone JB, editors. Methods in Plant Biochemistry: Enzymes of Primary Metabolism. Vol. 3. New York: Academic Press; 1990. pp. 315–324. [Google Scholar]
- Saito K. Biosynthesis of cysteine. In: Singh BK, editor. Plant Amino Acids: Biochemistry and Biotechnology. New York: Dekker; 1999. pp. 267–291. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shaul O, Galili G. Threonine overproduction in transgenic tobacco plants expressing a mutant desensitized aspartate kinase of Escherichia coli. Plant Physiol. 1992;100:1157–1163. doi: 10.1104/pp.100.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SSM, Zuo W-N, Tu HM. Molecular approaches for enhancing amino acid quality of plant proteins. In: Singh BK, Flores HE, Shannon JC, editors. Biosynthesis and Molecular Regulation of Amino Acids in Plants. Rockville, MD: American Society of Plant Physiologists; 1992. pp. 208–228. [Google Scholar]
- Tabe L, Higgins TJV. Engineering plant protein composition for improved nutrition. Trends Plant Sci. 1998;3:282–286. [Google Scholar]
- Thoen A, Rognes SE, Aarnes H. Biosynthesis of threonine from homoserine in pea-seedlings: 2. Threonine synthase. Plant Sci Lett. 1978;13:113–119. [Google Scholar]
- Thompson GA, Datko AH, Mudd SH. Methionine biosynthesis in Lemna: studies on the regulation of cystathionine gamma-synthase, O-phosphohomoserine sulfhydrylase, and O-acetyl sulfhydrylase. Plant Physiol. 1982;69:1077–1083. doi: 10.1104/pp.69.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove RM, Lea PJ, Miflin BJ. Intracellular localization of aspartate kinase and the enzymes of threonine and methionine biosynthesis in green leaves. Plant Physiol. 1983;71:780–784. doi: 10.1104/pp.71.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]