Abstract
The present work describes, for the first time, the changes that take place in the leaf apoplastic antioxidant defenses in response to NaCl stress in two pea (Pisum sativum) cultivars (cv Lincoln and cv Puget) showing different degrees of sensitivity to high NaCl concentrations. The results showed that only superoxide dismutase, and probably dehydroascorbate reductase (DHAR), were present in the leaf apoplastic space, whereas ascorbate (ASC) peroxidase, monodehydroascorbate reductase (MDHAR), and glutathione (GSH) reductase (GR) seemed to be absent. Both ASC and GSH were detected in the leaf apoplastic space and although their absolute levels did not change in response to salt stress, the ASC/dehydroascorbate and GSH to GSH oxidized form ratios decreased progressively with the severity of the stress. Apoplastic superoxide dismutase activity was induced in NaCl-treated pea cv Puget but decreased in NaCl-treated pea cv Lincoln. An increase in DHAR and GR and a decrease in ASC peroxidase, MDHAR, ASC, and GSH levels was observed in the symplast from NaCl-treated pea cv Lincoln, whereas in pea cv Puget an increase in DHAR, GR, and MDHAR occurred. The results suggest a strong interaction between both cell compartments in the control of the apoplastic ASC content in pea leaves. However, this anti-oxidative response does not seem to be sufficient to remove the harmful effects of high salinity. This finding is more evident in pea cv Lincoln, which is characterized by a greater inhibition of the growth response and by a higher rise in the apoplastic hydrogen peroxide content, O2.− production and thiobarbituric acid-reactive substances, and CO protein levels. This NaCl-induced oxidative stress in the apoplasts might be related to the appearance of highly localized O2.−/H2O2-induced necrotic lesions in the minor veins in NaCl-treated pea plants. It is possible that both the different anti-oxidative capacity and the NaCl-induced response in the apoplast and in the symplast from pea cv Puget in comparison with pea cv Lincoln contributes to a better protection of pea cv Puget against salt stress.
NaCl stress is a major factor limiting crop production because it affects almost all plant functions (Bohnert and Jensen, 1996). Therefore, it is important to understand how plants respond and adapt to such stress. Adaptation of the plant cells to high salinity involves osmotic adjustment and the compartmentation of toxic ions, whereas an increasing body of evidence suggests that high salinity also induces oxidative stress (Hernández et al., 1993, 1995, 1999; Gosset et al., 1996; Gómez et al., 1999; Savouré et al., 1999). Therefore, antioxidant resistance mechanisms may provide a strategy to enhance salt tolerance, and processes underlying antioxidant responses to salt stress must be clearly understood. In previous studies, we have suggested a pivotal role for subcellular compartmentation in antioxidant defense mechanisms under stress conditions, including senescence and NaCl stress (Jiménez et al., 1997, 1998a; del Río et al., 1998; Gómez et al., 1999).
In pea (Pisum sativum) plants, it has been demonstrated that the metabolism of chloroplasts and mitochondria under NaCl stress favored the formation of O2.− and H2O2 in two pea cultivars differing in NaCl sensitivity (Hernández et al., 1993, 1995). When the effect of salt stress on the antioxidant defenses was examined at the transcript levels of mitochondrial, chloroplast, and cytosolic enzymes, an increase in all the components of the ascorbate (ASC)-glutathione (GSH) cycle and superoxide dismutase (SOD) isozymes was only found in tolerant pea plants (Gómez et al., 1999; Hernández et al., 2000). Transcript levels for mitochondrial Mn-SOD, chloroplastic CuZn-SOD and phospholipid hydroperoxide GSH peroxidase, and cytosolic GSH reductase (GR) and ASC peroxidase (APX) were strongly induced in the salt-tolerant variety but not in the salt-sensitive one, suggesting that the induction of antioxidant defenses is one component of the tolerance mechanisms of peas to long-term salt treatment (Hernández et al., 2000).
The effects of both biotic and abiotic stress on the antioxidant systems of the apoplastic space have been studied by some authors, and results suggest that this compartment could be important in the plant cell response to SO2, O3, and pathogens (Dietz, 1996; Luwe, 1996; Vanacker et al., 1998a, 1998b). Likewise, it is known that the above-described elicitors are capable of inducing the synthesis of activated oxygen species (AOS) in the apoplast of plant cells and also modulate the level of cellular antioxidants as well as the levels of apoplastic antioxidant enzymes (Luwe, 1996; Ranieri et al., 1996; Blinda et al., 1997; Thordal-Christensen et al., 1997; Schraudner et al., 1998; Vanacker et al., 1998a, 1998b; Piqueras et al., 1999; Hernández et al., 2001). However, little is known about the capacity of salt to induce the synthesis of AOS in the apoplast and, in this case, the role played by apoplastic antioxidants. This information is important because in plant cells subject to salt stress, initial events most likely occur externally in the apoplasm-cell membrane space.
Nevertheless, relatively little information is available regarding the presence of the antioxidant enzymes and low-Mr antioxidants, such as ASC and GSH, in the apoplast of plant tissues (Vanacker et al., 1998a), and the results found are often contradictory. Thus, although previous studies have failed to detect the antioxidant enzymes of the ASC-GSH cycle in the apoplast (Castillo and Greppin, 1988; Polle et al., 1990; Luwe, 1996) Vanacker et al. (1998a, 1998b) recently described the presence not only of SOD activity, but also APX, monodehydroascorbate reductase (MDHAR), dehydroascorbate (DHA) reductase (DHAR), GR, and catalase in the apoplast from both barley (Hordeum vulgare) and oat (Avena sativa) leaves. In regard to the antioxidants ASC and GSH, it should be pointed out that although both ASC and DHA are apparently present in high concentrations in the leaf apoplast (Polle et al., 1990; Luwe, 1996; Ranieri et al., 1996; Vanacker et al., 1998a, 1998b), the presence of GSH is still uncertain, and little or no GSH has been found in the apoplast of plant cells (Luwe, 1996; Vanacker et al., 1998a, 1998b).
In this work, the presence of SOD and of the enzymes of the ASC-GSH cycle (APX, MDHAR, GR, and DHAR), as well as the ASC and GSH contents, were studied in the leaf apoplastic space of two pea cultivars, one sensitive (cv Lincoln) and the other relatively tolerant (cv Puget) to 70 mm NaCl. This study was carried out to characterize the antioxidant capacity in this cell compartment and also to observe its behavior in the face of possible oxidative perturbation induced by an NaCl stress situation. In both pea cultivars, salt stress induces oxidative effects in the apoplasts, although such effects are lower in the relatively NaCl-tolerant pea (cv Puget) cultivar than in the NaCl-sensitive (cv Lincoln) plants. Oxidative damage was shown by the appearance under high NaCl stress of highly localized O2.−/H2O2-induced necrotic lesions in minor veins, which resemble the microburst observed by other authors in response to pathogenic (biotic) stress situations.
RESULTS
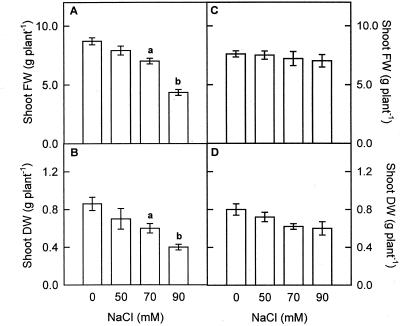
The growth of pea cv Lincoln plants, estimated as shoot fresh weight (Fig. 1A) and shoot dry weight (Fig. 1B), was not affected by 50 mm NaCl in the nutrient medium, but was reduced by about 30% and 50% in the presence of 70 and 90 mm NaCl, respectively, demonstrating that this pea cultivar is tolerant to 50 mm NaCl but sensitive to higher NaCl concentrations. In a previous study, the growth response of pea cv Puget, also estimated as shoot fresh and dry weight, among others parameters, seemed to not be significantly reduced by 70 and 90 mm NaCl (Hernández et al., 1999; Fig. 1, C and D), indicating that this pea cultivar is relatively NaCl tolerant.
Figure 1.
Plant growth response from pea cv Lincoln (A and B) and pea cv Puget (C and D) plants to increasing salinity, expressed as shoot fresh weight and shoot dry weight after 15 d of growth in hydroponic culture. Differences from control values were significant at: (a) P < 0.05 and (b) P < 0.01 according to Duncan's Multiple Range Test.
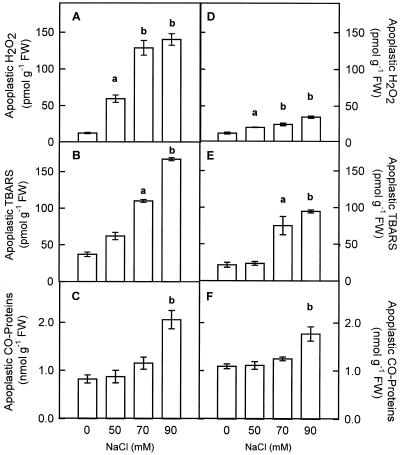
In pea cv Lincoln, the H2O2 content of the intercellular washing fluid (IWF) from NaCl-treated plants increased 4.8-, 10.0-, and 11.6-fold in plants treated with 50, 70, and 90 mm NaCl, respectively (Fig. 2A). The concentration of H2O2 in the apoplastic fluid ranged from 0.06 μm in control leaves to 0.70 μm in 90 mm NaCl-stressed pea leaves. The rise in H2O2 in 70 and 90 mm NaCl-treated plants was matched by an increase in lipid peroxidation (Fig. 2B), and protein oxidation at the higher NaCl concentration (Fig. 2C). In pea cv Puget plants, the H2O2 content in the IWF also increased with the severity of the stress but was limited to only 2.7-fold at 90 mm NaCl. Thus, the concentration of H2O2 in the apoplastic fluid ranged from 0.06 μm in control leaves to 0.20 μm in 90 mm NaCl-stressed plants (Fig. 2D). In pea cv Puget plants, 90 mm NaCl produced an increase in lipid peroxidation and protein oxidation (Fig. 2, E and F), although not so high as in pea cv Lincoln.
Figure 2.
Effects of salinity on the levels of apoplastic H2O2, lipid peroxidation estimated as apoplastic thiobarbituric acid-reactive substances (TBARS), and apoplastic carbonyl (CO)-proteins in pea leaves cv Lincoln (A, B, and C) and cv Puget (D, E, and F). Differences from control values were significant at: (a) P < 0.01 and (b) P < 0.001 according to Duncan's Multiple Range Test.
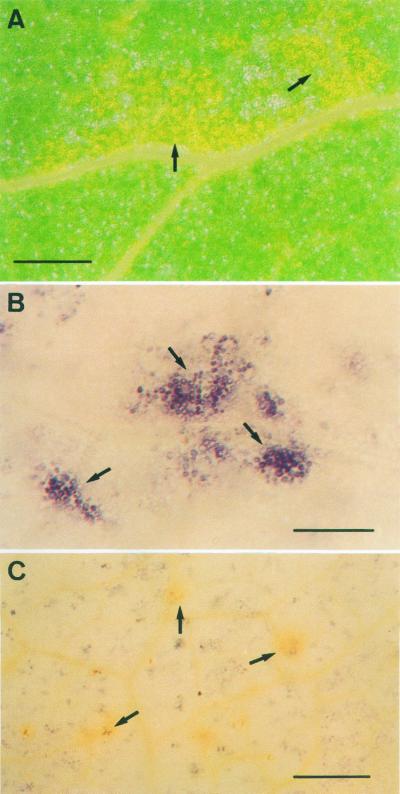
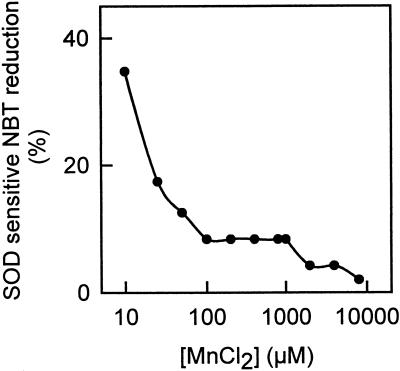
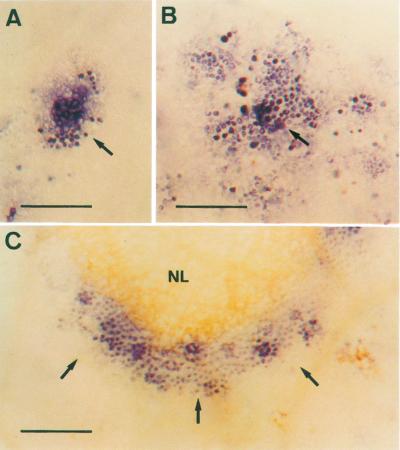
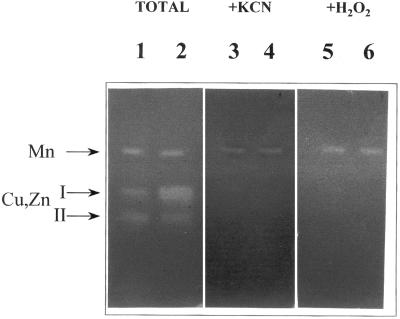
The treatment of pea cv Lincoln plants with 90 mm NaCl also produced the appearance of necrotic leaf lesions localized initially on minor veins. These necrotic lesions can be easily monitored during the first stages of injury by the bleaching of chlorophylls (Fig. 3A, arrows). As the lesions advance, they turn brown and their size becomes visible to the naked eye. No necrotic lesions were observed in control plants at any time of the culture period. When these salt-induced necrotic lesions (SINLs) of minor veins were stained with Nitroblue tetratolium (NBT) during the first stages of development, a blue/red wine staining appeared on the cell walls of mesophyll cells (Fig. 3B). This staining was due to the production of O2.− because it was totally suppressed by SOD (Fig. 3C, arrows), and by 10 mm MnCl2, a highly effective dismutating catalyst agent of O2.− (Fig. 4).
Figure 3.
Periveinal chlorotic lesions (arrows) in pea cv Lincoln leaves of plants treated with 90 mm NaCl for 14 d (A). Periveinal chlorotic lesions (arrows) in pea leaves of plants treated with 90 mm NaCl for 14 d, and stained with NBT in the absence (B) and in the presence (C) of 100 units mL−1 SOD. Bars = 250 μm.
Figure 4.
Effect of MnCl2 concentration on the scavenging of O2.− generated in a xanthine/xanthine oxidase reaction (at pH 7.6) and determined by the SOD-sensitive NBT reduction. NBT concentration in the reaction medium was 0.1 mg mL−1.
When O2.− production, as monitored by NBT, was followed with the degree of severity (judging from increased lesion size) of the SINLs (Fig. 5, A–C) in pea leaves, the formation of an O2.−-generating front was observed on the outer edge of the necrotic lesion (Fig. 5C, arrows). This O2.−-generating front was clearly distinguishable from the strictly local production of O2.−, which takes place during the first stages of lesion development (Fig. 5A). These results suggest that the SINLs advance as the O2.− production front advances, suggesting a key role for O2.− in the development of these SINLs.
Figure 5.
Periveinal chlorotic lesions (arrows) in pea cv Lincoln leaves of plants treated with 90 mm NaCl for 14 d and stained with NBT during the early (A), intermediate (B), and later (C) stages of development. The development rating was taken as the enlargement of the necrotic lesion. Bars = 250 μm.
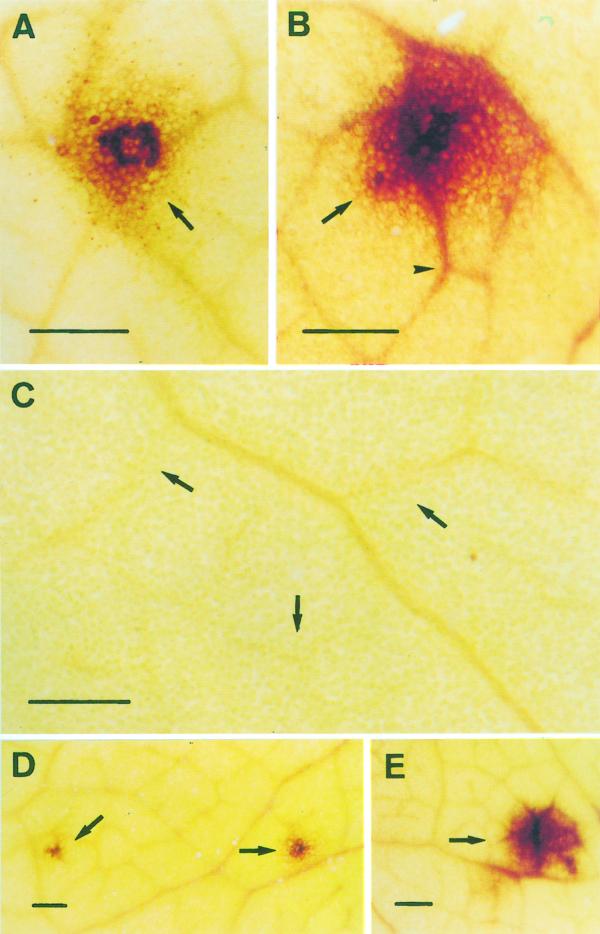
When stained with the diaminebenzydine (DAB) reagent to locate H2O2, the SINLs also showed a red-brown staining localized on the cell walls of mesophyll cells (Fig. 6A), which diffused rapidly through the minor veins of the leaf (Fig. 6B, arrows), probably due to the greater half-life time of H2O2 compared with O2.−. This staining seemed to be due to H2O2 because it was totally suppressed by 10 mm ascorbic acid (Fig. 6C, arrows). DAB-stainable AOS production was also observed in the relatively salt-tolerant cultivar (pea cv Puget), where the lesions were somewhat smaller in size (compare Fig. 6, D with E).
Figure 6.
Periveinal chlorotic lesions (arrows) in pea leaves of both pea cultivars, cv Lincoln (A–C and E) and cv Puget (D), treated with 90 mm NaCl for 14 d and stained with DAB during the early (A) and intermediate (B–E) stages of development. The development rating was taken as the enlargement of the necrotic lesion. Periveinal chlorotic lesions (arrows) in pea leaves of plants treated with 90 mm NaCl for 14 d stained with DAB in the presence (C) of 10 mm ascorbic acid. Bars = 250 μm. D (pea cv Puget) and E (pea cv Lincoln) showed the same magnification for comparison between the two cultivars.
The quantification of SOD activity in the apoplast of control and salt-stressed pea plants took into account corrections for cytosolic and chloroplastic protein contamination of the IWF during the infiltration procedure. In this way, the activity of the cytosolic and chloroplastic triose phosphate isomerase (TPI) was analyzed. The results showed that contamination was always less than 1.0% in control plants. However, the percentage of contamination increased with the severity of salt treatment, probably due to cellular injury, reaching about 1.54% in pea cv Lincoln and 2.38% in pea cv Puget plants treated with 90 mm NaCl (Table I). According to the results obtained with the inhibitors, H2O2 and KCN, CuZn-SOD activity seems to be present in the apoplastic space of control pea cv Lincoln (Fig. 7) and control pea cv Puget leaves (results not shown). This CuZn-SOD could not be clearly distinguished from the major cytosolic CuZn-SOD I present in pea leaves using native PAGE (Fig. 7) or isoelectrofocusing at different pH ranges (results not shown). Thus, the detection of apoplastic CuZn-SOD activity was carried out taking into account the different isozyme patterns shown by the symplastic and apoplastic fractions (Fig. 7). In leaf crude extract from pea cv Lincoln plants, three different SOD activity bands were detected, an Mn-containing SOD and two CuZn-containing SOD (named I and II in order of increased mobility), representing 14% to 15%, 34% to 36%, and 48% to 50%, respectively (Fig. 7). In pea plants, Mn-SOD is localized in mitochondria and peroxisomes, CuZn-SOD I in the cytosol, and CuZn-SOD II in chloroplasts (Hernández et al., 1999). In the apoplastic fraction, we detected the same number of SOD isozymes as in crude leaf extracts, but in the apoplast, mitochondrial and peroxisomal Mn-SOD and chloroplastic CuZn-SOD II only accounted for 4% to 5% and 33% to 35%, respectively, whereas cytosolic Cu,Zn-SOD represented 60% to 62% of the total SOD activity found in this compartment. By isoelectrofocusing of the apoplastic fraction, the percentage of CuZn-SOD I increased from 53% to 55% and that of CuZn-SOD II decreased to 45% from 47% in relation to the values in the symplastic fraction (data not shown).
Table I.
Contamination of the apoplastic extracts by the cytosolic and chloroplastic marker TPI (μmol min−1 g−1 fresh wt) and by chlorophyll (mg g−1 fresh wt)
| Treatment | TPI Activity | Chlorophyll Content |
|---|---|---|
| Control | ||
| Puget symplast | 13.45 ± 1.40 | 6.25 ± 0.59 |
| Puget apoplast | 0.14 ± 0.05 (1.03%) | 0.09 ± 0.02 (1.44%) |
| Lincoln symplast | 18.44 ± 1.34 | 6.45 ± 0.59 |
| Lincoln apoplast | 0.16 ± 0.04 (0.86%) | 0.08 ± 0.01 (1.24%) |
| 50 mm NaCl | ||
| Puget symplast | 15.16 ± 1.82 | 4.80 ± 0.34 |
| Puget apoplast | 0.206 ± 0.13 (1.34%) | 0.06 ± 0.01 (1.25%) |
| Lincoln symplast | 12.5 | 4.38 |
| Lincoln apoplast | 0.15 ± 0.03 (1.20%) | 0.05 ± 0.01 (1.14%) |
| 70 mm NaCl | ||
| Puget symplast | 12.85 ± 1.30 | 3.13 ± 0.24 |
| Puget apoplast | 0.225 ± 0.10 (1.72%) | 0.05 ± 0.03 (1.60%) |
| Lincoln symplast | 15.10 ± 1.64 | 3.22 ± 0.84 |
| Lincoln apoplast | 0.23 ± 0.09 (1.50%) | 0.04 ± 0.01 (1.22%) |
| 90 mm NaCl | ||
| Puget symplast | 10.58 ± 0.95 | 3.64 ± 0.15 |
| Puget apoplast | 0.258 ± 0.18 (2.38%) | 0.05 ± 0.02 (1.37%) |
| Lincoln symplast | 12.78 ± 0.70 | 2.66 ± 0.22 |
| Lincoln apoplast | 0.20 ± 0.08 (1.54%) | 0.04 ± 0.03 (1.48%) |
The percentage of activity in the apoplast (with respect to the total present in the whole leaf) is given in parentheses.
Figure 7.
SOD isozyme identification after native PAGE on 10% (w/v) acrylamide gels from crude extracts and apoplastic fractions from pea cv Lincoln leaves. 1, 3, and 5, Leaf crude extracts. 2, 4, and 6, Apoplastic fractions. Identification of SOD isoforms was performed by pre-incubation of gels with inhibitors. 1 and 2, Total activity (no inhibitors); 3 and 4, stained in the presence of 2 mm KCN; 5 and 6, stained in the presence of 5 mm H2O2.
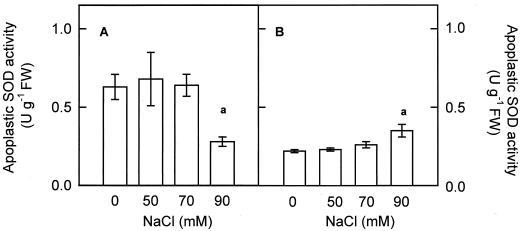
In control pea cv Lincoln leaves, apoplastic CuZn-SOD only represents 1% of total leaf SOD activity, this percentage being lower in pea cv Puget plants (0.35%). This apoplastic CuZn-SOD activity was not affected in pea plants of either cultivar treated with 50 and 70 mm NaCl but decreased significantly, to 54%, in pea cv Lincoln plants subjected to 90 mm NaCl (Fig. 8A). In contrast, a 90-mm NaCl treatment brought about a significant increase, by up to 60%, in the activity of apoplastic CuZn-SOD in pea cv Puget plants (Fig. 8B).
Figure 8.
Effect of NaCl concentration in the culture medium on the level of apoplastic SOD activity in pea cv Lincoln (A) and cv Puget (B) leaves, after corrections for chloroplastic and cytosolic contaminations. Differences from control values were significant at: (a) P < 0.05 according to Duncan's Multiple Range Test.
Following corrections for cytosolic and chloroplastic protein contamination, only DHAR activity was detected in the apoplastic space of both pea cultivars, where it represented 0.26% and 1.78% of the total leaf DHAR activity in pea cv Lincoln and pea cv Puget, respectively (Table II). p-CMPS-sensitive APX, p-CMPS-insensitive APX, MDHAR, and GR activities were apparently not located in the leaf apoplast of either cultivar because the values corresponding to these enzymes in the apoplast were possibly due to cytosolic and/or chloroplastic contaminations (Table II). Similar results were found in NaCl-treated plants, although in these cases, DHAR activity was mainly, and probably exclusively, due to chloroplastic and/or cytosolic contamination (Table II).
Table II.
Levels of ASC-GSH cycle enzymes and SOD activity in the symplast and apoplastic space from pea leaves (cv Puget and cv Lincoln)
| Activity
| ||||||
|---|---|---|---|---|---|---|
| Treatment | SOD | p-Chloromercurphenyl sulfonic acid (p-CMPS)-sensitive APX | p-CMPS-insensitive APX | MDHAR | DHAR | GR |
| Control | ||||||
| Puget symplast | 61.5 ± 4.5 | 6,264 ± 82 | nd | 527 ± 30 | 11.4 ± 2.5 | 473 ± 16 |
| Puget apoplast | 0.514 ± 0.06 (1.72%) | 14.6 ± 0.5 (0.23%) | nd | 3.53 ± 0.27 (0.66%) | 0.33 ± 0.05 (2.81%) | 3.14 ± 0.34 (0.65%) |
| Lincoln symplast | 65.9 ± 4.8 | 6,352 ± 320 | 330 ± 29 | 567 ± 18 | 47.9 ± 5.2 | 475 ± 0.4 |
| Lincoln apoplast | 1.21 ± 0.17 (1.8%) | 18.5 ± 0.8 (0.31%) | 3.11 ± 0.21 (0.86%) | 4.2 ± 0.37 (0.74%) | 0.55 ± 0.20 (1.12%) | 3.94 ± 0.54 (0.82%) |
| 50 mm NaCl | ||||||
| Puget symplast | 63.8 ± 7.9 | 6,454 ± 51 | nd | 584 ± 32 | 21.4 ± 3.1a | 523 ± 10a |
| Puget apoplast | 0.81 ± 0.01 (1.85%) | 21.7 ± 0.5 (0.33%) | nd | 3.64 ± 0.21 (0.62%) | 0.28 ± 0.06 (1.29%) | 3.54 ± 0.09 (0.36%) |
| Lincoln symplast | 67.1 ± 2.2 | 5,445 ± 117a | 240 ± 23 | 546 ± 94 | 53.7 ± 3.1 | 630 ± 2.5b |
| Lincoln apoplast | 1.50 ± 0.38 (2.59%) | 28.8 ± 0.56 (0.53%) | 2.72 ± 0.30 (1.13%) | 4.15 ± 0.41 (0.76%) | 0.54 ± 0.32 (1.03%) | 6.03 ± 0.19 (0.95%) |
| 70 mm NaCl | ||||||
| Puget symplast | 53.8 ± 5.9 | 6,363 ± 67 | nd | 501 ± 12 | 69.9 ± 4.3c | 645 ± 32b |
| Puget apoplast | 0.595 ± 0.02 (2.35%) | 16.6 ± 1.0 (0.26%) | nd | 2.57 ± 0.31 (0.51%) | 0.60 ± 0.10 (0.85%) | 6.36 ± 0.40 (0.98%) |
| Lincoln symplast | 57.4 ± 4.9 | 5,500 ± 50a | 299 ± 30 | 496 ± 6a | 69.3 ± 4.1a | 632 ± 22.6b |
| Lincoln apoplast | 1.53 ± 0.18 (2.59%) | 31.6 ± 2.0 (0.56%) | 3.97 ± 0.36 (1.32%) | 4.57 ± 0.50 (0.92%) | 0.67 ± 0.15 (0.91%) | 7.10 ± 0.60 (1.12%) |
| 90 mm NaCl | ||||||
| Puget symplast | 65.8 ± 11.2 | 5,674 ± 45b | nd | 626 ± 21.2a | 21.3 ± 5.4a | 572 ± 33a |
| Puget apoplast | 1.41 ± 0.04 (3.15%) | 19.4 ± 0.9 (0.34%) | nd | 4.19 ± 0.31 (0.66%) | 0.34 ± 0.04 (1.57%) | 5.40 ± 0.12 (0.94%) |
| Lincoln symplast | 69.5 ± 4.1 | 4,725 ± 58b | 301 ± 39 | 435 ± 30a | 77.3 ± 7.4b | 438 ± 11.3 |
| Lincoln apoplast | 1.38 ± 0.19 (1.93%) | 24.6 ± 1.8 (0.52%) | 2.83 ± 0.25 (0.86%) | 5.78 ± 0.61 (1.32%) | 0.71 ± 0.08 (0.92%) | 5.35 ± 0.10 (1.22%) |
APX, MDHAR, GR, and DHAR activities are expressed as nmol min−1 g−1 fresh wt. SOD is expressed as units g−1 fresh wt. The percentage of activity in the apoplast (with respect to the total present in the whole leaf) is given between parentheses. nd, Not determined.
Differences from control values were significant at P < 0.05 according to Duncan's Multiple Range Test.
Differences from control values were significant at P < 0.01 according to Duncan's Multiple Range Test.
Differences from control values were significant at P < 0.001 according to Duncan's Multiple Range Test.
The effect of salt stress on the “symplastic” enzymes was studied using the leaf residue resulting from the IWF extraction (Table II). DHAR activity increased in the “symplast” fraction of pea cv Lincoln plants treated with 70 and 90 mm NaCl (1.44- and 1.61-fold, respectively). GR activity showed a rise of about 30% at these NaCl concentrations, and MDHAR and p-CMPS-sensitive APX decreased in all the NaCl-treated plants (Table II). No effect of salinity on either “symplastic” p-CMPS-insensitive APX or SOD activity was observed (Table II). In pea cv Puget plants, DHAR and GR activity gradually and significantly increased with the severity of the stress although, unlike in pea cv Lincoln plants, MDHAR remained constant at 70 mm NaCl but was significantly induced at 90 mm NaCl, whereas p-CMPS-sensitive APX activity only fell at this NaCl concentration. No effect of NaCl on “symplastic” SOD activity was observed (Table II).
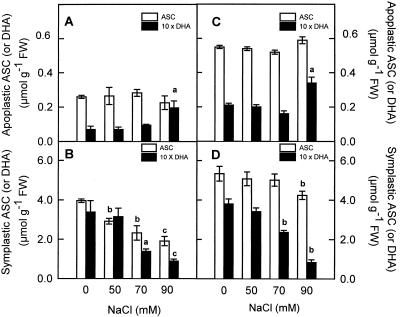
In control pea cv Lincoln leaves, the ASC content of the apoplast (0.12 mm) represented about 6% of the total ASC, whereas the apoplastic DHA content (3.6 μm) represented 2% of the total oxidized ASC present in leaves. In pea cv Puget plants, the ASC content of the apoplast (0.21 mm) was about 2-fold higher than in pea cv Lincoln, and represented by up to 9.5% of the total leaf ASC. In a similar manner, the apoplastic DHA content (7.1 μm) was higher in pea cv Puget than in pea cv Lincoln, representing 5.2% of the total leaf DHA. In neither pea cultivar did the ASC content change significantly in the leaf apoplast from NaCl-stressed plants (Fig. 9, A and C). However, DHA was observed to accumulate in the leaf apoplast of 90 mm NaCl-treated pea plants, particularly in the pea cv Lincoln (2.6-fold increase, from 3.6 to 9.1 μm; Fig. 9A) compared with the 1.5-fold increase (from 7.1 to 11.5 μm) seen in pea cv Puget (Fig. 9C). In both cultivars, the ASC to DHA ratio gradually decreased with the severity of the stress.
Figure 9.
Effect of NaCl concentration in the culture medium on apoplastic and “symplastic” ASC and DHA contents of pea cv Lincoln (A and B) and cv Puget (C and D) leaves. Differences from control values were significant at: (a) P < 0.05, (b) P < 0.01, and (c) P < 0.001 according to Duncan's Multiple Range Test.
As a result of the increasing salt stress, the ASC pool decreased progressively in the “symplastic” fraction from pea cv Lincoln leaves (by up to 52% in plants treated with the higher NaCl concentration; Fig. 9B). However, in the same conditions, the “symplastic” ASC content from pea cv Puget plants decreased by up to 20% (Fig. 9D). In both cultivars, the “symplastic” DHA content was not affected by treatment with 50 mm NaCl, although its content decreased progressively in plants treated with 70 and 90 mm NaCl (Fig. 9, B and D). As a result of these changes, the symplastic ASC to DHA ratio increased with the severity of the stress in both cultivars.
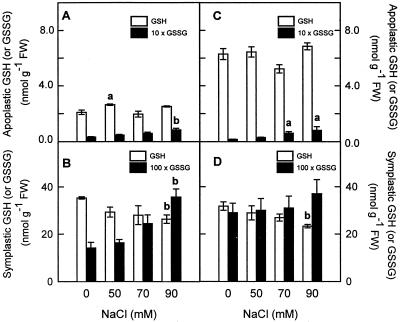
The apoplastic GSH content of pea cv Lincoln leaves represented about 5.6% of the total GSH pool in leaves, a percentage similar to that observed for ASC. The percentage of GSH in the leaf apoplast increased by up to 8.7% of the total GSH present in the leaf with the 50 mm NaCl treatment, but showed no statistical changes at higher salt concentration (Fig. 10A). The percentage for glutathione oxidized form (GSSG) in the leaf apoplast from pea cv Lincoln plants was about 18%, and only increased significantly (2.55-fold) in the leaf apoplast of pea plants treated with 90 mm NaCl (Fig. 10A). A higher percentage of GSH was found in the apoplast of pea cv Puget plants, ranging from 16% to 22%, and the values were nearly three times higher than in pea cv Lincoln (Fig. 10C). The percentage of GSSG in the apoplast of control pea cv Puget plants was lower (only 5.2%) than in pea cv Lincoln. However, at 70 and 90 mm NaCl, similar percentages were found in both cultivars, and a significant increase of GSSG was found in the apoplast of pea cv Puget plants treated with these salt levels (Fig. 10C).
Figure 10.
Effect of NaCl concentration in the culture medium on apoplastic and “symplastic” GSH and GSSG contents of pea cv Lincoln (A and B) and cv Puget (C and D) leaves. Differences from control values were significant at: (a) P < 0.05 and (b) P < 0.01 according to Duncan's Multiple Range Test.
The GSH content decreased significantly in the “symplast” from both pea cultivars only in plants treated with 90 mm NaCl (Fig. 10, B and D). However, an increase in the GSSG content was invariably observed in the “symplast” from pea cv Lincoln plants subjected to salt stress (as much as 2.52-fold at 90 mm NaCl; Fig. 10B), so that the “symplast” GSH to GSSG ratio decreased in NaCl-treated plant of this cultivar. However, no significant GSSG accumulation was observed in the symplast from pea cv Puget plants (Fig. 10D) and the GSH to GSSG ratio slightly decreased.
When the effect of salt stress on apoplastic p-CMPS-insensitive (class III) peroxidases was studied, the results showed that such activity in pea cv Lincoln plants, measured with both non-physiological (4-methoxy-α-naphthol and 3,3′,5,5′-tetra methylbenzidine) and physiological (coniferyl alcohol) substrates, increased in response to salt stress, although these changes were not statistically significant (Table III). The highest stimulation was attained with 70- and 90-mm NaCl treatments when 4-MN and coniferyl alcohol, respectively, were used as substrates. Similar results were obtained in the relatively salt-tolerant pea cultivar (data not shown). Isoenzyme patterns of apoplastic class III peroxidase only showed minor (nonsignificant) changes in response to salt treatment, and the effect seemed to be general for all the isoenzymes (results not shown).
Table III.
Effect of NaCl concentration in the culture medium on pea cv Lincoln leaf apoplastic class III peroxidase activity measured with 4-methoxy-α-naphthol (4-MN), 3,3′,5,5′-tetramethylbenzidine (TMB), and coniferyl alcohol, in the presence and in the absence of H2O2
| Treatment | 4-MN
|
TMB
|
Coniferyl Alcohol
|
|||
|---|---|---|---|---|---|---|
| +H2O2 | −H2O2 | +H2O2 | −H2O2 | +H2O2 | −H2O2 | |
| nKat g−1 fresh wt | ||||||
| Control | 2.60 ± 0.26 | 0.14 ± 0.01 | 17.7 ± 2.4 | 0.005 ± 0.001 | 16.6 ± 1.0 | nd |
| 50 mm NaCl | 2.90 ± 0.14 | 0.14 ± 0.03 | 18.4 ± 0.3 | 0.030 ± 0.007a | 15.2 ± 1.5 | nd |
| 70 mm NaCl | 3.83 ± 0.50 | 0.24 ± 0.01b | 19.3 ± 1.1 | 0.060 ± 0.010a | 19.3 ± 2.5 | nd |
| 90 mm NaCl | 3.04 ± 0.33 | 0.15 ± 0.05 | 13.0 ± 2.0, ns | 0.061 ± 0.020a | 23.2 ± 6.6, ns | nd |
nd, Not detectable.
Differences from control values were significant at P < 0.05 according to Duncan's Multiple Range Test.
Differences from control values were significant at P < 0.01 according to Duncan's Multiple Range Test.
DISCUSSION
A comparison of the growth response to salt stress shows that pea cv Lincoln is tolerant to 50 mm NaCl, although it is more sensitive to higher NaCl concentrations than pea cv Puget. The latter was previously designated as moderately tolerant to NaCl stress, when its growth response was compared with that reported for other leguminous plants, including different pea cultivars (Hernández et al., 1995, 1999; Gómez et al., 1999).
Evidence for the effects of salt stress-inducing changes in plant metabolism is well documented (Greenway and Munns, 1980). Salt stress, in addition to the known components of osmotic stress and ion toxicity, is also manifested as an oxidative stress, and all of these contribute to its deleterious effect (Gueta-Dahan et al., 1997). However, ion content and salt tolerance are not often correlated, and several studies indicate that acquisition of salt tolerance may also be a consequence of improving resistance to oxidative stress (Hernández et al., 1993, 1995, 1999; Gosset et al., 1996; Streb and Feierabend, 1996; Gueta-Dahan et al., 1997; Gómez et al., 1999).
Very few enzymes and metabolites have been shown unequivocally to be either absent or present in the apoplast (Dietz, 1996). Moreover, unavoidable contamination by cytosolic and chloroplastic components occurs. In this work, TPI was used both as a cytosolic and a chloroplastic marker to determine the degree of contamination of the apoplastic space by components originating in the symplast of plant cells because SODs and the enzymes of the ASC-GSH cycle are mainly accumulated in those subcellular compartments (Jiménez et al., 1997, 1998a; Ogawa et al., 1997; Foyer and Mullineaux, 1998; Gómez et al., 1999; Hernández et al., 2000).
In pea apoplast, values found for SOD activity agree with data for Scots pine (Pinus sylvestris) needles and oat and barley leaves (0.1%–2.5% of total activity; Streller and Winsgle, 1994; Vanacker et al., 1998a, 1998b). In agreement with reports for apoplastic CuZn-SOD from spinach leaves (Ogawa et al., 1996, 1997), the apoplastic CuZn-SOD isozyme detected in both pea cultivars here seems to be indistinguishable from the main cytosolic CuZn-SOD, at least under our experimental conditions.
The absence of APX activity in the apoplast of both pea cultivars agrees with what has been also reported for different plants (Polle et al., 1990; Durán Carril and Rodriguez-Bujan, 1999). Our results differ from those reported by Vanacker et al. (1998), who described the presence not only of SOD and APX activity in the apoplast of both barley and oat leaves, but also of MDHAR, DHAR, GR, and catalase. In our pea cultivars, similar percentages for these enzymes were observed in the apoplast (Table II). However, these activities were apparently due to cytosolic and/or chloroplastic contaminations, which ranged from 0.86% to 1.03% in control leaves to 1.54% to 2.38% in 90 mm NaCl-treated pea plants (cv Lincoln and cv Puget, respectively).
It is necessary to point out that the presence of DHAR in the apoplast of control pea leaves is still uncertain in both cultivars, if the total chlorophyll content of the apoplastic solution was considered as a symplastic marker instead of TPI activity. This fact is also supported by the absence of GR activity in the apoplast. No DHAR activity could be measured in the apoplastic space of beech (Fagus sylvatica) leaves (Luwe, 1996) and, recently, Morell et al. (1997) have reported that a certain pseudo-DHAR activity found in plants may be due to side reactions of proteins containing redox-active di-Cys sites. However, Foyer and Mullineaux (1998) expressed several consistent arguments in favor of the presence of DHA and DHAR activity in plant tissues. Moreover, the presence of a plasma membrane-associated MDHAR, which may play a role in the reduction of ASC in both cytosol and apoplast, has been described (Navas and Gómez-Díaz, 1995; Bérczi and Möller, 1998). All these results suggest that the presence of a “DHAR enzyme” in the apoplast fraction of pea leaves should be regarded with caution.
The ASC levels in the apoplast of spinach (0.65 mm), barley (0.016 mm), and oat leaves (0.01 mm; Takahama and Okini, 1992; Vanacker et al., 1998a, 1998b) agree well with the ASC concentration present in the pea leaf apoplast, which ranged from 0.12 to 0.22 mm. A different picture exists regarding GSH because little or no GSH has been detected in the apoplast of certain plant cells. This has been found in the apoplast from Picea abies needles (Polle et al., 1990) and in the apoplast of barley leaves (Vanacker et al., 1998b), where its concentration ranged from 6 to 6.5 μm. GSH concentration in pea leaf apoplast was lower and ranged from 0.8 to 1.2 μm for pea cv Lincoln and from 2.0 to 2.4 μm from pea cv Puget.
In response to NaCl stress conditions, the appearance of SINLs in the minor veins of 90 mm NaCl-treated plants supports the establishment of an oxidative stress situation in the apoplasts of both pea cultivars. The NaCl-induced oxidative stress situation is observed as highly localized areas of AOS (O2.− and H2O2) production in minor veins in both pea cultivars and is also manifested by increases in lipid peroxidation, H2O2 content, and protein oxidation. However, some differences between both pea cultivars were found because all the above oxidative effects were less pronounced in pea cv Puget (Fig. 6D) than in pea cv Lincoln (Fig. 6E) in 90 mm NaCl stress conditions, as reflected by the smaller necrotic lesions in pea cv Puget plants.
However, these areas of H2O2 production in pea leaves, as seen histochemically, never exceeded 0.25% of the total leaf area in 90 mm NaCl-stressed plants. Based on an H2O2 concentration of 0.70 μm in the whole-leaf apoplast of 90 mm NaCl-stressed pea cv Lincoln plants, this means that the local H2O2 concentration in the area of the SINL would reach 250 to 300 μm. This last value for the H2O2 content of SINLs fits in well with the fact that, although DAB can detect H2O2 in leaves at levels as low as 10 μm, strong color only develops with concentrations above 100 μm (Thordal-Christensen et al., 1997).
These SINLs emerge from periveinal zones with a capacity for sustaining net O2.− and H2O2 production in the first stages of development. These stained areas were sensitive to ASC, and therefore were indicative for H2O2 accumulation. The generation of necrotic lesions under NaCl stress resembles the leaf “microbursts” observed by other authors in response to pathogenic stress situations where H2O2 is accumulated (Alvarez et al., 1998; Orozco-Cardenas and Ryan, 1999). AOS accumulation, cell death, and lesion in the vicinity of leaf veins have been observed in ozone-treated tobacco leaves (Schraudner et al., 1998). In pea plants, the fact that the lesions generated under NaCl stress are associated with high levels of O2.− and H2O2 production provides strong evidence that these lesions may be part of an active hypersensitive-like response. These observations may be interpreted as indicating that in pea cv Lincoln the down-regulated apoplastic SOD activity together with the lower constitutive levels of reduced ASC and GSH, compared with that found in pea cv Puget, could contribute to allowing O2.− and H2O2 to destroy plant cells to a greater extent than in pea cv Puget, where the NaCl-induced lesions were less extensive.
The induction of apoplastic SOD in pea cv Puget might be responsible for increasing the apoplastic H2O2 content, although this metabolite, among others, could be directly scavenged by ASC, whose levels were 2-fold higher in this cultivar than in pea cv Lincoln.
It is important to note that the up-regulation of antioxidant enzymes, rather than the constitutive apoplastic level of SOD, among other factors, seems to be important in the tolerance of pea cv Puget to salt stress. This relationship was previously observed in other pea cultivars (Hernández et al., 1993, 1995, 2000; Gómez et al., 1999), and it was reported in different plants, including the pst1 Arabidopsis mutant (Tsugane et al., 1999). In addition, it has been reported that overexpression of SOD confers salt tolerance to rice (Oryza sativa) plants (Tanaka et al., 1999), further confirming that O2.− and derived AOS are involved in salt-induced damages. Leaf lesions under NaCl treatment were detected in mesophyll cells. A similar pattern of AOS accumulation was reported in ozone-treated plants (Schraudner et al., 1998), but a different pattern was observed in epidermal cells of barley following pathogen infection (Thordal-Christensen et al., 1997) and in tobacco following elicitor treatment (Allan and Fluhr, 1997).
Extracellularly secreted enzymes such as peroxidase are also know to catalyze the production of O2.− (Kawano and Muto, 2000). However, no significant changes in pea apoplastic p-CMPS-insensitive peroxidase were brought about by NaCl stress. On the other hand, a membrane NAD(P)H oxidase has been proposed as being responsible for the generation of AOS (O2.− and subsequent H2O2) in response to pathogens (Levine et al., 1994). There have been numerous reports indicating the production and involvement of AOS in cation-induced injuries (Cakmak, 2000; Quartacci et al., 2001). It was proposed that, analogous to the mammalian enzyme, it is likely that NADPH oxidase can be activated by cations (Cross et al., 1999). This enzyme, together with oxalate oxidase in the apoplast, could be the enzymatic source participating in AOS accumulation during NaCl stress, similar to that suggested under ozone stress (Schraudner et al., 1998). Further studies would determine the contribution of these enzymes to NaCl-induced O2.− generation.
In salt-treated pea plants, uptake of the oxidized ASC through the plasma membrane may represent an important step in the regeneration of apoplastic ASC. Such ASC regeneration has been described by Horemans et al. (1996, 1997), who demonstrated the existence of a carrier-mediated ASC/DHA transport system that preferentially translocates DHA from the apoplast to the cytosol and that probably exists to reduce this molecule in this cell compartment (Castillo and Greppin, 1988; Luwe et al., 1993; Horemans et al., 1997). This mechanism could be occurring in both pea cultivars, although its efficacy seems to be rather low in NaCl-stressed plants, particularly in pea cv Lincoln. Thus, in both cultivars, the lack of variation in total apoplastic ASC under NaCl stress was accompanied by a decrease in both the apoplastic ASC to DHA ratio and the ASC redox state, in both cases mainly due to the increase in the apoplastic DHA content. However, all these changes were less pronounced in pea cv Puget that in pea cv Lincoln, in which the increase in apoplastic DHA content was much higher under NaCl stress. Changes in the redox balance in the apoplastic antioxidant system have also been reported in response to ozone (Schraudner et al., 1998), pathogens (Thordal-Christensen et al., 1997), and heavy metals (Piqueras et al., 1999).
This suggested mechanism for apoplastic regeneration fits with the induction found in the symplastic DHAR and GR activities under NaCl stress, showing that “symplastic” ASC is regenerated using the GSH pool, although in pea cv Puget its regeneration could also involve MDHAR activity. This is in agreement with reports for other pea cultivars (Morán et al., 1994; Hernández et al., 2000). Thus, it seems that neither MDHAR nor DHAR limited the ASC content of the symplast or affected its reduction state, both of which seem to be determined by its rate of synthesis and degradation and/or its transport to other cell compartments. Similar to our findings in other pea cultivars (Hernández et al., 2000), it should be noted that symplastic DHA accumulation does not occur in either pea cv Lincoln or pea cv Puget.
GSH oxidation seems to occur at a higher rate in the symplast of pea cv Lincoln under NaCl stress because in such conditions a progressive increase in the symplastic GSSG content was observed. This fact could be related to a higher degree of induction of DHAR than of GR in the symplast, especially at 90 mm NaCl. Like ASC, constitutive apoplastic GSH content was much more elevated in pea cv Puget than in pea cv Lincoln plants, and showed no significant variations at higher salt concentrations, at which a decrease in both the GSH to GSSG ratio and the GSH redox state in this compartment occurred. The presence of GSSG in the apoplast may be the result of the oxidizing conditions existing in the cell wall, and of the interaction between GSH and the disulfides from membrane and apoplastic enzymes. This could explain why the GSSG level was higher in the apoplast than in the symplast. GSH synthesis seems to take place in the cytosol and in chloroplasts (Noctor et al., 1998), but the presence of a GSH transporter at the plasma membrane has been described (Jamaï et al., 1996). Therefore, this GSH transporter would retrieve GSSG for its reduction and recycling to the cytoplasm (Jamaï et al., 1996) and it seems to be similar to the previously described ASC/DHA transporter (Horemans et al., 1996).
In conclusion, salt stress produced an O2.−- and H2O2-mediated oxidative stress in the apoplast, which brought about visible NaCl injuries. A dual function of AOS, including O2.− and H2O2, in exacerbating damage and signaling the activation of defense responses has been described, firstly in pathogenesis (Dat et al., 2000), and more recently during plant responses to several abiotic stresses, including salt stress (Gueta-Dahan et al., 1997; Meneguzzo et al., 1999). Our results are in agreement with those previously reported, in which a lethal level of AOS damages the cell, whereas a moderate level of AOS enhances the adaptation to salt stress (Hernández et al., 1995; Gueta-Dahan et al., 1997). We suggest that NaCl is an abiotic elicitor of phytopathological and anti-oxidative defenses. In fact, together with AOS production and cell death, overlap in induced gene expression after pathogen and NaCl exposure has been observed (Knight et al., 1997; Delumeau et al., 2000). As has been reported under ozone stress, it seems that parts of the mechanisms involved in plant pathogen responses are also induced by NaCl stress, although in this case the appearance of necrotic leaf lesions is associated with sensitivity.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of pea (Pisum sativum L. cv Lincoln and cv Puget) plants supplied by Arnedo (Navarra, Spain) and Sharpes International Seeds Ltd. (Sleaford, UK), respectively, were surface sterilized and germinated in vermiculite with 0.5 mm CaSO4 for 1 week. Seedlings were grown in pots containing aerated nutrient solution in a growth chamber (ASL, Madrid) under optimal conditions for 7 d, as described by Hernández et al. (1999). After that time, plants were grown in a similar medium supplemented by different NaCl concentrations (0, 50, 70, and 90 mm) for 14 d.
Extraction of the IWF
For the recovery of the IWF, 20 g of pea leaves was soaked in deionized water, and subsequently vacuum infiltrated for 3 min at 1.0 KPa and 4°C with 50 mm K-phosphate buffer (pH 6.5) containing 0.2 m KCl and 0.1 mm CaCl2. For APX activity, 5 mm sodium ASC was added. Leaves were then quickly dried and centrifuged at 1,000g for 5 min at 4°C in a 25-mL syringe barrel placed in a centrifuge tube.
To assay enzymatic activities and carbonyl-protein content, the IWF fraction was concentrated about 10-fold using Centripep 10 tubes (Amicon, Bedford, MA) and prepurified by chromatography on Sephadex G-50 MPD10 columns (Pharmacia Biotech AB, Uppsala) equilibrated with 50 mm K-phosphate buffer (pH 6.5) with or without 5 mm sodium ASC. To determine H2O2 and lipid peroxidation, the IWF fraction obtained after centrifugation at 1,000g was used directly. For ASC and GSH analysis, leaves were infiltrated with cold metaphosphoric acid (2%, w/v), containing 0.1 mm bathophenanthroline-disulfonic acid and centrifuged as above. Apoplastic concentrations for H2O2 and other apoplastic metabolites are given without correction for any dilution due to infiltration of the air spaces.
Contamination by cytoplasmic and chloroplastic constituents was assessed by measuring the levels of TPI (Feierabend, 1975) and chlorophylls (Arnon, 1949). Glc-6-phosphate dehydrogenase was not used as cytosolic marker because it has been reported that only 10% of this enzyme is present in the cytosol fraction from pea plants (Corpas et al., 1998).
Leaf Enzyme Extraction
All operations were performed at 4°C. Leaf residues (2 g), which resulted from IWF extraction, were homogenized with a mortar and pestle in 4 mL of ice-cold 50 mm K-phosphate buffer, pH 7.8, 0.1 mm EDTA containing 5 mm Cys, 1% (w/v) polyvinyl pyrrolidone, 0.1 mm phenylmethylsulfonic fluoride, and 0.2% (v/v) Triton X-100. For APX activity, 20 mm sodium ASC was added. The “symplastic” homogenate was centrifuged at 14,000g for 20 min and the supernatant fraction was filtered through Sephadex G-50 PD10 columns (Pharmacia Biotech AB), equilibrated with the same buffer used for the homogenization, with or without 5 mm sodium ASC.
Determination of H2O2, Lipid Peroxidation, and Protein Oxidation
The H2O2 concentration in IWF was determined immediately after isolation by a peroxidase-coupled assay using 4-aminoantipyrine and phenol as donor substrates (Frew et al., 1983). The carbonyl content in oxidatively modified proteins was quantified using the 2,4-dinitrophenylhydrazone assay procedure (Levine et al., 1990). The extent of lipid peroxidation in apoplastic fluid was estimated by determining the concentration of substances reacting to thiobarbituric acid-reactive substances (Buege and Aust, 1978).
Enzymatic Activities of the ASC-GSH Cycle
APX, DHAR, MDHAR, and GR activities were assayed according to previously published protocols, as described by Jiménez et al. (1997). Enzyme activities were corrected for nonenzymatic rates and for interfering oxidations (Jiménez et al., 1997). For APX, the oxidation rate of ASC was estimated between 1 and 60 s after starting the reaction by the addition of H2O2. Correction was made for the low nonenzymatic oxidation of ASC by H2O2. APX was measured in the presence and absence of the specific inhibitor pCMPS (0.5 mm). pCMPS-sensitive APX activity was considered as being due to class I APX (EC 1.11.1.11), whereas pCMPS-insensitive APX activity was considered as due to class APX (EC 1.11.1.7), i.e. APX activity due to substrate-unspecific (guaiacol) class III peroxidases (Jiménez et al., 1998b). To determine MDHAR activity, monodehydroascorbate was generated by the ASC/ASC oxidase system. The rate of monodehydroascorbate-independent NADH oxidation (without ASC and ASC oxidase) was subtracted from the initial monodehydroascorbate-dependent NADH oxidation rate (with ASC and ASC oxidase). For DHAR activity, the reaction rate was corrected for the nonenzymatic reduction of DHA by GSH. A factor of 0.98 was considered to account for the small contribution to the absorbance by GSSG. Values due to GR activity were corrected for the small, nonenzymatic oxidation of NADPH by GSSG (Jiménez et al., 1997). The values of the ASC-GSH cycle enzymes determined in the apoplast fractions were corrected by the percentage of contamination caused by the cytosolic and chloroplastic marker, TPI.
Determination of the ASC/GSH Pool
Apoplastic ASC and DHA and apoplastic GSH and GSSG contents were directly determined in the IWF by HPLC, according to Jiménez et al. (1997). Reduced and oxidized ASC and GSH were extracted with 2% (w/v) metaphosphoric acid from the leaf material resulting from the IWF extraction, and measured as described by Jiménez et al. (1997).
SOD Activity
Total SOD activity was assayed, in the absence and in the presence of KCN, by the ferricytochrome c method using xanthine/xanthine oxidase as the source of O2.− radicals (McCord and Fridovich, 1969). SOD activity was corrected by the percentage of contamination caused by the cytosolic and chloroplastic marker, TPI. To separate SOD isozymes, non-denaturing PAGE and isoelectrofocusing were performed on 10% and 6% (w/v) acrylamide gels, respectively, using a mini protean II dual slab cell (Bio-Rad, Hercules, CA). The range of ampholytes (Pharmacia) used were: pH 3.5 to 5, pH 4.2 to 5.4, and pH 3.5 to 7.0. Samples were prefocused at 150 V for 30 min, and then focused at 250 V for 1 h 30 min. SOD isozymes were localized by the photochemical method of Weissiger and Fridovich (1973). Isoenzyme identification was performed by selective inhibition with KCN or H2O2 (Hernández et al., 1999). The percentage of activity for the different SODs was quantified by recording the transmittance of gels in a CS-9000 densitometer (Shimadzu, Kyoto).
Class III Peroxidase Activity and Isoenzyme Analysis
Class III peroxidase activity in IWF was determined in assays containing 50 mm Tris-acetate buffer (pH 5.0) and 0.5 mm H2O2, using the following electron donors: 0.1 mm coniferyl alcohol (ε262 = 9,600 m−1 cm−1), 0.1 mg mL−1 tetramethylbenzidine-HCl (ε652 = 39,000 m−1 cm−1), and 1.0 mm 4-methoxy-α-naphtol (ε595 = 21,600 m−1 cm−1; Ros Barceló, 1998). The reaction was initiated by adding enzyme. Controls were carried out in the absence of H2O2.
Class III peroxidase isoenzymes were separated by isoelectric focusing in 3.5 to 10.0 pH gradients as described by Ferrer and Ros Barceló (1999), and stained with 4-methoxy-α-naphtol for 1 h at 25°C using a reaction medium identical to that described above. Controls were carried out in the absence of H2O2.
Determination of Protein Content
Protein content was estimated according to Bradford (1976) using bovine serum albumin as standard.
Histochemical Detection of H2O2 and O2.− in Pea Leaves
The histochemical detection of H2O2 and O2.− in pea leaves was performed as described by Schraudner et al. (1998) with minor modifications. In the case of H2O2 we used an endogenous peroxidase-dependent in situ histochemical staining, in which leaf quarters were vacuum infiltrated with 0.1 mg mL−1 3,3′-diaminobenzidine in 50 mm Tris-acetate buffer (pH 5.0) and incubated at 25°C in the dark for 24 h. Controls were performed in the presence of 10 mm ascorbic acid.
The histochemical detection of O2.− was performed by infiltrating leaf quarters directly with 0.1 mg mL−1 NBT in 25 mm K-HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffer (pH 7.6) and incubating at 25°C in the dark for 2 h. Controls in the presence of both 10 mm MnCl2 and/or 100 units mL−1 SOD were performed. The O2.−-removing compounds (MnCl2 and SOD) were directly added to the infiltration buffer.
In both cases, leaf quarters were rinsed in 80% (v/v) ethanol for 10 min at 70°C, mounted in lactic acid:phenol:water (1:1:1, v/v), and photographed directly using an SZX 12 microscope (Olympus. Kyoto).
Footnotes
This work was supported by the Dirección General de Ensenñanza Superior e Investigación Científica, Spain (project no. PB95–004–02), by the Ministerio de Educación y Cultura, Spain (project no. PB–97–1042), by the European Union (project no. FAIR–CT–98–5020), and by the University of Murcia (postdoctoral fellowship to M.A.F.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010188.
LITERATURE CITED
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bérczi A, Möller IM. NADH-monodehydroascorbate oxidoreductase is one of the redox enzymes in spinach leaf plasma membranes. Plant Physiol. 1998;116:1029–1036. doi: 10.1104/pp.116.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinda A, Koch B, Ramanjulu S, Dietz KJ. De novo synthesis and accumulation of apoplastic proteins in leaves of heavy metal-exposed barley seedlings. Plant Cell Environ. 1997;20:969–981. [Google Scholar]
- Bohnert HJ, Jensen RG. Metabolic engineering for increased salt tolerance: the next step. Aust J Plant Physiol. 1996;23:661–667. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cakmak I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- Castillo FJ, Greppin H. Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. after ozone exposure. Environ Exp Bot. 1988;28:231–238. [Google Scholar]
- Corpas FJ, Barroso JB, Sandalio LM, Distefano S, Palma JM, Lupiañez JA, del Río LA. A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem J. 1998;330:777–784. doi: 10.1042/bj3300777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AR, Erichson R, Ellis BA, Curnutte JT. Spontaneous activation of NADPH oxidase in cell-free system: unexpected multiple effects of magnesium ion concentrations. Biochem J. 1999;338:229–233. [PMC free article] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jiménez A, López-Huertas E, Hernández JA. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998;116:1195–1200. doi: 10.1104/pp.116.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delumeau O, Morère-Le Paven MC, Montrichard F, Laval-Martin DL. Effects of short-term NaCl stress on calmodulin transcript levels and calmodulin-dependent NAD kinase activity in two species of tomato. Plant Cell Environ. 2000;23:329–336. [Google Scholar]
- Dietz KJ. Functions and responses of the leaf apoplast under stress. Prog Bot. 1996;58:221–254. [Google Scholar]
- Durán-Carril MV, Rodriguez Bujan C. Antioxidant systems in the leaf apoplast compartment of Pinus pinaster Ait. and Pinus radiata D. Don. plants exposed to SO2. Ann Appl Biol. 1998;133:455–466. [Google Scholar]
- Feierabend J. Developmental studies on microbodies in wheat leaves: III. On the photocontrol of microbody development. Planta. 1975;123:63–77. doi: 10.1007/BF00388061. [DOI] [PubMed] [Google Scholar]
- Ferrer MA, Ros Barceló A. Differential effects of nitric oxide on peroxidase and H2O2 production by the xylem of Zinnia elegans. Plant Cell Environ. 1999;22:891–897. [Google Scholar]
- Foyer CH, Mullineaux P. The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett. 1998;425:528–529. doi: 10.1016/s0014-5793(98)00281-6. [DOI] [PubMed] [Google Scholar]
- Frew J, Jones P, Scholes G. Spectophotometric determination of hydrogen peroxide and organic hydroperoxides at low concentrations in aqueous solution. Anal Chim Acta. 1983;155:130–150. [Google Scholar]
- Gómez JM, Hernández JA, Jiménez A, del Río LA, Sevilla F. Differential response of antioxidative enzymes of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Radic Res. 1999;31:S11–S18. doi: 10.1080/10715769900301261. [DOI] [PubMed] [Google Scholar]
- Gosset DR, Banks SW, Millhollon EP, Lucas MC. Antioxidant response to NaCl stress in a control and an NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiol. 1996;112:803–809. doi: 10.1104/pp.112.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance innonhalophytes. Annu Rev Plant Physiol. 1980;31:149–190. [Google Scholar]
- Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta. 1997;203:460–469. doi: 10.1007/s004250050215. [DOI] [PubMed] [Google Scholar]
- Hernández JA, Campillo A, Jiménez A, Alarcón JJ, Sevilla F. Response of antioxidant systems and leaf water relations to NaCl stress in pea plants. New Phytol. 1999;141:241–251. doi: 10.1046/j.1469-8137.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- Hernández JA, Corpas FJ, Gómez M, del Río LA, Sevilla F. Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant. 1993;89:103–110. [Google Scholar]
- Hernández JA, Jiménez A, Mullineaux PM, Sevilla F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ. 2000;23:853–862. [Google Scholar]
- Hernández JA, Olmos E, Corpas FJ, Sevilla F, del Río LA. Salt-induced oxidative stress in chloroplast of pea plants. Plant Sci. 1995;105:151–167. [Google Scholar]
- Hernández JA, Talavera JM, Martínez-Gómez P, Dicenta F, Sevilla F. Response of antioxidant enzymes to plum pox virus in two apricot cultivars. Physiol Plant. 2001;111:313–321. doi: 10.1034/j.1399-3054.2001.1110308.x. [DOI] [PubMed] [Google Scholar]
- Horemans N, Asard H, Caubergs RJ. Transport of ascorbate into plasma membrane vesicles of Phaseolus vulgaris L. Protoplasma. 1996;194:177–185. [Google Scholar]
- Horemans N, Asard H, Caubergs RJ. The ascorbate carrier of higher plant plasma membranes preferentially translocates the fully oxidized (dehidroascorbate) molecule. Plant Physiol. 1997;114:1247–1253. doi: 10.1104/pp.114.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaï A, Tommasini R, Martinoia E, Delrot S. Characterization of glutathione uptake in broad bean leaf protoplasts. Plant Physiol. 1996;111:1145–1152. doi: 10.1104/pp.111.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, del Río LA, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea (Pisum sativum L.) leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, Pastori GM, del Río LA, Sevilla F. On the role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998a;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, Ros Barceló A, Sandalio LM, del Río LA, Sevilla F. Characterization of mitochondrial and peroxisomal ascorbate peroxidase of pea leaves. Physiol Plant. 1998b;104:687–692. [Google Scholar]
- Kawano T, Muto S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. J Exp Bot. 2000;51:685–693. [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant Cell. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn B, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Luwe M. Antioxidants in the apoplast and symplast of beech (Fagus sylvatica L.) leaves: seasonal variations and responses to changing ozone concentration in air. Plant Cell Environ. 1996;19:321–328. [Google Scholar]
- Luwe M, Takahama U, Heber U. Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacea oleracea L.) leaves. Plant Physiol. 1993;101:969–976. doi: 10.1104/pp.101.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein. J Biol Biochem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Meneguzzo S, Navari-Izzo F, Izzo R. Antioxidative responses of shoots and roots of wheat to increasing NaCl concentrations. J Plant Physiol. 1999;155:274–280. [Google Scholar]
- Morán JF, Becana M, Iturbe-Ormaetxe I, Freschilla S, Klucas RV, Aparicio-Tejo P. Drought induces oxidative stress in pea plants. Planta. 1994;194:346–352. [Google Scholar]
- Morell S, Follmann H, De Tullio M, Häberlein I. Dehydroascorbate and dehydroascorbate reductase are phantom indicators of oxidative stress in plants. FEBS Lett. 1997;414:567–570. doi: 10.1016/s0014-5793(97)01074-0. [DOI] [PubMed] [Google Scholar]
- Navas P, Gómez-Díaz C. Ascorbate free radical and its role in growth control. Protoplasma. 1995;184:8–13. [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Ogawa K, Kanematsu S, Asada K. Intra- and extra-cellular localization of “cytosolic” Cu,Zn-superoxide dismutase in spinach leaf and hypocotyl. Plant Cell Physiol. 1996;37:790–799. [Google Scholar]
- Ogawa K, Kanematshu S, Asada K. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol. 1997;38:1118–1126. doi: 10.1093/oxfordjournals.pcp.a029096. [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemic via the octadecanoid pathway. Proc Natl Acad Sci USA. 1999;96:6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras A, Olmos E, Martínez-Solano JR, Hellín E. Cd-induced oxidative burst in tobacco BY2 cells: time curse, subcellular location and antioxidant response. Free Radic Res. 1999;31:S33–S38. doi: 10.1080/10715769900301291. [DOI] [PubMed] [Google Scholar]
- Polle A, Chakrabarti K, Schümann W, Rennenbreg H. Composition and properties of hydrogen peroxide decomposing systems in extracellular and total extracts from needles of Norway Spruce (Picea abies L., Karst.) Plant Physiol. 1990;94:312–319. doi: 10.1104/pp.94.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartacci MF, Cosi E, Navari-Izzo F. Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Exp Bot. 2001;52:77–84. [PubMed] [Google Scholar]
- Ranieri A, D'Urso G, Nali C, Lorenzini G, Soldatini GF. Ozone stimulates apoplastic antioxidant systems in pumpkin leaves. Physiol Plant. 1996;97:381–387. [Google Scholar]
- Ros Barceló A. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta. 1998;207:207–216. [Google Scholar]
- Savouré A, Thorin D, Davey M, Hua XJ, Mauro S, Van Montagu M, Inzé D, Verbruggen N. NaCl and CuZnSO4 treatments trigger distinct oxidative defense mechanism in Nicotiana plumbaginifolia L. Plant Cell Environ. 1999;22:387–396. [Google Scholar]
- Schraudner M, Moeder W, Wiese C, Van Camp W, Inzé D, Langebartels C, Sandermann H., Jr Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Streb P, Feierabend J. Oxidative stress-responses accompanying photoinactivation of catalase in NaCl-treated rye leaves. Bot Acta. 1996;109:125–132. [Google Scholar]
- Streller S, Wingsle G. Pinus sylvestris L. needles contain extracellular CuZn-superoxide dismutase. Planta. 1994;192:195–201. [PubMed] [Google Scholar]
- Takahama U, Okini T. Regulation of peroxidase-dependent oxidation of phenolic in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol. 1992;33:379–387. [Google Scholar]
- Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Kishitani S, Takabe T, Yokota S, Takabe T. Salt tolerance of transgenic rice overexpresing yeast mitochondrial Mn-SOD in chloroplasts. Plant Sci. 1999;148:131–138. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada KA. Recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell. 1999;11:1195–1206. doi: 10.1105/tpc.11.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H, Carver TLW, Foyer CH. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998a;117:1103–1114. doi: 10.1104/pp.117.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H, Harbinson J, Carver TLW, Foyer CH. Antioxidant defenses of the apoplast. Protoplasma. 1998b;205:129–140. [Google Scholar]
- Weissiger RA, Fridovich I. Superoxide dismutase: organelle specifity. J Biol Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]