Abstract
A novel protein elicitor (PaNie234) from Pythium aphanidermatum (Edson) Fitzp. was purified, microsequenced, and the corresponding cDNA was cloned. The deduced amino acid sequence contains a putative eukaryotic secretion signal with a proteinase cleavage site. The heterologously expressed elicitor protein without the secretion signal of 21 amino acids (PaNie213) triggered programmed cell death and de novo formation of 4-hydroxybenzoic acid in cultured cells of carrot (Daucus carota). Programmed cell death was determined using the tetrazolium assay and DNA laddering. Infiltration of PaNie213 into the intercellular space of leaves of Arabidopsis (Columbia-0, wild type) resulted in necroses and deposition of callose on the cell walls of spongy parenchyma cells surrounding the necrotic mesophyll cells. Necroses were also formed in tobacco (Nicotiana tabacum cv Wisconsin W38, wild type) and tomato (Lycopersicon esculentum Mill.) but not in maize (Zea mays), oat (Avena sativa), and Tradescantia zebrina (Bosse), indicating that monocotyledonous plants are unable to perceive the signal. The reactions observed after treatment with the purified PaNie213 were identical to responses measured after treatment with a crude elicitor preparation from the culture medium of P. aphanidermatum, described previously. The availability of the pure protein offers the possibility to isolate the corresponding receptor and its connection to downstream signaling-inducing defense reactions.
Plants are able to defend themselves successfully with a complex set of preformed structures and inducible reactions. The inducible reactions require the perception of either plant-derived (endogenous) or pathogen-derived (exogenous) signal molecules. These so-called elicitors are of diverse chemical nature and include proteins, peptides, glycoproteins, lipids, and oligosaccharides (Nürnberger, 1999). Elicitors trigger plant defense responses that are part of the basic or non-host resistance of plants (Nürnberger, 1999). Defense is often associated with localized hypersensitive cell death (Mittler et al., 1997) and the de novo formation of low-Mr antimicrobial compounds called phytoalexins (Hammond-Kosack and Jones, 1996). The reinforcement of cell wall constituents is also part of the defense response (Bruce and West, 1989). The structural and cultivar specificity of elicitors and their ability to trigger plant defense responses at very low concentrations strongly suggest the existence of receptors at the plasma membrane and a downstream signal transduction cascade (Ebel and Cosio, 1994). Despite many years of work, there are very few cases described in which the causal link between elicitors from oomycetes and putative receptors or high-affinity binding sites has unequivocally been linked to induced defense responses (Nennstiel et al., 1998). To study the elicitor-receptor interaction and the downstream signaling, pure elicitor molecules are necessary prerequisites.
Protein elicitors have been found in bacterial pathogens as well as in oomycetes and ascomycetes (Ebel and Cosio, 1994). Gram-negative phytopathogenic bacteria like Erwinia amylovora and Pseudomonas syringae secrete proteins, which induce a hypersensitive response, the so-called harpins (Wei et al., 1992; He et al., 1993). Bacterial flagellin and the corresponding receptor-like kinase were found more recently to induce defense responses (Felix et al., 1999; Gómez-Gómez and Boller, 2000).
In the order of Peronosporales, protein elicitors with a relative molecular mass of 10 kD and necrosis-inducing activity were identified and designated the elicitins (Ponchet et al., 1999). The race-specific Avr9-peptide from Cladosporium fulvum is responsible for the induction of active defense responses in tomato (Lycopersicon esculentum Mill.) cell cultures (May et al., 1996).
The cell wall of Phytophthora sojae contains a 42-kD glycoprotein that induces the activation of defense-related genes in parsley (Nürnberger et al., 1994). The active part of this glycoprotein is an internal peptide of 13 amino acids (Pep-13; Hahlbrock et al., 1995). From Phytophthora parasitica, an elicitor-active protein (Pp-elicitor) has been described recently that activates defense responses in parsley suspension cultures similar to those induced by the Pep-25 elicitor, containing the sequence of Pep-13 (Felbrich et al., 2000).
A 24-kD (Nep1) necrosis- and ethylene-inducing protein has been purified from culture filtrates of Fusarium oxysporum f. sp. erythroxyli (Bailey, 1995). When applied to weed species as a foliar spray it causes necrosis (Jennings et al., 2000).
Pythium aphanidermatum is a cosmopolitan pathogen with a wide host range causing economic losses on several important crops. P. aphanidermatum infects preferably juvenile tissues like seedling stems. Suspension-cultured hyphae of P. aphanidermatum release a variety of elicitor-active molecules into the culture medium. Among these elicitors are carbohydrates as well as proteins (Schnitzler 1992). In carrot (Daucus carota) cell cultures, and protoplasts derived from the cultured cells, these elicitors induce the de novo formation of 4-hydroxybenzoic acid (4-HBA). In intact cells, this compound is transferred to the cell wall and covalently linked to cell wall constituents, whereas in protoplasts lacking cell walls, the compound is secreted in a conjugated form into the culture medium (Schnitzler and Seitz, 1989). The carrot system described here reflects a non-host interaction or a basic resistance. The formation of active Phe ammonia-lyase (PAL) is necessary for 4-HBA synthesis and the elicitor induces the synthesis on a transcriptional level (Koch et al., 1998). The P. aphanidermatum-derived elicitor also induces programmed cell death in carrot cell cultures (Koch et al., 1998). Using an assay for loss of carrot cell viability, a 25-kD protein with elicitor activity was identified and partially purified (Koch et al., 1998). Using this crude elicitor preparation and specific inhibitors, several components of the signal transduction pathway have been identified. It was shown that an increase of cytoplasmic calcium concentration is essential for cell death induction and 4-HBA synthesis (Bach et al., 1993; Koch et al., 1998) and it was also demonstrated by these inhibitor experiments that G proteins are involved in signal transduction leading to programmed cell death, but not to 4-HBA accumulation (Koch et al., 1998).
To rule out effects of contaminating proteins and carbohydrates in the crude preparation, a pure elicitor protein is necessary. In the present paper, we describe the isolation of a cDNA encoding the elicitor protein (PaNie234) from P. aphanidermatum. The protein contains 234 amino acids and has a putative eukaryotic secretion signal harboring a proteinase cleavage site. The mature elicitor protein without the secretion signal (PaNie213) consists of 213 amino acids. PaNie213 has been heterologously expressed in Escherichia coli and can be detected by a rabbit antiserum raised against the elicitor protein. Using affinity chromatography, the His-tagged PaNie213 was purified and assayed for its elicitor activity in suspension-cultured carrot cells and by infiltration into leaves of dicotyledones Arabidopsis, tobacco (Nicotiana tabacum), tomato, and monocotyledons maize (Zea mays), oat (Avena sativa), and Tradescantia zebrina.
With the purified PaNie213 in hand, we were able to show that a single pure elicitor protein is sufficient to trigger multiple defense pathways.
RESULTS
To study elicitor-receptor interactions and the link to downstream defense reactions, a pure elicitor protein is necessary. Therefore, heterologous expression and purification of the elicitor from P. aphanidermatum was initiated.
Heterologous Expression of the His-Tagged Protein Elicitor from P. aphanidermatum (PaNie213)
The elicitor protein was purified from the culture medium of P. aphanidermatum with preparative SDS-PAGE as the final step. Because it was blocked at its N terminus, the protein was proteolytically digested and the released oligopeptides were microsequenced. On the basis of these sequences, degenerated primers were used to screen a cDNA library from P. aphanidermatum. The amino acid sequence is illustrated in Figure 1. By analyzing the sequence according to Nielsen et al. (1997), we can predict a putative eukaryotic secretion signal that is not present in PaNie213 and also reveal a putative proteinase cleavage site. This deduced amino acid sequence has no predicted transmembrane domain (Frishman and Argos, 1996). We ruled out the existence of a glycosyl residue using the DIG Glycan Double Labeling Kit (Boehringer Mannheim, Mannheim, Germany; data not shown). In Table I, the sequence of PaNie234 was compared with sequences in the National Center for Biotechnology Information database. Similar proteins with 70% to 84% similarity were found with F. oxysporum cv erythroxyli (accession no. AAC97382), P. sojae (accession no. AAK01636), P. parasitica (accession no. AAK19753), P. infestans (accession no. AAK25828), and the eubacterium B. halodurans (accession no. BAB04114). This may represent a novel family of elicitor proteins.
Figure 1.
Amino acid sequence of PaNie234 in the one letter code. A predicted secondary structure is given in the lower line (Frishman and Argos, 1996). H, Alpha helix; C, random coiled; E, extended strands; the arrowhead points to a proteinase cleavage site at the end of the eukaryotic secretory signal sequence (Nielsen et al., 1997). Underlined, Microsequenced oligopeptides; shadowed box, amino acid sequence used for degenerated primer (pep2rev) design.
Table I.
Comparison of deduced amino acid sequences of PaNie234 with other necrosis-inducing elicitor proteins
| Elicitor Proteins | Similarity in % to PaNie234 |
|---|---|
| P. aphanidermatum (25-kD protein elicitor) | 100.0 |
| Phytophthora infestans (necrosis-inducing protein NPP1) | 84.2 |
| P. parasitica (necrosis-inducing protein NPP1) | 83.8 |
| P. sojae (necrosis-inducing peptide) | 83.1 |
| Bacillus haloduransa (necrosis- and ethylene-inducing protein) | 78.2 |
| F. oxysporum f. sp. erythroxyli (necrosis- and ethylene-inducing peptide) | 70.4 |
Translated open reading frame (ORF) only; no activity assays were performed.
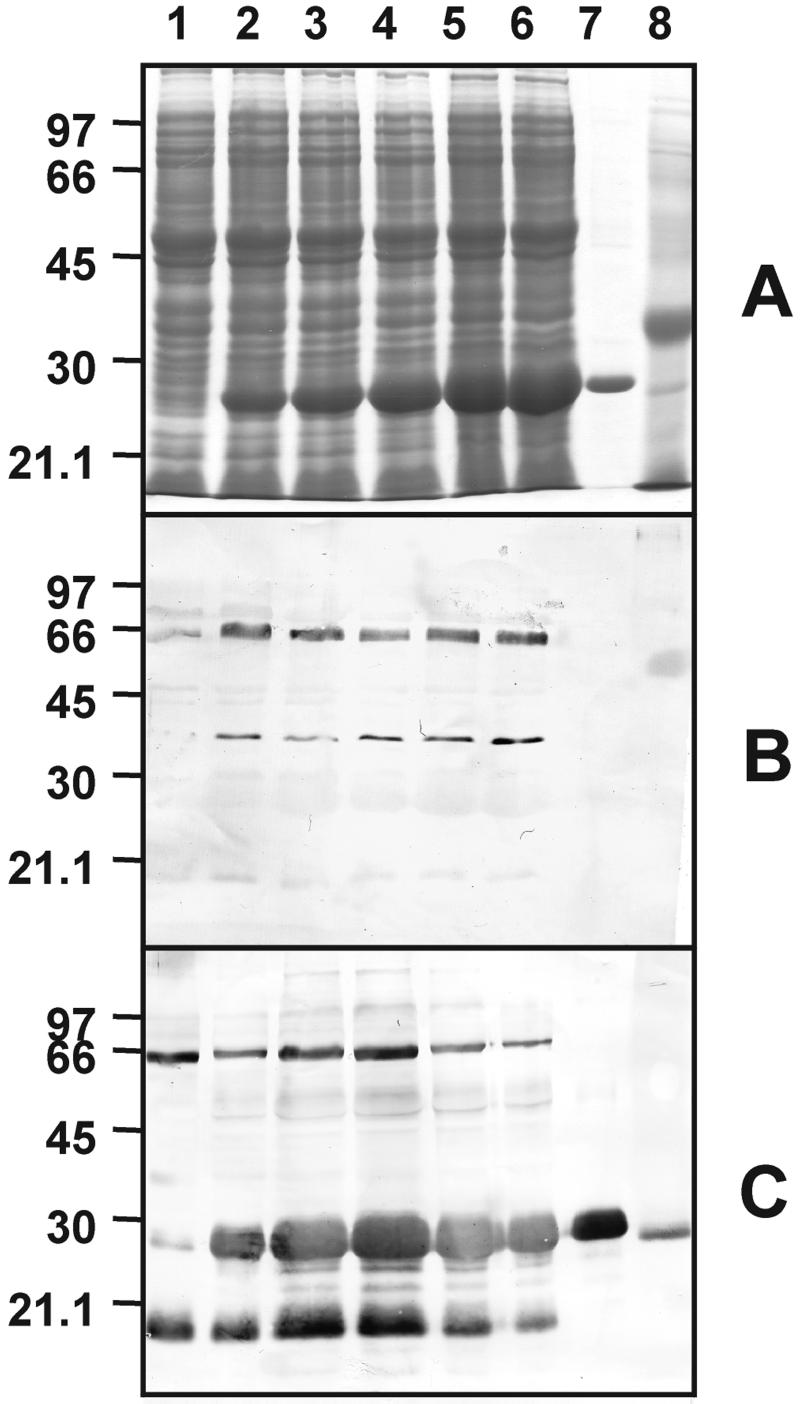
Appropriate oligopeptides were synthesized as antigens to raise an antiserum against the protein elicitor. In Figure 2, the heterologous expression and purification of PaNie213 is followed by SDS-PAGE and western blotting. The antiserum detects a single band after the final purification step.
Figure 2.
SDS-PAGE of fractions from purification of heterologously expressed C-terminal His-tagged PaNie213 in E. coli in comparison to the crude elicitor. A, Coomassie Blue-stained SDS-PAGE. Lane 1, Control; lanes 2 through 6, 1 to 5 h after induction with isopropylthio-β-galactoside; lane 7, purified PaNie213; lane 8, crude elicitor. B, Hybridization with pre-immuneserum (1:500); C, hybridization with PaNie213 antiserum (1:20,000).
The heterologous expression of C-terminal His-tagged PaNie213 in E. coli and purification using nickel-nitrilotriacetic acid agarose (Ni-NTA) resulted in a 25-kD protein that is the functional and mature part of the total PaNie234 secreted by the oomycete P. aphanidermatum into the culture medium. Purification leads to a single protein band with a molecular mass of 25 kD.
Genomic Organization
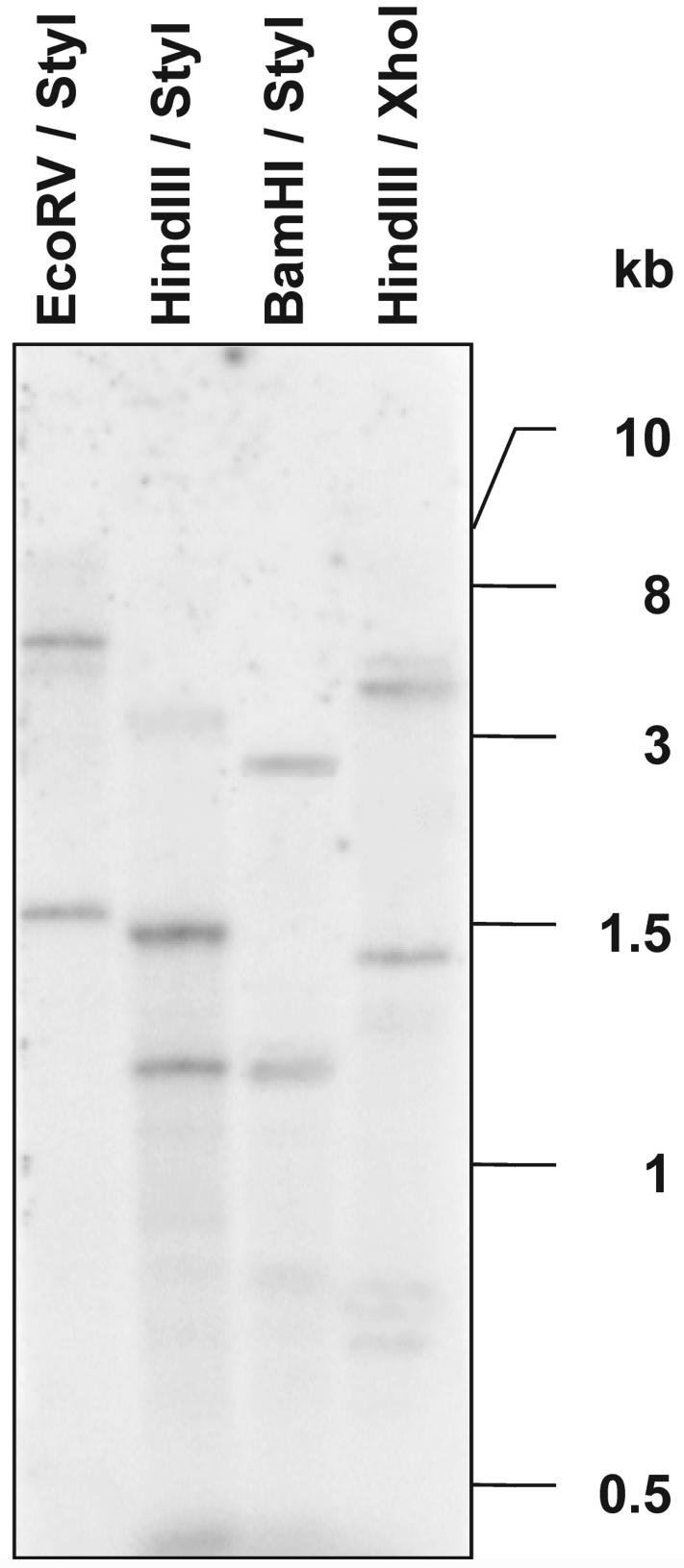
DNA gel blotting was performed to provide information on genomic organization (Fig. 3). Genomic DNA of P. aphanidermatum was digested with various combinations of restriction enzymes and probed with a cDNA clone containing the total ORF from PaNie213. The restriction enzymes used for digestions are not blocked by methylation sites on the DNA. Two strong bands were always present in all four lanes regardless of the enzyme combinations used. This is an indication that a multicopy gene is present, provided that no intron with a cleavage site exists. The absence of introns was demonstrated by comparing the product length after PCR on genomic DNA and cDNA. The resulting two molecules had identical lengths (data not shown).
Figure 3.
Southern-blot analysis of genomic DNA from P. aphanidermatum. Each of 30 μg of DNA was digested with the enzyme combination indicated and then separated on a 0.8% (w/v) agarose gel. The blot was probed with 32P-labeled PaNie213 ORF cDNA. The blots were washed with 0.1% (w/v) SDS and 0.2× SSC at 65°C.
Defense-Related Responses of Carrot Cell Cultures
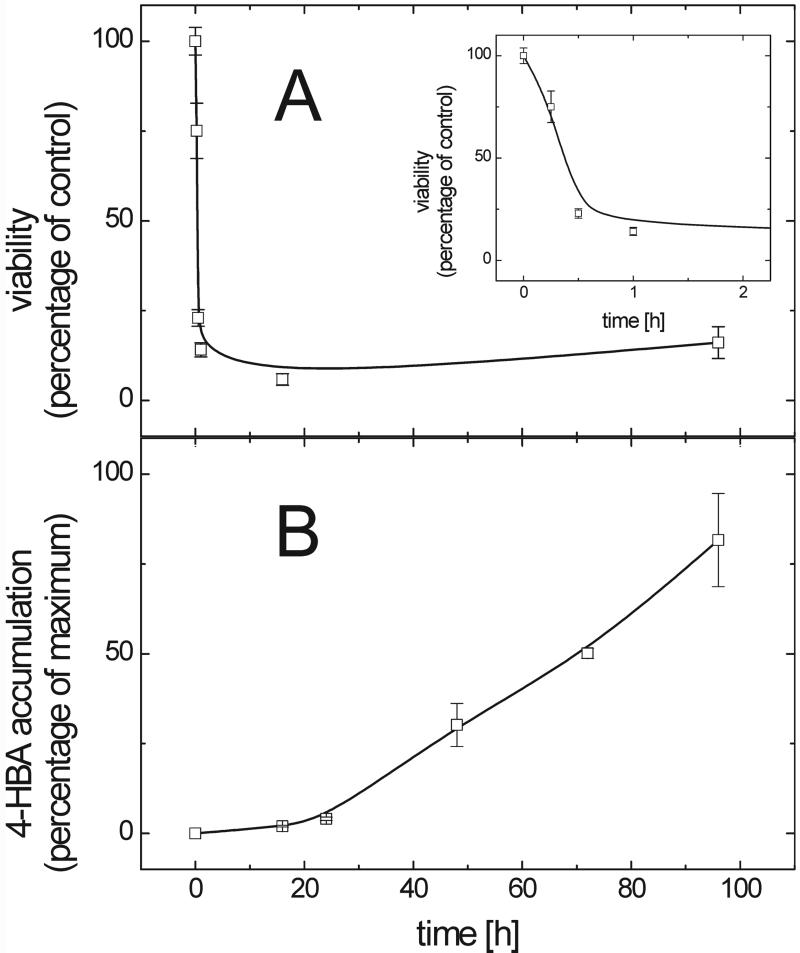
To show that PaNie213, a single pure elicitor protein, is sufficient to trigger multiple defense reactions, carrot cell cultures were treated with PaNie213. As can be seen in Figure 4A, the viability decreased rapidly within the first 30 min after elicitor application. This time course is nearly identical with that observed with a (NH4)2SO4-precipitated crude elicitor described previously (Koch et al., 1998). PaNie213 was active at very low protein concentrations, inducing marked viability losses (IC50 = 50 nm) and the accumulation of large amounts of 4-HBA (data not shown). However, to obtain maximum effects in the experiments depicted in Figure 4, final concentrations of 500 nm were applied.
Figure 4.
Time courses of elicitor-induced (500 nm PaNie213) viability changes and accumulation of 4-HBA in suspension-cultured carrot cells. A, The viability was monitored using the tetrazolium-assay and expressed as percentage of control (untreated cells). The inset shows the data for the first 2 h with an enlarged abscissa. B, Accumulation of cell wall-bound 4-HBA. Phenolic acids were released by saponification with 1 m NaOH from crude cell wall preparations and separated by HPLC (ODS Hypersil with a linear gradient of water:acetic acid (95:5; v/v) and methanol ranging from 10% to 50% (v/v) methanol over 30 min. 4-HBA was detected at 260 nm. The 4-HBA concentration is expressed as percentage of the highest accumulation of 4-HBA (100% = 10.6 μg mg−1 cell wall carbohydrates). Each data point represents the average of triplicates. Error bars represent sd.
The accumulation of 4-HBA was determined (Fig. 4B). As already demonstrated for the crude elicitor preparation (Schnitzler and Seitz, 1989; Koch et al., 1998), the pure PaNie213 also enhanced the accumulation of 4-HBA. Sixteen hours after the application of PaNie213 to carrot cells, 4-HBA was accumulated rapidly. As already shown previously, this process is preceded by an increase of PAL-mRNA (Koch et al., 1998). As previously shown, the 4-HBA could only be released from the carrot cell wall by alkaline hydrolysis indicating a covalent linkage of this compound to wall constituents (Schnitzler and Seitz, 1989). These results demonstrate that the pure elicitor and the crude preparation induce the same responses.
PaNie213-Induced Chromatin Fragmentation
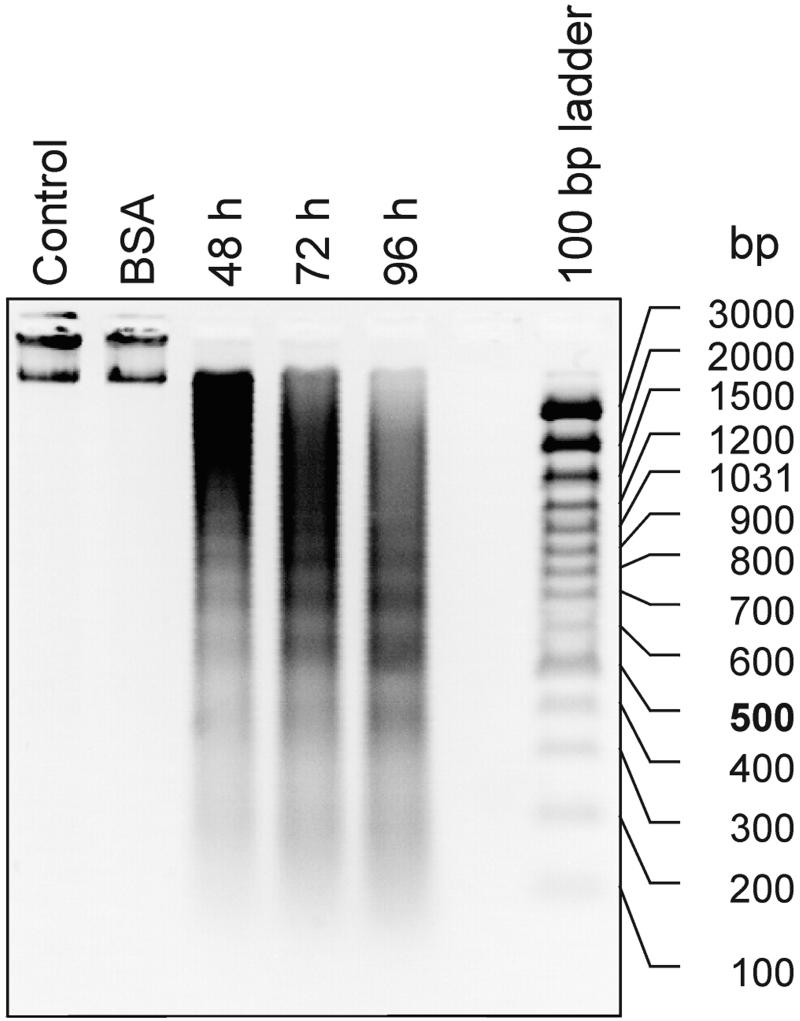
The fragmentation of nDNA is one of the best established criterion for confirming an elicitor-dependent programmed cell death during the hypersensitive response (Peitsch et al., 1993; Ryerson and Heath, 1996). Therefore, we isolated protoplasts from cultured carrot cells and treated them with PaNie213. These protoplasts responded to the pure elicitor protein PaNie213 in the same way that they did to the crude preparation, as described previously. In Figure 5, DNA laddering after treatment with PaNie213 is shown. The fragmentation of chromatin to multiples of 180 bp is already visible 48 h after the onset of elicitation and continues during the next 48 h. As shown by Koch et al. (1998), this active chromatin fragmentation is dependent on the import of external Ca2+, as was shown in a previous communication. G-proteins are involved in this active process as is shown by the fact that mastoparan and Mas-7 incubation mimic the elicitor effect (Koch et al., 1998).
Figure 5.
Elicitor–induced fragmentation of nDNA in protoplasts derived from suspension-cultured cell cultures of carrot. The DNA was extracted from protoplast at the indicated times and equal amounts were separated on a 1.2% (w/v) agarose gel and stained with ethidium bromide. The concentration of PaNie213 was 100 nm. In the controls, water or equal bovine serum albumin (BSA) concentrations were applied.
PaNie213 Treatment of Intact Plants
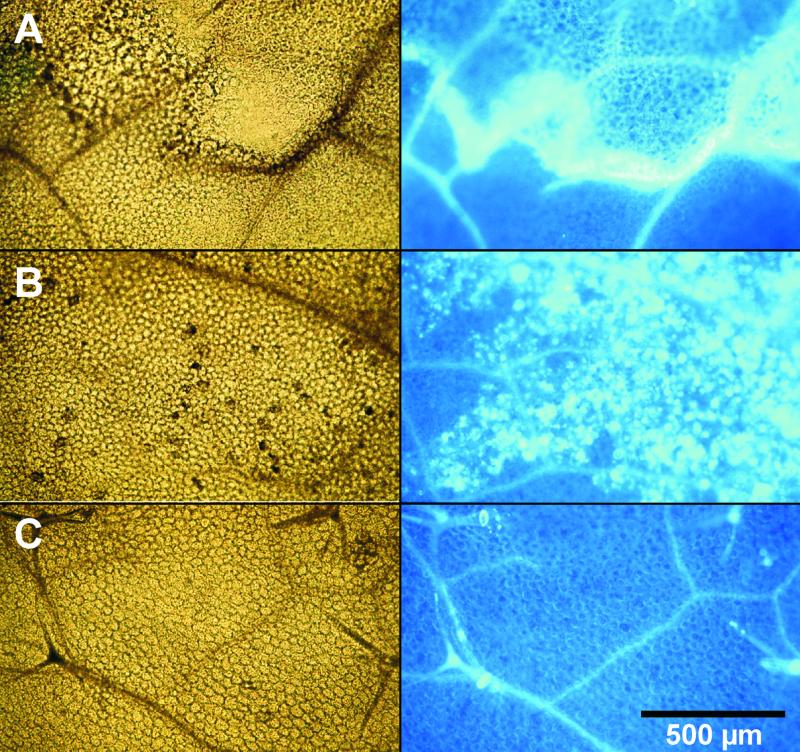
To study the effects of the pure elicitor protein on intact plant organs of genetically well defined systems, we treated leaves of Arabidopsis (Columbia-0 [Col-0], wild type [WT]), tobacco (W38; WT), and maize. In the first series (Fig. 6A), 5 μL of a PaNie213 solution (10 μm) was infiltrated into the intercellular space of Arabidopsis leaves through stomatal pores. This treatment resulted in the formation of clearly defined necrotic areas at the infiltration site (see also Fig. 7). The necrotic area is bordered by a ring of callose deposition on mesophyll cell walls (Fig. 6A). At lower elicitor concentrations (1 μm), no necrotic effects were observed; in contrast to the reaction seen at higher concentrations, callose is present only in a diffuse pattern (Fig. 6B). Equimolar protein concentrations of bovine serum albumin had no effect (Fig. 6C). The same is true for incubation with the corresponding buffer (data not shown).
Figure 6.
Elicitor-induced callose deposition in leaves of Arabidopsis (Col-0, WT). The 3-week-old leaves were infiltrated using a 1-mL syringe without a needle through stomatal pores with solutions of PaNie213 (5 μL of a 10-μm or 1-μm solution of PaNie213). As a control, equal concentration of BSA was applied. After an incubation period of 24 h, the chlorophyll was removed and the bleached leaves were stained with aniline blue and photographed in bright field (left) and under UV light (right). A, Ten micromolar PaNie213; B, 1 μm PaNie213; C, BSA control.
Figure 7.
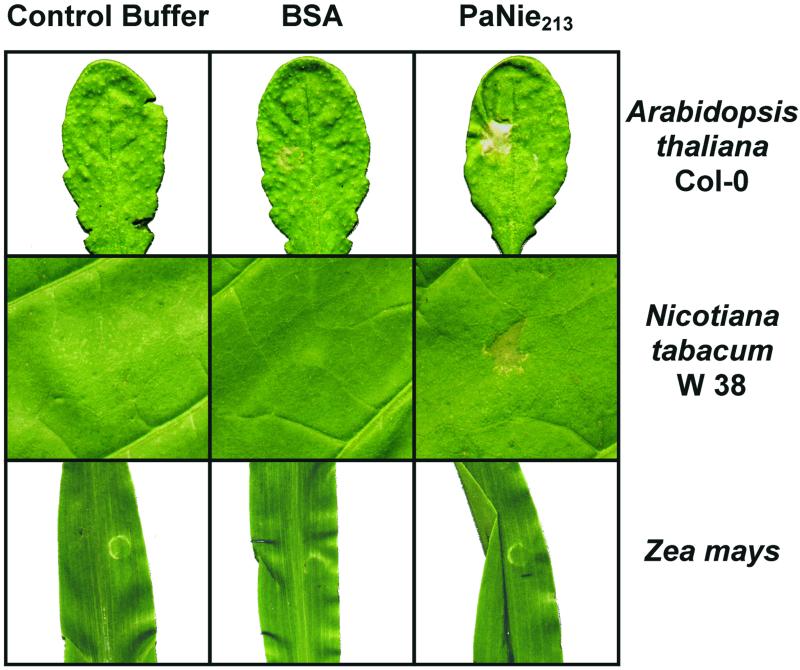
Comparison of effects of PaNie213 (10 μm) infiltrated (5 μL) into leaves of Arabidopsis (Col-0; 3-week-old plants), tobacco (W38, WT, 4-month-old plants), and maize (1-week-old plants). Buffer and BSA were used as controls. For infiltration, see Figure 5. The photographs were taken 48 h after elicitation.
In an additional series of experiments, the reactions of different plant species to PaNie213 were compared. Infiltration of a 10-μm elicitor solution into Arabidopsis leaves resulted in strong necrotic effects (Fig. 7), as did infiltration into tobacco, which responded more rapidly, already forming necrotic lesions after 6 h (data not shown). Tomato showed also necrotic lesions. Infiltration of maize failed to lead to a reaction even 72 h after the onset of elicitation (Fig. 7). Other monocotyledons like Avena sativa and T. zebrina (Bosse) also show no response to elicitation.
DISCUSSION
Here, we report a novel protein elicitor from the culture medium of the pathogenic oomycete P. aphanidermatum that triggers an array of defense responses in carrot cell cultures and in intact plants of Arabidopsis and tobacco.
The protein was purified by preparative SDS-PAGE (Koch et al., 1998). Sequence comparison of proteins in the National Center for Biotechnology Information database yielded similar sequences for various phytopathogenic fungi and the eubacterium B. halodurans (only the sequence was published but no physiological function of the deduced protein was described; see Table I) indicating a conserved gene family. Such a gene must have an indispensable function in the pathogen; otherwise, it would have been eliminated during evolution. Analysis of the genomic organization of P. aphanidermatum using Southern blot presents strong evidence for the existence of at least two copies of the PaNie gene.
To find the smallest peptide with elicitor activity, N-terminal truncated cDNAs missing 63 or 106 amino acids were expressed in E. coli. Both products did not show any elicitor activity in carrot cell cultures with regard to 4-HBA accumulation and loss of viability (data not shown). Because all attempts to produce C-terminal truncated peptides proved to be unsuccessful and therefore no physiological assays concerning elicitor activity of these truncated peptides could be performed. These experiments revealed that the entire PaNie213 is necessary for elicitor activity, suggesting that the intact secondary structure must be preserved for its activity. This is in contrast to the Pep-13 from P. sojae in which the elicitor active peptide was only 13 amino acides (Hahlbrock et al., 1995).
Rapid loss of viability and the induction of 4-HBA accumulation clearly demonstrates that a single protein elicitor (PaNie213) is sufficient to trigger both defense responses, namely programmed cell death and phytoalexin synthesis in suspension-cultured carrot cells.
This apparent contradiction between viability loss and the concomitant induction of de novo synthesis of 4-HBA suggests that the remaining viable cells have greatly elevated 4-HBA biosynthetic activity. In an earlier paper, we demonstrated that the transcription of PAL-mRNA proceeded even after cell death was initiated (Koch et al., 1998). Dying cells presumably can still be active 4-HBA producers. It has been shown for Lactuca sativa after infection with Bremia lactucae that dying cells are still able to synthesize defense-related compounds (Bennet et al., 1996). A second reasonable explanation would be that the tetrazolium assay as an indicator for initiated programmed cell death measures only the impairment of mitochondrial activity (Berridge and Tan, 1993).
A typical feature of programmed cell death is the digestion of the chromatin to nucleosomal fragments with multiples of 180 bp (Ryerson and Heath, 1996). Again, with the purified elicitor PaNie213 we obtained a DNA laddering that was also observed with the crude elicitor preparation (Koch et al., 1998).
In addition to the elicitor-triggered defense response of the carrot cell culture, responses of intact plants to this novel elicitor protein were examined. As previously shown, carrot leaves respond to injections of the crude elicitor preparation by senescence at the leaf tips and by the accumulation of 4-HBA and other wall-bound phenols (Koch et al., 1998).
To broaden our understanding of the elicitor action PaNie213 was applied to genetically well-defined systems like Arabidopsis and tobacco. Infiltration of Arabidopsis leaves resulted in necrotic lesions that are surrounded by a ring of cells with callose deposits at their cell walls. At the border of the necrosis, a higher concentration of brownish material was present that could be due to a reinforcement of these cell walls with wall-bound phenols. The callose deposits are thought to form a barrier between necrotic and healthy tissue (Vleeshouwers et al., 2000). It has been shown that 1,3-β-glucan synthase is merely activated by calcium and no de novo synthesis is necessary (for review, see Kauss, 1987).
These callose deposits appeared at a threshold concentration of 8 to 10 μm. At lower elicitor concentrations, the callose was distributed in a diffuse manner (see Fig. 6B). Similar to carrot cells, Arabidopsis also seems to react with multiple responses. Preliminary infiltration experiments with elicited Arabidopsis leaves showed a dose-dependent increase in camalexin accumulation measured according to Tsuji et al. (1992) and Thomma et al. (1999) by fluorescence and UV light detection after thin-layer chromatography and HPLC separation (M. Malcherowitz, H.U. Seitz, unpublished data).
Tobacco leaves infiltrated with PaNie213 also showed necroses. However, maize and other monocotyledons (see “Materials and Methods”) did not respond to this treatment by forming necroses. This is an indication that dicotyledonous and monocotyledonous plants respond differently to protein elicitors, demonstrated here for PaNie213. This has raised the question whether monocotyledonous plants are unable to perceive the elicitor signal, at least as expressed by rapid cell death. Jennings et al. (2000) reported similar behavior after spraying various weed plants with a protein isolated from culture filtrates of F. oxysporum.
In summary, we present strong evidence here that a single pure elicitor protein is sufficient to trigger multiple defense reactions in the cell culture system of carrot, Arabidopsis, and tobacco leaves. This study provides the basis for a better understanding of the recognition process and the causal connection with downstream signaling toward different defense reac-tions. The Arabidopsis system offers the possibility of taking a genetic approach to isolate a receptor protein and link it to the downstream signal pathway and the de novo synthesis of defense compounds.
MATERIALS AND METHODS
Culture Conditions for Cell Cultures of Carrot (Daucus carota) and for Pythium aphanidermatum (Edson) Fitzp.
Cell suspension cultures of carrot were cultivated as previously described (Noé et al., 1980). P. aphanidermatum was propagated in liquid media as previously described (Schnitzler and Seitz, 1989).
Treatment of Carrot Protoplasts with PaNie213 and Isolation of Genomic DNA (DNA Laddering)
The protoplasts were isolated from carrot cell cultures with a protocol described previously (Koch et al., 1998).
Purified protoplasts were counted in a Fuchs-Rosenthal hematocytometer. The suspension was brought to a cell titer of 2 × 105 protoplasts mL−1. The samples were incubated in aliquots of 10 mL in petri dishes at 26°C. The elicitor was applied directly after protoplast isolation.
After the incubation with PaNie213, the protoplasts were collected at 100g for 5 min and the supernatant was discarded. Lysis buffer (500 μL containing 100 mm Tris-HCl, pH 8.0, 100 mm NaCl, 20 mm EDTA, 2% [w/v] SDS, and 0.1% [v/v] 2-mercaptoethanol) was added and the mixture was incubated for 10 min at 65°C. After extraction in phenol:chloroform:isoamyl-alcohol (25:24:1, v/v), the aqueous phase was precipitated with ethanol (0.1 volume 3 m sodium acetate and 2.5 volumes of ethanol). The DNA was dissolved in Tris-EDTA buffer to a final concentration of 0.5 μg × μL−1. Equal amounts of DNA were separated on 1.2% (w/v) agarose gels by electrophoresis and stained with ethidium bromide.
Screening of cDNA and Microsequencing
Microsequencing of a purified protein from P. aphanidermatum with elicitor activity (Koch et al., 1998) yielded sequences of oligopeptides (peptide1, N′-AVINXDAVPVXPQPEPADXT-C′; and peptide2, N′- LGFTTSAGKQQPLIQWEQMTQAARD-C′) that were used to design a de- generated primer (degenerated primer derived from peptide2; pep2rev 5′-ATHCARTGGGARCARARGAC-3′). PCR (primer: pep2rev and T7 5′-GTAATACGACTCACTATAGGGC-3′) with cDNA from P. aphanidermatum as a template produced a 260-bp fragment which contained parts of the oligopeptide coding region. A second PCR on cDNA from P. aphanidermatum with pep2rev and a forward primer binds in front of the poly A tail (5′-GTCGACAGCACTTTACTGG-3′) led to a fragment that was used as an [α-32P]dCTP probe to screen a cDNA library of P. aphanidermatum. The cDNA library was established with the ZAP-cDNA Synthesis Kit (Stratagene, Heidelberg). A clone lacking the 5′-end was completed with 5′-Race (5′-Race System for Rapid Amplification of cDNA Ends, Version 2.0; GibcoBRL Life Technologies, Karlsruhe, Germany).
Heterologous Expression and Purification of the His-Tagged PaNie213
The pQE60 expression vector (Qiagen, Hilden, Germany) containing the ORF coding for PaNie213 was used for the heterologous expression of the elicitor protein with a C-terminal His tag in Escherichia coli (strain M15). An artificial translation initiation site was inserted using PCR-based mutagenesis, starting after the putative eukaryotic secretory signal sequence. This protein, coding for a protein of 213 amino acids, was designated PaNie213. For the PCR, we used the reverse primer 5′-GAGACCATGGCCGTGATCAACCATG-3′ and the forward primer 5′-CTCTGGATCCCTGGAAAAACGCCTTCACGAG-3′.
The following PCR conditions were chosen: 5 min at 94°C, cycling denaturation for 20 s at 94°C, annealing for 20 s at 56°C, and elongation for 90 s at 70°C using Pyrococcus furiosus DNA polymerase (Stratagene, La Jolla, CA).
Preparation and purification of PaNie213 under denaturing conditions was performed using the batch purification protocol for QIAexpressionist Ni-NTA technology (Qiagen). Transformed E. coli cells from a 1-L batch were induced with isopropylthio-β-galactoside (1 mm) for 4 h and then disrupted by ultrasonication (Micro Tip Sonifier B-12, Branson, Danbury, CT) in 20 mL of buffer B (Qiagen; 8 m urea, 0.1 m NaH2PO4, and 0.01 m Tris-HCl, pH 8.0). The lysate was added to 10 mL of Ni-NTA and incubated for 5 h at 4°C with gentle agitation. The matrix was washed three times stepwise with buffer C (urea; 0.1 m NaH2PO4, and 0.01 m Tris-HCl, pH 6.3) containing 20 mm imidazole with decreasing concentrations of urea (first step, 2 m; second step, 0.5 m; and third step, 0.1 m urea). Elution occurred following a final wash step (50 mm Na2HPO4/NaH2PO4 buffer, pH 8.0, 300 mm NaCl, and 300 mm imidazole) with 20 mL of 6 m guanidine-HCl and 0.2 m acetic acid. The elicitor protein PaNie213 was dialyzed against water. This protein was used for the elicitation of cell cultures and for infiltration into leaves.
Isolation of Genomic DNA of P. aphanidermatum and Southern-Blot Analysis
Genomic DNA was isolated according to Dellaporta et al. (1983) from mycelium (10 g fresh weight) frozen in liquid nitrogen and ground with mortar and pestle. DNA samples (30 μg) were digested with EcoR V and NdeI, HindIII and StyI, BamHI and StyI, HindIII and XhoI. The products were fractionated on 0.8% (w/v) agarose gel and then transferred onto a Hybond-N(+) membrane (Amersham Pharmacia Biotech, Freiburg, Germany). The PaNie213 ORF cDNA was labeled with [α-32P]dCTP and used as a probe. Hybridization was performed at 55°C in 0.33 m NaH2PO4/Na2HPO4, pH 7.0, 1 mm EDTA, and 7% (w/v) SDS for 16 h. The membranes were washed for at least 20 min with 0.1% (w/v) SDS, 0.2× SSC (20× SSC = 3 m NaCl, 0.3 m sodium citrate, pH 7.5) at 65°C and then subjected to autoradiography.
Preparation of the Antiserum and Immunoblotting
A synthetic oligopeptide (PaNIE201-214) was used for immunisation of a rabbit. The pre-immunesera and anti-sera were provided by BioTrend (Köln, Germany) and used for immunoblotting in a dilution of 1:20,000.
Determination of 4-HBA
The 4-HBA content of carrot cell walls was determined as previously described (Schnitzler and Seitz, 1989). Vanillic acid was used as an internal standard for the quantification of the 4-HBA concentration.
Tetrazolium Assay
The loss of viability of suspended carrot cells was measured at the indicated times after elicitor application. Relative viability was calculated as A555-655 * mg−1 fresh weight using the tetrazolium assay according to Koch et al. (1998).
In Situ Infiltration of Leaves with PaNie213
Plants were grown under constant greenhouse conditions (60% relative humidity, long day: 16 h at 22°C and 8 h at 18°C). Leaves of 3-week-old Arabidopsis plants (Col-0), 4-month-old tobacco (Nicotiana tabacum) plants (W38), tomato (Lycopersicon esculentum Mill.) plants, 1-week-old maize (Zea mays) plants, Tradescantia zebrina (Bosse) plants, and oat (Avena sativa) plants were infiltrated in situ with constant volumes (5 μL) of PaNie213 solutions. BSA and the corresponding buffer were used as controls. The solutions were injected into the intercellular space through the stomata pore using a 1-mL syringe without hypodermic needle. Leaves were harvested 24 h after infiltration to visualize callose deposition and after 48 h to monitor necrotic effects.
Analysis of Callose Deposition
To visualize callose deposition, seedlings were treated and stained as described by Gómez-Gómez et al. (1999) according to Currier and Strugger (1956). The tissue was fixed overnight in 1% (v/v) glutaraldehyde, 5 mm citric acid, 90 mm Na2HPO4 (pH 7.4). The chlorophyll was removed and the specimens were dehydrated in ethanol. The transparent leaves were transferred to 50% (v/v) ethanol and afterward equilibrated in 67 mm K2HPO4 (pH 12.0) and then stained for 1 h at room temperature in 0.1% (w/v) aniline blue dissolved in 67 mm K2HPO4 (pH 12.0). The stained leaves were transferred to a microscopic slide in 70% (v/v) glycerol and 30% (v/v) staining solution and examined under UV epifluorescence (Zeiss, Axioplan, Oberkochen, Germany). The callose deposits were visible as pale-blue fluorescence.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Se 229/19–1) and by the Land Baden-Württemberg (grant to W.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010350.
LITERATURE CITED
- Bach M, Schnitzler J-P, Seitz HU. Elicitor-induced changes in Ca2+ influx, K+ efflux, and 4-hydroxybenzoic acid synthesis in protoplasts of Daucus carotaL. Plant Physiol. 1993;103:407–412. doi: 10.1104/pp.103.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA. Purification of a protein from culture filtrates of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology. 1995;85:1250–1255. [Google Scholar]
- Bennet M, Gallagher M, Fagg J, Bestwick C, Paul T, Beale M, Mansfield J. The hypersensitive reaction, membrane damage and accumulation of autofluorescent phenolics in lettuce cells challenged by Bremia lactuca. Plant J. 1996;9:851–855. [Google Scholar]
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4, 5)-dimethylthiazol-2-yl)-2,5diphenyltetrazoliumbromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989;91:889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier H, Strugger S. Aniline blue and fluorescence microscopy of callose in bulb scales of Allium cepaL. Protoplasma. 1956;45:552–559. [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA-minipreparation: version II. Plant Mol Biol Res. 1983;1:19–21. [Google Scholar]
- Ebel J, Cosio EG. Elicitors of plant defense responses. Int Rev Cytol. 1994;148:1–36. [Google Scholar]
- Felbrich G, Blume B, Brunner F, Hirt H, Kroj T, Ligterink W, Romanski A, Nürnberger T. Phytophthora parasitica elicitor-induced reactions in cells of Petroselinum crispum. Plant Cell Physiol. 2000;41:692–701. doi: 10.1093/pcp/41.6.692. [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Frishman D, Argos P. Incorporation of non-local interactions in protein secondary structure prediction from the amino acid sequence. Protein Eng. 1996;9:133–142. doi: 10.1093/protein/9.2.133. [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. FLS2: an LRR Receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999;18:277–284. doi: 10.1046/j.1365-313x.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D, Logemann E, Nürnberger T, Paniske M, Reinold S, Sacks WR, Schmelzer E. Oligopeptide elicitor-mediated defense gene activation in cultured parsley cells. Proc Natl Acad Sci USA. 1995;92:4150–4157. doi: 10.1073/pnas.92.10.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YS, Huang H-C, Collmer A. Pseudomonas sysringea pv.syringae HarpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell. 1993;73:1255–1266. doi: 10.1016/0092-8674(93)90354-s. [DOI] [PubMed] [Google Scholar]
- Jennings JC, Apel-Birkhold PC, Bailey BA, Anderson JD. Induction of ethylene biosynthesis and necrosis in weed leaves by Fusarium oxysporumprotein. Weed Sci. 2000;48:7–14. [Google Scholar]
- Kauss H. Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol. 1987;38:47–72. [Google Scholar]
- Koch W, Wagner C, Seitz HU. Elicitor-induced cell death and phytoalexin synthesis in Daucus carotaL. Planta. 1998;206:523–532. [Google Scholar]
- May MJ, Hammond-Kosack KE, Jones JDG. Involvement of reactive oxygen species, gltathione metabolism, and lipid peroxidation in the Cf-gene dependent defense response of tomato cotyledons induced by race specific elicitors of Cladosporium fulvum. Plant Physiol. 1996;110:1367–1379. doi: 10.1104/pp.110.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Simon L, Lam E. Pathogen-induced programmed cell death in tobacco. J Cell Sci. 1997;110:1333–1344. doi: 10.1242/jcs.110.11.1333. [DOI] [PubMed] [Google Scholar]
- Nennstiel D, Scheel D, Nürnberger T. Characterization and partial purification of an oligopeptide elicitor receptor from parsley (Petroselinum crispum) FEBS Lett. 1998;431:405–410. doi: 10.1016/s0014-5793(98)00800-x. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;12:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Noé W, Langebartels C, Seitz HU. Anthocyanin accumulation and PAL activity in a suspension culture of Daucus carotaL. Inhibition by L-α-aminooxy-β-phenylpropionic acid and t-cinnamic acid. Planta. 1980;149:283–287. doi: 10.1007/BF00384567. [DOI] [PubMed] [Google Scholar]
- Nürnberger T. Signal perception in plant pathogen defense. Cell Mol Life Sci. 1999;55:167–182. doi: 10.1007/s000180050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Peitsch MC, Polzar B, Stephan H, Crompton P, MacDonald HR, Manherz HG, Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death) EMBO J. 1993;12:371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchet M, Panabières F, Milat M-L, Mikes V, Montillet J-L, Suty L, Triantaphylides C, Tirilly Y, Blein J-P. Are elicitins cryptograms in plant-oomycete communications? Cell Mol Life Sci. 1999;56:1020–1047. doi: 10.1007/s000180050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson DE, Heath MC. Cleavage of nuclear DNA into oligosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell. 1996;8:393–402. doi: 10.1105/tpc.8.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler J-P. Die Karotte Ein Modellsystem für Untersuchungen von Pflanze-Pathogen-Wechselwirkungen: Charakterisierung von pilzlichen Elicitoren, Isolierung elicitorreaktiver Protoplasten, Biosynthese der p-Hydroxybenzoesäure. PhD thesis. Germany: University of Tübingen; 1992. [Google Scholar]
- Schnitzler J-P, Seitz HU. Rapid responses of cultured carrot cells and protoplasts to an elicitor from the cell wall of Pythium aphanidermatum(Edson) Fitzp. Z Naturforsch. 1989;44:1020–1028. [Google Scholar]
- Thomma BPHJ, Nelissen I, Eggermont K, Broekaert WF. Deficiency in phytoalexin production causes enhanced susceptibilty of Arabidopsis thaliana to the fungus Alternaria brassicola. Plant J. 1999;19:163–171. doi: 10.1046/j.1365-313x.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Jackson EP, Gage DA, Hammerschmidt R, Somerville SC. Phytoalexin accumulation in Arabidopsis thaliana during hypersensitive reaction to Pseudomonas sysringae pv syringae. Plant Physiol. 1992;98:1304–1309. doi: 10.1104/pp.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeshouwers VGAA, van Dooijeweert W, Govers F, Kamoun S, Colon LT. The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta. 2000;210:853–846. doi: 10.1007/s004250050690. [DOI] [PubMed] [Google Scholar]
- Wei Z-M, Laby RJ, Zunoff CH, Bauer DW, He YS, Collmer A, Beer SV. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]