Abstract
Grape (Vitis vinifera cv Silvaner) vine plants were cultivated under shaded conditions in the absence of ultraviolet (UV) radiation in a greenhouse, and subsequently placed outdoors under three different light regimes for 7 d. Different light regimes were produced by filters transmitting natural radiation, or screening out the UV-B (280–315 nm), or screening out the UV-A (315–400 nm) and the UV-B spectral range. During exposure, synthesis of UV-screening phenolics in leaves was quantified using HPLC: All treatments increased concentrations of hydroxycinnamic acids but the rise was highest, reaching 230% of the initial value, when UV radiation was absent. In contrast, UV-B radiation specifically increased flavonoid concentrations resulting in more than a 10-fold increase. Transmittance in the UV of all extracted phenolics was lower than epidermal UV transmittance determined fluorimetrically, and the two parameters were curvilinearly related. It is suggested that curvilinearity results from different absorption properties of the homogeneously dissolved phenolics in extracts and of the non-homogeneous distribution of phenolics in the epidermis. UV-B-dependent inhibition of maximum photochemical yield of photosystem II (PSII), measured as variable fluorescence of dark-adapted leaves, recovered in parallel to the buildup of epidermal screening for UV-B radiation, suggesting that PSII is protected against UV-B damage by epidermal screening. However, UV-B inhibition of CO2 assimilation rates was not diminished by efficient UV-B screening. We propose that protection of UV-B inactivation of PSII is observed because preceding damage is efficiently repaired while those factors determining UV-B inhibition of CO2 assimilation recover more slowly.
Photosynthetic organisms form energy-rich compounds using the energy of the sun's visible radiation. When harvesting light, photosynthetic organs are inevitably exposed to the UV region of natural radiation. In general, UV radiation damages lipids, nucleic acids, and proteins in leaves of higher plants, and specifically targets the photosystem II (PSII) reaction center, Rubisco, chloroplast ATPase, and violaxanthin deepoxidase (Jordan, 1996; Vass, 1997).
To cope with UV radiation damage, plants have evolved a variety of mechanisms including: screening out UV radiation by accumulating UV-absorbing phenolic compounds in the leaf epidermis, repairing UV-induced DNA damage, and formation of antioxidants to scavenge peroxides and oxygen radicals (Bornman and Teramura, 1993; Jordan 1996). Increases in natural UV radiation due to decreased stratospheric ozone concentrations have stimulated research on mechanisms and maximum capacities for protection against UV exposure (Caldwell et al., 1998).
Studies with Arabidopsis mutants deficient in synthesis of phenolic sunscreens have demonstrated the essential role of epidermal screening in UV protection (Li et al., 1993; Lois and Buchanan, 1994; Landry et al., 1995; Booij-James et al., 2000; Mazza et al., 2000). In many higher plants, two classes of phenolics are involved in epidermal UV screening: the hydroxycinnamic acids, exhibiting a C6-C3 carbon skeleton; and the more complex flavonoids, which have a C15 backbone that synthesized from simple phenylpropanoids via chalcone synthase (Cockell and Knowland, 1999; Forkmann and Heller, 1999). Landry et al. (1995) indicated that in Arabidopsis, hydroxycinnamic acids are particularly effective in screening out UV-B radiation because they absorb effectively in the UV-B spectral region; in comparison, flavonoid absorbance peaks are often located in the UV-A range.
Many herbaceous plants do not have efficient UV protection per se but respond to high UV fluxes by stimulating flavonoid synthesis (Bornman and Teramura, 1993; Beggs and Wellmann, 1994). Beggs and Wellmann (1994) showed that flavonoid concentration in parsley cell cultures is particularly elevated by UV-B radiation and rather insensitive to visible light. Synthesis of hydroxycinnamic acids was considered, hitherto, to be largely unaffected by ambient radiation conditions (Bornman et al., 1997; Burchard et al., 2000). However, Mirecki and Teramura (1984) demonstrated that strong visible light effectively increased the absorbance of total phenolics: The obvious conclusion that visible light might specifically trigger synthesis of hydroxycinnamic acids has not been investigated.
We were interested, therefore, in how natural irradiances of visible and UV radiation might contribute to formation of epidermal screening for UV radiation. For UV screening, the physiologically relevant figure is not the concentration of phenolics but the percentage of UV radiation transmitted by the epidermis. Epidermal UV screening is assessed most frequently from absorbance of extracted phenolics. Absorbance of extracted phenolics, however, cannot be used as a simple measure of epidermal screening: The relation between epidermal transmittance and absorbance of phenolics frequently fails to exhibit the theoretically expected exponential relationship (Day, 1993; Day et al., 1994; Barnes et al., 2000). To determine epidermal transmittance, fiber optics are often utilized (Bornman and Vogelmann, 1988; Day et al., 1992; Cen and Bornman, 1993). However, this specialized technique is difficult to apply in the field. More simple methods were introduced recently that derive epidermal UV transmittance from intensities of UV-excited chlorophyll fluorescence from intact leaves (Sheahan, 1996; Bilger et al., 1997; Barnes et al., 2000; Burchard et al., 2000; Mazza et al., 2000; Markstädter et al., 2001). In this paper, we employ the latter technique for determining epidermal UV screening. To examine how effectively epidermal screening protects the leaf mesophyll from UV damage, we measured the state of the UV-B-sensitive PSII and also CO2 assimilation rates.
RESULTS
Light Climate
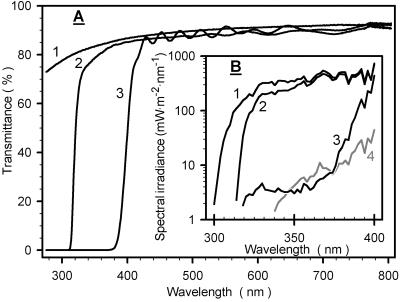
Figure 1A shows transmittance spectra of the three foil types under which plants were exposed outdoors: In the visible region, all foils exhibited high transmittances. The teflon foil was fairly transparent down to 290 nm. Because UV radiation at the earth's surface is restricted to wavelengths above 290 nm (Caldwell and Flint, 1997) the light climate under teflon foils contained the entire range of natural UV radiation (Fig. 1B) and, subsequently, will be denoted “vis + UVA + UVB.” The polyester and LEE 226 UV foils excluded most radiation below 320 or 400 nm, respectively. As a consequence, irradiance was depleted in UV-B (“vis + UVA” condition), or in the UV-B plus UV-A spectral range (“vis” condition). Figure 1B also demonstrates that light in the greenhouse was almost devoid of natural UV radiation.
Figure 1.
Light conditions. A, Transmittance spectra of the teflon (1), polyester (2), and LEE 226 UV foils (3) used to modify natural sunlight to produce “vis + UVA + UVB,” “vis + UVA,” and “vis” regimes, respectively. B, Spectral irradiances under the foils, and also in the greenhouse (4, gray line).
Under vis + UVA + UVB conditions, total UV irradiance (280–400 nm) at solar noon was 33 W m−2. Compared with vis + UVA + UVB, total UV irradiance was reduced to 80%, 10%, and 2% by vis + UVA, vis, and greenhouse conditions, respectively. Biologically effective irradicance (UVBE; calculated according to Caldwell et al., 1983) was 120 mW m−2 under vis + UVA + UVB conditions but was reduced below 1% of this value under all other conditions. For all three outdoor conditions, the photosynthetically active radiation at solar noon was 1,500 mMol m−2 s−1 and exceeded the greenhouse radiation intensity by a factor of 7.
The photosynthetically active radiation (PAR) during our outdoor exposure of 7 d was continuously recorded close to our experimental plot: mostly cloudless conditions, resulting in maximum daily PAR doses of 40 mol m−2, prevailed during most days; overcast skies reduced PAR doses to about 50% of the maximum value at d 4 and 7. UV-A and UV-B irradiances were recorded by the ELDONET dosimeter at the University of Erlangen (Germany; see “Materials and Methods”). Using ELDONET data and considering nonspecific attenuation of radiation by teflon foils, we calculated 0.9 mJ m−2 and 24 kJ m−2 for the maximum daily UV-A and UV-B doses, respectively.
Identity and Concentration of Phenolic Compounds
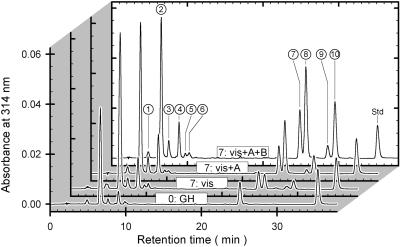
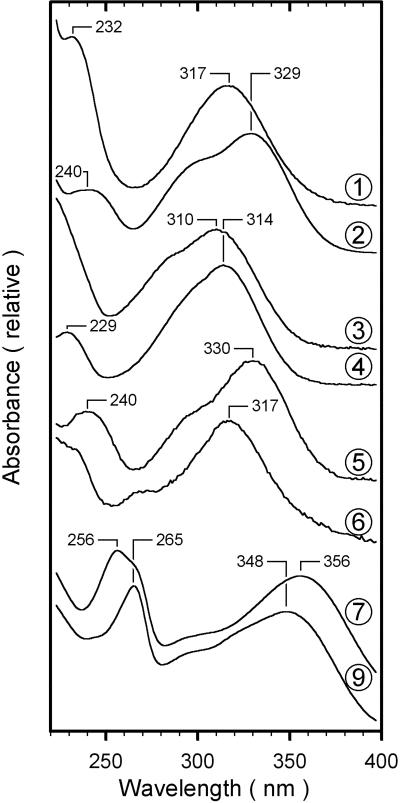
Figure 2 shows chromatograms of methanolic extracts from a greenhouse-grown leaf and from leaves exposed for 7 d to the three different outdoor conditions. Ten major peaks, labeled 1 through 10, accounted for about 92% and 96% of the chromatographically detected A314 and A360 nm, respectively. Spectroscopic properties divide the peaks into two groups (Fig. 3); first, peaks 1 through 6 exhibited maximum absorbance between 310 and 330 nm, which dropped to zero for wavelengths longer than 380 nm; second, peaks 7 through 10 were characterized by a maximum around 260 nm and another near 355 nm.
Figure 2.
Chromatography of phenolic compounds. HPLC analyses (unprocessed A314 versus retention time) of methanolic extracts from a greenhouse-grown leaf (0:GH), and from leaves exposed for 7 d to vis, vis + UVA, or vis + UVA + UVB conditions designated “7:vis,” “7:vis + A,” and “7:vis + A + B,” respectively, are shown. Absorbance spectra representing the peaks labeled 1 through 10 are depicted in Figure 3. Std, The internal standard, quercetin.
Figure 3.
Absorbance spectra of phenolic compounds. The absorbance spectra of chromatographically detected compounds, normalized to their long-wavelength maximum, are shown. Numbers identifying spectra refer to the peak numbers in Figure 2. The spectrum of compound 7 was indistinguishable from that of compound 8; the spectra of compounds 9 and 10 were also indistinguishable.
Based on the known spectral characteristics of phenolic compounds of grape (Vitis vinifera cv Silvaner) leaves (Cheban et al., 1976; Weber, 1992; Hmamouchi et al., 1996), we classified the chromophores giving rise to peaks 1 through 6 as hydroxycinnamic acids, and those of 7 through 10 as flavonoids. More detailed identification was carried out by comparing the hydrolytically released chromophores with authentic standards. Alkaline hydrolysis of leaf extracts produced five peaks of hydroxycinnamic acids, but trans-caffeic acid and trans-coumaric acid occurred at highest concentrations (not shown). This is consistent with Weber (1992) and Guidoni et al. (1997) who identified tartaroyl esters of trans-caffeic acid and trans-coumaric acid as the principal hydroxycinnamic acid derivatives in grape leaves. Based on spectral similarities with published data from grape (Singleton et al., 1978; Salgues et al., 1986) we suggest that peaks 2 and 4 in Figure 2 represent trans-caffeoyl tartaric acid and trans-coumaroyl tartaric acid, respectively. Two minor peaks obtained after alkaline hydrolysis were identified as cis-caffeic acid and cis-coumaric acid. We infer from the known spectroscopic and chromatographic behavior of phenolics from grape (Singleton et al., 1978; Weber, 1992; Karagiannis et al., 2000) that peaks 1 and 3 in Figure 2 correspond to the cis-isomers of peaks 2 and 4, respectively. The fifth peak released by alkaline hydrolysis included cis- and trans-ferulic acid: Based on spectroscopic data of Weber (1992), we tentatively conclude that peak 5 is derived from feruloyl tartaric acid. We were unable to identify the chromophore of peak 6.
Among the flavonoids, the absorbance spectra of peaks 7 and 8 were matching and indistinguishable from the spectrum of quercetin-3-O-galactoside. Also, peaks 9 and 10 exhibited matching absorbance spectra that agreed in wavelength positions of absorption peaks and spectral shape with kaempferol-3-O-7-O-diglycoside. Further, the only flavonoids released by acidic hydrolysis were the flavonols kaempferol and quercetin. Weber (1992) detected different O-glycosides of quercetin and kaempferol in grape leaves. As a consequence, we propose that in Figure 2 peaks 7 and 8 correspond to different quercetin-O-glycosides, and peaks 9 and 10 to different kaempferol-O-glycosides.
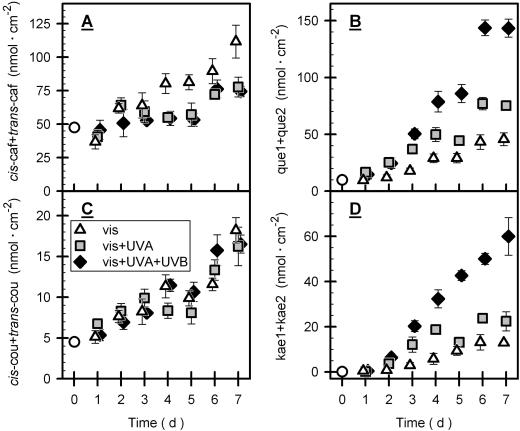
Caffeoyl tartaric acid concentration was increased 1.6-fold after 7 d under vis + UVA or vis + UVA + UVB conditions (Fig. 4A), but under visible light alone the increase was 2.3-fold. Concentration of coumaroyl tartaric acid was 10% to 20% of that of caffeoyl tartaric acid. Seven days of exposure under all three conditions produced an almost 4-fold increase in coumaroyl tartaric acid (Fig. 4C). The cis-isomer of caffeoyl tartaric acid always contributed less than 4% to total caffeoyl tartaric acid, but about 20% of total concentrations of coumaroyl tartaric acid consisted of the cis-isomer (data not shown).
Figure 4.
Concentration of phenolics during outdoor exposure. The figure depicts concentrations, normalized to leaf area, of the sum of cis- and trans-caffeoyl tartaric acid (A), and cis- and trans-coumaroyl tartaric acid (C) during exposure of grape leaves to different light conditions as defined in Figure 1. Data, normalized to leaf area, for quercetin (que1 + que2) and kaempferol (kae1 + kae2) are shown in B and D, respectively. que1 and que2 stand for HPLC peaks 7 and 8 (Fig. 2) representing different quercetin glycosides, and kae1 and kae2 correspond to peaks 9 and 10 (Fig. 2) representing different kaempferol glycosides. Here, and also in subsequent figures, triangles, squares, and diamonds symbolize data obtained under vis, vis + UVA and vis + UVA + UVB conditions, respectively. The white circles denote the initial data from greenhouse-grown vines measured immediately before outdoor exposure. For clarity, symbols of identical time intervals were slightly shifted relative to each other. Bars indicate ses of means (4 ≤ n ≤ 6). In the cases of small ses, bars are hidden by symbols. In A, vis data differed significantly those of the other treatments; in B and D, vis + UVA + UVB data differed significantly from those of the other treatments (see “Materials and Methods”).
Seven days of vis + UVA + UVB, vis + UVA, and vis exposure increased total quercetin concentration by a factor of 15, 8, and 5, respectively (Fig. 4B). Kaempferol contributed 33% to the total flavonol pool at most. Like quercetin, the kaempferol concentration was specifically enhanced by UV-B radiation (Fig. 4D).
Relation between Phenolic Compounds and Epidermal UV Screening
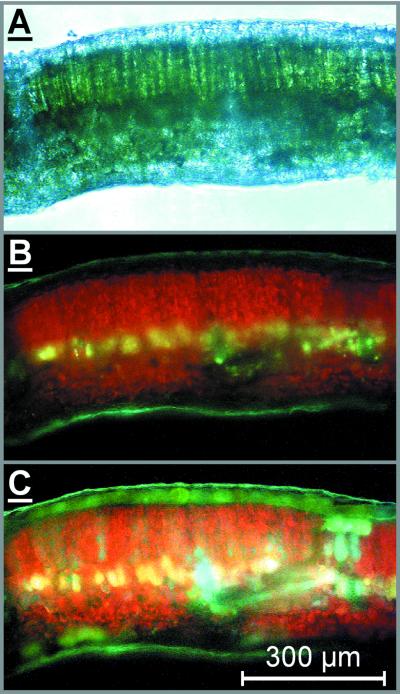
The distribution of phenolics in grape leaves was investigated by fluorescence microscopy (Fig. 5). Ammonia treatment resulted in conspicuous green fluorescence from the upper epidermis (compare Fig. 5, B with C) that was unevenly distributed, indicating accumulation of phenolics in the epidermal vacuoles (Day et al., 1993; Hutzler et al., 1998).
Figure 5.
Micrographs of a cross section from a grape leaf. The figure shows a light transmission image (A) and fluorescence images (B and C) either untreated (A and B) or ammonia treated (C) of the same cross section of a grape leaf acclimated to full sunlight. The presence of phenolic compounds in the upper epidermis is indicated by ammonia-enhanced green fluorescence (C).
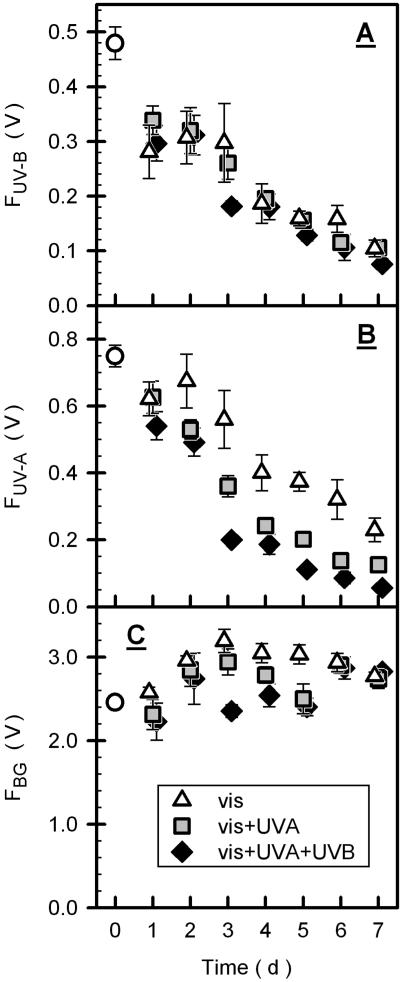
Chlorophyll fluorescence from intact leaves elicited by UV-B radiation (FUV-B) decreased continuously during outdoor exposure and reached about 20% of the initial value after 7 d under all light climates (Fig. 6A). UV-A-excited fluorescence (FUV-A) responded differently to the different light regimes: After 7 d of exposure to vis + UVA + UVB, vis + UVA, and vis conditions, FUV-A decreased to 8%, 17%, and 31% of the initial value, respectively (Fig. 6B). Compared with FUV-B and FUV-A, fluorescence excited by blue-green light (FBG) varied moderately during exposure (Fig. 6C).
Figure 6.
Intensity of chlorophyll fluorescence excited by UV or blue-green light. Unprocessed chlorophyll fluorescence at the F0 level excited by UV-B (FUV-B), UV-A (FUV-A), and blue-green radiation (FBG) is depicted in A, B, and C, respectively. See Figure 4 for comments on symbols and error bars. In B, vis data differed significantly from those of the other treatments (see “Materials and Methods”).
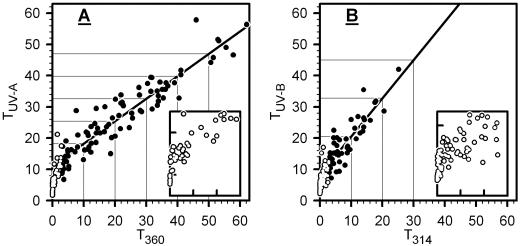
From fluorescence measurements, epidermal transmittance for UV-B and UV-A radiation (TUV-B and TUV-A, respectively) was derived as described in “Materials and Methods.” Prior to exposure, mean values of TUV-B and TUV-A were 20% and 36%, respectively; after 7 d, the TUV-B decreased to 3%, and the TUV-A to 2.5%, 5.5%, and 10% under UVB + UVA + vis, UVA + vis, and vis conditions, respectively. Most values for transmittance of extracted compounds at 314 nm (T314) and at 360 nm (T360) were smaller than the corresponding data for TUV-B and TUV-A, respectively, and dropped close to zero by the end of exposure (data not shown). The relationship between epidermal UV transmittance and transmittance of extracted phenolics is illustrated in Figure 7. For T360 > 3%, TUV-A was linearly related to T360. For T360 ≈ 40%, absolute values for TUV-A were similar to the corresponding T360; at values for T360 < 30%, TUV-A was higher than T360; below 3%, data deviated from the linear relation because TUV-A was markedly higher relative to T360. Also, TUV-B was linearly related to T314 when T314 was greater than 3%. In the linear range, TUV-B was always higher than T314. Deviations from linearity below T314 = 3% resulted from much higher TUV-B relative to T314.
Figure 7.
Relation between epidermal screening and UV absorption of phenolics. Data of individual leaves are shown. In A and B, we plot epidermal transmittance for UV-A radiation (TUV-A) against transmittance of extracted leaf phenolics at 360 nm (T360), and epidermal UV-B transmittance (TUV-B) against transmittance of extracted leaf phenolics at 314 nm (T314), respectively (extracted phenolics correspond to peaks 1–10 in Fig. 2). In A and B, the bold line results from linear regression to solid circles, that is for T360 > 3% and T314 > 3%. In A and B, coefficients of determinations are r2 = 0.874 and r2 = 0.750, and equations resulting from regression analyses are TUV-A = 11 + 0.72 × T360 and TUV-B = 8 + 1.22 × T360, respectively. White circles (T360 or T314 < 3%) are also shown at amplified scales in inserts (ordinate, 0%–12%; abscissa, 0%–3%).
Photosynthesic Parameters during Exposure
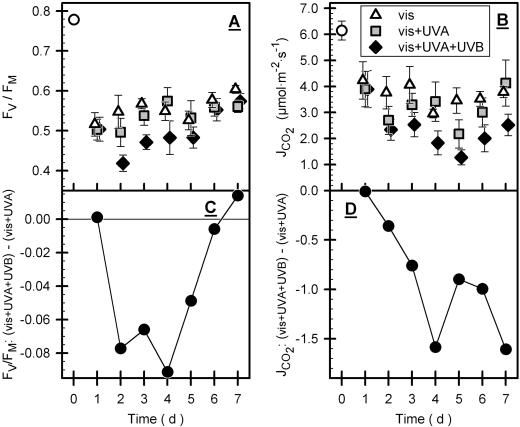
Greenhouse-grown leaves exhibited a normalized variable chlorophyll fluorescence (Fv/Fm) of 0.78, which dropped to 0.5 after 1 d of exposure for all three conditions (Fig. 8A). During further exposure, leaves under vis + UVA and vis conditions recovered slightly. Under vis + UVA + UVB conditions, Fv/Fm stayed below values of the other conditions during d 2 to 5; at the end of the experiment, Fv/Fm recovered to the data of vis + UVA and vis (Fig. 8, A and C). The light-saturated CO2 assimilation rate (JCO2) was decreased from 6.1 to about 4 μmol m−2 s−1 after 1 d of exposure under all conditions and tended to decrease during further exposure under all conditions (Fig. 8B). However, vis + UVA + UVB conditions depressed JCO2 significantly more than the other treatments. In contrast to Fv/Fm, UV-B-dependent inhibition of JCO2 is not recovered by the end of exposure (Fig. 8D).
Figure 8.
Photosynthetic parameters during exposure. Time courses of normalized variable chlorophyll fluorescence, Fv/Fm, of dark-adapted leaves and of light-saturated CO2 assimilation rate (JCO2) are shown in A and B, respectively. For both parameters, UV-B-dependent effects were calculated by subtracting mean values of vis + UVA + UVB minus mean values of vis + UVA conditions (C and D). See Figure 4 for comments on symbols and error bars. In A and B, vis + UVA + UVB data differed significantly from those of the other treatments (see “Materials and methods”).
DISCUSSION
In our grape leaves, the main hydroxycinnamic acids were coumaric acid and caffeic acid (Fig. 2), which were increased by all three exposure conditions (Fig. 4); therefore, high visible radiation stimulates synthesis of hydroxycinnamic acids in grape leaves. Hitherto, concentrations of hydroxycinnamic acids were considered to be independent from the plant's light environment (Bornman et al., 1997; Burchard et al., 2000); however, these studies designed to investigate UV-B action were not suited to recognize visible light effects. Because all our light regimes include the visible and far-red spectral range, we cannot differentiate between blue or red-light-sensing photoreceptors, which have both been shown to regulate phenolic biosynthesis (Beggs and Wellmann, 1994).
Caffeic acid, which represented most of the hydroxycinnamic acid fraction, was particularly enhanced by vis conditions, but under this same situation flavonoid concentrations were lowest (Fig. 4). Li et al. (1993) observed increased concentrations of hydroxycinnamic acids in an Arabidopsis mutant defective in the first step specific to flavonoid biosynthesis and suggested that the precursors from the blocked flavonoid pathway were shunted into synthesis of hydroxycinnamic acids. In accordance, we believe that the particular high caffeic acid concentrations under vis conditions result from relatively moderate rates of flavonoid biosynthesis that direct precursors toward synthesis of hydroxycinnamic acids.
In contrast to the stimulation of hydroxycinnamic acids by visible light, biosynthesis of kaempferol and quercetin was specifically increased by UV-B radiation (Fig. 4): This is consistent with the action spectrum for flavonoid synthesis in parsley cell cultures, which exhibits the maximum at 295 nm with little effectiveness for wavelengths > 320 nm (Beggs and Wellmann, 1994) and indicates that specific UV-B photoreception is involved in stimulation of flavonoid biosynthesis.
Fluorescence microscopy suggested the presence of considerable amounts of phenolic compounds in the upper epidermis of grape leaves (Fig. 5). Hence, the observed increase in leaf phenolics during exposure should improve epidermal UV screening, which we measured fluorometrically (Bilger et al., 1997). The reliability of the Bilger method was supported recently by correlation between fluorometric results and spectrophotometrically measured transmittance of epidermal peels from Vicia faba (Barnes et al., 2000; Markstädter et al., 2001); however, Barnes et al. (2000) pointed out that in some species, epidermal anthocyanins absorbing in the visible region can reduce fluorescence elicited by blue-green light (FBG). Because epidermal transmittance is calculated using FBG as the reference signal (see “Materials and Methods”), anthocyanin absorption would produce erroneous results; however, the FBG did not drop during vine exposure, nor did we detect anthocyanins by HPLC (Figs. 2 and 6). Therefore, anthocyanin absorption appears not to interfere with determinaton of epidermal transmittance.
Concomitant with chromatographically determined increase in phenolics during outdoor exposure, FUV-B and FUV-A decreased markedly, thus supporting the role of phenolic compounds in UV screening (compare Fig. 4 with Fig. 6). In contrast to enhancement of flavonoid concentration by UV radiation, specific effects of UV radiation on epidermal screening was only observed in the case of FUV-A: Under all conditions, FUV-B was similar (Fig. 6). The similar FUV-B probably arises from relatively moderate absorption of grape leaf flavonols in the UV-B spectral region (Fig. 3) and, hence, a predominant role of hydroxycinnamic acids in UV-B screening. The UV-dependent response of FUV-A is consistent with the data of Mazza et al. (2000), who measured in soybean (Fabaceae) chlorophyll fluorescence excited by broad-band UV radiation of leaves exposed to different light regimes in the field. That Mazza et al. (2000) also observed UV-B-dependent decrease in FUV-B could be explained by insignificant contribution of hydroxycinnamic acids to epidermal screening: Low contents of hydroxycinnamic acids were observed in the Fabaceae V. faba (Markstädter et al., 2001).
In most leaves, epidermal transmittance was higher than transmittance of total leaf phenolics (Fig. 7): This could be attributed to the fact that phenolics of the entire leaf exceed those of the epidermis because they also occur in the lower epidermis and in the mesophyll tissue (Weissenböck et al., 1986; Cen and Bornman, 1993; Day et al., 1996; Hutzler et al., 1998; Grammatikopoulos et al., 1999). The curved relationships between epidermal transmittance and transmittance of extracted phenolics (Fig. 7) could be explained by assuming that leaves with high total phenolic concentrations posses considerably more phenolics in the lower epidermis and the mesophyll relative to the upper epidermis than leaves exhibiting low phenolic concentrations. However, high phenolic concentrations in the abaxial epidermis are not consistent with fluorescence microscopy of sun-exposed grape leaves (Fig. 5). Further, studies demonstrating rather constant mesophyll concentrations over a wide range of total leaf concentrations of phenolics (Grammatikopoulos et al., 1999; Burchard et al., 2000) do not support an accumulation of mesophyll phenolics in grape leaves. Also, false correction for the scattering signals of the Xe-PAM fluorometer or an erroneous value for 100% transmittance (see “Materials and Methods”) could have produced the curved relationships; however, such artifacts can only account in part for curvilinearity as varying these experimental parameters did only moderately affect curvature.
We favor the view that curvilinearity in Figure 7 results to a great part from confinement of epidermal phenolics to the vacuoles (Fig. 5), leaving non-vacuolar cell spaces and anticlinal cell walls relatively transparent to UV radiation as has been shown by Day et al. (1993) and that gives rise to the so-called “sieve effect.” The lack of epidermal blue fluorescence in untreated samples (Fig. 5B) agrees with UV-transparent cell walls because blue fluorescence would indicate the presence UV-absorbing phenolics (Hutzler et al., 1998). In support of our suggestion, a comparable relationship between leaf transmittance and transmittance of homogenized leaf pigments was derived using a simple model that takes into account the sieve effect (McClendon and Fukshansky, 1990). Burchard et al. (2000) observed linearity between epidermal absorbance derived from fluorometry and HPLC-detected absorbance of epidermal phenolics in Secale cereale that might be related to the high amounts of UV-absorbing constituents of the Poaceae cell wall (Lichtenthaler and Schweiger, 1998), hence negating the sieve effect.
We utilized Fv/Fm to determine maximum photochemical quantum yield of PS II (Butler, 1978; Schreiber et al., 1994). PSII was inactivated under all exposure conditions, but a transient extra reduction occurred under UVB + UVA + vis conditions (Fig. 8). This extra reduction is consistent with susceptibility of PSII to UV-B radiation (Jordan, 1996; Vass, 1997), whereas decreased PSII activity under vis conditions can be attributed to reaction center damage by strong visible light (Baker et al., 1997; Melis, 1999).
Reduced JCO2 observed during exposure to the vis treatment (Fig. 8) could be related to PSII damage by visible light. As observed for Fv/Fm, the vis + UVA + UVB treatment caused an additional decrease in JCO2, relative to vis and vis + UVA conditions. The time course of the inhibitory effects of UV-B on Fv/Fm and JCO2 revealed, however, that these parameters are affected differently by UV-B. Compared with vis + UVA conditions, vis + UVA + UVB caused a further 20% decrease in Fv/Fm at most, but JCO2 was decreased by a further 50%. In addition, UV-B inhibition of Fv/Fm disappeared by the end of our experiment but UV-B-specific inhibition of JCO2 persisted. Because UV-B effects on PSII were small and transitory when compared with CO2 assimilation, we suggest that under natural light intensities UV-B inhibition of photosynthesis is not controlled by UV-B inhibition of PSII, as has also been proposed by Allen et al. (1998) and Xiong and Day (2001).
The extent of UV-B inhibition of Fv/Fm decreased with decreasing FUV-B, except during the 1st d of exposure (compare Fig. 6A with 8C), which supports the physiological significance of epidermal UV-B screening. The PSII reaction center is known as a target for UV-B inhibition (Renger et al., 1989) but it is also well known for its outstandingly high protein turnover rates that result in efficient PSII repair (Andersson, 1992); it has also been shown that natural UV-B radiation further stimulates PSII repair (Greenberg et al., 1989). Therefore, we explain the recovery of UV-B-dependent reduction of Fv/Fm by the combined action of epidermal UV-B screening and repair. This might explain why UV-B-dependent inactivation of PSII was hardly detectable under natural conditions. Krause et al. (1999) only recently demonstrated marked effects of UV-B radiation on PSII efficiency when plants grown in deep shade were exposed to tropical UV-B intensities that correspond to comparable conditions to those applied in this paper. Krause et al. (1999) interpreted high A282 of leaf extracts as an indicator for sufficient epidermal screening in shaded plants. Spectral analysis during HPLC of our leaf extracts, however, indicated that long-wavelength absorbance flanks of many compounds, measurable at 282 nm, do not extend significantly into the physiologically relevant UV range. Therefore, PSII inactivation observed by Krause et al. (1999) could well have been conducted with leaves inefficiently screened against UV-B radiation.
Inhibition of CO2 assimilation by UV-B could be due to limitation of stomatal gas exchange (Correia et al., 1999; Nogués et al., 1999) but calculated internal CO2 concentrations gave no indication of increased stomatal limitation (data not shown) and grape leaves are hypostomatal (Dürinig, 1980) so that stomata are exposed to only moderate UV intensities. Thus, UV-B does not appear to affect stomatal function. More likely, UV-B could have affected CO2 assimilation by inhibiting Rubisco activity (Strid et al., 1990; Huang et al., 1993; Rao et al., 1995). That UV-B inhibition of CO2 assimilation did not respond to buildup of epidermal screening suggests that recovery of the factors inhibited by UV-B is sluggish and preceding damage could not be as rapidly repaired as PSII.
CONCLUSION
Because plants in this study were grown under reduced UV radiation and low visible light intensities, extrapolation of our results to acclimation processes in the natural environment requires considerable care. Nonetheless, interesting information and questions have been generated: in grape leaves, synthesis of hydroxycinnamic acids was stimulated by strong visible light but flavonoid production was specifically enhanced by UV radiation. Hence, it remains to be clarified if plants grown under prolonged weather conditions of low visible light intensities posses diminished UV-B screening ability and, hence, increased susceptibility to UV-B damage when subsequently exposed to conditions of high UV fluxes under clear skies. Our data indicate that epidermal UV screening of leaves after short exposure to high natural radiation is sufficient to prevent UV-B-dependent reduction of PSII activity in the field. When photosynthetic performance was assessed by CO2 gas-exchange measurements, however, UV-B was inhibitory. For this reason, further investigations of UV-B effects on photosynthetic performance should not be confined to the study of PSII inhibition.
MATERIALS AND METHODS
Plants and Experimental Design
Two-year-old rooted grafted grape (Vitis vinifera cv Silvaner) vines were obtained from a local supplier in the winter of 1999 through 2000, stored at 1°C and 95% to 100% (v/v) relative humidity, and planted in pots of garden mulch in April 2000. The vines were grown in a shaded glasshouse, and fertilizer (Flory 3, Euflor, Munich) was applied twice a week. Plants were exposed to three different light conditions in the Botanical Garden of the University of Würzburg (49.8° N, 9.9° E) at an altitude of 200 m between August 12 and 18, 2000.
Different light regimes were provided by foils exhibiting different transmittance properties in the UV range (see Fig. 1 for transmittance spectra): teflon foils (Novofol, Siegsdorf, Germany), polyester foils “Melinex 400 Glasklar” (Pütz, Taunusstein, Germany), and “Lee 226 UV” foils (FFL-Rieger, Munich). Exposure of vines was performed in boxes (3 × 1 m) constructed with roofs and walls of these foils, and aligned with the longer axis in an east-west direction: The roof sloped from 1.8 m (north) to 1.2 m (south), and the northern wall remained open for ventilation; diffuse radiation from the north was minimized by shielding the open end with the appropriate foil (3.5 × 1.5 m) mounted at 45° and 20 cm from the box.
Transmittance spectra of our foils were measured in the turbid sample compartment of a UV4 spectrometer (Unicam, Cambridge, UK). Spectral irradiances below foils, and in the greenhouse, were measured with the OL 754-O-PMT optics head equipped with a dome window of an OL 754 UV-Visible Spectroradiometer (Optronic, Orlando, FL). The spectroradiometer was configured with 0.25-mm entrance and exit slits that produced a half bandwidth of ≤2 nm. Calibration was against a tungsten filament quartz halogen lamp that itself was calibrated against a standard light source (National Institute of Standards and Technology, Washington, DC). Spectra were recorded at solar noon ± 1 h under cloudless conditions during early August 2000. Natural PAR was continuously recorded by using an LI-190SZ quantum sensor (LI-COR, Lincoln, NE); PAR in the greenhouse and in exposure boxes were measured with an LI-189 quantum sensor (LI-COR). Daily courses of irradiance in the UV-A and UV-B range were obtained from the ELDONET dosimeter located at a distance of 80 km at the University of Erlangen (200-m altitude, 49.5° N, 11° E; Marangoni et al., 2000).
Each of the light regimes was represented by two boxes in each of which five plants were exposed. In total, 38 grapevine plants were investigated. Initial conditions were measured on August 11 with plants not assigned for outdoor exposure. 30 plants were transferred into our exposure boxes at 11 PM local summer time, on August 11. To provide comparable exposure conditions for differently orientated leaves, plants were rotated by 120° counterclockwise each hour from 9 am until 5 pm, resulting in three complete turns per day.
Only fully developed leaves of insertion levels 3 to 6, counted from the bottom to the top, were investigated. Five leaves per day for each light condition usually were sampled. Prior to the experiment, the sequence of leaf sampling was established using a random number generator. Daily measurements started at 4 pm by recording CO2 gas exchange of attached leaves. At 6 pm, these leaves were detached, the petiole dipped in tap water, and kept in darkness in humidified air, for at least 1.5 h. Thereafter, PSII photochemical yield was estimated by measuring variable chlorophyll fluorescence followed by establishing epidermal UV transmittance using UV-excited chlorophyll fluorescence (see below). Finally, leaf discs were punched out, frozen in liquid nitrogen, and stored at −80°C until chromatographic analysis of phenolic compounds.
CO2 Gas-Exchange Measurements
Leaf gas exchange was studied using a CO2 porometer (CQP-130, Walz, Effeltrich, Germany) which was set up in the greenhouse before outside exposure, and subsequently in a tent near the outdoor experimental site. Cuvette temperature was 28°C to 30°C, and relative humidity was 70% to 90% (v/v). Saturating photon flux density (around 700 μmol m−2 s−1) was supplied by an FL-440 lighting unit (Walz). CO2 measurements were done using a BINOS infrared gas analyser (Rosemount, Hanau, Germany) operating in differential mode, and a second BINOS in absolute mode was used to determine the CO2 concentration of the air flowing through the system (360–405 μL L−1). The upper half of the leaf was enclosed in the cuvette, and a reading was taken as soon as equilibrium was reached, which was typically after 2 to 4 min. All gas-exchange parameters subsequently were calculated following Von Caemmerer and Farquhar (1981).
Fluorescence Measurements
Maximum photochemical yield of PSII was measured as variable chlorophyll fluorescence of dark-adapted leaves at room temperature [Fv/Fm = (FM − F0)/FM; Schreiber et al., 1986) with a PAM-2000 fluorometer (Walz). Using fiber optics (Walz), the adaxial leaf side was examined. Minimum fluorescence (F0) was excited at 655 nm and 600 Hz modulation frequency, and maximum fluorescence (FM) was measured with 100-kHz modulation frequency. The FM was elicited by saturating flashes of 0.8-s duration from a built-in miniature halogen lamp.
Epidermal transmittance of UV radiation was determined as described earlier (Bilger et al., 1997) using an Xe-PAM fluorometer (Walz) equipped with a high-power xenon flash lamp (L4634, Hamamatsu, Herrsching, Germany). Constant chlorophyll fluorescence at the F0 level from dark-adapted samples was elicited by 2-Hz flashes. Using filters obtained by Walz, we recorded chlorophyll fluorescence at wavelength > 690 nm, elicited by UV-B (314 nm, bandwidth 24 nm), UV-A (360 nm, bandwidth 28 nm) or blue-green radiation (490 nm, bandwidth 165 nm). Fluorescence resulting from these excitation bands will be denoted as FUV-B, FUV-A, and FBG, respectively. In the absence of any sample, all three excitation wavebands produced small scattering signals that were subtracted from the corresponding leaf signal, prior to any data manipulation.
Epidermal transmittance for UV-B and UV-A radiation, denoted TUV-B and TUV-A, respectively, can be estimated by normalizing fluorescence quotients from intact leaves to fluorescence quotients from epidermis-free leaf mesophyll (Markstädter et al., 2001):
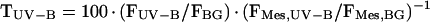 |
1 |
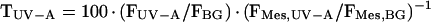 |
2 |
where FMES,UV-B, FMES,UV-A, and FMES,BG denote the fluorescence from mesophyll tissue excited by UV-B, UV-A, or blue-green light, respectively. In the case of grape leaves, stripping of the epidermis was not possible; hence, we determined values for FMES from the greenhouse-grown Pisum sativum mutant Argenteum, which possesses a loosely attached leaf epidermis.
HPLC
One liquid nitrogen-cooled leaf disc (1.3-cm diameter) together with 250 μL of extraction medium (50% [v/v] aqueous methanol containing 0.01% [w/v] phosphoric acid and 30 μg mL−1 quercetin as an internal standard) was reduced to a fine frozen suspension in a 5-mL teflon sample flask of a Mikro-Dismembrator II equipped with an agate grinding ball (B. Braun Melsungen, Melsungen, Germany), which had both been immersed in liquid nitrogen. The frozen suspension subsequently was thawed, centrifuged, and the supernatant collected. Pellets were extracted twice more at room temperature with 250 μL of extraction medium, respectively. The extract was clarified by further centrifugation before analysis on a 1100 Series chromatograph (Hewlett-Packard, Waldbronn, Germany) that includes a quaternary pump and a 1040M diode array detector.
Chromatograms were recorded at 314 and 360 nm, which represent the maximum transmittance of the UV-B and UV-A excitation window of our Xe-PAM fluorometer, respectively. Instrument control and data analysis were carried out using the HP ChemStation software. Separation of phenolics was on a 5-μm particle LiChrospher-100 RP18 column of 250-mm length and 4.6-mm i.d. thermostatted at 20°C (Knauer, Berlin) using a flow rate of 1 mL min−1. The injection volume was 10 μL. For separation of phenolics, the gradient program of Veit et al. (1992) was modified: Elution started with a linear decrease of solvent A (0.01% [w/v] H3PO4) from 80% (v/v) to 66% (v/v) with solvent B (methanol:0.1% [w/v] H3PO4 [9:1, v/v]) over a period of 7 min, followed by isocratic elution for 5 min. A decrease to 56% (v/v) of solvent A then occurred within 2 min, to 40% (v/v) during a further 18 min, and to 35% (v/v) during another 3 min. Finally, 100% solvent B was reached during a 2-min gradient followed by isocratic elution for 5 min. Starting conditions were restored during a 1-min gradient followed by column equilibration for 7 min; HPLC grade solvents (Fluka, Deisenhofen, Germany) were used.
For identification of phenolic chromophores, we compared hydrolytically released compounds with authentic markers; leaf extracts were evaporated under a nitrogen stream at 40°C. Hydrolyses were performed for 40 min in 1 n HCl at 90°C, or for 5 min under nitrogen gas in 1 n NaOH at 70°C: The latter reactions were stopped by adding equinormal amounts of HCl. Acid hydrolyzates were prepared for chromatography by evaporation under a nitrogen stream at 40°C and dissolving the residues in 50% (v/v) aqueous methanol. Alkaline hydrolyzates were extracted repeatedly with diethylether, which was then removed by evaporation; the residues were dissolved in 50% (v/v) methanol before analysis by HPLC.
Authentic markers of flavonols (kaempferol and quercetin) and trans-hydroxycinnamic acid derivatives (caffeic acid, coumaric acid, and ferrulic acid) were purchased from Fluka. Rutin [quercetin-3-O-(6-O-rhamnosyl) glucoside] was obtained from Carl Roth GmbH (Karlsruhe, Germany). Quercetin-3-O-galactoside and a kaempferol-3-O-7-O-diglycoside were a gift from Dr. Christiane Löffler (Universität Würzburg). Trans-coumaric acid propanoyl ester was provided by Dr. Claus Markstädter (Universität Würzburg).
Concentrations of trans-hydroxycinnamic acid derivatives were derived from the linear relationships existing between molar concentrations of the chromophore standards and A314. The relationships obtained with the pure trans-hydroxycinnamic acids were used to calculate concentrations of the respective tartaroyl esters in grape leaves (Weber, 1992) because we obtained identical calibration curves for trans-coumaric acid and trans-coumaric acid propanoyl ester. In addition, response factors for A314, as derived from data of Okamura and Watanabe (1981), were identical for caffeic acid and caffeoyl tartaric acid, and deviated by only 15% in the case of coumaric acid and coumarioyl tartaric acid.
Cis-isomers of the commercially obtained hydroxycinnamic acids, dissolved in 50% (v/v) methanol at a concentration of 250 μg mL−1, were enriched by 1-h illumination with natural sunlight or by TL12 fluorescent tubes (TTS-Product Service GmbH, Bad Sulza, Germany). After irradiation, molar ratios of cis- to trans-compounds (mrcis/trans) were determined by gas chromatography (Markstädter, 1994). For the same isomer mixture, the A314 of the trans- relative to the cis- compound (Artrans/cis) was determined by HPLC. Calibration factors (Fcis) for cis-caffeic acid and cis-coumaric acid were calculated according to:
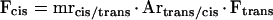 |
3 |
where Ftrans corresponds to the calibration factor determined for the respective trans-component.
Concentrations of flavonols were determined using A360. Calibration factors were obtained with kaempferol and quercetin standards. We used the factors of pure chromophores to calculate concentrations of glycosides from grape leaves (Weber, 1992) because identical response in HPLC of quercetin and its glycoside rutin indicated that glycosylation has an insignificant effect on flavonol absorbance.
Molar concentrations (cP) of a phenolic compound (P) were normalizedto leaf area using quercetin as an internal standard (free quercetin was not detected in grape leaves). The standard is present at a known concentration cStdEx in the extraction solvent. Concentration changes of extracted phenolics after extraction due to solvent evaporation or adsorption of phenolics to inner surfaces of test vessels can be corrected using Equation 4:
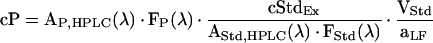 |
4 |
where AP,HPLC(λ) and FP(λ) represent the area of the HPLC peak of P at wavelength λ and the HPLC calibration factor at λ for P, respectively. The peak area and the calibration factor for the internal standard are designated AStd,HPLC(λ) and FStd(λ), respectively. VStd and aLF signify the total volume used for extraction of one leaf disc (i.e. 0.75 mL) and the extracted leaf disc area (1.33 cm2), respectively; the fraction VStd/aLF normalizes the true concentration of P in the extract to leaf area.
Removing HPLC calibration factors and replacing concentrations with absorbance terms, allows us to transform the AP,HPLC(λ) into in vivo absorbance of P, AP,LF(λ), according to Equation 5:
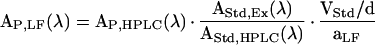 |
5 |
where AStd,Ex(λ) corresponds to the absorbance of the internal standard in the pure extraction solvent as determined with a UV/Vis spectrometer UV4 (Unicam). The parameter d denotes the optical path length of the extraction solvent in the spectrophotometer (1 cm); to account for the dependence of absorbance measurements on optical path length, the fraction of VStd/d is required to normalize the true absorbance of P in extracts to leaf area. Absorbance values were converted into transmittance using the known exponential relation between the two parameters.
Microscopy
Cross sections of freshly harvested leaves of 70- to 100-μm thickness were prepared using a hand microtome (Leica Instruments GmbH, Nuβloch, Germany). Samples were immediately examined in water and then in 0.5% (w/v) ammonia introduced under the coverslip using blotting paper (Hutzler et al., 1998). Transmission and fluorescence images were obtained using an Axioplan microscope (Zeiss, Oberkochen, Germany). In fluorescence microscopy, we used the Zeiss filter set number 5 that excites in the range from 395 to 440 nm and detects fluorescence at wavelengths > 470 nm. Images were recorded using a 3-CCD color video camera (INTAS Imaging Instruments, Göttingen, Germany).
Statistics
For statistical analyses, we utilized Sigma Stat for Windows Version 2.03 statistical software (SPSS, Munich). To test if any of the three different light conditions produced statistically significant differences, we used one-way analysis of variance. In the case of statistical significance (i.e. P < 0.05), the Student-Newman-Keuls method was performed to determine which data groups are different. Statistically significant differences were concluded for P values < 0.05. Handling and fitting of data was achieved using SigmaPlot scientific graphing software (SPSS).
ACKNOWLEDGMENTS
We thank Manuela Heidenfelder, Simone Geyer, Birgit Glück, Ingo Queck, and Ulrike Kunz for their excellent technical assistance. For providing us with grapevine plants, we are grateful to Josef Herrmann. We thank Jörg Peter Schnitzler for introducing us to HPLC of phenolic compounds, Claus Markstädter for stimulating discussions, and Bob Porra for help in preparing the manuscript.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. SFB 251) and by the state of Bavaria (BayFORKLIM and BayFORUV). J.K. received a fellowship from the Deutsche Forschungsgemeinschaft (Graduiertenkolleg: Pflanzen unter Stress) to support his visit to Würzburg.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010373.
LITERATURE CITED
- Allen DJ, Nogués S, Baker NR (1998) Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis? J Exp Bot 1775–1788
- Andersson B. Thylakoid membrane dynamics in relation to light stress and photoinhibition. In: Barber B, Guerrero MG, Medrano H, editors. Trends in Photosynthesis Research. Andover, UK: Intercept Ltd; 1992. pp. 71–86. [Google Scholar]
- Baker NR, Nogués S, Allen DJ. Photosynthesis and photoinhibition. In: Lumsden PJ, editor. Plants and UV-B: Responses to Environmental Change. Cambridge, NY: Cambridge University Press; 1997. pp. 95–111. [Google Scholar]
- Barnes PW, Searles PS, Ballaré CL, Ryel RJ, Caldwell MM. Non-invasive measurements of leaf epidermal transmittance of UV radiation using chlorophyll fluorescence: field and laboratory studies. Physiol Plant. 2000;109:274–283. [Google Scholar]
- Beggs CJ, Wellmann E. Photocontrol of flavonoid biosynthesis. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 733–751. [Google Scholar]
- Bilger W, Veit M, Schreiber L, Schreiber U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant. 1997;101:754–763. [Google Scholar]
- Booij-James IS, Dube SK, Jansen MAK, Edelman M, Mattoo AK. Ultraviolet-B radiation impacts light-mediated turnover of the photosystem II reaction center heterodimer in Arabidopsis mutants altered in phenolic metabolism. Plant Physiol. 2000;124:1275–1284. doi: 10.1104/pp.124.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornman JF, Reuber S, Cen Y-P, Weissenböck G. Ultraviolet radiation as a stress factor and the role of protective pigments. In: Lumsden J, editor. Plants and UV-B: Responses to Environmental Change. Cambridge, NY: Cambridge University Press; 1997. pp. 157–168. [Google Scholar]
- Bornman JF, Teramura AH. Effects of ultraviolet-B radiation on terrestrial plants. In: Young AR, editor. Environmental UV Photobiology. New York: Plenum Press; 1993. pp. 427–471. [Google Scholar]
- Bornman JF, Vogelmann TC. Penetration of blue and UV radiation measured by fiber optics in spruce and fir needles. Physiol Plant. 1988;72:699–705. [Google Scholar]
- Burchard P, Bilger W, Weissenböck G. Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ. 2000;23:1373–1380. [Google Scholar]
- Butler WL. Energy distribution in the photochemical apparatus of photosynthesis. Ann Rev Plant Physiol. 1978;29:345–378. [Google Scholar]
- Caldwell MM, Björn LO, Bornman JF, Flint SD, Kulandaivelu G, Teramura AH, Tevini M. Effects of increased solar ultraviolet radiation on terrestrial ecosystems. J Photochem Photobiol B Biol. 1998;46:40–52. [Google Scholar]
- Caldwell MM, Flint SD. Uses of biological spectral weighting functions and the need of scaling for the ozone reduction problem. Plant Ecol. 1997;128:67–76. [Google Scholar]
- Caldwell MM, Gold WG, Harris G, Ashurst CW. A modulated lamp system for solar UV-B (280–320 nm): supplementation studies in the field. Photochem Photobiol. 1983;37:479–485. doi: 10.1111/j.1751-1097.1983.tb04503.x. [DOI] [PubMed] [Google Scholar]
- Cen YP, Bornman J. The effect of exposure to enhanced UV-B radiation on the penetration of monochromatic and polychromatic UV-B radiation in leaves of Brassica napus. Physiol Plant. 1993;87:249–255. [Google Scholar]
- Cheban PL, Russo AG, Shvets SA, Mustyatsé GF. Phenolic compounds of vine leaves. Khim Prirodn Soedin. 1976;4:537–538. [Google Scholar]
- Cockell CS, Knowland J. Ultraviolet radiation screening compounds. Biol Rev Camb Phil Soc. 1999;74:311–345. doi: 10.1017/s0006323199005356. [DOI] [PubMed] [Google Scholar]
- Correia CM, Areal ELV, Torres-Pereira MS, Torres-Pereira JMG. Intraspecific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions. Field Crops Res. 1999;62:97–105. [Google Scholar]
- Day TA. Relating UV-B radiation screening effectiveness of foliage to absorbing-compound concentration and anatomical characteristics in a diverse group of plants. Oecologia. 1993;95:542–550. doi: 10.1007/BF00317439. [DOI] [PubMed] [Google Scholar]
- Day TA, Howells BW, Rice WJ. Ultraviolet absorption and epidermal-transmittance spectra in foliage. Physiol Plant. 1994;92:207–218. [Google Scholar]
- Day TA, Howells BW, Ruhland CT. Changes in growth and pigment concentrations with leaf age in pea under modulated UV-B radiation field treatments. Plant Cell Environ. 1996;19:101–108. [Google Scholar]
- Day TA, Martin G, Vogelmann TC. Penetration of UV-B radiation in foliage: evidence that the epidermis behaves as a non-uniform filter. Plant Cell Environ. 1993;16:735–741. [Google Scholar]
- Day TA, Vogelmann TC, DeLucia EH. Are some plant life forms more effective than others in screening out ultraviolet-B radiation? Oecologia. 1992;92:513–519. doi: 10.1007/BF00317843. [DOI] [PubMed] [Google Scholar]
- Dürinig H. Stomata frequency of leaves of Vitis spp. and cultivars. Vitis. 1980;19:91–98. [Google Scholar]
- Forkmann G, Heller W. Biosynthesis of flavonoids. In: Barton D, Nakanishi K, editors. Comprehensive Natural Products Chemistry. Vol. 1. Amsterdam: Elsevier; 1999. pp. 713–748. [Google Scholar]
- Grammatikopoulos G, Petropoulou Y, Manetas Y. Site-dependent differences in transmittance and UV-B-absorbing capacity of isolated leaf epidermes and mesophyll in Urginea maritima (L.) Baker. J Exp Bot. 1999;50:517–521. [Google Scholar]
- Greenberg BM, Gaba V, Canaani O, Malkin S, Mattoo AK, Edelmann M. Separate photosensitizers mediate degradation of the 32-kDa photosystem II reaction center protein in the visible and UV spectral regions. Proc Natl Acad Sci USA. 1989;86:6617–6620. doi: 10.1073/pnas.86.17.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidoni S, Mannini F, Ferrandino A, Argamante N, Di Stefano R. The effect of grapevine leafroll and rugose wood sanitation on agronomic performance and berry and leaf phenolic content of a nebbiolo clone (Vitis vinifera L.) Am J Enol Vitic. 1997;48:438–442. [Google Scholar]
- Hmamouchi M, Es-safi N, Lahrichi M, Fruchier A, Essassi EM. Flavones and flavonols in leaves of some Moroccan Vitis vinifera cultivars. Am J Enol Vitic. 1996;47:186–192. [Google Scholar]
- Huang L-K, He J, Chow WS, Whitecross MI, Anderson JM. Responses of detached rice leaves (Oryza sativa L.) to moderate supplementary ultraviolet-B radiation allow early screening for relative sensitivity to ultraviolet-B irradiation. Aust J Plant Physiol. 1993;20:285–297. [Google Scholar]
- Hutzler P, Fischbach R, Heller W, Jungblut TP, Reuber S, Schmitz R, Veit M, Weissenböck G, Schnitzler JP. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J Exp Bot. 1998;49:953–965. [Google Scholar]
- Jordan BR. The effects of ultraviolet-B radiation on plants: a molecular perspective. Adv Bot Res. 1996;22:97–162. [Google Scholar]
- Karagiannis S, Economou A, Lanaridis P. Phenolic and volatile composition of wines made from Vitis vinifera Cv. Muscat Lefko grapes from the island of Samos. J Agric Food Chem. 2000;48:5369–5375. doi: 10.1021/jf000459c. [DOI] [PubMed] [Google Scholar]
- Krause GH, Schmude C, Garden H, Koroleva OY, Winter K. Effects of solar ultraviolet radiation on the potential efficiency of photosystem II in leaves of tropical plants. Plant Physiol. 1999;121:1349–1358. doi: 10.1104/pp.121.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry LG, Chapple CCS, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995;109:1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 1993;5:171–179. doi: 10.1105/tpc.5.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Schweiger J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission in plants. J Plant Physiol. 1998;152:272–282. [Google Scholar]
- Lois R, Buchanan BB. Severe sensitivity to ultraviolet radiation in an Arabidopsis mutant deficient in flavonoid accumulation: II. Mechanisms of UV-resistance in Arabidopsis. Planta. 1994;194:504–509. [Google Scholar]
- Marangoni R, Gioffré D, Colombetti G, Lebert M, Häder D-P. ELDONET: European Light Dosimeter Network. J Photochem Photobiol B Biol. 2000;58:178–184. doi: 10.1016/s1011-1344(00)00117-2. [DOI] [PubMed] [Google Scholar]
- Markstädter C. Untersuchungen zur jahreszeitlichen Entwicklung der kutikulären Wachse von Fagus sylvatica L. PhD thesis. Germany: University of Kaiserslautern; 1994. [Google Scholar]
- Markstädter C, Queck I, Baumeister J, Riederer M, Schreiber U, Bilger W. Epidermal transmittance of leaves of Vicia faba for UV radiation as determined by two different methods. Photosynth Res. 2001;67:17–25. doi: 10.1023/A:1010676111026. [DOI] [PubMed] [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL. Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol. 2000;122:117–126. doi: 10.1104/pp.122.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon JH, Fukshansky L. On the interpretation of absorption spectra of leaves: II. The non-absorbed ray of the sieve effect and the mean optical pathlength in the remainder of the leaf. Photochem Photobiol. 1990;51:211–216. [Google Scholar]
- Melis A. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci. 1999;4:130–135. doi: 10.1016/s1360-1385(99)01387-4. [DOI] [PubMed] [Google Scholar]
- Mirecki RM, Teramura AH. Effects of ultraviolet-B irradiance on soybean: V. The dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol. 1984;74:475–480. doi: 10.1104/pp.74.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogués S, Allen DJ, Morison JIL, Baker NR. Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol. 1999;117:173–181. doi: 10.1104/pp.117.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura S, Watanabe M. Determination of phenolic cinnamates in white wine and their effect on wine quality. Agric Biol Chem. 1981;45:2063–2070. [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP. Differential response of photosynthetic pigments, rubisco activity and rubisco protein of Arabidopsis thaliana exposed to UVB and ozone. Photochem Photobiol. 1995;62:727–735. [Google Scholar]
- Renger G, Völker M, Eckert HJ, Fromme R, Hohm-Veit S, Gräber P. On the mechanism of photosystem II deterioration by UV-B irradiation. Photochem Photobiol. 1989;49:97–105. [Google Scholar]
- Salgues M, Cheynier V, Gunata Z, Wylde R. Oxidation of grape juice 2-S-glutathionyl caffeoyl tartaric acid by Botrytis cinerea laccase and characterization of a new substance: 2,5-di-S-gluthathionyl tartaric acid. J Food Sci. 1986;51:1191–1194. [Google Scholar]
- Schreiber U, Bilger W, Neubauer C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D, Caldwell MM, editors. Ecophysiology of Photosynthesis. Berlin: Springer-Verlag; 1994. pp. 49–70. [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Sheahan JJ. Sinapate esters provide greater UV-B attenuation than flavonoids in Arabidopsis thaliana (Brassicaceae) Am J Bot. 1996;83:679–686. [Google Scholar]
- Singleton VL, Timberlake CF, Lea AGH. The phenolic cinnamates of white grapes and wine. J Sci Food Agric. 1978;29:403–410. [Google Scholar]
- Strid A, Chow WS, Anderson JM. Effects of supplementary ultraviolet-B radiation on photosynthesis in Pisum sativum. Biochim Biophys Acta. 1990;1020:260–268. [Google Scholar]
- Vass I. Adverse effects of UV-B light on the structure and function of the photosynthetic apparatus. In: Pessarakli M, editor. Handbook of Photosynthesis. New York: Marcel Dekker, Inc.; 1997. pp. 931–949. [Google Scholar]
- Veit M, van Rensen I, Kirch J, Czygan F-C. HPLC analysis of phenolics and flavonoids in Arctostaphylos uva-ursi. Planta Med Suppl: 1992;58:A687. [Google Scholar]
- Von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Weber B. Phenolische Komponenten des Weinrebenblattes: Identität und phytopathologische Bedeutung. PhD thesis. University of Zürich; 1992. [Google Scholar]
- Weissenböck G, Hedrich R, Sachs G. Secondary phenolic products in isolated guard cell, epidermal cell and mesophyll cell protoplasts from Pea (Pisum sativum L.) leaves: distribution and determination. Protoplasma. 1986;134:141–148. [Google Scholar]
- Xiong FS, Day TA. Effect of solar ultraviolet-B radiation during springtime ozone depletion on photosynthesis and biomass production of Antarctic vascular plants. Plant Physiol. 2001;125:738–751. doi: 10.1104/pp.125.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]