Abstract
To investigate whether Cd induces common plant defense pathways or unspecific necrosis, the temporal sequence of physiological reactions, including hydrogen peroxide (H2O2) production, changes in ascorbate-glutathione-related antioxidant systems, secondary metabolism (peroxidases, phenolics, and lignification), and developmental changes, was characterized in roots of hydroponically grown Scots pine (Pinus sylvestris) seedlings. Cd (50 μm, 6 h) initially increased superoxide dismutase, inhibited the systems involved in H2O2 removal (glutathione/glutathione reductase, catalase [CAT], and ascorbate peroxidase [APX]), and caused H2O2 accumulation. Elongation of the roots was completely inhibited within 12 h. After 24 h, glutathione reductase activities recovered to control levels; APX and CAT were stimulated by factors of 5.5 and 1.5. Cell death was increased. After 48 h, nonspecific peroxidases and lignification were increased, and APX and CAT activities were decreased. Histochemical analysis showed that soluble phenolics accumulated in the cytosol of Cd-treated roots but lignification was confined to newly formed protoxylem elements, which were found in the region of the root tip that normally constitutes the elongation zone. Roots exposed to 5 μm Cd showed less pronounced responses and only a small decrease in the elongation rate. These results suggest that in cells challenged by Cd at concentrations exceeding the detoxification capacity, H2O2 accumulated because of an imbalance of redox systems. This, in turn, may have triggered the developmental program leading to xylogenesis. In conclusion, Cd did not cause necrotic injury in root tips but appeared to expedite differentiation, thus leading to accelerated aging.
Cd is an important environmental pollutant with high toxicity to animals and plants. It is released into the environment by traffic, metal-working industries, mining, as a by-product of mineral fertilizers, and from other sources (Nriagu and Pacyna, 1988). The regulatory limit of Cd in agricultural soils is 100 mg kg−1 soil, but regionally this threshold is exceeded (Salt et al., 1998). Heavy metal toxicity is also an important issue in reclamation of industrial sites. Cd accumulation causes reductions in photosynthesis, diminishes water and nutrient uptake (Sanita di Toppi and Gabbrielli, 1999), and results in visible symptoms of injury in plants such as chlorosis, growth inhibition, browning of root tips, and finally death (Kahle, 1993).
The question as to how Cd acts at the cellular level and how plants may defend themselves against this pollutants is receiving increasing attention. It has been shown that Cd induces the synthesis of phy-tochelatins (γ-glutamyl-Cys [γ-EC] peptides), which bind metals in the cytosol and sequester them in the vacuole (Rauser, 1995; Mehra and Tripathi, 2000). The precursor for phytochelatin synthesis is glutathione, whose cellular level was decreased after Cd exposure (Rauser, 1995; Zenk, 1996; Xiang and Oliver, 1998). Exposure to sublethal Cd concentrations resulted in the recovery of cellular glutathione concentrations and was accompanied by increased γ-EC synthetase and glutathione synthetase mRNA transcript levels (Xiang and Oliver, 1998).
Glutathione is the major non-protein thiol in plants and has many functions in plant metabolism. It is involved in the detoxification of heavy metals and xenobiotics and plays a role in gene activation and in the protection from oxidative stress (Lamoureux and Rusness, 1989; Bergmann and Rennenberg, 1993; Noctor and Foyer, 1998). As an antioxidant glutathione together with ascorbate and antioxidative enzymes, superoxide dismutases (SOD; EC 1.15.1.1), ascorbate peroxidases (APX; EC 1.11.1.11), and catalases (CAT; EC 1.11.1.6) controls the cellular concentrations of hydrogen peroxide (H2O2) and O2.− (Noctor and Foyer, 1998). Recycling of ascorbate and GSH is achieved by monodehydroascorbate radical reductase (MDAR; EC 1.6.5.4.), dehydroascorbate reductase (DAR; EC 1.8.5.1), and glutathione reductase (GR; EC 1.6.4.2).
Cd treatment affects the activities of antioxidative enzymes, but contrasting results have been reported. For example, in leaves of Cd-exposed Helianthus annuus plants, the activities of ascorbate-glutathione-related defense enzymes were decreased (Gallego et al., 1996). Roots and leaves of Phaseolus vulgaris as well as suspension cultures of tobacco (Nicotiana tabacum) cells contained elevated APX activities after Cd exposure (Chaoui et al., 1997; Piqueras et al., 1999). In Phaseolus aureus seedlings, Cd induced elevated guaiacol peroxidase (POD) but decreased CAT activities (Shaw, 1995). Cd also caused lipid peroxidation, suggesting that the tissues suffered from oxidative stress (Shaw, 1995; Gallego et al., 1996; Lozano-Rodriguez et al., 1997; Chaoui et al., 1997). The involvement of antioxidants in plant responses against Cd toxicity is unclear because Cd does not belong to the group of transition metals like copper, iron, and zinc, which may induce oxidative stress via Fenton-type reactions. It is possible that the observed changes in the antioxidant systems occurred as a result of unspecific cellular degradation processes. However, another possibility is that Cd triggers common defense pathways in plants cells like other biotic or abiotic environmental stresses. A joint initial event of these pathways is an accumulation of H2O2, which acts as a signaling molecule. In plant-pathogen interactions, H2O2 induces an orchestrated sequence of reactions involving the activation of peroxidases, the stimulation of secondary metabolism, structural changes such as lignin deposition, and eventually cell death (Alvarez and Lamb, 1997).
Because it is not known whether Cd induces common plant defense pathways, we investigated the sequence of physiological reactions, including H2O2 production, changes in ascorbate-glutathione-related antioxidant systems, secondary metabolism (peroxidases, phenolics, and lignification), developmental changes, and cell death, occurring in roots after Cd exposure. Scots pine (Pinus sylvestris) seedlings were chosen as a model system because forest ecosystems are particularly vulnerable to heavy metal pollution. The seedlings were exposed to 5 or 50 μm Cd in hydroponics and used to study physiological defense reactions and anatomical changes in root tips.
RESULTS
Growth Responses and Cd Accumulation
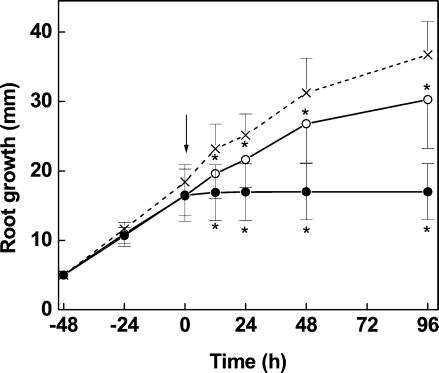
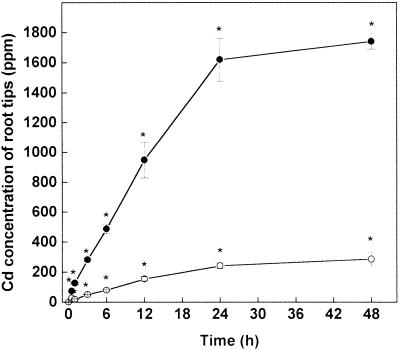
After acclimation to fresh medium, pine roots grew at a constant rate of about 6.4 mm d−1 (Fig. 1). Addition of Cd immediately affected root elongation. Exposure to Cd at 5 and 50 μm caused reductions in the growth rates of 20% and 90%, respectively, within 12 h. At that time the root tips contained Cd at about 150 and 1,000 μg g−1 dry weight (Fig. 2). The major accumulation of Cd occurred during the first 24 h after Cd exposure. Thereafter, neither roots exposed to Cd at 5 μm nor those exposed to Cd at 50 μm showed further significant changes in their Cd concentrations (Fig. 2). Roots exposed to the higher Cd concentration completely stopped growth after 12 h, whereas those treated with the lower Cd concentration continued to grow at a 20% diminished rate (Fig. 1).
Figure 1.
Growth of root tips of Scots pine seedlings in the absence (×, control) or presence (○, 5 μm; ●, 50 μm) of Cd. n = 20 replicates per sampling date (±sd). Asterisks indicates values that differ significantly from the control at P ≤ 0.05.
Figure 2.
Cd content in root tips of Scots pine seedlings treated with 5 μm Cd (○) or 50 μm Cd (●). One-centimeter-long root tips were used for analysis (n = 3–4 replicates per date, ±sd). The detection limit for Cd determination was 5 μg g−1 dry weight. Asterisks indicates values that differ significantly from the control at P ≤ 0.05.
Responses of Antioxidant Systems to Cd Exposure
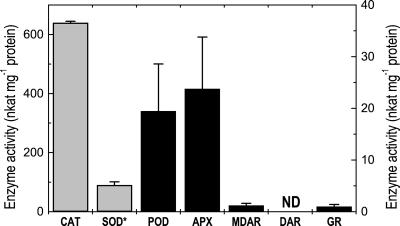
In root tips of control seedlings, activities of antioxidative enzymes were determined over an experimental period of 96 h in parallel with measurements in Cd-treated roots tips. In this period, enzyme activities of controls fluctuated only slightly around their means (Fig. 3). Enzymes involved in the removal of H2O2 and O2.− were generally by one or two orders of magnitude higher than those involved in the regeneration of antioxidants. DAR activities were not detected (Fig. 3).
Figure 3.
Activities of antioxidative enzymes in root tips of Scots pine seedlings. Root tips from control plants were harvested 6, 12, 24, 48, and 96 h after transfer to fresh medium and were used to determine antioxidative systems (n = 4 per sampling date). The bars indicate overall means from all sampling dates (n = 20, ±sd). SOD*, SOD activity is indicated in units. Gray bars, Left y axis; black bars, right y axis. ND, Not detected.
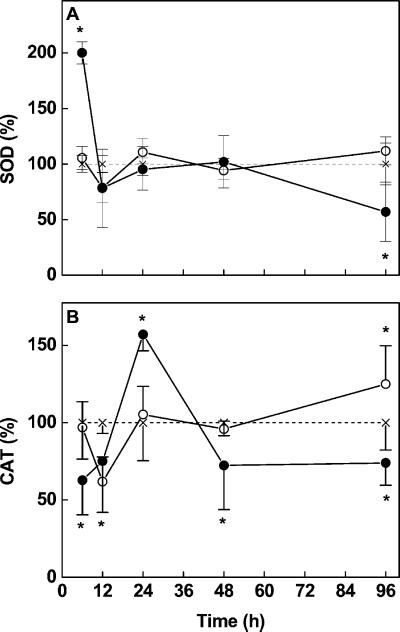
Treatment with high Cd concentrations (50 μm) initially resulted in doubling of SOD activities (Fig. 4A). However, this stimulation was only transient. After 12 h, SOD activities in roots treated with 50 μm Cd were again similar to those found in controls and decreased significantly at the end of the experiment. The rapid induction of SOD after 6 h in the 50 μm Cd treatment was not observed for transcripts of Cu/Zn-SOD (data not shown). Analysis of SOD activities in the presence of cyanide (5 mm) showed that Mn- and/or Fe-SOD activity contributed 18% of total SOD activity in controls and increased to 31% within 96 h after exposure to 50 μm Cd (data not shown). Exposure to 5 μm Cd had no significant effects on SOD activities (Fig. 4A).
Figure 4.
Activity of SOD (A) and CAT (B) in root tips of Cd-treated (○, 5 μm; ●, 50 μm) Scots pine seedlings. The activity was expressed relative to the activity in control plants (=100%, × with dashed line). Each value is the mean of four individual replicates (±sd). Asterisks indicate values that differ significantly from the control at P ≤ 0.05.
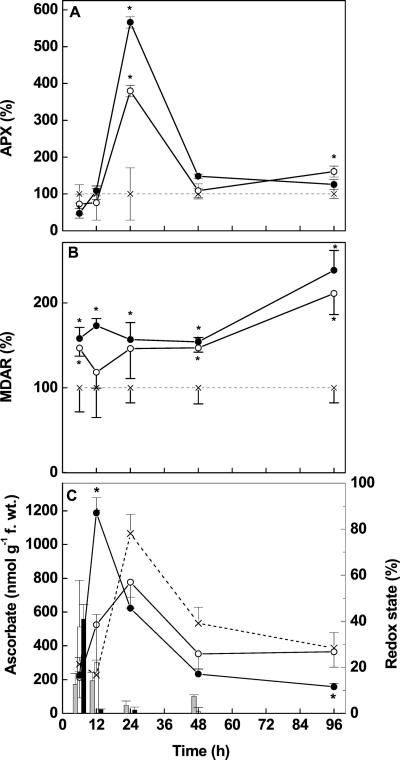
In contrast to SOD, which was stimulated or hardly affected by Cd, CAT and APX activities were initially significantly suppressed by Cd (Figs. 4B and 5A). After 24 h, the activities had recovered (CAT, 5 μm Cd) or were strongly increased (APX and CAT at 50 μm Cd; Figs. 4B and 5A). At later stages, the CAT and APX dropped back to activities similar to those found in controls.
Figure 5.
Activity of APX (A), MDAR (B), and concentrations of ascorbate + DHA (C) in root tips of controls (×) and Cd-treated (○, 5 μm; ●, 50 μm) Scots pine seedlings. Enzyme activities were expressed relative to the activity in control plants (=100%, dashed line). Each value is the mean of four individual replicates (±sd). Asterisks indicate values that differ significantly from the control at P ≤ 0.05. Bars indicate redox state (%) = (ascorbate × 100)/(ascorbate + DHA). Gray bar, Control, white bar, 5 μm Cd; black bar, 50 μm Cd.
“Total ascorbate,” defined as the sum of ascorbate + dehydroascorbate (DHA), was significantly increased after 12 h exposure of root tips to Cd at 5 and 50 μm compared with controls (Fig. 5C). A depletion of “total ascorbate” was observed in root tips of plants treated with the higher Cd concentration (Fig. 5C), whereas “total ascorbate” increased in controls. The fraction of reduced ascorbate in Cd-exposed roots was initially increased (from 13% in controls to 40% in the presence of 50 μm Cd, 6 h), but thereafter declined rapidly and dropped below the detection limit after 24 h of Cd treatment. In controls, ascorbate also decreased but was below the detection limit only after 96 h (Fig. 5C). The activities of MDAR increased 1.5- to 2-fold in response to both Cd treatments (Fig. 5B), suggesting Cd caused an increased redox cycling of ascorbate. DAR activities were generally below the detection limit (Fig. 3), and effects of Cd on DAR activities were not detected (data not shown).
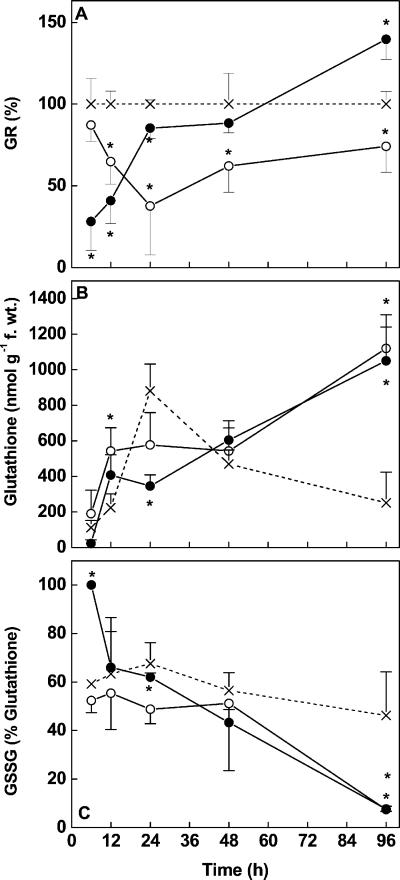
Cd at 50 μm caused a significant inhibition of GR activity (−70%) after 6 and 12 h (Fig. 6A). Recovery occurred within 24 h and after 96 h the GR activity was approximately 1.5-fold increased compared with controls. In the presence of 5 μm Cd, a significant inhibition of GR activity (−65%) was also observed. However, this suppression occurred delayed in comparison with roots treated with Cd at 50 μm; recovery to levels similar to those of controls was found after 96 h.
Figure 6.
Activity of GR (A), concentration of glutathione (B), and redox state of the glutathione pool (C) in root tips of controls (×) and Cd-treated (○, 5 μm; ●, 50 μm) Scots pine seedlings. The activity was expressed relative to the activity in control plants (=100%, dashed line). Each value is the mean of four individual replicates (±sd). Asterisks indicate values that differ significantly from the control at P ≤ 0.05.
Upon exposure to 50 μm Cd, the glutathione pool (GSH + oxidized glutathione [GSSG]) was almost completely depleted within 6 h and the remaining glutathione was oxidized (GSSG; Fig. 6, B and C). In Cd-treated roots, the increase in glutathione was initially accelerated as compared with controls but did not reach the high levels present in controls at 24 h (Fig. 6B). After 96 h, Cd-treated roots contained nearly four times higher concentrations of glutathione than controls (Fig. 6B). Despite fluctuations in the glutathione concentrations, the redox state of the glutathione pool remained relatively constant for most of the time accounting for 50% to 70% GSSG in root tips of control plants. After recovery from the initial stress in both Cd treatments, the fraction of GSSG decreased below 20% after 96 h (Fig. 6C).
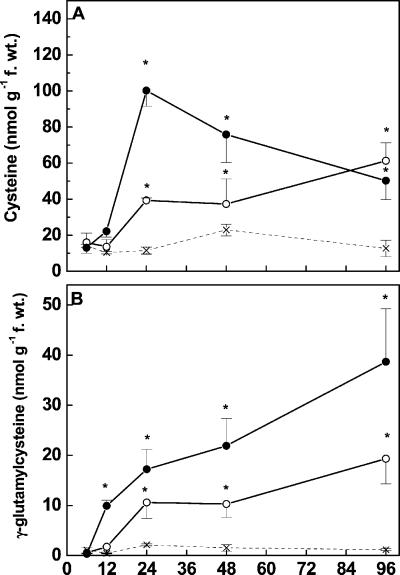
The effects of Cd on the precursors of glutathione synthesis, γ-EC and Cys, were also investigated (Fig. 7, A and B). In roots tips of control seedlings, the concentrations of these compounds were low (γ-EC: 1.26 ± 0.61 nmol g−1 fresh weight and Cys: 14.3 ± 4.47 nmol g−1 fresh weight) accounting 0.2% and 2.7% of the thiol pool. Both Cd concentrations caused significant decreases in γ-EC within 6 h (not seen due to the scaling of Fig. 7B). Thereafter, γ-EC concentrations increased extremely, reaching 10- and 20-fold higher concentrations in roots exposed for 12 h to Cd at 5 and 50 μm, respectively, than those present in controls (Fig. 7B). Elevated γ-EC concentrations were maintained in Cd-treated roots until the end of the experiment. Upon exposure to Cd, Cys concentrations also started to increase but with a delay of 12 h as compared with γ-EC (Fig. 7A). Maximum Cys concentrations were found in roots after 24 h exposure to Cd at 50 μm; thereafter, the concentrations decreased but maintained elevated levels for 96 h (Fig. 7A).
Figure 7.
Concentrations of Cys (A) and γ-EC (B) in root tips of controls (×) and Cd-treated (○, 5 μm; ●, 50 μm) Scots pine seedlings. Each value is the mean of four individual replicates (±sd). Asterisks indicate values that differ significantly from the control at P ≤ 0.05.
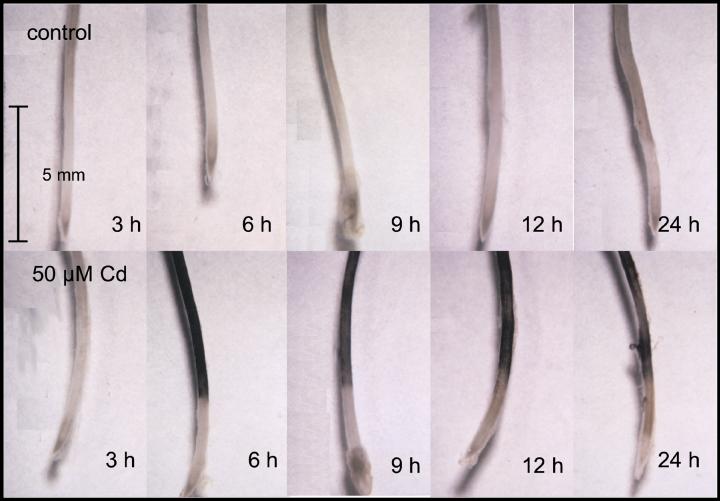
To investigate whether the inhibition of antioxidative enzymes, observed after Cd exposure, was accompanied by an increase in activated oxygen species, intact roots were stained histochemically for the presence of H2O2 (Fig. 8). Roots exposed 6, 9, and 12 h to 50 μm Cd showed significant accumulation of H2O2 compared with unstressed controls. After 24 h, the staining was less pronounced. At later stages, Cd had also caused browning of the roots tips, which would interfere with low H2O2 staining intensities.
Figure 8.
H2O2 in roots of 6-week-old seedlings of Scots pine grown in liquid culture. Roots of control plants and roots treated with 50 μm Cd were stained for H2O2 after exposure to Cd for the indicated times. Bar = 5 mm.
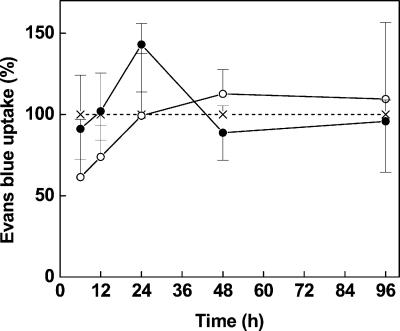
Despite the initial depletion of antioxidative systems and accumulation of H2O2 at 6 to 12 h after Cd exposure, these conditions did not result immediately in cell death (Fig. 9). Only at later stages of Cd exposure after 24 h, when ascorbate was hardly detected, cell death was slightly and only transiently increased (Fig. 9).
Figure 9.
Cell death indicated as Evans blue uptake by root tips of controls (×) and Cd-treated (○, 5 μm; ●, 50 μm) Scots pine seedlings. Each value is the mean of four individual replicates (±sd) and was expressed relative to controls.
Secondary Metabolism and Developmental Changes in Response to Cd
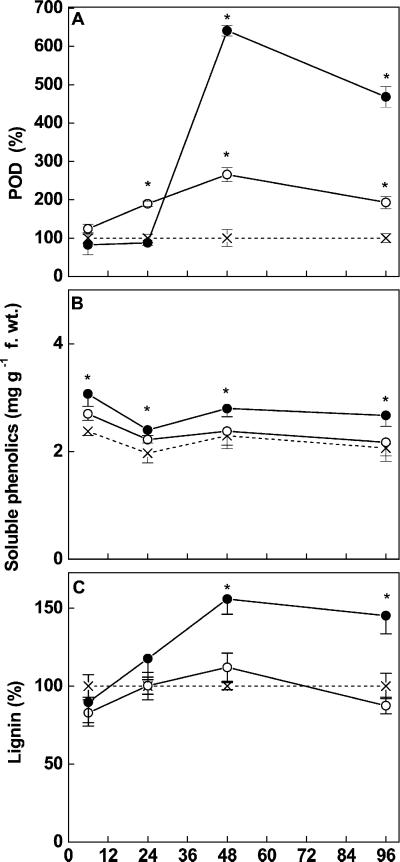
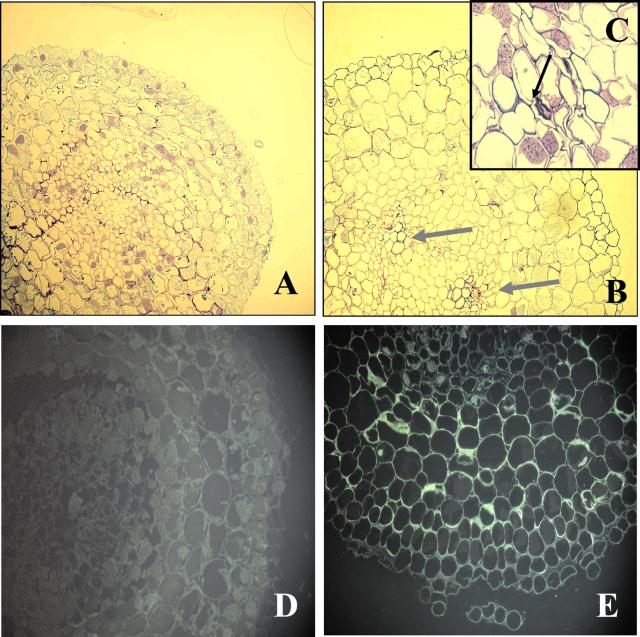
Cd treatment initially had no effect on nonspecific peroxidase activities (POD), i.e. peroxidases reducing H2O2 by the oxidation of aromatic substrates like phenolics or monolignols (Fig. 10A). A significant induction was found only after 48 h (Fig. 10A). The induction was less pronounced in roots of pine seedlings exposed to Cd at 5 μm as compared with those treated with Cd at 50 μm (Fig. 10A). It is interesting to note that the Cd-induced stimulation in POD activities was delayed as compared with APX or CAT activities and that initial reductions in POD activities did not occur (Fig. 10A). Soluble phenolics increased immediately after Cd exposure (Fig. 10B). The elevated levels were maintained in root tips exposed to Cd at 50 μm but not in those exposed to 5 μm (Fig. 10B). Histochemical analysis of roots tips showed that these newly formed phenolics were localized in the cytosol (Fig. 11E). The intense staining was not found in controls (Fig. 11D).
Figure 10.
Activity of POD and concentrations of soluble phenolics (B) and lignin (C) in root tips (1.5 cm) of controls (×) and Cd-treated (○, 5 μm; ●, 50 μm) Scots pine seedlings. The POD activity and lignin were expressed relative to controls (=100%, dashed line). Each value is the mean of four individual replicates (±sd). Asterisks indicate values that differ significantly from the control at P ≤ 0.05.
Figure 11.
Localization of lignin and suberin (A, control; B and C, 50 μm Cd) and phenolics (D, control; E, 50 μm Cd) in root tips of Scots pine seedlings. Ninety-six hours after exposure to Cd, cross sections were taken at distances from the root tip of 6 mm (A, B, D, and E) and 1.5 mm (C) and were stained with 1% (w/v) toluidine blue (A–C) or 0.1% (w/v) berberine-sulfate (D and E). Magnification, 20 × 12.5 (A, B, D, and E); 40 × 12.5 (C). Arrows indicate localization of protoxylem elements.
In root tips of seedlings exposed to 5 μm Cd, the lignin concentration was unaffected as compared with controls (136 ± 2.0 mg−1 dry weight, n = 16, ±sd), whereas treatment with 50 μm Cd resulted in significant increases in lignin (Fig. 10C). To find out the structural basis of this increased lignification, cross sections were stained with toluidine blue (Fig. 11, A–C). After 96 h of Cd exposure was complete, lignified protoxylem poles were found at a distance of 6,000 μm from the tip (Fig. 11B), whereas this part of untreated roots did not show any xylem-like differentiation (Fig. 11A). Further analysis revealed that first lignified protoxylem elements were detected already at a distance of 1,500 μm from the root tips in plants exposed for 96 h to 50 μm Cd (Fig. 11C).
DISCUSSION
Detoxification of Cd
It is well known that Cd inhibits growth (Godbold and Hüttermann, 1985; Arduini et al., 1996; Arisi et al., 2000) and affects glutathione metabolism (Rauser, 1995; Zenk, 1996; Xiang and Oliver, 1998; Arisi et al., 2000). In the present study, we showed that inhibition of root elongation was among the most sensitive responses to Cd exposure and faster than most of the other physiological reactions analyzed (Fig. 1) and preceded cell death (Fig. 9). Complete inhibition of root elongation occurred within 12 h at Cd concentrations in the root tip in the range between 112 and 560 nmol Cd g−1 fresh weight as judged from the 5 and 50 μm Cd treatments (Figs. 1 and 2). It is interesting that the root tips accumulated Cd only for about 24 h and maintained relatively stable levels later on, regardless of the concentration applied (Fig. 2). This observation suggests that Cd uptake by root tips is counterbalanced by transport to leaves within approximately 24 h. This finding is important because it shows that reactions occurring after 48 or 96 h were not caused directly by changes in the Cd concentrations of the tissue. This notion applies, for example, for the 4- and 30-fold increases in glutathione and γ-EC concentrations, respectively, occurring after 96 h Cd exposure (Figs. 6B and 7B). It is unlikely that these “late” thiol enhancements reflected a Cd defense mechanism. Arisi et al. (2000) also found that elevated glutathione concentrations in transgenic poplars overexpressing γ-EC synthetase activities did not protect from Cd toxicity. Perhaps the increases in thiols found in the present study at stages when Cd did not increase any longer were caused by enhanced sulfate assimilation. Such a response has been reported for Brassica juncea exposed to Cd (Lee and Leustek, 1999).
Six hours after Cd exposure, a significant depletion of glutathione initially was observed (Fig. 6B). This is a common response to Cd caused by an increased consumption of glutathione for phytochelatin production (Delhaize et al., 1989; Meuwly and Rauser, 1992; De Knecht et al., 1995; Schneider and Bergmann, 1995; Noctor et al., 1998; Xiang and Oliver, 1998). Because the synthesis of glutathione is demand driven, the low glutathione concentration might have triggered increased sulfur uptake and its own synthesis (May et al., 1998), thus resulting in elevated glutathione concentrations at later stages.
Cd Induces Oxidative Stress
Cd exposure initially resulted in severe oxidative stress because the low residual glutathione pool was completely oxidized (6 h, Fig. 6C) and H2O2 accumulated (Fig. 9). It is seemingly paradox that the redox state of ascorbate initially increased (Fig. 5C, 6 h) when H2O2 accumulated in the presence of 50 μm Cd. However, this is theoretically possible when APX is decreased (Polle, 2001). Such a situation occurred apparently after 6 h of 50 μm Cd exposure (Fig. 5A). Ascorbate was then consumed and DHA accumulated, perhaps because of shortage of reductant to maintain MDAR activities in vivo (Fig. 5B)
At the first glance, it may appear surprising that Cd, which is not a transition metal, may cause oxidative stress. However, Cd binds to thiol groups and thereby inactivates thiol-containing enzymes such as GR (Creissen and Mullineaux, 1995; Mullineaux and Creissen, 1997). The same inhibition mechanism may be possible for APX being sensitive to thiol reagents (Chen and Asada, 1989, 1992). In fact, we found that Cd simultaneously inhibited the systems involved in H2O2 removal, i.e. GSH/GR, CAT, and APX, and resulted in elevated activities of SOD, an enzyme, whose product is, beside O2, H2O2 (Figs. 6, A and B, 5A, and 4, A and B). Such a situation must inevitably lead to the accumulation of H2O2 (Polle, 2001).
Both glutathione and H2O2 play roles as signals for the regulation of stress enzymes (May et al., 1998; Noctor and Foyer, 1998). Accumulation of H2O2 is a general stress response, which has been observed in plants exposed to low temperature, heat, pathogens, and chilling (Doke et al., 1994; Levine et al., 1994; Mehdy, 1994; Prasad et al., 1994). H2O2 is a systemic signal for the induction of APX (Karpinski et al., 1999). In callus cultures of rice (Oryza sativa) embryos, H2O2 transiently induced mRNA for cytosolic APX (Morita et al., 1999). In the pine seedlings studied here, H2O2 accumulation was followed within few hours by recovery and significant increases in APX and CAT activities (Figs. 4B and 5A). GR activities also recovered, but interestingly, this recovery was faster in plants exposed to 50 μm than in those exposed to 5 μm Cd, suggesting that the decrease below a certain threshold of GR was necessary to induce the restoration. Xiang and Oliver (1998) found that the recovery was related to increased synthesis of GR protein because the transcript levels increased after Cd exposure. In contrast to APX gene expression, which is activated by H2O2, GR was not stimulated by H2O2 but by jasmonic acid (Xiang and Oliver, 1998). The different time courses for the induction of APX and GR activities observed here also support the finding that these enzymes are regulated by different stimuli.
The time courses of antioxidative responses after Cd exposure suggest that the following sequence of events may take place: Initially, Cd uptake leads to a depletion of glutathione and inhibits CAT, APX, and GR. This causes an accumulation of H2O2 and induces the synthesis of ascorbate and glutathione. H2O2 has been shown to act as a signaling molecule in the activation of cellular defenses including CAT and APX (Prasad et al., 1994; Karpinski et al., 1999). We have not investigated whether H2O2 production was augmented by the stimulation of plasma membrane-bound NADPH oxidases as in pathogen defense reactions (Doke et al., 1994; Alvarez and Lamb, 1997). However, this is likely to occur because Cd can induce an oxidative burst (Piqueras et al., 1999) and early pathogen defense reactions also involve elevated SOD activities (Doke et al., 1994) as observed here (Fig. 4A). The pathogen-related SOD induction is caused by increases in extracellular SOD activity (Doke et al., 1994). We currently cannot exclude such a response in Cd-exposed plants since we could not explain the increases in SOD by changes in the gene expression of the cytosolic CuZn-SOD, which is a major SOD in unstressed pine roots.
Cd Triggers Secondary Metabolism and Differentiation
H2O2 is a signaling intermediate in programmed cell death (Alvarez and Lamb, 1997), which is triggered by pathogen-derived elicitors and occurs as part of the normal developmental program of the plants, strictly controlled in committed cells during xylogenesis (Teichmann, 2001). Fungal elicitors induce H2O2-mediated oxidative cross linking of cell walls in processes not requiring transcription, translation, or activation of secondary metabolism (Bradley et al., 1992). Addition of H2O2 leads to increased mechanical strength and lowers the extensibility of plant cell walls (Schopfer, 1996). Such a rapid H2O2-mediated rigidification of cell walls would explain the fast abolishment of growth occurring in our study within 12 h, thus preceding the activation of peroxidases and lignification by more than 24 h (Fig. 10, A and C). To our knowledge, the induction of the phenylpropanoid metabolism upon Cd exposure has not been reported before. The activation of this pathway is also a typical event in pathogen defense and programmed cell death. Our results suggest that Cd in cells challenged by concentrations, which override the capacity for detoxification, may set off common plant defense pathways. In our system, the threshold was exceeded somewhere between 5 and 50 μm of externally applied Cd and was accompanied specifically by depletion of GSH in combination with failure of H2O2-consuming systems and transient increase in cell death (Fig. 9). Fojtova and Kovarik (2000) recently showed that Cd induced apoptotic changes in suspension cultures of tobacco cells. These changes were characterized by DNA fragmentation that occurred delayed about 48 h after Cd addition (50–100 μm). Once cells are committed to programmed cell death, the process cannot be reversed. In contrast to necrosis, programmed cell death is a strictly controlled process. In line with data on cell death (Fig. 9), we found no evidence for arbitrary injury in cross sections of roots after 96 h of Cd exposure (Fig. 11, B and E), which would have been expected for necrotic reactions. However, Cd appeared to induce the normal developmental program leading to xylogenesis because the formation of “normal” protoxylem elements was observed (Fig. 11B). Lignification is the final steøp in this process (Polle et al., 1997). However, in contrast to normal development, the lignified xylem elements were found here at a distance from the root tip, which normally constitutes the elongation zone.
The processes leading to lignification must be distinguished from those leading to the production of soluble phenolics. Cd caused an accumulation of soluble phenolics in the cytosol. However, this reaction was much faster than lignification (Fig. 10B) and spread over the whole cross section of the root (Fig. 11E). From the differences in temporal and spatial response patterns, it is clear that different signals or differences in the perceptibility of signals must have caused the rapid accumulation of phenolics on the one hand and delayed lignification on the other hand. However, which signals affected secondary metabolism in this distinct way is still unknown.
A further question is why phenolics were increased in response to Cd. Phenolics may contribute, together with ascorbate, to H2O2 destruction in the so-called phenol-coupled APX reaction (Polle et al., 1997), and thus protect from oxidative stress. However, this protection was probably limited by low concentrations of ascorbate present in roots (Fig. 5C). Whether phenolics have direct protective functions is unknown for Cd, but in cultured tobacco cells, phenolics protected from aluminum toxicity (Yamamoto et al., 1998).
Taken together, our results suggest that the inhibition of antioxidative systems by Cd promotes H2O2 production. This was probably the key event for the inhibition of elongation growth and might have set off a sequence of reactions leading to cell death. However, the latter response occurred seemingly only in committed cells, i.e. in those in which this process would normally occur. In conclusion, we suggest that Cd does not cause unspecific necrosis of root cells but expedites developmental and differentiation processes leading to accelerated aging.
MATERIALS AND METHODS
Culture of Plants and Growth Conditions
Seeds of Scots pine (Pinus sylvestris) were surface sterilized for 1 h in 30% (w/v) H2O2. After 3 d at 21°C in darkness, the seeds were germinated on sterile 1% (w/v) water-agar, pH 4.5, with a day/night regime of 16 h/8 h. The seedlings were grown at day/night conditions of 23°C/21°C air temperature, 16-h/8-h day length under white light of 200 μmol m−2 s−1 photosynthetic photon flux (Osram L18 W/21 lamps, Munich). After 3 weeks, the seedlings were transferred to aerated nutrient solutions containing the following nutrient elements: 300 μm NH4NO3, 100 μm Na2SO4, 200 μm K2SO4, 60 μm MgSO4, 130 μm CaSO4, 30 μm KH2PO4, 10 μm MnSO4, and 92 μm FeCl3; and 5 mL of a stock solution of micronutrients: 0.1545 g L−1 H3BO3, 0.012 g L−1 NaMoO4, 0.0144 g L−1 ZnSO4, and 0.0125 g L−1 CuSO4 per liter of nutrient solution. The pH was adjusted to 4.0. The solution was changed every 3 d. After 2 weeks of acclimation, the seedlings were treated with 5 or 50 μm CdSO4 for 96 h and sampled in regular intervals for analyses.
Root Length Measurements and Sampling
Two days before Cd exposure, roots were marked 5 mm behind the tip with water-resistant ink. Root lengths were measured daily under a binocular (Stemi SV 8, Zeiss, Oberkochen, Germany) with a measuring ocular (Zeiss CPL 10-fold). Measurements were performed on 20 plants per treatment and date.
Root samples for analysis of enzymes activities, antioxidants, phenolics, and lignin were collected after 6, 12, 24, 48, and 96 h of Cd treatment. Each sample consisted of 20 15-mm-long root tips. Activities of APX, MDAR, GR, and DHAR were measured in fresh extracts immediately after harvest. For analyses of further biochemical parameters, root tips were kept frozen at −80°C.
Each treatment was replicated four times. Statistical analyses were performed with Statgraphics using ANOVA followed by a least-significance difference test (STN, St. Louis).
Cd Determination
Root tips were cut 10 mm behind the tip, washed 15 min in 5 mm CaCl for exchange of apoplastic Cd (Rauser, 1987), dried to a constant weight, and wet ashed at 170°C in pure HNO3 for 12 h (Feldmann, 1974). Cd was determined by inductively coupled plasma-atomic emission spectroscopy with a standard method.
Enzyme Assays and Protein Determination
Root tips were powdered in liquid nitrogen. The powder (150 mg) was extracted 15 min at 4°C in 5 mL of cold extraction buffer (100 mm potassium phosphate, pH 7.8, 300 mg polyvinylpolypyrrolidone, 1% [v/v] Triton X-100, and 5 mm ascorbate). The extract was centrifuged (30 min, 48,000g, 4°C) and the supernatant was passed through a Sephadex G-25 column (PD-10 column, Pharmacia, Freiburg, Germany) and equilibrated with 100 mm potassium phosphate, pH 7.8. For stabilization of APX, the elution buffer contained 5 mm ascorbate. The enzyme activities were determined according to the following methods: SOD (McCord and Fridovich, 1969), CAT (Aebi, 1983), APX (Nakano and Asada, 1981), MDAR (Hossain et al., 1984), DAR (Dalton et al., 1986), GR (Foyer and Halliwell, 1976), and PODs (Pütter, 1970; modified after Polle et al., 1990). Soluble protein was determined in enzyme extracts with bicinchoninic acid reagent (Pierce, Munich) using bovine serum albumin as the standard. All solutions used for analytical and enzymatical investigations were prepared with double-ionized and ultrafiltrated water (Seralpur Delta UV/UF, USF Seral, Ransbach-Baumbach, Germany).
Extraction and Analysis of Antioxidants
For the extraction of water soluble antioxidants, 60 to 100 mg of frozen root tips were powdered in liquid nitrogen, mixed with 1 mL of 2% (w/v) meta-phosphoric acid containing 1 mm EDTA and 1 mg polyvinylpolypyrrolidone per mg of sample, and centrifuged (20 min, 4°C, 30,000g). The supernatant was used for analyses of antioxidants.
Ascorbate was determined at 268 nm after separation by capillary electrophoresis as described by Davey et al. (1996). The system consisted of a High-Performance Capillary Zone Electrophoresis (model P/ACE 5500 HPCE System, Beckman Instruments, Fullerton, CA) fitted with a UV-VIS diode array detector (P/ACE 5500 DAD, Beckman) and equipped with GOLD software (Beckman) for peak analysis. Injections were made for 5 s under hydrostatic (N2) pressure (0.5 pound per square inch) into a 57-cm fused silica capillary at a constant temperature of 25°C and a constant voltage of +25 kV. Separations were run for 10 min with 100 mm borate buffer, pH 9, as a carrier electrolyte. The capillary was subsequently conditioned for the next run with 0.1 m NaOH, water, and carrier electrolyte. The detection limit for ascorbate was 0.5 μg mL−1 under the present experimental conditions.
For determination of “total ascorbate,” DHA was reduced by dithiothreitol at pH 8.3 to 8.5 for 60 min at room temperature (Anderson et al., 1992). This was achieved by mixing 100 μL of sample with 150 μL of 60 mm dithiothreitol in 1 m 2-[N-cyclohexylamino] ethansulfonic acid. The mixture was analyzed as above and DHA was calculated by substracting ascorbate from “total ascorbate.”
Glutathione, γ-EC, and Cys were determined after reduction, derivatization with monobromobimanes, HPLC separation (Beckman System Gold, Munich, Germany) on a C-18 column, and detection with a fluorescence detector (RF-550, Shimadzu, Duisburg, Germany) after the method of Schupp and Rennenberg (1988). GSSG was determined in the same manner after removal of glutathione by alkylation with N-ethylmaleimide (Gorin et al., 1966) GSH was calculated as the difference of glutathione and GSSG. The redox state was defined as the GSSG content in percent of glutathione content.
Measurement of Cell Death
Cell death, indicated as loss of plasma membrane integrity, was measured spectrophotometrically as Evans blue uptake (Baker and Mock, 1994). After Cd treatment, three root tips (1 cm) were incubated in Evans blue solution (0.025% [w/v] Evans blue in water) for 30 min. After washing the roots for 15 min with water, the trapped Evans blue was released from the roots by homogenizing root tips with a microhomogenizer in 800 μL of a measuring solution (50% [v/v] MeOH and 1% [w/v] SDS). The homogenate was incubated for 15 min in a water bath at 50°C and centrifuged at 14,000g for 15 min. The optical density of the supernatant was determined at 600 nm and expressed on the basis of fresh mass.
Determination of Phenolics and Lignin
Frozen root tips (about 100 mg) were ground in liquid nitrogen, transferred into 1 mL of 50% (v/v) MeOH MeOH/H2O/H2O, incubated for 30 min on a shaker, and centrifuged at 48,000g. Five hundred microliters of extract was used for the determination of free phenolic compounds with the Folin-Ciocalteus reagent (Pritchard et al., 1997). Tannic acid was used as a standard. The pellet was subjected to several washing steps, modified after Strack et al. (1988): 2× 80% (v/v) MeOH/H2O, 1× water, 1× acetone, and hydrolyzed 1 h at 70°C with 1 m NaOH. The hydrolyzed pellet was washed with 80% (v/v) MeOH/ H2O (two times), distilled water and acetone, and dried. The resulting residue was used for lignin analysis with the thioglycolic acid method after Bruce and West (1989) as modified by Otter (1996).
Histochemical Staining of Roots for H2O2 and Phenolic Compounds
To detect H2O2, roots attached to the plants were stained for 30 to 45 min in KI/starch reagent (4% [w/v] starch and 0.1 m KI, pH 5.0; modified after Olson and Varner, 1993). Stained roots were photographed under a binocular (Stemi SV 8, Zeiss).
To analyze anatomical changes, roots were freeze dried and embedded for microscopy as described by Fritz (1989). Cross sections of roots (1 μm) were stained with 1% (w/v) toluidine blue in 0.1% (w/v) disodium tetraborate decahydrate for light microscopy (Axioplan microscope, Zeiss) or stained with 0.1% (w/v) berberine-sulfate in water for UV microscopy (UV filter UV-G365, Zeiss). The sections were photographed with a digital camera (Coolpix 990, Nikon, Tokyo).
ACKNOWLEDGMENTS
We are grateful to Claudia Rudolf and Karin Lange for excellent technical assistance.
Footnotes
This work was supported by the European Community (project no. FAIR3–CT961377; Metal Tolerant Ectomycorrhizal Fungi: Selection, Characterisation, and Utilisation for Restoration of Polluted Forests).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010318.
LITERATURE CITED
- Aebi H. Catalase. In: Bergmeyer H, editor. Methods of Enzymatic Analysis 3. Weinheim, Germany: Verlag Chemie; 1983. pp. 273–277. [Google Scholar]
- Alvarez ME, Lamb C. Oxidative burst mediated defense responses in plant disease resistance. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. New York: Cold Spring Harbor Laboratory Press; 1997. pp. 815–839. [Google Scholar]
- Anderson RG, Chevone BI, Hess JL. Seasonal variation in the antioxidant system of eastern white pine. Plant Physiol. 1992;98:501–508. doi: 10.1104/pp.98.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduini I, Godbold DL, Onnis A. Cadmium and copper uptake and distribution in Mediterranean tree seedlings. Physiol Plant. 1996;97:111–117. [Google Scholar]
- Arisi ACM, Mocquot B, Lagriffoul A, Mench M, Foyer CH, Jouanin L. Responses to cadmium in leaves of transformed poplars overexpressing gamma-glutamylcysteine synthetase. Physiol Plant. 2000;109:143–149. [Google Scholar]
- Baker CJ, Mock NM. An improved method for monitoring cell death in a cell suspension and leaf disk assays using Evans blue. Plant Cell Tissue Organ Cult. 1994;39:7–12. [Google Scholar]
- Bergmann L, Rennenberg H. Glutathione metabolism in plants. In: De Kok. LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE, editors. Sulfur Nutrition and Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 61–75. [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a prolin-rich plant cell wall protein: a novel rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of Castor bean. Plant Physiol. 1989;91:889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.) Plant Science. 1997;127:139–147. [Google Scholar]
- Chen G, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isoenzymes and their differences in enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Chen G, Asada K. Inactivation of ascorbate peroxidase by thiols requires hydrogen peroxide. Plant Cell Physiol. 1992;33:117–123. [Google Scholar]
- Creissen GP, Mullineaux PM. Cloning and characterization of glutathione reductase cDNAs and identification of two genes encoding the tobacco enzyme. Planta. 1995;197:422–425. doi: 10.1007/BF00202667. [DOI] [PubMed] [Google Scholar]
- Dalton D, Russell S, Hanus F, Pascoe G, Evans H. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA. 1986;83:3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MW, Bauw G, van Montagu M. Analysis of ascorbate in plant tissues by high-perfomance capillary zone electrophoreses. Anal Chem. 1996;239:8–19. doi: 10.1006/abio.1996.0284. [DOI] [PubMed] [Google Scholar]
- De Knecht JA, Van Baren N, Ten Bookum WM, Wong Fong Sang HW, Koevoets PLM, Schat H, Verkleij JAC. Synthesis and degradation of phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulagaris. Plant Sci. 1995;106:9–18. [Google Scholar]
- Delhaize E, Jackson PJ, Lujan LD, Robinson NJ. Poly(γ-glutamyl-cysteinyl) glycine synthesis in Datura innoxia and binding with cadmium: role in cadmium tolerance. Plant Physiol. 1989;89:700–706. doi: 10.1104/pp.89.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke N, Miura Y, Sanchez L, Kawakita K. Involvement of superoxide radical in signal transduction: responses to attack by pathogens, physical and chemical shocks and UV irradiation. In: Foyer C, Mullineaux PM, editors. Causes of Photo-oxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 177–198. [Google Scholar]
- Feldmann C. Perchloric acid procedure for wet-ashing organics for the determination of mercury (and other metals) Anal Chem. 1974;46:1606–1609. [Google Scholar]
- Fojtova M, Kovarik A. Genotoxic effect of cadmium is associated with apoptotic changes in tobacco cells. Plant Cell Environ. 2000;23:531–537. [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Fritz E. X-ray microanalysis of diffusible elements in plant cells after freeze-drying, pressure-infiltration with ether and embedding in plastic. Scanning Microsc. 1989;3:517–526. [Google Scholar]
- Gallego SM, Benavides MP, Tomaro ML. Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci. 1996;121:151–159. [Google Scholar]
- Godbold DL, Hüttermann A. Effect of zinc, cadmium and mercury on root elongation of Picea abies (Karst.) seedlings, and the significance of these metals to forest die-back. Environ Pollut. 1985;38:375–381. [Google Scholar]
- Gorin G, Martic PA, Doughty G. Kinetics of the reaction of N-ethylmaleimide with cysteine and some congeners. Anal Biochem Biophys. 1966;115:593–597. doi: 10.1016/0003-9861(66)90079-8. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. [Google Scholar]
- Kahle H. Response of roots of trees to heavy metals. Environ Exp Bot. 1993;33:99–119. [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- Lamoureux GL, Rusness DG. The role of glutathione and glutathione-S-transferases in pesticide metabolism, selectivity and medical aspects. In: Dolphin D, Poulson R, Avramovic O, editors. Enzymes and Cofactors, Series. IIIB. New York: Wiley Liss & Sons; 1989. pp. 153–196. [Google Scholar]
- Lee S, Leustek T. The effect of cadmium on sulfate assimilation enzymes in Brassica juncea. Plant Sci. 1999;141:201–207. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Lozano-Rodriguez E, Hernandez LE, Bonay P, Carpena-Ruiz RO. Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot. 1997;306:123–128. [Google Scholar]
- May MJ, Vernoux T, Leaver C, van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998;49:649–667. [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase: an enzyme function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RK, Tripathi RD. Phytochelatins and metal tolerance. In: Agrawal SB, Agrawal M, editors. Environmental Pollution and Plant Responses. Boca Raton, FL: CRC Press; 2000. pp. 367–382. [Google Scholar]
- Meuwly P, Rauser WE. Alteration of thiol pools in roots and shoots of maize seedlings exposed to cadmium: adaption and developmental cost. Plant Physiol. 1992;99:8–15. doi: 10.1104/pp.99.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Kaminaka H, Masumura T, Tanaka K. Induction of rice cytosolic ascorbate peroxidase mRNA by oxidative stress signaling. Plant Cell Physiol. 1999;40:417–422. [Google Scholar]
- Mullineaux P, Creissen GP. Glutathione reductase: regulation and role in oxidative stress. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. New York: Cold Spring Harbor Laboratory Press; 1997. pp. 667–713. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981;22:860–867. [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Renenberg H. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils with trace metals. Nature. 1988;333:134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Olson PD, Varner JE. Hydrogen peroxide and lignification. Plant J. 1993;4:887–892. [Google Scholar]
- Otter TJ. Untersuchung zur ligninbildung bei der fichte (Picea abies L., Karst.). PhD Thesis. Germany: Universität Freiburg; 1996. [Google Scholar]
- Piqueras A, Olmos E, Martinez-Solano JR, Hellin E. Cd-induced oxidative burst in tobacco BY2 Cells: time course, subcellular location and antioxidant response. Free Rad Res. 1999;31:33–38. doi: 10.1080/10715769900301291. [DOI] [PubMed] [Google Scholar]
- Polle A. Dissection of the superoxide dismutase-ascorbate-glutathione pathway by metabolic modeling: computer analysis as a step towards flux analysis. Plant Physiol. 2001;126:445–462. doi: 10.1104/pp.126.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A, Chakrabati K, Schürmann W, Rennenber H. Composition and properties of hydrogen peroxide decomposing systems in extracellular and total extracts from needles of Norway spruce (Picea abies L., Karst.) Plant Physiol. 1990;94:312–319. doi: 10.1104/pp.94.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A, Otter T, Sandermann H., Jr . Biochemistry and physiology of lignin synthesis. In: Rennenberg H, Escherich W, Ziegler H, editors. Trees: Contributions to Modern Tree Physiology. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 455–477. [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Steward CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard S, Peterson C, Runion GB, Prior S, Rogers H. Atmospheric CO2 concentration, N availibility, and water status affect patterns of ergastic substance deposition in longleaf pine (Pinus palustris Mill.) foliage. Trees. 1997;118:494–503. [Google Scholar]
- Pütter J. Peroxydasen. In: Bergmeyer H, editor. Methoden der Enzymatischen Analysen 1. Weinheim, Germany: Verlag Chemie; 1970. [Google Scholar]
- Rauser WE. Compartmental efflux analysis and removal of extracellular cadmium from roots. Plant Physiol. 1987;85:62–65. doi: 10.1104/pp.85.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE. Phytochelatins and related peptides: structure, biosynthesis, and function. Plant Physiol. 1995;109:1141–1149. doi: 10.1104/pp.109.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Sanita di Toppi L, Gabbrielli R. Response to cadmium in higher plants. Environ Exp Bot. 1999;41:105–130. [Google Scholar]
- Schneider S, Bergmann L. Regulation of glutathione synthesis in suspension cultures of parsley and tobacco. Bot Acta. 1995;108:34–40. [Google Scholar]
- Schopfer P. Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta. 1996;199:43–49. [Google Scholar]
- Schupp R, Rennenberg H. Diurnal changes in the glutathione content of spruce needles (Picea abies, L.) Plant Sci. 1988;57:113–117. [Google Scholar]
- Shaw BP. Effects of mercury and cadmium on the activities of antioxidative enzymes in the seedling of Phaseolus aureus. Bio Plant. 1995;37:587–596. [Google Scholar]
- Strack D, Heilemann J, Mömken M, Wray V. Cell wall-conjugated phenolics from Coniferae leaves. Phytochemistry. 1988;27:3517–3521. [Google Scholar]
- Teichmann T. The biology of wood formation: scientific challenges and biotechnological perspectives. In: Panadalai SG, editor. Recent Research Developments in Plant Physiology. Trivandrum, India: Research Signpost; 2001. pp. 269–284. [Google Scholar]
- Xiang C, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Hachia A, Hamada H, Matsumoto H. Phenylpropanoids as a protectant of aluminum toxicity in cultured tobacco cells. Plant Cell Physiol. 1998;39:950–957. [Google Scholar]
- Zenk MH. Heavy metal detoxification in higher plants: a review. Gene. 1996;179:21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]