Abstract
We have characterized the mechanism of action of four transgenes (AtBCB [Arabidopsis blue copper-binding protein], parB [tobacco {Nicotiana tabacum} glutathione S-transferase], NtPox [tobacco peroxidase], and NtGDI1 [tobacco GDP dissociation inhibitor]) that independently Al resistance on transgenic Arabidopsis. All four transgenic lines showed lower deposition of callose after Al treatment than the Landsberg erecta ecotype of Arabidopsis, confirming that the four genes function to ameliorate Al toxicity. Influx and efflux experiments of Al ions suggested that the AtBCB gene may suppress Al absorption, whereas expression of the NtGDI1 gene promotes a release of Al in the root tip region of Arabidopsis. The total enzyme activities of glutathione S-transferases or peroxidases in transgenic lines carrying either the parB or NtPox genes were significantly higher than in the Landsberg erecta ecotype of Arabidopsis, and these enzyme activities were maintained at higher levels during Al stress. Furthermore, lipid peroxidation caused by Al stress was repressed in these two transgenic lines, suggesting that overexpression of these two genes diminishes oxidative damage caused by Al stress. Al-treated roots of transgenic plants were also stained by 4′,6-diamino-2-phenylindole to monitor cell death caused by Al toxicity. The result suggested that cell death is repressed in the NtPox line. Analysis of F1 hybrids between the four transgenic lines suggests that more resistant transgenic plants can be constructed by combinations of these four genes.
Al is a major mineral constituent of soil and dissolves into soil solution as various ionic forms under low pH conditions. It is well known that these ions have toxic effects on root growth in plants (Kochian, 1995; Matsumoto, 2000). Many strategies for Al tolerance have been reported. Exudation of Al-chelating organic acids, such as malate, oxalate, or citrate, into the rhizosphere has been proposed as the most effective tolerance mechanism to avoid Al toxicity in many plants (Ryan et al., 1995). Overexpression of a bacterial citrate synthase gene in transgenic plants was reported to confer Al tolerance (de la Fuente et al., 1997), although this result was not able to be reproduced (Delhaize et al., 2001). Degenhardt et al. (1998) reported that the Al resistance in the Arabidopsis mutant (alr-104) is caused by an Al-induced increase in rhizosphere pH. A range of alternative tolerance mechanisms has also been proposed (Kochian, 1995), but it is not yet known how many genes contribute to Al resistance in tolerant plants.
Molecular genetic study has the potential to dissect Al resistance mechanisms. Over 20 genes induced by Al stress have been isolated from a range of plant species, including wheat (Triticum aestivum; Snowden and Gardner, 1993; Snowden et al., 1995; Cruz-Ortega et al., 1997; Hamel et al., 1998; Delhaize et al., 1999), tobacco (Nicotiana tabacum; Ezaki et al., 1995, 1996), and Arabidopsis (Sugimoto and Sakamoto, 1997; Richards et al., 1998). Most of these Al-induced genes are general stress-inducible genes, whose expression is turned on by oxidative stress, pathogen infection, phosphate starvation, heat shock, other metal stresses, and hormone treatments. It is therefore suggested that common gene induction mechanisms exist among these different stresses. However, the biological roles of Al-induced genes in Al stress and the induction mechanisms of these genes by Al stress are still unclear. We recently expressed plant Al-induced genes in both yeast (Saccharomyces cerevisiae) cells and Arabidopsis. The two genes, the Arabidopsis blue copper-binding protein gene (AtBCB) and a tobacco GDP dissociation inhibitor gene (NtGDI1), conferred Al resistance in yeast (Ezaki et al., 1999). These two genes and two others, a tobacco glutathione S-transferase (GST) gene (parB) and a tobacco peroxidase gene (NtPox), also ameliorated Al toxicity in Arabidopsis over a narrow range of Al concentrations (Ezaki et al., 2000). These four genes have different biochemical functions, suggesting that there are several different Al tolerance mechanisms in plants. Characterization of these genes in terms of their Al resistance mechanisms may be able to supply new strategies for Al resistance in plants in addition to the release of organic acids.
In this study, we investigate the resistance mechanisms of transgenic Arabidopsis lines expressing these four Al-induced genes. Furthermore, we show the feasibility of creating more resistant transgenic plants by combining pairs of the genes in F1 plants.
RESULTS
Determination of Callose Content in Al-Treated Roots
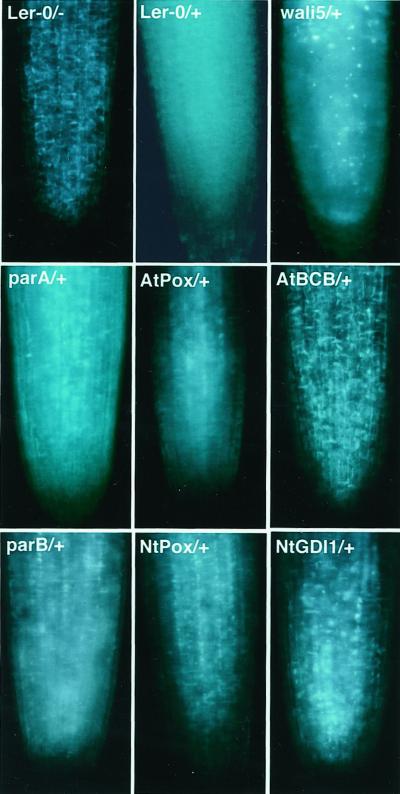
Callose deposition in plasma membranes and plasmodesmata is widely used as an indicator of Al toxicity in plants because callose accumulates in root tips after exposure to toxic levels of Al (Wissemeir et al., 1987; Zhang et al., 1994; Larsen et al., 1996; Sivaguru et al., 2000). Figure 1 shows callose deposition in root tip regions of seven transgenic Arabidopsis lines compared with the control Landsberg erecta (Ler) ecotype of Arabidopsis. Untreated Ler root tips showed a negligible level of fluorescence, whereas strong callose-specific fluorescence signals could be detected in whole root tip region after exposure to 100 μm Al for 6 h. Compared with Al-treated control plants, callose accumulation in the root tip region was slightly lower in the parB or NtGDI1 lines and much lower in the NtPox or AtBCB lines. The transgenic plants carrying the wali5 or parA genes, which are not Al resistant (Ezaki et al., 2000), showed a similar strength of fluorescence signals to the control line. These results are consistent with our previous results showing that Al toxicity is diminished in these four transgenic plants compared with the control. An exception to the pattern was noted for the AtPox transgenic line, which did not show significant Al resistance (Ezaki et al., 2000). It showed a lower callose staining in the root tip region. Therefore, this line may be an exception to previous results that suggested callose content in Al-treated roots is a good indicator for Al toxicity (Larsen et al., 1996).
Figure 1.
Microscopic observation of callose deposition in the Al-treated root tip region of Ler and the transgenic lines. All samples were visualized by staining with aniline blue. −, Without Al for 6 h; +, with 100 μm Al for 6 h.
Al Contents in the Root Tip Region of the AtBCB or NtGDI1 Lines
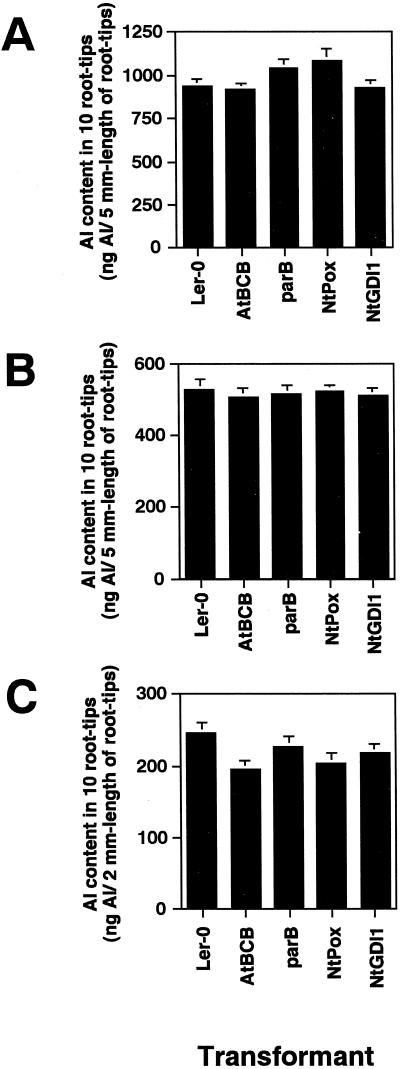
We previously examined Al content of root tip regions of the transgenic plants using morin staining (Ezaki et al., 2000). To monitor the Al content in the root region of the resistant lines more precisely, an atomic absorption spectrophotometer was used in three preliminary experiments: (a) detection of Al content in 5-mm root tip segments after 100 μm Al treatment for 12 h, (b) detection of Al content in 5-mm segments after 50 μm Al treatment, and (c) detection of Al content in 2-mm segments after 50 μm Al treatment. There was no difference in Al content between the transgenic lines and control under conditions a and b (Fig. 2, A and B), whereas small differences could be seen between the tested lines when 2-mm root tip segments were used (Fig. 2C; condition c). These results are consistent with our previous morin staining, which showed a clear difference between the resistant plants and Ler in the root tip region (0–0.5 mm) but minimal difference in the upper region (>1-mm region). Because the difference in Al content between the resistant lines and the control was only seen in the root apex, treatment condition c was used to characterize the functions of the AtBCB and NtPox genes in Al toxicity.
Figure 2.
Al content in the root tip regions of the resistant lines and a control line after Al treatments. A, Al content in a 5-mm-long region of root tip after 100 μm Al treatment for 12 h; B, Al content in a 5-mm-long region of root tip after 50 μm Al treatment for 12 h; C, Al content in a 2-mm-long region of root tip after 50 μm Al treatment for 12 h. Al content in each of the 10 plants is shown. Error bars are calculated from three independent experiments.
Our previous influx and efflux experiments of yeast transformants overexpressing the AtBCB or NtGDI1 genes suggested that the former gene can function in suppression of Al uptake and the latter provides an increase in Al efflux (Ezaki et al., 1999). We therefore measured Al content in the root tips region (0–2-mm length) of transgenic Arabidopsis carrying either of these two genes during the Al uptake period and the recovery period. The AtBCB transgenic plant showed slightly lower Al uptake than Ler, whereas Al influx of the NtGDI1 is similar to that of Ler as seen in Figure 2C (Fig. 3A). The other two resistant lines, expressing the parB and NtPox genes, also showed a similar Al influx to Ler in their uptake periods (data not shown). Al uptake in all tested plants was linear for the first 24 h, suggesting that there was no saturation in our Al influx experiment (data not shown). The decrease rate of Al in the AtBCB line was very similar to that in Ler in the recovery period, whereas the NtGDI1 line clearly showed a faster decrease of Al than the two other lines tested (Fig. 3B). Because the AtBCB line showed a slightly lower Al uptake as described above, the Al content in this line was always lower than that in Ler (approximately 15%–20% lower content in both uptake and recovery period). In contrast, the reduction of Al content in the NtGDI1 line was only seen in the Al release period.
Figure 3.
Al content in the root tip region of the AtBCB and NtGDI1 lines. Seven-day-old seedlings of each line were used for the Al uptake experiment (100 μm Al treatment for 12 h as Al uptake period, A) and recovery experiment (100 μm Al treatment for 12 h and continuous growth for another 12 h without Al condition as Al release period, B). Error bars are calculated from three independent experiments.
Enzyme Activities of GST and Peroxidase Are Increased in Transgenic Lines
Two of the Al-resistant genes, parB and NtPox, encode antioxidation enzymes, a GST and a moderate anionic peroxidase, respectively. To confirm that the transgene-encoded enzymes were being expressed, total activities of peroxidases and GSTs were determined in the soluble fractions of whole roots of parB or NtPox lines (Table I). Compared with control plants (Ler), approximately 1.4 times higher total peroxidase or total GST activities were detected in these lines because of a constitutive overexpressing of either of these genes. Reduction of enzyme activity by Al treatment was seen in all of tested lines, but much higher enzyme activities (approximately 2.0 times and 1.7 times higher activity in parB and in NtPox lines than in Ler, respectively) were retained in these lines than in control after 100 μm Al treatment for 6 h. These results indicate that overexpression of the two genes in transgenic lines contributes to a maintenance of the total enzyme activity under the Al treatment.
Table I.
Total enzyme activity of GST or peroxidase in the parB and NtPox lines
| Enzyme | Tested Line | Total Enzyme Activitya
|
|
|---|---|---|---|
| −AI (6 h) | +100 μm AI (6 h) | ||
| ng μg−1 total protein | |||
| GST | Ler-0 | 0.094 ± 0.008 | 0.052 ± 0.015 |
| parB | 0.129 ± 0.011 | 0.104 ± 0.015 | |
| Peroxidase | Ler-0 | 6.39 ± 0.68 | 3.42 ± 0.25 |
| NtPox | 8.72 ± 0.80 | 5.81 ± 0.42 | |
Each total enzyme activity was determined using three independent samples and shown as mean ± se.
Content of Lipid Peroxides in Root Region of the Transgenic Lines
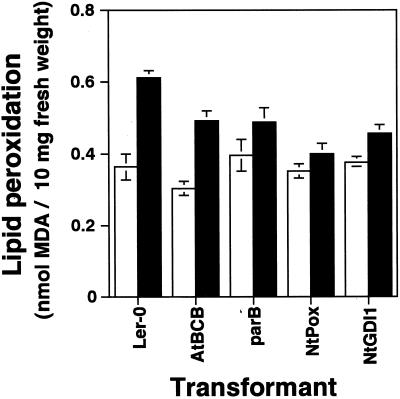
Staining of Al-treated roots with 2′,7′-dichloro fluorescein diacetate (H2DCFDA) had suggested that Al treatment causes oxidative damage and that there is a reduction of oxidative stress in the root tips of the four Al-resistant transgenic plants, especially in the NtPox lines (Ezaki et al., 2000). To confirm these results, peroxidation of phospholipids in the whole root region of the four transgenic plants and Ler was determined by measuring malon dialdehyde (MDA), which is the final product of lipid peroxidation (Fig. 4). There was no significant difference in the basal levels of MDA produced by untreated roots of the five tested lines. An increase of MDA could be seen in all tested lines after an exposure to 100 μm Al treatment for 6 h. However, the degree of induction varied between lines: 1.7 times in Ler, 1.6 times in the AtBCB line, 1.2 times in the parB and the NtGDI1 lines, and 1.1 times in the NtPox line. The two lines expressing parB and NtPox showed much lower inductions than Ler, consistent with their overexpression of antioxidation enzymes.
Figure 4.
Determination of lipid peroxidation caused by Al treatment in whole root region of Ler, AtBCB, parB, NtPox, or NtGDI1 lines. The content of lipid peroxides was estimated as MDA. Black and white bars represent with 100 μm Al treatment or without Al treatment for 6 h, respectively.
The NtPox Transgenic Line Can Avoid Cell Death at High Levels of Al Toxicity
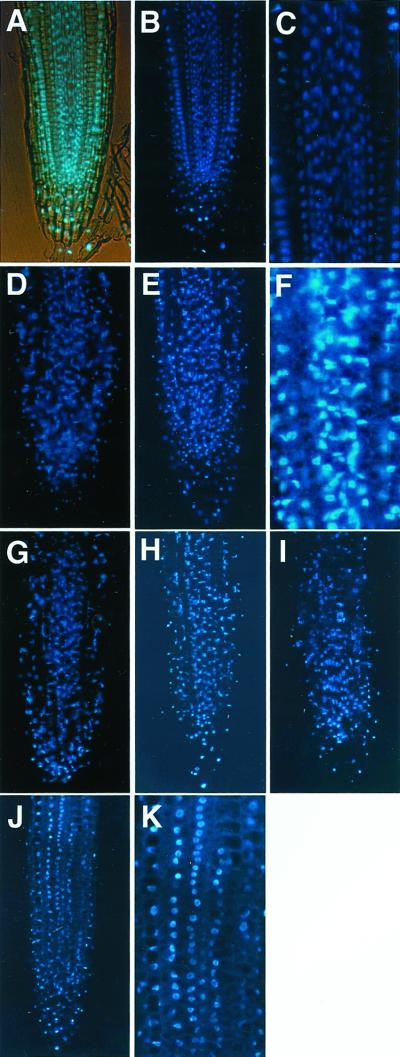
It is known that salt stress (Katsuhara, 1997) and Al stress (Yamaguchi et al., 1999) cause a degradation of DNA molecules and an apoptosis-like cell death. To investigate whether Al treatment can cause damage in nuclei leading to cell death in our four Al-resistant lines, these four lines and Ler were exposed to Al stress and stained with 4′,6-diamino-2-phenylindole (DAPI). Without Al treatment, most of the nuclei showed normal alignment and were round in shape with smooth boundaries in roots (Fig. 5, A–C for control line; transgenic lines showed the same patterns, data not shown). There was no change under 25 μm Al treatment, but deformed nuclei could be observed after 50 μm Al treatment for 24 h, and some degradation of nucleus was clearly detected by 100 μm Al treatment for 24 h (Fig. 5, D and E). A number of small DAPI-specific fluorescence particles also could be seen in 100 μm Al-treated roots (Fig. 5F), suggesting that some decomposition of nuclei had occurred. Al concentrations higher than 100 μm (150 and 200 μm) were applied to the control roots, but there was no clear difference in DNA damage between the three concentrations (data not shown).
Figure 5.
Microscopic observation of cell death caused by Al stress. A through F, Ler; G, AtBCB line; H, parB line; I, NtGDI1 line; J and K, NtPox line. Ler roots were treated for 24 h with various concentration of Al: 0 μm (A–C), 50 μm (D), and 100 μm (E and F). Transgenic lines were also treated with 100 μm Al for 24 h (G–K). A, Simultaneous light and fluorescent image; C, F, and K, magnified pictures of Al-treated root of Ler and the NtPox line.
The four transgenic lines were also exposed to 50 and 100 μm Al for 24 h. At 50 μm Al, there was no difference between the lines or compared with untreated control Ler plants (data not shown). At 100 μm Al, lines expressing the AtBCB, parB, and NtGDI1 genes showed nuclei damage (Fig. 5, G–I). In contrast, whereas some of the roots showed levels of damage similar to that of controls, approximately 60% to 70% of the roots derived from the NtPox line showed a lower damage after treatment with 100 μm Al (Fig. 5J). Compared with 100 μm Al-treated root tips of Ler, their nuclei were kept round in shape, and a much lower number of DAPI-specific particles could be observed (Fig. 5K).
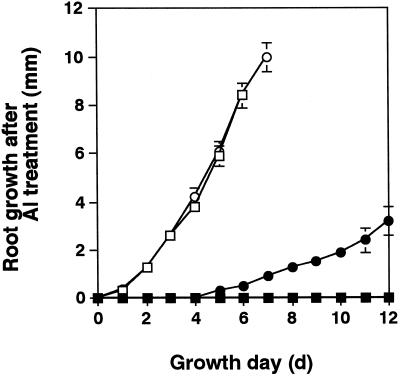
To clarify whether the Al-treated roots still had the capability to elongate their roots, the four transgenic lines and Ler were treated with 100 μm Al for 24 h, then cultured for a further 7 to 12 d without Al, and their root growth was determined. The AtBCB, parB, and NtGDI1 lines had their root elongation irreversibly inhibited by the 100 μm Al treatment, as did Ler (data not shown). In contrast, the NtPox line showed a small recovery of root elongation after a 4- to 5-d time lag (Fig. 6).
Figure 6.
Recovery of root growth after Al treatment. Young (4-d-old) seedlings of the NtPox line (circles) and the Ler control line (squares) were treated with 100 μm Al (black symbols) or without Al (white symbols) for 24 h and then cultured under an Al-free condition. Root elongation was determined every 24 h and is shown here as the sum of means of root growth per 24 h (n = 10). Error bars are also calculated from 10 tested plants.
Together, these results suggest that the NtPox line may differ from the other transgenic lines in its capacity to avoid some of the DNA damage caused by Al toxicity.
Construction of New Al-Resistant Transgenic Plants
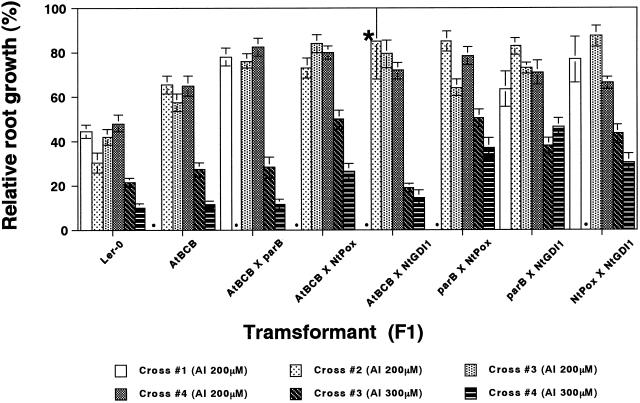
The levels of Al resistance conferred by these four transgenic plants were small, and differences in resistance were only observed over a narrow range of concentrations (Ezaki et al., 2000). If each gene operates by a different mechanism for Al resistance, it is expected that F1 plants carrying pairs of genes should show a higher Al-resistant phenotype than parental plants carrying a single resistant gene. To investigate this possibility, all six pairs of hybrids between the resistant lines were constructed: AtBCB × parB, AtBCB × NtPox, AtBCB × NtGDI1, parB × NtPox, parB × NtGDI1, and NtPox × NtGDI1. The hybrid crosses were independently performed three to four times, and the constructed F1 lines were tested for their Al sensitivity using Ler and the AtBCB line as controls.
Figure 7 shows the results of root elongation tests in the presence of Al. The results show that most of the F1 plants are more resistant to 200 μm Al than the AtBCB line. In particular, all three F1 lines containing the AtBCB gene showed a higher resistance to 200 μm Al than the parental AtBCB line. At 300 μm Al, the three F1 lines containing the NtPox transgene (AtBCB × NtPox, parB × NtPox, and NtPox × NtGDI) and the parB × NtGDI1 line all showed clearly higher tolerance than the single transgene lines. Comparison of these results with those obtained previously for the single lines with NtPox, NtGDI1, and parB transgenes (Ezaki et al., 2000) confirms that there is an increased level of tolerance for each of the corresponding double transgenic lines for these transgenes.
Figure 7.
Sensitivity tests of the constructed transgenic F1 lines for Al stress. Plants were tested for their sensitivity to 0, 200, or 300 μm Al toxicity according to a vertical mesh transfer technique described previously (Ezaki et al., 2000). Error bars represent se values (n = 20 for all except for one cross of AtBCB × NtGDI1 shown by the asterisk, n = 8). Three or four independent crossbreeds were performed for each cross, and F1 seeds were used for Al sensitivity tests (crosses #1 and #2 were tested at 200 μm, and crosses #3 and #4 were tested at both 200 and 300 μm). We show here all results of Al sensitivity tests. Dots show untested crosses. The lines carrying the parB, NtPox, or NtGDI1 genes were not tested in this experiment as control lines carrying a single resistant gene because our previous sensitivity tests have already shown them to have similar Al sensitivity to the line expressing the AtBCB gene (Ezaki et al., 2000).
DISCUSSION
Because different assays measure different parameters of Al toxicity, multiple estimations by different methods are necessary to judge Al sensitivity. In this study, we investigated the Al-resistant mechanisms of four transgenic lines carrying the AtBCB, parB, NtPox, or NtGDI1 genes, and differences in the mechanism of tolerance conferred by each of the genes could be revealed using a series of different assays. From this point of view, we suggest that the NtPox is the most attractive gene to confer Al resistance to plants because it rated higher than the control Ler line in most of the assays used to date. The NtPox line showed higher relative root growth in Al (Ezaki et al., 2000), lower morin staining (Ezaki et al., 2000), lower H2DCFDA staining (Ezaki et al., 2000), lower deposition of callose in the root tip region, lower deposition of lipid peroxides, lower disintegration of nuclei, and better recovery after Al toxicity.
We determined the Al content in the root tip regions (2- or 5-mm region) of the Al-resistant lines and the control line in Al influx experiments and found that the differences of Al content between them were small. Although our morin staining experiment of the Al-treated roots showed a much clearer difference between the two groups, suggesting that the four transgenic plants accumulate lower Al than Ler in the root tip region, this difference was seen most clearly within the 0.5-mm region of the tips, including the cell division and cell elongation zones (Ezaki et al., 2000). One simple explanation of the difference between the results is that Al ions are absorbed into the whole root region in Arabidopsis, but the biological effects of Al ions in each part of the root may be different. We have determined the Al content in the whole root region by using an atomic absorption spectrophotometer and found that the Al content in whole roots is almost the same among the sensitive and resistant Arabidopsis plants (data not shown). We speculate that the content of Al ions in the root tip region (0–0.5-mm region) is the most important and critical factor for Al sensitivity in Arabidopsis. Diminishing Al content in this region, rather than in whole root, is much more important for Al resistance. Similar results have been found in wheat.
The difference in Al content during the uptake and recovery periods among the tested lines (the AtBCB, NtGDI1, and Ler) was small but reproducible. We have not yet clarified the precise function of these genes in Al stress, but these results suggest that overexpression of AtBCB may suppress Al uptake and that overexpression of NtGDI1 can promote a decrease of Al content in the root tip region. Because similar increases in influx or efflux could be observed in the yeast transformant carrying either of these two genes (Ezaki et al., 1999), we propose that each of these genes has a similar function in control of Al content in both plant and yeast.
There is more than 80% similarity in the total amino acid sequence between the AtGDI (Arabidopsis GDI gene) and NtGDI1 genes, and the AtGDI gene can complement the sec19 mutation of yeast, which is related to the vesicle transport system (Ueda et al., 1996). Therefore, there is a possibility that overexpression of our NtGDI1 gene is also directly or indirectly related to stimulation of a vesicle transport system in plant cells. One simple possibility is that NtGDI1 protein functions for a transportation or localization of cell membrane protein(s) that are involved in an efflux system for Al ions. Complementation of the sec19 mutation of yeast by an overexpression of the NtGDI1 gene may be one of the ways to confirm this possibility. Otherwise, we should measure Al content of the medium over the same time frame. Another possibility is that Figure 3B may not represent Al efflux, but actually dilution of Al concentration attributable to differences in growth of root tip region (especially in the 0–0.5-mm region) under Al conditions. In this case, overexpression of the NtGDI1 gene in Arabidopsis and/or yeast may cause for a decrease of Al content by a promotion of cell division and/or cell elongation.
An explanation of the Al resistance mechanism in AtBCB lines is more difficult. Van Gysel et al. (1993) reported that the AtBCB gene encodes a negatively light-regulated, cupredoxin-like protein and proposed that it is involved in electron transfer reactions in the cell membrane region. The AtBCB gene shows 86% amino acid identity with horseradish (Armoracia rusticana) umecyanin, whose function has not yet been clarified. However, because horseradish umecyanin was isolated from roots bound to peroxidase, Van Driesche et al. (1995) suggested the possibility that the horseradish umecyanin and peroxidase function together in the root cell membrane region. The very high similarity in amino acid sequence between AtBCB and umecyanin suggests a possibility that the AtBCB protein also has a similar function in Arabidopsis roots. Drew and Gatehouse (1994) further reported that the pea (Pisum sativum) blue copper-binding protein is correlated with lignin deposition in pod endocarp. The last enzymatic step in lignin biosynthesis involves oxidative polymerization of free radicals catalyzed by cell wall peroxidase in plants. Therefore, another possibility is that the AtBCB protein and umecyanin may be related to lignin metabolism in the cell membrane region. We speculate that the AtBCB gene can function in the protection of cell walls or cell membranes from Al toxicity via electron transfer reactions and that Al uptake can be restricted in the AtBCB line. To confirm this hypothesis, we determined the content of phenolic compounds and lignin in both of the Al-treated and untreated root tips of the AtBCB line and Ler (0–5-mm region). Their contents were slightly decreased by Al treatment, but there was no significant difference under the Al-treated and untreated conditions between the two lines (data not shown). These results indicated that it is unlikely that the AtBCB protein effects quantitative change in lignin metabolism, but it still cannot be excluded that the AtBCB protein causes a qualitative change. Further work is necessary to characterize the function of the AtBCB gene in Al stress.
Compared with Ler, the transgenic plants overexpressing the NtPox or parB genes showed higher activity of each enzyme without Al, and they can maintain a higher level of enzyme activity during Al treatment in their root regions. We also found that the transgenic lines carrying these genes accumulate lower levels of lipid peroxides in root region than Ler by Al stress. We propose that the high enzyme activities in transgenic plants probably diminish lipid peroxidation caused by Al stress. These results are consistent with our previous results of H2DCFDA staining after Al treatment indicating that oxidative damage caused by Al treatment was diminished especially in the NtPox line (Ezaki et al., 2000). We therefore propose that they act to restrict lipid peroxidation in cell membrane regions and help to maintain membrane function. We also suppose that cell membranes of these lines, especially the NtPox line, probably can restrict the influx of Al ions into the cytosol to a low level and maintain (or recover) root cell viability and/or root growth. We have not yet characterized precisely whether the oxidative damage is a cause or a result of Al stress, but these results suggest that overexpression of antioxidation enzymes is an effective way to prevent Al toxicity in Arabidopsis. In our determination of lipid peroxides in root regions, the NtGDI1 line also showed a low increase of MDA by Al treatment. Because it is unlikely that an overexpression of the NtGDI1 genes can directly repress lipid peroxidation in the cell membrane region, we suppose that the lower induction was derived from a secondary effect of the overexpression.
It has been reported that fragmentation of DNA occurs after exposure to Al, and a programmed cell death has been proposed in Al toxicity (Yamaguchi et al., 1999). In our study, we observed a conformational change of nuclei and small DAPI-specific particles in the Al-treated roots of the control line, consistent with the idea that apoptosis-like cell death via DNA fragmentation is occurring during Al stress. Similar damage can be induced in barley (Hordeum vulgare) roots by sodium stress (Katsuhara and Kawasaki, 1996; Katsuhara, 1997). Compared with Ler, there was a lower damage in nuclei of the NtPox line under 100 μm Al treatment. Furthermore, our root elongation assay after 100 μm Al treatment indicated that the NtPox line can gradually recover its root cell viability after a 4- to 5-d time lag. These results suggested that the Al-resistant phenotype of the NtPox line may be essentially different from those of the other three lines and that the threshold for Al toxicity is higher in the NtPox line than in the other three. The reduced disintegration of nuclei and the ability of this line to survive Al treatment suggest that apoptosis-like cell death may specifically be reduced in this transgenic line as its unique resistant mechanism.
In this study, we constructed transgenic plants with pairs of Al resistance genes, which had higher resistance than the individual genes alone. These results suggested that the genes have additive effects on Al tolerance. Secretion of organic acids is a useful resistant mechanism for several important crops, such as wheat, maize (Zea mays), snapbean (Phaseolus vulgaris), taro (Colocasia esculenta), and so on. However, it is clear that alternative mechanisms for Al tolerance are possible (Wenzl et al., 2001). We believe that characterization of Al-induced genes and how they affect the level of tolerance to Al toxicity will be a profitable source of new mechanisms for Al resistance in plants.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All transgenic Arabidopsis lines [AtBCB(5-1), parB(3-1), NtPox(6-2), NtGDI1(5-11), wali5(8-11), AtPox(4-1), and parA(10-1)] and the control line (Ler) used in this study were described previously (Ezaki et al., 2000). Plants were grown under fluorescent illumination (approximately 50 μE m−2 s−1, 16 h of light, and 8 h of darkness) at 22°C. A modified Murashige and Skoog medium (Murashige and Skoog, 1962), one-sixth Murashige and Skoog, in which Suc was 10 g L−1 but Murashige and Skoog salts and B5 vitamins were 6 times diluted, was used for plant growth and for Al treatment. The pH of the medium was adjusted to 4.0 for all Al treatments.
Al Treatments
All Al treatments in this study except for the Al sensitivity test (Fig. 7) were performed under sterile hydroponic conditions using plant growth racks. Sterilized seeds were incubated at 4°C for 4 d and then plated on a nylon mesh square cup (mesh size, 300 μm; cup size, 4-cm width × 4-cm length × 1-cm height). This square cup was kept floating with a sponge supporter on 130 mL of one-sixth Murashige and Skoog medium without Al in a plant growth rack to support plant growth. Young seedlings grown for 6 to 7 d (with 15–25-mm-length roots) were used for the various Al treatments. After Al treatments, plants were washed several times with an excess of distilled water, and then root tips or whole roots were used for analyses.
For the Al uptake experiment and recovery experiment, young (7-d-old) seedlings were treated with 100 μm Al for 12 h as an uptake period (uptake experiment), washed well with an excess of distilled water, and then transferred to fresh one-sixth Murashige and Skoog medium without Al for 12 h (recovery experiment). Sampling of 20 root tips (2-mm length) was performed every 3 h in each experiment, and the collected roots were stored at −80°C until analyzed.
Microscopic Observations
Callose production in Al-treated roots was visualized by staining with aniline blue as described by Larsen et al. (1996). The root region of 7-d-old seedlings was exposed to one-sixth Murashige and Skoog medium containing 100 μm Al for 5 h, fixed with formaldehyde under vacuum condition, and then stained with 0.1% (w/v) aniline blue. A fluorescent microscope, MPM800 (Carl Zeiss, Oberkochen, Germany), was used for observation.
Detection of Al Content in Root Region
Root tips of Al-treated roots (2- or 5-mm length) were excised and disrupted by treatment with acid (HNO3/H2SO4, 1:1, v/v) at 100°C for 5 h. Al content was determined by a polarized Zeeman atomic absorption spectrophotometer (Z-8270; Hitachi, Tokyo).
Total Peroxidase and GST Activities
Whole roots of Al-treated plants (100 μm Al treatment for 6 h) were frozen in liquid nitrogen, ground with pestle and mortar, and then suspended in 10 mm phosphate-buffered saline (pH 6.0). The ratio of buffer volume to sample volume was almost 1:1 (v/v). The homogenate was centrifuged at 15,000g at 4°C for 20 min, and the supernatant was used for enzyme analyses as a soluble fraction. Total protein content of each sample was determined by the Bradford reagent assay (Bradford, 1976). Enzyme activities of total peroxidase and GST were determined by the methods described by Wakiuchi et al. (1971) and Edwards and Owen (1986), respectively.
Detection of Lipid Peroxidation
Lipid peroxides were determined as MDA by the thiobarbituric method described previously (Ono et al., 1995).
Determination of DNA Degradation
Cell death caused by Al treatments was monitored by DAPI staining (Katsuhara, 1997). After replacement of water with ethanol, fixed roots were embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany), cut to 15-μm-thick sections, and stained with DAPI. Conformational changes of the nuclei were observed under UV fluorescence.
Root Elongation Assay
To investigate recovery from Al toxicity (Fig. 6), Al-treated plants were tested in a root elongation assay. Young plants were grown in one-sixth Murashige and Skoog medium for 4 d in a nylon square mesh cup. The medium was changed to a fresh one-sixth Murashige and Skoog medium containing 0 μm (control) or 100 μm Al (Al treatment), and the seedlings were treated for 24 h. After the treatments, 10 plants were transferred to a 3-mm-thick plastic plate with a square of nylon mesh (30-μm mesh) and three squares of 1-mm-thick chromatography paper (3MM CHR, Whatman, Maidstone, UK) on it in this order. The nylon mesh and 3MM papers were previously saturated well with a fresh one-sixth Murashige and Skoog medium without Al. The transferred plants were cultured for another 7 or 12 d in a growth box. The position of each root apex was marked on the nylon mesh by a pencil every 24 h and elongation of root length of each plant was directly measured by a ruler, and a mean per 1 d for 10 plants was calculated. The root elongation shown in Figure 6 is a sum of the means of root growth per 1 d for 10 plants.
In the case of the new constructed transgenic plants (Fig. 7), Al sensitivity tests were performed according to the vertical mesh transfer technique, which we used previously (Ezaki et al., 2000). Root growth (the length between the root apex and bending point) of 20 plants was measured for each treatment group during the 2-d Al treatment.
ACKNOWLEDGMENTS
We thank Dr. Richard C. Gardner for his comments concerning our manuscript and Ms. Yuka Ezaki for her technical supports in the experiments.
Footnotes
This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences (to H.M.) and by the Ministry of Education, Culture, Sports, Science and Technology, the Ohara Foundation for Agricultural Sciences [Grant-in-Aid for Scientific Research (A)(2) no. 11306006 to H.M. and Grant-in-Aid for Scientific Research (C)(2) no. 13660066 to B.E.].
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010399.
LITERATURE CITED
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cruz-Ortega R, Cushman JC, Ownby JD. cDNA clones encoding 1,3-β-glucanase and a fimbrin-like cytoskeletal protein are induced by Al toxicity in wheat roots. Plant Physiol. 1997;114:1453–1460. doi: 10.1104/pp.114.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV. Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 1998;117:19–27. doi: 10.1104/pp.117.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente JM, Ramirez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Hebb DM, Richards KD, Lin JM, Ryan PR, Gardner RC. Cloning and expression of a wheat (Triticum aestivum L.) phosphatidylserine synthase cDNA. Overexpression in plants alters the composition of phospholipids. J Biol Chem. 1999;274:7082–7088. doi: 10.1074/jbc.274.11.7082. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Hebb DM, Ryan PR. Expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiol. 2001;125:2059–2067. doi: 10.1104/pp.125.4.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew JE, Gatehouse JA. Isolation and characterization of a pea pod cDNA encoding a putative blue copper protein correlated with lignin deposition. J Exp Bot. 1994;45:1873–1884. [Google Scholar]
- Edwards R, Owen WJ. Comparison of glutathione S-transferases of Zea mays responsible for herbicide detoxification in plants and suspension-cultured cells. Planta. 1986;169:208–215. doi: 10.1007/BF00392316. [DOI] [PubMed] [Google Scholar]
- Ezaki B, Gardner RC, Ezaki Y, Matsumoto H. Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 2000;122:657–655. doi: 10.1104/pp.122.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki B, Sivaguru M, Ezaki Y, Matsumoto H, Gardner RC. Acquisition of aluminum tolerance in Saccharomyces cerevisiae by expression of the BCB or NtGDI1 gene derived from plants. FEMS Microbiol Lett. 1999;171:81–87. doi: 10.1111/j.1574-6968.1999.tb13415.x. [DOI] [PubMed] [Google Scholar]
- Ezaki B, Tsugita S, Matsumoto H. Expression of a moderately anionic peroxidase is induced by aluminum treatment in tobacco cells: possible involvement of peroxidase isozymes in aluminum ion stress. Physiol Plant. 1996;96:21–28. [Google Scholar]
- Ezaki B, Yamamoto Y, Matsumoto H. Cloning and sequencing of the cDNAs induced by aluminum treatment and Pi starvation in tobacco cultured cells. Physiol Plant. 1995;93:11–18. [Google Scholar]
- Hamel F, Breton C, Houde M. Isolation and characterization of wheat aluminum-regulated genes: possible involvement of aluminum as a pathogenesis response elicitor. Planta. 1998;205:531–538. doi: 10.1007/s004250050352. [DOI] [PubMed] [Google Scholar]
- Katsuhara M. Apoptosis-like cell death in barley roots under salt stress. Plant Cell Physiol. 1997;38:1091–1093. [Google Scholar]
- Katsuhara M, Kawasaki T. Salt stress induced nuclear and DNA degradation in meristematic cells of barley roots. Plant Cell Physiol. 1996;37:169–173. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Larsen PB, Tail CY, Kochan LV, Howell SH. Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol. 1996;110:743–751. doi: 10.1104/pp.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Ono K, Yamamoto Y, Hachiya A, Matsumoto H. Synergistic inhibition of growth by Al and iron of tobacco (Nicotiana tabacum L.) cells in suspension culture. Plant Cell Physiol. 1995;36:115–125. [Google Scholar]
- Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC. Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol. 1998;116:409–418. doi: 10.1104/pp.116.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated efflux of malate from apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- Sivaguru M, Fujiwara T, Sanaj J, Baluska F, Yang Z, Osawa H, Maeda T, Mori T, Volkmann D, Matsumoto H. Aluminum-induced 1,3-β-d-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata: a new mechanism of aluminum toxicity in plants. Plant Physiol. 2000;124:991–1005. doi: 10.1104/pp.124.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden KC, Gardner RC. Five genes induced by aluminum in wheat (Triticum aestivum L.) roots. Plant Physiol. 1993;103:855–861. doi: 10.1104/pp.103.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden KC, Richards KD, Gardner RC. Aluminum-induced genes: induction by toxic metals, low calcium, and wounding and pattern of expression in root tips. Plant Physiol. 1995;107:341–348. doi: 10.1104/pp.107.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Sakamoto W. Putative phospholipid hydroperoxide glutathione peroxidase gene from Arabidopsis thaliana induced by oxidative stress. Genes Genet Syst. 1997;72:311–316. doi: 10.1266/ggs.72.311. [DOI] [PubMed] [Google Scholar]
- Ueda T, Matsuda N, Anai T, Tsukaya H, Uchimiya H, Nakano A. An Arabidopsis gene isolated by a novel method for detecting genetic interaction in yeast encodes the GDP dissociation inhibitor of Ara4 GTPase. Plant Cell. 1996;8:2079–2091. doi: 10.1105/tpc.8.11.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driesche G, Dennison C, Sykes AG, Van Beeumen JV. Heterogeneity of the covalent structure of the blue copper protein umecianin from horseradish roots. Protein Sci. 1995;4:209–227. doi: 10.1002/pro.5560040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gysel A, Montagu MV, Inze D. A negatively light-regulated gene from Arabidopsis thaliana encodes a protein showing high similarity to blue copper-binding proteins. Gene. 1993;136:79–85. doi: 10.1016/0378-1119(93)90450-h. [DOI] [PubMed] [Google Scholar]
- Wakiuchi N, Matsumoto H, Takahashi E. Changes of some enzyme activity of cucumber during ammonium toxicity. Physiol Plant. 1971;24:248–253. [Google Scholar]
- Wenzl P, Patino GM, Chaves AL, Mayer JE, Rao IM. The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol. 2001;125:1473–1484. doi: 10.1104/pp.125.3.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissemeir AH, Klotz F, Horst WJ. Aluminum induced callose synthesis in roots of soybean (Glycine max L.) J Plant Physiol. 1987;129:487–492. [Google Scholar]
- Yamaguchi Y, Yamamoto Y, Matsumoto H. Cell death process initiated by a combination of aluminum and iron in suspension-cultured tobacco cells (Nicotiana tabacum): apoptosis-like cell death mediated by calcium and proteinase. Soil Sci Plant Nutr. 1999;45:647–657. [Google Scholar]
- Zhang G, Hoddinott J, Taylor GJ. Characterization of 1,3-β-d-glucan (callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. Plant Physiol. 1994;144:229–234. [Google Scholar]