Abstract
A reverse genetic strategy was used to isolate Arabidopsis plants containing “knockout” mutations in AKT1 and AKT2, two members of a K+ channel gene family. Comparative studies of growth and membrane properties in wild-type and mutant seedlings were performed to investigate the physiological functions of these two related channels. The growth rates of plants supplied with rate-limiting concentrations of K+ depended on the presence of AKT1 but not AKT2 channels. This result indicates that AKT1 but not AKT2 mediates growth-sustaining uptake of K+ into roots, consistent with the expression patterns of these two genes. K+-induced membrane depolarizations were measured with microelectrodes to assess the contribution each channel makes to the K+ permeability of the plasma membrane in three different organs. In apical root cells, AKT1 but not AKT2 contributed to the K+ permeability of the plasma membrane. In cotyledons, AKT1 was also the principal contributor to the K+ permeability. However, in the mesophyll cells of leaves, AKT2 accounted for approximately 50% of the K+ permeability, whereas AKT1 unexpectedly accounted for the remainder. The approximately equal contributions of AKT1 and AKT2 in leaves detected by the in vivo functional assay employed here are not in agreement with previous RNA blots and promoter activity studies, which showed AKT2 expression to be much higher than AKT1 expression in leaves. This work demonstrates that comparative functional studies of specific mutants can quantify the relative contributions of particular members of a gene family, and that expression studies alone may not reliably map out distribution of gene functions.
The most abundant inorganic solute in plant cells is K+. The transport in and out of cells of this essential element is a highly regulated process mediated by specific transporters within the plasma membrane (Maathuis et al., 1997; Chrispeels et al., 1999). Present at concentrations on the order of 100 mm, K+ serves as an osmoticum important to turgor pressure, and may act as an essential cofactor for certain enzymes. Its abundance contributes to the electrolyte character of cytoplasm and affects electrostatic interactions between charged entities such as proteins and other biopolymers. The transport of K+ helps set the electric potential difference across the plasma membrane, which powers the transport of other substances. Because K+ serves such fundamental functions throughout the plant, understanding the molecular mechanisms of its uptake and redistribution is an important goal. Progress in this regard may also spawn novel strategies for improving plant mineral nutrition and fertilizer application in the field.
The first isolation of Arabidopsis genes encoding plasma membrane K+ channels by complementation of yeast K+ uptake mutants marked a major step toward this goal (Anderson et al., 1992; Sentenac et al., 1992). The transport properties displayed by AKT1 and KAT1 channels expressed in heterologous systems (Schachtman et al., 1992; Bertl et al., 1995, 1997; Gaymard et al., 1998a) indicated that they probably function in plants as K+ uptake pathways in the tissues that express them; primarily guard cells in the case of KAT1 and root cells for AKT1 (Nakamura et al., 1995; Lagarde et al., 1996). Comparisons of genome sequences revealed that AKT1 and KAT1 are members of a family of plant channels similar in structure and sequence to the shaker superfamily of animal voltage-dependent K+ channels (Anderson et al., 1992; Sentenac et al., 1992).
The field now faces the challenge of ascribing physiological functions to the various other family members. One standard approach to this problem has been to determine the tissue expression pattern for particular family members, at the protein level with isoform-specific antibodies, or at the mRNA level with gene-specific hybridization probes or promoter-reporter gene constructs. Hypotheses about function then are based on the observed expression patterns. For example, KAT1 is primarily expressed in guard cells so it is logical to propose that it encodes the well-studied inward-rectifying K+ channels that mediate stomatal opening. Although this strategy has proven useful, it does not directly indicate the distribution of activity for each family member's gene product.
A more direct means of determining the function of specific gene family members is to isolate null mutants for each of the genes and then to assess the phenotypes of these homozygous “knockout” plants by performing in planta assays of the encoded protein's catalytic function. This “reverse genetic” strategy relies on a PCR-based method of screening DNA pools from large numbers of T-DNA-mutagenized plants to isolate individuals containing a mutation in the gene of interest (Krysan et al., 1999). A plant with an insertion in the AKT1 gene was isolated previously and used to study the function of the channel it encodes (Hirsch et al., 1998). Consistent with the AKT1 expression pattern, electrophysiological experiments revealed that root cells of the knockout mutant, akt1-1, lacked inward-rectifying K+ channel activity, displayed significantly reduced plasma membrane K+ permeability, and grew more slowly than wild type on media containing rate-limiting concentrations of K+. These results indicated that AKT1 mediates K+ uptake into roots in parallel with one or more as-yet-unidentified, NH4+-sensitive transporters (Hirsch et al., 1998; Spalding et al., 1999).
Among the several shaker-like channel genes identified in plants is AKT2 (Cao et al., 1995; Ketchum and Slayman, 1996), a K+ channel related to AKT1 but whose mRNA is predominantly located in the phloem of stems and leaves (Marten et al., 1999; Lacombe et al., 2000). Here, we report the isolation of an akt2 mutant by reverse genetics and the generation of an akt1Δ2Δ double mutant. Using this set of channel mutants and a microelectrode-based technique for assessing K+ permeability of the plasma membrane, we were able to quantitatively map the distribution of AKT1 and AKT2 activities in roots, cotyledons, and leaves of Arabidopsis seedlings. The degree to which growth rate depends on each of these channels was also assessed. Our results demonstrate that insight into the “division of labor” between members of a gene family can be obtained by combining reverse genetics and assays of in vivo function.
RESULTS
Mutant Isolation and Identification
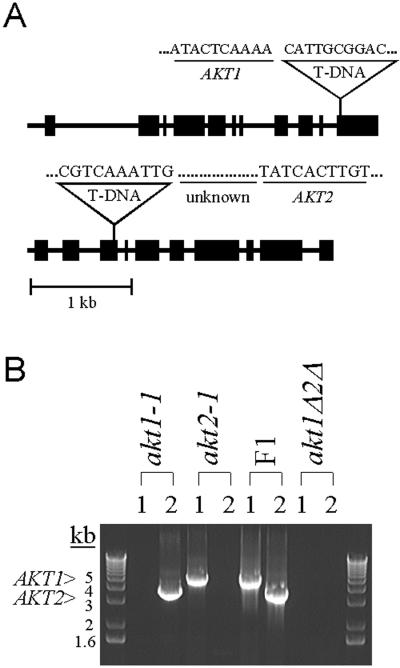
To study the function of the AKT1 and AKT2 genes, plants containing T-DNA insertion alleles of each were isolated. Sequence analysis revealed that in both cases the T-DNA disrupted the gene within the coding region, as shown in Figure 1A. The akt1-1 allele contains a T-DNA insert in the 3′ end of the gene, in a region predicted to encode a regulatory domain in the C terminus. Plants homozygous for this mutation appear to lack AKT1 activity completely (Hirsch et al., 1998). The akt2-1 allele contains a T-DNA insert in the third exon of the coding sequence, a region predicted to encode the third transmembrane domain of the protein (Fig. 1A). Because the T-DNA disrupted the gene before the pore-forming region would be transcribed, the akt2-1 allele is not expected to make functional protein. A double mutant homozygous for both the akt1-1 and akt2-1 mutations was constructed as described in “Materials and Methods.” Figure 1B shows that the akt1-1 and akt2-1 parental lines did not contain genomic DNA capable of producing AKT1- and AKT2-specific PCR products, respectively. Plants of the F1 generation were heterozygous at each locus, as expected (Fig. 1B). An akt1Δ2Δ double-mutant line was identified in an F2 population as an individual lacking wild-type copies of AKT1 and AKT2 (Fig. 1B).
Figure 1.
T-DNA insertion mutations in the AKT1 and AKT2 genes, and the construction of a double mutant. A, Schematic diagrams of the akt1-1 and akt2-1 alleles indicating locations of the T-DNA insertions and sequences of the gene/T-DNA junctions. Forty-nine bases of unknown origin separate the T-DNA border and AKT2 sequence. Large and small boxes represent exons and introns, respectively. T-DNA is not drawn to scale. B, Identification of single and double mutants in a segregating population by analysis of PCR products. Ethidium bromide-stained gel showing the presence or absence of AKT1 and AKT2 PCR products in akt1-1 and akt2-1 parents, the F1 heterozygote, and F2 akt1Δ2Δ double mutant. Lanes marked 1 should not contain a product if the akt1-1 T-DNA insert was present. Lanes marked 2 should not contain a product if the akt2-1 T-DNA insert was present. The double mutant lacked both wild-type genes.
Contributions of AKT1 and AKT2 to Growth Rate
When grown on soil replete with nutrients, neither of the single mutants nor the double mutant displayed an overt phenotype. Previous work had demonstrated that under specific conditions, growth of akt1-1 seedlings was strongly impaired relative to wild type (Hirsch et al., 1998; Spalding et al., 1999). Experiments were performed to determine if a similar phenotype would become apparent in akt2-1 seedlings when they were grown in conditions that made K+ uptake the rate-limiting step in the growth process, i.e. at concentrations less than 1,000 μm and in the presence of NH4+. Examinations of akt1Δ2Δ plants under these same conditions would test for genetic interactions between the two channel genes. For example, evidence of one channel compensating for the lack of another could be obtained if the double mutant was affected in ways not explicable by the sum of the single-mutant phenotypes.
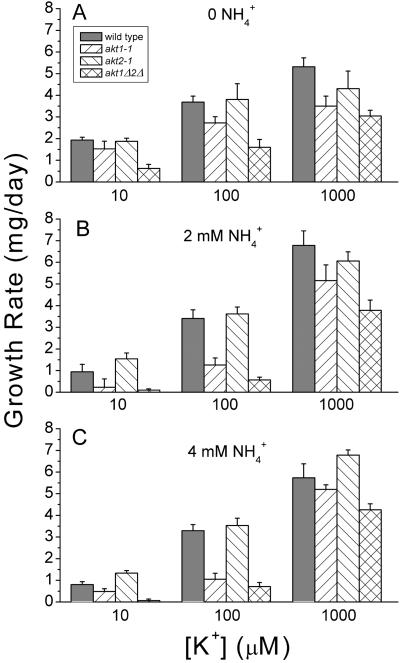
Figure 2A shows that in the absence of NH4+ the growth rate of wild-type seedlings increased as [K+]ext increased between the concentrations of 10 and 1,000 μm. This established the important point that under these conditions, the growth rate of seedlings was limited by the availability of K+. Loss of either AKT1 or AKT2 had little effect on growth rate at any of the K+ concentrations when NH4+ was absent. Figure 2B shows that addition of NH4+ greatly inhibited the growth rate of akt1-1 plants without affecting growth of akt2-1 seedlings. As found previously, increasing [K+]ext ameliorated the inhibitory effect of NH4+, consistent with the notion that NH4+ inhibits a non-AKT1 K+ uptake pathway by competing for a K+ binding site (Spalding et al., 1999). It is presumable that because akt2-1 seedlings possess a functional AKT1 channel, their growth is not sensitive to NH4+ and is similar to or greater than wild type under all combinations of K+ and NH4+ (Fig. 2, A–C). Taken together, these results indicate that AKT1, but not AKT2, contributes to the ability of seedlings to take up K+ when its availability limits growth of seedlings.
Figure 2.
The K+ dependence and NH4+ inhibition of growth rate in seedlings. A, 0 NH4+. B, 2mM NH4+. C, 4 mM NH4+. Each bar represents the mean of three independent trials ± se.
The growth rate of the akt1Δ/2Δ double mutant was examined under the same conditions to determine if the absence of AKT2 was of more consequence in a genetic background lacking AKT1 than it was in a wild-type background. A consistent exacerbation of the akt1-1 phenotype by the akt2-1 mutation was observed but the small effect was not statistically significant. A reasonable conclusion to be drawn from these data is that AKT2 does not contribute importantly to seedling growth rate on K+-limiting media even in the absence of AKT1, and even when growth is made more dependent on AKT1 by the presence of NH4+. For the most part, the mutations in these two related channels act independently of each other.
Contribution of AKT2 to Root K+ Permeability
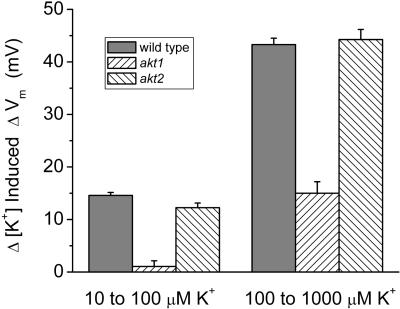
The lower growth rate of akt1-1 seedlings was correlated with a reduction in the K+ permeability of the plasma membrane of root cells, measured by patch clamping (Hirsch et al., 1998) and by in vivo measurements of K+-induced changes in membrane potential (ΔVm; Spalding et al., 1999). The electrophysiological and growth rate phenotypes were consistent with previous studies of RNA abundance and promoter strength, which showed AKT1 expression occurring primarily, but not exclusively, in the root (Basset et al., 1995; Lagarde et al., 1996). The K+ permeability of root cell membranes was expected to be normal in akt2-1 plants because the akt2-1 mutation did not affect growth rate in K+-limiting conditions (Fig. 2C) and its expression pattern was previously shown not to include roots (Cao et al., 1995; Ketchum and Slayman, 1996; Marten et al., 1999; Lacombe et al., 2000). To test this, we compared the ΔVm induced by changes in [K+]ext in root cells of the wild-type and two mutants. As shown in Figure 3 and consistent with previous reports, the akt1-1 mutation essentially eliminated the permeability detected in the low concentration range and dramatically reduced it in the higher concentration range. In contrast, and consistent with its mRNA expression pattern, the akt2-1 mutation did not affect the K+ permeability of the root cell plasma membrane. Thus, AKT1 is the most important contributor to the K+ permeability of the plasma membrane of roots bathed in ammoniacal media containing between 10 and 100 μm K+.
Figure 3.
K+ permeability of apical root cells in wild-type and mutant seedlings. Values are mean depolarizations in response to shifts in [K+]ext from 10 to 100 μm, and from 100 to 1,000 μm for between six and eight independent trials. Error bars represent se of the mean.
Contributions of AKT1 and AKT2 to Cotyledon K+ Permeability
Cotyledons were also examined to determine which of the two AKT channels contributed more to the K+ permeability of the plasma membrane in these photosynthetic organs. Figure 4 demonstrates that the small ΔVm induced by shifting [K+]ext from 10 to 100 μm in wild-type cotyledons was essentially eliminated in akt1-1 cotyledons. The akt2-1 mutation had no significant effect on the membrane potential response to this same K+ shift. The higher K+ shift (100–1,000 μm) induced a small depolarization in akt1-1 cotyledons and a response in akt2-1 cotyledons that was 65% of the wild-type value. Thus, the K+ permeability of cotyledon cells behaves as the sum of a large AKT1 component and a small AKT2 component.
Figure 4.
K+ permeability of cotyledon cells in wild-type and mutant seedlings. Values are mean depolarization in response to shifts in [K+]ext from 10 to 100 μm, and from 100 to 1,000 μm for between six and eight independent trials. Error bars represent se of the mean.
Contributions of AKT1 and AKT2 to Leaf K+ Permeability and Growth
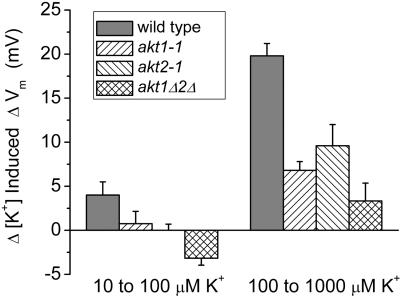
The K+ permeability of mesophyll cells was assessed in leaves by the same method of measuring K+-induced changes in Vm. The ΔVm induced by 10-fold shifts in [K+]ext in wild-type leaves, shown in Figure 5, were significantly smaller than the responses measured in roots or cotyledons, consistent with previous demonstrations that the K+ permeability of mesophyll cells is relatively low (Bei and Luan, 1998). Although smaller in magnitude than the AKT1-dependent response of root cells, the ΔVm measured in leaf mesophyll probably also reflects the activity of plasma membrane K+ channels, which were characterized by patch-clamp studies (Spalding et al., 1992; Spalding and Goldsmith, 1993). Shifts of [K+]ext from 10 to 100 μm induced a small response of 4 mV in wild-type leaves. The responses of akt1-1 and akt2-1 mutants to the same treatment were almost undetectable. Mesophyll cell membranes of the double mutant responded with a slight hyperpolarization, indicating that deletion of both AKT channels and the scarcity of K+ created a situation in which the permeability of one or more other ions exceeded that of K+. Chloride ions are likely candidates and the theory supporting this interpretation was recently explained in Spalding et al. (1999).
Figure 5.
K+ permeability of leaf mesophyll cells in wild-type and mutant seedlings. Values are mean depolarization in response to shifts in [K+]ext from 10 to 100 μm, and from 100 to 1,000 μm for between six and eight independent trials. Error bars represent se of the mean.
Shifts from 100 to 1,000 μm K+ induced a 20-mV response in wild-type leaves. The responses measured in akt1-1 and akt2-1 leaves were only 7 and 10 mV, respectively. Given the level of uncertainty associated with the values, the two mutant responses should be considered equivalent to each other. The still smaller ΔVm response measured in akt1Δ2Δ plants indicates that deleting both channels further impaired K+ permeability. One may conclude that AKT1 and AKT2 channels contribute approximately equally to the K+ permeability of the plasma membrane in leaf mesophyll cells. A minor non-AKT component is evidenced by the residual response detected in the double mutant.
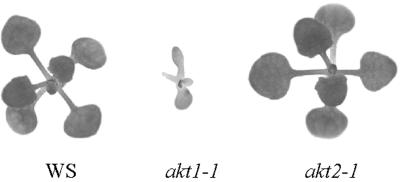
The finding that loss of AKT2 impaired the K+ permeability of leaf mesophyll cells raised a question about the effect of the akt2-1 mutation on leaf growth under K+-limiting conditions. A determination of this effect required growing plants on a sterile medium having a defined ionic composition until they developed true leaves. Between 18 and 22 d of growth on a complete agarose medium containing 100 μm K+ and 2 mm NH4+, the average growth rate of wild-type seedlings was 4.14 ± 1.6 mg d−1. At this stage of development, the increase in mass is largely due to expansion of the first five or six leaves (Fig. 6). Although the majority of akt2-1 plants resembled wild-type plants grown in the same conditions (Fig. 6), the growth rate of akt2-1 plants (6.99 ± 2.16 mg d−1) was consistently higher than wild type during this 4-d period. The substantial variability encountered within a genotype in these experiments is probably due to the negative effects of completely enclosed chambers and an agarose medium over an extended period, relative to growth in pots on soil. For comparison, akt1-1 plants grew only 0.64 ± 0.26 mg d−1 during this same time period. The double mutant was similar to akt1-1 seedlings. Thus, impairing the uptake ability of the plant root impairs growth when K+ is limiting growth rate but a significant impairment of mesophyll K+ permeability by the akt2-1 mutation does not impair leaf growth in the same conditions.
Figure 6.
Phenotypes of wild-type and mutant plants at the leaf development stage. Photographs of representative individual plants grown for 22 d on agarose medium containing 100 μm K+ and 2 mm NH4+. Leaf growth and development is not affected by the akt2-1 mutation under the same K+-limiting condition that strongly inhibits akt1-1 growth.
DISCUSSION
Permeability of the limiting membrane to K+ is a feature of all cells, prokaryotic and eukaryotic. It would seem that in some fundamental way it is important to cells that the membrane separating inside from out be permeable to K+. Especially in the case of plants, one is able to point to specific physiological processes assisted by this permeability, such as nutrient uptake and osmoregulation (Maathuis et al., 1997), but it is possible that even more fundamental or vital reasons for the ubiquity of K+ permeability will be revealed in the future. Interdisciplinary studies that combine genetics and (electro) physiology may provide the next level of understanding. The recent success of approaches in which a channel protein is ablated through genetic mutation and the physiological consequences assessed with physiological assays gives reason to be optimistic (Gaymard et al., 1998b; Hirsch et al., 1998; Geleen et al., 2000; Sunkar et al., 2000).
A combination of “knockout” mutations and electrophysiological assays of function allowed us here to decipher how two members of the AKT family act together to control the K+ permeability of a plasma membrane. In the root, the AKT1 channel predominates. Plants grown on agar plates depend on K+ uptake by roots, so it is understandable why the akt1-1 mutation impairs growth under K+-limiting conditions. The predominance of AKT1 expression in roots (Lagarde et al., 1996) is consistent with the electrophysiological phenotype of the root and the growth impairment observed in akt1-1 seedlings. However, it is more difficult to reconcile the low AKT1 mRNA levels or promoter activity in leaves (Lagarde et al., 1996; Lacombe et al., 2000) with the major effect of the akt1-1 mutation on the K+ permeability of the mesophyll cells. Based on RNA blots, AKT2 would be expected to dominate the permeability of the leaf mesophyll plasma membrane. Instead, the results presented here indicate that AKT1 and AKT2 contribute independently and approximately equally in leaves. Thus, it would appear that posttranscriptional regulation of AKT1 or AKT2 equalizes their contributions. This insight into the operation of proteins encoded by paralogous genes is a unique result of combining reverse genetics and quantitative assays of function.
Posttranslational regulation of activity is very common in channels. Some types rarely adopt their open-state conformation until an event such as ligand binding, phosphorylation, or a change in membrane potential increases many fold their probability of opening. An abundant channel may not contribute to the permeability of the membrane to the extent indicated by its mRNA or protein levels if it is rarely open. Likewise, a rare channel could contribute more than expected if its open probability were high. It is possible that AKT1, expected to be much the less abundant of the two channels, has a relatively high open probability, which would increase its contribution to membrane permeability. Patch-clamp experiments performed with mesophyll protoplasts isolated from wild-type and mutant plants could test this possibility. An independent explanation is that protein levels of AKT1 and AKT2 are not proportional to their mRNA concentrations. This could be tested with immunohistochemical methods.
It is prudent to consider how the functional assays used here are affected by the leaf's anatomy. To make measurements in leaves, the tip of the electrode was advanced through the organ until a highly negative and stable Vm was obtained. Although the electrode tip could not be visualized during this process, it was probably located inside a mesophyll cell because the first cell encountered (epidermis) rarely produced a stable recording and cells associated with the vascular bundles are far fewer and smaller than mesophyll cells. Any path for current to flow between the electrode tip and the grounded bathing solution must cross a plasma membrane. Shifts in [K+]ext produce a ΔVm that is proportional to the K+ permeability of the plasma membrane(s) in the least resistive of all such paths. That membrane is probably the plasma membrane of the impaled mesophyll cell because it separates the cytoplasm from a low-resistance, apoplastic path to the bathing solution. (Although the electrode tip was undoubtedly located in the vacuole in the majority of recordings, this does not affect the interpretation of ΔVm; shifts in [K+]ext would not alter the tonoplast voltage and therefore its effect on the measured ΔVm can be considered negligible [Bates et al., 1982].) In this simplest case, the measured ΔVm reflects the K+ permeability of the impaled cell. However, the existence of plasmodesmatal connections between mesophyll cells has the effect of producing a weighted average of the ΔVm responses of adjacent cells, the contribution of each cell to the measured value being weighted by the extent to which it is electrically coupled to the impaled cell (Spanswick, 1972). In the extreme case of perfect intercellular coupling, the measured ΔVm would be the average response of all connected mesophyll cells. The real situation undoubtedly lies somewhere between the extremes of nonexistent and perfect coupling so the recorded ΔVm may be viewed as the average response of a few interconnected mesophyll cells.
The AKT2 gene, previously also referred to as AKT3 (the nomenclature is explained by Lacombe et al. [2000]) is not expressed uniformly throughout the leaf. Reporter gene constructs and mRNA measurements indicate that AKT2 expression is considerably higher in phloem than mesophyll of Arabidopsis leaves (Marten et al., 1999; Lacombe et al., 2000). Did an electrode located in the mesophyll detect membrane responses in cells of other leaf tissues such as the phloem and therefore give a distorted view of the degree to which the two channels contribute to mesophyll membrane properties? If there were significant intercellular coupling between phloem and mesophyll cells, the AKT2 contribution in mesophyll would be overestimated and the AKT1 contribution detected here would be even more out of line with its expression level. Also, if the mesophyll and phloem had extensive symplastic connections, the active concentration of sugars into the latter tissue could not occur, at least by the currently accepted mechanisms. The most straightforward interpretation of the data in Figure 5 is that the K+ permeability of the mesophyll of Arabidopsis leaves, on average, is approximately equally determined by AKT1 and AKT2, despite their very different mRNA expression levels.
A scenario that could complicate the present assessment of channel contributions is that expression of AKT family members change when one is mutated. There is no evidence that such compensation occurs in roots or cotyledons because removing AKT1 channels has a large effect, whereas loss of AKT2 has little or no effect. AKT1 appears to be important in these organs, and AKT2 does not appear to compensate for the loss of AKT1. The results obtained with leaves (Fig. 5) also hold no evidence that any consistent compensatory changes in expression of these two genes occurred. That is not to say no changes in gene expression occur as a result of a particular channel mutation. A very appropriate use of current DNA microarrays would be to determine the impact of the mutations presented here on genome-wide expression profiles. Suites of genes related to K+ nutrition and the control of membrane permeability may be revealed.
An electrophysiological phenotype of the akt2-1 mutant was uncovered here, although a corresponding impairment of growth was not observed. When grown on nutrient-replete soil, all of the mutants studied here resembled the wild type. This indicates that neither channel is required for leaf growth under those conditions. When grown in K+-limiting conditions to the leaf-producing stage, akt2-1 and wild-type plants were similar (Fig. 6). The 50% reduction in K+ permeability caused by the akt2-1 mutation apparently is not sufficient to impair growth. In fact, in the presence of NH4+, the lack of AKT2 activity appears to be somewhat beneficial. The possibility that AKT1 provides an activity required for leaf expansion is not readily tested with these mutants because akt1-1 seedlings do not reach the leaf expansion stage in K+-limiting conditions, presumably because the uptake ability of their roots has been greatly impaired.
Perhaps loss of AKT2 does not affect leaf expansion because AKT1 can provide the necessary uptake function, or perhaps AKT2 does not function primarily as a K+ uptake mechanism in leaves analogous to the AKT1 function in roots. A link between K+ permeability and photosynthesis in leaf mesophyll cells has long been established (Jeschke, 1976) and previous patch-clamp studies of Arabidopsis mesophyll cells indicated that AKT2-like K+ channels at the plasma membrane are activated by photosynthesis, possibly via ATP (Spalding and Goldsmith, 1993). It has not yet been determined whether photosynthesis benefits from the increase in K+ permeability it causes. The new mutants described here may prove to be useful in studies of the importance of K+ permeability to photosynthesis. They may also be used to genetically test the suggestion that AKT2 activity is important to sugar translocation in phloem (Lacombe et al., 2000). There are undoubtedly other K+-dependent physiological processes that will be better understood by studying channel “knockout” mutants with appropriate functional assays.
MATERIALS AND METHODS
Identification of T-DNA Mutant Lines
The akt1-1 and akt2-1 plants were isolated from a T-DNA mutagenized population of Arabidopsis (a combination of the Arabidopsis Biological Resource Center and DuPont collections) using a PCR-based, reverse genetic strategy (Krysan et al., 1996). DNA sequencing of PCR-amplified fragments that spanned the insertion site determined the position of the T-DNA insert in each gene.
Plants homozygous for the akt1-1 allele were crossed to plants homozygous for the akt2-1 allele. Individuals of the F1 generation were grown and allowed to self-fertilize to produce a population of F2 plants in which the two mutant alleles were independently segregating. The genotypes of individual F2 plants were determined by examining ethidium bromide-stained products of PCR amplifications performed on genomic DNA using the following AKT1 and AKT2 specific primers: AKT1-5′, gttgcaatcaatatctcacttcaaatctc; AKT1-3′, ggaaaaacttgttgtagtcagtagcagac; AKT2-5′, atctctcattttcttctgcttcacattcc; and AKT2-3′, caatctcagctccatctcattcgtcacc.
This process identified akt1Δ2Δ plants, which were homozygous for both mutations. When grown on media replete with nutrients, neither of the single mutants nor the double mutant displayed an overt phenotype.
Electrical Measurements
Measurements of membrane potential in roots, cotyledons, and leaf mesophyll cells were made with an intracellular microelectrode as previously described for root cells (Hirsch et al., 1998). Leaves 1 to 4, or cotyledons, were excised from 7- to 14-d-old plants, mounted on 1% (w/v) agarose containing 10 μm K+ and complex medium (Spalding et al., 1999), immersed in identical liquid medium, and allowed to recover overnight. Cells were impaled near the edge of the leaf. Experiments proceeded with shifts in external potassium concentration ([K+]ext) only if the membrane potential (Vm) stabilized at a value more negative than −175 mV. (The resting potentials of the mutants and wild-type plants were not significantly different.) If a few basic assumptions are adopted, changes in membrane potential (ΔVm) due to shifts in [K+]ext are indicative of the relative permeability of the plasma membrane to K+ (Spalding et al., 1999).
Growth Rate Measurements
To measure growth rate, 25 surface-sterilized seeds each of akt1-1, akt2-1, akt1Δ2Δ, and the Arabidopsis Wassilewskija ecotype were sown on a complete nutrient medium (Spalding et al., 1999) containing 0.8% (w/v) agarose in square petri plates such that all four genotypes were represented on each of two plates. After 4 d of growth, one plate was harvested and the fresh weights of each genotype group were measured to the nearest 0.1 mg. The second plate was harvested after 8 d of growth, the seedlings were weighed, and the difference between the 4- and 8-d masses was divided by 4 to obtain a growth rate value in units of milligrams per day for 25 seedlings. This experiment was repeated at least three times to obtain the averages presented. Potassium was varied as additions of 10-, 100-, and 1,000-μm concentrations of KCl to the nominally K+-free nutrient medium. The concentration of NH4+ was varied from 0 to 2 mm by adding NH4H2PO4, and to 4 mm by adding additional NH4Cl.
To measure the growth rates of older plants, nine seeds were sown on 50 mL of the complete medium (100 μm KCl and 2 mm NH4Cl) in transparent plastic “magenta” boxes (100 × 65 × 65 mm). Duplicate boxes with seeds were placed in a growth chamber set to deliver 16-h-light/8-h-dark cycles. The culture boxes were sealed to prevent evaporation, which could alter critical ion concentrations of the medium during the course of the experiment. The mass of the nine seedlings was determined after 18 or 22 d of growth and growth rates calculated from the differences as stated above. The average rate of growth during these 4 d was primarily due to expansion of the five or six leaves wild-type seedlings had produced at this stage in development. The experiment was repeated three times per genotype.
Footnotes
This work was supported by the National Science Foundation (Career Award no. IBN–9734478 to E.P.S. and grant no. DBI–9872638 to M.R.S.) and by the Department of Energy/National Science Foundation/U.S. Department of Agriculture Collaborative Research in Plant Biology Program (grant no. BIR–9220331) at the University of Wisconsin.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010193.
LITERATURE CITED
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset M, Conejero G, Lepetit M, Fourcroy P, Sentenac H. Organization and expression of the gene coding for the potassium transport system AKT1 of Arabidopsis thaliana. Plant Mol Biol. 1995;29:947–958. doi: 10.1007/BF00014968. [DOI] [PubMed] [Google Scholar]
- Bates GW, Goldsmith MHM, Goldsmith TH. Separation of tonoplast and plasma membrane potential and resistance in cells of oat coleoptiles. J Membr Biol. 1982;66:15–23. [Google Scholar]
- Bei QX, Luan S. Functional expression and characterization of a plant K+ channel gene in a plant cell model. Plant J. 1998;13:857–865. doi: 10.1046/j.1365-313x.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- Bertl A, Anderson JA, Slayman CL, Gaber RF. Use of Saccharomyces cerevisiae for patch-clamp analysis of heterologous membrane proteins: characterization of Kat1, an inward-rectifying K+ channel from Arabidopsis thaliana, and comparison with endogenous yeast channels and carriers. Proc Natl Acad Sci USA. 1995;92:2701–2705. doi: 10.1073/pnas.92.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A, Reid JD, Sentenac H, Slayman CL. Functional comparison of plant inward-rectifier channels expressed in yeast. J Exp Bot. 1997;48:405–413. doi: 10.1093/jxb/48.Special_Issue.405. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ward JM, Kelly WB, Ichida AM, Gaber RF, Anderson JA, Uozumi N, Schroeder JI, Crawford NM. Multiple genes, tissue specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995;109:1093–1106. doi: 10.1104/pp.109.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Crawford NM, Schroeder JI. Proteins for transport of water and mineral nutrients across the membranes of plant cells. Plant Cell. 1999;11:661–675. doi: 10.1105/tpc.11.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F, Cerutti M, Horeau C, Lemaillet G, Urbach S, Ravallec M, Devauchelle G, Sentenac H, Thibaud J-B. The baculovirus/insect cell system as an alternative to Xenopus oocytes. J Biol Chem. 1998a;271:22863–22870. doi: 10.1074/jbc.271.37.22863. [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud J-B, Sentenac H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998b;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- Geleen D, Lurin C, Bouchez D, Frachisse JM, Lelievre F, Barbier-Brygoo H, Maurel C. Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J. 2000;21:259–267. doi: 10.1046/j.1365-313x.2000.00680.x. [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Jeschke WD. Ionic relations of leaf cells. In: Luttge U, Pitman MG, editors. Encyclopedia of Plant Physiology, N.S. 2: Transport in Plants II, Part B. Berlin: Springer; 1976. pp. 160–194. [Google Scholar]
- Ketchum KA, Slayman CW. Isolation of an ion channel gene from Arabidopsis thaliana using the H5 signature sequence from voltage-dependent K+ channels. FEBS Lett. 1996;378:19–26. doi: 10.1016/0014-5793(95)01417-9. [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR. T-DNA as an insertional mutagen in Arabidopsis. Plant Cell. 1999;11:2283–2290. doi: 10.1105/tpc.11.12.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Tax F, Sussman MR. Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc Natl Acad Sci USA. 1996;93:8145–8150. doi: 10.1073/pnas.93.15.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud J-B. A shaker-like channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell. 2000;12:837–851. doi: 10.1105/tpc.12.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Gaymard F, Astruc S, Grignon C. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J. 1996;9:195–203. doi: 10.1046/j.1365-313x.1996.09020195.x. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Ichida AM, Sanders D, Schroeder JI. Roles of higher plant K+ channels. Plant Physiol. 1997;114:1141–1149. doi: 10.1104/pp.114.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten I, Hoth S, Deeken R, Ache P, Ketchum KA, Hoshi T, Hedrich R. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc Natl Acad Sci USA. 1999;96:7581–7586. doi: 10.1073/pnas.96.13.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WLJ, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Spalding EP, Goldsmith MHM. Activation of K+ channels in the plasma membrane of Arabidopsis by ATP produced photosynthetically. Plant Cell. 1993;5:477–484. doi: 10.1105/tpc.5.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD. Potassium uptake supporting plant growth in the absense of AKT1 channel activity: inhibition by ammonium and stimulation by sodium. J Gen Physiol. 1999;113:909–918. doi: 10.1085/jgp.113.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Slayman CL, Goldsmith MHM, Gradmann D, Bertl A. Ion channels in Arabidopsis plasma membrane: transport characteristics and involvement in light-induced voltage changes. Plant Physiol. 1992;99:96–102. doi: 10.1104/pp.99.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick RM. Electrical coupling between cells of higher plants: a direct demonstration of intercellular communication. Planta. 1972;102:215–227. doi: 10.1007/BF00386892. [DOI] [PubMed] [Google Scholar]
- Sunkar R, Kaplan B, Bouche N, Arazi T, Dolev D, Talke IN, Maathuis FJM, Sanders D, Bouchez D, Fromm H. Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J. 2000;24:533–542. doi: 10.1046/j.1365-313x.2000.00901.x. [DOI] [PubMed] [Google Scholar]