Abstract
Regulation of Ca2+ transport determines the duration of a Ca2+ signal, and hence, the nature of the biological response. Ca2+/H+ antiporters such as CAX1 (cation exchanger 1), play a key role in determining cytosolic Ca2+ levels. Analysis of a full-length CAX1 clone suggested that the CAX1 open reading frame contains an additional 36 amino acids at the N terminus that were not found in the original clone identified by suppression of yeast (Saccharomyces cerevisiae) vacuolar Ca2+ transport mutants. The long CAX1 (lCAX1) could not suppress the yeast Ca2+ transport defects despite localization to the yeast vacuole. Calmodulin could not stimulate lCAX1 Ca2+/H+ transport in yeast; however, minor alterations in the 36-amino acid region restored Ca2+/H+ transport. Sequence analysis suggests that a 36-amino acid N-terminal regulatory domain may be present in all Arabidopsis CAX-like genes. Together, these results suggest a structural feature involved in regulation of Ca2+/H+ antiport.
Calcium (Ca2+) levels in the cytosol fluctuate in response to growth, development, and environmental perturbations (Sanders et al., 1999; Curran et al., 2000). The degree and duration of the cytosolic Ca2+ “spike” determines the plant's biological response. Like all eukaryotes, plants utilize transporter systems to meticulously control Ca2+ concentrations in different cellular compartments (Bush, 1995; Harper et al., 1998; Sze et al., 2000). Influx of Ca2+ to the cytosol occurs as a “downhill” transport through Ca2+ channels (Chung et al., 2000). Ca2+/H+ antiporters along with Ca2+-ATPases regulate the active Ca2+ efflux from the cytosol (Ueoka-Nakanishi et al., 1999; Chung et al., 2000; Hirschi, 2001). The mechanisms of Ca2+-ATPase regulation have begun to emerge recently (Curran et al., 2000; Hwang et al., 2000b); however, little is known about regulation of other plant Ca2+ transporters (Sze et al., 2000).
Ca2+/H+ exchange helps to establish the concentration gradient of Ca2+ across the tonoplast (vacuolar membrane; Schumaker and Sze, 1985; Blumwald and Poole, 1986). Two Arabidopsis genes, CAX1 (cation exchanger 1), and CAX2 were identified by their ability to sequester Ca2+ into vacuoles in Saccharomyces cerevisiae mutants deleted in vacuolar Ca2+ transport (Hirschi et al., 1996; Mäser et al., 2001). In Arabidopsis, the high-affinity, high-capacity Ca2+/H+ transporter CAX1, and a closely related gene, CAX3 (HCX1), are both highly expressed in response to exogenous Ca2+, whereas the low-affinity Ca2+/H+ transporter CAX2 is not induced by exogenous Ca2+ (Hirschi, 1999, Hirschi et al., 2000; Shigaki and Hirschi, 2000). Aside from these findings, nothing is known regarding the regulation of these Ca2+/H+ transporters.
The plant Ca2+-ATPases may serve as a useful prototype for potential regulatory mechanisms that may be utilized among plant Ca2+ transporters. The Arabidopsis endoplasmic reticulum Ca2+-ATPase, ACA2, can be activated or repressed by regulatory molecules binding to the N terminus (Harper et al., 1998; Hwang et al., 2000a). Calmodulin binding to the N terminus causes activation, whereas the pump can be inhibited by a Ca2+-dependent protein kinase (CDPK) phosphorylation at Ser-45 (Hwang et al., 2000b). Thus, the pump can be activated or repressed by different sensors that are responding to alterations in cytosolic Ca2+. In addition, N-terminal truncations of ACA2, the plasma membrane (PM) Ca2+-ATPase SCA1 (soybean; Glycine max), and the vacuolar Ca2+-ATPases ACA4 (Arabidopsis) and BCA1 (cauliflower; Brassica oleracca) are required for these gene products to suppress yeast mutants defective in vacuolar Ca2+ transport (Harper et al., 1998; Chung et al., 2000; Geisler et al., 2000; Malmström et al., 2000). These studies suggest that Ca2+ pumps in plants contain important regulatory domains at the N terminus.
To investigate the potential N-terminal regulatory domains within CAX1, we obtained a full-length cDNA clone of CAX1. This long CAX1 (lCAX1) clone contained additional coding sequences at the N terminus that were not present in the clone characterized by function in yeast (Hirschi et al., 1996). We expressed, localized, and determined the transport properties of lCAX1 when expressed in yeast. We then modified the N terminus of lCAX1 in an attempt to modify transport activity in yeast. Sequence analysis suggests that the Arabidopsis CAX transporters contain N-terminal amino acids not found in the previously cloned CAX1 and CAX2. These findings offer insights into the regulation of Ca2+/H+ antiport.
RESULTS
Identification of lCAX1 cDNA
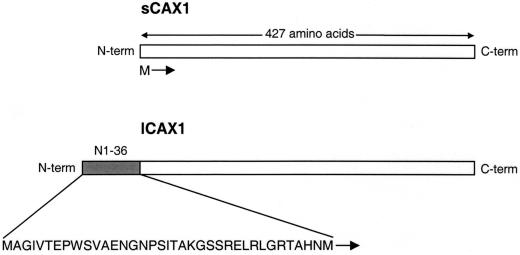
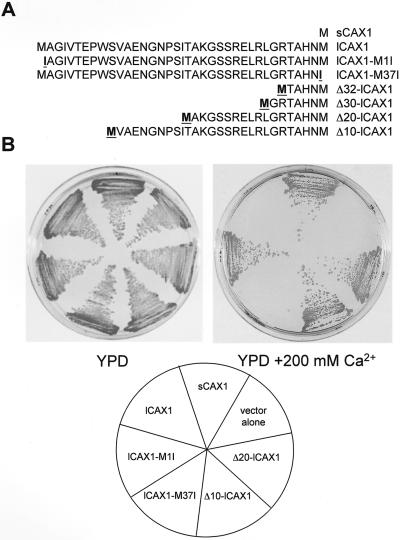
A BLAST search of the GenBank sequence database using the CAX1 cDNA sequence (Hirschi et al., 1996) identified an expressed sequence tag (EST) clone (accession no. BE038949) that had a high level of similarity with the CAX1 sequence. As shown in Figure 1, the EST codes for a predicted open reading frame identical to all 427 amino acids of CAX1 except for an additional 36 amino acids at the N terminus. This EST was obtained and fully sequenced to verify the fidelity of the open reading frame. We have named this cDNA long CAX1 (lCAX1) in order to differentiate it from the shorter CAX1 (sCAX1) previously cloned. The lCAX1 sequence is consistent with the predicted open reading frame of CAX1 from the genomic database (accession no. AC003028).
Figure 1.
Structure of lCAX1 compared with that of sCAX1. lCAX1 contains an additional 36 amino acids at the N terminus. This domain (shaded) has been named N1-36. The sequence of the first 37 amino acids of lCAX1 is shown. The start Met for sCAX1 is also shown. Following the identification of a sequencing error, the original nucleotide sequence of sCAX1 deposited in the GenBank database was recently amended, as previously described (Shigaki and Hirschi, 2000), thereby changing the length of the sCAX1 open reading frame from 459 amino acids to 427 amino acids. This altered open reading frame is identical to amino acids 37 to 463 of lCAX1.
lCAX1 Cannot Suppress a Yeast Mutant Defective in Vacuolar Ca2+ Transport
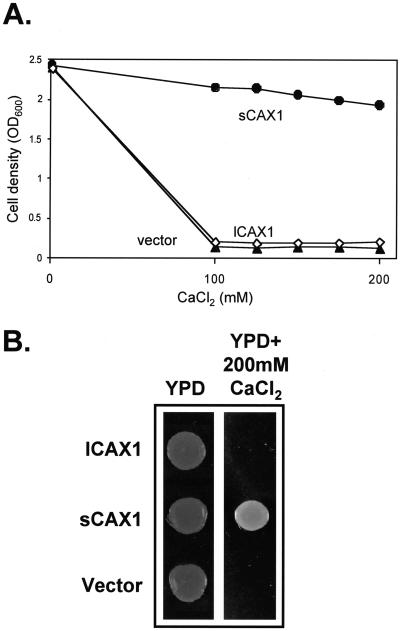
To test the function of lCAX1 in yeast, the lCAX1 cDNA was inserted into a high-copy yeast expression plasmid under the control of the glyceraldehyde-3-phosphate dehydrogenase promoter (Nathan et al., 1999) and expressed in the yeast mutant K667 (Cunningham and Fink, 1996). This strain lacks the endogenous vacuolar Ca2+-ATPase PMC1 and vacuolar Ca2+/H+ antiporter VCX1 and thus is defective in vacuolar Ca2+ transport, making it unable to grow on high-Ca2+ media (Cunningham and Fink, 1996). This mutation can be suppressed by VCX1, Arabidopsis sCAX1 and CAX2, and mung bean (Vigna radiata) VCAX1 (Cunningham and Fink, 1996; Hirschi et al., 1996; Ueoka-Nakanishi et al., 2000). We examined the growth of K667 yeast cells expressing sCAX1, lCAX1, or the vector alone in liquid medium containing a range of CaCl2 concentrations from 100 to 200 mm. The lCAX1-expressing strain was unable to suppress the Ca2+ sensitivity of K667, whereas the sCAX1-expressing strain successfully rescued the growth defect of K667 (Fig. 2A). The growth of lCAX1-expressing strains was indistinguishable from that of the vector control-transformed yeast (Fig. 2A). In a similar manner, when growth of these yeast strains was compared on solid media containing 200 mm CaCl2, no growth was visible for the lCAX1-expressing strain, whereas sCAX1 suppressed the growth defect (Fig. 2B). We tested the possibility that lCAX1 was not being expressed in the transformed K667 yeast strain. Although a specific antibody to lCAX1 was unavailable, the transcription of lCAX1 in yeast was demonstrated by reverse transcriptase (RT)-PCR using specific primers designed against the lCAX1 sequence. The presence of lCAX1-specific transcripts in lCAX1-transformed yeast, but not from vector-alone or sCAX1-expressing yeast (data not shown), confirmed that the lCAX1 mRNA was transcribed in K667.
Figure 2.
A, Ca2+ tolerance assay of K667 mutant yeast-expressing vector alone (▴), sCAX1 (●), or lCAX1 (⋄). Yeast strains were grown in selection media overnight at 30°C and diluted to an optical density at 600 nm (OD600) of 1.0, then inoculated into fresh yeast peptone dextrose (YPD) media containing a range of CaCl2 concentrations from 100 to 200 mm. Yeast cells were grown for 16 h at 30°C in flat-bottomed 24-well dishes. Cell density was determined by measurements at OD600. B, K667 yeast strains expressing vector alone, sCAX1, and lCAX1 were grown in selection media overnight at 30°C and diluted to an OD600 of 1.5, then spotted onto YPD media alone and YPD media containing 200 mm CaCl2. Yeast growth on YPD alone and YPD with 200 mm CaCl2 was photographed after 1 and 3 d, respectively.
lCAX1 Is Localized at the Vacuolar Membrane in Yeast
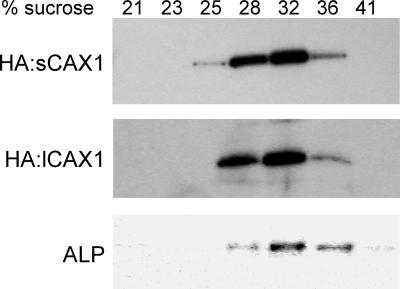
The inability of lCAX1 to suppress yeast mutants defective in vacuolar Ca2+ transport could be due to localization of the protein to a different membrane. The extended N terminus may encode a signal peptide, although none were identified using various motif prediction programs (data not shown). To identify the cellular location of lCAX1 in yeast, an epitope-tagged variant was generated by the fusion of a triple copy of hemagglutinin (HA) to the N terminus (HA:lCAX1). An N-terminal HA epitope tag has been used previously to identify the cellular location of VCX1 in yeast and transgenic Arabidopsis (Cunningham and Fink, 1996; Hirschi et al., 2001), and to confirm the localization of sCAX1 to the vacuolar membrane of yeast (T. Shigaki and K.D. Hirschi, unpublished data). The HA epitope tag did not appear to disrupt antiport function because both HA:VCX1 and HA:sCAX1 had the ability to suppress the Ca2+ sensitive phenotype of K667 (Cunningham and Fink, 1996; T. Shigaki and K.D. Hirschi, unpublished data). In addition, an N-terminal green fluorescence protein tag has successfully been used to identify the localization of VCAX1 in transgenic tobacco (Nicotiana tobacum; Ueoka-Nakanishi et al., 2000). As shown in Figure 3, western-blot analysis of yeast membranes fractionated on Suc gradients showed that sCAX1 cofractionated with vacuolar membranes. HA:lCAX1 was detected only in the 28% to 36% (w/w) Suc fractions and its distribution corresponded with that of HA:sCAX1 and the yeast vacuolar membrane marker ALP.
Figure 3.
Intracellular localization of HA:sCAX1 and HA:lCAX1 in K667 mutant yeast. Yeast microsomal membranes were extracted and fractionated through a 15% to 50% (w/w) Suc gradient and 1-mL fractions were collected. Approximately 2 μg of protein from each of the seven fractions from 21% to 41% (w/w) Suc were separated by SDS-PAGE, blotted, then subjected to western-blot analyses using the anti-HA monoclonal antibody (HA:sCAX1 and HA:lCAX1) and an antibody against a yeast vacuolar membrane marker alkaline phosphatase (ALP).
Inability of lCAX1 to Transport 45Ca in Yeast
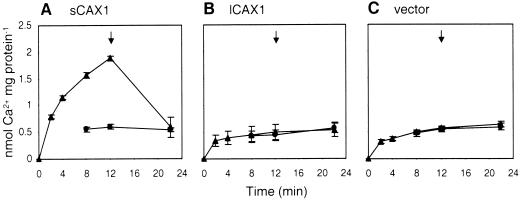
It is presumable that lCAX1 failed to suppress yeast strains deficient in vacuolar Ca2+ transport due to this transporter's inability to drive Ca2+ transport into the yeast vacuole. To directly test Ca2+/H+ antiport activity, we isolated yeast endomembrane vesicles purified from sCAX1, and lCAX1-expressing K667 cells. The capacity for ΔpH-dependent Ca2+ uptake was measured by a filtration assay. As shown in Figure 4A, Ca2+/H+ transport by sCAX1 measured using 10 μm 45CaCl2 was consistent with previous results (Hirschi et al., 1996), but no Ca2+ transport was demonstrated for lCAX1 when assayed with a range of Ca2+ concentrations from 1 to 100 μm (Fig. 4B; data not shown). The presence of the Ca2+-binding protein calmodulin also had no effect on lCAX1-mediated Ca2+ transport (data not shown).
Figure 4.
Time course of ΔpH-dependent 10-μm 45Ca2+ transport into endomembrane-enriched vesicles prepared from K667 mutant yeast expressing either A, sCAX1; B, lCAX1; or C, vector alone. Ca2+ transport was determined in the absence (▴) or presence (●) of 5 μm gramicidin. Ca2+ transport in the presence of gramicidin was not determined for the first two time points. All time course experiments were performed in the presence of 0.1 mm NaN3, 0.2 mm Na orthovanadate, and 1 mm ATP. The Ca2+ ionophore A23187 (5 μm) was added at the times indicated (arrow). Results are the average (±se) of three independent experiments.
Truncations of the lCAX1 N-Terminal Region Restore Activity
To determine which amino acid residues were involved in inhibition of lCAX1 activity, a number of lCAX1 mutants were generated by PCR-based site-directed mutagenesis (Fig. 5A). The introduction of a point mutation converting Met-37 to Ile-37 (lCAX1-M37I) did not restore growth of K667 on 200 mm CaCl2 (Fig. 5B), indicating that the lack of lCAX1 activity was not due to the presence of two Met residues in close proximity (Met-1 and Met-37). A truncated variant of lCAX1 lacking the first 36 residues was generated by converting Met-1 into Ile (lCAX1-M1I), so that translation would be initiated at Met-37, thereby converting lCAX1 to sCAX1. Removal of all 36 residues restored the activity of lCAX1, as demonstrated by suppression of the K667 phenotype on 200 mm CaCl2, making it comparable to the growth of the sCAX1-expressing cells (Fig. 5B). In an attempt to delineate any potential regulatory domain in N1-36, various N-terminal truncated variants of lCAX1 were generated, lacking either the first 10, 20, 30, or 32 amino acid residues (Δ10-lCAX1, Δ20-lCAX1, Δ30-lCAX1, and Δ32-lCAX1; Fig. 5A). All of the truncated forms of lCAX1 were able to suppress the K667 mutant, and growth was found to be indistinguishable from sCAX1 and lCAX1-M1I (Fig. 5B; data not shown).
Figure 5.
The ability of lCAX1 mutants with structural alterations at the N-terminal tail to suppress the Ca2+-sensitive growth phenotype of K667 mutant yeast. A, Schematic representation of the first 37 amino acids of lCAX1 summarizing the point mutations and truncations that were generated. Highlighted Ile residues indicate a substitution from Met. Highlighted Met residues indicate the addition of a Met that was created to initiate translation following truncation. B, Growth analysis of K667 mutant yeast expressing sCAX1, lCAX1, lCAX1-M1I, lCAX1-M37I, Δ20-lCAX1, Δ10-lCAX1, and vector alone. The yeast strains were streaked onto either plates containing YPD alone or YPD supplemented with 200 mm CaCl2, then grown at 30°C for 2 d.
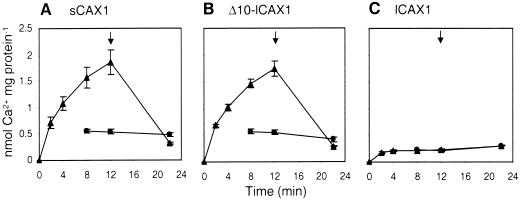
To confirm that the Ca2+ tolerance observed for the Δ10-lCAX1-expressing yeast strain was due to a restoration of Ca2+ transport activity, 45Ca2+/H+ transport was measured from endomembrane vesicles obtained from Δ10-lCAX1-expressing yeast cells. Ca2+/H+ transport by Δ10-lCAX1 was measured using 10 μm 45CaCl2, and was comparable to the activity observed for sCAX1-expressing cells, whereas transport was absent for lCAX1 (Fig. 6).
Figure 6.
Time course of ΔpH-dependent 10-μm 45Ca2+ transport into endomembrane-enriched vesicles prepared from K667 mutant yeast expressing either A, sCAX1; B, Δ10-lCAX1; or C, lCAX1. Ca2+ transport was determined in the absence (▴) or presence (●) of 5 μm gramicidin. Ca2+ transport in the presence of gramicidin was not determined for the first two time points. All time course experiments were performed in the presence of 0.1 mm NaN3, 0.2 mm Na orthovanadate, and 1 mm ATP. The Ca2+ ionophore A23187 (5 μm) was added at the times indicated (arrow). Results are the average (±se) of two independent experiments.
Other CAX-Like Genes Contain Similar N-Terminal Domains
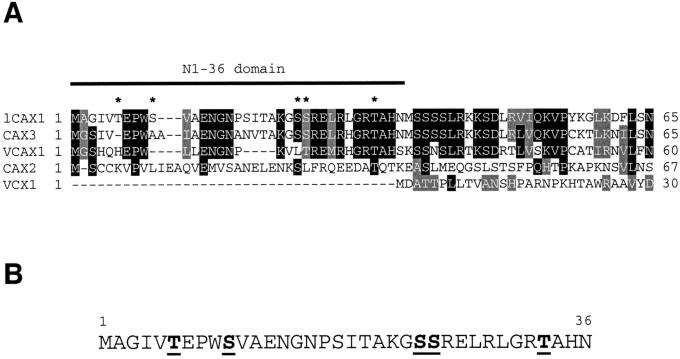
The N-terminal 36-amino acid sequence of lCAX1 (N1-36) was compared with the sequences of other CAX-like genes. This analysis found that VCAX1 of mung bean (Ueoka-Nakanishi et al., 1999) and Arabidopsis CAX3 (Shigaki and Hirschi, 2000) also possess similar N-terminal sequences (Fig. 7A). In addition, the genomic database suggests that the endogenous CAX2 (accession no. AB024034) may contain a 42-amino acid extension not found in the clone that suppressed the yeast vacuolar Ca2+ transport mutant (Hirschi et al., 1996). However, the sequence of VCX1 from S. cerevisiae does not appear to contain an extended N terminus (Fig. 7A). The N1-36 domain of lCAX1 shares significant sequence similarity with the extended N termini of most of these genes, with the highest similarity found between lCAX1 and CAX3 (Fig. 7A). This gene has previously been shown to share 77% identity at the amino acid level with the entire sCAX1 sequence (Shigaki and Hirschi, 2000). As shown in Figure 7A, 24 of the 36 amino acids in the N1-36 domain are shared between lCAX1 and CAX3.
Figure 7.
A, Partial amino acid sequence alignment of the N-terminal tail region of various CAX-like genes from Arabidopsis (lCAX1, CAX2, and CAX3), mung bean (VCAX1), and S. cerevisiae (VCX1). The aligned sequences correspond to the entire N-terminal tails up until the first predicted transmembrane domain. The N1-36 region of lCAX1 is underlined. Alignments were performed using ClustalW 1.8 (Baylor College of Medicine Software Programs). Identical residues are shaded in black and similar residues are shaded in gray. Gaps introduced to maximize the alignment are denoted by hyphens. An asterisk denotes a putative phosphorylated residue (see B). The deduced amino acid sequence of CAX2 used here was derived from the extracted sequence of the genomic clone (accession no. AB024034). B, Amino acid sequence of the N1-36 domain of lCAX1 highlighting putative phosphorylation sites. Putative phosphorylation sites were determined using the prediction software NetPhos (Blom et al., 1999) and from analyzing known binding sites of CDPKs.
DISCUSSION
Regulation of Ca2+ signals is contingent upon the precise control of transporters and channels that modulate the amount of Ca2+ in the cytosol (McAinsh and Hetherington, 1998). Ca2+/H+ antiporters are part of the ensemble of transporters that help modulate the duration of these Ca2+ signaling events (Ueoka-Nakanishi et al., 2000). However, the mechanisms by which the plant Ca2+/H+ antiporters are regulated are unknown (Sze et al., 2000; Hirschi, 2001). The Arabidopsis CAX1 gene was identified previously as the putative vacuolar Ca2+/H+ antiporter due to the gene product's ability to suppress yeast mutants defective in vacuolar Ca2+ transport (Hirschi et al., 1996). Ectopic expression of this CAX1 gene product in tobacco causes alterations in Ca2+ homeostasis and stress sensitivities, which implies that regulated expression of Ca2+/H+ antiporter activity is a vital component of plant responses to the environment (Hirschi, 1999). Analysis of the Arabidopsis genome and ESTs suggested that the endogenous CAX1 may contain 36 amino acids at the N terminus not present in the initial clone of CAX1. For the sake of clarity in this report, we have termed the original clone short CAX1 (sCAX1), and the CAX1 cDNA clone containing the 36-amino acid N-terminal region long CAX1 (lCAX1). This 36-amino acid region has been termed N1-36 or the regulatory region. In the future, we will refer to lCAX1 as CAX1 and sCAX1 will become the constitutively activated form of CAX1.
The lCAX1 clone was unable to suppress yeast mutants defective in vacuolar Ca2+ transport (Fig. 2, A and B). Using RT-PCR, we demonstrated that lCAX1 was transcribed in yeast (data not shown). Thus, the failure to suppress the yeast mutations was not due to a failure to transcribe the Arabidopsis gene. Furthermore, HA-tagged sCAX1 and lCAX1 both colocalize to the yeast vacuolar membrane (Fig. 3). This suggests that the failure of lCAX1 to suppress these mutations was not due to altered localization of the protein in yeast. This also indicates that the N terminus is not necessary for vacuolar membrane localization. We then isolated endomembrane vesicles from yeast cells expressing lCAX1. The failure to suppress the yeast mutants was not due to lower activity of Ca2+/H+ transport, but rather it appeared to be due to the complete absence of endomembrane Ca2+/H+ activity. The level of transport activity in lCAX1 cells was comparable to levels found in yeast membranes expressing vector controls (Fig. 4).
These findings suggested that the N-terminal region of lCAX1 acts as an autoinhibitory domain for Ca2+/H+ transport activity in yeast. The Arabidopsis Ca2+-ATPase, ACA2, has been shown previously to have regulatory elements at the N-terminal autoinhibitory region (Harper et al., 1998; Hwang et al., 2000a). Only ACA2 cDNA clones with truncations at the N terminus are able to suppress yeast mutants defective in endomembrane Ca2+ transport. A calmodulin-binding sequence is present within the first 36 residues of the N-terminal domain of ACA2, and the full-length gene product demonstrates calmodulin-stimulated Ca2+ transport in yeast (Harper et al., 1998; Hwang et al., 2000a). This indicates that the N terminus of ACA2 acts as a calmodulin-regulated autoinhibitory domain. A CDPK-binding site is present in the N terminus of ACA2 that phosphorylates a Ser residue near the calmodulin-binding site. This CDPK activity inhibits ACA2 activity (Hwang et al., 2000b). Thus, ACA2 is regulated between calmodulin stimulation and CDPK inhibition. In a manner analogous to the Ca2+-ATPases, much evidence suggests that plant PM H+-ATPases are regulated by a C-terminal autoinhibitory domain (for review, see Palmgren, 2001). For example, the Arabidopsis H+-ATPase AHA2 is activated by the binding of a 14-3-3 protein to the autoinhibitory domain and this binding is induced by a protein kinase-mediated phosphorylation of a specific Thr residue in this domain (Palmgren, 2001).
Like ACA2, lCAX1 was derepressed by alterations in the N terminus. Deletions of the first 10, 20, 30, or 32 amino acids at the N terminus caused the gene product to suppress yeast mutants defective in vacuolar Ca2+ transport (Figs. 5 and 6; data not shown). Because Δ10-lCAX1 was able to suppress the mutant yeast phenotype (Fig. 5) and transport Ca2+ as efficiently as sCAX1 (Fig. 6), it might be concluded that the first 10 amino acids of lCAX1 are critical for its activity and that the putative autoinhibitory domain is present within this region. In an alternate manner, deletion of the first 10 amino acids may significantly alter the structure of the N-terminal tail and therefore perturb its regulatory activity. Therefore, further experiments are required to determine exactly which of the first 36 amino acids are required for autoinhibition. Work on the Ca2+-ATPases suggests that the N-terminal deletions are more active than the calmodulin-stimulated full-length clones (Hwang et al., 2000a). If this is true of CAX1, then sCAX1 may be more active than any modified form of the endogenous protein. We are currently testing the hypothesis that the altered Ca2+ homeostasis exhibited in transgenic tobacco plants expressing sCAX1 is also the fortuitous consequence of expressing a constitutively activated form of the transporter (Hirschi, 1999).
Alterations in the N terminus can alter the function of many proteins. For example, these results with CAX1 are analogous to work done in mammalian systems with the soluble insulin-like growth factor-I (IGF-1). In these studies, the addition or subtraction of N-terminal residues conferred increased activity onto the growth regulator (Tomas et al., 1997). With plant Ca2+ transporters, there has been some evidence suggesting that they can be modulated by protein cleavage (Askerlund, 1996). Trypsin treatment resulted in cleavage of the calmodulin-binding domain from the N terminus of the cauliflower Ca2+-ATPase BCA1 and subsequently activated this protein (Askerlund, 1996; Malmström et al., 2000). It is conceivable that CAX1 may be regulated through such events.
Unlike ACA2, the lCAX1 gene product was not activated by addition of exogenous calmodulin (data not shown). Sequence analysis of the 36-amino acid regulatory region did not identify a calmodulin-binding site; however, there are several putative CDPK-binding sites (Fig. 7B). These results suggest that CAX1 is not up-regulated by calmodulin, and that the transporter may be activated by CDPKs or other regulatory molecules. There are numerous examples in animal studies where protein kinases directly activate Ca2+ transport function (Enyedi et al., 1996).
This study suggests that sCAX1 suppression of the vacuolar transport mutants is due to a truncated cDNA and a second Met codon fortuitously found in CAX1. It is unclear whether the truncated sCAX1 is an artifact of the original yeast suppression screen and is therefore a partial-length cDNA. In an alternate manner, there may be splice variants of CAX1 in Arabidopsis; therefore, sCAX1 and lCAX1 may be products of alternative splicing. An informative example of this type of regulation occurs in mammalian PM-type Ca2+-ATPases, which exhibit alternative splicing that alters the presence of the calmodulin regulatory domains (Penniston and Enyedi, 1998). We have shown previously that the CAX1 RNA levels in Arabidopsis increase in response to exogenous Ca2+ in the media (Hirschi, 1999). Using primers specific for lCAX1, we have performed RT-PCR experiments on RNA from exogenous metal-treated Arabidopsis tissues and demonstrated that the CAX1 RNA corresponds to the lCAX1 sequence (data not shown). These preliminary studies suggest that lCAX1 is the predominant form of CAX1 found in Arabidopsis.
It is interesting that sequence data demonstrate that CAX2 may contain an additional N-terminal sequence that codes for amino acids not found in the cDNA that suppresses yeast vacuolar Ca2+ transport mutants (Fig. 7A). Based on this sequence analysis, CAX3, a homolog of CAX1, also contains a putative N-terminal regulatory region very similar to that present on CAX1. The mung bean Ca2+/H+ antiporter, VCAX1, also contains a putative N-terminal regulatory domain, but this region does not repress Ca2+ transport activity in yeast (Fig. 7A; Ueoka-Nakanishi et al., 2000). This finding suggests that mung bean may regulate Ca2+/H+ transport in a different manner than Arabidopsis. Comparisons between lCAX1 and VCAX1 may offer further insight into the important sequences required for lCAX1 autoinhibition (Fig. 7A). Yeast VCX1, however, does not possess an extended N terminus. In yeast, the function of VCX1 is inhibited by the Ca2+/calmodulin-dependent protein phosphatase, calcineurin. Calcineurin is believed to regulate VCX1 activity at the posttranslational level (Cunningham and Fink, 1996) and it is likely that this involves a direct protein-protein interaction, although the possible site of calcineurin binding to VCX1 is unknown.
Some of the regulatory mechanisms of other cation/H+ antiporters from various species have begun to be elucidiated. The mammalian Na+/H+ exchanger, NHE1, contains a calmodulin-binding autoinhibitory domain that reduces the affinity of this transporter for H+ (Wakabayashi et al., 1997). In addition, protein kinase-dependent regulation has been observed for both Na+/H+ and Na+/Ca2+ exchangers in animal cells (Iwamoto et al., 1998; Haworth et al., 1999). A long hydrophilic C-terminal tail has recently been shown to be important for the activity of a Na+/H+ antiporter from Synechocystis sp. PCC 6803 as truncation of this tail significantly reduced antiporter activity (Hamada et al., 2001). The vacuolar Na+/H+ antiporter from S. cerevisiae, NHX1, contains a putative N-terminal regulatory domain. This region does not act as an autoinhibitor but has been suggested to be a cleavable signal peptide and is absent from the Arabidopsis homologue AtNHX1 (Darley et al., 2000). The regulation of plant Ca+/H+ antiporters by N-terminal autoinhibition as proposed in this study appears to be a novel mechanism of regulation for cation/H+ antiporters.
Growth, development, and adaptation require dynamic oscillations in cytosolic Ca2+ levels (Navazio et al., 2000; Hirschi, 2001). In this study, we demonstrate that the activity of Ca2+/H+ transporters may be modulated by changes at the N terminus of these proteins. This regulation may occur through RNA splicing, protein cleavage, or regulation by activators or suppressors binding to this regulatory domain. By further analysis of the mechanisms of CAX1 regulation, namely important components of Ca2+ ion homeostasis, the molecules that are directing the Ca2+ traffic may be identified.
MATERIALS AND METHODS
DNA Manipulation of lCAX1 cDNA
lCAX1 cDNA (EST clone, accession no. BE038949) in pBluescript (Stratagene, La Jolla, CA) was obtained from Dr. Hans Bohnert (University of Arizona, Tucson). The 5′ and 3′ ends were sequenced to confirm that the clone was full length. lCAX1 mutant variants (lCAX1-M1I and lCAX1-M37I) were produced using the type IIS restriction enzyme-based site-directed mutagenesis method (Shigaki and Hirschi, 2001). Specific point mutations were generated by PCR using the mutagenic primers lCAX1-M1I forward (5′-GAA TTC CGT CTC GAG AAA TAG CGG GAA TCG TGA CAG AG-3′), lCAX1-M1I reverse (5′-GAA TTC CGT CTC TTT CTC TAC TGA CTC AAA ACT TTG-3′), lCAX1-M37I forward (5′-GAA TTC CGT CTC ACA ACA TAT CTT CTT CTT CTT TGA GGA-3′), and lCAX1-M37I reverse (5′-GAA TTC CGT CTC TGT TGT GAG CGG TTC TTC CAA GTC-3′). All primers contained the type IIS restriction enzyme site BsmBI as underlined. Truncated variants of lCAX1 (Δ10-lCAX1, Δ20-lCAX1, Δ30-lCAX1, and Δ32-lCAX1) were generated by PCR using the forward primers Δ10-lCAX1 (5′-CGC GGA TCC ATG GTA GCT GAG AAC GGA AAC CCA-3′), Δ20-lCAX1 (5′-CGC GGA TCC ATG GCG AAA GGA TCG AGC AGA GAA-3′), Δ30-lCAX1 (5′-CGC GGA TCC ATG GGA AGA ACC GCT CAC AAC ATG-3′), Δ32-lCAX1 (5′-CGC GGA TCC ATG GCT CAC AAC ATG TCT TCT TC-3′), and the CAX1 reverse primer (5′-AAC GAG CTC TTA AGA TGA GAA AAC TCC TCC TCC TGT TGC A-3′). A BamHI site (underlined) was generated into each forward primer and a SacI site (underlined) was generated into the reverse primer. Three tandem copies of the HA epitope (YPYDVPDYA) were used to produce an in-frame fusion of HA to the 5′ end of lCAX1 by PCR, generating HA:lCAX1. The forward primer (5′-GAT TAC GCT GCT CAG TGC ATG GCG GGA ATC GTG ACA-3′) and reverse primer (5′-TGT CAC GAT TCC CGC CAT GCA CTG AGC AGC GTA ATC-3′), which both code for the last six amino acids of HA and the first six amino acids of lCAX1, were used to generate the fusion, then an HA-specific forward primer (5′-GAA TTC TCT AGA ATG GGC CGC ATC TTT TAC CCA TAC GAT-3′) and the CAX1 reverse primer were used to amplify the entire construct. An XbaI site (underlined) was generated in the HA forward primer. All PCR amplifications were performed using the high-fidelity Expand polymerase kit (Roche, Mannheim, Germany). Amplification was performed by initially heating at 94°C for 5 min, followed by 30 cycles at 94°C for 1 min, 60°C for 1 min, and 68°C for 4 min, followed by a final 10-min extension at 68°C. The mutant and epitope-tagged lCAX1 constructs were cloned into pGEM-T Easy (Promega, Madison, WI) for sequencing and propagation in Escherichia coli DH5α. All mutant and epitope-tagged lCAX1 constructs were fully sequenced to confirm the presence of the expected sequence and to check for the presence of unwanted PCR-generated mismatches. The wild-type, mutant, and epitope-tagged lCAX1 constructs were subcloned into the yeast (Saccharomyces cerevisiae) expression vector piHGpd (Nathan et al., 1999) for the expression in yeast.
Yeast Transformation, Growth, and Manipulations
The Saccharomyces cerevisiae strain K667 (MATa ade2-1 can1-100 his3-11, 15 leu2-3, and 112 trp1-1 ura3-1 cnb1::LEU2 pmc1::TRP1 vcx1Δ; Cunningham and Fink, 1996) was used. Yeast cells were transformed using the lithium acetate method and selected on synthetic complete minus His (SC-His) media (Sherman et al., 1986). For Ca2+ tolerance assays, yeast were grown at 30°C for 1 to 3 d on solid YPD medium containing 2% (w/v) Difco yeast extract, 1% (w/v) bacto-peptone, and 2% (w/v) dextrose, and supplemented with 200 mm CaCl2 (Hirschi et al., 1996). For liquid Ca2+ tolerance assays, yeast strains were grown to OD600 of 1.0 in SC-His medium at 30°C, then inoculated into YPD medium supplemented with 100 to 200 mm CaCl2, and finally grown for 16 h, shaking at 30°C, in 24-well flat-bottomed plates. Cell growth was then determined by OD600 measurements.
RT-PCR
Total RNA was extracted from yeast using the acid phenol extraction procedure as described by Ausubel et al. (1998). RT-PCR was performed by standard methods using a specific forward primer against lCAX1 (5′-TCT CAG AAT TTA CAA AGT TTT GAG TCA-3′) and the CAX1 reverse primer (see above). First-strand cDNA was produced using the Superscript II reverse transcription kit (Gibco-BRL, Gaithersburg, MD), then PCR was performed as described above.
Membrane Fractionation and Western Analysis of HA-Tagged lCAX1
Microsomal membranes were prepared from yeast expressing HA:lCAX1-piHGpd and HA:SCAX1-piHGpd, essentially as described by Hwang et al. (2000a), with a few modifications. Transformants were inoculated into 500 mL of SC-His and grown to stationary phase. The cells were pelleted by centrifugation at 4,000g for 5 min, then washed with 10 mL of ice-cold water, and finally resuspended in 10 mL of glass bead buffer {10% [w/v] Suc, 20 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.5, 2 mm EGTA, 1 mm MgSO4, and 1 mm dithiothreitol [DTT]}. The washed cells were resuspended in 2 mL of glass bead buffer with 0.2 mm phenylmethylsulfonyl fluoride (PMSF), 5 mm benzamidine, 1 μg mL−1 leupeptin, and 5 μg mL−1 pepstatin. To break cells, an equal volume of dry glass beads was added to cells and vortexed for 3 min (30 s × six times) at 4°C. The samples were centrifuged at 5,000g for 5 min and the supernatants were collected. The process was repeated three times and the supernatants were combined. For Suc gradient fractionation, 1 mL of supernatants from the broken yeast cells was layered onto a 15% to 50% (w/w) continuous Suc gradient and centrifuged at 25,000g at 4°C for 16 h. Membrane fractions were stored at −80°C. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
Immunoblots were performed and the HA epitope was detected essentially as described previously (Hirschi et al., 2001). Protein fractions were separated by SDS-PAGE on a 12% (w/v) gel and transferred to polyvinylidene fluoride membrane (Pall Gelman, Ann Arbor, MI). The blots were blocked in 5% (w/v) nonfat dried milk in phosphate-buffered saline with Tween (PBS-T; 10 mm NaH2PO4/NaOH, pH 7.2, and 150 mm NaCl with 0.1% [v/v] Tween 20) for 1 h, and then reacted with a 1:3,000 dilution of anti-HA monoclonal primary antibody (Berkeley Antibody Co., Richmond, CA) in PBS-T for 1 h at room temperature. The vacuolar membrane marker antibody ALP against the yeast vacuolar ALP (Molecular Probes, Eugene, OR) was used at a 1:250 dilution. The blots were washed in PBS-T before incubating for 1 h in PBS-T containing a 1:10,000 dilution of horseradish peroxidase-coupled anti-mouse secondary antibody (Amersham, Little Chalfont, UK). The blots were then washed in PBS-T. ECL Plus reagents (Amersham) were used to develop the blots, which were then exposed to Hyperfilm photographic film (Amersham).
Preparation of Endomembrane Vesicles
Endomembrane vesicles were prepared as previously described (Nakanishi et al., 2001), with a few modifications. Transformants were grown in 50 mL of SC-His media at 30°C for 2 d, then inoculated into 1 L of YPD medium. The cells were pelleted by centrifugation at 4,000g for 5 min, then washed with 0.1 m Tris-HCl, pH 9.4, 50 mm 2-mercaptoethanol, and 0.1 m Glc at 30°C for 10 min. Spheroplasts were produced by incubating the cells at 30°C for 1 h in 0.05% (w/v) Zymolyase 20T (Seikagaku Kogyo, Tokyo), 0.9 m sorbitol, 0.1 m Glc, 50 mm Tris-MES [2-(N-morpholino)-ethanesulfonic acid], pH 7.6, 5 mm DTT, 0.043% (w/v) YPD, and 0.25× dropout mix (Sherman et al., 1986). The suspension was centrifuged at 3,000g for 10 min and washed with 1 m sorbitol. Spheroplasts were resuspended in 50 mm Tris-ascorbate, pH 7.6, 1.1 m glycerol, 1.5% (w/v) polyvinylpyrrolidone 40,000, 5 mm EGTA, 1 mm DTT, 0.2% (w/v) bovine serum albumin, 1 mm PMSF, and 1 mg L−1 leupeptin and homogenized with a glass homogenizer (Wheaton Science Products, Millville, NJ). The homogenate was centrifugated at 2,000g for 10 min and the supernatant was then centrifuged at 120,000g for 30 min. The microsomal pellet was resuspended in 15% (w/w) Suc solution (containing 10 mm Tris-MES, pH 7.6, 1 mm EGTA, 2 mm DTT, 25 mm KCl, 1.1 m glycerol, 0.2% [w/v] bovine serum albumin, 1 mm PMSF, and 1 mg L−1 leupeptin) and layered onto a 35% (w/w) Suc solution, then centrifuged at 150,000g for 30 min. Endomembrane-enriched vesicles were collected at the interface and diluted in 5 mm Tris-MES, pH 7.6, 0.3 m sorbitol, 1 mm DTT, 1 mm EGTA, 0.1 m KCl, 1 mm PMSF, 1 mg L−1 leupeptin, and 5 mm MgCl2. The membranes were centrifuged at 150,000g for 30 min and resuspended in 5 mm Tris-MES, pH 7.6, 0.3 m sorbitol, 1 mm DTT, 1 mm PMSF, and 1 mg L−1 leupeptin. The membrane vesicles were stored at −80°C until use.
Ca2+ Transport Assay
Time-dependent 45Ca2+/H+ transport into endomembrane vesicles was measured using the filtration method (Hwang et al., 1997). Membrane vesicles (30–40 μg mL−1) were incubated in a reaction mixture containing 200 mm Suc, 25 mm HEPES-bis(tris[hydroxymethyl]methylamino) propane (pH 7.5), 50 mm KCl, 0.1 mm NaN3, and 0.2 mm Na orthovanadate. Vacuolar H+-translocating ATPase-catalyzed H+ transport was initiated by the addition of 1 mm MgSO4 and 1 mm ATP. The vesicles were allowed to reach steady state with respect to the pH gradient for 5 min at 25°C. 45Ca2+ uptake was initiated by the addition of 1 to 100 μm 45Ca2+ (6 mCi mL−1; American Radiolabeled Chemicals, St. Louis). At the times indicated, 70-μL aliquots of the reaction mix were removed and filtered through premoistened 0.45 μm pore-size cellulose acetate GS type filters (Millipore, Bedford, MA) and washed with 2 mL of ice-cold wash buffer (250 mm Suc, 2.5 mm HEPES-bis(tris[hydroxymethyl]methylamino) propane, pH 7.5, and 0.2 mm CaCl2). The filters were air dried and radioactivity was determined by liquid scintillation counting. The ΔpH-dependent component of 45Ca2+ uptake was determined in the presence of 5 μm gramicidin. For some experiments, 45Ca2+/H+ transport was measured in the presence of 0.5 to 5 μm bovine brain calmodulin (Sigma, St. Louis).
ACKNOWLEDGMENTS
We are grateful to Ning-hui Cheng, Toshiro Shigaki, and Coimbatore Sreevidya for critical reading of this manuscript. We are thankful to Heven Sze and Zhongyi Wu for helping us establish the Ca2+ transport assay in our lab and to Heven Sze for useful comments. We are also very grateful to Toshiro Shigaki for the HA:sCAX1-piHGpd plasmid.
Footnotes
This work was supported by the U.S. Department of Agriculture/Agricultural Research Service (under Cooperative Agreement No. 58–6250–6001) and by the National Institutes of Health (grant nos. CHRC 5 P30 and 1R01 GM57427).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010409.
LITERATURE CITED
- Askerlund P. Modulation of an intracellular calmodulin-stimulated Ca2+-pumping ATPase in cauliflower by trypsin: the use of Calcium Green-5N to measure Ca2+ transport in membrane vesicles. Plant Physiol. 1996;110:913–922. doi: 10.1104/pp.110.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates/Wiley Interscience; 1998. [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ. Kinetics of Ca2+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris L. Plant Physiol. 1986;80:727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Chung WS, Lee SH, Kim JC, Heo WD, Kim MC, Park CY, Park HC, Lim CO, Kim WB, Harper JF. Identification of a calmodulin-regulated soybean Ca2+-ATPase (SCA1) that is located in the plasma membrane. Plant Cell. 2000;12:1393–1407. doi: 10.1105/tpc.12.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran AC, Hwang I, Corbin J, Matinez S, Rayle D, Sze H, Harper JF. Autoinhibition of a calmodulin-dependent calcium pump involves a structure in the stalk that connects the transmembrane domain to the ATPase catalytic domain. J Biol Chem. 2000;275:30301–30308. doi: 10.1074/jbc.M002047200. [DOI] [PubMed] [Google Scholar]
- Darley CP, van Wuytswinkel OCM, van der Woude K, Mager WH, De Boer AH. Arabidopsis thaliana and Saccharomyces cerevisiae NHX1 genes encode amiloride sensitive electroneutral Na+/H+ exchangers. Biochem J. 2000;351:241–249. doi: 10.1042/0264-6021:3510241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A, Verma AK, Filoteo AG, Penniston JT. Protein kinase C activates the plasma membrane Ca2+ pump isoform 4b by phosphorylation of an inhibitory region downstream of the calmodulin-binding domain. J Biol Chem. 1996;271:32461–32467. doi: 10.1074/jbc.271.50.32461. [DOI] [PubMed] [Google Scholar]
- Geisler M, Frangne N, Gomes E, Martinoia E, Palmgren MG. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000;124:1814–1827. doi: 10.1104/pp.124.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada A, Hibino T, Nakamura T, Takabe T. Na+/H+ antiporter from Synechocystis species PCC 6803, homologous to SOS1, contains an aspartic residue and long C-terminal tail important for the carrier activity. Plant Physiol. 2001;125:437–446. doi: 10.1104/pp.125.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang JF, Palmgren MG, Sze H. A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J Biol Chem. 1998;273:1099–1106. doi: 10.1074/jbc.273.2.1099. [DOI] [PubMed] [Google Scholar]
- Haworth RS, Sinnett-Smith J, Rozengurt E, Avkiran M. Protein kinase D inhibits plasma membrane Na+/H+ exchanger activity. Am J Physiol. 1999;277:C1202–C1209. doi: 10.1152/ajpcell.1999.277.6.C1202. [DOI] [PubMed] [Google Scholar]
- Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD. Vacuolar H+/Ca2+ transport: who's directing the traffic? Trends Plant Sci. 2001;6:100–104. doi: 10.1016/s1360-1385(00)01863-x. [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ. Expression of Arabidopsis CAX2 in tobacco: altered metal accumulation and increased manganese tolerance. Plant Physiol. 2000;124:125–134. doi: 10.1104/pp.124.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Miranda ML, Wilganowski NL. Phenotypic changes in Arabidopsis caused by expression of a yeast Ca2+/H+ antiporter. Plant Mol Biol. 2001;46:57–65. doi: 10.1023/a:1010620227913. [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Zhen R-G, Cunningham KW, Rea PA, Fink GR. CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Harper JF, Liang F, Sze H. Calmodulin activation of an endoplasmic reticulum-located calcium pump involves an interaction with the N-terminal autoinhibitory domain. Plant Physiol. 2000a;122:157–168. doi: 10.1104/pp.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Ratterman DM, Sze H. Distinction between endoplasmic reticulum-type and plasma membrane-type Ca2+ pumps. Plant Physiol. 1997;113:535–548. doi: 10.1104/pp.113.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sze H, Harper JF. A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc Natl Acad Sci USA. 2000b;97:6224–6229. doi: 10.1073/pnas.97.11.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Pan Y, Nakamura TY, Wakabayashi S, Shigekawa M. Protein kinase C-dependent regulation of Na+/Ca2+ exchanger isoforms NCX1 and NCX3 does not require their direct phosphorylation. Biochemistry. 1998;37:17230–17238. doi: 10.1021/bi981521q. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Hetherington AM. Encoding specificity in Ca2− signaling systems. Trends Plant Sci. 1998;3:32–36. [Google Scholar]
- Malmström S, Åkerlund H-E, Askerlund P. Regulatory role of the N-terminus of the vacuolar Ca2+-ATPase in cauliflower. Plant Physiol. 2000;122:517–526. doi: 10.1104/pp.122.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi KD, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D. Phylogenetic relationships within cation-transporter families of Arabidopsis thaliana. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Saijo T, Wada Y, Maeshima M. Mutagenic analysis of functional residues in putative substrate-binding site and acidic domains of vacuolar H+-pyrophosphatase. J Biol Chem. 2001;276:7654–7660. doi: 10.1074/jbc.M009743200. [DOI] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. Identification of SSF1, and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc Natl Acad Sci USA. 1999;96:1409–1414. doi: 10.1073/pnas.96.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc Natl Acad Sci USA. 2000;97:8693–8698. doi: 10.1073/pnas.140217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:817–845. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- Penniston JT, Enyedi A. Modulation of the plasma membrane Ca2+ pump. J Membr Biol. 1998;165:101–109. doi: 10.1007/s002329900424. [DOI] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker KS, Sze H. Ca2+/H+ antiport system driven by the proton electrochemical gradient of a tonoplast H+-ATPase from oat roots. Plant Physiol. 1985;79:1111–1117. doi: 10.1104/pp.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- Shigaki T, Hirschi KD. Characterization of CAX-like genes in plants: implications for functional diversity. Gene. 2000;257:291–298. doi: 10.1016/s0378-1119(00)00390-5. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi KD (2001) Use of class IIS restriction enzymes for site directed mutagenesis: variations on Phoenix mutagenesis. Anal Biochem (in press) [DOI] [PubMed]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- Tomas FM, Walton PE, Dunshea FR, Ballard FJ. IGF-I variants which bind poorly to IGF-binding proteins show more potent and prolonged hypoglycaemic action than native IGF-I in pigs and marmoset monkeys. J Endocrinol. 1997;155:377–386. doi: 10.1677/joe.0.1550377. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Nakanishi Y, Tanaka Y, Maeshima M. Properties and molecular cloning of a Ca2+/H+ antiporter in the vacuolar membrane of mung bean. Eur J Biochem. 1999;262:417–425. doi: 10.1046/j.1432-1327.1999.00377.x. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Tsuchiya T, Sasaki M, Nakanishi Y, Cunningham KW, Maeshima M. Functional expression of mung bean Ca2+/H+ antiporter in yeast and its intracellular localization in the hypocotyl and tobacco cells. Eur J Biochem. 2000;267:3090–3098. doi: 10.1046/j.1432-1033.2000.01343.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S, Ikeda T, Iwamoto T, Pouyssegur J, Shigekawa M. Calmodulin-binding autoinhibitory domain controls “pH-sensing” in the Na+/H+ exchanger NHE1 through sequence-specific interaction. Biochemistry. 1997;36:12854–12861. doi: 10.1021/bi9715472. [DOI] [PubMed] [Google Scholar]