Abstract
Heat stress inhibits photosynthesis by reducing the activation of Rubisco by Rubisco activase. To determine if loss of activase function is caused by protein denaturation, the thermal stability of activase was examined in vitro and in vivo and compared with the stabilities of two other soluble chloroplast proteins. Isolated activase exhibited a temperature optimum for ATP hydrolysis of 44°C compared with ≥60°C for carboxylation by Rubisco. Light scattering showed that unfolding/aggregation occurred at 45°C and 37°C for activase in the presence and absence of ATPγS, respectively, and at 65°C for Rubisco. Addition of chemically denatured rhodanese to heat-treated activase trapped partially folded activase in an insoluble complex at treatment temperatures that were similar to those that caused increased light scattering and loss of activity. To examine thermal stability in vivo, heat-treated tobacco (Nicotiana rustica cv Pulmila) protoplasts and chloroplasts were lysed with detergent in the presence of rhodanese and the amount of target protein that aggregated was determined by immunoblotting. The results of these experiments showed that thermal denaturation of activase in vivo occurred at temperatures similar to those that denatured isolated activase and far below those required to denature Rubisco or phosphoribulokinase. Edman degradation analysis of aggregated proteins from tobacco and pea (Pisum sativum cv “Little Marvel”) chloroplasts showed that activase was the major protein that denatured in response to heat stress. Thus, loss of activase activity during heat stress is caused by an exceptional sensitivity of the protein to thermal denaturation and is responsible, in part, for deactivation of Rubisco.
Photosynthesis is sensitive to inhibition by moderate heat stress (Berry and Björkman, 1980). Inhibition of photosynthesis at high temperature is often attributed to inactivation of membrane-associated proteins, particularly the oxygen-evolving complex of photosystem II (Havaux, 1993; Heckathorn et al., 1998; Murakami et al., 2000). However, measurements of chlorophyll fluorescence in intact leaves have shown that, although the light reactions of photosynthesis are certainly disrupted at very high temperatures (Weis and Berry, 1988; Havaux, 1993; Pastenes and Horton, 1996), photosynthetic electron transport continues to function uninterruptedly at the moderately high temperatures that inhibit CO2 fixation (Weis, 1981a; Law and Crafts-Brandner, 1999; Crafts-Brandner and Law, 2000). Of the various reactions associated with CO2 fixation, the one that appears to be most sensitive to inhibition by heat is the activation of Rubisco by Rubisco activase (Crafts-Brandner and Salvucci, 2000).
Rubisco activase is an AAA+ (ATPases associated with a variety of cellular activities) protein (Neuwald et al., 1999) that facilitates the ATP-dependent removal of sugar phosphates from Rubisco active sites (Portis, 1995; Salvucci and Ogren, 1996). This action frees the active site of Rubisco for spontaneous carbamylation by CO2 and metal binding, prerequisites for activity. We previously showed that the rate of Rubisco deactivation (i.e. decarbamylation and sugar phosphate binding) increases at high temperature, exceeding the ability of activase to maintain Rubisco in an active state (Crafts-Brandner and Salvucci, 2000). These effects were attributed to the relatively low temperature optimum of activase, its marked thermal lability, and possibly to perturbation of protein-protein interactions between Rubisco and activase.
In a study with wheat and cotton leaves, Feller et al. (1998) showed that activase associates into large molecular mass complexes and forms insoluble aggregates at temperatures that inhibit Rubisco activation but not photosynthetic electron transport. Rokka et al. (2001) reported more recently that activase associates with the thylakoid membrane in heat-stressed spinach (Spinacia oleracea) due to changes in the conformation of activase. These effects are indicative of a change in activase structure and suggest that inhibition of activase activity by moderate heat stress was caused by an exceptional sensitivity of activase to thermal denaturation. To determine if thermal denaturation of activase is a causative factor in heat inhibition of photosynthesis, we examined the thermal stability of activase in vitro and in intact protoplasts and chloroplasts and compared activase with two other soluble stromal proteins, Rubisco and phosphoribulokinase (i.e. ribulose-5-P kinase). Rubisco catalyzes the carboxylation reaction and is the enzyme that is modulated by activase via a physical interaction (Wang et al., 1992). Phosphoribulokinase catalyzes the synthesis of ribulose 1,5-bisphosphate, the 5C sugar-phosphate substrate for CO2 fixation (for review, see Miziorko, 2000). Like activase, phosphoribulokinase is a nuclear-encoded chloroplast stromal protein that requires ATP for activity. The results show that activase is extraordinarily sensitive to thermal denaturation both in vitro and in vivo. The exceptional sensitivity of activase to thermal denaturation is responsible, in part, for inhibition of Rubisco activation at high temperature.
RESULTS
Temperature Response of Rubisco and Activase Activities
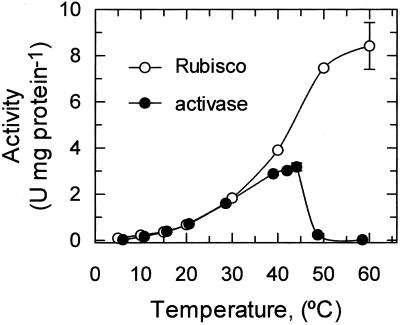
The temperature response of Rubisco and activase were examined previously over the range of temperatures from 25°C to 50°C (Crafts-Brandner and Salvucci, 2000). To compare the thermal stability of these enzymes, we extended the temperature range and directly compared the specific activities of the two enzymes at each temperature (Fig. 1). The rate of carboxylation by isolated Rubisco increased with increasing temperature from 5°C to 60°C. The optimum temperature for activity was at least 60°C and perhaps even higher. However, measurements of Rubisco activity were not conducted at temperatures higher than 60°C because decreases in both the solubility of CO2 and the affinity of Rubisco for CO2 with temperature (Jordan and Ogren, 1984) make it difficult to provide saturating concentrations of CO2 for activity and carbamylation at very high temperatures.
Figure 1.
The effect of temperature on the activities of Rubisco and activase. The carboxylase activity of purified tobacco Rubisco (○) and the ATPase activity of purified, recombinant tobacco activase (●) were measured separately at the indicated temperatures.
In contrast to the response of Rubisco activity, ATP hydrolysis by activase exhibited a well-defined temperature optimum of about 44°C (Fig. 1). ATPase activity increased with increasing temperature from 5°C to 44°C, but decreased abruptly at temperatures exceeding 45°C. The specific activities of carboxylation by Rubisco and ATP hydrolysis by activase coincidentally were almost identical through the range of temperatures from 5°C to 30°C. This relationship was not maintained at temperatures higher than about 30°C because of the marked difference in temperature optima between activase and Rubisco.
Thermal Unfolding/Aggregation of Activase and Rubisco Measured by Light Scattering
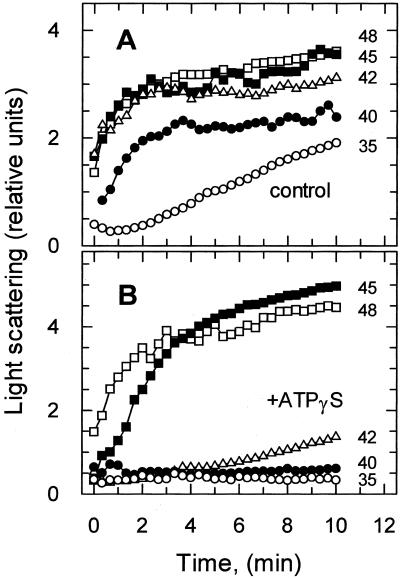
Light scattering was used to monitor thermal unfolding/aggregation of isolated activase and Rubisco. Time course experiments showed that light scattering increased markedly when activase was incubated at elevated temperatures (Fig. 2). The precise temperature at which this increase occurred was affected by the presence of the non-hydrolyzable substrate analog, ATPγS. In the absence of ATPγS, light scattering by activase increased markedly over the 10-min time course at temperatures of 35°C and higher. In the presence of ATPγS, light scattering by activase increased over a similar time course, but only when temperatures exceeded 42°C.
Figure 2.
Kinetics of activase unfolding/aggregation at elevated temperature. At time zero, purified activase was added to assays at 35°C (○), 40°C (●), 42°C (▵), 45°C (▪), and 48°C (□) in the absence (control) and presence (+ATPγS) of 0.75 mm ATPγS. Light scattering was measured over a 10-min time course.
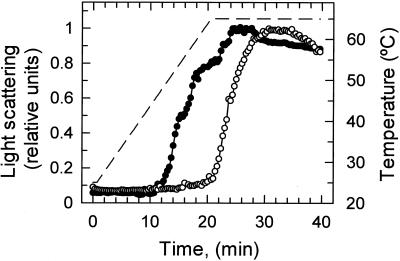
When experiments similar to those described above were conducted with Rubisco, light scattering remained at a constant low level at temperatures that caused marked increases in activase light scattering (data not shown). To compare thermal unfolding/aggregation of Rubisco and activase directly, light scattering was measured for each of these proteins during a time course in which temperature was increased continuously from 25°C to 65°C over 20 min and then held constant at 65°C for an additional 20 min (Fig. 3). In the presence of ATPγS, light scattering by activase increased early in the time course when temperatures were about 47°C. In similar experiments conducted in the absence of ATPγS, light scattering by activase increased even earlier in the time course when temperatures were about 40°C (data not shown). Light scattering by Rubisco also increased markedly when the protein was heated, but the increase did not occur until late in the time course, several minutes after the temperature had reached the maximum of 65°C.
Figure 3.
Time course of activase and Rubisco unfolding/aggregation during an increase in temperature. At time zero, purified activase (●) or Rubisco (○) were added to assays at 25°C. Over the next 20 min, the temperature of the assays (−) was increased to 65°C and then held constant at this temperature for an additional 20 min. Light scattering was measured over the entire time course. Light scattering by activase was measured in the presence of 0.75 mm ATPγS.
Thermal Unfolding of Isolated Activase and Rubisco Measured by Aggregation with Rhodanese
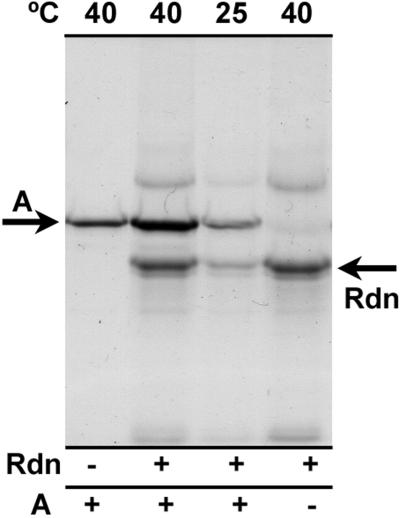
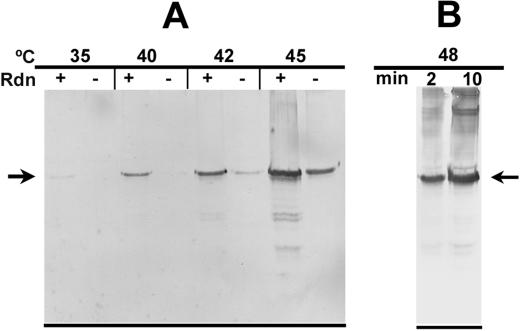
Because of its propensity to associate with partially folded proteins, rhodanese aggregation has been used as a way to improve detection of thermal unfolding of proteins either in the isolated state or as components of crude mixtures from cells (Kim et al., 1992). In the present study, a rhodanese-trapping assay was used to monitor the extent of thermal unfolding that occurred for purified activase (Fig. 4). After incubation at 40°C in the absence of ATPγS, activase aggregated in a complex that could be recovered by brief centrifugation. Addition of rhodanese immediately following heat treatment of activase greatly increased the amount of activase that aggregated as an insoluble complex. The amount of activase recovered in the insoluble aggregate was much lower at 25°C (Fig. 4) and was unaffected by the addition of rhodanese (data not shown). Thus, chemically denatured rhodanese trapped thermally unfolded activase in a heterologous complex that was recoverable by centrifugation.
Figure 4.
Enhanced aggregation of purified activase after thermal denaturation and trapping with rhodanese. Purified activase was incubated at the indicated temperatures in the presence of 0.75 mm ATPγS. After 10 min, chemically denatured rhodanese or guanidine-HCl alone was added to the reactions and the reactions were incubated for 5 min at 23°C. Aggregated protein was collected by centrifugation, separated by SDS-PAGE, and visualized by staining with Coomassie Brilliant Blue. The arrows labeled A and Rdn indicate the positions of activase (42 kD) and rhodanese (33.3 kD), respectively, on the gel.
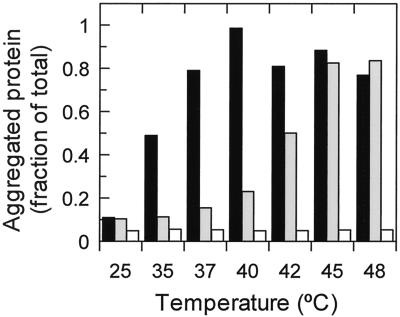
The rhodanese-trapping assay was used in a quantitative way to determine the effect of temperature and ATPγS on activase unfolding (Fig. 5). In the presence of ATPγS, rhodanese aggregated with activase when added to activase that had been incubated at temperatures higher than about 40°C (Fig. 5). Aggregation with rhodanese occurred at much lower temperatures in the absence of ATPγS. For example, 50% of the activase aggregated with rhodanese after incubation at 35°C in the absence of ATPγS and 42°C in the presence of ATPγS. Aggregation of heat-treated Rubisco was minimal below 50°C (Fig. 5) and even at temperatures as high as 60°C (data not shown). However, after 10 min at 65°C, 100% of the Rubisco aggregated as an insoluble complex both in the presence and absence of rhodanese (data not shown).
Figure 5.
Effect of temperature and ATPγS on rhodanese trapping of heat-treated activase and Rubisco. Purified activase (black and shaded bars) or purified Rubisco (white bars) were incubated at the indicated temperatures in the absence (black and white bars) and presence (shaded bars) of 0.75 mm ATPγS. After 10 min, chemically denatured rhodanese was added to the reactions and the reactions were incubated for 5 min at 23°C. Aggregated protein was collected by centrifugation, separated by SDS-PAGE and visualized by staining with Coomassie Brilliant Blue. The amount of activase and Rubisco that aggregated was determined by densitometry using known amounts of the purified proteins as standards.
Thermal Unfolding of Activase, Phosphoribulokinase, and Rubisco in Vivo
The rhodanese-trapping assay was modified for estimating thermal unfolding in vivo. For these experiments, intact tobacco (Nicotiana rustica cv Pulmila) protoplasts were first exposed to various temperatures in the light and then instantaneously lysed with detergent in the presence of rhodanese. The extent of unfolding that occurred for specific proteins was indicated by the amount of target protein recovered in the insoluble aggregate as measured by immunoblot analysis. To minimize protein unfolding after lysis (i.e. during incubation with rhodanese), protoplasts were lysed in prechilled tubes and the tubes placed at a constant temperature of 23°C. This procedure cooled the solutions during lysis and incubation with rhodanese (see “Materials and Methods”). Also, the use of the detergent, Triton X-100, caused rapid lysis of the chloroplast and protoplast and eliminated interactions between soluble proteins and membranes. Control experiments showed that over 95% of the protoplasts were intact prior to lysis based on the amount of soluble protein (i.e. 3.7%–5.3% of the total soluble protein) and activase (below detection) present in the supernatant after the heat treatment. The amount of soluble protein released from protoplasts incubated at 48°C was similar to the amount released from protoplasts at 25°C (data not shown), indicating that the protoplasts remained intact during the heat treatment.
After incubation of intact protoplasts in the light, the amount of activase that precipitated following lysis of the protoplasts increased with increasing incubation temperature (Fig. 6). At the higher temperatures, a portion of the activase aggregated as an insoluble complex even in the absence of rhodanese. However, a much greater amount of activase was present in the pellet fractions when rhodanese was present during lysis. That rhodanese increased the amount of activase that precipitated suggests that rhodanese trapped partially folded activase that would have otherwise remained soluble. The amount of activase that precipitated with rhodanese was at the limit of detection when the temperature used for incubation of the protoplasts was 35°C. In contrast, the amount of activase associated with rhodanese increased markedly when the protoplast incubation temperature was 45°C.
Figure 6.
Enhanced aggregation of thermally denatured activase from heat-treated tobacco protoplasts by trapping with rhodanese. A, Intact protoplasts were incubated for 10 min at the indicated temperatures and then lysed in detergent in the presence (+) and absence (−) of rhodanese. B, Intact protoplasts were incubated for 2 and 10 min at 48°C and then lysed with detergent in the presence of rhodanese. After 5 min at 23°C, aggregated protein was collected by centrifugation, separated by SDS-PAGE, and the activase polypeptides were visualized by immunoblotting. The arrows on the blot indicate the position of the 42-kD activase polypeptide.
As described above, protoplast solutions were cooled during cell lysis and rhodanese incubation to minimize thermal unfolding after release of the proteins. Thus, it seemed likely that most, if not all, of the protein unfolding was occurring during the 10-min incubation of the intact protoplasts rather than during the brief interval of high temperature immediately following lysis of the protoplast. To address this issue experimentally, protoplasts were incubated at 48°C for 2 and 10 min prior to lysis with detergent in the presence of rhodanese. Two minutes was chosen for this experiment because it was the minimum time required for the protoplast incubation solution to reach 48°C. Immunoblot analysis of the aggregated proteins showed that a much greater amount of activase was associated with rhodanese after the longer incubation time (Fig. 6B). These results indicated that thermal unfolding was occurring primarily in the intact protoplasts rather than after lysis.
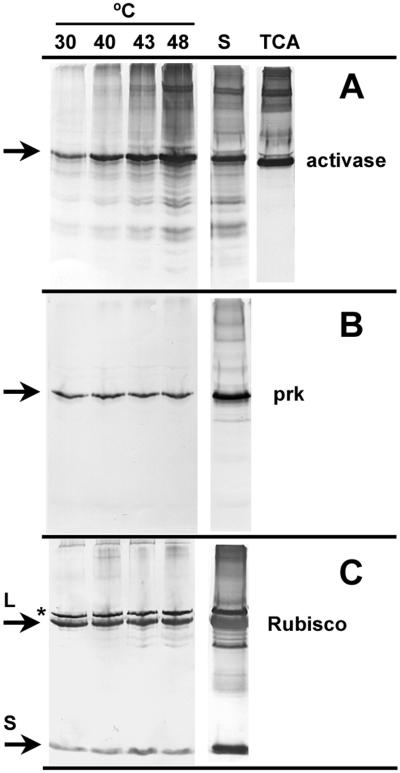
Rhodanese-trapping and immunoblot analysis were used to compare the thermal stability of activase directly with the thermal stabilities of two other chloroplast proteins involved in CO2 fixation (Fig. 7). As shown above, the amount of activase that aggregated in an insoluble complex increased markedly with increasing temperature (Fig. 7A). In contrast, immunoblot analysis of aliquots of the aggregates showed that the amounts of phosphoribulokinase and Rubisco that aggregated with rhodanese was low and relatively unaffected by incubation temperature from 30°C to 48°C (Fig. 7, B and C). It is interesting that proteolytic products and higher molecular mass species of activase were present in the insoluble aggregate, particularly at the two highest temperatures. In contrast, proteolytic products of Rubisco were observed primarily in the soluble fraction.
Figure 7.
Effect of temperature on thermal aggregation of activase, phosphoribulokinase, and Rubisco from heat-treated tobacco protoplasts. Intact protoplasts were incubated for 10 min at the indicated temperatures and then lysed with detergent in the presence of rhodanese. After 5 min at 23°C, aggregated protein was collected by centrifugation and separated by SDS-PAGE. Proteins in the soluble portion of the extract from the 48°C incubation (S) and in protoplasts that were incubated at 48°C and then lysed directly in TCA were also separated by SDS-PAGE after diluting 3-fold. Individual polypeptides were visualized by immunoblotting using antibodies to activase (A), phosphoribulokinase (prk, B), and Rubisco (C). The arrows indicate the position of activase (42 kD), phosphoribulokinase (43 kD), and the large (L, 53 kD) and small (S, 14 kD) subunits of Rubisco on the blots. The polypeptide labeled with an asterisk is an unidentified chloroplast protein that cross-reacts with Rubisco antibodies.
Immunoblot analysis of the soluble extracts, diluted 3-fold compared with the insoluble aggregate, showed that the amount of phosphoribulokinase and Rubisco that precipitated in the rhodanese aggregate was relatively low compared with the amount that remained soluble in the protoplast lysate (Fig. 7). A similar analysis with activase was difficult to interpret because of extensive proteolysis of activase in the soluble protoplast extract. However, the amount of activase in the total protoplast lysate could be determined without interference from proteolysis by lysing protoplasts in 10% (w/v) trichloroacetic acid (TCA). Comparison of the relative amounts of activase in the insoluble aggregate with the total amount present in the entire extract showed that unfolded activase represented greater than 30% of the total activase in the protoplasts at the two highest temperatures. Extraction of protoplasts in TCA also showed that proteolytic degradation of activase occurred after lysis and not in the intact protoplast. In contrast, high molecular mass species of activase were present in the TCA lysates, an indication that they were formed in the intact protoplast, i.e. prior to lysis and incubation with rhodanese.
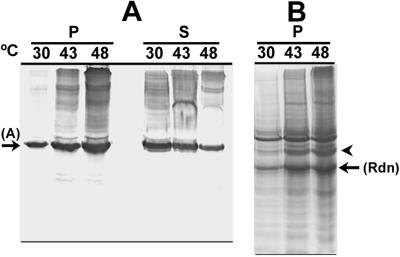
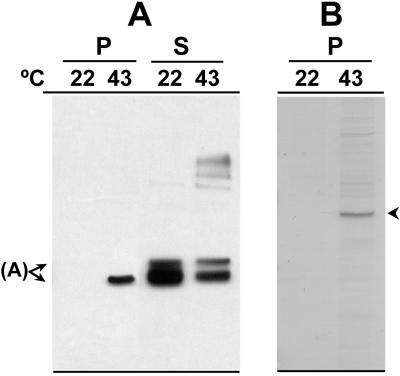
Thermal unfolding of activase also occurred when intact tobacco chloroplasts were heated (Fig. 8). Compared with 30°C, the amount of activase associated with rhodanese as an insoluble aggregate increased markedly at 43°C and 48°C. When compared with the amount of activase that remained in solution, the amount that aggregated represented a considerable portion of the total activase in the chloroplasts. In contrast, the relative amounts of phosphoribulokinase and Rubisco present in the insoluble aggregate were similar at 30°C and 48°C and represented less than 10% of the total amount of these proteins in chloroplasts (data not shown). The insoluble aggregate that formed with rhodanese after incubation of intact chloroplasts at 43°C and 48°C also contained high molecular mass species of activase.
Figure 8.
Effect of temperature on thermal aggregation of activase in tobacco chloroplasts. Intact tobacco chloroplasts were incubated for 10 min at the indicated temperatures and then lysed with detergent in the presence of rhodanese. After 5 min at 23°C, aggregated protein was collected by centrifugation and separated by SDS-PAGE. Proteins in the soluble portion of the extracts were also separated by SDS-PAGE after diluting 3-fold. A, Activase in the aggregates (P) and in the soluble fractions (S) was visualized by immunoblotting. B, Total proteins in the aggregates were visualized by staining with Coomassie Brilliant Blue. The arrows labeled A and Rdn indicate the position of rhodanese (33.3 kD) and activase (42 kD) on the blots. The 42-kD polypeptide that was submitted for sequencing is indicated by the arrowhead.
Measurement of soluble protein and activase in the supernatant following re-isolation of chloroplasts showed that heating for 10 min decreased the integrity of the chloroplasts. The amount of soluble protein released increased from 6.7% at 25°C to 13.6 and 17.9% at 43°C and 48°C, respectively. The amount of activase released was 8% at 25°C and 10% at 43°C and 48°C, far less than the amount that aggregated at these temperatures (Fig. 8). Thus, thermal denaturation of activase occurred primarily inside the chloroplast because most of the chloroplasts were intact and only a minor amount of activase was present in the media.
Proteolytic degradation of activase was minimal in chloroplast compared with protoplast lysates. As a consequence, it was possible to determine the relative abundance of proteins in the rhodanese aggregates from heat-treated tobacco chloroplasts. Staining of protein blots with Coomassie blue showed that a 42-kD polypeptide was the major rhodanese-trapped protein that increased in abundance in the pellet fraction after incubating chloroplasts at elevated temperature (Fig. 8B). This polypeptide comigrated with activase on protein blots (Fig. 8A). To determine if the stained band corresponded to activase, the section of the blot containing the stained 42-kD polypeptide was sequenced by Edman degradation. The sequence of this polypeptide, EEKDADPKKQTDG/SR, matched the sequence of the N terminus of tobacco activase (Wang et al., 1992).
Thermal Aggregation of Activase in Pea (Pisum sativum cv “Little Marvel”) Chloroplasts
In a previous study, Osteryoung and Vierling (1994) reported that a 41-kD polypeptide was the major in vitro translation product of poly(A+) RNA that redistributed from the soluble to the pellet fraction in heat-treated pea chloroplasts. The similarity of this result with those for tobacco chloroplasts prompted us to determine if the thermally labile 41-kD polypeptide in pea chloroplasts was activase. For these experiments, activase aggregation was assayed by incubating isolated chloroplasts at various temperatures and then examining the distribution of the endogenous activase polypeptides in soluble and pellet fractions following detergent extraction, i.e. the identical conditions used previously (Osteryoung and Vierling, 1994). These conditions did not include rhodanese trapping.
In contrast with tobacco, in which only a single form of activase was detected, immunoblot analysis of chloroplast extracts from pea revealed the presence of two activase polypeptides of approximately 41 and 44 kD (Fig. 9A; see also Salvucci et al., 1987). After treatment of the chloroplasts at 22°C, both forms of activase remained associated with the soluble fraction (Fig. 9A). After 10 min at 43°C, all of the larger activase polypeptide was recovered in the soluble fraction, but a significant proportion of the smaller form was detected as an insoluble aggregate in the pellet. In addition, several high-molecular mass species that reacted with the activase antibodies were present in the soluble fraction at 43°C but not at 22°C. A pattern of activase distribution similar to that observed at 43°C also occurred in isolated pea chloroplasts treated at 38°C, and in whole plants subjected to abrupt heat stress (data not shown). These results suggest that activase behavior in isolated chloroplasts reflects the in planta condition.
Figure 9.
Effect of temperature on thermal aggregation of activase in pea chloroplasts. Intact pea chloroplasts were incubated for 10 min at the indicated temperatures and then lysed with detergent. Aggregated protein was collected by centrifugation and separated by SDS-PAGE. Proteins in the soluble portion of the extracts were also separated by SDS-PAGE. A, Activase in the aggregates (P) and in the soluble fractions (S) were visualized by immunoblotting. The split arrows labeled A indicate the position of 44- and 41-kD activase polypeptides. B, Total proteins in the aggregates were visualized by staining with Coomassie Brilliant Blue. The 41-kD polypeptide that was submitted for sequencing is indicated by the arrowhead.
Coomassie Blue staining of the pellet fractions from pea chloroplasts revealed a significant increase in the presence of an insoluble, 41-kD polypeptide at 43°C (Fig. 9B, arrow). The amino terminal sequence of this polypeptide, AAEIEPEKQLDGDRWR, resembles the N terminus of activase from other higher plant species (Wang et al., 1992), thus confirming its identity as Rubisco activase. Taken together, these findings indicate that pea activase, like tobacco activase, is particularly sensitive to thermal aggregation. In pea chloroplasts (Fig. 9) and also leaves (data not shown), this sensitivity was particularly acute for the smaller activase polypeptide.
DISCUSSION
High temperatures disrupt the forces that normally stabilize protein folding, causing proteins to denature and lose catalytic activity (Darby and Creighton, 1993). Measurements of the thermal stability of ATP hydrolysis have shown that activase is very susceptible to thermal inactivation, particularly in the absence of ATP or the non-hydrolyzable analog, ATPγS (Fig. 1; see also Robinson and Portis, 1989; Crafts-Brandner et al., 1997; Eckardt and Portis, 1997; Crafts-Brandner and Salvucci, 2000). In comparison, Rubisco is considerably more heat stable, exhibiting a temperature optimum for activity that is nearly 20°C higher than the optimum for activase (Fig. 1; see also Crafts-Brandner and Salvucci, 2000). This difference is remarkable considering that Rubisco is unable to function in vivo without activase (Salvucci et al., 1985).
Thermal denaturation of proteins involves loss of tertiary structure, which often exposes hydrophobic domains that are normally buried in the active enzyme (Darby and Creighton, 1993). The interaction of hydrophobic residues can cause proteins to associate into aggregates that can be detected by light scattering (Horowitz and Criscimagna, 1986). The exposed side chains of two different partially folded proteins can also interact producing heteroprotein aggregates composed of the two different proteins. An example of relevance to the present study is rhodanese, a protein that has been used extensively as model to study protein folding (Horowitz and Criscimagna, 1986; Mendoza et al., 1991). During refolding, chemically denatured rhodanese can aggregate with itself or with other partially folded proteins, forming insoluble complexes that can be readily isolated by centrifugation (Horowitz and Criscimagna, 1986; Kim et al., 1992).
In the present study, increases in activase light scattering and aggregation with rhodanese exhibited similar temperature responses in the presence of ATPγS, occurring at the same temperatures that inhibited catalytic activity. In a similar manner, light scattering, rhodanese aggregation (this study), and thermal inactivation of enzyme activity (Robinson and Portis, 1989; Crafts-Brandner et al., 1997) all increased at much lower temperatures when activase was heated in the absence of ATP or ATPγS. Taken together, these results indicate that inactivation of activase activity by temperature was caused by a loss of the structural integrity of the protein. It is interesting that Rubisco, which was much more thermally stable than activase, showed minimal aggregation with rhodanese at temperatures below 60°C and exhibited an increase in light scattering and aggregation only when subjected to prolonged incubation at 65°C. Similar results concerning the thermal stability of Rubisco have been reported by others based on measurements of circular dichroism, differential scanning calorimetry, and fluorescence (Tomimatsu and Donovan, 1981; Dolahka-Angelova et al., 2000).
Because of the self-associating properties of activase (Salvucci, 1992; Wang et al., 1993), loss of structural integrity at high temperature probably involves a two-stage process. In the first stage, activase subunits dissociate from the active, highly associated n = 16 state (Lilley and Portis, 1997) to a less associated n = 1 to 4 state (Wang et al., 1993). In the second stage, partial unfolding of monomeric subunits or subunits within the lower ordered oligomers exposes hydrophobic domains that interact nonproductively to form insoluble aggregates.
The propensity of rhodanese to aggregate with partially folded proteins provided the basis of a method for examining the thermal stability of proteins in vivo. Rhodanese trapping of proteins released from heat-treated tobacco protoplasts and chloroplasts showed that thermal denaturation of activase in vivo occurred at temperatures that were similar to those that denatured the isolated enzyme. Comparisons with Rubisco and phosphoribulokinase demonstrated that: (a) activase was considerably more sensitive to thermal denaturation and proteolysis than the two other soluble chloroplast proteins, and (b) a considerable portion of the total activase protein was denatured at temperatures that did not denature other soluble chloroplast proteins. In fact, N-terminal sequencing of the polypeptide that increased in abundance in the aggregate after lysis of heat-treated chloroplasts indicated that, of the more abundant chloroplast proteins, activase was the protein that was most obviously affected by high temperature.
Although rhodanese trapping increased the amount of activase in the insoluble complex, some of the activase released from heat-treated tobacco chloroplasts, protoplasts, and wheat and cotton leaves was insoluble even in the absence of rhodanese (Figs. 6A; Feller et al., 1998). In pea, the thermally labile 41-kD chloroplast polypeptide that was identified in the present study as Rubisco activase aggregates in an insoluble complex in heat-treated chloroplast even without rhodanese trapping (Osteryoung and Vierling, 1994; Fig. 9). Thus, thermally denatured activase either self-aggregates or aggregates with other chloroplast proteins in situ, causing activase to redistribute to the pellet fraction. Aggregation and cross-linking of activase with other activase molecules or with other chloroplast proteins may also explain the presence of high-molecular mass polypeptides that cross-react with activase antibodies in heat-stressed leaves (Feller et al., 1998), protoplasts, and chloroplasts (this study). Candidate proteins for these interactions with activase include the chloroplast chaperonins, a group of proteins that bind partially folded proteins (Vierling, 1991). In fact, previous studies have shown that the small chloroplast chaperonin, HSP21, parallels activase in its redistribution from the soluble to the pellet fraction in heat-stressed chloroplasts (Osteryoung and Vierling, 1994). However, HSP21 is inducible (Osteryoung and Vierling, 1994), whereas aggregation of activase and the formation of high molecular species requires no induction (Feller et al., 1998) and occurs even in chloroplasts isolated from unstressed plants. Thus, although HSP21 may associate with thermally denatured activase, aggregation of activase does not depend on its presence.
During preparation of this manuscript, Rokka et al. (2001) reported that activase associates with the thylakoid membrane in heat-treated spinach leaf discs. The redistribution to the membrane fraction was attributed to changes in the conformation of activase and possibly to specific binding of activase to the thylakoid membrane-bound polysomes. However, the sedimentation behavior of activase after treatment with Triton X-100 and N-dodecyl-β,d-maltoside is also more consistent with self-aggregation and cosedimentation of the activase complex with the densest components of the thylakoid membrane. In the present study, activase was insoluble even though the chloroplasts were lysed with sufficient detergent to completely solubilize the thylakoid membranes (see also Osteryoung and Vierling, 1994). Thus, activase does not require thylakoid membranes to redistribute to the pellet fraction after heat treatment. Rokka et al. (2001) discount the possibility of self-aggregation based on the failure to observe aggregation of activase in heated stromal extracts. However, the propensity of activase to bind rhodanese suggests that, in addition to self-aggregation, thermally denatured activase could also bind nonspecifically to components of the thylakoids or specifically to stromal proteins such as the Hsps. Nonspecific binding to the thylakoids may stabilize activase during periods of heat stress and promote self-aggregation of activase.
The activation state of Rubisco in leaves (Law and Crafts-Brandner, 1999), and isolated chloroplasts (Weis, 1981a, 1981b) decreases under moderate (i.e. 30°C–40°C) heat stress and this decrease is closely associated with a decrease in photosynthetic activity. The decrease in Rubisco activation that occurs at high temperature appears to be caused by an inability of activase to keep pace with a faster rate of Rubisco deactivation (Crafts-Brandner and Salvucci, 2000). Rubisco activation in tobacco protoplasts decreased at temperatures higher than about 30°C (M.E. Salvucci, unpublished data), the same point in the temperature response where the activities of isolated Rubisco and activase began to deviate (Fig. 1). The first indications of thermal denaturation of activase both in vitro and in vivo occurred at temperatures near the optimum for ATP hydrolysis (i.e. 42°C–44°C) and denaturation was extensive at temperatures above the optimum. Because activase physically interacts with Rubisco, minor changes in its structural integrity or oligomeric state could affect its ability to interact productively with Rubisco. A disruption of activase-Rubisco interactions would explain the inability of activase to maintain Rubisco in an active state at temperatures between 30°C and 44°C, i.e. elevated temperatures that are at or below the temperature optimum for ATP hydrolysis. In an alternate manner, activase activity may simply be inadequate to offset the faster rate of Rubisco deactivation at these temperatures (Crafts-Brandner and Salvucci, 2000). At temperatures higher than the optimum for ATP hydrolysis, thermal denaturation of activase was extensive. As a consequence, the marked decrease in Rubisco activation at temperatures greater than 44°C is almost certainly caused by loss of activase activity per se and disruption of activase-Rubisco interactions.
Compared with other chloroplast proteins, activase was extraordinarily sensitive to thermal denaturation. In pea, which has two forms of activase, the shorter form was considerably more sensitive to thermal denaturation and aggregation than the longer form. Similar results were reported for the two forms of activase in spinach leaf discs (Rokka et al., 2001). These results are consistent with the relative temperature sensitivities of the two forms of spinach activase in vitro (Crafts-Brandner et al., 1997), but inconsistent with the findings of Kallis et al. (2000), which showed that the two forms of activase in Arabidopsis exhibit similar thermal sensitivities. In Arabidopsis plants subjected to abrupt heat stress, both forms of activase exhibited similar patterns of thermal aggregation (K.W. Osteryoung and E. Vierling, unpublished data). Thus, species variability may exist in the relative sensitivities of the two activase polypeptides to thermal denaturation.
When heat stress is imposed rapidly, as in the present study, activase rapidly loses structural integrity, probably overwhelming the constitutive chaperonin system. However, if heat stress is imposed slowly photosynthesis can acclimate, requiring higher temperatures for inhibition of CO2 fixation (Berry and Björkman, 1980; Weis and Berry, 1988) and for deactivating Rubisco (Law and Crafts-Brandner, 1999). The mechanistic basis for photosynthetic acclimation is unknown, but could involve stabilization of activase structure by chaperonins to prevent activase from participating in unproductive associations with other activase molecules or with Rubisco or other chloroplast proteins. In an alternate manner, de novo synthesis of more thermally stable forms of activase (Sánchez de Jiménez et al., 1995; Law and Crafts-Brandner, 2001), modifications that improve the thermal stability of existing forms of activase or changes in expression that increase the ratio of the longer, more thermally stable form of activase (Crafts-Brandner et al., 1997) could also provide mechanisms for photosynthetic acclimation to high temperature. A more permanent way of increasing the thermal stability of photosynthesis may be to transform plants with additional copies of activase or with an activase that is engineered to be more thermally stable. Modification of activase structure to improve its thermal stability will require changes that do not compromise the ability of activase to interact with Rubisco.
MATERIALS AND METHODS
Chemicals
Rhodanese and other biochemicals were purchased from Sigma Chemical Co. (St. Louis). Sodium [14C]bicarbonate was obtained from Amersham-Pharmacia (Piscataway, NJ). Adenosine 5′-O-(3-thiotriphosphate) (ATPγS), macerase, and cellulysin were purchased from CalBiochem-Novobiochem Corp (San Diego). Antibodies for phosphoribulokinase, Rubisco, and activase have been described previously (Salvucci et al., 1987; Crafts-Brandner et al., 1990; Feller et al., 1998).
Plant Material and Protoplast Isolation
Tobacco (Nicotiana rustica cv Pulmila) plants were grown in an air-conditioned greenhouse under natural lighting with a day/night temperature regime of 28°C/24°C. Plants were transferred to the laboratory at the end of the night period for isolation of protoplasts. Protoplasts were isolated from leaves by enzymatic digestion and purified by centrifugation through Percoll as described previously (Salvucci and Anderson, 1987). Chloroplast were isolated from protoplast by lysis through a 20-μm net and were purified by centrifugation through 40% (v/v) Percoll (Mills and Joy, 1980). Chloroplasts were isolated from pea (Pisum sativum cv “Little Marvel”) plants as described previously (Osteryoung and Vierling, 1994).
Protein Isolation and Enzyme Assays
Rubisco was isolated from tobacco leaves as described previously (Crafts-Brandner and Salvucci, 2000). The mature form of tobacco activase was produced in and purified from Escherichia coli as described previously (van de Loo and Salvucci, 1997). The temperature response of Rubisco activity was determined for the isolated enzyme by measuring the incorporation of 14CO2 into acid stable products using 30 mm NaH14CO3 (Crafts-Brandner and Salvucci, 2000). The temperature response of ATP hydrolysis by activase was determined spectrophotometrically as described previously (Crafts-Brandner and Salvucci, 2000).
Light Scattering Measurements
Thermal unfolding was monitored by measuring light scattering as described previously (Jakob et al., 1995) using a Jobin Yvon SPEX Fluor-Max 2 (Edison, NJ) spectrofluorometer. Reactions were conducted in a quartz microcuvette in a total volume of 400 μL at the temperatures indicated in the text. The temperature of the solution was controlled using a thermostatted cuvette holder connected to a circulating water bath and was monitored using a type-T thermocouple. Reactions contained 100 mm Tricine-NaOH, 10 mm MgCl2, 1 mm NaHCO3, 0.15 mm dithiothreitol, and 15 μg of either Rubisco (0.51-μm protomer) or activase (0.89-μm protomer). Where indicated, ATPγS was added to the reactions at a final concentration of 0.75 mm.
Rhodanese Aggregation Assays
An aggregation assay using chemically denatured rhodanese (Kim et al., 1992) was also used to measure thermal denaturation of isolated activase and Rubisco and to estimate thermal denaturation of Rubisco, phosphoribulokinase, and activase in vivo. For experiments with isolated proteins, 25 μg of either Rubisco (0.71 μm protomer) or activase (1.19 μm protomer) in 500 μL of the reaction mixture described above for light scattering measurements were incubated in microfuge tubes in a temperature-controlled water bath. After 10 min at a given temperature, the microfuge tubes were removed from the water bath and 10 μL of 5 mg mL −1 rhodanese in 6 m guanidine-HCl was added to the reactions with mixing. After 5 min at room temperature, the tubes were centrifuged for 4 min at 13,000g and the supernatants were removed. Pelleted material was immediately frozen and stored at −80°C. Preliminary experiments using increasing amounts of rhodanese at 60°C showed that this amount of rhodanese was sufficient to precipitate all of the activase.
For estimates of thermal denaturation in vivo, aggregation with rhodanese was used to trap unfolded proteins that were released from heat-treated protoplasts and chloroplasts. Intact protoplasts (50 μg chlorophyll mL−1) were illuminated in thin-walled glass test tubes with 1,000 μmol photons m−2 s−1 in 0.45 m sorbitol, 40 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-KOH, pH 7.6, and 1 mm NaHCO3. Protoplasts were stirred during the incubation and the solution was gassed continuously with air containing 370 μL CO2 l−1 and 21% (v/v) O2. Intact chloroplasts (50 μg chlorophyll mL −1) were illuminated in 28 mm HEPES-KOH, pH 7.6, 0.3 m sorbitol, 2.5 mm EDTA, 1 mm NaHCO3 and 0.15 mm KH2PO4. After 10 min, 900 μL of protoplasts or chloroplasts was transferred to prechilled (i.e. 4°C) microfuge tubes containing 45 μL of 5% (v/v) Triton X-100, 5 m guanidine-HCl, and, where indicated, 180 μg of rhodanese. The solution was immediately mixed and then incubated in a water bath at 23°C. Measurements with a type-T thermocouple showed that the solution temperature decreased markedly upon transfer to the chilled microfuge tube. For example, at an incubation temperature of 48°C, the temperature of the solution decreased to 34.2°C 15 s after transfer and mixing. After 5 min of incubation at 23°C, the solution was centrifuged for 4 min at 13,000g and the supernatants were removed, supplemented with acetone to 80% (v/v), and stored overnight at −20°C to precipitate protein. Pelleted material was immediately frozen and stored at −80°C. Preliminary experiments with purified activase showed that Triton X-100 had no effect on the extent of rhodanese-activase aggregation when included with rhodanese at concentrations as high as 1% (v/v; data not shown). The detergent:chlorophyll ratio used for chloroplast lysis was 50:1, sufficient to completely solubilize the thylakoid membranes (Osteryoung and Vierling, 1994).
Proteins in the frozen pellets from the rhodanese aggregation assays were dissolved in 100 μL of buffer containing SDS and dithiothreitol (Salvucci et al., 1998) on ice and the solution was immediately heated for 3 min at 90°C. Proteins in the TCA- and acetone-supplemented supernatants were collected by centrifugation, taken to dryness, and then dissolved in 300 μL of buffer plus SDS and dithiothreitol and heated as described above. Polypeptides were separated by SDS-PAGE (Salvucci et al., 1998). For assays involving purified activase and Rubisco, polypeptides were electrophoresed on 15- × 15-cm 11% (w/v) polyacrylamide gels and were visualized by staining with Coomassie Brilliant Blue R-250. The amount of Rubisco and activase protein in each lane was determined by whole-band analysis using an image acquisition densitometer. For standards, known amounts of activase or Rubisco were electrophoresed on the same gel. For protoplast and chloroplast experiments, polypeptides were electrophoresed on 11% (w/v) polyacrylamide minigels (Salvucci et al., 1998), transferred to a polyvinylidene difluoride membrane (Immobilon PSQ, Millipore, Bedford, MA), and either probed with monospecific antibodies (Salvucci et al., 1998) or stained with Coomassie Brilliant Blue R-250. Sections of the blots containing stained bands were excised and the sequences of the polypeptides were determined by Edman degradation at the Protein Sequence Facility at Arizona State University.
Aggregation Assays with Pea Chloroplasts
Pea chloroplasts subjected to control and heat stress treatments were processed into soluble and pellet fractions and the fractions analyzed by electrophoresis and immunoblotting as described (Osteryoung and Vierling, 1994), except that 10% (w/v) polyacrylamide gels were used for SDS-PAGE. Immunoreactive bands were identified using antibodies raised in mice against spinach (Spinacia oleracea) activase (Salvucci et al., 1987) and visualized by chemiluminescent detection using anti-mouse secondary antibody conjugated to horseradish peroxidase (Amersham-Pharmacia).
For amino-terminal sequence analysis of the thermally labile pea chloroplast polypeptide, the Triton-insoluble chloroplast fraction was suspended in SDS-PAGE sample buffer containing 0.1 mm sodium thioglycolic acid. Solubilized polypeptides were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore), and the membrane stained as described (Osteryoung et al., 1992). Edman degradation of the 41-kD polypeptide was performed at the Macromolecular Structure Facility, University of Arizona.
Miscellaneous
The chlorophyll content of protoplasts and chloroplasts was determined in 80% (v/v) acetone as described previously (Osteryoung and Vierling, 1994). The intactness of protoplasts and chloroplasts after heating was evaluated by determining the amount of soluble protein and activase released. Intact protoplasts and chloroplasts were incubated in the light as described above and then re-isolated by centrifugation at 270g for 4 min and 800g for 2 min, respectively. Protein in the supernatant was determined by a dye-binding assay (Bradford, 1976). Soluble protein in the pellet was determined after suspending the pellet in water, freezing at −80°C to lyse the organelles, and centrifuging the suspension for 10 min at 10,000g to remove membranes. Activase in the supernatants and pellets was determined by immunoblot analysis as described above.
ACKNOWLEDGMENT
The authors thank Connie Chamberlain for technical assistance.
Footnotes
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010357.
LITERATURE CITED
- Berry JA, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–259. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Law RD. Effect of heat stress on the inhibition and recovery of the ribulose-1,5-bisphosphate carboxylase/oxygenase activation state. Planta. 2000;212:67–74. doi: 10.1007/s004250000364. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA. 2000;97:13430–13435. doi: 10.1073/pnas.230451497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME, Egli DB. Changes in ribulose bisphosphate carboxylase/oxygenase and ribulose 5-phosphate kinase abundances and photosynthetic capacity during leaf senescence. Photosynth Res. 1990;23:223–230. doi: 10.1007/BF00035013. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, van de Loo FJ, Salvucci ME. The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol. 1997;114:439–444. doi: 10.1104/pp.114.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby NJ, Creighton TE. Protein Structure. Oxford: Oxford University Press; 1993. [Google Scholar]
- Dolahka-Angelova P, Ali SA, Demirevska-Kepova K, Stoeva S, Voelter W. Purification, characterization and thermostability of ribulose 1,5,-bisphosphate carboxylase/oxygenase from barley leaves. Z Naturforsch. 2000;55c:611–619. [Google Scholar]
- Eckardt NA, Portis AR., Jr Heat denaturation profiles of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and Rubisco activase and the inability of Rubisco activase to restore activity of heat-denatured Rubisco. Plant Physiol. 1997;113:243–248. doi: 10.1104/pp.113.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci ME. Moderately high temperature inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 1998;116:539–546. doi: 10.1104/pp.116.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993;16:461–467. [Google Scholar]
- Heckathorn SA, Downs CA, Sharkey TD, Coleman JS. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 1998;116:439–444. doi: 10.1104/pp.116.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz P, Criscimagna NL. Low concentrations of guanidinium chloride expose apolar surfaces and cause differential perturbation in catalytic intermediates of rhodanese. J Biol Chem. 1986;261:15652–15658. [PubMed] [Google Scholar]
- Jakob U, Lilie H, Meyer I, Bucher J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase: implication for heat shock in vivo. J Biol Chem. 1995;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL. The CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase: dependence on ribulosebisphosphate concentration and temperature. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- Kallis RP, Ewy RG, Portis AR., Jr Alteration of the adenine nucleotide response and increased Rubisco activation activity of Arabidopsis Rubisco activase by site-directed mutagenesis. Plant Physiol. 2000;123:1077–1086. doi: 10.1104/pp.123.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Lee YJ, Corry PM. Employment of a turbidometric assay system to measure heat-induced protein aggregation. J Therm Biol. 1992;17:297–303. [Google Scholar]
- Law RD, Crafts-Brandner SJ. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1999;120:173–181. doi: 10.1104/pp.120.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ. High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Arch Biochem Biophys. 2001;386:261–267. doi: 10.1006/abbi.2000.2225. [DOI] [PubMed] [Google Scholar]
- Lilley RMcC, Portis AR., Jr ATP hydrolysis activity and polymerization state of ribulose-1,5-bisphosphate carboxylase oxygenase activase. Plant Physiol. 1997;114:605–613. doi: 10.1104/pp.114.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza JA, Lorimer GH, Horowitz PM. Intermediates in the chaperonin-assisted refolding of rhodanese are trapped at low temperature and show a small stoichiometry. J Biol Chem. 1991;266:16973–16976. [PubMed] [Google Scholar]
- Mills WR, Joy KW. A rapid method for isolation of purified, physiologically active chloroplasts, used to study the intracellular distribution of amino acids in pea leaves. Planta. 1980;148:75–83. doi: 10.1007/BF00385445. [DOI] [PubMed] [Google Scholar]
- Miziorko HM. Phosphoribulokinase: current perspectives on the structure/function basis for regulation and catalysis. Adv Enzymol Relat Areas Mol Biol. 2000;74:95–127. doi: 10.1002/9780470123201.ch3. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K. Trienoic fatty acids and plant tolerance to high temperature. Science. 2000;287:476–479. doi: 10.1126/science.287.5452.476. [DOI] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Osteryoung KW, Sticher L, Jones RL, Bennett AB. In vitro processing of tomato proteinase inhibitor I by barley microsomal membranes: a system for analysis of cotranslational processing of plant endomembrane proteins. Plant Physiol. 1992;99:378–382. doi: 10.1104/pp.99.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Vierling E. Dynamics of small heat shock protein distribution within the chloroplasts of higher plants. J Biol Chem. 1994;269:28676–28682. [PubMed] [Google Scholar]
- Pastenes C, Horton P. Resistance of photosynthesis to high temperature in two bean varieties (Phaseolus vulgaris L.) Photosynth Res. 1996;62:197–203. [Google Scholar]
- Portis AR., Jr The regulation of Rubisco by Rubisco activase. J Exp Bot. 1995;46:1285–1291. doi: 10.1093/jxb/erm240. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Portis AR., Jr Adenosine triphosphate hydrolysis by purified Rubisco activase. Arch Biochem Biophys. 1989;268:93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Rokka A, Zhang L, Aro E-M. Rubisco activase: an enzyme with a temperature-dependent dual function? Plant J. 2001;25:463–471. doi: 10.1046/j.1365-313x.2001.00981.x. [DOI] [PubMed] [Google Scholar]
- Salvucci ME. Subunit interactions of Rubisco activase: Polyethylene glycol promotes self-association, stimulates ATPase and activation activities and enhances interactions with Rubisco. Arch Biochem Biophys. 1992;298:688–696. doi: 10.1016/0003-9861(92)90467-b. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Anderson JC. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987;85:66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Ogren WL. The mechanism of Rubisco activase: insights from studies of the properties and structure of the enzyme. Photosynth Res. 1996;47:1–11. doi: 10.1007/BF00017748. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Portis AR, Jr, Ogren WL. A soluble chloroplast protein catalyzes ribulosebisphosphate carboxylase/oxygenase activation in vivo. Photosynth Res. 1985;7:193–201. doi: 10.1007/BF00037012. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Werneke JM, Ogren WL, Portis AR., Jr Purification and species distribution of Rubisco activase. Plant Physiol. 1987;84:930–936. doi: 10.1104/pp.84.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Wolfe GH, Hendrix DL. Purification and properties of an unusual NADPH-dependent ketose reductase from the silverleaf whitefly. Insect Biochem Mol Biol. 1998;28:357–363. [Google Scholar]
- Sánchez de Jiménez E, Medrano L, Martínez-Barajas E. Rubisco activase, a possible new member of the molecular chaperone family. Biochemistry. 1995;34:2826–2831. doi: 10.1021/bi00009a012. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y, Donovan JW. Effect of pH, Mg2+, CO2 and mercurials on the circular dichroism, thermal stability and light scattering of ribulose 1,5-bisphosphate carboxylases from alfalfa, spinach and tobacco. Plant Physiol. 1981;68:808–813. doi: 10.1104/pp.68.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo FJ, Salvucci ME. Activation of ribulose-1,5-bisphosphate carboxylase/oxygenase involves Rubisco activase Trp16. Biochemistry. 1997;35:8143–8148. doi: 10.1021/bi9604901. [DOI] [PubMed] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Weis E. Reversible heat-inactivation of the Calvin cycle: a possible mechanism of temperature regulation of photosynthesis. Planta. 1981a;151:33–39. doi: 10.1007/BF00384234. [DOI] [PubMed] [Google Scholar]
- Weis E. The temperature-sensitivity of dark-inactivation and light-activation of the ribulose-1,5-bisphosphate carboxylase in spinach chloroplasts. FEBS Lett. 1981b;129:197–200. [Google Scholar]
- Weis E, Berry JA. Plants and high temperature stress. In: Long SP, Woodward FI, editors. Plants and Temperature. Cambridge, UK: Society of Experimental Botany; 1988. pp. 327–346. [PubMed] [Google Scholar]
- Wang ZY, Ramage RT, Portis AR., Jr Mg2+ and ATP or adenosine-5′-[γ-thio]-triphosphate (ATPγS) enhances intrinsic fluorescence and induces aggregation which increases the activity of spinach Rubisco activase. Biochim Biophys Acta. 1993;1202:47–55. doi: 10.1016/0167-4838(93)90061-u. [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Snyder GW, Esau BD, Portis AR, Jr, Ogren WL. Species-dependent variation in the interaction of substrate-bound ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and Rubisco activase. Plant Physiol. 1992;100:1858–1862. doi: 10.1104/pp.100.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]