Abstract
With the aim of analyzing their protective function against chilling-induced injury, the pools of glutathione and its precursors, cysteine (Cys) and γ-glutamyl-Cys, were increased in the chilling-sensitive maize (Zea mays) inbred line Penjalinan using a combination of two herbicide safeners. Compared with the controls, the greatest increase in the pool size of the three thiols was detected in the shoots and roots when both safeners were applied at a concentration of 5 μm. This combination increased the relative protection from chilling from 50% to 75%. It is interesting that this increase in the total glutathione (TG) level was accompanied by a rise in glutathione reductase (GR; EC 1.6.4.2) activity. When the most effective safener combination was applied simultaneously with increasing concentrations of buthionine sulfoximine, a specific inhibitor of glutathione synthesis, the total γ-glutamyl-Cys and TG contents and GR activity were decreased to very low levels and relative protection was lowered from 75% to 44%. During chilling, the ratio of reduced to oxidized thiols first decreased independently of the treatments, but increased again to the initial value in safener-treated seedlings after 7 d at 5°C. Taking all results together resulted in a linear relationship between TG and GR and a biphasic relationship between relative protection and GR or TG, thus demonstrating the relevance of the glutathione levels in protecting maize against chilling-induced injury.
Chilling induces oxidative stress (Prasad et al., 1994) during which reactive oxygen species, including hydrogen peroxide (H2O2), are accumulated in concentrations higher than necessary for normal metabolism. The H2O2 excess can be removed by catalase and the ascorbate-glutathione pathway (Prasad et al., 1994; Willekens et al., 1995; Noctor et al., 1998b). In the latter pathway, reduced glutathione (GSH) functions as a reductant of dehydroascorbate via dehydroascorbate reductase, thus forming ascorbate and oxidized glutathione (GSSG; Foyer and Halliwell, 1976; Alscher et al., 1997; May et al., 1998; Noctor and Foyer, 1998; Noctor et al., 1998b).
Several lines of evidence indicate a qualitative involvement of glutathione in the protection of plants from low temperature. An increase in the total glutathione (TG) level in white pine during winter (Anderson et al., 1992) and with increasing altitude in alpine plants (Wildi and Lütz, 1996) was explained by assuming that GSH was used as an antioxidant to protect against low temperature-induced injuries. This assumption was supported by studies carried out under controlled conditions in which cold treatment increased the TG content (Vierheller and Smith, 1990; Brunner et al., 1995; O'Kane et al., 1996; Badiani et al., 1997; Zhao and Blumwald, 1998; Kingston-Smith et al., 1999). At low, nonfreezing temperatures, the GSH content and GSH to GSSG ratio were higher in tolerant genotypes of tomato, Sorghum bicolor, and wheat compared with sensitive ones (Walker and McKersie, 1993; Badiani et al., 1997; Kocsy et al., 2000a), indicating that the maintenance of a high GSH to GSSG ratio contributes to improved chilling tolerance or cold hardening.
Because GSH is oxidized to GSSG during the detoxification of H2O2, appropriate glutathione reductase (GR; EC 1.6.4.2) activity is necessary to regenerate the reduced form (Foyer et al., 1997). The GR activity increased correspondingly during winter in Picea abies growing in the field (Esterbauer and Grill, 1978). In growth chamber experiments, low temperature treatment increased the GR activity in the leaves of spinach (de Kok and Oosterhuis, 1983), in the roots of jack pine (Zhao and Blumwald, 1998), and in Arabidopsis callus (O'Kane et al., 1996). The induction of an increase in GR activity by paclobutrazol in a chilling-sensitive maize (Zea mays) genotype was accompanied by better chilling tolerance (Pinhero et al., 1997).
More information about the contribution of GSH and GR to low temperature tolerance in plants was obtained when genotypes differing in chilling stress tolerance were compared or when the GSH content and GR activity of plants was manipulated by genetic engineering. Tolerant genotypes of tomato and maize accumulated more GSH during chilling and had constitutively higher GR activity than sensitive ones (Walker and McKersie, 1993; Kocsy et al., 1996, 1997), corroborating the involvement of GSH and GR in increased stress tolerance. Genetic studies of GSH accumulation and GR activity during cold hardening in wheat also showed the involvement of both parameters in low temperature stress tolerance (Kocsy et al., 2000a). The overexpression of γ-glutamyl-Cys (γEC) synthetase in the cytoplasm (Noctor et al., 1996) or in the chloroplast (Noctor et al., 1998a) increased the GSH level in poplar, but did not improve tolerance against paraquat-induced oxidative stress (Noctor et al., 1998b), indicating that high GSH levels alone may not be sufficient to confer increased chilling tolerance. On the other hand, poplars containing enhanced foliar GSH pools and also overexpressing GR in the chloroplasts had increased tolerance against low temperature-induced photoinhibition (Foyer et al., 1995). Although Noctor et al. (1998a) and Zhu et al. (1999) found no negative effects of enhanced GSH accumulation, Creissen et al. (1999) observed chlorosis or necrosis in tobacco overexpressing γEC synthetase and containing increased GSH levels. A possible explanation for these injuries could be the oxidation state of the γEC pool (Creissen et al., 2000).
Although a role of GSH besides the removal of reactive oxygen species was described in several other important physiological processes, like stress signaling (Wingate et al., 1988; Foyer et al., 1997), detoxification of toxic products of lipid peroxidation (Mullineaux et al., 1998), thiol-disulfide exchange reactions (Kunert and Foyer, 1993), and the stabilization of enzymes requiring reduced thiol groups for activity (Foyer et al., 1994), the possible role of its precursors was not investigated. GSH synthesis proceeds in two steps: Cys and Glu are combined to form γEC by γEC synthetase, then Gly is added to the dipeptide by GSH synthetase (Rennenberg and Brunold, 1994; Brunold and Rennenberg, 1997). Cys formation is controlled by the GSH level because GSH functions as an electron donor for adenosine 5′-phosphosulfate (APS) reductase, a key enzyme of assimilatory sulfate reduction (Rennenberg and Brunold, 1994; Leustek and Saito, 1999; Suter et al., 2000).
The effect of decreasing GSH levels on chilling tolerance was analyzed previously in a chilling-tolerant maize genotype (Kocsy et al., 2000b). In the present paper, GSH levels were increased in a chilling-sensitive maize genotype using herbicide safeners, and decreased by the simultaneous application of buthionine sulfoximine (BSO), an inhibitor of GSH synthesis (Griffith and Meister, 1979). Because alterations in the ratio of reduced to oxidized Cys and γEC could be involved in signaling chilling stress, these thiols were measured in addition to GSH, GSSG, and GR.
RESULTS
Increase in Total Thiol Levels and GR Activity by Herbicide Safener Combinations
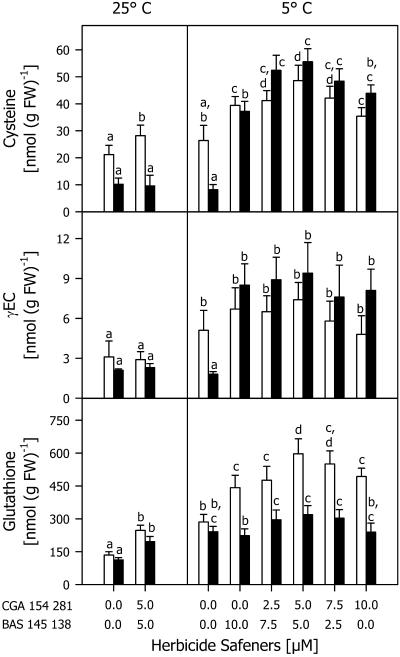
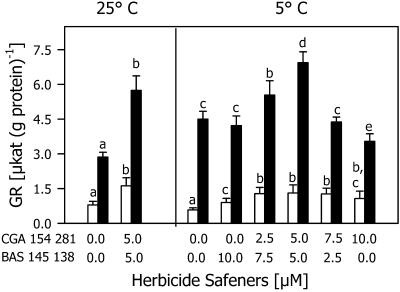
Cys formation and GSH synthesis were induced by two herbicide safeners: CGA 154 281 [4-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzooxazine (benoxacor)] and BAS 145 138 {1-dichloroacetyl-hexahydro-3,3,8 δα-trimethyl-pyrrolo-[1,2-α]-pyrimidine-6-(2H)-one-(dicyclonone)}. When both safeners were applied at a concentration of 5 μm, an increase in total Cys was measured in the shoots and in TG in both shoots and roots of the seedlings cultivated at 25°C (Fig. 1). After 7 d at 5°C, the level of total Cys and TG in the shoots and roots was higher in safener-treated seedlings compared with the untreated ones (Fig. 1). The greatest effect of the safeners on the TG level was detected when both were applied at a concentration of 5 μm (Fig. 1). Total Cys increased 2- and 6-fold in shoots and roots, respectively, whereas the TG level doubled in the shoots and increased only slightly in the roots. The total γEC level, however, greatly increased in the roots but not in the shoots (Fig. 1). Safeners also increased the activity of GR at 25°C and 5°C as shown in Figure 2. The greatest effect on GR activity, with an increase of 100% and 50% in the shoots and roots, respectively, was detected when both safeners were used at a concentration of 5 μm.
Figure 1.
Total Cys, γEC, and glutathione contents in shoots (white bars) and roots (black bars) of maize seedlings cultivated with different combinations of the herbicide safeners CGA 154 281 and BAS 145 138 at 25°C for 4 d, then at 5°C for 7 d (5°C). Controls (25°C) were cultivated at 25°C during the whole experimental period. Mean values of six measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
Figure 2.
GR activity in shoots (white bars) and roots (black bars) of maize seedlings cultivated with different combinations of the herbicide safeners CGA 154 281 and BAS 145 138 at 25°C for 4 d, then at 5°C for 7 d (5°C). Controls (25°C) were cultivated at 25°C during the whole experimental period. Mean values of six measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
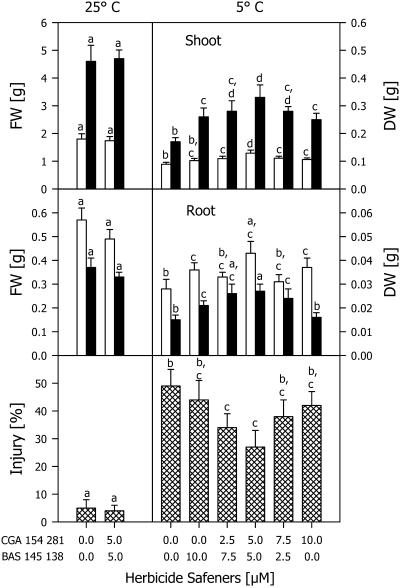
In seedlings cultivated at 25°C throughout, the two herbicide safeners affected neither fresh weight nor dry weight when both were applied at a concentration of 5 μm (Fig. 3). During chilling for 7 d, only small visible injuries were detected. During subsequent cultivation at 25°C for 7 d, however, the leaves partially or totally withered and turned brown. The relative injury of the shoots was decreased when they were cultivated with different concentrations of the two herbicide safeners. A maximum protective effect was detected when both safeners were applied at 5 μm (Fig. 3): The relative injury to the shoots was about 2-fold lower and the dry weight of the seedlings was 2-fold higher than those of the controls. The effect of the safeners on fresh weight was also significant, but not so pronounced as in the case of the two other parameters presented in Figure 3.
Figure 3.
Shoot and root fresh weight (FW, white bars) and dry weight (DW, black bars) and relative injury of maize seedlings cultivated with different combinations of the herbicide safeners CGA 154 281 and BAS 145 138 at 25°C for 4 d, then at 5°C for 7 d, and subsequently at 25°C for an additional 7 d (5°C). Controls (25°C) were cultivated at 25°C during the whole experimental period. Mean values of 12 measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
Inhibition of GSH Synthesis by BSO
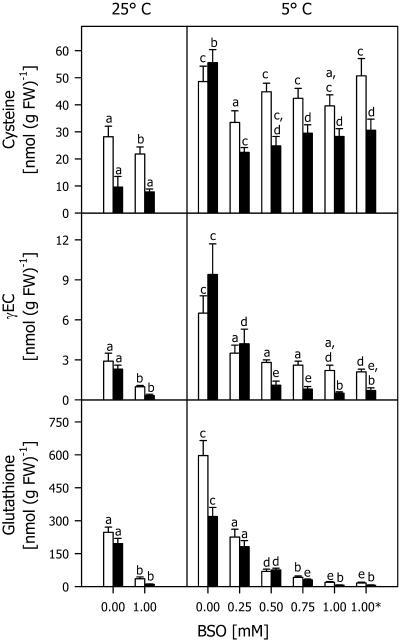
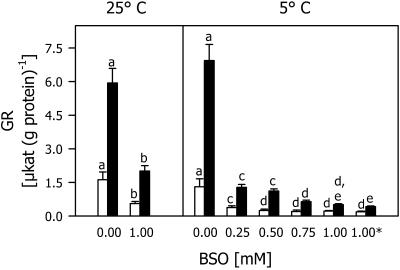
When applied at different concentrations in the presence of 5 μm each of two herbicide safeners, BSO decreased TG and total γEC levels at 25°C and 5°C compared with seedlings treated only with safeners (Fig. 4). At 5°C, even the lowest BSO concentration of 0.25 mm induced a great decrease in the total thiol level both in roots and shoots. At higher BSO concentrations, the total Cys content did not change significantly, whereas total γEC and TG contents decreased. The effect of adding BSO 1 d before the safeners was not different than that of simultaneous addition.
Figure 4.
Total Cys, γEC, and glutathione contents in shoots (white bars) and roots (black bars) of maize seedlings cultivated with different BSO concentrations in the presence of 5 μm each of the safeners CGA 154 281 and BAS 145 138 at 25°C for 4 d, then at 5°C for 7 d (5°C). Asterisk, Indicates an experiment in which the two herbicide safeners were added 1 d after BSO. Controls (25°C) were cultivated at 25°C during the whole experimental period. Mean values of six measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
The simultaneous application of BSO and safeners also decreased the GR activity compared with controls treated only with safeners both at 25°C and 5°C (Fig. 5). Even the lowest BSO concentration applied induced a great reduction in GR activity. With increasing BSO concentrations, the GR gradually decreased in small steps. When the safeners were added 1 d after BSO, there was no difference in GR activity at the end of chilling compared with simultaneous addition.
Figure 5.
GR activity in shoots (white bars) and roots (black bars) of maize seedlings cultivated with different BSO concentrations in the presence of 5 μm each of the safeners CGA 154 281 and BAS 145 138 at 25°C for 4 d, then at 5°C for an additional 7 d (5°C). Asterisk, Indicates an experiment in which the two safeners were added 1 d after BSO. Controls (25°C) were cultivated at 25°C during the whole experimental period. Mean values of six measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
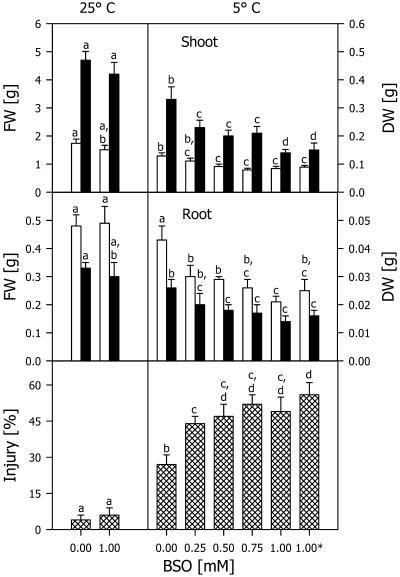
The highest BSO concentration (1 mm) applied did not inhibit the growth of the maize seedlings at 25°C (Fig. 6), but at 5°C even the lowest BSO concentration (0.25 mm) resulted in a great reduction in the fresh weight of the roots and the dry weight of the shoots and in a pronounced increase in the relative injury to the shoots. Increasing the BSO concentration led to increased injury. When the safeners were added 1 d after BSO, no difference in the growth parameters was observed compared with simultaneous application (Fig. 6).
Figure 6.
Shoot and root fresh weight (FW, white bars) and dry weight (DW, black bars) and relative injury of maize seedlings cultivated with BSO at different concentrations in the presence of 5 μm each of the safeners CGA 154 281 and BAS 145 138 at 25°C for 4 d, then at 5°C for 7 d, and subsequently at 25°C for an additional 7 d (5°C). Asterisk, Indicates an experiment in which the two herbicide safeners were added 1 d after BSO. Controls (25°C) were cultivated with corresponding additions at 25°C during the whole experimental period. Mean values of 12 measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
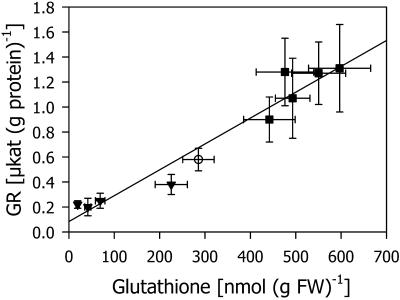
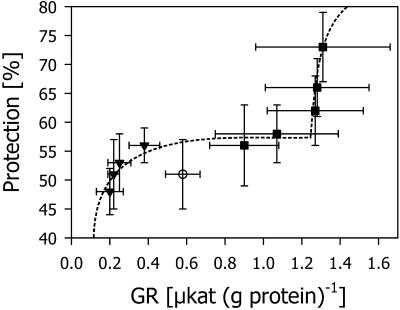
The consistent parallel changes in GR activity with increasing and decreasing TG levels prompted us to analyze a possible correlation between these two parameters. A linear relationship was found between the TG and GR levels in shoots (Fig. 7). With increasing GR activity, the relative protection of the shoots increased according to a biphasic curve (Fig. 8). Because of the linear relation between GR activity and TG, a biphasic curve was also obtained when relative protection was plotted against TG (data not shown). The first phase corresponds to the Michaelis-Menten saturation kinetics, which is consistent with previously published results (Kocsy et al., 2000b) in which the TG level of a chilling-tolerant maize genotype was decreased using increasing levels of BSO. In the second phase, increasing GR levels contributed to additional protection.
Figure 7.
Effect of TG levels on GR activity in shoots and roots of maize seedlings. The values for TG were taken from Figures 1 and 4, and those for GR activity were taken from Figures 2 and 5. The TG levels were manipulated by treatment with different concentrations of the herbicide safeners CGA 154 281 and BAS 145 138 (square) or simultaneous treatment with 5 μm each of two herbicide safeners and different concentrations of BSO (triangle). Controls (circle) were cultivated on nutrient solution without additions.
Figure 8.
Changes in the relative protection from chilling injury at different levels of GR activity in shoots and roots of maize seedlings. The values for GR activity were taken from Figures 2 and 5, and those for protection were calculated from Figures 3 and 6. The symbols correspond to those in Figure 7.
Effect of Safeners and BSO on Thiol Pools, Their Redox State, and GR Activity
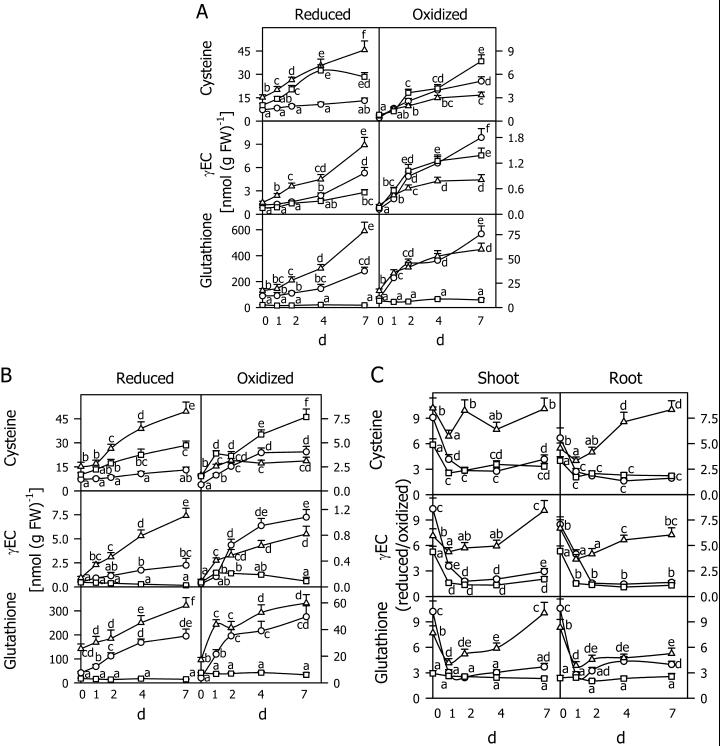
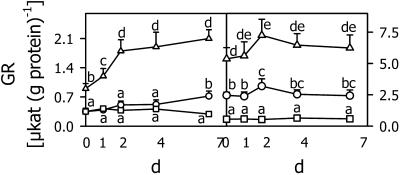
The time course of changes in reduced and oxidized thiols and their ratio (Fig. 9) was measured together with GR activity (Fig. 10) during a 7-d chilling period at 5°C. Figure 9, A and B, show that during chilling all thiols, both in reduced and oxidized form, increased in shoots and roots of the safener-treated and control seedlings. The level of GSH in the shoots of the controls was about one-half of that in the safener-treated seedlings, whereas GSSG was at similar levels in both treatments (Fig. 9A). BSO treatment constantly induced low levels of GSH and GSSG (Fig. 9, A and B) and a low ratio between GSH and GSSG (Fig. 9C). Due to the inhibition of γEC synthetase by BSO, the Cys and cystine levels were increased in shoots (Fig. 9A) and roots (Fig. 9B). The ratio of reduced to oxidized forms of the thiols rapidly decreased at the beginning of the chilling period (Fig. 9C). Subsequently, it increased slowly in the controls, but did not reach the original level. In contrast, in safener-treated seedlings the ratios of reduced to oxidized thiols corresponded to the original levels in the roots and shoots after 7 d at 5°C. Figure 10 shows the time course of GR activity in maize seedlings exposed to 5°C for 7 d. In the shoots and roots of safener-treated plants, enhanced enzyme activity was detected even at the beginning of the chilling period. It rapidly increased during the first and especially the 2nd d at 5°C, but did not change significantly during the 5 subsequent d. BSO treatment established a low, constant level of GR activity in shoots and roots. In the control plants, the enzyme activity increased gradually in the shoots, but did not change in the roots after an initial increase.
Figure 9.
Reduced and oxidized Cys, γEC, and glutathione contents in shoots (A) and roots (B) and the ratio of reduced to oxidized thiols in shoots and roots (C) of maize seedlings cultivated at 5°C for 7 d with no chemical (circle), 1 mm BSO (square), or 5 μm each of the herbicide safeners CGA 154 281 and BAS 145 138 (triangle). Before the measurements, the seedlings were grown at 25°C for 4 d in the presence of these chemicals. Mean values of six measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
Figure 10.
GR activity in shoots and roots of maize seedlings cultivated at 5°C for 7 d with no chemical (circle), 1 mm BSO (square), or 5 μm each of the herbicide safeners CGA 154 281 and BAS 145 138 (triangle). Before the measurements, the seedlings were grown at 25°C for 4 d in the presence of these chemicals. Mean values of six measurements from three independent experiments ± sd are presented. Values carrying different letters are significantly different at P ≤ 0.05.
DISCUSSION
A syndrome of defense reactions is activated in plants exposed to chilling temperatures (Kocsy et al., 2000b). Because of their function in the ascorbate-GSH pathway, the contribution of GSH and GR to protecting plants against chilling-induced oxidative stress was previously discussed in several publications (Badiani et al., 1993; Walker and McKersie, 1993; Kocsy et al., 1996; 1997; 2000b; O'Kane et al., 1996; Alscher et al., 1997; Foyer et al., 1997; Noctor and Foyer, 1998; Zhao and Blumwald, 1998; Leipner et al., 1999). The biphasic relationship between GR activity or TG and the relative protection of maize from chilling-induced injury presented here is an important new finding. It is tempting to speculate that the physiological basis for these two phases consists in two enzymatic reactions involving GSH.
Even at very low GR activity and TG levels, the relative protection was still more than 45% and high levels of both GR activity and TG only resulted in 75% relative protection (Fig. 8). These results indicate that protective systems besides the ascorbate-GSH pathway (Kocsy et al., 2000b) are involved in preventing chilling damage at low GR activity and TG levels, whereas other protective systems do not operate at optimal efficiency at high levels of both GR activity and TG.
In agreement with previously published results (Noctor et al., 1998b), TG predominantly consisted of GSH (Fig. 9), and even after 7 d of chilling stress, GSSG made up only 9%, 29%, and 20% of the TG contained in safener-treated, BSO-treated, and control shoots, respectively. The use of GSH values instead of TG for plotting Figure 7 therefore would result in a comparable linear correlation with GR activity. The quantitative analysis reported here was possible because: (a) the chilling tolerance of a sensitive genotype could be gradually increased by a combination of herbicide safeners that gradually increased TG levels and GR activity, and (b) the simultaneous addition of increasing concentrations of BSO and safeners decreased TG accumulation and GR activity and made the plants gradually more chilling sensitive. These results are consistent with those obtained with the chilling-tolerant maize genotype Z7 (Kocsy et al., 2000b) in the following respect: (a) BSO treatment at different concentrations gradually decreased TG, GR activity, and chilling tolerance; and (b) the addition of γEC at increasing concentrations to BSO-treated plants gradually increased TG, GR activity, and chilling tolerance. It should be stressed, however, that γEC addition only restored the GSH levels in the shoots to 50% of that in the untreated controls and reduced the rate of relative chilling injury from 50% to 25%, compared with 10% in the untreated controls.
The increased GR activity may result in the improvement of chilling tolerance in maize primarily because of the reduction of the GSSG produced in the ascorbate-GSH pathway (Foyer and Halliwell, 1976). In addition, GR has an important function in the synthesis of Cys, a precursor molecule for GSH production, because in this pathway GSSG is formed during the reduction of APS (Suter et al., 2000).
It has been assumed that GSH (Wingate et al., 1988) or GSSG (Wingsle and Karpinski, 1996) or changes in the ratio between GSH and GSSG (Foyer et al., 1997) may function as signals for adapting gene expression to a stress situation. The time courses presented in Figures 9 and 10 suggest that the GSH level could have a signaling function during chilling in the shoots of control seedlings not treated with chemical. The rapid change in the GSH to GSSG ratio could induce a rapid increase in GR activity in the shoots and roots of safener-treated seedlings and in the roots of control seedlings (Fig. 10). Changes in the redox state of the GSH precursors may also be involved in these signaling pathways through their effect on GSH synthesis. In the BSO-treated seedlings, there was no change in either GSH or GSH/GSSG and correspondingly no effect on GR activity was observed.
The improvement induced in the chilling tolerance of maize by safeners may be due to their activating effect on GSH synthesis even during the pretreatment before chilling. This assumption was corroborated by the greater GSH content detected in safener-treated seedlings compared with the control before the beginning of the chilling period. The safener-induced increase in the GSH content can be explained by increased Cys synthesis due to higher APS reductase activity (Farago and Brunold, 1990, 1994), a key enzyme of sulfate assimilation (Suter et al., 2000), and by an increased activity of γEC synthetase, the key enzyme of GSH formation (Farago and Brunold, 1994).
On the basis of the results presented here and previously (Kocsy et al., 2000b), it seems reasonable to assume that the genetic or chemical manipulation of GSH synthesis may be used to increase chilling tolerance in maize. It remains to be shown, however, if such manipulation increases the survival of maize seedlings under field conditions sufficiently to make it of agronomic interest.
MATERIALS AND METHODS
Plant Material and Treatments
Maize (Zea mays) kernels of the highly chilling-sensitive (Stamp et al., 1983; Kocsy et al., 1996) maize inbred line Penjalinan were germinated between two layers of damp paper under a photoperiod of 12 h at 25°C for 3 d. The seedlings were cultivated on nutrient solution as described previously (Kocsy et al., 2000b). The plants were cultivated under a 12-h photoperiod at 300 μmol m−2 s−1, 25°C, and 60% (v/v) relative humidity for 4 d in a growth chamber (Conviron PGR-15, Controlled Env. Ltd., Winnipeg, MN). One-week-old seedlings were treated with a concentration range (0–0, 0–10, 2.5–7.5, 5–5, 7.5–2.5, and 10–0 μm) of the two herbicide safeners BAS 145 138 and CGA 154 281. For the induction of GSH synthesis, the seedlings were cultivated with the different safener combinations for 4 d at 25°C, then at 5°C for 7 d, and subsequently at 25°C for 7 d (Farago and Brunold, 1994). BSO, an inhibitor of γEC synthetase (Griffith and Meister, 1979), the first enzyme in GSH synthesis (Rennenberg and Brunold, 1994), was applied at concentrations of 0, 0.25, 0.5, 0.75, and 1 mm in the presence of the most effective safener combination (5 μm of each) for 4 d before chilling. In a control experiment, 1 mm BSO was added 1 d before the safener combination. The culture medium was routinely replaced after the chilling phase. For biochemical analysis, the plant material was harvested at the end of the chilling period, and for the measurement of growth parameters and the relative injury to the shoots at the end of the recovery phase. Injury to the plants was scored on a scale from 0 (completely dried out, no growth) to 5 (no injury, very good growth) at the end of the recovery period (Kocsy et al., 2000b).
For the determination of the reduced and oxidized thiols, the seedlings were treated with no chemical, 1 mm BSO, or 5 μm of each safener at 25°C for 4 d, then chilled at 5°C for 7 d. Sampling for the determination of thiols and GR was done after 0, 1, 2, 4, and 7 d of chilling.
GR and Protein Assay
The plant material was homogenized in 0.1 m Na-K-phosphate buffer, pH 7.5 (1:5, w/v), containing 0.2 mm diethylenetriamine pentaacetic acid and 4% (w/v) polyvinylpolypyrrolidone in an ice-cooled glass homogenizer and centrifuged at 30,000g for 10 min at 4°C. The supernatant was used for measuring GR activity and protein content.
GR activity was measured according to Smith et al. (1988). The assay mixture contained 100 mm Na-K-phosphate (pH 7.5), 0.2 mm diethylenetriamine pentaacetic acid, 0.75 mm 5,5′dithiobis(2-nitrobenzoic acid), 0.1 mm NADPH, 0.5 mm GSSG, and 50 μL of plant extract in a total volume of 1 mL. Ten micromolar dithiothreitol (DTT) was also added to the reaction mixture to obtain fully reduced DTNB, i.e. maximum GR within the constraints of the assay.
Proteins were determined according to Bradford (1976) using bovine serum albumin as the standard. The reaction mixture contained 200 μL of protein assay reagent (Bio-Rad, Munich) and 5 μL of extract in a total volume of 1 mL.
Determination of Cys, γEC, and GSH
The plant material was extracted at a ratio of 1:10 (w/v) in 0.1 m HCl containing 1 mm Na2EDTA in an ice-cooled glass homogenizer. The extracts were filtered through viscose fleece and centrifuged for 30 min at 30,000g and 4°C.
For the determination of the total thiol content, 400 μL of supernatant was added to 600 μL of 0.2 m 2-[cyclohexylamino]ethane sulfonic acid (pH 9.3) and reduced with 100 μL of a freshly prepared 400 mm NaBH4 solution. The mixture was kept on ice for 20 min. For derivatization, 330 μL of this mixture was added to 15 μL of 15 mm monobromobimane and kept in the dark at room temperature for 15 min. The reaction was stopped with 250 μL of 5% (v/v) acetic acid.
When determining reduced and oxidized thiols for the measurement of total disulphides, the reduction was carried out with 3 mm DTT instead of NaBH4. For the detection of oxidized thiols, 600 μL of 0.2 m 2-[cyclohexylamino]ethane sulfonic acid (pH 9.3) was added to 400 μL of extract, and the free thiols were blocked with 30 μL of 50 mm N-ethylmaleimide (Kranner and Grill, 1996). The excess of N-ethylmaleimide was removed by extracting five times with equal volumes of toluene, after which 300 μL of extract was reduced with 30 μL of 3 mm DTT. Derivatization was done as described for total thiols and the reaction was stopped with 250 μL of 0.25% (v/v) methane sulfonic acid.
The samples were analyzed as described by Schupp and Rennenberg (1988) as modified by Rüegsegger and Brunold (1992) using reverse-phase HPLC and fluorescence detection. Recovery was determined according to Kocsy et al. (2000b).
Statistics
Data of six measurements (12 for growth parameters) from three independent experiments were compared using two-component analysis of variance (Excel 97, Microsoft, Redmond, WA). The significance of the differences was tested using the Student's t test, and mean differences were compared pair wise with the Tukey test (Systat for Windows, Version 5, SPSS Science, Chicago).
ACKNOWLEDGMENTS
Thanks are due to the following colleagues of the Institute for Plant Sciences of the University of Berne (Berne, Switzerland): Karl Hilti and Lea Kamber for preliminary experiments with safeners, Rita Hintermann for her help in preparing this manuscript, and Michael Stalder for his technical work. We are indebted at the Agricultural Research Institute of the Hungarian Academy of Sciences (Martonvásár, Hungary) to László Stéhli, Márta Csollány, and Apollónia Horváth for their technical work and Barbara Harasztos for correcting the manuscript linguistically. The authors are grateful to Peter Stamp (Institute for Plant Sciences, Federal Technical High School, Zürich) for providing the seed material.
Footnotes
This work was supported by the Swiss National Science Foundation, by the European Union (project OPTIMISTICK), by the Hungarian Scientific Research Fund (grant nos. OTKA F025190, F026236, and M28074), by the Hungarian Committee for Technological Development (grant no. OMFB–02579/2000), and by two János Bolyai Research Grants.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010107.
LITERATURE CITED
- Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant. 1997;100:224–233. [Google Scholar]
- Anderson JV, Chevone BI, Hess JL. Seasonal variation in the antioxidant system of eastern white pine needles. Plant Physiol. 1992;98:501–508. doi: 10.1104/pp.98.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani M, Paolacci AR, D'Annibale A, Sermanni GG. Antioxidants and photosynthesis in the leaves of Triticum durum L. seedlings acclimated to low, non-chilling temperature. J Plant Physiol. 1993;142:18–24. [Google Scholar]
- Badiani M, Paolacci AR, Fusari A, D'Ovidio R, Scandalios JG, Porceddu E, Sermanni GG. Non-optimal growth temperatures and antioxidants in the leaves of Sorghum bicolor (L.) Moench.: II. Short-term acclimation. J Plant Physiol. 1997;151:409–421. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brunner M, Kocsy G, Rüegsegger A, Schmutz D, Brunold C. Effect of chilling on assimilatory sulfate reduction and glutathione synthesis in maize. J Plant Physiol. 1995;46:743–747. [Google Scholar]
- Brunold C, Rennenberg H. Regulation of sulfur metabolism in plants: first molecular approaches. Prog Bot. 1997;58:164–186. [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kular B, Leykand N, Reynolds H, Pastori G, Wellburn F, Baker H, Wellburn A. Elevated glutathione biosynthesis causes increased oxidative stress. Plant Cell. 1999;11:1277–1291. doi: 10.1105/tpc.11.7.1277. ; erratum Creissen G, Firmin J, Fryer M, Kular B, Leykand N, Reynolds H, Pastori G, Wellburn F, Baker H, Wellburn A et al. (2000) Plant Cell 12: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok LJ, Oosterhuis FA. Effects of frost-hardening and salinity on glutathione and sulfhydryl levels and on glutathione reductase activity in spinach leaves. Physiol Plant. 1983;58:47–51. [Google Scholar]
- Esterbauer H, Grill D. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. Plant Physiol. 1978;61:119–121. doi: 10.1104/pp.61.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago S, Brunold C. Regulation of assimilatory sulfate reduction by herbicide safeners in Zea mays L. Plant Physiol. 1990;94:1808–1812. doi: 10.1104/pp.94.4.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago S, Brunold C. Regulation of thiol content in maize roots by intermediates and effectors of glutathione synthesis. J Plant Physiol. 1994;144:433–437. [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Plants. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J Biol Chem. 1979;102:42–51. [PubMed] [Google Scholar]
- Kingston-Smith AH, Harbinson J, Foyer CH. Acclimation of photosynthesis, H2O2 content and antioxidants in maize (Zea mays) grown at sub-optimal temperatures. Plant Cell Environ. 1999;22:1071–1083. [Google Scholar]
- Kocsy G, Brunner M, Rüegsegger A, Stamp P, Brunold C. Glutathione synthesis in maize genotypes with different sensitivity to chilling. Planta. 1996;198:365–370. [Google Scholar]
- Kocsy G, Owttrim G, Brander K, Brunold C. Effect of chilling on the diurnal rhythm of enzymes involved in protection against oxidative stress in a chilling tolerant and a chilling sensitive maize genotype. Physiol Plant. 1997;99:249–254. [Google Scholar]
- Kocsy G, Szalai G, Vágújfalvi A, Stéhli L, Orosz G, Galiba G. Genetic study of glutathione accumulation during cold hardening in wheat. Planta. 2000a;210:295–301. doi: 10.1007/PL00008137. [DOI] [PubMed] [Google Scholar]
- Kocsy G, von Ballmoos P, Suter M, Rüegsegger A, Galli U, Szalai G, Galiba G, Brunold C. Inhibition of glutathione synthesis reduces chilling tolerance in maize. Planta. 2000b;211:528–536. doi: 10.1007/s004250000308. [DOI] [PubMed] [Google Scholar]
- Kranner I, Grill D. Determination of glutathione and glutathione disulphide in lichens: a comparison of frequently used methods. Phytochem Anal. 1996;7:24–28. [Google Scholar]
- Kunert KJ, Foyer CH. Thiol/disulphide exchange in plants. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser W, editors. Sulfur Nutrition and Assimilation in Higher Plants: Regulatory, Agricultural and Environmental Aspects. The Hague, The Netherlands: SPB Academic Publishers; 1993. pp. 339–351. [Google Scholar]
- Leipner J, Fracheboud Y, Stamp P. Effect of growing season on the photosynthetic apparatus and leaf antioxidative defenses in two maize genotypes of different chilling tolerance. Environ Exp Bot. 1999;42:129–139. [Google Scholar]
- Leustek T, Saito K. Sulfate transport and assimilation in plants. Plant Physiol. 1999;120:637–643. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implication for environmental sensing and plant development. J Exp Bot. 1998;49:649–667. [Google Scholar]
- Mullineaux PM, Karpinski S, Jimenez A, Cleary SP, Robinson C, Creissen GP. Identification of cDNAs encoding plastid-targeted glutathione peroxidase. Plant J. 1998;13:375–379. doi: 10.1046/j.1365-313x.1998.00052.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Foyer CH. Manipulation of glutathione and amino acid biosynthesis in the chloroplast. Plant Physiol. 1998a;118:471–482. doi: 10.1104/pp.118.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998b;49:623–647. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert KJ, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane D, Gill V, Boyd P, Burdon R. Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta. 1996;198:371–377. doi: 10.1007/BF00620053. [DOI] [PubMed] [Google Scholar]
- Pinhero RG, Rao MV, Paliyath G, Murr DP, Fletcher RA. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol. 1997;114:695–704. doi: 10.1104/pp.114.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg H, Brunold C. Significance of glutathione metabolism in plants under stress. Prog Bot. 1994;55:144–156. [Google Scholar]
- Rüegsegger A, Brunold C. Effect of cadmium on γ-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp R, Rennenberg H. Diurnal changes in the glutathione content of spruce needles (Picea abies L.) Plant Sci. 1988;57:113–117. [Google Scholar]
- Smith IK, Vierheller TL, Thurne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Stamp P, Geisler G, Thiraporn R. Adaptation to sub- and supraoptimal temperatures of inbred maize lines differing in origin with regard to seedling development and photosynthetic traits. Physiol Plant. 1983;58:62–68. [Google Scholar]
- Suter M, von Ballmoos P, Kopriva S, Op den Camp R, Schaller J, Kuhlemeier C, Schürmann P, Brunold C. Adenosine 5′-phosphosulfate sulfotransferase and adenosine 5′-phosphosulfate reductase are identical enzymes. J Biol Chem. 2000;275:930–936. doi: 10.1074/jbc.275.2.930. [DOI] [PubMed] [Google Scholar]
- Vierheller TL, Smith IK. Effect of chilling on glutathione reductase and total glutathione in soybean leaves (Glycine max (L) Merr) In: Rennenberg H, Brunold C, de Kok LJ, Stulen I, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishers; 1990. pp. 261–265. [Google Scholar]
- Walker MA, McKersie BD. Role of the ascorbate-glutathione antioxidant system in chilling resistance of tomato. J Plant Physiol. 1993;141:234–239. [Google Scholar]
- Wildi B, Lütz C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996;19:138–146. [Google Scholar]
- Willekens H, Inzé D, Van Montagu M, Van Camp W. Catalase in plants. Mol Breed. 1995;1:207–228. [Google Scholar]
- Wingate VPM, Lawton MA, Lamb CJ. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 1988;87:206–210. doi: 10.1104/pp.87.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingsle G, Karpinski S. Differential redox regulation of glutathione reductase and CuZn-superoxide dismutase gene expression in Pinus sylvestris L. needles. Planta. 1996;198:151–157. doi: 10.1007/BF00197598. [DOI] [PubMed] [Google Scholar]
- Zhao S, Blumwald E. Changes in oxidation-reduction state and antioxidant enzymes in the roots of jack pine seedlings during cold acclimation. Physiol Plant. 1998;104:134–142. [Google Scholar]
- Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N. Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol. 1999;119:73–79. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]