Abstract
Oxygen electrode and fluorescence studies demonstrate that linear electron transport in the freshwater alga Chlamydomonas reinhardtii can be completely abolished by abrupt hyperosmotic shock. We show that the most likely primary site of inhibition of electron transfer by hyperosmotic shock is a blockage of electron transfer between plastocyanin (PC) or cytochrome c6 and P700. The effects on this reaction were reversible upon dilution of the osmolytes and the stability of plastocyanin or photosystem (PS) I was unaffected. Electron micrographs of osmotically shocked cells showed a significant decrease in the thylakoid lumen volume. Comparison of estimated lumenal width with the x-ray structures of plastocyanin and PS I suggest that lumenal space contracts during HOS so as to hinder the movement of docking to PS I of plastocyanin or cytochrome c6.
The effects of high osmotic potentials on primary photosynthetic processes are of great interest because they are thought to influence the ability of plants to survive desiccation and salt stress. It has been known for some time that photosynthesis can be inhibited by hyperosmotic shock (HOS), and several studies have been made on the effects of such conditions on the primary processes of photosynthesis (for review, see Kirst, 1990). However, the nature of the primary lesions to photosynthesis caused by HOS has remained elusive. To date, the most intensive studies on the responses of green algae to HOS have been performed on marine species, in particular Duneliella salina (e.g. Wiltens et al., 1978; Satoh et al., 1983; Gilmour et al., 1984). Because such species are found in waters of variable salinity, they are expected to have robust osmoregulatory systems. On the other hand, freshwater algae are likely to respond differently to salt or osmotic stresses. Because of the detailed genetic information and its ability to be transformed, the fresh water Chlorophyte, Chlamydomonas reinhardtii, has become an important laboratory species, particularly in studies of photosynthesis; thus, understanding its ecophysiology has become critical. Considerable work has been done on the effects of HOS on cyanobacteria species, such as Anacystis nidulans and Synechococcus sp. PCC 7942 (Grodzinski and Colman, 1973; Fulda et al., 1999; Allakhverdiev et al., 2000a), which pointed to direct effects of HOS on the photosynthetic reaction centers, particularly photosystem (PS) II (Allakhverdiev et al., 2000b). However, direct comparisons of the effects with those in freshwater green algae may be difficult considering their evolutionary and physiological differences.
Previous research has shown that overall photosynthetic capacity of C. reinhardtii is severely inhibited by HOS (Reynoso and Gamboa, 1982; Berkowitz et al., 1983; Gamboa et al., 1985; Neale and Melis, 1989; Kirst, 1990; Endo et al., 1995; León and Galván, 1995). Furthermore, Neale and Melis (1989) have shown that the photosynthetic apparatus of C. reinhardtii cells is significantly more susceptible to photoinhibition or photodamage during osmotic stress, probably as a result of osmotic-induced inhibition of electron transfer and repair processes. Endo et al. (1995) have attempted to pinpoint the site of HOS-induced inhibition of photosynthetic processes in C. reinhardtii. They concluded, based mainly on fluorescence and 820-nm absorbance assays, that hyperosmotic stress inhibits PS II while inducing a state transition and stimulating cyclic electron flow (Endo et al., 1995).
Our work builds on the experiments of Endo et al. (1995), and allows us to propose a detailed mechanism for the inhibition of photosynthesis by hyperosmotic stress in C. reinhardtii. We have concluded that the largest effect of abrupt hyperosmotic stress is to block the transfer of electrons between plastocyanin (PC) or cytochrome c6 and P700. It is likely that this is caused by desiccation and subsequent flattening of the thylakoid membrane system, resulting in obstruction of PC or cytochrome c6 mobility and access to their docking sites on PS I. Other effects noted by earlier workers, such as decreases in maximum fluorescence levels (Fm), apparent increases in cyclic electron transfer under moderate osmotic stress, and inhibition of O2 evolution, can be ascribed to modification of the thylakoid granal structure caused either by a state transition or by high salt-induced dissociation of the granal stacks.
RESULTS
Continuous Light-Induced Redox Changes
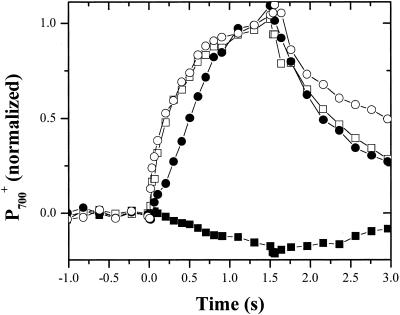
Illumination of whole cells of C. reinhardtii with weak (approximately 100 μmol photons m−2 s−1, 640-nm peak emission) continuous red light in the absence of osmotic stress (Fig. 1) resulted in only small changes in the A820, which are associated predominantly with the formation of P700+ (for review, see Klughammer and Schreiber, 1991). After inhibition of the cytochrome b6f complex by addition of 10 μm DBMIB, the same illumination regime resulted in sigmoidal oxidation kinetics of P700 leading, as reflected in a large absorbance increase at 820 nm, to complete oxidation in approximately 2 s. These results are similar to those of previous studies (e.g. Vredenberg and Duysens, 1965; Malkin, 1968; Marsho and Kok, 1970) and can be understood as follows. During normal steady-state illumination with relatively weak light, the rate of P700 oxidation is slower than its rate of rereduction; thus, the amount of P700+ remains low. Upon inhibition of plastoquinol (PQH2) oxidation with DBMIB, P700+ accumulated in the light after a short lag phase, during which P700+ is rereduced by PC, cytochrome f, and the Rieske iron-sulfur center. The lag phase ends when these components become nearly fully oxidized, and P700+ then accumulates.
Figure 1.
Inhibition of PC to P700+ electron transfer by HOS. C. reinhardtii cells were incubated in the dark for 20 min in the presence (white symbols) or absence (black symbols) of 0.3 m Suc and in the presence (circles) or absence (squares) of 10 μm 2,5-dibromo-3-methyl-6 isopropyl-p-benzoquinone (DBMIB). Changes in P700 absorbance (i.e. −ΔI/I) during steady-state illumination (approximately 100 μmol photons m−2 s−1) were measured at 820 nm. Illumination began at the zero time point and continued for 1.5 s. All values were normalized the maximum 820-nm absorbance change in the presence of DBMIB (white squares) and then plotted as a function of time.
Upon addition of 0.3 m Suc, P700+ accumulated in weak light even in the absence of DBMIB (Fig. 1). P700+ was rereduced only slowly (half time [t1/2] approximately 500 ms) upon the light-dark transition. Addition of 10 μm DBMIB had little effect on the oxidation or rereduction kinetics. These results are in contrast to those of Endo et al. (1995) who reported an actual acceleration of P700+ rereduction upon addition of 0.1 m ethylene glycol. As discussed below, the differences in the Endo et al. (1995) data are likely due to osmolyte concentrations that only partially inhibited PS I rereduction. A close examination of Figure 1 reveals that addition of high concentrations of Suc completely eliminated the lag phase before the onset of P700 oxidation. This led us to hypothesize that osmotic stress inhibits the flow of electrons between PC and P700+. These experiments were repeated with a range of osmolytes, including Glc, Fru, sorbitol, NaCl, KCl, and K2HPO4 (data not shown). The effects of these osmolytes on P700 redox kinetics were nearly identical, although the concentrations required to achieve full effects varied (see below).
Flash-Induced Redox changes of Cytochrome f and P700
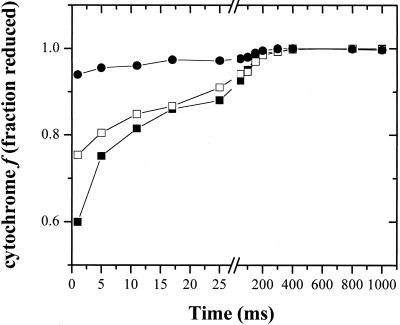
Figure 2 shows flash-induced redox kinetics of cytochrome f. In the absence of osmotic stress, a single turnover flash resulted in typical transient cytochrome f oxidation kinetics as previously observed (e.g. Rich and Bendall, 1981; Jones and Whitmarsh, 1985, 1987; Cramer et al., 1987; Kramer et al., 1990; Joliot and Joliot, 1992). The oxidation phase was essentially complete prior to the first measuring flash at 1 ms after actinic flash illumination. Rereduction occurred with the turnover of the cytochrome b6f complex with a half-time of about 10 ms. Twenty minutes after the addition of 0.08 m potassium phosphate, a small but reproducible decrease in the extent of cytochrome f oxidation was observed (Fig. 2). The remaining photooxidized cytochrome f was rereduced with kinetics nearly identical to those of the control, suggesting that the uninhibited fraction functioned normally. Upon addition of 0.16 m potassium phosphate (Fig. 2), cytochrome f oxidation was nearly completely inhibited. Similar results were obtained with a wide range of osmolytes, including Suc, Glc, NaCl, and KCl (data not shown), although the concentrations required for inhibition differed (see below). These data further support a hyperosmotic stress-induced blockage in electron transfer between PC and P700+.
Figure 2.
Inhibition of cytochrome f oxidation by HOS. Dark-adapted cells were incubated for 20 min in darkness in the presence of 0.01 mm (black squares), 0.08 m (white squares), or 0.16 m (black circles) potassium phosphate, pH 7.0. Single-turnover flash-induced cytochrome f absorbance signals (i.e. −ΔI/I) were monitored as described in “Materials and Methods.” Chlorophyll concentrations were between 25 and 50 μg chlorophyll mL−1 for all assays. Data from each preparation were scaled to the total photooxidizable cytochrome f concentration in the presence of DBMIB, as described in “Materials and Methods,” and it was assumed that all cytochrome f was reduced in the darkness, prior to flash excitation. Note break in time axis at 27 ms.
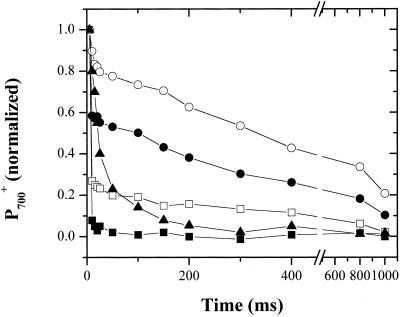
Figure 3 shows kinetics of saturating single-turnover flash-induced redox changes of P700 without addition or 20 min after addition of a range of Glc concentrations. The absorbance changes were scaled to the maximum extent of P700 absorbance changes obtained after illumination with a series of 10 closely spaced (10 Hz) actinic flashes in the presence of 10 μm DBMIB (data not shown). In the control, P700 was nearly completely rereduced within the first 5 ms after flash excitation. A small slow phase remained, which we attribute to a fraction of PS I centers inaccessible to PC. After addition of 0.2 m Glc, a significant increase in slower phases of P700+ rereduction appeared. The half-time for this slow phase was approximately 400 ms. After addition of higher Glc concentrations, the extent of this slow phase increased dramatically, and nearly full inhibition of P700+ rereduction occurred with 0.3 m Glc. We interpret this data as reflecting a progressive inhibition of PC to P700+ electron transfer. As with the cytochrome f redox kinetics, the uninhibited fraction appears to function normally.
Figure 3.
Inhibition of P700+ reduction by HOS. Cells were incubated for 20 min in darkness in Tris-acetate-photosphate (TAP) medium (see “Materials and Methods”) containing no Glc (black squares), 0.2 m Glc (white squares), 0.25 m Glc (black circles), 0.3 m Glc (white circles), or 0.3 m Glc, 1 μm phenazine methosulfate (PMS), and 1 mm ascorbate (black triangles). Single-turnover flash-induced 703 to 730 absorbance (i.e. −ΔI/I) changes were measured as described in “Materials and Methods” and normalized against the maximal changes observed in control cells containing 10 μm DBMIB.
Kinetics and Concentration Dependence of HOS Effects on Electron Transfer
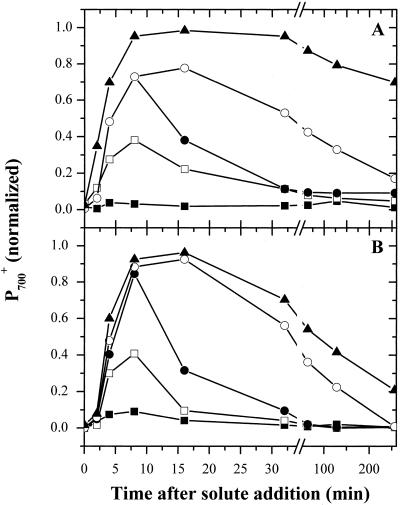
Figure 4 shows the kinetics of the onset of inhibition of P700+ rereduction following addition of Suc (Fig. 4A) or KCl (Fig. 4B). The half-times for inhibition were fairly constant, at about 2 to 5 min, regardless of the final extent of inhibition. This is consistent with a pseudo-first order reaction such as that expected for the movement of water across membranes in response to osmotic potential differences. However, these half-times were considerably longer than the 5 to 10 s measured for the initial fluxes of water from micro-algae following hyperosmotic challenge (Kirst, 1990). This suggests that blockage of P700+ rereduction may occur subsequent to the actual dehydration of the cell and may reflect secondary reorganization of the thylakoid membranes (see below).
Figure 4.
Time course of the inhibition of P700+ reduction by HOS. A, Dark-adapted cells were mixed with Suc to final concentrations of 0.15 (black squares), 0.25 (white squares), 0.3 (white circles), and 0.4 (black triangles) m Suc. Ten minutes after the addition of Suc, part of the 0.3 m Suc incubation was centrifuged and resuspended in fresh TAP medium (black circles). At various times following Suc addition, kinetic traces of 703 to 730 absorbance changes (as shown in Fig. 3) were collected. The extents of P700+ signals at 10 ms after a single actinic, normalized to the maximal extent of P700+ obtained after 10 actinic flashes in the presence of DBMIB are presented. B, The same experiment was repeated with KCl at final concentrations of 0.1 m (black squares), 0.15 m (white squares), 0.2 m (white circles), 0.25 m (black triangles), and 0.2 m (diamonds). Ten minutes after the addition of KCl, part of the sample was centrifuged and resuspended in fresh TAP medium (black circles).
Figure 4 also shows that the rapid phase of P700+ rereduction recovered at least partially on the minutes time scale after hyperosmotic stress, presumably by osmo-adjustment processes (for review, see Kirst, 1990). León and Galván (1995) showed that glycerol synthesis is a major component of osmo-adjustment in C. reinhardtii and the time scale we observe for P700 recovery after addition of KCl appears to be consistent with their estimates of the accumulation of this osmolyte. Recovery after the addition of high concentrations of Suc was considerably slower than after the addition of KCl. This is not altogether surprising because movements of ions across the plasma membrane appears to be an early reaction to osmotic or salt stress (for review, see Kirst, 1990) and the intracellular concentrations of these ions might be different under the two types of stress. This is supported by the recent observations of Munnik et al. (2000), which show that in Chlamydomonas moewusii the accumulation and dissipation of second messenger molecules in response to HOS occurs with kinetics similar to the inhibition and recovery of P700+ reduction reported here.
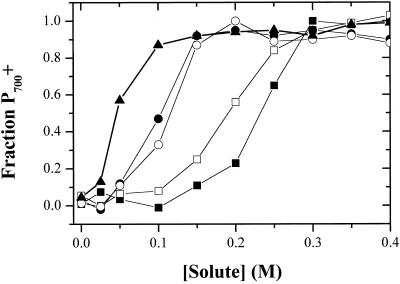
Figure 5 shows the osmolyte concentration dependence of the maximal extent of inhibition of P700+ rereduction (measured as above) with five different osmolytes. Small increases in osmolyte concentrations had little effect on P700 rereduction kinetics, but inhibition appeared abruptly at around 0.05 to 0.1 m, with half-inhibitory concentrations appearing at 0.075 to 0.1 and 0.1 to 0.2 m for salts and organic osmolytes respectively. Inhibition by K2HPO4 appeared at even lower concentrations, as expected for its 3-fold ionization. Thus, these data are consistent with predominantly osmotic effects. However, we noted that different organic osmolytes had small but reproducible differences in half inhibitory concentrations. This is evident in the approximately 25% difference in half-inhibitory concentrations for Glc and Suc. While it is expected that the plasma and chloroplast membranes are differentially permeable to salt, none of the organic osmolytes appears to be transported into various species of Chlamydomonas (Harris, 1989). We do not have a simple explanation for these smaller differences. In addition, the half-inhibitory concentrations for various osmolytes varied from culture to culture by as much as 10% to 20%, but varied much less between assays using a single culture (data not shown). This suggests that physiological state may play a significant role in determining susceptibility of the cells to HOS, as would be expected if the internal osmotic balance of the cell varied with growth conditions. Moreover, the results correlate reasonably well with the observations of Berkowitz et al. (1983), that CO2 fixation in inhibited as osmolyte (in this case mannitol) concentrations are increased, at a threshold of about 0.25 m.
Figure 5.
Concentration dependence of inhibition of P700+ reduction. Dark-adapted cells were incubated in the dark in the presence of Suc (black squares), Glc (white squares), KCl (black circles), NaCl (white circles), and K2HPO4 (black triangles). After 20 min, flash-induced 703 to 730 absorbance changes (i.e. −ΔI/I) were measured. The fractions P700+ remaining oxidized 10 ms after a saturating flash is plotted against the concentration of solute. Absorbance values were normalized to the maximum value after a series of 10 flashes in the presence of 10 μm DBMIB.
Endo et al. (1995) reported that inhibition of C. reinhardtii photosynthetic electron transport by HOS was primarily caused by inhibition of PS II reactions, which seem to agree with more recent reports of greater PSII sensitivity to HOS in Synechococcus sp. PCC 7942 (Allakhverdiev et al., 2000a, 2000b). On the contrary, we found that water to CO2 electron transfer (measured as light-induced oxygen evolution in intact cells in the presence of NaHCO3) was nearly completely (>95%) inhibited by addition of 0.4 m Suc, whereas water to dichlorophenol indophenol (DCPIP) electron transfer was only inhibited to about 50% (data not shown). This demonstrated that, although PS II turnover was affected by HOS, the largest effect on whole chain electron transport in Chlamydomonas sp. was elsewhere, consistent with our suggestion that it results from blockage of PC to P700 electron transfer.
The Nature of the Lesion between PC and P700+
We considered several possible sources for the blockage of electron transfer between PC and P700+. One possibility was that HOS elevated the redox poise of the photosynthetic electron transfer chain such that no electrons were available to rereduce P700+. To test this, we added a low (1 μm) concentration of the redox mediator, PMS, and 1 mm of the reductant, sodium ascorbate. This combination is expected to set the redox poise of the cell so that the high potential chain components (i.e. the Rieske iron-sulfur center, cytochrome f, PC, and P700) are fully reduced in the dark. Figure 3 contrasts the flash-induced P700 redox kinetics in cells osmotically stressed with 0.3 m Glc in the absence and presence of the artificial redox poising system. The addition of PMS/ascorbate dramatically increased the rereduction rate in the shocked cells, indicating that is was able to penetrate the membranes and shuttle electrons to the high-potential chain components. On the other hand, the redox mediator was unable to restore the rapid (microsecond) phase of P700+ rereduction. This demonstrated that PMS bypassed, rather than restored, the normal P700+ rereduction by PC. Addition of PMS/ascorbate to control cells had little effect on the rereduction kinetics (data not shown). Thus, we conclude that HOS-induced inhibition of P700+ rereduction is not caused by the establishment of an oxidizing redox poise.
Another possible explanation is that HOS leads to the near complete destruction of PC. To test this hypothesis, we extracted PC from intact cells by repeated freezing and thawing cycles, which results in nearly 100% PC recovery (S. Merchant, personal communication). We then assayed the total PC content by measuring the oxidized-reduced difference spectrum (see “Materials and Methods” for details). The peak absorbance change at 597 nm was used to quantify the total concentration of PC. These data were normalized to the total concentration of P700 present in the extracted sample, determined before extraction by the light-induced 703- to 730-nm absorbance change in the presence of DBMIB, as described in “Materials and Methods.” Using this technique, we estimated from three separate experiments 2.7 ± 0.02 mol of PC per mol PS I reaction center to be present in untreated cells. Addition of Suc to 0.4 m or KCl to 0.3 m had no significant effect on the total extractable redox-active PC content; relative yields of 2.6 ± 0.02 and 2.8 ± 0.02 mol PC per mol PSI were obtained, respectively. Furthermore, removal of Suc (Fig. 4A) or other osmolytes (not shown), by mild centrifugation and resuspension in TAP medium, led to a relatively rapid (on the minutes time scale) and nearly complete recovery of P700+ rereduction kinetics. This time scale is likely inconsistent with resynthesis of PC. Taken together, these data led us to the conclusion that PC is not destroyed by hyperosmotic stress, but is nevertheless rendered unable to interact with P700.
We next tested whether the effects of hyperosmotic stress were specific to an interaction of PC with PS I. Cells were grown in copper-free TAP medium using acid-washed glassware, as described previously (Harris, 1989), to prevent the synthesis of PC, but induce the synthesis of cytochrome c6 (sometimes referred to as cytochrome c552; Merchant and Bogorad, 1986). Cells grown in this way showed a large bleaching at 552 nm associated with cytochrome c6 oxidation upon illumination in the presence of DBMIB (data not shown). Hyperosmotic stress-induced inhibition of P700+ rereduction, measured as the extent of P700+ remaining 10 ms after a saturating single turnover actinic flash, was nearly identical between cells grown in TAP medium with or without Cu2+ (data not shown). Thus, we conclude that the inhibition is not specifically associated with the interaction between PC and P700, but to a more general phenomenon.
Ultrastructural Changes Associated with Osmotic Stress
To explore whether HOS disrupted thylakoid ultrastructure, we performed electron microscopy on control and hyperosmotically stressed cells. Extensive efforts to obtain highly resolved frozen sections were not successful because the high concentrations of solutes affected the freezing process. Therefore, we relied on standard embedding and fixation procedures. We argue that these procedures, though susceptible to artifacts, can yield interpretable results. Two separate sets of fixations were performed with different cultures and between 20 and 50 thin sections were examined from each fixation.
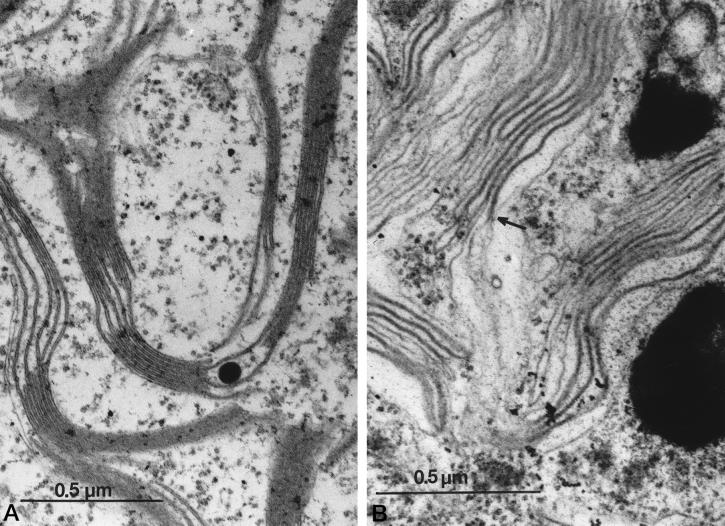
Control cells (Fig. 6A) displayed typical thylakoid membrane structures with well-defined stacked and unstacked regions and clearly visible lumenal spaces separating membranes. There are two major effects of Suc HOS (Fig. 6B): first, stacking was nearly completely disrupted; and second, in most regions, lumenal space (i.e. seen as a region of low electron density between the two thylakoid membranes) was diminished, giving the appearance of a single, thick membrane. We interpret this as indicating that, upon addition of osmolytes, the lumenal space contracted, bringing the two thylakoid membranes closer together. Similar ultrastructural changes were reported for strong HOS to Dunaliella salina (Trezzi et al., 1965). The fact that the thylakoid structures observed under HOS were two separate membranes appressed against each other is demonstrated in many thin sections where the thick aggregate membrane pair is observed to split into two typically sized thylakoid membranes. An example of this phenomenon is shown in Figure 6B (see arrow). Intermediate HOS levels produced intermediate effects (not shown). Addition of 0.1 m Suc did not significantly alter the stacking properties of the thylakoids, whereas addition of 0.2 m Suc resulted in nearly complete unstacking and significant compression of the lumenal space.
Figure 6.
HOS-induced changes in thylakoid ultrastructure. Cells were fixed in the absence (A) or presence (B) of 0.3 m Suc. Thin sections of the cells embedded in SPURRS were negatively stained with uranyl acetate and the images were obtained using a JEOL JEM 1200-EX microscope (JEOL, Ltd., Tokyo). The arrow in B shows a transition point where a pair of thylakoid membranes become appressed against each other. See text for details.
We made simple, rough estimates of the width of the thylakoid double membrane structure to test whether a significant change in the lumen volume (or width) had occurred upon application of hyperosmotic stress. An estimated width of approximately 156 ± 4 Å was obtained for the thylakoid thickness in control cells, very similar to the estimate obtained earlier (Murakami and Packer, 1970; Whitmarsh, 1986). After incubation for 30 min in 0.3 m Suc, the thickness had decreased to approximately 115 ± 6 Å. Keeping in mind the inherent limitations of these measurements, and assuming that the thylakoid membranes themselves maintained a constant thickness of 50 Å (see Murakami and Packer, 1970; Whitmarsh, 1986), then our data suggest that the thickness of the lumenal space decreased from about 50 to 15 Å upon application of HOS. High-resolution x-ray structures of PC show that it has dimensions of roughly 40 × 32 × 28 Å (Sykes, 1991; Redinbo et al., 1993). Comparison of these dimensions with the estimated width of the lumenal space suggest that osmotic stress may hinder the movement of PC and/or prevent PC docking to PS I (see below and Albertsson, 1982; Haehnel, 1984). Murakami and Packer (1970) observed a similar reduction in lumen volume upon illumination of spinach (Spinacia oleracea) thylakoids in the presence of phenyl mercuric actetate, a sulfhydryl-active reagent. They attributed the observed ultrastructural changes as reflecting acidification of the lumen and accompanying movement of phenyl mercuric actetate. It should be noted, however, that because these authors added PMS to catalyze PS I-dependent cyclic electron transfer, their light-induced acidification would not be sensitive to blockage of electron transfer from PC to P700 (see our results with PMS, above).
Effects of HOS on Chlorophyll a Fluorescence Kinetics
In this section, we aimed to determine if inhibition of P700+ reduction is the “primary” lesion in abrupt hyperosmotic stress. To do this, we compared the effects of osmolytes on P700+ reduction with their effects on fluorescence induction kinetics, which reflect overall linear electron transfer. The interpretation of chlorophyll a fluorescence kinetics has been extensively reviewed elsewhere (e.g. Briantais et al., 1986; Krause and Weis, 1991; Govindjee, 1995; Genty and Harbinson, 1996; Kramer and Crofts, 1996; Lavergne and Briantais, 1996). Chlorophyll a fluorescence yields are controlled by the competition for excitons trapped by the antenna complexes between fluorescence decay and all other excitation decay processes. In a control, dark-adapted sample, most excitation energy is consumed by photochemistry, thus lowering fluorescence yield through a process termed photochemical quenching (qP). When PS II centers are in their open states, fluorescence yield is low, whereas when they are blocked, particularly by the reduction of the primary quinone electron acceptor in PS II (QA), fluorescence yield increases. Thus, chlorophyll assays were used as indicators of the efficiency or turnover rate of PS II. In addition to qP, various regulatory or inhibitory processes can lower fluorescence yield, and these are generally termed non-qP and include contributions from such processes as qE quenching (dissipation of excitation energy by processes related to the energization of the thylakoid membrane) and state transitions (dissociation of antenna complexes from the PS II centers leads to a decrease in the yield of variable fluorescence).
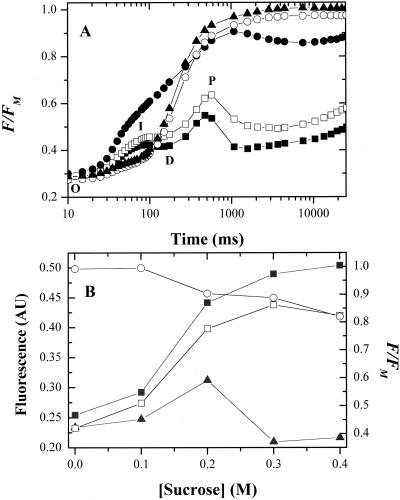
Figure 7A shows selected fluorescence induction curves for dark-adapted C. reinhardtii cells suspended in TAP growth medium alone (control) or 20 min after addition of various concentrations of Suc. In general, our fluorescence results were similar to those of F.-A. Wollman (unpublished data). Experiments were repeated after addition of 10 μm atrazine (data not shown) to block the secondary quinone electron acceptor of PS II (QB) site to obtain maximal fluorescence yields (Fm) at each concentration of Suc. Illumination of the control led to a fluorescence induction curve typical of normal C. reinhardtii cells (e.g. Govindjee and Satoh, 1986). The generally accepted model ascribes the initial rise (the O-I phase) to a fraction of PS II centers in which the QB site is inactive, either because it does not contain plastoquinone (PQ; as expected for the so-called non-B centers; for review, see Lavergne and Briantais, 1996), or because QB is prereduced in the dark. Because the normal oxidant for QA is unavailable, QA− rapidly accumulates upon illumination. The second induction phase (D-P phase) corresponds to an increase in the relative rate of QA reduction by PS II turnover over the rate of QA− oxidation and is controlled by downstream processes, including turnover of the cytochrome b6f complex and PS I. Fs was eventually reached, reflecting the balance of competing oxidation and reduction processes of the induced photosynthetic apparatus and down-regulatory processes that quench or decrease fluorescence (e.g. state transitions, qE quenching, heat dissipation through the xanthophyll cycle, etc.). In the presence of atrazine (10 μm), fluorescence induction was predominantly monophasic, rising rapidly to Fm; in essence, the D-P and subsequent phases were replaced with a large O-I phase (data not shown).
Figure 7.
Effects HOS on chlorophyll a fluorescence induction curves. A, Cells were dark adapted for 20 min in TAP medium containing no Suc (black squares), 0.1 (white squares), 0.2 (black circles), 0.3 (white circles), or 0.4 (black triangles) m Suc. Chlorophyll a fluorescence yield changes were measured under continuous illumination. In each case, curves were normalized to the maximum fluorescence (Fm) of the corresponding treatments containing 10 μm atrazine. Fluorescence yield, expressed in units of fractional fluorescence, F/Fm, is plotted against time after the start of illumination. B, The dependence on Suc concentration of steady-state (taken at 16.5 s after onset of illumination) variable fluorescence levels, presented in arbitrary units, steady-state fluorescence level (Fs, white squares) and maximum fluorescence levels (Fm, white circles). The steady-state fluorescence yield is also depicted; Fs normalized to Fm (black squares), expressed in fractional fluorescence units, F/Fm. Also shown is the dependence of fluorescence yield at the peak of phase I (taken at 90 ms after the start of illumination), Fi, normalized to Fm (black triangles).
Addition of Suc had multiple effects on the fluorescence induction process. The most dramatic effect was a rise in the level of Fs (or Fs/Fm of the normalized curves, see below) that appeared above about 0.1 m Suc with half-effective concentrations of about 0.175 m (Fig. 7B). At 0.4 m Suc, Fs was essentially equal to the maximal fluorescence value obtained in the same osmotic treatments in the presence of atrazine, and thus oxidation of QA appeared to be nearly completely inhibited by this level of HOS. Such an effect would be expected if electron transfer were inhibited at the level of PC to P700+ electron transfer.
HOS also affected Fm measured in the presence of atrazine (Fig. 7B). Fm decreased as Suc concentration increased above 0.1 m, and we interpret this as reflecting a state 2-like transition accompanying the unstacking of the thylakoid membranes, as previously observed by Endo et al. (1995; see “Discussion”).
The concentration dependence of HOS-induced changes in Fs/Fm was nearly identical to that of its effects on P700+ reduction (compare Fig. 7B with Fig. 5). Fluorescence changes induced with KCl and NaCl also followed the concentration dependencies observed in Figure 5 (data not shown). Thus, we conclude that although the state transition effect observed by Endo et al. (1995) may occur at lower solute concentrations, blockage of PC to P700+ electron transport is likely to have greater physiological impact, at least at higher concentrations.
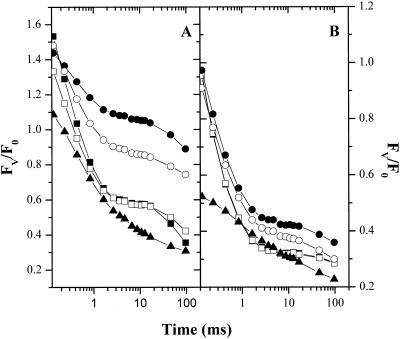
In the presence of moderate concentrations of Suc (0.2 m), the early O-I phase of fluorescence induction appeared to be enhanced and elongated so that the O-I and D-P phases overlapped (Fig. 7A). This effect was repeatable at intermediate concentrations of different solutes, although the effect appeared at lower concentrations when salts were added. Higher concentrations (0.3–0.4 m Suc) almost completely suppressed the O-I phase. This is illustrated in Figure 7B, where the relative fluorescence yield of the I phase, Fi/Fm, (where Fi is the variable fluorescence 90 ms after shutter opening) reaches a peak value at about 0.2 m after which it decreased to levels below the control. We surmised that the rise in the O-I phase might reflect an HOS-induced reduction of the PQ pool, leading to a slowing of QA oxidation. In this case, stronger HOS leads to a net oxidation of the pool. To test this idea, we measured single turnover flash-induced fluorescence kinetics (Fig. 8). In the control, flash excitation induced typical fluorescence decay kinetics (for review, see Kramer et al., 1990; Renger et al., 1995; Kramer and Crofts, 1996). A rapid rise phase, corresponding to the formation of the P680QA− state, occurred during the actinic flash and was unresolved with our instrument. A slower rise phase in the tens of microseconds was evident in an increase in fluorescence yield between the first and second measuring pulses at 50 and 110 μs after actinic flash excitation, respectively. This phase is related to the turnover of the S states of the oxygen evolving complex and probably reflects the reduction of P680+ in equilibrium with the YZ/YZ· couple as the oxygen evolving complex turns over. The subsequent decay of high fluorescence yield reflects the transfer of electrons from QA− to QB. The process is multiphasic, probably reflecting QA− oxidation in centers with the QB site either unoccupied or occupied by PQ, as well as a fraction of centers unable to reduce QB (i.e. non-B centers) and the equilibrium of electron transfer between the QA−QB and QAQB− states (see Taoka et al., 1983; Crofts et al., 1993). In control cells, the most rapid phase had a half-time of about 300 μs, consistent with previous estimates in C. reinhardtii (e.g. Crofts et al., 1993). After addition of 0.2 to 0.3 m Suc (Fig. 8A) a significant fraction of the rapid QA− to QB electron transfer was replaced by a very slow QA− reoxidation phase, reflecting a blockage in electron transfer at the level of QB. Addition of 100 μm p-benzoquinone, which effectively oxidizes the PQ pool (for review, see Bulté and Wollman, 1990), restored rapid QA oxidation (Fig. 8B). This data is consistent with our conclusion from fluorescence induction kinetics that moderate concentrations of solutes induce reduction of the PQ pool. Addition of higher concentrations of Suc (e.g. 0.4 m, Fig. 8A) appeared to suppress this reduction, as evidenced by the large rapid fluorescence decay phase. We suggest that severe HOS can also inhibit processes that lead to PQ reduction in the dark (Godde and Trebst, 1980; Bennoun, 1982; Finazzi and Rappaport, 1998). Addition of p-benzoquinone also significantly lowered the flash-induced fluorescence yields, particularly at high concentrations of Suc, probably reflecting the well-known fluorescence quenching properties of quinones (for review, see Vasil'ev et al., 1998).
Figure 8.
Effects of HOS on single-turnover flash-induced fluorescence changes. A, Cells were incubated in darkness in TAP medium in the presence of no Suc (black squares), 0.1 (white squares), 0.2 (black circles), 0.3 (white circles), or 0.4 (black triangles) m Suc. After 20 min, flash-induced fluorescence kinetics were measured as described in “Materials and Methods.” For each trace, FV (variable fluorescence) was normalized to F0 (baseline fluorescence of the dark-adapted sample) and FV/F0 was plotted against time (on a log10 scale). B, The samples in A were subsequently treated with a final concentration of 100 μm p-benzoquinone to oxidize PQH2 and, after a minimum 5-min dark adaptation, the flash-induced fluorescence kinetics experiments were repeated. Symbols are as in A.
DISCUSSION
Our data strongly suggest that abrupt HOS primarily affects photosynthesis by inhibiting reduction of P700+ (Figs. 1, 3, and 5) and the oxidation of cytochrome f (Fig. 2). The lack of sigmoidal P700 photooxidation kinetics (Fig. 1) and the inhibition of rapid P700+ rereduction after a flash (Fig. 3) show that the blockage occurs between P700 and PC. By demonstrating that the rereduction rate of P700+ can be partially restored by addition of PMS/ascorbate (Fig. 3), we were able to rule out the possibility that HOS led to an oxidizing redox poise, which prevented P700+ rereduction. Two lines of evidence led us to conclude that neither PS I nor PC was destroyed by HOS. First, and most directly, the content of extractable, redox-active PC was not decreased by HOS (data not shown). Second, the effects of such stress were reversed upon removal of excess osmolytes (Fig. 4A) at a rate incompatible with resynthesis of PC. The inhibition process was not restricted to the specific interaction of PC with PS I because cells grown without copper, thus expressing cytochrome c6, were inhibited at very similar osmotic stresses (data not shown). To a lesser extent, HOS also inhibited electron transport through PS II. Electron transport from water to DCPIP was decreased to about 50% of control rates upon addition of 0.4 m Suc (data not shown). However, at these solute concentrations, nearly complete inhibition of PS I reduction (Figs. 4 and 5) was observed, leading us to conclude that the effects on PS I reduction are likely to have larger physiological impact.
Ultrastructural measurements suggest that the average thylakoid width was decreased from approximately 160 to 115 Å upon addition of 0.3 m Suc. Assuming that the thylakoid lipid bilayer has a thickness of approximately 50 Å (Murakami and Packer, 1970; Whitmarsh, 1986), we estimated that the lumenal cavity was compressed from 60 to about 15 Å by osmotic pressure. Because PC has a minimal diameter of 28 Å (Sykes, 1991; Redinbo et al., 1993), and the 4-Å resolution crystal structure of PS I from Synechococcus elongatus shows protrusions extending up to about 20 Å into the lumen (Schubert et al., 1997), we conclude that the mobility of PC and its docking to its binding site on PSI should be severely hindered under these conditions. The possibility that lateral diffusion of PC could be restricted by the narrowness of the thylakoid membranes, as well as by lumenal extrusions of integral membrane proteins, was explicitly proposed by Whitmarsh (1986). This is somewhat at odds with earlier work on isolated higher plant thylakoids, where the general effect of neutral solute addition was to increase the rates of PC interaction with PS I (Haehnel, 1980, and references within). This effect was attributed to a decrease in lumen volume and consequent increase in PC concentrations. We propose that the properties of the lumenal faces of thylakoid membranes and associated membrane proteins may differ between C. reinhardtii and higher plants, preventing, in the case of higher plants, the lumenal space from over-narrowing.
Our model also explains the sigmoidal dependence of inhibition on solute concentrations. Relatively small osmotic changes should have little effect on P700+ reduction, as long as the width of the lumen remains greater than that the diameter of PC. However, a sharp transition should occur at the point where the lumen contracts beyond the point where PC can readily pass to its docking site on PS I.
Fluorescence induction assays (Fig. 7) strongly support our hypothesis that blockage of PC-P700+ electron transfer is the predominant site of inhibition of linear electron transfer, although a state 2-like transition, reflected in a lowering of Fm (Fig. 7B), likely contributes to the effects. HOS also affects the redox state of the PQ pool (Figs. 7A and 8). Moderate HOS induces a reduction of the PQ pool, whereas stronger shocks appear to lead to a net oxidation. There is significant data to support the operation of a “chlorespiratory pathway” in green algae such as C. reinhardtii (Godde and Trebst, 1980; Bennoun, 1982; Finazzi and Rappaport, 1998). We suggest that the reduction is caused by suppression of the PQH2 oxidation reactions as might be expected if they involved an aqueous mobile redox carrier such as PC. We suspect that the net oxidation of the PQ pool at stronger HOS levels is caused by suppression of the reductase reactions.
The data of Endo et al. (1995), which shows an apparent increase in the rate of P700+ rereduction, can also be reconciled with ours. In our hands, full inhibition of P700+ rereduction required 0.15 m or higher concentrations of NaCl, whereas the experiments of Endo et al. (1995) were performed with 0.1 m (approximately half inhibitory). About 50% of P700+ rereduction was slowed (see Fig. 6 in Endo et al., 1995). The observation that the remaining, uninhibited P700+ reduction phase was accelerated may be due to reduction of the PQ pool that occurs, in our hands, at intermediate levels of inhibition. Based on the quenching of Fm, Endo et al. (1995) suggested that a state 2 transition was induced in C. reinhardtii by osmotic shock. Our ultrastructural studies show that unstacking of grana occurs during hyperosmotic stress. In parallel, we observe a lowering of maximal fluorescence yields in the presence of atrazine (Fig. 7B). This would also be consistent with our data showing that the PQ pool was reduced at intermediate concentrations of osmolytes. This, in turn, could lead to the activation of redox-sensitive kinases responsible for regulating state transitions (for review, see Melis, 1996).
In conclusion, we have demonstrated that HOS affects photosynthesis in C. reinhardtii, by dehydration of the lumenal cavity, leading to physical blockage of PC and cytochrome c6 mobility and docking to PS I. Compression of the lumenal cavity is accompanied by unstacking of the grana, which in turn likely increases the non-qP of chlorophyll a fluorescence. We also have observed only a relatively minor inhibition of PS II electron transport, the cause of which remains to be clarified. The effects of HOS in C. reinhardtii are distinct from those in Synechococcus sp. PLL 7942 (Allakhverdiev et al., 2000b). If our interpretation holds true, it would represent, to our knowledge, the first known case where inhibition of a biochemical reaction was caused by physical hindrance from a biological membrane.
MATERIALS AND METHODS
Biological Materials and Growth Conditions
Chlamydomonas reinhardtii CC125 (obtained from Chlamydomonas Genetics Center, Duke University, Durham, NC) was grown photoheterotrophically under approximately 50 μmol photons m−2 s−1 white light from fluorescent tubes in TAP medium at pH 7.0, as described previously (Harris, 1989). Log phase cultures were used either directly, or after concentration by low-speed centrifugation (3 min, 1,000 rpm; Sorvall SLA-1500 rotor, DuPont, Wilmington, DE) followed by resuspension in a small volume of fresh TAP. Cells were allowed to recover after concentration for at least 30 min prior to experimentation. Unless specified, all chemicals used were reagent grade quality from Fisher Scientific Co. (Pittsburgh) or Sigma Chemical Co. (St. Louis).
Spectrophotometric Assays
Flash or continuous light-induced changes in the redox state of P700 were probed by observing time-resolved absorbance changes at 820 nm or between 703 and 730 nm (703- to 730-nm absorbance changes), using an instrument based on those described previously (Kramer and Crofts, 1990; Kramer and Sacksteder, 1998). Saturating single-turnover actinic flashes were provided by a xenon flashlamp (approximately 2 J total energy output per pulse, 5-μs duration), filtered with two layers of a red filter (Schott RG665, Schott Glass Technologies, Duryea, PA). Continuous light was provided by a set of seven red (640 nm) light-emitting diodes (LEDs; HLPM-8103, Agilent, Palo Alto, CA), providing approximately 110 μmol photons m−2 s−1. The concentration of cells varied somewhat between experiments, from about 25 to 50 μg chlorophyll mL−1. Data from each preparation was scaled to the total photooxidizable P700 concentration, determined by measuring the extent of the 703- to 730-nm absorbance change upon illumination in the presence of 10 μm DBMIB to block electron transfer through the cytochrome b6f complex.
Flash-induced absorbance changes associated with the redox state of cytochrome f were measured at 545, 554, and 572 nm and deconvoluted using the procedure described previously (Kramer and Sacksteder, 1998). In some experiments, DBMIB was added to final concentration of 10 μm to block reduction of cytochrome f by PQH2.
PC was extracted from whole cells by five cycles of freezing (at −20°C) and thawing, followed by centrifugation for 30 min at 10,000g to remove cellular debris. Extracts were either oxidized in the presence of 100 μm sodium ferricyanide or reduced in the presence of 5 mm sodium ferrocyanide. The concentration of ferrocyanide used reduced essentially all PC (addition of 5 mm sodium ascorbate had little additional effect on the peak A597) without reducing significant amount of soluble c-type cytochrome. PC content was then calculated from the oxidized-minus-reduced difference spectra of extracts, using an extinction coefficient of 9.8 mm−1 cm−1 at 597 nm (Hiyama and Ke, 1972). The content of P700 was determined by measuring the extents of the light-induced absorbance changes at 703 to 730 nm in the presence of 10 μm DBMIB, using an extinction coefficient of 64 mm−1 cm−1 (Hiyama and Ke, 1972).
Oxygen Evolution
Steady-state rates of oxygen evolution in intact C. reinhardtii cells at 25 μg chlorophyll mL−1 were measured polarographically (Allen and Holmes, 1986), using a water-jacketed (20°C) Clark electrode O2 electrode, constructed in-house. Cells were dark adapted for 20 min in the presence or absence of added osmolytes, during which time dark respiratory rates were assessed. Cells were then illuminated with saturating white light (approximately 800 μmol photons m−2 s−1) filtered through a dilute solution of cupric sulfate to remove excess heat. PS II turnover was assayed as O2 evolution in the presence of 100 μm DCPIP. All samples were assayed in 20 mm Tris-HCl, pH 7.4, with 1 mm sodium azide to inhibit endogenous catalase activity.
Chlorophyll a Fluorescence Kinetics
Single-turnover pulsed fluorescence changes and fluorescence induction kinetics were measured using a microsecond-resolution kinetic fluorimeter based on that described previously (Kramer, 1990; Kramer et al., 1995). The pulsed beam (pulse width of 3 μs) had an emission peak of 650 nm, was provided by a bank of seven LEDs (HLMP-8104, 637-nm peak emission). The light was filtered through a 635-nm 70-nm band pass interference filter (635DF70, Omega Optical, Brattleboro, VT) to block infrared emissions. Fluorescence excited by the LED pulses was detected by a photodiode (Hamamatsu 1773, Hamamatsu Corp., Bridgewater, NJ) protected by two layers of Kodak Wratten 89B filter (Eastman-Kodak, Rochester, NY) covered by one layer of red glass filter (Schott RG695, Schott Glass Technologies). A flash sufficiently intense and short (5 μs at half intensity) to provide a single turnover of >95% of PS II centers was provided by a xenon lamp filtered through a blue color glass filter (BG-23 Schott glass filter, Schott Glass Technologies). Continuous actinic illumination (50 μmol photons m−2 s−1) was provided by a shuttered halogen lamp filtered through one layer of a blue glass filter (Corning 4-96) and an infrared reflecting filter (OBHM, Optical Coating Laboratory, Los Angeles).
Electron Microscopy
C. reinhardtii cells in TAP medium or treated in TAP medium with 0.3 m Suc for 30 min were fixed and embedded for electron microscopy by standard techniques. Cells were fixed in 2% (w/v) paraformaldehyde, 2% (w/v) glutaraldehyde, and 50 mm PIPES (1,4-piperazinediethanesulfonic acid), pH 7.2, overnight at 4°C. They were then postfixed in 1% (w/v) OsO4 for 2 h at room temperature and then rinsed three times with 50 mm HEPES [4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid] buffer, pH 7.2. The fixed cells were then progressively dehydrated in a series of ethanol concentrations from 30% to 100% (w/v). Following dehydration, the cells were infiltrated with SPURRS (Sigma Chemical Co.):ethanol mix, gradually increasing the ratio SPURRS:ethanol from 1:3 (w/v) to undiluted over a period of 2 d, followed by embedding overnight at 70°C. Embedded cells were then sectioned with a Reichert 2 microtome (Leica Microsystems, Bannockburn, IL) and the resulting thin sections were collected on formvar coated copper grids and stained for 15 min with a 2% (w/v) solution of uranyl acetate followed by 15 min with a 1% (w/v) solution of lead citrate. Electron microscopy was performed with a JEOL JEM 1200-EX microscope. Efforts to obtain satisfactory frozen sections of C. reinhardtii cells were unsuccessful because high concentrations of osmolytes significantly affected the freezing process and thus the resolution of the resulting sections.
For estimates of thylakoid width, areas on the images of thylakoids were randomly selected by positioning a transparent (overhead projector) film with a series of 20 cross-hatched lines. Points of intersection between these hatches or recognizable thylakoid membranes were measured. The thickness of thylakoid (which includes both membranes and the lumen space) was estimated using a Vernier caliper and scaled using appropriate magnification factors. This approach was very similar to those of Murakami and Packer (1970), who used densitometry of the electron micrographs to estimate thylakoid thickness. Cases where the two-thylakoid membranes diverged significantly (as in Fig. 6, arrow) were ignored. Furthermore, because some thylakoid membranes were not always identifiable, e.g. membranes running nearly parallel to the plane of the thin section would not readily be identified as thylakoids, it was not possible to obtain true representative measurements of thylakoid geometry.
ACKNOWLEDGMENTS
The authors express their gratitude to Christine M. Davitt, Valerie Lynch-Holm, and Dr. Vincent Franceschi for expert assistance with the electron microscopy experiments, and to Dr. Francís-André. Wollman for important discussions and access to unpublished data.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 9635306577), by the Plant Biochemistry Research Training Center (postdoctoral fellowship no. DE–FG06–94ER20160 to J.A.C.), and by the U.S. Department of Energy (grant no. DE–FG03–98ERZ0299).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010328.
LITERATURE CITED
- Albertsson P-A. Interaction between the lumenal sides of the thylakoid membrane. FEBS Lett. 1982;149:186–190. [Google Scholar]
- Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000a;123:1047–1056. doi: 10.1104/pp.123.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev SI, Sakamoto A, Nishiyama Y, Murata N. Inactivation of photosystems I and II in response to osmotic stress in Synechococcus: contributions of water channels. Plant Physiol. 2000b;122:1201–1208. doi: 10.1104/pp.122.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JF, Holmes NG. Electron transport and redox titration. In: Hipkins MF, Baker NR, editors. Photosynthesis: Energy Transduction, A Practical Approach. Oxford: IRL Press Limited; 1986. pp. 103–141. [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA. 1982;79:4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GA, Chen C, Gibbs M. Stromal acidification mediates in vivo water stress inhibition of nonstomatal-controlled photosynthesis. Plant Physiol. 1983;72:1123–1126. doi: 10.1104/pp.72.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briantais J-M, Vernotte C, Krause G, Weis E. Govindjee, J Amesz, DC Fork, eds, Light Emission by Plants and Bacteria. New York: Academic Press; 1986. Chlorophyll a fluorescence of higher plants: chloroplasts and leaves; pp. 539–583. [Google Scholar]
- Bulté L, Wollman F-A. Stabilization of states I and II by p-benzoquinone treatment of intact cells of Chlamydomonas reinhardtii. Biochim Biophys Acta. 1990;1016:253–258. [Google Scholar]
- Cramer WA, Widger WR, Black MT, Girvin M. Structure and function of photosynthetic bc1 and b6f complexes. In: Barber J, editor. The Light Reactions. Amsterdam: Elsevier; 1987. pp. 447–493. [Google Scholar]
- Crofts AR, Baroli I, Kramer D, Taoka S. Kinetics of electron transfer between QA and QB in wild type and herbicide-resistant mutants of Chlamydomonas reinhardtii. Z Naturforsch. 1993;48:259–266. [Google Scholar]
- Endo T, Schreiber U, Asada K. Suppression of quantum yield of photosystem II by hyperosmotic stress in Chlamydomonas reinhardtii. Plant Cell Physiol. 1995;36:1253–1258. [Google Scholar]
- Finazzi G, Rappaport F. In vivo characterization of the electrochemical proton gradient generated in darkness in green algae and its kinetic effects on cytochrome b6f turnover. Biochemistry. 1998;37:9999–10005. doi: 10.1021/bi980320j. [DOI] [PubMed] [Google Scholar]
- Fulda S, Huckauf J, Schoor A, Hagemann M. Analysis of stress responses in the cyanobacterial strains Synechococcus so. PCC 6803, and Synechococcus sp. PCC 7418: osmolyte accumulation and stress protein synthesis. J Plant Physiol. 1999;154:240–249. [Google Scholar]
- Gamboa A, Alfaro J, Reynoso T. Taurine induction of cation tolerance in Chlamydomonas reinhardtii. Comp Biochem Physiol. 1985;81A:491–493. [Google Scholar]
- Genty B, Harbinson J. Regulation of light utilization for photosynthetic electron transport. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 67–99. [Google Scholar]
- Gilmour DJ, Hipkins MF, Boney AD. The effect of osmotic stress and ion stress on the primary processes of photosynthesis. J Exp Bot. 1984;35:18–27. [Google Scholar]
- Godde D, Trebst A. NADH as electron donor for the photosynthetic membrane of Chlamydomonas reinhardtii. Arch Microbiol. 1980;127:245–252. [Google Scholar]
- Govindjee Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol. 1995;22:20–29. [Google Scholar]
- Govindjee, Satoh K. Fluorescence properties of chlorophyll b- and chlorophyll c-containing algae. In: Govindjee X, Amesz J, Fork DC, editors. Light Emission by Plants and Bacteria. Orlando, FL: Academic Press; 1986. pp. 497–530. [Google Scholar]
- Grodzinski B, Colman B. Loss of photosynthetic activity in two blue-green algae as a result of osmotic stress. J Bacteriol. 1973;115:456–458. doi: 10.1128/jb.115.1.456-458.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel W. Electron transfer from plastocyanin to P700. FEBS Lett. 1980;111:79–82. [Google Scholar]
- Haehnel W. On the lateral electron transport between the two light reactions in spinach chloroplasts. In: Sybesma C, editor. Advances in Photosynthesis Research. Martinus Nijhoff/Dr. W. The Hague, The Netherlands: Junk Publishers; 1984. pp. 545–548. [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Hiyama T, Ke B. Difference spectra and extinction coefficients of P700. Biochim Biophys Acta. 1972;267:160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Joliot P, Joliot A. Electron transfer between photosystem II and the cytochrome b/f complex: mechanistic and structural implications. Biochim Biophys Acta. 1992;1102:53–61. [Google Scholar]
- Jones RW, Whitmarsh J. Origin of the electrogenic reaction in the chloroplast cytochrome b/f complex. Biochem J. 1985;9:119–127. [Google Scholar]
- Jones RW, Whitmarsh J. Inhibition of electron transfer in the cytochrome b/f complex by NQNO and DBMIB: evidence for two quinone binding sites. In: Biggens J, editor. Progress in Photosynthesis Research. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1987. pp. 441–473. [Google Scholar]
- Kirst GO. Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:21–53. [Google Scholar]
- Klughammer C, Schreiber U. Analysis of light-induced absorbance changes in the near-infrared spectral region. Z Naturforsch. 1991;46c:233–244. [Google Scholar]
- Kramer DM. Parallel instrumental approaches to the study of the bioenergetics of photosynthesis in chloroplasts and intact plants. PhD thesis. Champaign, IL: University of Illinois-Urbana; 1990. [Google Scholar]
- Kramer DM, Crofts AR. Demonstration of a highly-sensitive portable double-flash kinetic spectrophotometer for measurement of electron transfer reactions in intact plants. Photosynth Res. 1990;23:231–240. doi: 10.1007/BF00035014. [DOI] [PubMed] [Google Scholar]
- Kramer DM, Crofts AR. Control of photosynthesis and measurement of photosynthetic reactions in intact plants. In: Baker N, editor. Photosynthesis and the Environment: Advances in Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Press; 1996. pp. 25–66. [Google Scholar]
- Kramer DM, DiMarco G, Loreto F. Contribution of plastoquinone quenching to saturation pulse-induced rise in chlorophyll fluorescence in leaves. In: Mathis P, editor. Photosynthesis: From Light to Biosphere. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 147–150. [Google Scholar]
- Kramer DM, Robinson HR, Crofts AR. A portable multi-flash kinetic fluorimeter for measurement of donor and acceptor reactions of photosystem 2 in leaves of intact plants under field conditions. Photosynth Res. 1990;26:181–193. doi: 10.1007/BF00033131. [DOI] [PubMed] [Google Scholar]
- Kramer DM, Sacksteder CA. A diffused-optics flash kinetic spectrophotometer (DOFS) for measurements of absorbance changes in intact plants in the steady-state. Photosynth Res. 1998;56:103–112. doi: 10.1023/A:1017906626288. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Lavergne J, Briantais J-M. Photosystem II heterogeneity. In: Ort DR, Yocum CF, editors. Oxygenic Photosystem: The Light Reactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 265–287. [Google Scholar]
- León R, Galván F. Metabolic pathway for glycerol synthesis under osmotic stress in the fresh water alga Chlamydomonas reinhardtii. Plant Physiol Biochem. 1995;33:213–218. [Google Scholar]
- Malkin S. Kinetic studies on electron-transport components in isolated chloroplasts: I. The effect of the pool of electron carriers between the two photosystems in P700 changes. Biochim Biophys Acta. 1968;162:392–401. doi: 10.1016/0005-2728(68)90125-4. [DOI] [PubMed] [Google Scholar]
- Marsho TV, Kok B. Interactions between electron transport components in chloroplasts. Biochim Biophys Acta. 1970;223:240–250. doi: 10.1016/0005-2728(70)90181-7. [DOI] [PubMed] [Google Scholar]
- Melis A. Excitation energy transfer: functional and dynamic aspects of Lhc (cab) proteins. In: Ort DR, Yokum CF, editors. Oxygenic Photosynthesis: The Light Reactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 523–538. [Google Scholar]
- Merchant S, Bogorad L. Regulation by copper of the expression of plastocyanin and cytochrome c552 in Chlamydomonas reinhardtii. Mol Cell Biol. 1986;6:462–469. doi: 10.1128/mcb.6.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Meijer HJG, Riett R, Hirt H, Franck W, Bartels D, Musgrave A. Hyperosmotic stress stimulated phospholipase D activity and elevated the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J. 2000;22:147–154. doi: 10.1046/j.1365-313x.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Packer L. Protonation and chloroplast membrane structure. J Cell Biol. 1970;47:332–351. doi: 10.1083/jcb.47.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale PJ, Melis A. Salinity-stress enhances photoinhibition of photosynthesis in Chlamydomonas reinhardtii. J Plant Physiol. 1989;134:619–622. [Google Scholar]
- Redinbo MR, Cascio D, Choukair MK, Rice D, Merchant S, Yeates TO. The 1.5-Å crystal structure of plastocyanin from the green alga Chlamydomonas reinhardtii. Biochemistry. 1993;32:10560–10567. doi: 10.1021/bi00091a005. [DOI] [PubMed] [Google Scholar]
- Renger G, Eckert HJ, Bergmann A, Bernarding J, Liu B, Napiwotzki A, Reifarth F, Eichler HJ. Fluorescence and spectroscopic studies of exciton trapping and electron transfer in photosystem II of higher plants. Aust J Plant Phys. 1995;22:167–181. [Google Scholar]
- Reynoso T, Gamboa A. Salt tolerance in the freshwater alga Chlamydomonas reinhardtii: effect of proline and taurine. Comp Biochem Physiol. 1982;73A:95–99. [Google Scholar]
- Rich PR, Bendall DS. Electron and proton transfer in the plastoquinol-plastocyanin oxidoreductase. In: Palmieri F, editor. Vectorial Reactions in Electron and Ion Transport in Mitochondria and Bacteria. Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press; 1981. pp. 187–190. [Google Scholar]
- Satoh K, Smith CM, Fork DC. Effects of salinity on primary processes of photosynthesis in the red algae. Plant Physiol. 1983;73:643–647. doi: 10.1104/pp.73.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W-D, Klukas O, Krauβ N, Saenger W, Fromme P, Witt HT. Photosystem I of Synechococcus elongus at 4 Å resolution: comprehensive structural analysis. J Mol Biol. 1997;272:741–769. doi: 10.1006/jmbi.1997.1269. [DOI] [PubMed] [Google Scholar]
- Sykes AG. Plastocyanin and blue copper proteins. Struct Bond. 1991;75:175–224. [Google Scholar]
- Taoka S-I, Robinson HH, Crofts AR. Kinetics of the reaction of the two electron gate of photosystem II: studies of the competition between plastoquinone and inhibitors. In: Inoue Y, Crofts AR, Govindjee X, Murata N, Renger G, Satoh K, editors. The Oxygen Evolving System of Photosynthesis. Tokyo: Academic Press Inc.; 1983. pp. 369–381. [Google Scholar]
- Trezzi F, Galli MG, Bellini E. L'osmo-resistenza di Dunaliella salina richerche ultrastrutturali. G Bot Ital. 1965;72:255–263. [Google Scholar]
- Vasil'ev S, Wiebe S, Bruce D. Non-photochemical quenching of chlorophyll fluorescence in photosynthesis: 5-hydroxy-1,4-naphthoquinone in spinach thylakoids as a model for antenna based quenching mechanisms. Biochim Biophys Acta. 1998;1363:147–156. doi: 10.1016/s0005-2728(97)00096-0. [DOI] [PubMed] [Google Scholar]
- Vredenberg WJ, Duysens LNM. Light-induced changes in absorbancy and fluorescence of chlorophyllous pigments associated with the pigment systems 1 and 2 in blue-green algae. Biochim Biophys Acta. 1965;94:355–370. doi: 10.1016/0926-6585(65)90044-0. [DOI] [PubMed] [Google Scholar]
- Whitmarsh J. Mobile electron carriers in thylakoids. In: Staehlin LA, Arntzen CJ, editors. Encyclopedia of Plant Physiology. Berlin: Springer; 1986. pp. 508–527. [Google Scholar]
- Wiltens J, Schreiber U, Vidaver W. Chlorophyll fluorescence induction: an indicator of photosynthetic activity in marine algae undergoing dessication. Can J Bot. 1978;56:2787–2794. [Google Scholar]