Abstract
Biotin synthase, encoded by the bio2 gene in Arabidopsis, catalyzes the final step in the biotin biosynthetic pathway. The development of radiochemical and biological detection methods allowed the first detection and accurate quantification of a plant biotin synthase activity, using protein extracts from bacteria overexpressing the Arabidopsis Bio2 protein. Under optimized conditions, the turnover number of the reaction was >2 h−1 with this in vitro system. Purified Bio2 protein was not efficient by itself in supporting biotin synthesis. However, heterologous interactions between the plant Bio2 protein and bacterial accessory proteins yielded a functional biotin synthase complex. Biotin synthase in this heterologous system obeyed Michaelis-Menten kinetics with respect to dethiobiotin (Km = 30 μm) and exhibited a kinetic cooperativity with respect to S-adenosyl-methionine (Hill coefficient = 1.9; K0.5 = 39 μm), an obligatory cofactor of the reaction. In vitro inhibition of biotin synthase activity by acidomycin, a structural analog of biotin, showed that biotin synthase reaction was the specific target of this inhibitor of biotin synthesis. It is important that combination experiments using purified Bio2 protein and extracts from pea (Pisum sativum) leaf or potato (Solanum tuberosum) organelles showed that only mitochondrial fractions could elicit biotin formation in the plant-reconstituted system. Our data demonstrated that one or more unidentified factors from mitochondrial matrix (pea and potato) and from mitochondrial membranes (pea), in addition to the Bio2 protein, are obligatory for the conversion of dethiobiotin to biotin, highlighting the importance of mitochondria in plant biotin synthesis.
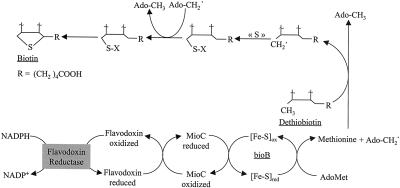
Biotin acts as a cofactor for a small number of enzymes involved in carboxylation, decarboxylation, and transcarboxylation reactions that are concerned with fatty acid and carbohydrate metabolism (Knowles, 1989; Alban et al., 2000). Biotin biosynthesis has been widely investigated in bacteria such as Escherichia coli or Bacillus sphaericus through combined biochemical and genetic studies (Eisenberg, 1987; Gloeckler et al., 1990). The most fascinating reaction in this pathway, as well as the most complex, is undoubtedly the last one, i.e. the insertion of a sulfur atom between the unactivated methyl and methylen carbon atoms adjacent to the imidazolinone ring of dethiobiotin (DTB). Biotin synthase (the product of bioB gene), an iron-sulfur containing protein, is involved in this reaction. Although extensively studied during the past decade, the catalytic mechanism of the last step of biotin synthesis is not fully understood, and all the components involved in this reaction are not identified. Nevertheless, it was established that this conversion reaction requires S-adenosyl-Met (Ado-Met) acting as a radical-forming molecule responsible for the homolytic cleavage of the C-H bonds at the carbons to be functionalized, and a physiological reduction system consisting, in E. coli, of flavodoxin, flavodoxin (ferredoxin)-NADP+ reductase, NADPH, and possibly another FMN-containing flavoprotein, MioC (Scheme S1; Ifuku et al., 1992, 1994; Sanyal et al., 1994, 1996; Birch et al., 1995; Guianvarc'h et al., 1997; Shaw et al., 1998; Birch et al., 2000). In bacteria, Cys is very likely the initial source of the sulfur atom for the reaction (Birch et al., 1995). However, recent work provides evidence that the immediate sulfur donor is biotin synthase itself (Tse Sum Bui et al., 1998; Gibson et al., 1999).
Scheme 1.
Initial information on biotin synthesis and transport in plants came from analysis of the bio1 biotin auxotrophic mutant of Arabidopsis that requires biotin at a critical stage of embryogenesis (Schneider et al., 1989; Shellhammer and Meinke, 1990). Bio1 mutation can be complemented genetically by the E. coli bioA gene that codes for 7,8-diaminopelargonic acid aminotransferase, the second enzyme in the E. coli biotin biosynthetic pathway (Patton et al., 1996b). Radiotracer studies of a biotin-overexpressing strain of lavender (Lavandula vera) cells grown in the presence of [3H]pimelic acid demonstrated that the pathway of biotin synthesis in bacteria is conserved in plants and that the reaction catalyzed by biotin synthase may proceed in two distinct steps involving 9-mercaptodethiobiotin as an intermediate (Baldet et al., 1993b). Since then, a second biotin auxotroph of Arabidopsis has subsequently been identified. Arrested embryos from this bio2 mutant are defective in the final step of biotin synthesis, i.e. the conversion of DTB to biotin (Patton et al., 1998). Molecular characterization of the biosynthetic pathway has dealt primarily with the biotin synthase gene. A cDNA corresponding to this gene from Arabidopsis (called bio2) has been isolated by functional complementation of a bioB biotin auxotroph mutant of E. coli (Baldet and Ruffet, 1996), and gene expression characterized (Patton et al., 1996a; Weaver et al., 1996). Purified recombinant Arabidopsis biotin synthase is a homodimer of 41.6-kD subunits with a reddish color and has an absorbance spectrum characteristic of a protein with [2Fe-2S] clusters (Baldet et al., 1997a). Finally, immunological analyses with antibodies raised against the purified recombinant protein demonstrated a mitochondrial location for the plant biotin synthase (Baldet et al., 1997a).
Here, we present the first biochemical characterization of a plant biotin synthase activity, using a heterologous system comprising the recombinant Arabidopsis biotin synthase and accessory proteins from E. coli. On the other hand, using a plant-reconstituted system, we demonstrate that besides the bio2 gene product, mitochondrial proteins and/or unidentified factors are required for the plant biotin synthase reaction.
RESULTS
Arabidopsis Biotin Synthase Reaction in a Heterologous System
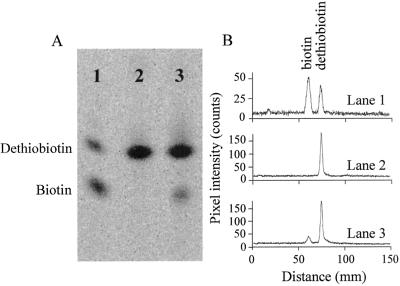
Studies presented in this article were performed using an E. coli strain overproducing bio2 gene product (biotin synthase) from Arabidopsis (Baldet et al., 1997a). When bacteria were grown under optimum overexpression conditions (see “Materials and Methods”), the polypeptide accounted for 2% to 4% of the bacterial soluble proteins, according to the preparations, as judged by ELISA quantitation, using affinity-purified anti-Bio2 antibodies. Biotin synthase activity from Arabidopsis was detected using both the radiochemical and the microbiological methods detailed in “Materials and Methods.” Figure 1 shows the results of a typical in vitro assay for biotin synthase, in protein extracts from genetically engineered bacteria, with [3H]DTB as the source of radioactive label. Detection of [3H]biotin was by TLC and PhosphorImager analysis. This experiment demonstrated biotin production by a protein extract from BL21 E. coli cells overproducing Bio2 from Arabidopsis (Fig. 1, lane 3). In contrast, a protein extract from untransformed BL21 strain proved unable to synthesize biotin from DTB (Fig. 1, lane 2; Fig. 2). Therefore, biotin synthesis was strictly dependent on the presence of recombinant Bio2 protein in the assay. The lack of detectable biotin synthase activity in the host bacterial strain, in vitro, could be explained by the fact that although it carried the wild-type chromosomal genes for biotin synthesis, these were repressed under the conditions of our experiments (Eisenberg, 1973; Alban, 2000). Furthermore, because of the weakness of biotin synthase activities reported in cell-free assay systems of bacterial origin, all biotin synthase reactions from these sources were obtained only with extracts of BioB-overproducing strains (Ifuku et al., 1992; Sanyal et al., 1994, 1996; Birch et al., 1995; Guianvarc'h et al., 1997; Shaw et al., 1998).
Figure 1.
Detection of biotin synthase activity in the in vitro heterologous system. A, Biotin synthase activity was measured by the conversion of [3H]DTB to [3H]biotin (“Materials and Methods”). Substrate and product were separated by thin-layer chromatography (TLC) and detected by phosphorimaging Analysis. Lane 1, DTB and biotin standards; lane 2, assay with 1.5 mg of protein extract from untransformed BL21 E. coli strain; lane 3, assay with 1.5 mg of protein extract from BL21 E. coli cells overproducing Bio2 from Arabidopsis. Reaction mixtures were incubated for 2 h. B, Quantitative analysis of radioactive spots in A, using ImageQuant software (Molecular Dynamics, Sunnyvale, CA), by the area quantitation method with peak finder.
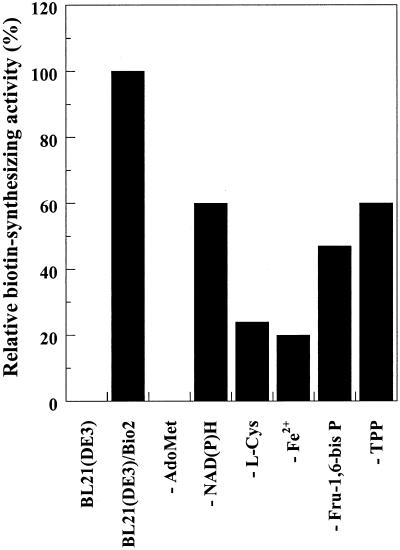
Figure 2.
Optimization of the biotin synthase reaction medium from the in vitro heterologous system. Biotin synthase activity was measured by the conversion of [3H]DTB to [3H]biotin. Biotin formed was determined by TLC analysis and phosphorimaging quantitation (“Materials and Methods”). Complete reaction medium consisted of 1.5 mg of protein extract from BL21 E. coli cells overproducing Bio2 from Arabidopsis, 10 mm dithiothreitol (DTT), 0.5 mm Fe(NH4)2(SO4)2, 1 mm NADPH, 10 mm KCl, 0.2 mm Ado-Met, 5 mm Fru-1,6-bisP, 0.5 mm l-Cys, 0.1 mm thiamin pyrophosphate (TPP), 20 mm l-Asn, and 150 μm [3H]DTB in 50 mm Tris-HCl, pH 8 [lane BL21(DE3)/Bio2]. The effect of omissions, as indicated, was then investigated. An assay with complete reaction medium except for substitution of the protein extract from Bio2-overproducing strain by a protein extract from untransformed bacteria, was run as negative control [lane BL21(DE3)]. The data are from a representative experiment repeated three times. Fe2+ = Fe(NH4)2(SO4)2.
To precisely quantify the activity of biotin synthase from Arabidopsis in our in vitro system, we have first undertaken optimization of the assay. Defined in vitro reaction mixtures reported in the literature for bacterial systems are rather inconsistent and still a matter of debate. Required small molecules in these assays include, in addition to the substrate DTB, Ado-Met, NAD(P) H, Fe2+, l-Cys, DTT, and, according to the cases, KCl, Fru-1,6-bisP, l-Asn, and/or TPP (Sanyal et al., 1994, 1996; Birch et al., 1995; Guianvarc'h et al., 1997; Shaw et al., 1998; Tse Sum Bui et al., 1998). In our hands, biotin synthase activity was initially measured in a reaction mixture termed “complete,” i.e. comprising all the above components. Then, the effect of not supplying each of these compounds individually was investigated (Fig. 2). The reaction was found to be strictly dependent on Ado-Met. This compound is probably not the initial sulfur donor in the reaction, but rather acts as a source of deoxyadenosyl radical, which would be implicated in the activation of the C-H bonds on DTB, as demonstrated in bacterial systems (Guianvarc'h et al., 1997). l-Cys and Fe2+, although not absolutely necessary, highly stimulated biotin synthase reaction, up to five times. Na2S could efficiently replace l-Cys in the assay (not shown). NADH or NADPH, TPP, and Fru-1,6-bisP also enhanced biotin formation, albeit to lower extents (Fig. 2). The physiological relevance of Fru-1,6-bisP is not fully understood. Nevertheless, because it had a significant positive effect on biotin synthase activity, it was maintained in our assay. The influence of TPP is also controversial among different groups (Birch et al., 1995; Sanyal et al., 1996; Shaw et al., 1998). TPP is a potential initial source of sulfur for the biotin synthase activity in vitro, and possibly somehow participates in the reaction together with l-Cys. Alternatively, TPP may act indirectly, having a positive effect on enzymes, in the bacterial extract, which are necessary for biotin formation by Arabidopsis biotin synthase. It is interesting that Birch et al. (2000) reported the identification of a new flavoprotein in E. coli, encoded by mioC gene, which is essential for E. coli biotin synthase activity, in vitro (see Scheme S1). This protein required TPP to stabilize its activity. Finally, KCl and l-Asn, reported to have stimulating effects in bacterial systems, had no effect in our assay, and were subsequently omitted. Thus, after optimization of the concentrations of these low-Mr components required for activity, all our analyses led to reaction medium composition detailed in “Materials and Methods.”
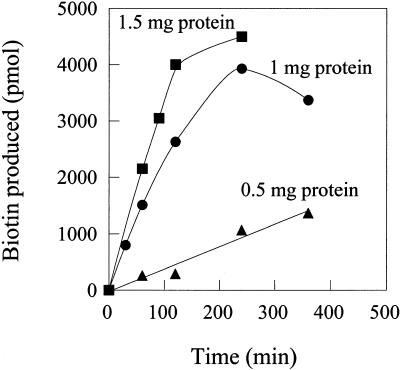
To further optimize the assay conditions for biotin synthase activity, the linearity of each assay with respect to incubation time and protein concentrations were established, using both radiochemical (Fig. 3) and microbiological detection methods (not shown), with similar results. Best kinetics were obtained using Bio2-containing protein extract concentrations of at least 10 mg mL−1. Under these optimum concentration conditions, activity was linear for at least 3 h and then slowed down to reach a plateau after 4- to 6-h incubation times (Fig. 3). From the kinetic results in Figure 3, and assuming that 2% of protein in the extract was biotin synthase from Arabidopsis, as determined by ELISA quantitation, and a molecular mass for the monomer of 41.6 kD, we calculated that up to 7 mol biotin mol Bio2 monomer−1 were synthesized, under optimized conditions over a 6-h incubation period. The catalytic center activity of the enzyme was also estimated from these data to be approximately 2.5 to 3 h−1. These parameter values, albeit unusually low for an enzyme activity, are somewhat higher than those reported for bacterial biotin synthase reactions, in both cell-free extract and well-defined systems (0.04–0.08 h−1 for B. sphaericus, Méjan et al., 1995; 0.5–1 h−1 for E. coli, Sanyal et al., 1994; Shaw et al., 1998).
Figure 3.
Biotin synthase reaction time course in the in vitro heterologous system. Biotin synthase activity was assayed under optimized assay conditions, in the presence of 0.5, 1, and 1.5 mg of protein extract from BL21 E. coli cells overproducing Bio2 from Arabidopsis, respectively, in a total volume of 100 μL. Biotin produced was determined by TLC analysis and phosphorimaging quantitation (conversion of [3H]DTB to [3H]biotin).
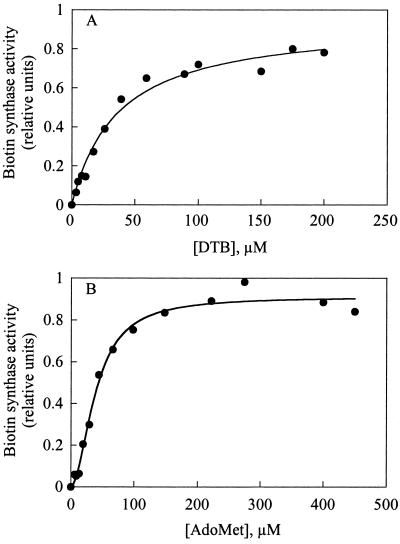
Kinetic parameters for d-DTB and Ado-Met, the two obligatory substrates for the reaction, were determined under these optimized assay conditions. Assays were conducted by varying the concentration of one of the substrates while keeping the other substrates and cofactors at saturating levels. Under these conditions, recombinant Arabidopsis biotin synthase obeyed Michaelis-Menten kinetics with respect to DTB (Fig. 4). The apparent Km for DTB in the biotin synthase reaction was found to be 30 ± 5 μm. This value is one order of magnitude higher than that measured by Sanyal et al. (1994) for E. coli biotin synthase reaction in vitro, but well agrees with optimized DTB concentrations determined for this reaction, reported in other studies (Sanyal et al., 1996; Kiyasu et al., 2000; Tse Sum Bui et al., 2000). Arabidopsis biotin synthase exhibited an exceptional sensitivity to changes in Ado-Met concentrations (kinetic cooperativity). Hill plot provides a simple means of evaluating this cooperativity (Hill coefficient = 1.9; Fig. 4). The apparent K0.5 value for Ado-Met, calculated from the data in Figure 4B and Hill equation (Segel, 1975), was 39 ± 2 μm. There are no published kinetic constants for Ado-Met, but this value is consistent with Ado-Met concentrations required to saturate biotin synthase reactions in bacterial systems (for example, see Shaw et al., 1998; Tse Sum Bui et al., 2000).
Figure 4.
Effect of DTB and Ado-Met on the activity of biotin synthase in a protein extract from E. coli cells overproducing Bio2 from Arabidopsis (in vitro heterologous system). Biotin synthase activity was measured under optimized conditions by varying the concentrations of one of the substrates (DTB or Ado-Met) while keeping the other substrates and cofactors at saturating levels (see “Materials and Methods”). Biotin produced was determined by a turbidimetric microbiological method using Lactobacillus plantarum. From the curves, apparent Km value for d-DTB (A) and K0.5 for Ado-Met (B) were determined. The lines represent nonlinear regressions to the Michaelis-Menten equation (A) or the Hill equation (B), using KaleidaGraph, as described in “Materials and Methods.”
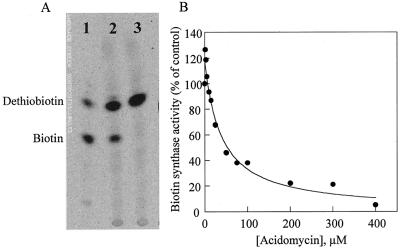
The effect of acidomycin, a structural analog of biotin, with antibiotic properties, was investigated on biotin synthase reaction (Fig. 5). Previous studies performed in vivo using cultured lavender cells indicated that this compound specifically blocked biotin synthesis at the level of biotin synthase reaction (Baldet et al., 1993b). When included at a concentration of 400 μm, acidomycin completely inhibited in vitro biotin formation driven by Bio2 from Arabidopsis (Fig. 5A). Under the optimized assay conditions described in “Materials and Methods,” biotin synthase reaction was inhibited in a concentration-dependent manner, and 50% inhibition (IC50) occurred at about 35 μm acidomycin (Fig. 5B). This value well correlated with inhibitor concentrations necessary to obtain a sensitive herbicidal effect in planta (Baldet, 1993; Baldet et al., 1993b). Acidomycin may prove, therefore, to be a good tool for studying biotin synthase reaction mechanism further.
Figure 5.
Effect of acidomycin on the activity of biotin synthase in a protein extract from E. coli cells overproducing Bio2 from Arabidopsis (in vitro heterologous system). Biotin synthase activity was measured under optimized conditions with 1.5 mg of protein extract. Reaction mixtures were incubated for 2 h. A, Biotin formed was determined by TLC analysis and phosphorimaging quantitation (conversion of [3H]DTB to [3H]biotin). Lane 1, DTB and biotin standards; lane 2, assay in the absence of acidomycin; lane 3, assay in the presence of 400 μm acidomycin. B, Effect of increasing concentrations of acidomycin on biotin synthase activity. Biotin produced was determined by a turbidimetric microbiological method using L. plantarum.
To further characterize the enzymatic activity of the bio2 gene product from Arabidopsis, a three-step protocol was devised to purify it from E. coli/Bio2 cells. The procedure, derived from our previously published protocol (Baldet et al., 1997a), consisted in two anion-exchange chromatographies followed by a cation-exchange chromatography. Thus, typically 4 to 5 mg of at least 95% pure recombinant biotin synthase was obtained from 1 L of cell culture. Data in Table I show that after the very first purification step, protein fractions containing Bio2 protein lost biotin synthase activity. However, when a protein extract from a wild-type E. coli strain, which alone had no detectable biotin synthase activity in vitro, was added to the fractions, biotin synthase activity was restored, albeit partially (Table I). These results demonstrated that plant biotin synthase required additional protein factors for activity, as did its bacterial counterparts, and that accessory proteins from E. coli were competent to play this function in our reconstituted heterologous system. Under the conditions detailed in the legend of Table I, only one-half the initial biotin synthase specific activity (on a per milligram Bio2 protein basis) was recovered in a reaction medium containing purified Bio2 protein and bacterial accessory proteins from a crude extract. This feature could be explained, at least in large part, by iron-sulfur cluster degradation in the course of biotin synthase purification, despite systematic saturation of chromatographic buffers with argon, as noticed by progressive bleaching of the red color of the protein. The determination of protein-bound iron by chemical and inductively coupled plasma analyses yielded, respectively, 0.5 and 0.7 mol iron/monomer, confirming that the purified enzyme was cluster deficient. Cluster degradation has also been observed for anaerobic ribonuleotide reductase-activating enzyme, another iron-sulfur enzyme utilizing Ado-Met as a source of deoxyadenosyl radical, where there was a close correspondence between activity and iron content (Mulliez et al., 1993).
Table I.
Purification of recombinant Bio2 protein from Arabidopsis
| Purification Step | Estimated Purity | Biotin Synthesized
|
|
|---|---|---|---|
| − | + | ||
| % | nmol h−1 mg−1 Bio2 | ||
| Protein extract | 2 | 60 | – |
| EMD-DEAE | 10 | 0 | 45 |
| DEAE-Sepharose | 20 | 0 | 35 |
| CM-Sepharose | 95 | 0 | 32 |
Purity of the enzyme fraction after each step of purification was estimated by ELISA quantitation. Protein was determined with the protein assay (Bio-Rad Laboratories, Munich) up to the DEAE-Sepharose column and thereafter according to Scopes (1974). Biotin synthase activity was measured under optimized conditions, with the equivalent of 0.5 nmol Bio2 protein from protein fractions, in the absence (−) or in the presence (+) of 1 mg of protein extract from untransformed BL21 E. coli strain. Biotin produced was determined with L. plantarum. Note that activities are expressed in nmol h−1 mg−1 Bio2 protein present in the protein extract (− = not tested).
Arabidopsis Biotin Synthase Reaction in a Reconstituted Plant System
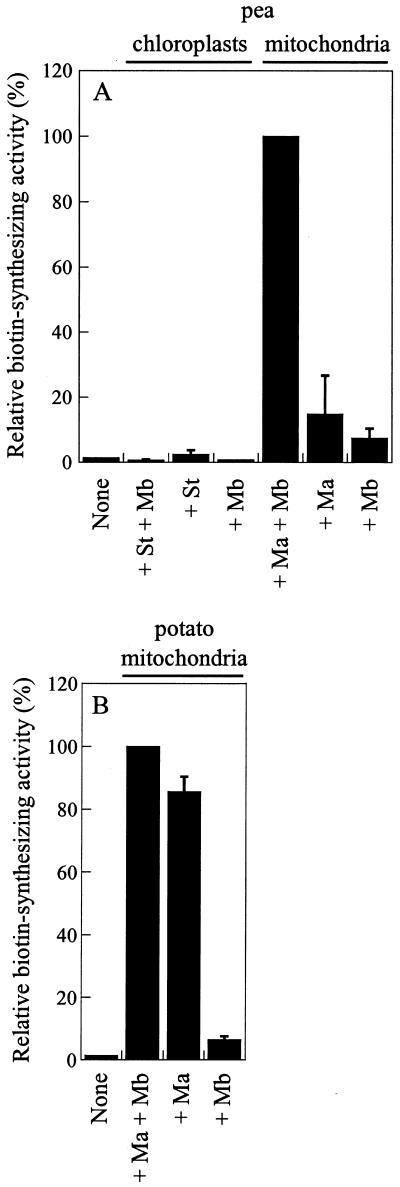
As a prerequisite in the identification of the plant factors other than Bio2 involved in biotin synthase reaction, we have undertaken combination experiments using purified recombinant Arabidopsis Bio2 protein and various plant extracts (Fig. 6). Because Bio2 sequence presented a typical organelle-targeting presequence (Baldet and Ruffet, 1996; Patton et al., 1996a; Weaver et al., 1996), and immunological studies localized Bio2 protein into mitochondria (Baldet et al., 1997a), purified organelle fractions from pea leaf cells (chloroplasts and mitochondria) and potato tubers (mitochondria) were used for these experiments. As suspected, none of the plant extracts (see “Materials and Methods”) alone exhibited any detectable levels of biotin-synthesizing activity (not shown). As it is commonly observed in non-biotin synthase overproducing bacteria, biotin synthase activity is probably too low in plants to be detected by currently developed in vitro systems. In the presence of purified Bio2 from Arabidopsis, only mitochondrial fractions could elicit biotin formation in the assay (Fig. 6). Added separately in the reaction medium, mitochondrial matrix and membranes from pea leaves had only limited effects on biotin synthase reaction driven by Bio2. In association, these fractions acted synergistically on activity (Fig. 6A). It is interesting that in potato tuber mitochondria, required factors for biotin synthase reaction were found to be soluble (Fig. 6B). These results demonstrated that one or more unidentified factors from mitochondrial matrix (pea and potato) and from mitochondrial membranes (pea), in addition to the bio2 gene product, are obligatory for the conversion of DTB to biotin in plants. Also, this specific mitochondrial localization of biotin synthase accessory components is well consistent with previous immunological detection of Bio2 protein in matrix space of plant mitochondria (Baldet et al., 1997a).
Figure 6.
Activation of purified recombinant biotin synthase by plant organelle extracts (plant-reconstituted system). A, Biotin synthase activity was measured under optimized conditions with 0.5 nmol purified Bio2 protein from Arabidopsis alone (none), or in the presence of 0.5 mg of protein extracts from isolated pea (Pisum sativum) leaf organelle (chloroplasts and mitochondria) subfractions, added separately or in combination, as indicated on the figure. B, Biotin synthase activity was measured under optimized conditions with 0.5 nmol purified Bio2 protein from Arabidopsis, in the presence of 0.5 mg of protein extracts from isolated potato (Solanum tuberosum) tuber mitochondria subfractions, added separately or in combination, as indicated in the figure. Biotin produced was determined by a turbidimetric microbiological method using L. plantarum. The error bars represent sds from three independent measurements, and when not shown fall within the column. St, Stroma; Mb, membranes; Ma, matrix).
To determine the possible involvement of the mitochondrial inner membrane respiratory chain from pea mitochondria as a reducing system for biotin synthase reaction, we have investigated the effect of various substrates and of an inhibitor of electron transport in plant mitochondria. In contrast with the biotin synthase reaction in the heterologous system (Fig. 2), activity was strictly dependent upon the addition of NADPH or NADH in the reaction medium in the plant-reconstituted system (not shown). It is possible that under our assay conditions, plant mitochondria, contrary to bacteria, were not able to regenerate the endogenous pool of protein-bound reduced pyridine dinucleotides. Succinate could not substitute for these electron donors in the assay, suggesting that the succinate dehydrogenase-associated electron chain did not participate in the biotin synthase reaction. This situation markedly contrasts with that found in B. sphaericus, where electrons involved in biotin synthesis appeared to flow through succinate dehydrogenase in vivo (Fujisawa et al., 1994). Moreover, antimycin A, used at a concentration known to block complex III of the mitochondrial respiratory chain (Douce, 1985), had no impact on biotin synthase reaction in the plant-reconstituted system (not shown).
DISCUSSION
We report here, to our knowledge, the first biochemical characterization of a plant biotin synthase reaction. Heterologous interactions between a plant recombinant Bio2 protein and bacterial proteins yield a functional biotin synthase complex, in good agreement with the successful functional complementation approach, using an E. coli bioB mutant, employed to isolate the bio2 gene product from Arabidopsis (Baldet and Ruffet, 1996). The turnover number of the reaction was >2 h−1 in the heterologous system with unfractionated protein extract from Bio2-overproducing strain and still >1 h−1 in the heterologous system comprising purified Bio2 protein (calculated from data in Table I). It appears from our results that biotin synthase from Arabidopsis acts as a catalyst and not, as suggested in bacteria, as a simple reactant (Gibson et al., 1999; Kiyasu et al., 2000).
The relative low levels of biotin synthase measured in our in vitro systems may reflect the limited proportion of recombinant Bio2 protein having a functional iron-sulfur cluster. Biotin synthase cluster is extremely labile, particularly when exposed to oxygen (Sanyal et al., 1994; Tse Sum Bui et al., 1998; Ollagnier de Choudens et al., 2000). In addition, there is convincing evidence that, in bacteria, the [2Fe-2S]-containing form of biotin synthase is a direct consequence of aerobic isolation and that conversion to [4Fe-4S] forms occurs under strict anaerobiosis and reducing conditions (Duin et al., 1997; Tse Sum Bui et al., 1999; Ollagnier de Choudens et al., 2000; Ugulava et al., 2000). [4Fe-4S]-containing forms of biotin synthase would be responsible for activity. If this also holds true for plant biotin synthase, it is possible that under our assay conditions, in addition to cluster deficiency evidenced by measured iron content in purified enzyme, only a partial conversion to a [4Fe-4S] cluster form of the enzyme does occur. Specific activities related to this fraction of active enzyme would be much higher than those calculated in this report.
Another explanation for low biotin synthase activity would be that one of the components of biotin synthase reaction is limiting or missing. Recent studies demonstrated that the iron-sulfur cluster of E. coli biotin synthase is very likely the immediate sulfur donor for the DTB to biotin conversion in vitro (Tse Sum Bui et al., 1998; Gibson et al., 1999). If this also holds true for plants remains to be demonstrated. Cys desulfurases, like NifS from Azotobacter vinelandii and Klebsiella pneumoniae or IscS from E. coli, that are involved in mobilization of sulfur for metallocluster formation and/or repair, have been found to stimulate to various extents biotin synthase reaction in E. coli (Kiyasu et al., 2000; Tse Sum Bui et al., 2000). We recently have isolated a full-length cDNA from Arabidopsis encoding a putative Cys desulfurase (GenBank accession no. AF229854; A. Picciocchi and C. Alban, unpublished data). Sorting programs identified the N-terminal sequence of this protein as a putative mitochondrial targeting sequence. Moreover, recent studies indicated that plant mitochondria might contain a complex iron-sulfur cluster assembly machinery similar to that previously found in yeast (Saccharomyces cerevisiae; Kushnir et al., 2001). Whether all or part of its components participates or not in the plant biotin synthase reaction remains to be established.
Low levels of biotin synthase activities in in vitro systems may also reflect the very small needs of biotin in plants and microorganisms (Eisenberg, 1973; Shellhammer and Meinke, 1990). However, plants, in contrast to bacteria, accumulate rather high concentrations of biotin in their cells, whose role is still enigmatic (Baldet et al., 1993a). This observation may suggest the lack of a strong regulatory mechanism of de novo biotin synthesis in plants compared with bacteria.
Data presented in this article also demonstrate that in a plant-reconstituted system, only mitochondrial fractions were competent in biotin synthase activation, well matching previous localization of plant biotin synthase (Baldet et al., 1997a). Nevertheless, despite the strong positive effect of the membrane fraction from pea mitochondria on the activity, our results suggest that the mitochondrial respiratory chain is not part of the reducing system for biotin synthase reaction. Moreover, in potato mitochondria, no membrane-associated factor is needed for biotin synthase activity. Therefore, we postulate that the physiological electron transfer system required for plant biotin synthase reaction is more probably similar to the NADPH/flavodoxin/flavodoxin reductase system found in E. coli (for an explanation, see Scheme S1). However, at present, no flavodoxin has been documented in higher plants (Mayhew and Tollin, 1993; Arabidopsis Genome Initiative, 2000).
Further identification and characterization of the mitochondrial biotin synthase accessory components (proteins and other factors), which are in progress in our laboratory, will be determinant in the aim of elucidating the reaction mechanism of the plant biotin synthase complex. In particular, the determination of catalytic efficiency of the plant enzyme in a well- and fully defined system will then be possible. Such characterization will also be helpful in our understanding of regulation of biotin synthesis in plant cells.
MATERIALS AND METHODS
Reagents
d-[8,9-3H]Biotin (42 Ci mmol−1) was purchased from Amersham (Saclay, France). (+)-[6(R),9-3H]DTB (2 mCi mmol−1) was kindly synthesized by Dr. Laurent Besse (Aventis CropScience) by desulfuration of d-biotin with Raney nickel in the presence of tritium gas. (±)-Acidomycin was synthesized at the Research Center Vitry-Alforville (Aventis Pharma, Vitry, France) by following the protocol of McLamore et al. (1953). Lactobacilli Man-Rogosa-Sharpe broth, Micro Inoculum broth, and dehydrated biotin assay medium were from Difco (Detroit, MI). Isopropylthio-β-d-galactoside was from Bioprobe Systems (Montreuil, France). All other biochemicals were obtained from Sigma Chimie SARL (La Verpillère, France) and were the purest grade available.
Plant Material and Bacterial Strains
Pea (Pisum sativum cv Douce Provence) was grown as described previously (Baldet et al., 1997b). Potato (Solanum tuberosum) tubers were obtained from a local market. The Escherichia coli BL21(DE3) strain, containing the plasmid pET11a/9-BS that carries the Arabidopsis bio2 cDNA coding sequence, and the plasmid pBkat37, which carries the molecular chaperones GroES and GroEL genes required for the correct folding of the overexpressed enzyme, were used as a source of recombinant plant biotin synthase (BL21/Bio2; Baldet et al., 1997a). The biotin auxotroph Lactobacillus plantarum (ATCC 8014) was obtained from the American Type Culture Collection (Manassas, VA).
Expression and Purification of Recombinant bio2 Gene Product
E. coli cells overexpressing Arabidopsis biotin synthase were grown at 37°C in Luria-Bertani medium with the addition of 50 μg mL−1 of kanamycin and streptomycin for plasmids stability (Baldet et al., 1997a). When A600 reached 0.6, 1 mm isopropylthio-β-d-galactoside was added and growth was continued at 28°C for 6 h. Cells were collected by centrifugation, resuspended in 50 mm Tris-HCl, pH 8.0, 10% (v/v) glycerol, 1 mm DTT, 1 mm phenylmethanesulphonyl fluoride, 5 mm 6-aminocaproic acid, and 1 mm benzamidine-HCl (buffer A), and disrupted by sonication for 5 min at 0°C. After a 20-min centrifugation at 40,000g to remove cell debris, the supernatant was desalted on PD10 Sephadex G25(M) columns (Pharmacia, Saclay, France). The resulting protein extract was concentrated (50–100 mg mL−1) using Macrosep-10 tubes (Filtron, Colgnière, France) and stored aliquoted at −80°C until use for biotin synthase activity measurements, or processed immediately for enzyme purification.
Purification of the recombinant Arabidopsis biotin synthase to near homogeneity was performed essentially by the method described by Baldet et al. (1997a). All subsequent purification steps were carried out at 4°C with argon-saturated buffers to preserve the enzyme iron-sulfur cluster from oxidation. During the purification steps, Bio2 protein was chased as a protein band (44 kD) on SDS-PAGE gels. In brief, soluble proteins were loaded onto a Fractogel EMD-DEAE 650 m column (2.6 × 35 cm; Merck, Fontenay-sous-Bois, France) that had been equilibrated with buffer A, and eluted with a linear gradient of KCl from 0 to 0.3 m (500 mL) in buffer A. The purest fractions were combined, concentrated with Macrosep-10 tubes, desalted on a PD10 Sephadex G25(M) column, and loaded onto a DEAE-Sepharose Fast Flow column (2.6 × 12 cm; Pharmacia) that had been equilibrated with buffer A. Proteins were eluted with a linear gradient of KCl from 0 to 0.3 m (400 mL) in buffer A. Fractions containing Bio2 protein were combined and desalted on PD10 Sephadex G25(M) columns that had been equilibrated with 50 mm [2-bis(hydroxyethyl)amino]-2-(hydroxymethyl)-1-propane-1,3-diol]HCl, pH 6.6, 10% (v/v) glycerol, and 1 mm DTT (buffer B). Sample was then applied onto a CM-Sepharose Fast Flow column (1.6 × 10 cm; Pharmacia) that had been equilibrated with buffer B. Proteins were eluted with a linear gradient of KCl from 0 to 0.3 m (100 mL) in buffer B. The biotin synthase containing fractions (>95% pure as judged by SDS-PAGE) were combined, concentrated, and stored aliquoted at −80°C.
In Vitro Assays for Biotin Synthase Activity
Biotin synthase was assayed by measuring the conversion of DTB to biotin. The optimized reaction mixture, in a final volume of 100 μL, contained 50 mm Tris-HCl, pH 8.0, 2 mm DTT, 0.5 mm Fe(NH4)2(SO4)2, 1 mm NADPH, 0.2 mm Ado-Met, 5 mm Fru-1,6-bisP, 0.5 mm l-Cys, 0.1 mm TPP, and 1 to 1.5 mg of a protein extract from E. coli cells overproducing Bio2 from Arabidopsis. Alternatively, 0.5 nmol of purified Bio2 protein plus 1 mg of a protein extract from untransformed E. coli cells or 0.5 to 1 mg protein from plant organelle extracts, as sources of accessory proteins, were used in the assays. Mixtures were pre-incubated in Eppendorf tubes (Eppendorf Scientific, Westbury, NY) sealed with a rubber septum and degassed for 30 min at room temperature under a continuous stream of wet nitrogen. Reactions were initiated by the addition of 150 μm cold (+)-DTB or (+)-[3H]DTB (0.15 Ci mmol−1) and lasted for 30 min to up to 6 h at 37°C. Reactions were stopped by heating to 80°C for 5 min. Precipitated proteins were eliminated by centrifugation and the amount of biotin formed was determined in the supernatant, either by a turbidimetric microbiological method using L. plantarum (Maeland and Sandnes, 1999) or by TLC analysis (Birch et al., 1995). In this last situation, samples were lyophilized, resuspended in 50 μL of methanol:water:acetic acid (65:25:10, v/v), and 5 μL was loaded with cold biotin as carrier onto a silica gel high performance TLC plate (Merck). Plates were developed with chloroform:methanol:formic acid (17:3:0.2, v/v) and then exposed to a tritium phosphor screen (Molecular Dynamics) for 24 h. Radiolabeled biotin and DTB standards of known activity were run in parallel in the same plates for spot identification and absolute quantitation. Exposed screens were quantitated using a Storm 820 PhosphorImager and ImageQuant software (Molecular Dynamics). The intensity of the spots was quantitatively analyzed by the area quantitation method with peak finder. In early experiments, the radioactivity in each compound was determined by scintillation counting of the spots scraped from the TLC plates after PhosphorImager analysis to validate the phosphorimaging quantitation procedure. Kinetic data were fitted to the appropriate rate equations by nonlinear regression analyses using the KaleidaGraph program (Synergy Software, Reading, PA).
Preparation of Purified Chloroplasts and Mitochondria
Pea leaf chloroplasts and mitochondria from pea leaves and potato tubers were purified using Percoll gradients as described by Mourioux and Douce (1981) and Douce et al. (1987), respectively. Intact chloroplasts were lysed (Tissot et al., 1997) and the suspension was centrifuged (72,000g, 20 min): The pellet and the supernatant comprised the chloroplast membranes (envelope membranes and thylakoids) and the soluble fraction (stroma), respectively. Intact mitochondria were lysed (Tissot et al., 1997) and the suspension was centrifuged (100,000g, 20 min): The pellet and the supernatant comprised the mitochondrial membranes and the soluble fraction (matrix), respectively. Chloroplast stroma and mitochondrial matrix were concentrated (20–30 mg protein mL−1) using Jumbosep-3 tubes (Filtron).
Analytical Methods
Protein concentration was determined either by the method of Bradford (1976) using Bio-Rad protein-assay reagent, with γ-globulin as a standard, or by measuring the A205 (Scopes, 1974). The amount of Bio2 protein in protein extracts was measured by an ELISA assay using affinity-purified rabbit antibodies raised against the recombinant protein (Baldet et al., 1997a) and purified protein as a standard. Protein-bound iron was determined both by the method of Doeg and Ziegler (1962) and by inductively coupled plasma atomic emission spectroscopy in the “Service Central d'Analyze du Centre National de la Recherche Scientifique” (Vernaison, France).
ACKNOWLEDGMENTS
We wish to thank Michèle Quémin for her assistance in plant mitochondria isolation. We are grateful to Drs. Renaud Dumas, Dominique Job, and Michel Matringe for critical reading of the manuscript.
Footnotes
This work was supported by Aventis CropScience, by the Centre National de la Recherche Scientifique, by the Institut National de la Recherche Agronomique, and in part by the Ministère de l'Education Nationale, de la Recherche, et de la Technologie (grant no. 98 C 0328).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010346.
LITERATURE CITED
- Alban C. Is plant holocarboxylase synthetase a bifunctional enzyme? C R Acad Sci Paris. 2000;323:681–688. doi: 10.1016/s0764-4469(00)01223-3. [DOI] [PubMed] [Google Scholar]
- Alban C, Job D, Douce R. Biotin metabolism in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:17–47. doi: 10.1146/annurev.arplant.51.1.17. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Baldet P. Quelques observations sur le métabolisme de la biotine chez les plantes supérieures. PhD thesis. France: University of Grenoble; 1993. [Google Scholar]
- Baldet P, Alban C, Axiotis S, Douce R. Localization of free and bound biotin in cells from green pea leaves. Arch Biochem Biophys. 1993a;303:67–73. doi: 10.1006/abbi.1993.1256. [DOI] [PubMed] [Google Scholar]
- Baldet P, Alban C, Douce R. Biotin synthesis in higher plants: purification of bio B gene product equivalent from Arabidopsis thaliana overexpressed in Escherichia coli and its subcellular localization in pea leaf cells. FEBS Lett. 1997a;419:206–210. doi: 10.1016/s0014-5793(97)01458-0. [DOI] [PubMed] [Google Scholar]
- Baldet P, Alban C, Douce R. Biotin synthesis in higher plants. Methods Enzymol. 1997b;279:327–339. doi: 10.1016/s0076-6879(97)79037-2. [DOI] [PubMed] [Google Scholar]
- Baldet P, Gerbling H, Axiotis S, Douce R. Biotin biosynthesis in higher plant cells: identification of intermediates. Eur J Biochem. 1993b;217:479–485. doi: 10.1111/j.1432-1033.1993.tb18267.x. [DOI] [PubMed] [Google Scholar]
- Baldet P, Ruffet ML. Biotin synthesis in higher plants: isolation of a cDNA encoding Arabidopsis thaliana bioB-gene product equivalent by functional complementation of a biotin auxotroph mutant bioB105 of Escherichia coli K12. C R Acad Sci Paris. 1996;309:99–106. [PubMed] [Google Scholar]
- Birch OM, Fuhrmann M, Shaw NM. Biotin synthase from Escherichia coli, an investigation of the low molecular weight and protein components required for activity in vitro. J Biol Chem. 1995;270:19158–19165. doi: 10.1074/jbc.270.32.19158. [DOI] [PubMed] [Google Scholar]
- Birch OM, Hewitson K, Fuhrmann M, Burgdorf K, Baldwin JE, Roach PL, Shaw NM. MioC is an FMN-binding protein that is essential for Escherichia coli biotin synthase activity in vitro. J Biol Chem. 2000;275:32277–32280. doi: 10.1074/jbc.M004497200. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Doeg KA, Ziegler DM. Simplified methods for the estimation of iron in mitochondria and submitochondrial fractions. Arch Biochem Biophys. 1962;97:37–40. doi: 10.1016/0003-9861(62)90041-3. [DOI] [PubMed] [Google Scholar]
- Douce R. Mitochondria in Higher Plants: Structure, Function, and Biogenesis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Douce R, Bourguignon J, Brouquisse R, Neuburger M. Isolation of plant mitochondria: general principles and criteria of integrity. Methods Enzymol. 1987;148:403–415. [Google Scholar]
- Duin EC, Lafferty ME, Crouse BR, Allen RM, Sanyal I, Flint DH, Johnson MK. [2Fe-2S] to [4Fe-4S] cluster conversion in Escherichia coli biotin synthase. Biochemistry. 1997;36:11811–11820. doi: 10.1021/bi9706430. [DOI] [PubMed] [Google Scholar]
- Eisenberg MA. Biotin: biogenesis, transport, and their regulation. Adv Enzymol. 1973;38:317–372. doi: 10.1002/9780470122839.ch7. [DOI] [PubMed] [Google Scholar]
- Eisenberg MA. Biosynthesis of biotin and lipoic acid. In: Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M, Umbarger ME, editors. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. New York: American Society of Microbiology; 1987. pp. 544–550. [Google Scholar]
- Fujisawa A, Abe T, Hara N, Ohori M, Shiozaki S, Izumi Y. Biotin production by bioB transformants of a Bacillus sphaericus mutant resistant to 1-(2′-tenoyl)-3,3,3-trifluoroacetone, an electron transport inhibitor. Biosci Biotech Biochem. 1994;58:1018–1022. [Google Scholar]
- Gibson KJ, Pelletier DA, Turner IM., Sr Transfer of sulfur to biotin from biotin synthase (BioB protein) Biochem Biophys Res Commun. 1999;254:632–635. doi: 10.1006/bbrc.1998.9991. [DOI] [PubMed] [Google Scholar]
- Gloeckler R, Ohsawa I, Speck D, Ledoux C, Bernard S, Zinsius M, Villeval D, Kisou T, Kamogawa K, Lemoine Y. Cloning and characterization of the Bacillus sphaericus genes controlling the bioconversion of pimelate into dethiobiotin. Gene. 1990;87:63–70. doi: 10.1016/0378-1119(90)90496-e. [DOI] [PubMed] [Google Scholar]
- Guianvarc'h D, Florentin D, Tse Sum Bui B, Nunzi F, Marquet A. Biotin synthase, a new member of the family of enzymes which use S-adenosylmethionine as a source of deoxyadenosyl radical. Biochem Biophys Res Comm. 1997;236:402–406. doi: 10.1006/bbrc.1997.6952. [DOI] [PubMed] [Google Scholar]
- Ifuku O, Kishimoto J, Haze S-I, Yanagi M, Fukushima S. Conversion of dethiobiotin to biotin in cell-free extracts of Escherichia coli. Biosci Biotech Biochem. 1992;56:1780–1785. doi: 10.1271/bbb.56.1780. [DOI] [PubMed] [Google Scholar]
- Ifuku O, Koga N, Haze S, Kishimoto J, Wachi Y. Flavodoxin is required for conversion of dethiobiotin to biotin in Escherichia coli. Eur J Biochem. 1994;224:173–178. doi: 10.1111/j.1432-1033.1994.tb20009.x. [DOI] [PubMed] [Google Scholar]
- Kiyasu T, Asakura A, Nagahashi Y, Hoshino T. Contribution of cysteine desulfurase (NifS protein) to the biotin synthase reaction of Escherichia coli. J Bacteriol. 2000;182:2879–2885. doi: 10.1128/jb.182.10.2879-2885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JR. The mechanism of biotin-dependent enzymes. Ann Rev Biochem. 1989;58:195–221. doi: 10.1146/annurev.bi.58.070189.001211. [DOI] [PubMed] [Google Scholar]
- Kushnir S, Babiychuk E, Storozhenko S, Davey MW, Papenbrock J, De Rycke R, Engler G, Stephan UW, Lange H, Kispal G. A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell. 2001;13:89–100. doi: 10.1105/tpc.13.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeland A, Sandnes K. Determination of biotin in low-temperature fish-meal processed from different species by means of a microbiological method using Lactobacillus plantarum as test organism. J Sci Food Agric. 1999;79:1298–1300. [Google Scholar]
- Mayhew SG, Tollin G. General properties of flavodoxins. In: Müller F, editor. Chemistry and Biochemistry of Flavoenzymes. Boca Raton, FL: CRC Press; 1993. pp. 389–426. [Google Scholar]
- McLamore WM, Celmer WD, Bogert VV, Pennington FC, Sobin BA, Solomons IA. Structure and synthesis of a new thialidone antibiotic. J Am Chem Soc. 1953;75:105–108. [Google Scholar]
- Méjan A, Tse Sum Bui B, Florentin D, Ploux O, Izumi Y, Marquet A. Highly purified biotin synthase can transform dethiobiotin into biotin in the absence of any other protein, in the presence of photoreduced deazaflavin. Biochem Biophys Res Commun. 1995;217:1231–1237. doi: 10.1006/bbrc.1995.2900. [DOI] [PubMed] [Google Scholar]
- Mourioux G, Douce R. Slow passive diffusion of orthophosphate between intact isolated chloroplasts and suspending medium. Plant Physiol. 1981;67:470–473. doi: 10.1104/pp.67.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulliez E, Fontecave M, Gaillard J, Reichard P. An iron-sulfur center and a free radical in the active anaerobic ribonucleotide reductase of Escherichia coli. J Biol Chem. 1993;268:2296–2299. [PubMed] [Google Scholar]
- Ollagnier de Choudens S, Sanakis Y, Hewitson KS, Roach P, Baldwin JE, Münck E, Fontecave M. Iron-sulfur center of biotin synthase and lipoate synthase. Biochemistry. 2000;39:4165–4173. doi: 10.1021/bi992090u. [DOI] [PubMed] [Google Scholar]
- Patton DA, Johnson M, Ward ER. Biotin synthase from Arabidopsis thaliana. cDNA isolation and characterization of gene expression. Plant Physiol. 1996a;112:371–378. doi: 10.1104/pp.112.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DA, Schetter AL, Franzmann LH, Nelson K, Ward ER, Meinke DW. An embryo-defective mutant of Arabidopsis disrupted in the final step of biotin synthesis. Plant Physiol. 1998;116:935–946. doi: 10.1104/pp.116.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DA, Volrath S, Ward ER. Complementation of an Arabidopsis thaliana biotin auxotroph with an Escherichia coli biotin biosynthetic gene. Mol Gen Genet. 1996b;251:261–266. doi: 10.1007/BF02172516. [DOI] [PubMed] [Google Scholar]
- Sanyal I, Cohen G, Flint DH. Biotin synthase: purification, characterization as a [2Fe-2S] cluster protein, and in vitro activity of the Escherichia coli bioB gene product. Biochemistry. 1994;33:3625–3631. doi: 10.1021/bi00178a020. [DOI] [PubMed] [Google Scholar]
- Sanyal I, Gibson J, Flint DH. Escherichia coli biotin synthase: an investigation into the factors required for its activity and its sulfur donor. Arch Biochem Biophys. 1996;326:48–56. doi: 10.1006/abbi.1996.0045. [DOI] [PubMed] [Google Scholar]
- Schneider T, Dinkins R, Robinson K, Shellhammer J, Meinke DW. An embryo-lethal mutant of Arabidopsis thaliana is a biotin auxotroph. Dev Biol. 1989;131:161–167. doi: 10.1016/s0012-1606(89)80047-8. [DOI] [PubMed] [Google Scholar]
- Scopes RK. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974;59:277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. New York: John Wiley and Sons; 1975. [Google Scholar]
- Shaw NM, Birch OM, Tinschert A, Venetz V, Dietrich R, Savoy L-A. Biotin synthase from Escherichia coli: isolation of an enzyme-generated intermediate and stoichiometry of S-adenosylmethionine use. Biochem J. 1998;330:1079–1085. doi: 10.1042/bj3301079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellhammer J, Meinke D. Arrested embryos from the bio1 auxotroph of Arabidopsis thaliana contain reduced levels of biotin. Plant Physiol. 1990;93:1162–1167. doi: 10.1104/pp.93.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot G, Douce R, Alban C. Evidence for multiple forms of biotin holocarboxylase synthetase in pea (Pisum sativum) and in Arabidopsis thaliana: subcellular fractionation studies and isolation of a cDNA clone. Biochem J. 1997;323:179–188. doi: 10.1042/bj3230179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse Sum Bui B, Escalettes F, Chottard G, Florentin D, Marquet A. Enzyme-mediated sulfide production for the reconstitution of [2Fe-2S] clusters into apo-biotin synthase of Escherichia coli: sulfide transfer from cysteine to biotin. Eur J Biochem. 2000;267:2688–2694. doi: 10.1046/j.1432-1327.2000.01284.x. [DOI] [PubMed] [Google Scholar]
- Tse Sum Bui B, Florentin D, Fournier F, Ploux O, Méjean A, Marquet A. Biotin synthase mechanism: on the origin of sulphur. FEBS Lett. 1998;440:226–230. doi: 10.1016/s0014-5793(98)01464-1. [DOI] [PubMed] [Google Scholar]
- Tse Sum Bui B, Florentin D, Marquet A, Benda R, Trautwein AX. Mössbauer studies of Escherichia coli biotin synthase: evidence for reversible interconversion between [2Fe-2S]2+ and [4Fe-4S]2+ clusters. FEBS Lett. 1999;459:411–414. doi: 10.1016/s0014-5793(99)01300-9. [DOI] [PubMed] [Google Scholar]
- Ugulava NB, Gibney BR, Jarrett JT. Iron-sulfur cluster interconversions in biotin synthase: dissociation and reassociation of iron during conversion of [2Fe-2S] to [4Fe-4S] clusters. Biochemistry. 2000;39:5206–5214. doi: 10.1021/bi9926227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Yu F, Wurtele ES, Nikolau BJ. Characterization of the cDNA and gene coding for the biotin synthase of Arabidopsis thaliana. Plant Physiol. 1996;110:1021–1028. doi: 10.1104/pp.110.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]