Abstract
Based on work with cotton fibers, a particulate form of sucrose (Suc) synthase was proposed to support secondary wall cellulose synthesis by degrading Suc to fructose and UDP-glucose. The model proposed that UDP-glucose was then channeled to cellulose synthase in the plasma membrane, and it implies that Suc availability in cellulose sink cells would affect the rate of cellulose synthesis. Therefore, if cellulose sink cells could synthesize Suc and/or had the capacity to recycle the fructose released by Suc synthase back to Suc, cellulose synthesis might be supported. The capacity of cellulose sink cells to synthesize Suc was tested by analyzing the Suc phosphate synthase (SPS) activity of three heterotrophic systems with cellulose-rich secondary walls. SPS is a primary regulator of the Suc synthesis rate in leaves and some Suc-storing, heterotrophic organs, but its activity has not been previously correlated with cellulose synthesis. Two systems analyzed, cultured mesophyll cells of Zinnia elegans L. var. Envy and etiolated hypocotyls of kidney beans (Phaseolus vulgaris), contained differentiating tracheary elements. Cotton (Gossypium hirsutum L. cv Acala SJ-1) fibers were also analyzed during primary and secondary wall synthesis. SPS activity rose in all three systems during periods of maximum cellulose deposition within secondary walls. The Z. elegans culture system was manipulated to establish a tight linkage between the timing of tracheary element differentiation and rising SPS activity and to show that SPS activity did not depend on the availability of starch for degradation. The significance of these findings in regard to directing metabolic flux toward cellulose will be discussed.
A model based on molecular, biochemical, and immunolocalization data from cotton (Gossypium hirsutum L. cv Acala SJ-1) fibers suggested that cellulose synthesis depends on the coordinated activity of the enzymes Suc synthase (SuSy; E.C. 2.4.1.13) and cellulose synthase. SuSy catalyzes the reaction:
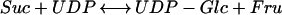 |
A particulate form of SuSy (P-SuSy) acting degradatively was proposed to channel UDP-Glc, the substrate for cellulose polymerization, to cellulose synthase in the plasma membrane of cotton fibers synthesizing secondary walls with high cellulose content (Amor et al., 1995; Haigler et al., 2001). This model was generalized by electron microscopic immunolocalization of SuSy close to the plasma membrane at patterned sites of secondary wall cellulose synthesis in differentiating tracheary elements (TEs) (Salnikov et al., 2001). High SuSy activity has also been observed in the zone of differentiating TEs of trees (Hauch and Magel, 1998; Uggla et al., 2001). In addition, mutant or transgenic plants of maize (Zea mays), carrot (Daucus carota), and potato (Solanum tuberosum) that are deficient in SuSy gene expression showed reduced cellulose content (63%–72% of wild type) and phenotypes (dwarfing, degenerated, or swollen cells) consistent with reduced cellulose content in primary walls (Carlson and Chourey, 1996; Tang and Sturm, 1999; Haigler et al., 2001).
The model for SuSy-mediated cellulose synthesis suggests that Suc is the preferred substrate for cellulose synthesis, at least during secondary wall deposition, as was directly demonstrated in cotton fibers (Pillonel et al., 1980; Amor et al., 1995). Therefore, the availability of Suc in the cell would affect the rate of cellulose synthesis (Fig. 1). Some of the required Suc might be synthesized within cellulose sink cells after initial hydrolysis and intracellular cycling of carbon from translocated Suc (Hill et al., 1995; Thorpe and Minchin, 1996). In addition, SuSy-mediated channeling of UDP-Glc to the cellulose synthase results in the concomitant release of a Fru molecule that can be advantageously cycled back to Suc (Delmer, 1999).
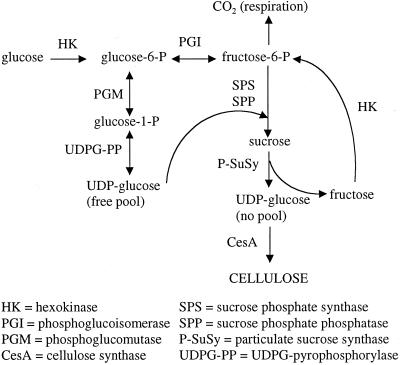
Figure 1.
Diagram placing SPS in the context of cellulose synthesis. P-SuSy is shown as channeling UDP-Glc to the cellulose synthase (Amor et al., 1995; Haigler et al., 2001). This channeled UDP-Glc is labeled “no pool,” in contrast to the “free pool” of UDP-Glc that would support general metabolism and Suc synthesis within cellulose sink cells. Cofactors for enzymatic reactions are omitted from this diagram.
We hypothesized that Suc synthesis mediated by Suc phosphate synthase (SPS) would be prominent in cellulose sink cells. SPS (E.C. 2.4.1.14) catalyzes the reaction:
 |
Suc 6′-phosphate phosphatase then produces Suc, pulling the reaction in the synthetic direction. The regulatory role of SPS in leaf Suc synthesis is well established. SPS is also active in other Suc-synthesizing organs or tissues including those adapting to cold or drought, fruits, etiolated cotyledons, germinating seeds, sugarcane (Saccharum officinarum) stems, and beet (Beta vulgaris) roots (Huber and Huber, 1996; Quick and Schaffer, 1996; Winter and Huber, 2000). However, only a few studies of SPS activity in heterotrophic tissues have any possible relationship to secondary wall deposition or cellulose synthesis. In concentric rings of wood from Robinia pseudoacacia trees, SPS activity was low during the growing season in all tissues (including differentiating TEs) except the heartwood (Hauch and Magel, 1998). In 30-μm sections from the cambial zone of Pinus sylvestris trees, SPS activity was highest in the phloem and present in substantial but variable amounts in differentiating TEs (Uggla et al., 2001). Because SuSy rather than SPS activity peaked in the zone of the differentiating TEs in both cases, no connection was made between SPS activity and cellulose synthesis. Antisense SPS potato plants with 32% of wild-type SPS activity showed decreased daytime flux to Suc in leaves and to cell walls and other cell fractions in tubers (Geigenberger and Stitt, 2000). However, cellulose was not analyzed as a separate wall component, and the observations were attributed to the generalized effect of SPS on Suc available for translocation.
In this research, SPS activity was analyzed during development of three heterotrophic systems representing cellulose sinks because they store large amounts of carbon in cellulose within secondary walls: (a) cotton fibers (Haigler et al., 1991), (b) cultured mesophyll cells of Zinnia elegans that were induced to form TEs with cellulose-rich secondary walls (Fukuda and Komamine, 1980), and (3) etiolated hypocotyls of kidney beans (Phaseolus vulgaris) that contained differentiating TEs. The Z. elegans culture system was particularly precise and manipulable, thereby allowing a tight linkage to be established between rising SPS activity and high-rate cellulose synthesis. In this system, (a) the TEs undergo autolysis in less than 10 h after secondary wall deposition commences, (b) the timing and characteristics of TE differentiation varied in different media, (c) TE differentiation could be induced in starch-depleted cells, and (d) a noninductive medium allowed cells to divide and expand via primary wall synthesis without TE differentiation.
RESULTS
SPS Activity Increased in Parallel with the Increasing Rate of Cellulose Synthesis during the Primary to Secondary Wall Transition in Cotton Fibers
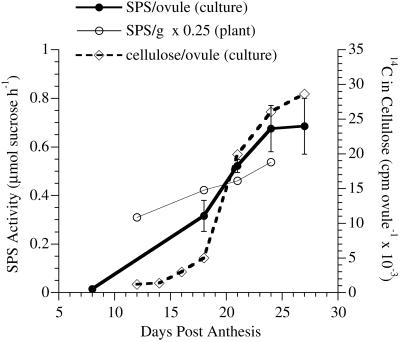
In fibers on ovules cultured in vitro, SPS activity rose 45-fold from a very low level at 8 DPA during rapid fiber elongation via primary wall synthesis to a high level during secondary wall synthesis (Fig. 2; adapted from Tummala, 1996). The level increased during secondary wall synthesis after 18 DPA until 24 DPA in culture, then plateaued. This rapid increase was paralleled by the rapidly increasing rate of cellulose synthesis from exogenous [14C]Glc in cultured ovules (Fig. 2; adapted from Martin, 1999). In plant-grown fibers, SPS activity also increased at the primary to secondary wall transition, which occurred between 12 (representing pure primary wall synthesis) and 18 (including secondary wall synthesis) DPA under warm temperatures (Thaker et al., 1989). Extractable SPS activity continued to increase as secondary wall synthesis in plant-grown fibers continued through 24 DPA, although normalization of the data from plant-grown fibers per gram of fiber dry weight suppressed the magnitude of the increase (see discussion).
Figure 2.
Changes in SPS activity compared with the rate of cellulose synthesis during cotton fiber differentiation. Data are shown for the SPS activity in cultured fibers (normalized per the amount of fibers on one ovule) with error bars showing se of the means from each separate extract. Data for plant-grown fibers were normalized per gram of fiber and multiplied by 0.25 to achieve a better match with the y axis scale from cultured fibers. In this case, error bars representing se of the means from each separate extract are not visible. A graph of the cellulose synthesis rate in cultured fibers (normalized per the amount of fibers on one ovule) is included for comparison to the SPS data from cultured fibers. The graphs of SPS activity are adapted from figures in a thesis (Tummala, 1996). The graph of cellulose synthesis rate is adapted from a figure in a dissertation (Martin, 1999), and it has also been published elsewhere in combination with other data (Haigler et al., 2001).
SPS Activity Increased in Correlation with TE Differentiation in Cultured Z. elegans Cells
No Suc production occurred under the SPS assay conditions when Fru was substituted for Fru-6-P (data not shown). Therefore, the Suc synthesis observed was attributable to SPS, not to SuSy acting synthetically. If no detectable Suc formed under SPS assay conditions including abundant exogenous Fru, none would have been formed by SuSy from endogenous Fru in the tissue extract.
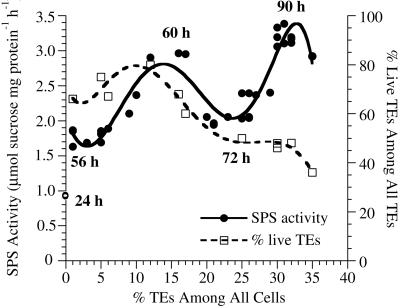
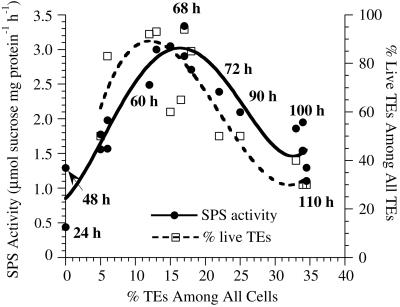
In complex inductive medium, SPS activity was low at 24 h when TE differentiation had not yet occurred, then it showed two peaks during TE differentiation (Fig. 3). The first peak at 60 h corresponded to the highest percentage of living TEs in the culture. The trough at 72 h corresponded to the autolysis of these first differentiated TEs as determined by staining with Evans blue, with another wave of TE differentiation occurring at 90 h. The percent living TEs remained on a plateau during the second peak of SPS activity because there was only a 5% to 7% increase in total TEs during this period, and the first differentiated TEs constituted a high background number of dead TEs. However, the later differentiating TEs are very large (Fig. 4), which is correlated with the high SPS activity. Note that SPS activity dropped again as the large TEs began to die (data points at 35% total TEs). In simplified medium, cell division was suppressed and only one peak of differentiation of small TEs was observed. Both SPS activity and percent living TEs peaked at about 68 h (Fig. 5). The lack of a second peak of SPS activity correlated with the absence of late-differentiating large TEs in simplified medium.
Figure 3.
Changes in SPS activity and the percentage of live TEs throughout the time course of TE differentiation in complex medium. SPS activity peaked two times corresponding to two successive waves of TE differentiation. Although the total percent TEs increased throughout the time course, around 60 h the first peak in SPS activity correlated with a first peak in the percentage of living TEs. Autolysis of the first differentiated TEs occurred by 72 h, when a lower level of SPS activity was observed. As additional large TEs differentiated after 72 h in complex medium (see Fig. 4), another peak in SPS activity occurred. As those TEs began to autolyze, the SPS activity declined (see data for 35% total TEs). Here and in Figures 5 and 6, the mean of three replicate assays for SPS activity (and percent living TEs, if applicable) is presented as one data point. se of the means for SPS activity are omitted to increase clarity of the graphs; they averaged about 4.3% of the mean.
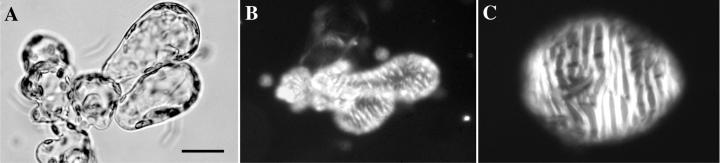
Figure 4.
Illustration of the stages of differentiation of Z. elegans cells in complex medium. A, Zero-hours mesophyll cells observed in bright-field microscopy; B, small TEs differentiated at 60 h observed by polarization microscopy; C, larger TEs differentiated at 90 h observed by polarization microscopy. Cultures in simplified medium did not form large TEs at 90 h. The micrographs are black and white digital reprints of the scanned original color slides. Bar = 20 μm.
Figure 5.
Changes in SPS activity and the percentage of live TEs throughout the time course of TE differentiation in simplified medium. SPS increased in correlation with one peak of differentiation of small TEs at 68 h and declined as the TEs autolyzed.
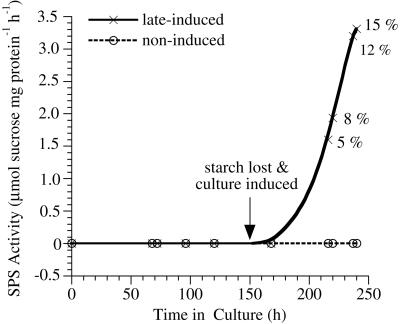
To check for possible endogenous cycles of SPS activity in cultured cells, Z. elegans mesophyll cells were cultured in the noninductive medium that allowed cell division and cell expansion but not TE differentiation. In these cells, SPS activity was undetectable up to 240 h in culture (Fig. 6). To check for a possible obligatory relationship of increasing SPS activity to the degradation of starch in differentiating TEs (A. Roberts and Haigler, unpublished data), cells were cultured in noninductive medium for 7 d until they depleted their starch. Subsequently, TE differentiation was induced by addition of cytokinin, and SPS activity increased within 2 d and continued to rise in correlation with the increasing percent of TEs (Fig. 6).
Figure 6.
Changes in SPS activity in noninductive medium before and after the addition of an inducing concentration of cytokinin. In the absence of TE induction, SPS activity remained undetectable. The culture was allowed to deplete its starch before the cytokinin was added to certain aliquots at 150 h to produce the late-induced culture. Percentages beside the data points for the late-induced culture are the percentage of TEs among all cells counted at that time point. All unlabeled data points correspond to 0% TEs.
SPS Activity Increased with Maximum Dry Weight Accumulation and Xylem Synthesis in Etiolated Bean Hypocotyls
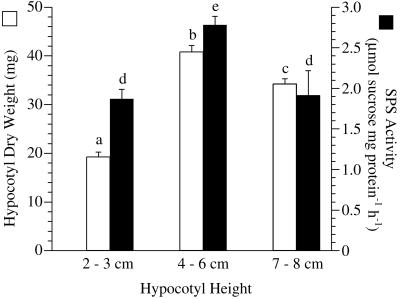
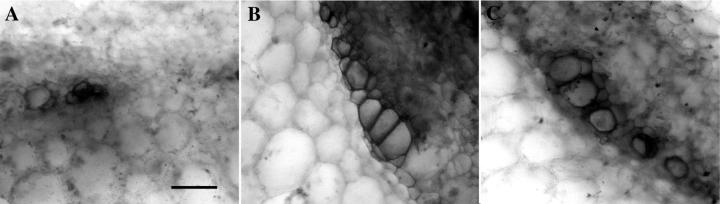
Etiolated bean hypocotyls achieved maximum dry weight at 4- to 6-cm height (Fig. 7). Dry weight decreased thereafter even though elongation continued. Extractable SPS activity followed the same pattern (Fig. 7), peaking when the largest vascular bundles were detected in 4- to 6-cm hypocotyls (compare Fig. 8, A with B). Even though the hypocotyls continued to elongate, additional xylem elements were not added in any observable quantity to the vascular bundles (compare Fig. 8, B with C). The micrographs represent the maximum vascular tissue that was observed at the base of the hypocotyls of each height class.
Figure 7.
Changes in SPS activity (black bars) and dry weight (white bars) over the time course of elongation in etiolated bean hypocotyls. Hypocotyls in three height classes, 2 to 3 cm, 4 to 6 cm, and 7 to 8 cm were analyzed. Histogram bars represent the means of six separate determinations containing three hypocotyls each, and error bars are se of the mean. a through c and d, e, Significantly different groups for hypocotyl weight and SPS activity, respectively. Significance was established by P < 0.001 in both cases, and the test statistic, F, was 86.01 for hypocotyl weight (n = 36 in each group) and 25.19 for SPS activity (n = 6 in each group).
Figure 8.
Safranin-stained cross-sections of etiolated hypocotyls in three height classes showing the extent of xylem differentiation. Height classes shown are: A, 2 to 3 cm; B, 4 to 6 cm; and C, 7 to 8 cm. Representative micrographs of the maximum size of vascular bundles at the base of the hypocotyl are shown in each case. The micrographs are black and white digital reprints of the scanned original color slides. Bar = 30 μm.
DISCUSSION
These results show that increased SPS activity is consistently correlated with high-rate cellulose synthesis and secondary cell wall deposition in cotton fibers, TEs, and etiolated hypocotyls. Cellulose synthesis mediated by P-SuSy (Amor et al., 1995; Haigler et al., 2001) implies that the ability to synthesize Suc within cellulose sink cells might enhance the rate of cellulose synthesis. In cultured cotton ovules supplied only with exogenous Glc, Suc is synthesized within fibers (Carpita and Delmer, 1981; Martin, 1999), and SPS activity rose along with the increasing rate of cellulose synthesis around 15 DPA (Fig. 2; Tummala, 1996; Martin, 1999). Maximum rates of elongation and primary wall deposition occurred at 6 to 14 DPA, and 15 DPA represents the primary to secondary wall transition (Carpita and Delmer, 1981; Haigler et al., 1991). The increase in SPS activity was not culture induced because similar results were obtained in plant-grown fibers connected to the Suc translocation stream (Fig. 2). Although there is a functional symplastic connection through plasmodesmata at the fiber foot (Ryser, 1992; Ruan et al., 1997), it is possible that some Suc is hydrolyzed before or after import into plant-grown fibers. When the data in Figure 2 are adjusted for an approximate 3-fold increase in the wall weight of plant-grown fibers between 12 and 24 DPA (Stewart, 1986), the increase in SPS activity was over 5-fold in plant-grown fibers. A similar 4.8-fold increase is predicted for cultured ovules (interpolating 0.14 μmol Suc ovule−1 h−1 at 12 DPA).
Differentiating TEs also had increased SPS activity, which declined with TE autolysis (Figs. 3, 5, and 6), even though Suc was the exogenous carbon source. The varying times of high SPS activity (60, 68, 90, and 240 h) argue against any endogenous rhythm explaining the results. This is reinforced by variable culture starting times—cultures leading to composite graphs were arbitrarily started and harvested at different times between 9 and 12 am (Fig. 3, 5, and 6). Nevertheless, SPS activity changed in a predictable manner in correlation with the percent living TEs in the culture at harvest time. The different patterns of high SPS activity in complex and simplified inductive media also were paralleled by two or one peak(s) in the percent living TEs, respectively (compare Figs. 3 and 5).
SPS activity was not detected in Z. elegans cells dividing and expanding via primary wall synthesis in noninductive medium or in mesophyll cells freshly isolated for culture (Fig. 6). The mesophyll cells were isolated from small, first leaves (about 1–1.5 cm long; 6 h into the photoperiod) that were still acting as sinks (Harn et al., 1993). (In contrast, expanded Z. elegans leaves had SPS activity of 3.09 μmol Suc mg protein−1 h−1; data not shown.) The noninductive medium differed from the complex inductive medium only by its lower level of TE-inducing cytokinin (Fukuda and Komamine, 1980). Therefore, high SPS activity is tightly correlated with secondary wall synthesis in Z. elegans cells. SPS activity in etiolated bean hypocotyls also peaked at the stage of maximum dry weight accumulation and maximum differentiation of xylem bundles, both of which involve extensive cellulose deposition (Figs. 7 and 8).
All three heterotrophic systems had high SPS activity (2.8–3.5 μmol Suc mg protein−1 h−1), emphasizing the important role of SPS in cellulose sink cells. In cotton cv Coker 312, three comparative values for Vmax SPS activity (μmol Suc mg protein−1 h−1 obtained after identical extraction and assay as described for Z. elegans cultures) were for: (a) 24-DPA plant-grown fibers, 3.0; (b) 24-DPA cultured fibers, 3.5; and (c) recently expanded leaves (fifth down from the apex harvested from greenhouse-grown plants at 4 pm in sunlight and major veins discarded), 1.5 (data not shown). High rates of cellulose synthesis occur at 24 DPA in both cultured and plant-grown fibers.
SPS activity has been associated with the synthesis of Suc during starch mobilization in several systems (e.g. Geigenberger and Stitt, 1991; Hauch and Magel, 1998; Langenkämper et al., 1998; Chavez-Barcenas, et al., 2000), but the increases shown here were not dependent on concomitant starch degradation. Cotton fibers do not store or degrade starch during secondary wall deposition, as confirmed in many electron micrographs (C.H. Haigler, M.J. Grimson, and V.V. Salnikov, unpublished data). Starch degradation occurs in earlywood differentiating before tree source leaves develop (Hill et al., 1995) and in differentiating Z. elegans TEs (A. Roberts and C.H. Haigler, unpublished data). However, noninduced Z. elegans cultures depleting their starch over 170 h showed no detectable SPS activity, and SPS activity rose in the starch-depleted cultures only when TEs were induced by cytokinin addition (Fig. 6). Because the etiolated hypocotyls contained a ring of starch around the xylem (data not shown), SPS activity cannot be separated from starch degradation in this system. However, it is possible that the final hypocotyl dry weight decrease is due to a “last-chance” degradation of starch to support elongation growth, and SPS activity declined along with the dry weight decrease (Fig. 7).
The data presented here make it plausible to suggest that SPS activity may help to regulate sink strength in cellulose sink cells. SPS could control the amount of Fru-6-P pulled toward Suc and cellulose synthesis and away from respiration or a cycle between Fru-6-P and the triose phosphates that is characteristic of heterotrophic plant tissues including wood (Hill et al., 1995). This hypothesis is supported by the observation that, in cotton fibers, the pyrophosphate:Fru-6-phosphate 1-phosphotransferase (the PPi-dependent phosphofructokinase) that is an entry point to respiration and that participates in the Fru-6-P/triose phosphate cycle has similar activity during most of primary and secondary wall synthesis (Wäfler and Meier, 1994). In contrast, SPS activity increases at the transition to secondary wall synthesis. There is also evidence that up-regulated SPS activity under the control of a constitutive promoter in transgenic cotton plants leads to increased cellulose deposition in fiber secondary walls, at least under some environmental conditions (Haigler et al., 2000a, 2000b, 2000c). However, the relative contributions of possible changes in source and sink strength to the phenotype of the transgenic cotton plants are still being investigated.
If some translocated Suc is cleaved by invertases or soluble SuSy before the use of the carbon for cellulose synthesis, SPS could assume a very important role in supplying Suc for cellulose synthesis. Although Suc breakdown and resynthesis might appear energetically wasteful, such a cycle has been documented in several heterotrophic systems and may allow precise response of the direction of flux to sink demand (for review, see Huber and Huber, 1996). Such a cycle could also help to regulate the extent and timing of cellulose synthesis, which is a large, mostly irreversible, carbon sink that must be carefully regulated in interaction with the environment in evolutionarily successful plants (Haigler et al., 2001).
MATERIALS AND METHODS
Cotton (Gossypium hirsutum cv Acala SJ-1) Plant Growth and Culture
For in vitro fiber culture, flowers of cotton (Cotton Germplasm Bank, College Station, TX) were collected 1 DPA from plants grown in the greenhouse. Ovules were excised and cultured at constant 34°C (the optimum for in vitro cultures; Beasley, 1977) as previously described (Haigler et al., 1991). Ovules were floated on a medium containing Glc as the sole carbon source because exogenous Suc causes ovule browning and callus, rather than fibers, to form on the ovules (Beasley, 1971; C.H. Haigler and L.K. Martin, unpublished data). Ovules originating from four to six flowers on each culture day were collected with their attached fibers on 8, 18, 21, 24, and 27 DPA for SPS assay. Ovules with attached fiber were collected on 12, 14, 16, 18, 21, 24, and 27 DPA for determination of cellulose synthesis rate from exogenous [14C]Glc as previously described (Roberts et al., 1992). These time points were chosen to include both primary and secondary wall deposition in cotton fibers; at warm temperatures, the transition between these two phases begins around 15 DPA (Haigler et al., 1991). Bolls for assay of SPS in plant-grown fibers were collected at 12, 18, 21, and 24 DPA from plants growing in a growth chamber held at constant 28°C, which is an optimum temperature for cotton vegetative growth (Burke et al., 1988) and fiber cellulose synthesis (Roberts et al., 1992).
TE Differentiation in Culture
Mesophyll cells isolated from the first true leaves of Zinnia elegans L. var. Envy (Bodger Seeds Ltd., El Monte, CA) were induced to differentiate into TEs in the dark as previously described with Suc as the carbon source (Fukuda and Komamine, 1980). Cultures represented in the data were begun at various times between 9 and 12 am. The extent and timing of TE differentiation were manipulated by use of three kinds of media. Two of the media, one complex (Fukuda and Komamine, 1980) and one simplified (Roberts and Haigler, 1992), contained sufficient cytokinin (0.2 mg L−1 6-benzylaminopurine) to induce TE differentiation. The complex inductive medium supported cell division and the production of two sizes of TEs, with differentiation of small TEs peaking at about 60 h and differentiation of larger TEs peaking at about 90 h. The simplified inductive medium suppressed cell division and eliminated the differentiation of large TEs at 90 h. The third medium used did not induce TE differentiation; it differed from the complex inductive medium only by having a lower level of cytokinin (Fukuda and Komamine, 1980). This noninductive medium also allowed cells to be maintained in culture until they lost visible starch grains about 7 d after culture as detected by absence of staining with I2KI (2% [w/v] KI and 0.2% [w/v] I2; Gahan 1984). Late TE differentiation was induced in starch-depleted cells by raising the cytokinin concentration to 0.2 mg L −1.
Determining Percent Differentiation and Percent Live TEs in Z. elegans Cultures
Polarization microscopy (for early stage TEs) and bright-field microscopy (for late stage TEs) were used to count TEs among all cells in the culture over the time course of differentiation. Sensitive polarization methods (BH-2 microscope, Olympus, Tokyo) allowed the detection of cellulosic thickenings by their birefringence before they became visible by bright field microscopy. Although the fluorescent brightener Tinopal LPW (CibaGeigy, Greensboro, NC) would have detected TEs (Falconer and Seagull, 1985) approximately 2 h earlier than polarization microscopy (C.H. Haigler, unpublished data) by binding to their patterned cellulosic thickenings, it was not used because polarization microscopy was simpler and adequate to perceive the trends observed in these experiments.
Because it was hypothesized that changes in SPS activity in these cultures would correlate with the percentage of living TEs and not with arbitrary times after the culture, the progress of TE differentiation was monitored prior to SPS assay. At prospective harvest times, the percentage of total TEs and dead TEs was determined in all the available flasks. Percent TEs was calculated as: [total TEs/(total TEs + other cells) × 100]. Evans blue, a dye that permeates only dead cells, was used to quantify TE autolysis as previously described (Roberts and Haigler, 1989). Percent live TEs among all TEs was calculated as: [(total TEs − autolyzed TEs)/total TEs × 100]. Sets of three flasks with similar extent of TE differentiation (±1% for TEs and live TEs) were combined by low-speed centrifugation (20g, 2 min) prior to extraction of SPS.
Growth and Developmental Analysis of Etiolated Hypocotyls
Kidney beans (Phaseolus vulgaris) were purchased from the grocery store and germinated in the dark at 28°C to 30°C in potting soil. They grew into etiolated seedlings characterized by hyperelongation and lack of chlorophyll and leaf development. The etiolated hypocotyls still contained differentiating TEs to support water conduction.
Short (2–3 cm), medium (4–6 cm), and tall (7–8 cm) hypocotyls (about 3, 4–5, and 7–8 d after germination, respectively) were analyzed for the extent of xylem development and dry weight. Hand sections were cut with a razor blade from the base, middle, and top of several hypocotyls in each height class. The sections were stained with safranin (1% [w/v] for anatomical observations) or I2KI (for starch), and examined in the light microscope to compare the relative amounts of xylem as the hypocotyls elongated. Micrographs were taken by adhering stained sections to the bottom of a slide in a small amount of water and looking through the slide, which enhanced clarity of the anatomy (method of J. Varner, personal communication). Representative areas showing maximum amounts of xylem near the base of the hypocotyls were photographed. Thirty-six hypocotyls of each size were stripped of roots and cotyledons, dehydrated in a 60°C oven for 3 d, and weighed.
Assay of SPS in Cotton Fiber
For both cultured and plant-grown fibers, seeds with attached fibers were snap frozen in liquid nitrogen, frozen fibers were removed from seeds by scraping with an ultracold spatula, and the isolated fibers were ground with a pestle to a fine powder under liquid nitrogen prior to SPS assay. All the fibers of 10 cultured ovules or all the fibers from one locule of the boll were ground together as one sample. SPS assay methods were adapted from those previously published (Kerr et al., 1987; Copeland, 1990). Cotton fiber powder was quickly weighed while frozen, transferred to a 12-mL centrifuge tube, thawed in extraction buffer {50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH. 7.4; 10 mm MgCl2; 1 mm EDTA; 1 mm EGTA; 10% [v/v] glycerol; and 0.1% [v/v] Triton X-100}, and mixed with buffer by aid of a plastic pestle. Cellular debris was pelleted in a 20-s spin (15,000 rpm), and the supernatant was used to assay SPS. SPS assay proceeded in 70 μL of reaction mixtures for 5 min at 34°C in: 50 mm HEPES, pH 7.4; 8 mm UDP-Glc; 4 mm Fru-6-P; 20 mm Glc-6-P; 10 mm MgCl2; 1 mm EDTA; 0.4 mm EGTA; 4% (v/v) glycerol; and 0.04% (v/v) Triton X-100]. Forty-two microliters of SPS assay buffer (50 mm HEPES, pH 7.4; 10 mm MgCl2; 1 mm EDTA; 13.36 mm UDP-Glc; 6.67 mm Fru-6-P; and 33.3 mm Glc-6-P) was prewarmed to the assay temperature and added to tissue supernatant (28 μL) to make the final assay mixture. High substrate concentrations and the presence of the activator Glc-6-P define conditions for assay of Vmax SPS activity (Winter and Huber 2000). Three reaction tubes and three blanks (to normalize for possible different amounts of endogenous Suc) were run for each sample. One normal NaOH was added to the blanks before the plant extract. After 5 min, 1 n NaOH was added to stop the reaction, followed by boiling for 10 min to destroy unreacted hexoses. Twelve molar HCL was added to hydrolyze Suc-P and Suc into Fru and Glc, 0.1% (w/v) resorcinol in 100% (v/v) ethanol was added to react with Fru, and A520 of the pink reaction product was measured. A Suc standard curve was run in parallel.
Each cultured fiber extraction was performed two (for 8 and 27 DPA) to six (for 18, 21, and 24 DPA) times, and each extract was assayed with four replications. Assays of cultured fibers were normalized to activity per the unit of fibers growing on one ovule (per ovule). Values for plant-grown fibers are means from six separate extractions (from two sets of plants grown 6 months apart) with each extract assayed in triplicate. Assays of plant-grown fibers were normalized to the fresh weight of the fiber sample (per gram), which reduces the magnitude of the apparent increase over the developmental time course because of the increasing weight of inert, secondary wall cellulose. Dry weight of plant-grown cotton fibers typically increases about 3-fold between 12 and 24 DPA (Stewart, 1986).
Assay of SPS in Z. elegans Cells and Bean Hypocotyls
Three similar flasks of Z. elegans cells in medium were combined and washed three times in 10 mL each of 0.2 m mannitol by repeated centrifugation (20g, 2 min) to remove exogenous Suc. An equal volume of SPS extraction buffer (2× concentrated) was added to the cell pellet, and the dense slurry of cells was frozen by drops in liquid nitrogen. The frozen cells could be stored at −80°C for at least 30 d without detrimental effects on SPS activity levels. Immediately prior to SPS assay, the cells were ground while frozen to a fine powder. A prechilled 1.5-mL microfuge tube was half-filled with frozen powder, and 1 mL of extraction buffer at 4°C was added. (For consistent results, the frozen tissue was not allowed to thaw before addition of extraction buffer.) The tube was vortexed for 20 s and stored on ice until the other samples (usually four) being assayed in parallel were ready (≤10 min waiting for any sample). All samples were again vortexed for 20 s, then microfuged (15,000 rpm; 20 s; 4°C). The supernatant was removed to another prechilled tube followed by two times repetition of the microcentrifugation step. The final supernatant was used for SPS assay and for quantitation of protein (Bio-Rad Protein Assay, Hercules, CA).
Bean hypocotyls were grouped into height classes, frozen in liquid nitrogen, and stored intact at −80°C prior to SPS assay. Immediately prior to SPS assay, three similar hypocotyls were ground together to form one sample. The sample was processed as described for Z. elegans cells.
SPS activity for Z. elegans cells and hypocotyls was measured as described for cotton fibers with minor changes: (a) 2% (w/v) polyvinylpolypyrrolidone was added to the extraction buffer, (b) substrate concentrations in the SPS assay were increased to 6 mm Fru-6-P and 10 mm UDP-Glc, and (c) the reactions were run for 10 min. (We proved that the reaction is linear for at least 12.5 min.) Each tissue extract (itself a combination of three culture flasks or three hypocotyls) was assayed in triplicate.
To check if SuSy acting synthetically under our assay conditions was increasing the apparent SPS activity in Z. elegans cells, Fru was substituted in equal amounts for Fru-6-P and an assay was run in parallel with the SPS assay on the same sample (68-h Z. elegans cells with high percentage TEs).
Statistical Analysis
Groups were analyzed for similarity or difference by one-way ANOVA with randomization (1,000 iterations) by use of a subroutine written by R.E. Strauss for Matlab (Natik, MA). The subroutine is called “pairwise” (http://www.biol.ttu.edu/Faculty/FacPages/Strauss/Matlab/matlab.htm).
ACKNOWLEDGMENTS
We thank Jyothi Tummala and Kirt Martin for contributions of data in Figure 2, Scott Holaday and Tahhan Jaradat for contributions of expertise on SPS assay, Scott Holaday for helpful comments on the manuscript, Brett Kiedaisch for contributions of expertise on Z. elegans cultures, and Richard E. Strauss for assistance with statistical analyses.
Footnotes
This work was supported by the Howard Hughes Medical Institute (through the Undergraduate Biological Sciences Education Program), by the Texas Advanced Research Program (grant no. 003644–095), and by Cotton Incorporated (Raleigh, NC).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010424.
LITERATURE CITED
- Amor Y, Haigler CH, Wainscott M, Johnson S, Delmer DP. A membrane-associated form of sucrose synthase and its potential role synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CA. Improvements in fiber yield and quality: test tube cotton. Calif Agric. 1971;25:6–8. [Google Scholar]
- Beasley CA. Temperature-dependent response to indoleacetic acid is altered by NH4+ in cultured cotton ovules. Plant Physiol. 1977;59:203–206. doi: 10.1104/pp.59.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JJ, Mahan JR, Hatfield JL. Crop-specific thermal kinetic windows in relation to wheat and cotton biomass production. Agron J. 1988;80:553–556. [Google Scholar]
- Carpita NC, Delmer DP. Concentration and metabolic turnover of UDP-glucose in developing cotton fibers. J Biol Chem. 1981;256:308–315. [PubMed] [Google Scholar]
- Carlson SJ, Chourey P. Evidence for plasma membrane-associated forms of sucrose synthase in maize. Mol Gen Genet. 1996;252:303–310. doi: 10.1007/BF02173776. [DOI] [PubMed] [Google Scholar]
- Chavez-Barcenas AT, Valdez-Alarcon JJ, Martinez-Trujillo M, Chen L, Xoconostle-Cazares B, Lucas WJ, Herrera-Estrella L. Tissue-specific and developmental pattern of expression of the rice sps1 gene. Plant Physiol. 2000;124:641–653. doi: 10.1104/pp.124.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland L. Enzymes of sucrose metabolism. Methods Plant Biochem. 1990;3:73–85. [Google Scholar]
- Delmer DP. Cellulose biosynthesis: exciting times for a difficult field of study. Ann Rev Plant Physiol Mol Biol. 1999;50:245–276. doi: 10.1146/annurev.arplant.50.1.245. [DOI] [PubMed] [Google Scholar]
- Falconer MM, Seagull RW. Immunofluorescent and Calcofluor white staining of developing tracheary elements in Zinnia elegans L. suspension cultures. Protoplasma. 1985;125:190–198. [Google Scholar]
- Fukuda H, Komamine A. Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol. 1980;65:57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahan PB. Plant Histochemistry and Cytochemistry: An Introduction. London: Academic Press; 1984. pp. 239–240. [Google Scholar]
- Geigenberger P, Stitt M. A “futile” cycle of sucrose synthesis and degradation is involved in regulating partitioning between sucrose, starch and respiration in cotyledons of germinating Ricinus communis L. seedlings when phloem transport is inhibited. Planta. 1991;185:81–90. doi: 10.1007/BF00194518. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M. Diurnal changes in sucrose, nucleotides, starch synthesis and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J. 2000;23:795–806. doi: 10.1046/j.1365-313x.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- Haigler CH, Cai W, Martin LK, Tummala J, Anconetani R, Gannaway JG, Jividen GJ, Holaday AS. Mechanisms by which fiber quality and fiber and seed weight can be improved in transgenic cotton growing under cool night temperatures. In: Dugger CP, Richter DA, editors. Proceedings of the 2000 Beltwide Cotton Conference, 4–8 January, San Antonio, TX. National Cotton Council, Memphis. 2000a. pp. 483–484. [Google Scholar]
- Haigler CH, Hequet EF, Krieg DR, Strauss RE, Wyatt BG, Cai W, Jaradat T, Srinivas NG, Wu C, Jividen GJ. Transgenic cotton with improved fiber micronaire, strength, length, and increased fiber weight. In: Dugger CP, Richter DA, editors. Proceedings of the 2000 Beltwide Cotton Conference, 4–8 January, San Antonio, TX. National Cotton Council, Memphis. 2000b. p. 483. [Google Scholar]
- Haigler CH, Holaday AS, Wu C, Wyatt BG, Jividen GJ, Gannaway JG, Cai WX, Hequet EF, Jaradat TT, Krieg DR. Proceedings of Plant Biology 2000, July 15–19, San Diego, CA. Rockville, MD: American Society of Plant Physiologists; 2000c. Transgenic cotton over-expressing sucrose phosphate synthase produces higher quality fibers with increased cellulose content and has enhanced seedcotton yield (abstract no. 477) [Google Scholar]
- Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin LK, Delmer DP. Carbon partitioning to cellulose synthesis. Plant Mol Biol. 2001;47:29–51. [PubMed] [Google Scholar]
- Haigler CH, Rao NR, Roberts EM, Huang JY, Upchurch DP, Trolinder NL. Cultured cotton ovules as models for cotton fiber development under low temperatures. Plant Physiol. 1991;95:88–96. doi: 10.1104/pp.95.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harn C, Khayat E, Daie J. Expression dynamics of genes encoding carbon metabolism enzymes during sink to source transition of developing leaves. Plant Cell Physiol. 1993;34:1045–1053. [Google Scholar]
- Hauch S, Magel E. Extractable activities and protein content of sucrose-phosphate synthase, sucrose synthase and neutral invertase in trunk tissues of Robinia pseudoacacia L. are related to cambial wood production and heartwood formation. Planta. 1998;207:266–274. [Google Scholar]
- Hill SA, Waterhouse JS, Field EM, Switsur VR, ap Rees T. Rapid cycling of triose phosphates in oak stem tissue. Plant Cell Environ. 1995;18:931–936. [Google Scholar]
- Huber SC, Huber JL. Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:431–444. doi: 10.1146/annurev.arplant.47.1.431. [DOI] [PubMed] [Google Scholar]
- Kerr PS, Kalt-Torres W, Huber SC. Resolution of two molecular forms of sucrose-phosphate synthase from maize, soybean, and spinach leaves. Planta. 1987;170:515–519. doi: 10.1007/BF00402985. [DOI] [PubMed] [Google Scholar]
- Langenkämper G, McHale R, Gardner RC, MacRae E. Sucrose-phosphate synthase steady-state mRNA increases in ripening kiwifruit. Plant Mol Biol. 1998;36:857–869. doi: 10.1023/a:1005964812161. [DOI] [PubMed] [Google Scholar]
- Martin LK. Cool-temperature-induced changes in metabolism related to cellulose synthesis in cotton fibers. PhD thesis. Lubbock, Texas: Texas Tech University; 1999. [Google Scholar]
- Pillonel C, Buchala AJ, Meier H. Glucan synthesis by intact cotton fibers fed with different precursors at the stages of primary and secondary wall formation. Planta. 1980;149:306–312. doi: 10.1007/BF00384571. [DOI] [PubMed] [Google Scholar]
- Quick WP, Schaffer AA. Sucrose metabolism in sources and sinks. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. New York: Marcel Dekker; 1996. pp. 115–156. [Google Scholar]
- Roberts AW, Haigler CH. Rise in chlorotetracycline fluorescence accompanies tracheary element differentiation in suspension cultures of Zinnia. Protoplasma. 1989;152:37–45. [Google Scholar]
- Roberts AW, Haigler CH. A simplified medium for in vitro tracheary element differentiation in mesophyll suspension cultures from Zinnia elegans. Plant Cell Tissue Organ Cult. 1992;28:27–35. [Google Scholar]
- Roberts EM, Nunna RR, Huang JY, Trolinder NL, Haigler CH. Effects of cycling temperatures on fiber metabolism in cultured cotton ovules. Plant Physiol. 1992;100:979–986. doi: 10.1104/pp.100.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y-L, Chourey PS, Delmer DP, Perez-Grau L. The differential expression of sucrose synthase in relation to diverse patterns of carbon partitioning in developing cotton seed. Plant Physiol. 1997;115:375–385. doi: 10.1104/pp.115.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser U. Ultrastructure of the epidermis of developing cotton (Gossypium) seeds: suberin, pits, plasmodesmata, and their implications for assimilate transport into cotton fibers. Am J Bot. 1992;79:14–22. [Google Scholar]
- Salnikov VV, Grimson MJ, Delmer DP, Haigler CH. Sucrose synthase localizes to cellulose synthesis sites in tracheary elements. Phytochemistry. 2001;57:823–833. doi: 10.1016/s0031-9422(01)00045-0. [DOI] [PubMed] [Google Scholar]
- Stewart JM. Integrated events in the flower and fruit. In: Mauney JR, Steward JM, editors. Cotton Physiology. The Cotton Foundation, Memphis. 1986. pp. 261–300. [Google Scholar]
- Tang GQ, Sturm A. Antisense repression of sucrose synthase in carrot (Daucus carota L.) affects growth rather than sucrose partitioning. Plant Mol Biol. 1999;41:465–479. doi: 10.1023/a:1006327606696. [DOI] [PubMed] [Google Scholar]
- Thaker VS, Saroop S, Vaishnav PP, Singh YD. Genotypic variations and influence of diurnal temperature on cotton fiber development. Field Crops Res. 1989;22:1–13. [Google Scholar]
- Thorpe MR, Minchin PEH. Mechanisms of long- and short-distance transport from sources to sinks. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. New York: Marcel Dekker; 1996. pp. 261–282. [Google Scholar]
- Tummala J. Response of sucrose phosphate synthase activity to cool temperatures in cotton fibers. MS thesis. Lubbock: Texas Tech University; 1996. [Google Scholar]
- Uggla C, Magel E, Moritz T, Sundberg B. Function and dynamics of auxin and carbohydrates during early wood/late wood transition in Scots pine. Plant Physiol. 2001;125:2029–2039. doi: 10.1104/pp.125.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäfler U, Meier H. Enzyme activities in developing cotton fibers. Plant Physiol Biochem. 1994;32:697–702. [Google Scholar]
- Winter H, Huber S. Regulation of sucrose metabolism in higher plants: localization and regulation of key enzymes. Crit Rev Plant Sci. 2000;19:31–67. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]