Abstract
The aromas of fruits, vegetables, and flowers are mixtures of volatile metabolites, often present in parts per billion levels or less. We show here that tomato (Lycopersicon esculentum Mill.) plants transgenic for a heterologous Clarkia breweri S-linalool synthase (LIS) gene, under the control of the tomato late-ripening-specific E8 promoter, synthesize and accumulate S-linalool and 8-hydroxylinalool in ripening fruits. Apart from the difference in volatiles, no other phenotypic alterations were noted, including the levels of other terpenoids such as γ- and α-tocopherols, lycopene, β-carotene, and lutein. Our studies indicate that it is possible to enhance the levels of monoterpenes in ripening fruits by metabolic engineering.
In addition to the four basic flavors—sweet, sour, salty, and bitter—that humans recognize in foodstuffs, aromas also have an important influence on people's choice of foods. Food aromas are perceived in humans by the nasal olfactory epithelium, a relatively small area of the mucous-covered inner surface of the nasal cavity. The threshold for human perception of a volatile molecule can be as low as 0.007 μg L−1 in water (Buttery, et al., 1971). Thus, unique combinations of volatiles, as well as the specific proportions of each of the volatile components, determine aroma properties of fruits and other foods (Thomson, 1987).
Most of the research on fruit and vegetable breeding carried out during the last few decades has focused on obtaining desirable agronomic characteristics such as resistance to environmental stresses, pests, and pathogens (Stevens and Rick, 1986). Breeding for improved flavor of fruits such as tomatoes (Lycopersicon esculentum Mill.) has mainly been directed toward controlling sugar to acid ratios and improving texture and storage characteristics of the products (Jones and Scott, 1983; Stevens and Rick, 1986). Tomatoes lacking a characteristic or distinctive “tomato” aroma have often given rise to public complaints about the quality of the produce. Nevertheless, conventional breeding to improve the aromas of agricultural products is often impeded by the large number of genes involved in aroma formation, the significant environmental and developmental effects on aroma, and the lack of consistent, simple, and cheap methodologies to probe both aroma preferences of the public and the chemistry involved.
Although many specific flavor and aroma compounds have been identified in fruits and vegetables, the enzymes and genes controlling their production and their pattern of inheritance have scarcely been studied and are therefore little understood. However, based on studies in many plants, including tomato, it is known that volatile compounds found in fruits are mainly derived from three biosynthetic pathways (Croteau and Karp, 1991). The formation of the hedonically important short-chain aldehydes and alcohols, such as cis-3-hexenol or n-hexanal, takes place through the action of lipases, hydroperoxide lyases, and cleavage enzymes on lipid components, followed by the action of alcohol dehydrogenases (Galliard and Matthew, 1977). Other prominent flavor compounds such as eugenol, phenethyl alcohol, and guaiacol are derived from the shikimic acid pathway (Croteau and Karp, 1991). Nor-isoprenoids, such as β-ionone and geranylacetone, are probably produced by degradation of longer terpenoids such as β-carotene and lycopene, respectively (Stevens, 1970), whereas monoterpenes such as linalool are formed directly from geranyl diphosphate (GPP; Pichersky et al., 1995) via the isoprenoid pathway.
The cultivated tomato is a popular and highly consumed food worldwide. The wide acceptability of tomatoes and their products is not only related to their nutritional value and versatility, but also to their particular flavor and aroma. The characteristic taste of tomatoes and their products is due not only to reducing sugars (Fru and Glc) and free acids (mainly citric), but to a large extent to more than 400 different volatile compounds they contain. These volatile components include acyclic, cyclic, and heterocyclic hydrocarbons, alcohols, phenols, ethers, aldehydes, ketones, carboxylic acids, esters, and lactones, as well as nitrogen, sulfur, and halogen-containing compounds (Petro-Turza, 1986; Buttery et al., 1987, 1989, 1990; Linforth et al., 1994; Maul et al., 1998). As is often the case with the aromas of other fruits and vegetables, it is difficult to relate the aroma of tomato to the presence or absence of a single compound because some of the key flavorants, which have extremely low threshold values, are present in minute amounts. In addition, masking of flavors and synergism between components are also common.
Despite the general lack of knowledge about fruit aroma biosynthesis, modification of tomato aroma utilizing genetic engineering methodologies has recently been attempted by two groups. Wang et al. (1996) modified the oxidation pattern of lipids that degrade into aroma compounds by overexpressing a yeast Δ9-desaturase in transgenic tomatoes, with the result that the concentration of some short-chain alcohols and aldehydes increased 2- to 3-fold. In a second case, transgenic tomato plants were obtained that overexpressed a nonspecific tomato alcohol dehydrogenase gene in fruits, again affecting the levels and ratios of aroma-determining short-chain aldehydes and alcohols (Speirs et al., 1998; Prestage et al., 1999). In both cases, the changes in aroma volatiles were found to impact flavor discrimination by humans, who judged the fruit to be “more ripe.”
One of the 10 most important volatile compounds that influences the quality of the flavor of tomatoes and their products is the acyclic monoterpene alcohol, linalool (Buttery et al., 1971, 1990). Linalool, present in fresh tomatoes in ranges between 1 and 20 μg g−1 (Buttery et al., 1988), imparts a sweet, floral, alcoholic note and it is also a major component of the scent of flowers of many species (Dobson, 1993; Knudsen et al., 1993). Linalool is also present in many edible fruits, including guava (Psidium guajava), peach (Prunus persica), plum (Prunus domestica), pineapple (Ananas comosus), and passionfruit (Passiflora edulis; Bernreuther and Schreier, 1991). Linalool has a chiral center at C3 (see Fig. 1), and two enantiomeric forms are found in nature: S-linalool is found, for example, as a major constituent of the essential oils of coriander (Coriandrum sativum L. family Apiaceae) seed, palmarosa [Cymbopogon martinii var martinii (Roxb.) Wats., family Poaceae], and sweet orange (Citrus sinensis Osbeck, family Rutaceae) flowers (Ravid et al., 1985; Casabianca et al., 1998). R-linalool is present in lavender (Lavandula officinalis Chaix, family Lamiaceae), laurel (Laurus nobilis, family Lauraceae), and sweet basil (Ocimum basilicum, family Lamiaceae), among others (Ravid et al., 1985, 1997; Casabianca et al., 1998). Each enantiomer evokes different neural responses in humans, and therefore is classified as possessing a distinct scent (Sugawara et al., 2000).
Figure 1.
Diversion of the existing plastid terpenoid pathway leading to carotenoids into the production of S-linalool in ripening tomatoes by the expression of the C. breweri LIS transgene. DOXP, Deoxy-d-xylulose 5-phosphate; GPPS, geranyl diphosphate synthase; GGPPS, geranylgeranyl diphosphate synthase.
The potential of genetic engineering of the terpenoid pathway for the nutritional improvement of plant foodstuffs as well as for the improvement of flavor has already been noted (Lewinsohn, 1996; Haudenschild and Croteau, 1998). Manipulation of later steps of the plastid terpenoid pathway has resulted in the generation of transgenic canola (Brassica napus) seed (Shewmaker et al., 1999), rice (Oryza sativa; Romer et al., 2000), and tomato (Lycopersicon esculentum; Ye et al., 2000) containing high concentrations of provitamin A (β-carotene). In addition, tocopherol (vitamin E) metabolism has also been modified, resulting in changes in the α-tocopherol to γ-tocopherol ratio in transgenic Arabidopsis seeds (Shintani and DellaPenna, 1998). Modification of the cytosolic sesquiterpene metabolism by the overexpression of a cotton farnesyl diphosphate synthase has led to increased levels of artemisinin, an antimalarial sesquiterpene in Artemisia annua (Chen et al., 2000). Also, novel sesquiterpenes have been obtained in transgenic tobacco (Nicotiana tabacum) plants, cell cultures, and bacterial cells by overexpressing heterologous sesquiterpene synthase genes (Hohn and Plattner, 1989; Hohn and Ohlrogge, 1991; Zook et al., 1996). However, the successful increase in the concentration of an aroma-determining terpene by means of genetic engineering has not yet been reported.
The gene that codes for S-linalool synthase (LIS), the enzyme responsible for the presence of the monoterpene S-linalool in the scent of Clarkia breweri flowers, has been isolated and characterized (Dudareva et al., 1996). The LIS enzyme uses GPP as a substrate (Fig. 1). Because GPP is also an intermediate (although possibly enzyme bound; see “Discussion”) in the pathway leading to carotenoids, and because a substantial amount of synthesis and accumulation of lycopene and other carotenoid pigments takes place during tomato ripening (Ronen et al., 1999), we tested the hypothesis that expressing C. breweri LIS in the fruit during ripening would divert a portion of the isoprenoid pool available in the plastids to the production of S-linalool (Fig. 1), thus increasing its concentration in the ripe fruit and possibly improving the fruit aroma.
RESULTS
Initial Screen of Transgenic Plants
We chose two tomato varieties with weak aroma, UC82B (a processing line) and CB3 (a fresh market line), for plant transformation. The fruits of these lines virtually lack linalool, thus facilitating our analyses. The first generation of kanamycin-resistant, regenerated tomato plants (T1) were analyzed by Southern blots using the entire LIS cDNA clone as a probe. Several transgenic lines of both CB3 and UC82B were identified that contained one to four copies of LIS (data not shown). A preliminary analysis of the presence of volatiles in ripe fruit was also conducted, and the majority of the plants that contained the LIS transgene also had elevated linalool levels. Plants with the highest levels of linalool (representing four independent transformants from CB3 and six independent transformants from UC82B) were selfed to obtain the T2 generation, and all subsequent generations were also obtained by selfing. All generations from T2 onwards were analyzed for the presence of the LIS transgene using a PCR reaction (described in “Materials and Methods”). All transgenic plants that showed the presence of LIS also showed elevated levels of linalool and linalool derivatives in the fruit, and all their siblings that did not show the presence of the LIS gene, as well as a non-transformed control, did not (see below). In most T2 progenies, a 3:1 ratio was observed between plants with elevated levels of linalool and those without, indicating the presence of LIS at a single locus. However, in a few cases, all progeny plants examined (10 or fewer) had the LIS gene, suggesting multiple insertion sites. In subsequent generations, lines were established that bred true for the presence of LIS, as well as lines that continued to segregate.
Changes in Volatile Profiles of T3 Transgenic Tomatoes
The most abundant volatiles in ripe fruits of the UC82B variety are the phenolic derivatives guaiacol, phenylethyl alcohol, 4-vinylphenol, 4-vinyl-2-methoxy phenol, methyl salicylate, and methylbenzophenone (Table I), each of these components averaging between 50 and 200 ng g−1 fresh weight and ranging from 0 to 390 ng g−1 fresh weight in individual fruits. Their levels in transgenic UC82B lines were slightly more elevated in general, but not significantly different, as judged by the Tukey-Kramer analysis of variance at P < 0.05. Lower levels (4–50 ng g−1 fresh weight) of eugenol, vanillin, and vanillic acid were also found. Fruits from some transgenic UC82B lines showed increased levels of several monoterpenes, such as myrcene, limonene, β-ocimene, and geranial, and a decrease in nor-isoprenes such as geranylacetone compared with the control plants, but these differences were not statistically significant with the exception of limonene. The most striking feature found in fruits from all the transgenic plants was the presence of relatively high levels of linalool (average 187 ng g−1 fresh weight, range 123–258 ng g−1 fresh weight). Linalool was totally lacking in the non-transformed controls (Table I). In addition, the appearance of substantial 8-hydroxylinalool levels (range 94–147 ng g−1 fresh weight) was also noted in the transgenic UC82B fruits. One unknown compound with major mass fragments at m/z 71 (100%), 55 (15%), 69 (9%), 72 (6%), 96 (5.5%), and 81 (5.4%), a second unknown with the major fragmentation ions of 87 (100%), 71 (79%), 82 (72%), 55 (56%) 83 (52%), and 98 (42%), and low levels of a third compound tentatively identified as acetoxylinalool by its mass spectrum were also noted in transgenic UC82B plants but were lacking in the controls.
Table I.
Volatiles in LIS-transgenic and control tomato fruits
| Volatile Detected | Detection Method | UC82B
|
CB3
|
||
|---|---|---|---|---|---|
| Transformed | Control | Transformed | Control | ||
| ng g−1/fresh wt | |||||
| Aliphatic acids, aldehydes, and alcohols | |||||
| Pentanoic acid | Retention time (RT), mass spectrometry (MS) | 20 (0–61) | 12 (0–20) | ||
| Hexanoic acid | RT, MS | 48 (0–103) | Traces | 66 (0–103) | Traces |
| Decanol | RT, MS | 25 (0–76) | 2.8 (0–5) | – | – |
| Nonanoic acid | RT, MS | Traces | 2 (0–5) | 5 (0–15) | 8 (0–13) |
| 2,4-Heptadienal | RT, MS | – | – | Traces | 8 (0–25) |
| Dodecanoic acid | RT, MS | 27 (10–57) | 10 (8–14) | Traces | 16 (0–25) |
| Tetradecanoic acid | RT, MS | 24 (0–58) | Traces | 17 (0–30) | 28 (15–40) |
| Oxygenated and nonoxygenated monoterpenes | |||||
| Myrcene | RT, MS | 20 (0–60) | 10 (0–29) | 17 (0–51) | Traces |
| Limonene | RT, MS | 97 (64–130) | 1 (0–2) | – | – |
| β-Ocimene | RT, MS | 20 (0–61) | Traces | – | – |
| γ-Terpinene | RT, MS | 7 (0–22) | Traces | – | – |
| Terpinolene | RT, MS | 11 (0–34) | Traces | – | – |
| Linaloola | RT, MS | 187 (123–258) | 0 | 456 (165–833) | 0 |
| Geranial | RT, MS | 4 (0–11) | 1 (0–2.5) | 0.5 (0–2) | 4 (2–6) |
| Acetoxylinalool | MS | 5 (0–14) | 0 | – | – |
| 8-Hydroxylinalool | RT, MS | 113 (94–147) | 0 | 345 (199–504) | 0 |
| Phenolic derivatives | |||||
| Benzaldehyde | RT, MS | 13 (0–39) | Traces | 8 (0–25) | Traces |
| Benzyl alcohol | RT, MS | 96 (48–187) | 50 (36–71) | 167 (120–217) | 77 (56–105) |
| Guaiacol | RT, MS | 128 (54–178) | 43 (0–65) | 537 (54–1026) | 252 (16–391) |
| 2-Phenylethyl alcohol | RT, MS | 165 (40–307) | 120 (100–134) | 206 (133–249) | 123 (80–200) |
| Benzoic acid | RT, MS | 38 (0–101) | 38 (6–100) | 5 (0–15) | 7 (0–22) |
| Methyl salicylate | RT, MS | 167 (50–249) | 58 (0–94) | 205 (129–317) | 173 (53–251) |
| 4-Vinylphenol | RT, MS | 147 (55–310) | 75 (35–145) | 179 (139–254) | 87 (44–115) |
| 4-Vinyl 2-methoxyphenol | MS | 130 (0–282) | 92 (29–195) | 297 (112–659) | 77 (47–96) |
| Salicylic acid | MS | – | – | 2 (0–7) | Traces |
| Eugenol | RT, MS | 45 (0–91) | 33 (22–50) | 28 (24–34) | 16 (11–19) |
| Vanillin | RT, MS | 3 (0–8) | 2 (0–4) | 4 (0–11) | 7 (4–8) |
| 2-Methoxyhydroquinone | MS | – | – | 7 (0–20) | 17 (0–36) |
| 4-Methoxy-2-methylphenol | MS | 7 (0–12) | 10 (9–13) | – | – |
| Acetovanillone | RT, MS | – | – | 30 (19–39) | 28 (18–42) |
| Vanillic acid | MS | – | – | 10 (7–23) | Traces |
| Benzophenone | MS | 15 (0–32) | 5 (0–7) | 14 (0–31) | 7 (6–7) |
| Homovanillic acid | MS | 26 (0–54) | 15 (8–28) | 48 (22–94) | 18 (0–34) |
| Methylbenzophenone | MS | 67 (51–94) | 16 (10–21) | 67 (42–87) | 48 (32–65) |
| Coumaric acid | MS | – | – | Traces | 13 (0–38) |
| Nor-isoprenes | |||||
| Geranylacetone | RT, MS | Traces | 5 (3–9) | Traces | 3 (0–7) |
| β-Ionone | MS | – | – | Traces | 3 (0–10) |
| 6-Methyl-2-hepten-1-one | RT, MS | Traces | 6 (0–17) | – | – |
| Other | |||||
| 3-(Methylthio)-1-propanol | RT, MS | – | – | 44 (25–81) | 59 (17–129) |
| 2-Isobutylthiazole | MS | Traces | 13 (0–22) | Traces | 22 (18–28) |
| Furaneol | RT, MS | 5 (0–16) | 33 (22–44) | – | – |
| Butyl butyrate | MS | 109 (91–128) | 52 (49–56) | 46 (0–98) | 47 (29–64) |
| Dihydroactindiolide | MS | 7 (0–11) | 10 (9–13) | Traces | 6 (4–7) |
| Unknown 1 | MS | 60 (0–112) | 0 | 425 (0–922) | 0 |
| Unknown 2 | MS | 135 (37–290) | 0 | – | – |
Means and ranges of three independent determinations are shown. One fruit from three plants, each originating from an independent transformation event, was analyzed for each variety.
Bold indicates identified linalool and linalool derivatives.
The pattern of volatiles found in variety CB3 (non-transgenic) was very similar to that found in UC82B, except for the absence of furaneol, and some monoterpenes such as limonene, γ-terpinene, and β-ocimene. Again, a striking feature was the appearance of linalool in all transgenic plants that were scored positive by the PCR reaction, as well as the presence of 8-hydroxylinalool. Unknown 1 was also present in the transgenic CB3 fruit but absent from the control. A typical chromatogram displaying the analysis of m/z of 93 (corresponding to monoterpenes such as linalool) produced in fruits of a transgenic CB3 line is shown in Figure 2.
Figure 2.
Presence of S-linalool and 8-hydroxylinalool in LIS-transgenic tomato fruits. Fresh tomato fruits were extracted with tert-butyl methyl ether (MTBE) and analyzed by gas chromatography-MS using an HP5 column as described in “Materials and Methods.” The trace obtained for the m/z = 93 typical for linalool and other monoterpene derivatives is shown. Top, An extract from a typical transgenic CB3 fruit; bottom, non-transformed control.
Analysis of Linalool and Linalool Derivatives Made by the Transgenic Tomatoes
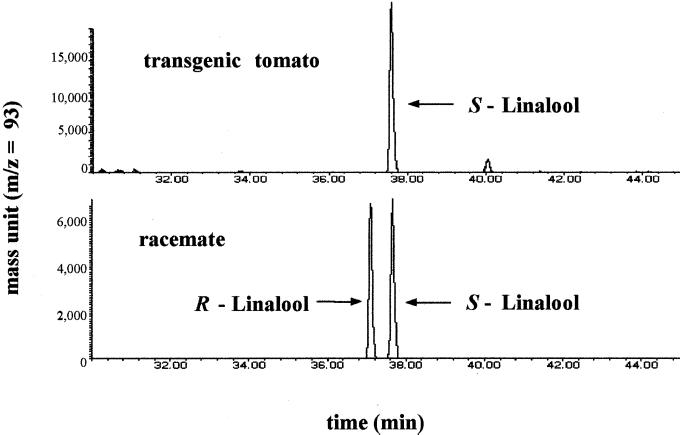
The C. breweri LIS gene catalyzes the formation of enantiomerically pure S-linalool from the general monoterpene precursor GPP (Pichersky et al., 1995). Because linalool is a tertiary allylic alcohol, it may readily racemize, especially in acidic environments (Casabianca et al., 1998) such as those that prevail in ripe tomato fruits. Therefore, we examined the chirality of the linalool accumulated in LIS-transgenic tomatoes by chiral gas chromatography using a modified β-cyclodextrin phase (Ravid et al., 1997). It is interesting that the linalool that accumulated in the LIS-transgenic plants was enantiomerically pure, accumulating exclusively as the enantiomer S-linalool (Fig. 3). The apparent lack of racemization might reflect a process of compartmentalization that separates linalool from the acid environment of the fruit flesh. In many plants, linalool accumulates in compartmentalized secretory structures such as glandular trichomes (Werker et al., 1993) or is emitted into the surrounding environment (Pichersky et al., 1994). It is presently unknown how S-linalool is accumulated in ripening tomato fruit tissues.
Figure 3.
Enantiomeric composition of the linalool accumulated in LIS-transgenic tomatoes. The same extract utilized in Figure 2 was analyzed on a chiral Restek Rt-βDEXsm column (top). The separation of a racemic (synthetic) mixture of linalool under the same conditions is shown (bottom).
The accumulation of 8-hydroxylinalool in the transgenic tomato fruits can be explained by allylic hydroxylation, a common reaction in monoterpene metabolism (Croteau and Karp, 1991). Our results suggest that such a hydroxylating activity is present in ripening tomato fruits, although the endogenous substrate is presently unknown. We do not have a simple explanation for the increase in limonene (and possibly in other terpene volatiles) in some of the LIS-transgenic plants. In C. breweri, LIS is responsible for the production of S-linalool only.
Time Course of Accumulation of Linalool and 8-Hydroxylinalool
In tomatoes and other fruits, aroma volatiles are normally accumulated late during maturation (Baldwin et al., 1991; Shalit et al., 2001). The E8 promoter that was used to direct the expression of LIS has been shown previously to be activated at this stage (Good et al., 1994). Therefore, we examined the accumulation of linalool and 8-hydroxylinalool at various stages of fruit maturation (Fig. 4). At the “green” stage, the levels of S-linalool and 8-hydroxylinalool in fruits were negligible, but in later stages of development they reached up to 0.4 and 0.2 μg g−1 fresh weight, respectively. The levels of linalool and 8-hydroxylinalool were higher in transgenic CB3 tomato fruits than in transgenic UC82B (Fig. 4). In both lines, fruits from the non-transformed control plants were devoid of both linalool and 8-hydroxylinalool (Fig. 4).
Figure 4.
Accumulation of S-linalool (squares) and 8-hydroxylinalool (circles) in transgenic and control tomatoes during fruit maturation. A, CB3 variety; B, UC82B variety. White symbols represent transgenic plants and black symbols represent non-transformed controls. Each data point represents an average and the se of data obtained from two to seven different fruits analyzed separately, but originating from the same plants.
Effect of LIS Expression on the Accumulation of Nonvolatile Terpenoids
Several nutritionally important metabolites in tomato fruits are synthesized via the terpenoid pathway. They include plant hormones, pigments, and vitamins such as gibberellins, lycopene, and tocopherols (Croteau et al., 2000). The results of manipulation of the terpenoid pathway might have negative effects on related terpenoid accumulation and on the growth and development of the plants due to hormonal imbalance (Fray et al., 1995). Therefore, it was of interest to investigate the effect of the metabolic diversion of the terpenoid pathway to S-linalool on the accumulation of other terpenoids in tomato fruits. The results are shown in Table II. The levels of lycopene, other carotenoids, and of tocopherols were unaffected as judged by the Tukey-Kramer analysis of variance at P < 0.05 in the LIS-transgenic plants as compared with controls. Because the levels of these latter compounds are normally at least an order of magnitude higher than the levels of S-linalool that were produced, it appears that the small fraction of the metabolic flow through the terpenoid pathway that was diverted into S-linalool caused only a small (<0.3%), nonsignificant decrease in the accumulation of nonvolatile terpenoids.
Table II.
Levels of S-linalool and other terpenoids in LIS-transgenic and control ripe tomato fruits
| Plant Line | γ-Tocopherol | α-Tocopherol | Lutein | Lycopene | β-Carotene | S-Linalool |
|---|---|---|---|---|---|---|
| μg g−1 fresh wt (± se) | ||||||
| CB3 LIS-transgenic | 10.45 (0.31) | 8.49 (0.82) | 2.28 (0.24) | 66.51 (5.50) | 5.53 (0.56) | 0.31 (0.01) |
| CB3 Controls | 11.07 (0.09) | 7.02 (0.09) | 1.99 (0.21) | 63.75 (14.91) | 3.00 (0.48) | 0.00 (0.00) |
| UC82B LIS-transgenic | 11.83 (0.34) | 7.45 (0.75) | 1.62 (0.50) | 76.60 (1.11) | 3.35 (0.60) | 0.25 (0.05) |
| UC82B Controls | 10.40 (0.98) | 6.40 (1.82) | 2.15 (0.13) | 91.02 (24.63) | 4.80 (0.14) | 0.00 (0.00) |
Transformation was verified by PCR. Means and se of two to five independent determinations from one fruit from plants containing high S-linalool levels that originated in one transformation event for each variety and compared with controls.
DISCUSSION
We have used the C. breweri LIS gene under the control of the E8 promoter to direct the production of S-linalool and its accumulation in ripening tomato fruits, and the measurements presented here clearly indicate that it is possible to substantially increase the amount of an aroma compound in such fruits. However, although the changed aroma of the intact fruit could be easily detected by the human nose (data not shown), we have not yet carried out taste tests with humans, so the actual effect on human preference for the transgenic fruit is unknown.
In plastids, isopentenyl diphosphate and dimethyl allyl diphosphate are now known to be synthesized through the Rohmer pathway (Rohmer, 1999; Bohlmann et al., 2000; Croteau et al., 2000) via deoxy-d-xylulose 5-phosphate (Fig. 1). GPP is used by the plastids for further synthesis of monoterpenes involved in plant defense or as pollinator attractors (Dudareva et al., 1996; Turner et al., 1999; Croteau et al., 2000). Geranylgeranyl diphosphate (GGPP) is synthesized by the chloroplasts of all green plants as a precursor to carotenoids involved in photosynthesis, and in chromoplasts, such as those found in ripening tomato fruits, for the synthesis of the carotenoid pigment lycopene (Giuliano et al., 1993; Ronen et al., 1999). GGPP synthase, the enzyme that catalyzes the formation of GGPP, uses one molecule of dimethyl allyl diphosphate and three molecules of isopentenyl diphosphate (Fig. 1) and the two intermediates, GPP and farnesyl diphosphate, are believed to be mostly enzyme bound (Ogura et al., 1972; Dogbo and Camara 1987; Bohlmann et al., 2000). Our findings indicate that sufficient levels of the substrate GPP might escape from the GGPP synthase enzyme and are available for the action of LIS activity in ripe transgenic tomatoes. This further indicates that part of the metabolic flow normally committed to the biosynthesis of lycopene and other carotenoids can be recruited for S-linalool formation without any major adverse effect on the accumulation of the nutritionally important lycopene and tocopherols.
The promoter of the tomato E8 gene was selected for expressing the C. breweri LIS gene in transgenic tomato because this promoter is relatively well characterized, and has been successfully used previously for expressing transgenes in tomato fruits (Good et al., 1994; Sandhu et al., 2000). Although this gene is also expressed in flowers (mainly in anther tissues), the E8 gene is expressed late during ripening, and is expressed uniformly throughout the ripening fruit tissues (Deikman et al., 1992; Kneissl and Deikman 1996), a pattern of expression ideally suited to attempting to engineer flavor biosynthesis.
The potential of genetic engineering for the improvement of aroma and taste properties of agricultural products is underexploited. We have described the utilization of a floral gene, normally involved in fragrance production, in an attempt to improve the aroma of tomato fruits. With the discovery of other genes encoding key enzymes involved in the production of volatile aroma chemicals, the potential to utilize genetic engineering for the manipulation of crops is very promising. Because many aroma compounds are derived from ubiquitous metabolic pathways, diverting existing biosynthetic pathways into the production of unique scent compounds may result in fruits, vegetables, and grains with enhanced or modified aromas and better overall taste. Moreover, because for many volatile compounds only small amounts are needed to affect a change in flavor, a careful choice of genes and promoters used for the genetic engineering will likely result in little or no effect on primary metabolism.
MATERIALS AND METHODS
Vector Construction and Plant Transformation
The Clarkia breweri LIS coding region (Dudareva et al., 1996) was cloned into a binary vector capable of replicating in both Escherichia coli and Agrobacterium tumefaciens (McBride and Summerfelt 1990). The LIS gene was flanked by the tomato (Lycopersicon esculentum Mill.) E8 promoter (Deikman and Fischer, 1988) and the tml gene 3′ end (Barker et al., 1983). The vector also contains a neomycin phosphotransferase (nptII) gene driven by the cauliflower mosaic virus 35S promoter for selection of transformed plant cells.
The binary vector was introduced into A. tumefaciens strain LBA4404 and the resulting bacteria were used for transformation of tomato varieties UC82B and CB3, essentially as described by McBride and Summerfelt (1990).
Plant Growth
Transgenics and control seeds were sown in seedling trays (Polyvid, Mishmar HaNegev, Israel; 37 mm cell−1 and 128 cells tray−1) filled with a 1:1 (v/v) mixture of peat:vermiculite in a controlled greenhouse and irrigated daily until three true leaves appeared. They were then transferred to 20-L pots containing the same mixture. Plants were drip irrigated for 10 min, 10 times a day and fertilized through the irrigation system twice a week with 0.2% (w/v) N:P:K (5:3:8).
DNA Extraction and PCR Analysis
DNA was extracted according to Tai and Tanksley (1990). One-hundred milligrams of actively growing leaf tissue was frozen in liquid N2, ground with a polypropylene tip, and extracted with 300 μL of extraction buffer as described. To check for the presence of LIS in transgenic plants, a PCR with two oligonucleotides specific for the C. breweri LIS cDNA was carried out. The oligonucleotides were chosen so that the amplified fragment would be 1.1 kb in length. The PCR reaction was performed by mixing 100 mm Tris-HCl (pH 8.3), 500 mm KCl, 2.5 mm MgCl2, 1 unit of Taq DNA polymerase (Advanced Biotechnologies Co., Epson, Surrey, UK), 0.2 mm of each dNTP (MBI Fermentas Co., Vilnius, Lithuania), 100 ng of genomic DNA, and 50 ng each of the following primers (Sigma Chemical Co., St. Louis): sense primer number 212419, 5′ GTT GGT TCA CCA TCA TGT TCC 3′, and antisense primer number 171319, 5′ CTA CAA AAT CCC ATG TC 3′ in a total volume of 20 μL. Amplification was performed in a PTC-100 PCR machine (MJ Research Inc., Watertown, MA) under the following regime: 5 min at 94°C (1 min at 95°C, 2 min at 45°C, and 2 min at 72°C) repeated 31 times, followed by 7 min at 72°C. Each reaction was electrophoresed in 1.2% (w/v) agarose gel using 89 mm Tris and 89 mm boric acid with the addition of 2 mm EDTA (Tris-borate/EDTA) buffer (Sambrook et al., 1989). PBR322 DNA treated with Alw44I (ApaLI) and MvaI (BstNI) was used as size markers.
Metabolite Analyses
Extraction of Volatile Metabolites from Tomato Fruits
Fully mature tomato fruits were analyzed, except when indicated. Approximately 30 to 50 g fresh peeled tomato fruits were cut into small (approximately 0.5 cm3) pieces and homogenized with a mortar and pestle. The paste was extracted by vigorous shaking with a vortex apparatus for 1 h with 60 to 100 mL of MTBE and with 10 μg of disobutyl benzene added as an internal standard. The phases were separated in a separatory funnel and the upper ethereal phase was dried with anhydrous Na2SO4 (4 cm thick, in a funnel and concentrated with a gentle stream of N2 utilizing a Turbo Vap II evaporator (Zymark Corp., Hopkinton, MA) until the volume reached 0.5 mL.
Analysis and Identification of Volatile Metabolites
Samples consisting of 1 μL of the concentrated MTBE extracts (see above) were analyzed on a GCD gas chromatograph (Hewlett-Packard, Waldbronn, Germany) equipped with a HP5 (30 m × 0.25 mm) fused-silica capillary column. Helium (1 mL min−1) was used as a carrier gas with splitless injection. The injector temperature was 250°C and the detector temperature was 280°C. Conditions used were as follows: Initial temperature was 70°C for 2 min, followed by a ramp of 70°C to 200°C at a rate of 4°C/min, and 10 additional min at 200°C. Masses between 45 and 450 m/z were recorded. Identification of the main components was done by co-injection and comparison of the electron-ionization-MS obtained with authentic standards and complemented with computerized libraries (Lewinsohn et al., 1998; Shalit et al., 2001).
Enantiomeric Determination of Linalool Produced in Transgenic Tomatoes
Chiral separations were performed on the same GCD gas chromatograph equipped with an alkylated β-cyclodextrin (Restek, Bellefonte, PA; Rt-βDEXsm) fused silica capillary column (30-m × 0.25-mm i.d.; 0.25-μm film thickness). Injector and detector temperatures were 230°C. Initial temperature was kept at 55°C for 1 min, then raised to 200°C at a rate of 1°C min−1. Helium was used as a carrier gas at a flow rate of 1 mL min−1. A split ratio of 1:50 was used. Masses between 45 and 450 m/z were recorded. Racemic linalool was purchased from Roth Chemical Co. (Karlsruhe, Germany). (−)-R-linalool isolated from sweet basil (Ocimum basilicum) was from our collection (Ravid et al., 1985, 1997).
Determination of Tocopherol and Carotenoid Levels
Fresh ripe fruits were homogenized with a blender and a 200-μL aliquot of the homogenate was extracted with 3 mL of acetone containing 60 μg δ-tocopherol as an internal standard (Ronen et al., 1999). The samples were sonicated (cavitator ultrasonic cleaner, Mettler Corp., Anaheim, CA) for 10 min until the tomato tissues were colorless and then 1 mL of a 12% (w/v) NaCl solution and 4 mL of dichloromethane were added. After extraction, the lower organic phase was dried with Na2SO4 and concentrated to dryness using a Savant Speed Vac centrifuge. The remaining solids were resuspended in acetonitrile:dichloromethane:methanol, triethylamine (75:20:5, 0.05, v/v) and analyzed with a Alliance photodiode array HPLC machine (Hewlett-Packard) utilizing the gradient system described previously (Tadmor et al., 2000). The levels of lycopene, β-carotene, and lutein, as well as α- and γ- tocopherol, were quantified based on calibration curves with authentic standards (Lycored, Beer Sheba, Israel; Sigma)
ACKNOWLEDGMENTS
We thank Michael Minkoff and Midiab Diabath for growing the plants.
Footnotes
This work was supported in part by the U.S. Israel Binational Agricultural Research and Development fund (grant no. IS–2709–96). This is publication no. 105/2001 of the Agricultural Research Organization, Bet Dagan Israel.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010293.
LITERATURE CITED
- Baldwin EA, Nisperos-Carriedo MO, Moshonas MG. Quantitative analysis of flavor and other volatiles and for certain constituents of two tomato cultivars during ripening. J Am Soc Hortic Sci. 1991;116:265–269. [Google Scholar]
- Barker RF, Idler KB, Thompson DV, Kemp JD. Nucleotide sequence of the T-DNA region from the Agrobacterium tumefaciens octopine Ti plasmid pTi15955. Plant Mol Biol. 1983;2:335–350. doi: 10.1007/BF01578595. [DOI] [PubMed] [Google Scholar]
- Bernreuther A, Schreier P. Multidimensional gas chromatography/mass spectrometry: a powerful tool for the direct chiral evaluation of aroma compounds in plant tissues: II. Linalool in essential oils and fruits. Phytochem Anal. 1991;2:167–170. [Google Scholar]
- Bohlmann J, Gershenzon J, Aubourg S. Biochemical, molecular, genetic and evolutionary aspects of defense-related terpenoid metabolism in conifers. In: Romeo JT, Ibrahim R, Varin L, De Luca V, editors. Evolution of Metabolic Pathways: Recent Advances in Phytochemistry. Vol. 34. Oxford: Elsevier Science; 2000. pp. 109–150. [Google Scholar]
- Buttery RG, Siefert RM, Guadagni DG, Ling LC. Characterization of additional volatile components of tomato. J Agric Food Chem. 1971;19:524–529. [Google Scholar]
- Buttery RG, Teranishi R, Flath RA, Ling LC. Fresh tomato volatiles: composition and sensory studies. In: Teranishi R, Buttery RG, Shahidi F, editors. Flavor Chemistry, Trends and Developments, ACS Symposium Series 388. Washington, DC: American Chemical Society; 1989. pp. 213–222. [Google Scholar]
- Buttery RG, Teranishi R, Ling LC. Fresh tomato aroma volatiles: a quantitative study. J Agric Food Chem. 1987;35:540–544. [Google Scholar]
- Buttery RG, Teranishi R, Ling LC, Flath RA, Stern DJ. Quantitative studies on origins of fresh tomato aroma volatiles. J Agric Food Chem. 1988;36:1247–1250. [Google Scholar]
- Buttery RG, Teranishi R, Ling LC, Turnbaugh JG. Quantitative and sensory studies on tomato paste volatiles: J Agric Food Chem. 1990;38:336–340. [Google Scholar]
- Casablanca H, Graff JB, Faugier V, Fleig F, Grenier C. Enantiomeric distribution studies of linalool and linalyl acetate: a powerful tool for authenticity control of essential oils. J High Resolution Chromatogr. 1998;21:107–112. [Google Scholar]
- Chen DH, Ye HC, Li GF. Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Science. 2000;155:179–185. doi: 10.1016/s0168-9452(00)00217-x. [DOI] [PubMed] [Google Scholar]
- Croteau R, Karp F. Origin of natural odorants. In: Muller PM, Lamparsky D, editors. Perfumes: Art, Science and Technology. London: Elsevier Applied Science; 1991. pp. 101–126. [Google Scholar]
- Croteau R, Kutchan TM, Lewis NG. Natural products (secondary metabolites) In: Buchanan B, Gruissem W, Jones R, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 1250–1318. [Google Scholar]
- Deikman J, Fischer RL. Interaction of a DNA binding factor with the 5′-flanking region of an ethylene-responsive fruit ripening gene from tomato. EMBO J. 1988;7:3315–3320. doi: 10.1002/j.1460-2075.1988.tb03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deikman J, Kline R, Fischer RL. Organization of the ripening and ethylene regulatory regions in a fruit-specific promoter from tomato (Lycopersicon esculentum) Plant Physiol. 1992;100:2013–2017. doi: 10.1104/pp.100.4.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson HEM. Floral volatiles in insect biology. In: Bernays E, editor. Insect-Plant Interactions. Vol. 5. Boca Raton, FL: CRC Press; 1993. pp. 47–81. [Google Scholar]
- Dogbo O, Camara B. Purification of isopentenyl pyrophosphate isomerase and geranylgeranyl pyrophosphate synthase from Capsicum chromoplasts by affinity chromatography. Biochim Biophys Acta. 1987;920:140–148. [Google Scholar]
- Dudareva N, Cseke L, Blanc VD, Pichersky E. Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell. 1996;8:1137–1148. doi: 10.1105/tpc.8.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. [Google Scholar]
- Galliard T, Matthew JA. Lipoxygenase-mediated cleavage of fatty acids to carbonyl fragments in tomato fruits. Phytochemistry. 1977;16:339–343. [Google Scholar]
- Good X, Kellogg JA, Wagoner W, Langhoff D, Matsumura W, Bestwick RK. Reduced ethylene synthesis by transgenic tomatoes expressing S-adenosylmethionine hydrolase. Plant Mol Biology. 1994;26:781–790. doi: 10.1007/BF00028848. [DOI] [PubMed] [Google Scholar]
- Giuliano G, Bartley GE, Scolnik PA. Regulation of carotenoid biosynthesis during tomato development. Plant Cell. 1993;5:379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild CD, Croteau RB. Molecular engineering of monoterpene production. Genet Eng. 1998;20:267–280. doi: 10.1007/978-1-4899-1739-3_14. [DOI] [PubMed] [Google Scholar]
- Hohn TM, Ohlrogge JB. Expression of a fungal sesquiterpene cyclase gene in transgenic tobacco. Plant Physiol. 1991;97:460–462. doi: 10.1104/pp.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn TM, Plattner RD. Expression of the trichodiene synthase gene of Fusarium sporotrichioides in Escherichia coli results in sesquiterpene production. Arch Biochem Biophys. 1989;275:92–97. doi: 10.1016/0003-9861(89)90353-6. [DOI] [PubMed] [Google Scholar]
- Jones RA, Scott SJ. Improvement of tomato flavor by genetically increasing sugar and acid contents. Euphytica. 1983;32:845–855. [Google Scholar]
- Kneissl ML, Deikman J. The tomato E8 gene influences ethylene biosynthesis in fruit but not in flowers. Plant Physiol. 1996;112:537–547. doi: 10.1104/pp.112.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen JT, Tollsten L, Bergstrom G. Floral scents: a check list of volatile compounds isolated by headspace techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Lewinsohn E. Molecular biology for the improvement of medicinal and aromatic plants. In: Craker LE, Nolan L, Shetty K, editors. Proceedings of the International Symposium on Medicinal and Aromatic Plants. Acta Hortic 426: 443–466. 1996. [Google Scholar]
- Lewinsohn E, Dudai N, Tadmor Y, Katzir I, Ravid U, Putievsky E, Joel DM. Histochemical localization of citral accumulation in lemongrass leaves (Cymbopogon citratus (DC.) Stapf., Poaceae) Ann Bot. 1998;81:35–39. [Google Scholar]
- Linforth RST, Savary I, Pattenden B, Taylor AJ. Volatile compounds found in expired air during eating of fresh tomatoes and in the headspace above tomatoes. J Sci Food Agric. 1994;65:241–247. [Google Scholar]
- Maul F, Sargent SA, Balaban MO, Baldwin EA, Huber DJ, Sims CA. Aroma volatile profiles from ripe tomatoes are influenced by physiological maturity at harvest: an application for electronic nose technology. J Am Soc Hortic Sci. 1998;123:1094–1101. [Google Scholar]
- McBride KE, Summerfelt KR. Improved binary vectors for Agrobacterium-mediated plant transformation. Plant Mol Biol. 1990;14:269–276. doi: 10.1007/BF00018567. [DOI] [PubMed] [Google Scholar]
- Ogura K, Shinka T, Seto S. The purification and properties of geranylgeranyl pyrophosphate synthetase from pumpkin fruit. J Biochem. 1972;72:1101–1108. doi: 10.1093/oxfordjournals.jbchem.a129997. [DOI] [PubMed] [Google Scholar]
- Petro-Turza M. Flavor of tomato and tomato products. Food Rev Int. 1986;2:309–351. [Google Scholar]
- Pichersky E, Lewinsohn E, Croteau R. Purification and characterization of S-linalool synthase, an enzyme involved in the production of floral scent in Clarkia breweri. Arch Biochem Biophys. 1995;316:803–807. doi: 10.1006/abbi.1995.1107. [DOI] [PubMed] [Google Scholar]
- Pichersky R, Raguso RA, Lewinsohn E, Croteau R. Floral scent production in Clarkia (Onagraceae): I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol. 1994;106:1533–1540. doi: 10.1104/pp.106.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestage S, Linforth RST, Taylor AJ, Lee E, Speirs J, Schuch W. Volatile production in tomato fruit with modified alcohol dehydrogenase activity. J Sci Food Agric. 1999;79:131–136. [Google Scholar]
- Ravid U, Putievsky E, Katzir I, Lewinsohn E. Enantiomeric composition of linalol in the essential oils of Ocimum species and in commercial basil oils. Flavour Fragrance J. 1997;12:293–296. [Google Scholar]
- Ravid U, Putievsky E, Weinstein V, Ikan R. Determination of the enantiomeric composition of natural flavouring agents by 1H-NMR spectroscopy. In: Baerheim Svendsen A, Scheffer JJC, editors. Essential Oils and Aromatic Plants. Dordrecht, The Netherlands: Martinus Nijhoff/Dr. W. Junk Publ.; 1985. pp. 135–138. [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Römer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley PM. Elevation of the provitamin A content of transgenic tomato plants. Nature Biotechnol. 2000;18:666–669. doi: 10.1038/76523. [DOI] [PubMed] [Google Scholar]
- Ronen G, Cohen M, Zamir D, Hirschberg J. Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J. 1999;17:341–351. doi: 10.1046/j.1365-313x.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sandhu JS, Krasnyanski SF, Domier LL, Korban SS, Osadjan MD, Buetow DE. Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response. Transgenic Res. 2000;9:127–135. doi: 10.1023/a:1008979525909. [DOI] [PubMed] [Google Scholar]
- Shalit M, Katzir N, Tadmor Y, Larkov O, Burger Y, Shalechet F, Lastochkin E, Ravid U, Amar O, Edelstein M. Acetyl CoA: alcohol acetyl transferase activity and aroma formation in ripening melon fruits. J Agric Food Chem. 2001;49:794–799. doi: 10.1021/jf001075p. [DOI] [PubMed] [Google Scholar]
- Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY. Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J. 1999;20:401–412. doi: 10.1046/j.1365-313x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Shintani D, DellaPenna D. Elevating the vitamin E content of plants through metabolic engineering. Science. 1998;282:2098–2100. doi: 10.1126/science.282.5396.2098. [DOI] [PubMed] [Google Scholar]
- Speirs J, Lee E, Holt K, Yong-Duk K, Scott NS, Loveys B, Schuch W. Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavor aldehydes and alcohols. Plant Physiol. 1998;117:1047–1058. doi: 10.1104/pp.117.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MA. Relatioship between polyene-carotene content and volatile compound composition in tomatoes. J Am Soc Hortic Sci. 1970;95:461–464. [Google Scholar]
- Stevens MA, Rick CM. Genetics and breeding. In: Atherton JG, Rudich J, editors. The Tomato Crop. NY: Chapman & Hall; 1986. pp. 35–109. [Google Scholar]
- Sugawara Y, Hara C, Aoki T, Sugimoto N, Masujima T. Odor distinctiveness between enantiomers of linalool: difference in perception and responses elicited by sensory test and forehead surface potential wave measurement. Chem Senses. 2000;25:77–84. doi: 10.1093/chemse/25.1.77. [DOI] [PubMed] [Google Scholar]
- Tadmor Y, Larkov O, Meir A, Minkoff M, Lastochkin E, Edelstein M, Levin S, Wong J, Rocheford T, Lewinsohn E. Reversed-phase high performance liquid chromatographic determination of vitamin E components in maize kernels. Phytochem Anal. 2000;11:370–374. [Google Scholar]
- Tai TH, Tanksley SD. A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. Plant Mol Biol Rep. 1990;8:297–303. [Google Scholar]
- Thomson DMH. The meaning of flavour. In: Birch GG, Lindley NG, editors. Developments in Food Flavours. London: Elsevier; 1987. pp. 1–21. [Google Scholar]
- Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999;120:879–886. doi: 10.1104/pp.120.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chin CK, Ho CT, Hwang CF, Polashock JJ, Martin CE. Changes of fatty acids and fatty acid derived flavor compounds by expressing the yeast Δ-9 desaturase gene in tomato. J Agric Food Chem. 1996;44:3399–3402. [Google Scholar]
- Werker E, Putievsky E, Ravid U, Dudai N, Katzir I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae) Ann Bot. 1993;71:43–50. [Google Scholar]
- Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- Zook M, Hohn T, Bonnen A, Tsuji J, Hammerschmidt R. Characterization of novel sesquiterpenoid biosynthesis in tobacco expressing a fungal sesquiterpene synthase. Plant Physiol. 1996;112:311–318. doi: 10.1104/pp.112.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]