Abstract
In situ measurements of alternative respiratory pathway activity are needed to provide insight into the energy efficiency of plant metabolism under various conditions in the field. The only reliable method at present to measure alternative oxidase (AOX) activity is through measurement of changes in δ18O(O2), which to date has only been used in laboratory environments. We have developed a cuvette system to measure partitioning of electrons to AOX that is suitable for off-line use and for field experiments. Plant samples are enclosed in airtight cuvettes and O2 consumption is monitored. Gas samples from the cuvette are stored in evacuated gas containers until measurement of δ18O(O2). We have validated this method using differing plant material to assess AOX activity. Fractionation factors were calculated from δ18O(O2) measurements, which could be measured with an accuracy and precision to 0.1‰ and 0.3‰, respectively. Potential sources of error are discussed and quantified. Our method provides results similar to those obtained with laboratory incubations on-line to a mass spectrometer but greatly increases the potential for adoption of the stable isotope method.
Measurements of partitioning of respiratory electron flow between the energy-conserving cytochrome oxidase (COX) pathway and the energy-inefficient alternative oxidase (AOX) pathway were initially quantified by assessing the effect on O2 consumption of titrations with specific inhibitors of both respiratory pathways (see Møller et al., 1988). However, titration with inhibitors is associated with a number of problems. The most important is the assumption that inhibition of the alternative pathway would not affect the cytochrome pathway; this is not necessarily true (Wilson, 1988; Millar et al., 1995; Ribas-Carbo et al., 1995). Therefore, AOX activity may be underestimated by titration. This problem was overcome by the noninvasive stable isotope method, which was initially developed by Guy et al. (1989) and has been perfected during the last decade (Robinson et al., 1992; Ribas-Carbo et al., 1997; Gonzàlez-Meler et al., 1999; Henry et al., 1999). This method relies on the different discrimination against the 18O isotope by COX and AOX. Although inhibitors are used to measure the discrimination factor (D) of either oxidase, the actual measurement does not depend on inhibitors.
The present on-line method (Robinson et al., 1992; Gonzàlez-Meler et al., 1999) uses a cuvette for incubation of plant material that is directly connected to a gas chromatograph and an isotope ratio mass spectrometer (IRMS), operating in continuous flow mode. This approach allows rapid and accurate measurements but has a few limitations. In particular, it is restricted to the very few laboratories worldwide that can acquire and maintain a dedicated on-line system. There is a great need to extend the use of the stable isotope approach to assess the role of AOX in the dissipation of excess reducing power under unfavorable growth conditions, such as low temperatures (Stewart et al., 1990; Vanlerberghe and McIntosh, 1992; Purvis and Shewfelt, 1993; Gonzàlez-Meler et al., 1999; Ribas-Carbo et al., 2000a), high light (Millenaar et al., 2000; Ribas-Carbo et al., 2000b; Noguchi et al., 2001), drought (Collier and Cummins, 1992), a low supply of minerals (Rychter and Mikulska, 1990; Theodorou and Plaxton, 1993; Parsons et al., 1999; Gonzàlez-Meler et al., 2001), and wounding or infection (Lennon et al., 1997; Simons et al., 1999). Thus, it was timely to develop a system with which cuvette headspace could be sampled and stored for transfer to laboratories where IRMSs are available. Very recently, an off-line stable isotope system for plant respiration was described by Noguchi et al. (2001), but their design is still quite complex and costly and is not suitable for measurements in the field. Here, we discuss the development and testing of a very low cost off-line cuvette system that does not require an on-line IRMS and can be used for field assessment of electron partitioning to the alternative path.
RESULTS AND DISCUSSION
Inhibitor Titrations
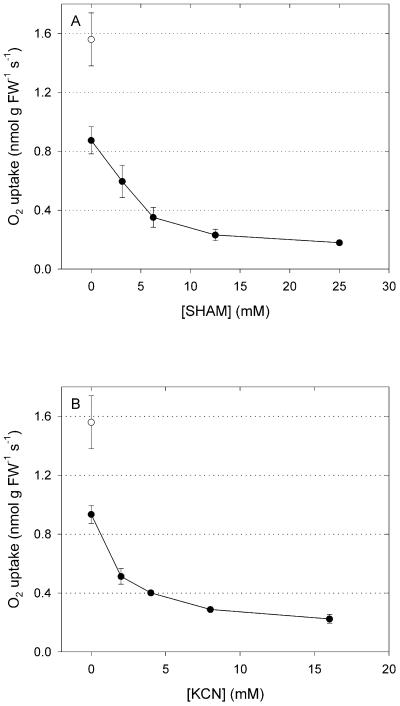
Inhibitor titrations were carried out to demonstrate inhibitor penetration and action. Respiration of Griselinia littoralis leaves could not be inhibited by soaking in inhibitor solutions or by feeding of inhibitors through the petiole. Addition of 5 mL of highly concentrated (200 mm) KCN solutions buffered at pH 6, 7, or 8 to the cuvettes did not substantially decrease respiration until 10 h of incubation and respiration rates were still decreasing after 30 h of incubation (results not shown). Therefore, leaves had to be infiltrated with inhibitor solutions to inhibit respiration. Leaf slices were infiltrated with a series of mixtures of KCN and salicylhydroxamic acid (SHAM). Increasing concentrations of both inhibitors decreased respiration to a minimum of 10% of that of the control (Fig. 1). A residual respiration of 10% of the uninhibited rate is similar to previous results for leaves (e.g. Azcón-Bieto et al., 1983; Atkin et al., 1993; Lennon et al., 1997). Because slicing leaves in smaller parts and infiltrating at lower pressure did not further decrease residual O2 uptake, this may be associated with non-respiratory O2-consuming processes. Such residual respiration is not necessarily present in the absence of inhibitors (Ribas-Carbo et al., 1997). Based on these results, 25 mm SHAM and 16 mm KCN were used to obtain the baseline data for the isotope fractionation measurements. The unusually high concentration of 16 mm KCN needed to fully inhibit COX may be explained by volatilization of cyanide during infiltration and the subsequent 2-h period of evaporation prior to the measurements. We see no obvious way to prevent this loss of cyanide.
Figure 1.
Titration of G. littoralis leaf respiration with inhibitors. A, Titration with SHAM in the presence of 16 mm KCN. B, Titration with KCN in the presence of 25 mm SHAM. Values are means ± se of three (SHAM) or two (KCN) measurements of 10 g of leaf material. ○, Uninhibited respiration (n = 2). FW, Fresh weight.
Isotope Fractionation Measurements
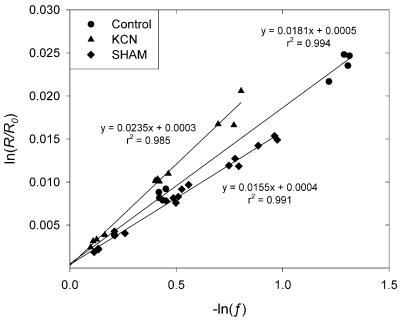
Fractionation factors (D; ‰) were obtained from regressions of ln(R/R0) against −ln(f) (Guy et al., 1989; Fig. 2) and were transformed to standardized fractionation factors (Δ; ‰; Guy et al., 1989). We found a mean standardized fractionation factor Δ of 18.5‰ for uninhibited respiration of leaves of G. littoralis (Table I). Inhibition of the cytochrome path increased Δ to 24.1‰ (or 25.3‰ when one significant [P < 0.05] outlier was omitted), whereas inhibition of the alternative path decreased Δ to 15.7‰ (Table I). The fractionation factor for residual respiration was 20.6‰ (Table I). These Δ values are somewhat lower than those found earlier for leaf material for other species (Robinson et al., 1995; Ribas-Carbo et al., 2000a). To determine whether the rather low values obtained were due to our system, we also measured isotope fractionation of the species Crassula argentea, for which fractionation factors have already been published (Robinson et al., 1995). We found a fractionation factor of 20.0 ± 1.34‰, which is similar to the value of 21.4‰ reported by Robinson et al. (1995), indicating that the low Δ values are probably specific for G. littoralis. Note, however, that Δ values of uninhibited respiration may vary with growth conditions. From the Δ values it could be calculated that 33% of the electron flow was partitioned to the alternative pathway in uninhibited G. littoralis leaves sampled at midday in July.
Figure 2.
Discrimination against 18O of uninhibited and inhibitor-treated G. littoralis leaves (D = regression coefficient × 1,000). Regressions were calculated from pooled data of various independent experiments.
Table I.
Fractionation factors (Δ, ‰) of G. littoralis and C. argentea leaf samples
| Δ | se | n | |
|---|---|---|---|
| ‰ | |||
| G. littoralis | |||
| Control | 18.5b | 0.53 | 9 |
| KCN | 24.1c | 0.92 | 12 |
| Outlier omitted | 25.3c | 0.53 | 11 |
| SHAM | 15.7a | 0.37 | 18 |
| SHAM + KCN | 20.6abc | 1.73 | 4 |
| Large slices | 17.2ab | 1.06 | 8 |
| Small slices | 18.8b | 0.04 | 3 |
| Static | 20.1b | 0.51 | 5 |
| Shaken | 20.2b | 0.81 | 7 |
| C. argentea | 20.0 | 1.34 | 14 |
se of the regression coefficient; n, number of data points in the regression. Different superscript letters indicate significant difference at P < 0.01, as calculated from a Student's t test of the ses of differences in regression coefficients (Sokal and Rohlf, 1995).
The most appropriate measure for both good precision and accuracy are the se values of the regression coefficient (Table I), which were comparable with what has been reported before (Ribas-Carbo et al., 1995, 1997; Robinson et al., 1995; Lennon et al., 1997). The r2 values of the regressions between ln(R/R0) and −ln(f) ranged from 0.985 for the KCN treatment to 0.994 for untreated leaves (Fig. 2). Omitting the significant (P < 0.05) outlier from the KCN treatment regression increased its r2 to 0.996. Although the r2 values of control and SHAM treatments are slightly lower than the value of 0.995 considered as a minimum acceptable value for a reliable estimation of Δ (Ribas-Carbo et al., 1995, 1997; Lennon et al., 1997; Gonzàlez-Meler et al., 1999; Henry et al., 1999), this does not imply that our measurements are unreliable. First, the minimum value of 0.995 applies to a specific experimental design in which six subsequent samples are taken from a single cuvette. With our method, three samples were taken per cuvette and the regressions were calculated from pooled data of different independent experiments and therefore include changes between leaf samples and possible changes over time due, for example, to changes in weather conditions. Second, although r2 is a useful statistic for evaluation of a regression, it is not an index of significance by itself.
Accuracy and Precision of the Stable Isotope Technique
Repeated analysis of individual air samples over a 6-month period has shown accuracy and precision of our δ18O measurements to be 0.1‰ and 0.3‰, respectively (n = 56; 23.5‰ ± 0.3‰). This is significantly smaller than the 8.4‰ difference in δ18O between SHAM-inhibited and KCN-inhibited respiration observed in our O2 depletion experiments or even the smallest treatment difference of 2.8‰ between control and SHAM treatment (Table I). Such precision and accuracy is comparable with that produced with on-line systems (e.g. Robinson et al., 1995).
Possible Contamination of the Samples with Outside Air
Because O2 is more abundant in the atmosphere than in the gas samples and the atmospheric O2 has a more 18O-depleted isotopic signature than the O2 remaining in the experimental cuvettes, ingress of air during the procedure may reduce δ18O(O2) and thus lead to an underestimation of the isotope fractionation. We have quantified the extent of leakage at various steps in the procedure.
Leakage of Air into Cuvette during Respiration Measurements
To measure the leakage of air into the cuvettes, the cuvettes were purged with O2-free N2 gas, the balloons were filled with air-saturated salt solution, and the O2 concentrations were monitored over a 30-h period. On average, the apparent rate of O2 entry into the cuvette was only 0.12% of the rate of O2 depletion by 10 g of leaves and 1.6% of the extremely low O2 uptake rate of 10 g of leaves inhibited with SHAM plus KCN.
18O Contamination during Storage in Exetainers and through the Introduction of the Sample into the Vacuum Preparation Line
Although the Exetainers (PDZ Europa, Sandbach, Cheshire, UK) were at near atmospheric pressure once the air samples were introduced, longer term storage might cause contamination because of diffusion of O2 into the sample. To test the long-term integrity of storing samples in Exetainers, O2-free N2 samples were stored for 6 months in our Exetainers (n = 10). Likewise, to test whether any air was introduced into the vacuum line when the sample was injected, the needle of an empty syringe was pushed through the septum of the vacuum line and the line was further operated as if a true sample was present (n = 5). The blanks from either of these processes were smaller than the minimal amount of O2 needed for a measurement of δ18O(O2), despite the IRMS utilizing the liquid nitrogen-cooled cold finger to cryo-focus the sample.
Effects of Slicing and Infiltration of Leaf Material
Although leaf slices were allowed to transpire excess water, to address concerns that the slicing and infiltration of the leaves could have affected the 18O discrimination, experiments were undertaken to compare isotope fractionation and respiration rates between infiltrated leaf slices and untreated leaf slices of the same size. The difference in Δ of infiltrated (16.3 ± 0.45‰) and noninfiltrated (17.0 ± 1.11‰) leaf slices was far below the detection limit (i.e. the lsd of Δ of untreated leaves) of that experiment (3‰). The Δ values for both treatments were somewhat lower than in subsequent measurements of uninhibited respiration, which may be explained by changes in growth conditions between the experiments. To test the effects of slicing, leaf pieces of the standard 10- × 10-mm size were compared with those about 4 times as large as usual. Any effect of the extent of slicing on Δ was less than the detection limit of this experiment of 2‰ (Table I).
Testing for Possible Errors Due to Boundary Layer Effects
Another possible source of error is an underestimation of D resulting from diffusion limitation of respiration (Angert and Luz, 2001). Large amounts of leaf material per cuvette are needed to reduce incubation time as much as possible. For the slowly respiring G. littoralis leaves, 10 g of leaf material was the minimum amount needed to deplete 50% of the available O2 in the 150-mL cuvettes within 24 h, which necessitated stacked heaps of leaf slices. To test whether the stacking of leaf slices introduced resistance to diffusion, the cuvettes were filled with twice the usual amount (20 g) of leaf material, and during the course of O2 uptake, one-half of the cuvettes were vigorously shaken every 30 min to break up boundary layers and mix all air in the cuvette; the other cuvettes were not touched. After 8 h, air samples were collected and analyzed as described above. Shaking did not significantly affect D values (Table I).
Effects of Buildup of CO2 and Depletion of O2 on Respiration Rates
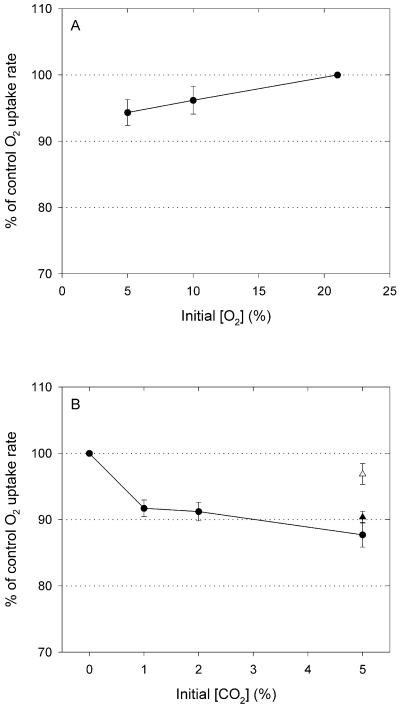
Respiration rates may be reduced by high concentrations of CO2 (e.g. Gonzàlez-Meler et al., 1996; Drake et al., 1999) or low concentrations of O2 (e.g. Gale et al., 1992; Ribas-Carbo et al., 1994). The effect of O2 depletion on respiration rates was measured by exposing the leaf material in the cuvettes to a range of O2 concentrations: 21%, 10%, and 5% (v/v) in random order. The 5% (v/v) O2 concentration is lower than the lowest remaining O2 concentration in our experiments (i.e. 5.7% [v/v]). Respiration rates tended to be lower at low O2 concentrations, but differences were small, i.e. at 5% (v/v) O2 the respiration rate was reduced to 94% of that at 21% (v/v) O2 (Fig. 3A). Because the Km of AOX is much higher than that of COX, this effect of O2 depletion might decrease fractionation. Similarly, the effect of CO2 buildup was tested by purging the cuvettes every 2 h with air containing one of four different concentrations of CO2. High concentrations of CO2 decreased respiration, mainly via its effect on the cytochrome pathway (Fig. 3B), as was found by Gonzàlez-Meler et al. (1996). Although this may also have lead to an underestimation of D, there was no evidence for nonlinearity during the course of the experiments. For example, the high r2 of 0.994 and the small se of the regression coefficient of 0.53 for the control treatment (Fig. 2, Table I) both indicate that the variation in D between subsequent samples due to O2 depletion or CO2 accumulation must have been very small. However, because the effect of CO2 accumulation is strongest at the lower CO2 concentrations (Fig. 3B; Gonzàlez-Meler et al., 1996), the cytochrome path may have been inhibited to the same extent in all subsequent samples, and therefore D may have been underestimated. Because O2 depletion and CO2 accumulation affect respiration rates and therefore may influence apparent D values, we agree with other authors that CO2 accumulation should be avoided (Gonzàlez-Meler et al., 1999) and O2 depletion should be restricted to an agreed minimum of 50% of the amount initially available, i.e. −ln(ƒ) = 0.7 (Guy et al., 1989; Robinson et al., 1995).
Figure 3.
A, Influence of the initial O2 concentration on O2 uptake rates. B, Effects of initial CO2 concentration on O2 uptake. White and black triangles represent measurements on leaf samples infiltrated with KCN and SHAM, respectively. Values are means ± se of 10 to 15 measurements.
Limitation of Respiration by Carbohydrate Supply
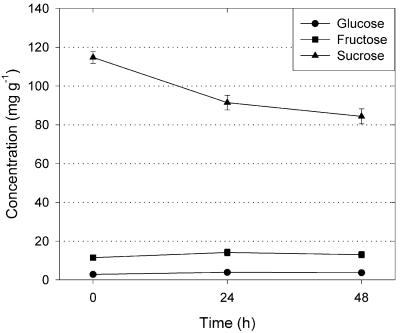
Concentrations of Suc, Glc, and Fru were compared between leaf samples that were taken before and after respiration measurements of 48 h, which is longer than our normal incubation period of 24 h. Initial Suc concentrations were 115 mg g−1 dry leaf mass and decreased only 27% after a 48-h period in the cuvettes (Fig. 4). The concentrations of Glc and Fru remained constant (Fig. 4). We therefore conclude that carbohydrate supply did not limit respiration in our measurements.
Figure 4.
Changes over time in concentrations of Glc, Fru, and Suc in leaf samples of G. littoralis. Values are means ± se of four (0 h) to 16 (24 h) measurements.
Suggestions for Further Improvement of the Method
Although the long incubation period of the measurements presented in this paper did not introduce significant problems (see above), more rapid measurements would allow the study of short-term effects of treatments and would increase throughput. Increasing the ratio of plant material volume to cuvette volume by means of minimizing the dead volume spaces in the cuvette will reduce incubation time. Some means of mixing may be needed to avoid problems with air diffusion at very high densities of plant material. Mixing may be achieved by using cuvettes with flexible walls, but such a system is more difficult to make airtight. To allow smaller sample size, the cuvettes could be miniaturized. The O2 sensor could be placed outside the cuvette, but the housing of the Figaro O2 sensor is slightly permeable to O2 and hence an O2-impermeable coating might need to be applied. To avoid inhibition of respiration, CO2 can be scrubbed by adding CO2 absorbent to the cuvette, but because this will inevitably decrease the air pressure inside the cuvette, the pressure change should be monitored or compensated for. The extent of the inhibitory effect of O2 depletion should be minimized by restricting sampling to cases in which >50% of the initial amount of O2 remains (−ln(f) < 0.7; e.g. Guy et al., 1989; Robinson et al., 1995). Replacing the vacuum preparation system with a continuous flow gas chromatography system would reduce the risk of contamination with outside air or potential problems with conversion of O2 to CO2 and allows the use of an autosampler, which increases throughput. We are investigating increasing the integrity of long-term (>6 months) storage of samples in Exetainers by sealing the cap in black wax (wax W100, Apiezon Products, Manchester, UK), which can be chipped off to expose the septum prior to analyses.
CONCLUSIONS
The offline approach described in this paper offers a very flexible alternative to the existing on-line method to measure electron flow to AOX. We found a mean Δ of 18.5‰ for uninhibited respiration of leaves of G. littoralis, and this value was neither affected by the degree of slicing of the leaves nor by the infiltration/evaporation procedure. The stacking of leaf material inside the cuvette did not diffusion limit respiration, and carbohydrate concentrations were not limiting respiration either. Our method yielded similar Δ values for C. argentea as found before with the on-line gas chromatography-IRMS technique. The O2 depletion and CO2 accumulation during the course of the experiment decreased respiration rates but did not seem to affect discrimination to a large extent. The technique we have developed has the resolution to distinguish differences in respiratory metabolism that might have functional significance to the adaptation of plants to stressful environments. This will provide an affordable alternative to the on-line system that would allow widespread adoption of the stable isotope method by laboratories where the study of AOX activity is not a core research activity.
MATERIALS AND METHODS
Plant Material
Most measurements were made on leaves of Griselinia littoralis (Raoul.), an evergreen shrub growing outside in Dundee, UK. G. littoralis was chosen because the large evergreen shrub provided a constant supply of homogenous leaf material and is comparable with other species growing in the field. Leaves were harvested at midday, the midrib was removed, and for most experiments leaf halves were sliced into parts of approximately 10 × 10 mm to facilitate infiltration with inhibitors. To find out whether our technique yielded similar results as an on-line gas phase system, we also measured isotope fractionation of leaves of the species Crassula argentea (Thunb.; = Crassula ovata [Mill.]), for which fractionation factors have already been published (Robinson et al., 1995). The C. argentea plants were grown in a greenhouse and measurements were made on intact leaves. All measurements were made during the period July through September 2000, except for the measurements of the effect of infiltration, which were made in May 2000.
Respiration Measurements
Leaf samples were enclosed in airtight cuvettes and allowed to deplete O2. During the course of the O2 depletion, gas samples were extracted using gastight syringes and transferred to pre-evacuated 10-mL Exetainers for subsequent measurement of δ18O(O2) at Scottish Universities Environmental Research Centre, East Kilbride (Glasgow, UK). Exetainers were used only once to prevent leakage due to wear of the septa. The glass cuvettes (Fig. 5) had a volume of approximately 150 mL and were sealed with a rubber lid and equipped with a galvanic O2 sensor (KE25, Figaro Engineering Inc., Osaka), which has an accuracy of 1% of full scale (21% [v/v] O2). To correct O2 values for possible variations in temperature and pressure, a thermocouple and an absolute pressure sensor (SDX-A, Sensortechnics GmbH, Düsseldorf, Germany) were also included in each cuvette. The signals from these sensors were monitored using a DL2e data logger (Delta-T Devices, Cambridge, UK), connected to a personal computer. Cuvettes were kept at a constant temperature in a thermostatic water bath. Pressure changes due to depletion of the gas volume by sampling could be compensated for by means of a rubber balloon inside the cuvette that was filled with a solution of 5% (w/v) NaCl and connected to a reservoir that was at atmospheric pressure (Fig. 5). This pressure equilibration prevented contamination of air samples during extraction and possible changes in respiration rate or Δ because of pressure changes. Cuvette air volumes were calculated from the increase in pressure after injecting 30 mL of air into the cuvette.
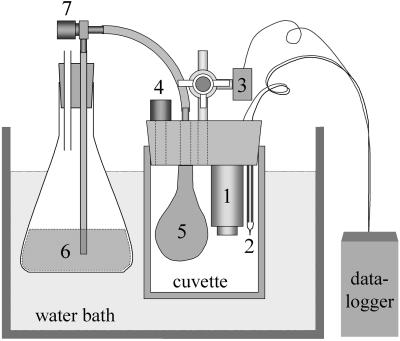
Figure 5.
Drawing of the off-line cuvette system for O2 measurements and air sampling with full-pressure equilibration. Cuvettes are equipped with an O2 sensor (1), a thermocouple (2), and a sensor for absolute pressure (3), which is connected to a three-way valve. Signals from these sensors are collected by a data logger. Gas samples are extracted with a syringe via the sample port (4). The pressure equilibration consists of a balloon (5) filled with salt solution, which is connected to an Erlenmeyer flask with salt solution (6) that has an opening to keep the pressure at atmospheric pressure. Pressure equilibration may be omitted by closing an in-line valve (7). The whole system is placed in a thermostatic water bath.
Gas Mixtures
Where particular gas mixtures were required, these were obtained using digital mass flow controllers (Teledyne Hastings, Hampton, VA). Gas mixtures were stored for up to 1 h until required in poly(vinyl chloride) beach balls, which have a very low permeability to gases (Parsons, 1989). Gases were obtained from Air Products (Walton-on-Thames, UK).
Stable Isotope Analysis
Approximately 10 mL of gas was extracted from the Exetainers using a gastight syringe and introduced into a vacuum line through a butyl rubber septum. Condensable gases were cryogenically removed, and the remaining O2 was quantitatively converted to CO2 in a graphite furnace, aided by a platinum catalyst (Bottinga and Craig, 1969). The product CO2 was cryogenically purified from the remaining noncondensable gases prior to measurement of δ18O(CO2) on a VG Sira 10 dual inlet IRMS (Analytical Precision Ltd., Cheshire, UK). Local air samples were collected and run approximately every 10 samples to assess the accuracy and precision of the vacuum line extraction process. Isotopic integrity of the IRMS was maintained by daily checks with two calibrated laboratory standard bottle gases. From each cuvette, three samples were taken over time, and data of different independent experiments were pooled. Ds were obtained from the slope of an unconstrained regression of ln(R/R0) against −ln(f), where R is the isotopic ratio in a given sample, R0 is the isotopic ratio in an initial reference sample, and f is the fraction of O2 remaining unconsumed (see Henry et al., 1999). Because both ln(R/R0) and −ln(f) are subject to error, normal regression would yield biased results. Therefore, model II regressions were calculated using the reduced major axis method (Sokal and Rohlf, 1995).
Inhibitor Titrations
G. littoralis leaves were sampled at midday and were cut into pieces of about 10 × 10 mm and pieces were mixed. For each measurement, 10 g of leaf material were infiltrated under reduced pressure (40 kPa) with either water or solutions of KCN or SHAM or a combination of these inhibitors in water. Concentrations of KCN ranged from 0 to 16 mm, and SHAM concentrations ranged from 0 to 50 mm. Because the free acid form of SHAM does not readily dissolve in water above concentrations of 25 mm, 5 mmol of SHAM were first dissolved in 5 mL of 1 m KOH, and after addition of 90 mL of demineralized water, the pH was readjusted to 6.0 with 25% (w/v) HCl, after which the volume was finally adjusted to 100 mL to yield a 50 mm SHAM solution. This way no organic solvents such as ethanol, methoxyethanol, or dimethyl sulfoxide, which possibly influence respiration, were needed. All solutions were freshly prepared and their pH was adjusted to 6.0. After infiltration, the leaf slices were blotted dry with filter paper, and excess water was allowed to evaporate until the initial mass of 10 g was reached again, which took approximately 2 h. Leaf slices were enclosed in a cuvette and measurements were made as described above.
Carbohydrate Analysis
Extraction
Leaf material was rapidly dried in a microwave (Popp et al., 1996) and subsequently dried in an oven for 24 h at 70°C. Dried material was ground in a ball mill and 50 to 100 mg were suspended in 1.5 mL of extraction buffer {100 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 20 mm MgCl2, and 1 mm EDTA, pH 7.4}. The sample was kept on ice for 10 min; 400 μL of the suspension was added to 600 μL of preheated 80% (v/v) ethanol, kept at 76°C for 20 min, then cooled on ice for 5 min, and centrifuged at 11,600g (13,000 rpm) for 3 min. The supernatant containing soluble sugars was stored at −20°C until assay.
Sugar Assay
All sugars were first enzymatically converted to Glc-6-P, and the equimolar production of NADPH after subsequent conversion of Glc-6-P to 6-P-gluconate was measured spectrophotometrically at 340 nm in 96-well microtiter plates using an automatic microplate reader (MR 5000; Dynatech Laboratories, Billinghurst, UK). Concentrations of Glc were derived by monitoring the increase in A340 after addition of 0.5 unit of hexokinase and 1.4 units of Glc-6-P dehydrogenase to 10 μL of sugar extract in 200 μL of an assay buffer (100 mm imidazole, 10 mm MgCl2, 1.65 mm ATP, and 0.625 mm NADP; pH 6.9). Concentrations of Fru were derived from the subsequent increase in absorbance after addition of 0.35 unit of phospho-Glc isomerase, and Suc concentrations were obtained by subsequent addition of 10 units of β-fructosidase. Readings of absorbance were recorded only after a stable value had been achieved. The assay was calibrated using analytical grade sugar standards obtained from BDH (Poole, Dorset, UK). Enzymes were obtained from Boehringer (Roche Diagnostics, Lawes, UK) except for phospho-Glc isomerase, which was obtained from Sigma-Aldrich (Poole, UK).
ACKNOWLEDGMENTS
We are grateful to Dr. Miquel Ribas-Carbo for helpful discussions and critical comments. We thank Shona McInroy for her help with the sugar analyses and Dr. Brian Eddy for the use of his microplate reader.
Footnotes
This work was supported by the UK National Environmental Research Council (grant no. 9/04503).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010372.
LITERATURE CITED
- Angert A, Luz B. Fractionation of oxygen isotopes by root respiration: implications for the isotopic composition of atmospheric O2. Geochim Cosmochim Acta. 2001;65:1695–1701. [Google Scholar]
- Atkin OK, Cummins WR, Collier DE. Light induction of alternative pathway capacity in leaf slices of Belgium endive. Plant Cell Environ. 1993;16:231–235. [Google Scholar]
- Azcón-Bieto J, Lambers H, Day DA. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiol. 1983;72:598–603. doi: 10.1104/pp.72.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinga Y, Craig H. Oxygen isotope fractionation between CO2 and water, and the isotopic composition of marine atmospheric CO2. Earth Planet Sci Lett. 1969;5:285–295. [Google Scholar]
- Collier DE, Cummins WR. Stimulation of respiration during plant water deficits in sand-grown Arnica alpina. In: Lambers H, Van der Plas LHW, editors. Biochemical and Physiological Aspects of Plant Respiration. The Hague, The Netherlands: SPB Academic Publishing bv; 1992. pp. 541–546. [Google Scholar]
- Drake BG, Azcón-Bieto J, Berry J, Bunce J, Dijkstra P, Farrar J, Gifford RM, Gonzàlez-Meler MA, Koch G, Lambers H. Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant Cell Environ. 1999;22:649–657. [Google Scholar]
- Gale J, Reuveni J, Mayer AM. An oxygen gradient method for determining the partitioning of respiration between its cytochrome and alternative pathway components, with application to Lemna gibba fronds under different temperatures. In: Lambers H, Van der Plas LHW, editors. Biochemical and Physiological Aspects of Plant Respiration. The Hague, The Netherlands: SPB Academic Publishing bv; 1992. pp. 433–442. [Google Scholar]
- Gonzàlez-Meler MA, Giles L, Thomas RB, Siedow JN. Metabolic regulation of leaf respiration and alternative pathway activity in response to phosphate supply. Plant Cell Environ. 2001;24:205–215. [Google Scholar]
- Gonzàlez-Meler MA, Ribas-Carbo M, Giles L, Siedow JN. The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiol. 1999;120:765–772. doi: 10.1104/pp.120.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Ribas-Carbo M, Siedow JN, Drake BG. Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol. 1996;112:1349–1355. doi: 10.1104/pp.112.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC. Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta. 1989;177:483–491. doi: 10.1007/BF00392616. [DOI] [PubMed] [Google Scholar]
- Henry BK, Atkin OK, Day DA, Millar AH, Menz RI, Farquhar GD. Calculation of the oxygen isotope discrimination factor for studying plant respiration. Aust J Plant Physiol. 1999;26:773–780. [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN. The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol. 1997;115:783–791. doi: 10.1104/pp.115.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Atkin OK, Lambers H, Wiskich JT, Day DA. A critique of the use of inhibitors to estimate partitioning of electrons between mitochondrial respiratory pathways in plants. Physiol Plant. 1995;95:523–532. [Google Scholar]
- Millenaar FF, Roelofs R, Gonzàlez-Meler MA, Siedow JN, Wagner AM, Lambers H. The alternative oxidase in roots of Poa annua after transfer from high-light to low-light conditions. Plant J. 2000;23:623–632. doi: 10.1046/j.1365-313x.2000.00832.x. [DOI] [PubMed] [Google Scholar]
- Møller IM, Berczi A, Van der Plas LHW, Lambers H. Measurement of the activity and capacity of the alternative pathway in intact plant-tissues: identification of problems and possible solutions. Physiol Plant. 1988;72:642–649. [Google Scholar]
- Noguchi K, Go CS, Terashima I, Ueda S, Yoshinari T. Activities of the cyanide-resistant respiratory pathway in leaves of sun and shade species. Aust J Plant Physiol. 2001;28:27–35. [Google Scholar]
- Parsons R. Physiological regulation of nitrogen fixation in soybean root nodules. PhD thesis. Canberra: Australian National University; 1989. [Google Scholar]
- Parsons HL, Yip JYH, Vanlerberge GC. Increased respiratory restriction during phosphate-limited growth in transgenic tobacco cells lacking alternative oxidase. Plant Physiol. 1999;121:1309–1320. doi: 10.1104/pp.121.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp M, Lied W, Meyer J, Richter A, Schiller P, Schwitte H. Sample preservation for determination of organic compounds: microwave versus freeze-drying. J Exp Bot. 1996;47:1469–1474. [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant-tissues? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Aroca R, Gonzàlez-Meler MA, Irigoyen JJ, Sanchez-Diaz M. The electron partitioning between the cytochrome and alternative respiratory pathways during chilling recovery in two cultivars of maize differing in chilling sensitivity. Plant Physiol. 2000a;122:199–204. doi: 10.1104/pp.122.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Azcon-Bieto J, Siedow JN. The reaction of the plant mitochondrial cyanide-resistant alternative oxidase with oxygen. Biochim Biophys Acta. 1994;1188:205–212. [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN. Electron partitioning between the cytochrome and alternative pathways in plant-mitochondria. Plant Physiol. 1995;109:829–837. doi: 10.1104/pp.109.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Lennon AM, Robinson SA, Giles L, Berry JA, Siedow JN. The regulation of electron partitioning between the cytochrome and alternative pathways in soybean cotyledon and root mitochondria. Plant Physiol. 1997;113:903–911. doi: 10.1104/pp.113.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Robinson SA, Gonzàlez-Meler MA, Lennon AM, Giles L, Siedow JN, Berry JA. Effects of light on respiration and oxygen isotope fractionation in soybean cotyledons. Plant Cell Environ. 2000b;23:983–989. [Google Scholar]
- Robinson SA, Ribas-Carbo M, Yakir D, Giles L, Reuveni Y, Berry JA. Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen-isotope discrimination. Aust J Plant Physiol. 1995;22:487–496. [Google Scholar]
- Robinson SA, Yakir D, Ribas-Carbo M, Giles L, Osmond CB, Siedow JN, Berry JA. Measurements of the engagement of cyanide-resistant respiration in the crassulacean acid metabolism plant Kalanchoe-daigremontiana with the use of online oxygen isotope discrimination. Plant Physiol. 1992;100:1087–1091. doi: 10.1104/pp.100.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychter AM, Mikulska M. The relationship between phosphate status and cyanide-resistant respiration in bean roots. Physiol Plant. 1990;79:663–667. doi: 10.1111/j.1399-3054.1990.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Simons BH, Millenaar FF, Mulder L, Van Loon LC, Lambers H. Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiol. 1999;120:529–538. doi: 10.1104/pp.120.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. Ed 3. New York: W.H. Freeman; 1995. [Google Scholar]
- Stewart CR, Martin BA, Reding L, Cerwick S. Seedling growth, mitochondrial characteristics, and alternative respiratory capacity of corn genotypes differing in cold tolerance. Plant Physiol. 1990;92:761–766. doi: 10.1104/pp.92.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou ME, Plaxton WC. Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol. 1993;101:339–344. doi: 10.1104/pp.101.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Lower growth temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiol. 1992;100:115–119. doi: 10.1104/pp.100.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SB. The switching of electron flux from the cyanide-insensitive oxidase to the cytochrome pathway in mung-bean (Phaseolus aureus L) mitochondria. Biochem J. 1988;249:301–303. doi: 10.1042/bj2490301. [DOI] [PMC free article] [PubMed] [Google Scholar]