Abstract
Techniques for fast noninvasive control of neuronal excitability will be of major importance for analyzing and understanding neuronal networks and animal behavior. To develop these tools we demonstrated that two light-activated signaling proteins, vertebrate rat rhodopsin 4 (RO4) and the green algae channelrhodospin 2 (ChR2), could be used to control neuronal excitability and modulate synaptic transmission. Vertebrate rhodopsin couples to the Gi/o, pertussis toxin-sensitive pathway to allow modulation of G protein-gated inward rectifying potassium channels and voltage-gated Ca2+ channels. Light-mediated activation of RO4 in cultured hippocampal neurons reduces neuronal firing within ms by hyperpolarization of the somato-dendritic membrane and when activated at presynaptic sites modulates synaptic transmission and paired-pulse facilitation. In contrast, somato-dendritic activation of ChR2 depolarizes neurons sufficiently to induce immediate action potentials, which precisely follow the ChR2 activation up to light stimulation frequencies of 20 Hz. To demonstrate that these constructs are useful for regulating network behavior in intact organisms, embryonic chick spinal cords were electroporated with either construct, allowing the frequency of episodes of spontaneous bursting activity, known to be important for motor circuit formation, to be precisely controlled. Thus light-activated vertebrate RO4 and green algae ChR2 allow the antagonistic control of neuronal function within ms to s in a precise, reversible, and noninvasive manner in cultured neurons and intact vertebrate spinal cords.
Amajor challenge in understanding the relationship between neural activity and development and between neuronal circuit activity and specific behaviors is to be able to control the activity of large populations of neurons or regions of individual nerve cells simultaneously. Recently, it was demonstrated that neuronal circuits can be manipulated by expressing mutated ion channels or G protein-coupled receptors (GPCRs). For example, the regional expression of a genetically modified K+ channel in Drosophila was able to reduce the excitability of targeted cells (i.e., muscle, neurons, photoreceptors) (1). Silencing of cortical neurons was achieved by binding of the peptide allostatin to its exogenously expressed receptor (2). Recently, Zemelman et al. (3) elegantly demonstrated that light activation of the protein complex, encoded by the Drosophila photoreceptor genes (i.e., arrestin-2, rhodopsin, and G protein α subunit), could induce action potential firing of hippocampal neurons. Activation and deactivation of neuronal firing could also be achieved when ligand-gated ion channels, such as the capsaicin receptor, menthol receptor, purinergic receptors, or light-controllable K+ channel blockers, were used to control firing in hippocampal neurons (4, 5). However, the application of these techniques to control neuronal function especially in neural circuits and living animals is limited by their relatively slow time course, the complexity of the constructs to be expressed, or the requirement to apply and wash out ligands. To overcome these limitations, we developed molecular probes that could hyperpolarize or depolarize cells on a ms time scale and be used in intact vertebrate systems to examine behavior.
To produce hyperpolarization of the somato-dendritic membrane or inhibition of synaptic transmitter release, the GPCR rat rhodopsin 4 (RO4), a member of the vertebrate rhodopsin family (6), that acts via the Gi/o pathway to regulate excitability by increasing somato-dendritic K+ and decreasing presynaptic Ca2+ conductances in neurons, was used. To depolarize the cell membrane, channelrhodopsin (ChR2) from the green algae Chlamydomonas reinhardtii, a cation selective channel directly gated by light (7), was expressed to produce a high Na+ conductance. The properties of these light-activated switches were extensively characterized and shown to be useful for modulating neuronal excitability and synaptic transmission in cultured hippocampal neurons. They were then introduced into the embryonic chick spinal cord and shown to be capable of controlling spontaneous rhythmic activity in isolated cords and living embryos.
Materials and Methods
Plasmid Constructs. For construction of ChR2(1-315)-GFP, RO4, and muscarinic acetylcholine receptor (mAChR)-M2 expression constructs and SinRep(nsP2S726)dSP-EGFP carrying RO4 and ChR2(1-315) virus constructs see Supporting Text, which is published as supporting information on the PNAS web site. Sindbis virus vector SinRep(nsP2S726) and helper DH-BB were kindly provided by P. Osten (Max Planck Institute for Medical Research, Heidelberg) (8) and RO4 by A. Huber (University of Karlsruhe, Karlsruhe, Germany) (GenBank accession no. Z46957) (9).
Cell Culture. Culturing, maintaining, and transfection of human embryonic kidney (HEK) 293 cells (tsA201 cells) and low-density and autaptic hippocampal neurons were performed as described (10, 11). To detect the distribution of RO4 and ChR2, neurons were transfected by using the calcium phosphate method (12).
Viral Production and Infection. Sindbis pseudovirions were prepared according to Invitrogen's directions (Sindbis Expression System). Viral titer was ≈1 × 108 unit per ml stocked in -80°C. For neuronal infection, viral solution was added to cultured hippocampal neurons on coverslips in 24-well plates. Expression was detected after 10 h and reached maximal expression after 24 h.
Immunocytochemistry and Image Aquisition. For transfection, immunostaining, and image acquisition of hippocampal neurons and spinal cord whole mounts see Supporting Text.
Application of Retinal to RO4- or ChR2-Expressing Cells. Bath application of all-trans retinal [100 nM (Sigma)] 2 min before the experiment was sufficient for light activation of both proteins in all preparations tested, i.e., HEK293 cells, cultured hippocampal neurons, and isolated chicken spinal cord. Exogenous application of retinal compounds was not required for light-mediated activation of RO4 and ChR2 in chicken embryos in ovo. See Supporting Text for other retinal compounds tested.
Electrophysiology and Data Analysis. All whole-cell patch-clamp recordings were performed as described (7, 11, 13). Recording solutions and conditions are given in Supporting Text.
Illumination of patches was achieved with a TILL Photonics (Planegg, Germany) Polychrome II monochromator containing a 75-W xenon short arc lamp with an output of 250-690 nm and 475 nm was used to excite ChR2 or RO4. The light intensity was 1 × 10-6 W measured by power meter (Coherent, Santa Clara, CA), and the light source was controlled by the EPC9. Light and perfusion traces were programmed in pulse software.
Spinal Cord Preparation and Measurements. In ovo electroporation, imaging of motor axons, recording of spontaneous bursting episodes in isolated spinal cord preparations, and the quantification of unit activity were as described by Hanson and Landmesser (14).
Statistical significance throughout the experiments was tested with ANOVA by using igor software. Standard errors are given as mean ± SEM.
Results
Vertebrate Rhodopsin Can Be Used to Inhibit Neuronal Excitability and Synaptic Transmission. Vertebrate rhodopsin couples to the G protein transducin, the α subunit of which belongs to the Gi subfamily (15), thus raising the possibility that mammalian rhodopsins would couple to other Gi/o family members. In neurons, the pertussis toxin (PTX)-sensitive Gi/o pathway activates G protein inward rectifying potassium channels (GIRKs) and inhibits presynaptic voltage-gated Ca2+ channels (16). GIRK channels are predominantly expressed on dendrites where they can hyperpolarize neurons (17). Presynaptic Ca2+ channels control transmitter release and inhibiting them via Gi/o-coupled receptors inhibits Ca2+ influx and transmitter release (18).
To determine whether vertebrate rhodopsin could be used as a light-activated switch to reduce neuronal excitability postsynaptically and transmitter release presynaptically, RO4 was coexpressed with either GIRK channel subunits 1 and 2 or the P/Q-type Ca2+ channel consisting of the α12.1, β1b, and α2δ subunits. The mAChR M2 (mAChR-M2) was also expressed to serve as a positive control for G protein modulation of GIRK and presynaptic Ca2+ channels via Gi/o-PTX-sensitive GPCRs, because it modulates both GIRK and P/Q-type Ca2+ channels in vivo and in heterologous expression systems (17, 19). We first demonstrated in HEK cells that both of these channels were modulated by light activation of RO4 in a manner very similar to their modulation via mAChR-M2.
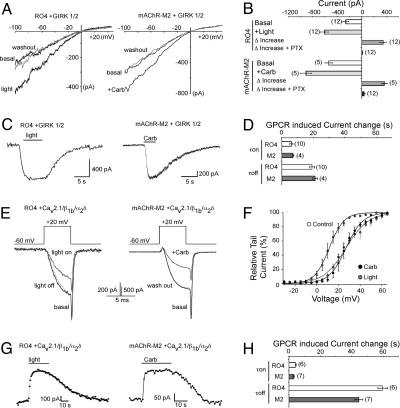
Activation of the GPCRs by either light or the AChR agonist carbachol (Carb) increased GIRK-mediated K+ currents by comparable amounts (Fig. 1 A and B) and with comparable activation and deactivation kinetics (Fig. 1 C and D). Importantly, light activation of RO4 was blocked by prior application of PTX, indicating that activation of GIRK channels by vertebrate rhodopsin is mediated via PTX-sensitive pathways (Fig. 1B). The amount of desensitization during long light or ligand exposure times was modest and comparable between the two [8.7 ± 0.8% (n = 4) for mAChR-M2 and 8.7 ± 1.1% (n = 4) for RO4], indicating that R04 can be activated by light over long time periods. When RO4 and mAChR-M2 were coexpressed with the P/Q-type Ca2+ channel, light caused reversible inhibition of the Ca2+ currents (Fig. 1 E and G and Fig. 5, which is published as supporting information on the PNAS web site). Light or Carb caused a similar shift in the voltage dependence of activation to more depolarized potentials (Fig. 1F). In addition, the G protein inhibition caused by light was reversed by high positive prepulses applied shortly before a test pulse (Fig. 5) over a voltage range between -10 and +65 mV (data not shown) similar to the inhibition caused by Carb. Furthermore, light-mediated channel inhibition was inhibited by PTX (Fig. 5). The time constants for onset of inhibition and reversal of inhibition were also comparable between RO4 and mAChR-M2 (τon = 3-7 s, τoff ≈ 20-60 s, Fig. 1 G and H). Thus, vertebrate rhodopsin modulates GIRK and P/Q-type Ca2+ channels via PTX-sensitive pathways with similar efficacy and activation and deactivation kinetics as the mAChR.
Fig. 1.
Vertebrate rhodopsin modulates GIRK and P/Q-type Ca2+ channels via Gi/o-PTX-sensitive pathways. (A) K+ current traces of GIRK1/2 channels coexpressed with RO4 or mAChR-M2 in HEK293 cells before, during, and after light stimulation (Left) or 10 μM Carb application (Right). Currents were elicited by 500-ms voltage ramps from -100 to +50 mV. (B) Comparison of the GPCR-induced current increase in the presence and absence of 5 nmol PTX. (C) Time course traces of GPCR-mediated activation of GIRK currents. GIRK currents were recorded at -60 mV. (D) Comparison of the time constants of the GPCR-induced GIRK current changes before and after GPCR activation. (E) Ba2+ current traces of P/Q-type Ca2+ channels (α12.1, β1b, and α2δ subunits) coexpressed with RO4 or mAChR-M2 in HEK293 cells before, during, and after light stimulation (Left) or 10 μM Carb application (Right). (F) GPCR-induced depolarizing shift in the voltage dependence of activation curve of P/Q-type Ca2+ currents. Currents were elicited from a holding potential of -60 mV by 5-ms-long, 5-mV voltage steps from -10 to +65 mV. Relative tail currents were plotted against the voltage pulses. (G) Time course traces of GPCR-mediated inhibition of P/Q-type Ca2+ currents. Ba2+ currents were elicited by voltage pulses from -60 to +20 mV and measured every s. (H) Comparison of the time constants of the GPCR-induced P/Q-type channel current changes before and after GPCR activation. Throughout all experiments number in parentheses indicate the number of experiments and statistical significance as indicated (*, P < 0.05; **, P < 0.01, ANOVA).
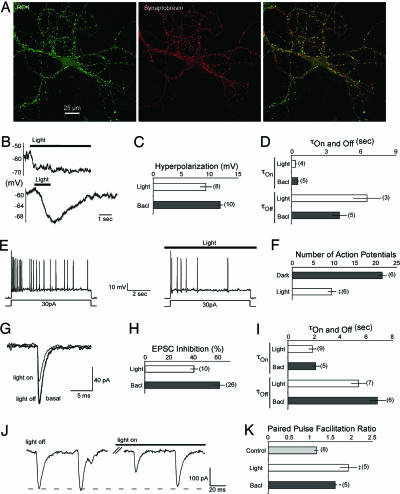
Because RO4 activates GIRKs, which control excitability postsynaptically, and inhibits Ca2+ channels of the Cav2 family, which trigger transmitter release presynaptically, we next investigated in cultured hippocampal neurons whether light activation of RO4 could hyperpolarize neurons somato-dendritically to decrease their firing as well as inhibit presynaptic Ca2+ influx to modulate short-term synaptic plasticity such as paired-pulse facilitation. Exogenously expressed RO4 was localized somato-dendritically and transported to 70-80% of the synaptic sites where it colocalized with the presynaptic neuronal marker synaptobrevin II (Fig. 2A). Light activation of RO4 induced a 9-mV hyperpolarization within ms comparable to the hyperpolarization induced by activation of endogenous GABAB receptors by 50 μM baclofen (Fig. 2 B and C). The hyperpolarization was stable during light application (measured up to 30 s) but was rapidly reversed when the light was switched off (Fig. 2 B and D). The time constants for hyperpolarization and repolarization were much faster than in HEK293 cells (compare Figs. 2D and 1C) probably because of the effect of endogenous proteins, such as RGS proteins, which accelerate the GTPase activity of the G proteins. These observations are comparable to the described actions of Gi/o-coupled receptors on membrane changes in neurons (20). More importantly, the hyperpolarization induced by light was capable of reducing the number of action potentials produced during a depolarizing current pulse (Fig. 2 E and F).
Fig. 2.
Functional expression and characterization of vertebrate rhodopsin in cultured hippocampal neurons. (A) Colocalization of RO4 and synaptobrevin in cultured hippocampal neurons. (Left) Fluorescence patterns of neurons from low-density hippocampal cultures transfected with RO4 reveal a punctate staining. RO4 was detected with an anti-RO4 antibody and visualized with an Alexa 488-coupled secondary antibody. (Center) Hippocampal cells were stained with an antisynaptobrevin II antibody and visualized with an Alexa 568-coupled secondary antibody. (Right) Overlay of RO4 and synaptobrevin II staining. Yellow indicates colocalization. (B) RO4 induced voltage change during a long (Upper) and short (Lower) light pulse. (C) Average GPCR (RO4, GABAB)-induced hyperpolarization of cultured hippocampal neurons. Throughout the experiments GABAB receptors were activated by application of 50 μM baclofen (Bacl). (D) Time course of GPCR (RO4, GABAB)-induced hyperpolarization and recovery from hyperpolarization after switching off the light or washing out baclofen. (E) Voltage traces of current-induced (30 pA) neuronal firing of cultured hippocampal neurons before and during light activation of RO4. (F) Comparison of the number of action potentials measured after current injection for a neuron before and during light activation of RO4. (G) Comparison of EPSC amplitude before, during, and after light application for EPSCs measured in autaptic hippocampal cultures expressing RO4. EPSCs in autaptic hippocampal neurons were elicited by 2-ms voltage pulses from -60 to +10 mV. (H) Comparison of GPCR (RO4, GABAB)-induced EPSC inhibition measured in autaptic hippocampal neurons. (I) Time constants of GPCR (RO4, GABAB)-induced EPSC inhibition and release from inhibition. EPSCs were elicited every 5 s as described in G. (J) Autaptic EPSC traces elicited by 2-ms voltage pulses from -60 to +10 mV separated by 50 ms (20-Hz stimulation) before and after light activation of RO4. (K) Comparison of paired-pulse facilitation before and after GPCR (RO4, GABAB) activation for a 20-Hz stimulation protocol. The amplitude of the second EPSC was compared with the first EPSC.
Because RO4 appeared to be localized at synapses and inhibits P/Q-type Ca2+ channels in HEK293 cells, we investigated whether light activation of RO4 could be used to control presynaptic function. We analyzed facilitation properties before and after light application and compared these to the effect of activating the GABAB receptor with baclofen (Fig. 2 G-K). Light activation of RO4 reduced the excitatory postsynaptic current (EPSC) amplitude by 40% compared with 60% when the GABAB receptor was activated (Fig. 2 G and H), presumably because of a reduction in quantal content (21). The time constants for these effects were comparable for both receptors [τon = 0.3-0.6 s, τoff ≈ 4-6 s (Fig. 2I)]. As would be expected if this reduction of EPSC amplitude was caused by a reduction in quantal content, paired-pulse facilitation for both receptor types was increased (Fig. 2 J and K). Taken together, these results show that light activation of RO4 can be used to control cell excitability via hyperpolarization of the somato-dendritic membrane as well as presynaptically via reduction of transmitter release.
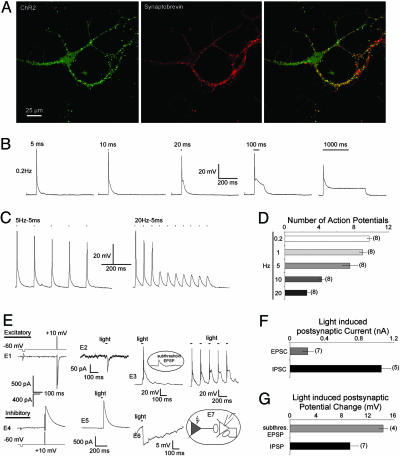
Green Algae ChR2 Can Be Used to Precisely Drive Neuronal Firing on a Fast (ms) Time Scale. ChRs are microbial type rhodopsins with an intrinsic light-gated cation conductance. ChR1 from C. reinhardii is specific for protons (22), whereas ChR2 is a less selective cation channel with conductance for H+ ≫ Na+ > K+ > Ca2+. Because the conductance of ChR2 is higher than that of ChR1 and the C terminally truncated version of ChR2(1-315) is as active as the full-length protein, all experiments were carried out with the ChR2(1-315) fragment fused to GFP at the C-terminal end of ChR2(1-315) (7). To test whether the ChR2 can act to depolarize cells when activated by light, ChR2(1-315) was first expressed and extensively characterized in HEK293 cells (Fig. 6, which is published as supporting information on the PNAS web site). Light activation of ChR2 was found to cause depolarizations of 10-25 mV within 10 ms, with repolarization occurring within 200 ms. Thus ChR2 should be capable of depolarizing neurons sufficiently to elicit action potentials.
When exogeneously expressed in hippocampal neurons, ChR2 appeared to localize both somato-dendritically and at 50-70% of the synaptic sites defined by synaptobrevin 2 immunostaining (Fig. 3A). A 5-ms light activation was sufficient to elicit action potentials in >90% of the experiments performed, whereas longer light exposure led to continuous subthreshold depolarization of the neurons (Fig. 3B). When stimulated at 5 Hz most stimuli elicited action potentials, but as the frequency of stimulation was increased, the proportion that triggered subthreshold EPSPs increased (Fig. 3 C and D). We next tested whether presynaptically expressed ChR2 was capable of triggering synaptic transmission on postsynaptic neurons. Pairs of hippocampal neurons were analyzed, in which a GFP-ChR2 expressing neuron synapsed with a ChR2-negative neuron that had formed autapses on its own soma (Fig. 3E, E7 diagram). We found that inhibitory postsynaptic currents (IPSCs) as well as EPSCs could be successfully triggered by light activation of the presynaptic neuron (Fig. 3E). The light-activated currents were different in amplitude than the autaptic currents elicited by electrically stimulating the postsynaptic neuron (Fig. 3E), indicating that they are mediated through different neuronal contacts. In three of seven experiments light-activated postsynaptic EPSCs were sufficient to trigger somato-dendritic firing up to 20 Hz. In the remaining four experiments subthreshold EPSPs were observed (Fig. 3E, E3). Light-induced postsynaptic IPSCs caused somato-dendritic hyperpolarization (Fig. 3E, E6). As expected the IPSC/EPSC amplitudes and degree of hyperpolarization or depolarization varied between analyzed neuronal pairs, as they would depend on the amount of synaptic contacts formed between the presynaptic and postsynaptic neuron (Fig. 3 F and G).
Fig. 3.
Functional expression and characterization of green algae ChR2 in cultured hippocampal neurons. (A) Colocalization of ChR2 and synaptobrevin in cultured hippocampal neurons. (Left) Fluorescence patterns of neurons from low-density hippocampal cultures transfected with GFP-ChR2 reveal a punctate staining. (Center) Hippocampal cells were stained with an antisynaptobrevin II antibody and visualized with an Alexa 568-coupled secondary antibody. (Right) Overlay of GFP-ChR2 and synaptobrevin II staining. Yellow indicates colocalization. (B) Voltage traces of ChR2-induced neuronal firing of cultured hippocampal neurons for light stimuli with increasing duration. (C) Voltage traces of ChR2-induced neuronal firing of cultured hippocampal neurons for light stimuli with different frequencies. (D) Number of action potentials measured in neurons expressing ChR2. Action potentials were elicited by a train of 10 stimuli for different light stimulation frequencies with a light duration of 5 ms. (E) Light activation of ChR2 expressed in excitatory (Upper) or inhibitory (Lower) presynaptic neurons induce activation or inhibition in the paired postsynaptic neurons. (E1 and E4) EPSC (Upper) or IPSC (Lower) were elicited by a 2-ms voltage pulse from -60 to +10 mV in the postsynaptic autaptic neuron. (E2 and E5) Light activation of the excitatory and inhibitory presynaptic cells expressing ChR2 induced EPSC (Upper) or IPSC (Lower) on the postsynaptic, autaptic neurons. (E3) Presynaptically (excitatory) light induced spiking or subthreshold depolarization (Inset) of the postsynaptic neuron after a single 5-ms light pulse (Left) or a 10-Hz/5-ms light stimulation protocol (Right). Five light pulses were applied. (E6) Presynaptically (inhibitory) light induced hyperpolarization of the postsynaptic neurons after a single 5-ms light pulse. (E7) Schematic diagram of the neuronal circuit analyzed. Gray indicates the presynaptic neuron expressing ChR2. (F) Average amplitude of the light induced EPSCs or IPSCs. (G) Average amplitude of the light-induced hyperpolarization (IPSP) or depolarization (EPSP), when the depolarization was not sufficient to trigger an action potential.
Activation of RO4 and ChR2 Can Be Used to Control Spontaneous Activity in Isolated Intact Spinal Cords and Living Embryos. Our next goal was to show that these light-sensitive proteins could be used to control circuit behavior in whole animal preparations. Early embryonic chick spinal cords exhibit rhythmic episodes of spontaneous bursting activity, which are generated by recurrent excitatory connections between motoneurons and GABAergic and glycinergic interneurons, all of which are excitatory at this stage of development (23-25). Recently, it has been shown that the normal pattern and frequency of this early spontaneous activity is required for appropriate motor axon pathfinding in the chick (14) and for the development of cord circuits that enable appropriate flexor-extensor and right-left phasing during locomotor-like activity in the mouse (26).
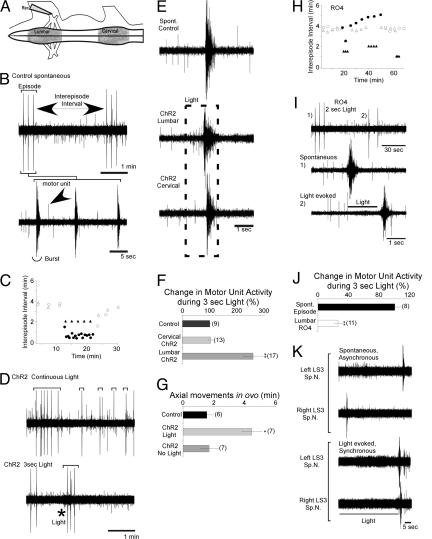
To assess whether such network activity, especially the frequency of spontaneous bursting episodes, could be controlled noninvasively by light, constructs for GFP-ChR2 or GFP-RO4 under the control of the CMV promoter were electroporated into the spinal cords of stage 16 (embryonic day 2-3) chick embryos in ovo. At stage 26 (embryonic day 4.5-5) isolated spinal cord-hindlimb preparations were made, and the constructs were found to be expressed in many neurons including motor and interneurons (Fig. 7, which is published as supporting information on the PNAS web site) and could be expressed selectively in lumbar or cervical cord by varying the electroporation protocol. Suction electrode recordings from lumbar motor nerves (Fig. 4 A and B) revealed that as in control embryos the electroporated embryos exhibited episodes consisting of several bursts every 4 min (Fig. 4 B and C) (25). Thus the electroporation protocol and expression of these constructs over several days did not appear to have any adverse effects on the development of the cord circuits responsible to generating this activity. The asynchronous firing of individual motoneurons between bursts and between episodes could also be detected (Fig. 4B, arrow). When exposed to continuous light (Fig. 4C, •) the inter-episode intervals in this cord, electroporated at the lumbar level with ChR2, were shortened to <1 min. They were, however, less rhythmic than control spontaneous episodes and consisted of single bursts (Fig. 4D Upper). In contrast, the application of a 3-s light pulse was able to elicit a normal three-burst episode shortly after a spontaneous episode (Fig. 4D Lower), and such pulses when repeated could drive episodes at precise frequencies, in the example shown (Fig. 4C, ▴) at 2-min intervals. The expanded time base traces (Fig. 4E) show that light first elicited an increase in lumbar motor unit firing that subsequently resulted in a burst very similar to spontaneous episodes in nonelectroporated embryos. However, when expression of ChR2 was restricted to the cervical cord, lumbar motor nerve recordings revealed that it was also possible to drive episodes in the lumbar cord by light without a previous increase in lumbar unit activity, by generating episodes that propagated from the cervical level (Fig. 4 E and F). Thus light, as has been previously shown for electrical stimulation (24, 25), can be used to elicit episodes either by activation of local lumbar interneurons and motoneurons or activation of neurons many cord segments distant.
Fig. 4.
RO4 and ChR2 can be used to regulate the frequency of spontaneous rhythmic activity in isolated embryonic chick spinal cords and living embryos. (A) Diagram of isolated chicken spinal cord preparation showing the position of the recording suction electrode; regions electroporated with either ChR2 or RO4 are shown in gray. (B) Electrical recording from motor nerve of ChR2 lumbar-electroporated embryo showing two control episodes in the absence of light (Upper) with an expanded time base trace of a single episode shown (Lower). Bursts of many motor axons firing synchronously and individual motor axons firing asynchronously are noted. (C) Plot of the intervals (in min) between bursting episodes from a lumbar electroporated ChR2 embryo subjected to a long interval of continuous light (circles) or 3-s pulses of light (triangles); filled symbols indicate episodes elicited in the presence of light, and open circles indicate episodes occurring in the absence of light. (D) Electrical recordings showing episodes (denoted by brackets) occurring during several minutes of continuous light (Upper) or elicited by a 3-s pulse of light at the position of the asterisk (Lower). (E) Comparison of unit activity preceding bursts that occurred spontaneously in a nonelectroporated embryo (Top) or were elicited by light when ChR2 was expressed selectively in the lumbar cord (Middle) or cervical cord (Bottom). Time of light exposure is indicated by dashed line. (F) Bar graph of the percent change in motor unit activity occurring in control embryo and one electroporated at cervical or lumbar level during a 3-s exposure to light. (G) The frequency of axial movements of stage 25-26 embryos in ovo, 3 days after ChR2 was electroporated into cervical cord segments, in the presence or absence of 475 nM light. (H) Plot of intervals between bursting episodes in embryos electroporated with RO4 at lumbar level when exposed to a long interval of continuous light (circles) or 3-s light pulses at different repetition rates (triangles); filled symbols indicate episodes occurring in the presence of light, open symbols indicate those that occurred in the absence of light. (I) Activation of RO4 by brief light pulses triggers bursting episodes. (Top) After a spontaneous episode (no. 1) a 2-s light pulse was able to trigger a premature bursting episode (no. 2); both are shown on expanded time bases in Middle and Bottom, respectively (see text for more detail). (J) Bar graph of change in motor unit activity in the period preceding the first burst of a spontaneous episode or one evoked by light activation of RO4. (K) Light activation of RO4 can synchronize the bursting behavior of spinal cord motoneurons. Right and left sides of a RO4 lumbar electroporated cord exhibit independent (asynchronous) rhythms when they are surgically separated at the midline (top pair of traces) However, the bursts triggered after the cessation of a light stimulus results in their synchronization (bottom pair of traces). LS3, lumbar segment 3; Sp.N., spinal nerve.
To asses whether light could be used to drive rhythmic activity in intact embryos in ovo, axial movements, which are precisely correlated with electrically recorded episodes of activity (14), were videotaped under red light that did not activate the cervically electroporated ChR2. When several light pulses of the wavelength necessary to activate ChR2 were given through a window in the shell, each elicited a clear movement episode. Furthermore, a significant increase in the frequency of axial movements could be maintained by continuous application of light over several minutes (Fig. 4G). These observations indicate that the light switches can act in intact animal preparations without application of all-trans retinal (see Discussion) and that the light used is able to penetrate through the amnion and layers of tissue to activate the spinal cord neurons.
Because light activation of RO4 hyperpolarized hippocampal neurons, we next explored whether it could be used to suppress spontaneous bursting activity. During continuous light, the interval between spontaneous episodes increased only modestly in cords with lumbar expression of RO4 (Fig. 4H, •). This finding was not entirely unexpected because regions of cord not electroporated with RO4 would still be able to depolarize and contribute to the excitation required to elicit a bursting episode (see ref. 23) for details of episode generation). Surprisingly, however, a 2-s pulse of light actually elicited a premature episode (4I, 2) 1 min after a spontaneous episode (Fig. 4I, 1). Yet when 1-, 1.5-, or 2-s pulses of light were given, lumbar motor unit activity was suppressed during the light and the episode was triggered only when the light was switched off (Fig. 4I, 2). During the light exposure asynchronous firing of motoneurons was also suppressed (Fig. 4 I Bottom and J). Thus, while the activation of RO4 in intact cord circuits could affect excitability by the activation of other G protein-coupled pathways, for example, by activating glycine receptors that are excitatory at this stage, our results suggest that in the embroynic day 5 chick cord hyperpolarization of the transfected neurons predominates. We propose that such hyperpolarization of cells within the circuit (27), possibly by relieving the inactivation of voltage-gated Na+ channels, enhances the probability that these cells will fire together, when the light is extinguished and thus provides another means for synchronizing bursting episodes within the circuit. Thus light activation of RO4 could precisely drive episodes at 1-, 1.5-, or 2-s intervals (Fig. 4H, ▴). In addition, when the connections between the right and left sides of the cord are surgically severed, the episodes on the two sides occur asynchronously, but can be synchronized by light activation of RO4 (Fig. 4K).
Discussion
This study has shown that vertebrate rhodopsin RO4 and green algae ChR2 can be used to control neuronal function when activated by light. RO4 acted postsynaptically to hyperpolarize neurons and inhibit action potential firing and presynaptically to reduce transmitter release. We also demonstrated that ChR2 could function somato-dendritically to depolarize neurons and cause action potential firing. Whether it is transported to the presynaptic terminal where currents generated by it could modulate transmission remains to be determined. However, the transport of RO4 to presynaptic sites, where it was capable of modulating presynaptic function (transmitter release and paired-pulse facilitation), suggest that it will be a useful tool for studying G protein-mediated effects at the vertebrate presynaptic terminal in the ms time range and will provide a means for precise temporal activation and deactivation of presynaptic G proteins. Such precise activation is not possible with activating GPCRs with ligands, because washout, transport, or degradation of the ligands is slow. It is likely that ms activation of presynaptic terminal G proteins will lead to insights into the presynaptic function of G proteins, and in particular for events involved in short-term synaptic plasticity and modulation of transmitter release.
ChR2, which appears to be the protein of choice for increasing excitability and firing of neurons, was also very recently characterized in neurons by Boyden et al. (28). This group, also using the truncated version of ChR2, was able to control firing with light pulses up to 30 Hz. We observed that light stimulation frequencies >5 Hz led to a decrease in the success rate of action potential firing, probably because of the use-dependent decrease in ChR2 currents combined with a frequency-dependent increase in Na+ channel inactivation. The 5-Hz stimulation protocol, which we found resulted in a high success rate in eliciting trains of action potentials, is in agreement with the 200-ms time it takes to recover from the ChR2-induced depolarization (Fig. 6). Thus the extent to which a neuron will be able to precisely follow the frequency of light pulses will probably depend on the membrane properties of the different classes of neurons.
A potential concern related to the use of light-activated switches is the extent to which the light will penetrate tissues. However, we demonstrated here that the applied light was sufficient to activate both isolated spinal cords and intact embryonic day 5-6 chick embryos inside the egg, where light was applied through a window in the shell. Furthermore, the fact that light stimuli could be applied to the chick cords over many hours without altering the pattern or frequency of the spontaneous rhythmic activity in the absence of light suggests that the light has not damaged the complex cord circuits required for generating this activity. Taken together, our experiments thus demonstrate that neuronal circuits within intact embryos can be controlled by a noninvasive technique without the need for any chemical compounds.
Thus, the light switches we have developed should provide important tools for characterizing cell and network function in living animals or tissue. Placing these switches under the control of specific promoters will enable one to control the activity of specific subsets of neurons and thus determine their role in complex behaviors, as, for example, defining the roles of subclasses of interneurons and motoneurons in locomotion. Besides their utility for basic characterization of neuronal circuit function and behavior, these proteins will provide additional tools for developing externally, light-controlled molecular machines to circumvent disease or trauma-induced alterations in nervous system excitability, such as after spinal cord injuries, heart arrhythmia, and Parkinson's disease.
Supplementary Material
Acknowledgments
We thank Dr. E. Deneris for reading the manuscript and Dr. B. Ovryn for help with the early studies on RO4. This work was supported by National Institutes of Health Grants NS0447752 and NS42623 (to S.H.) and National Institutes of Health Grants NS19640 and NS23678 (to L.T.L.).
Author contributions: X.L., D.V.G., M.D.M., L.T.L., and S.H. designed research; X.L., D.V.G., M.G.H., J.H., M.D.M., H.C., P.H., and L.T.L. performed research; X.L., D.V.G., M.G.H., J.H., M.D.M., H.C., and P.H. contributed new reagents/analytic tools; X.L., D.V.G., M.G.H., and S.H. analyzed data; and X.L., D.V.G., M.G.H., H.C., P.H., L.T.L., and S.H. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: ChR, channelrhodopsin; GPCR, G protein-coupled receptor; mAChR, muscarinic acetylcholine receptor; GIRK, G protein inward rectifying potassium channel; PTX, pertussis toxin, RO4, rat rhodopsin 4; HEK, human embryonic kidney; Carb, carbachol; EPSC, excitatory postsynaptic current; IPSC, inhibitory postsynaptic current.
References
- 1.White, B. H., Osterwalder, T. P., Yoon, K. S., Joiner, W. J., Whim, M. D., Kaczmarek, L. K. & Keshishian, H. (2001) Neuron 31, 699-711. [DOI] [PubMed] [Google Scholar]
- 2.Lechner, H. A., Lein, E. S. & Callaway, E. M. (2002) J. Neurosci. 22, 5287-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zemelman, B. V., Lee, G. A., Ng, M. & Miesenbock, G. (2002) Neuron 33, 15-22. [DOI] [PubMed] [Google Scholar]
- 4.Zemelman, B. V., Nesnas, N., Lee, G. A. & Miesenbock, G. (2003) Proc. Natl. Acad. Sci. USA 100, 1352-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R. H. (2004) Nat. Neurosci. 7, 1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber, A., Sander, P. & Paulsen, R. (1996) J. Biol. Chem. 271, 11710-11717. [DOI] [PubMed] [Google Scholar]
- 7.Nagel, G., Szellas, T., Huhn, W., Kateriya, S., Adeishvili, N., Berthold, P., Ollig, D., Hegemann, P. & Bamberg, E. (2003) Proc. Natl. Acad. Sci. USA 100, 13940-13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, J., Dittgen, T., Nimmerjahn, A., Waters, J., Pawlak, V., Helmchen, F., Schlesinger, S., Seeburg, P. H. & Osten, P. (2004) J. Neurosci. Methods 133, 81-90. [DOI] [PubMed] [Google Scholar]
- 9.Dodge, J., Fulton, A. B., Parker, C., Hansen, R. M. & Williams, P. (1996) Invest. Ophthalmol. Visual Sci. 37, 1951-1956. [PubMed] [Google Scholar]
- 10.Bekkers, J. M. & Stevens, C. F. (1991) Proc. Natl. Acad. Sci. USA 88, 7834-7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittemann, S., Mark, M. D., Rettig, J. & Herlitze, S. (2000) J. Biol. Chem. 275, 37807-37814. [DOI] [PubMed] [Google Scholar]
- 12.Park, M., Penick, E. C., Edwards, J. G., Kauer, J. A. & Ehlers, M. D. (2004) Science 305, 1972-1975. [DOI] [PubMed] [Google Scholar]
- 13.Li, X., Hummer, A., Han, J., Xie, M., Melnik-Martinez, K., Moreno, R. L., Buck, M., Mark, M. D. & Herlitze, S. (2005) J. Biol. Chem. 280, 23945-23959. [DOI] [PubMed] [Google Scholar]
- 14.Hanson, M. G. & Landmesser, L. T. (2004) Neuron 43, 687-701. [DOI] [PubMed] [Google Scholar]
- 15.Downes, G. B. & Gautam, N. (1999) Genomics 62, 544-552. [DOI] [PubMed] [Google Scholar]
- 16.Hille, B. (1994) Trends Neurosci. 17, 531-536. [DOI] [PubMed] [Google Scholar]
- 17.Mark, M. D. & Herlitze, S. (2000) Eur. J. Biochem. 267, 5830-5836. [DOI] [PubMed] [Google Scholar]
- 18.Reid, C. A., Bekkers, J. M. & Clements, J. D. (2003) Trends Neurosci. 26, 683-687. [DOI] [PubMed] [Google Scholar]
- 19.Mark, M. D., Wittemann, S. & Herlitze, S. (2000) J. Physiol. (London) 528, 65-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrengruber, M. U., Doupnik, C. A., Xu, Y., Garvey, J., Jasek, M. C., Lester, H. A. & Davidson, N. (1997) Proc. Natl. Acad. Sci. USA 94, 7070-7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittman, J. S. & Regehr, W. G. (1997) J. Neurosci. 17, 9048-9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel, G., Ollig, D., Fuhrmann, M., Kateriya, S., Musti, A. M., Bamberg, E. & Hegemann, P. (2002) Science 296, 2395-2398. [DOI] [PubMed] [Google Scholar]
- 23.Wenner, P. & O'Donovan, M. J. (2001) J. Neurophysiol. 86, 1481-1498. [DOI] [PubMed] [Google Scholar]
- 24.Hanson, M. G. & Landmesser, L. T. (2003) J. Neurosci. 23, 587-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milner, L. D. & Landmesser, L. T. (1999) J. Neurosci. 19, 3007-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers, C. P., Lewcock, J. W., Hanson, M. G., Gosgnach, S., Aimone, J. B., Gage, F. H., Lee, K. F., Landmesser, L. T. & Pfaff, S. L. (2005) Neuron 46, 37-49. [DOI] [PubMed] [Google Scholar]
- 27.McCobb, D. P., Best, P. M. & Beam, K. G. (1990) J. Neurosci. 10, 2974-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. (2005) Nat. Neurosci. 8, 1263-1268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.