Thrombotic thrombocytopenic purpura (TTP) is a rapidly fatal illness, first described by Moschowitz in 1924 (1), that is characterized by anemia, a low platelet count (thrombocytopenia), and microvascular thrombosis. The anemia is caused by hemolysis and is associated with sharply pointed red cell fragments, termed schistocytes, that look as though they might have been sliced with a knife. The thrombocytopenia is caused by platelet deposition in thrombi, which leads to tissue damage that can produce renal failure, stroke, myocardial infarction, and acute respiratory distress. TTP often attacks otherwise healthy adults, with a preference for females of childbearing age. Without treatment the mortality rate exceeds 90%, but plasma exchange therapy has reduced the death rate to approximately 25% (2, 3).
Until recently, the pathogenesis of TTP was obscure and the efficacy of plasma exchange therapy was unexplained. The thrombi in TTP are rich in platelets and von Willebrand factor (VWF) but relatively poor in fibrin (4), reflecting minimal participation of the classical blood clotting cascades. VWF is required for normal platelet adhesion (Fig. 1), and exaggerated VWF adhesive activity has been proposed to cause TTP (5). Breakthroughs reported during the last year support this model. A new metalloprotease that cleaves VWF, ADAMTS13, was cloned (6–8), and mutations in the ADAMTS13 gene were shown to cause a congenital form of TTP (9). In this issue of PNAS, Kokame et al. (10) report new ADAMTS13 mutations and characterize the recombinant mutant proteases, giving us our first glimpses of ADAMTS13 structure–function relationships and discovering unexpected heterogeneity at the ADAMTS13 locus.
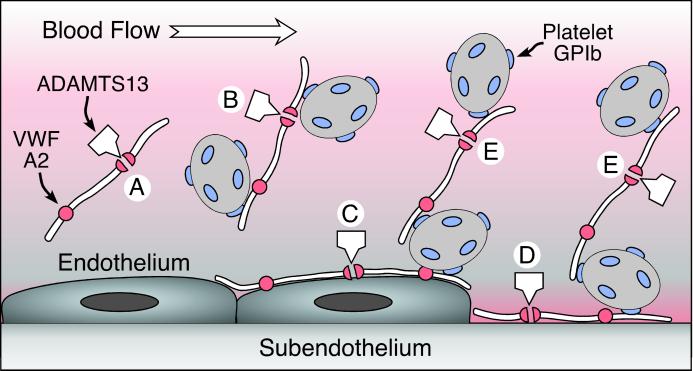
Figure 1.
Possible sites of ADAMTS13 action on VWF. VWF is released from endothelial cells as unusually large multimers that may diffuse into the circulation (A and B) or adhere to the endothelial cell surface (C). VWF also binds to connective tissue exposed at sites of vascular injury (D). Under conditions of high fluid shear stress, platelets can adhere to VWF in solution (B) or on surfaces (C and D) through the platelet glycoprotein Ib (GPIb) receptor. VWF also may recruit platelets to previously adhering platelets (E). ADAMTS13 cleaves a Tyr–Met bond in the A2 domain of the VWF subunit, severing the multimer. This reaction is slow for VWF in solution (A) but occurs rapidly when platelets adhere to VWF under high fluid shear conditions in suspension (B) or on surfaces (C–E), presumably as a consequence of conformational changes induced by tensile force on the VWF multimer. Failure of this mechanism appears to cause TTP.
The first hint of VWF involvement in TTP was reported 20 years ago and, as often is the case, the crucial insight was suggested by careful observation of rare patients. In 1982, Joel Moake et al. (5) found “unusually large” VWF multimers in blood plasma from four patients with chronic relapsing TTP, particularly when their disease was in remission. The plasma VWF multimers were similar to the very large multimers secreted by cultured endothelial cells, suggesting that unusually large VWF multimers persisted after secretion into the blood because the patients lacked a VWF depolymerase, possibly a protease or a disulfide reductase. Congenital or acquired deficiency of the depolymerase might cause TTP, and plasma exchange therapy might provide a fresh supply of depolymerase or remove an inhibitor.
Until now, no mutation had been characterized sufficiently to establish the corresponding mechanism of disease.
Recent discoveries are consistent with this early proposal. In back-to-back papers, Furlan et al. (11) and Tsai (12) described a plasma metalloprotease activity that shortens VWF multimers by cleaving a single Tyr–Met bond within domain A2 of the VWF subunit. The enzyme cleaved VWF very slowly in plasma (Fig. 1), but the reaction was greatly accelerated by fluid shear stress or by mild denaturation of VWF with urea or guanidine hydrochloride. Shortly thereafter, deficiency of this VWF cleaving protease, whether congenital or caused by an acquired antibody inhibitor, was shown to be strongly associated with TTP (13–16). Last year, the VWF cleaving protease was purified to homogeneity, sequenced, and recognized as a new member of the ADAMTS protease family, which is named for the combination of A Disintegrin-like and Metalloprotease with Thrombospondin-1 repeats (6, 17, 18). Full-length cDNA clones for ADAMTS13 were obtained rapidly (7, 8). Simultaneously, the ADAMTS13 gene was identified by genomewide linkage analysis in families affected by congenital TTP, and 12 distinct mutations were characterized in seven unrelated families (9). Interestingly, no patient had definitive null mutations on both alleles, suggesting that total ADAMST13 deficiency may be lethal.
ADAMTS13 consists of a signal peptide, a propeptide, and a typical reprolysin-like or adamalysin-like metalloprotease domain, followed by a disintegrin-like motif, a thrombospondin-1 repeat, a characteristic cysteine-rich and spacer domain, seven additional thrombospondin-1 repeats, and two CUB domains. Aside from the metalloprotease domain, the purpose of the domains is not known. However, all are conserved among ADAMTS13 from human, mouse (7, 9), and Japanese pufferfish (Takifugu rubripes) (unpublished observations), suggesting that they are essential for some function. Based on the role of their homologues in other proteins, these domains are candidates to interact with cell surfaces, extracellular matrix, protein cofactors, or VWF. The mutations described so far are distributed from the metalloprotease domain through the first CUB domain with no obvious mutational hotspot (9) and therefore could illuminate the function of several structural domains. Until now, however, no mutation had been characterized sufficiently to establish the corresponding mechanism of disease.
Kokame et al. (10) studied two unrelated patients from Japan with Upshaw–Schulman syndrome, which refers to inherited TTP with a chronic relapsing course that often begins in the neonatal period (19, 20). Neither patient had detectable plasma ADAMTS13 activity, suggesting the presence of inactivating mutations on both alleles. One patient was homozygous for a Q449X nonsense mutation. The second patient had three candidate mutations: Q448E and C508Y on the maternal allele and R268P on the paternal allele. Q448E was reported previously as a polymorphism, although the effect of the substitution on function was not determined. In addition, the asymptomatic father had the mutation P475S on his other ADAMTS13 allele and plasma ADAMTS13 activity of only 5.6%, suggesting that the substitution P475S also impairs ADAMTS13 function.
The properties of the expressed recombinant ADAMTS13 mutants accounted for the patients' severe functional deficiency. Constructs with Glu or Gln at position 448 had similar activity, indicating that Q448E is a polymorphism without clear functional significance. Constructs with Glu-268 or Tyr-508 accumulated within the cell and were not secreted. However, the truncated Q449X and full-length P475S mutants were secreted at the expected sizes and had extremely low proteolytic activity toward VWF. Thus, substitution or truncation within the cysteine-rich domain of ADAMTS13 can impair activity without affecting secretion. This result is consistent with a role for the cysteine-rich domain in VWF recognition, although proper folding of the metalloprotease domain has not been demonstrated for the mutant proteins.
When the prevalence of these mutations was investigated among healthy controls, the results were surprising. Allele frequencies for Q448E were 81% Gln and 21% Glu, consistent with a polymorphism, and the Upshaw–Schulman mutations R268P, Q449X, and C508Y were not detected in 364 normal individuals. However, the P475S mutation, which severely impairs ADAMTS13 function, had a frequency of 5%—-one asymptomatic homozygote was identified and 9.6% of the sampled population was heterozygous, a value sufficiently high that balanced selection might be entertained as a possible explanation. This unexpected finding certainly will prompt additional studies of ADAMTS13 activity and polymorphisms. If confirmed in other populations, a high frequency of ADAMTS13 deficiency could have significant implications for medical practice. For example, low ADAMTS13 activity might reduce the risk of hemorrhage in certain settings, moderate the phenotype of von Willebrand disease, or exacerbate the platelet-rich thrombosis associated with myocardial infarction and ischemic stroke. In addition, patients using the platelet function inhibitors ticlopidine or clopidogrel rarely develop autoantibodies to ADAMTS13 and TTP (21, 22), and ADAMTS13 polymorphisms could influence the risk of this complication.
The new data of Kokame et al. (10) fit well with an emerging model for how VWF and ADAMTS13 normally cooperate to regulate platelet adhesion and prevent TTP (Fig. 1). A few elements of this scenario require additional experimental testing, but it explains many clinical and biochemical observations. Unusually large VWF multimers are secreted by endothelial cells and may enter the circulation or remain bound to the cell surface as long strings that can be several millimeters in length (23). VWF in solution is cleaved slowly by ADAMTS13, but the rate of cleavage increases under conditions of high fluid shear stress that are characteristic of the microcirculation (24), especially if platelets are available to bind and increase the tensile force on the VWF multimer (25, 26). Unusually large VWF multimers bind platelets more tightly than typical plasma VWF (27) and therefore may be more susceptible to cleavage by ADAMTS13 when sheared in the presence of platelets. Platelets adhere to VWF presented on endothelial cells, but ADAMTS13 rapidly cleaves the VWF and releases the platelets (23). Platelets also adhere to VWF that is bound to extracellular matrix (28), and platelets can be recruited to a growing thrombus by a similar VWF-dependent mechanism (29); these processes would be inhibited by ADAMTS13 cleavage of VWF. Deficiency of ADAMTS13 increases shear-induced platelet aggregation (25, 26) and causes TTP (9, 10, 13–16), possibly by allowing the uncontrolled growth of thrombi rich in platelets and VWF (4). Desmopressin, a vasopressin analog, releases unusually large VWF multimers from endothelial cells in vivo and is used to treat bleeding in patients with hemophilia A or von Willebrand disease. As might be expected if VWF were a critical ADAMTS13 substrate, desmopressin promptly induced TTP symptoms in a patient with Upshaw–Schulman syndrome (30). Patients with congenital ADAMTS13 deficiency can be treated with plasma infusions every 2 or 3 weeks because ADAMTS13 has a long circulatory half-life and levels >5% of normal appear sufficient to prevent symptoms (31). Plasma exchange is effective for autoimmune TTP, probably because it removes some of the inhibitory antibodies and enables the administration of large amounts of exogenous ADAMTS13.
The unusually large VWF model of TTP pathogenesis is appealing, but it requires VWF to be the major physiological substrate of ADAMTS13 and this assumption as yet has only indirect support. Although ADAMTS13 clearly inhibits VWF function in vitro, it might prevent TTP by cleaving other unknown proteins in vivo. Genetic approaches could establish the relevance of VWF cleavage by ADAMTS13. For example, a mutation in VWF that made it resistant to ADAMTS13 might cause TTP, but no such human mutation has been identified. Conversely, ADAMTS13 deficiency does cause TTP, and if VWF is the principal substrate then severe von Willebrand disease should be protective. No instance of such a dual defect has been found yet in humans, but the combination might be produced in genetically modified mice. In the meantime, the catastrophic phenotype of human ADAMTS13 deficiency highlights the biological importance of ADAMTS13 in controlling microvascular thrombosis, which occurs in many disorders besides TTP. The high prevalence of heterozygous ADAMTS13 deficiency found by Kokame et al. (10) suggests that genetic variation at the ADAMTS13 locus may also modulate the risk of thrombosis in other cardiovascular diseases. These and other potential clinical correlations will be examined carefully during the next few years.
Footnotes
See companion article on page 11902.
References
- 1.Moschcowitz E. Proc NY Pathol Soc. 1924;24:21–24. [Google Scholar]
- 2.Amorosi E L, Ultmann J E. Medicine. 1966;45:139–159. [Google Scholar]
- 3.Rock G A, Shumak K H, Buskard N A, Blanchette V S, Kelton J G, Nair R C, Spasoff R A. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 4.Asada Y, Sumiyoshi A, Hayashi T, Suzumiya J, Kaketani K. Thromb Res. 1985;38:469–479. doi: 10.1016/0049-3848(85)90180-x. [DOI] [PubMed] [Google Scholar]
- 5.Moake J L, Rudy C K, Troll J H, Weinstein M J, Colannino N M, Azocar J, Seder R H, Hong S L, Deykin D. N Engl J Med. 1982;307:1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 6.Fujikawa K, Suzuki H, McMullen B, Chung D. Blood. 2001;98:1662–1666. doi: 10.1182/blood.v98.6.1662. [DOI] [PubMed] [Google Scholar]
- 7.Zheng X, Chung D, Takayama T K, Majerus E M, Sadler J E, Fujikawa K. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 8.Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. J Biochem. 2001;130:475–480. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- 9.Levy G G, Nichols W C, Lian E C, Foroud T, McClintick J N, McGee B M, Yang A Y, Siemieniak D R, Stark K R, Gruppo R, et al. Nature (London) 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 10.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, et al. Proc Natl Acad Sci USA. 2002;99:11902–11907. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlan M, Robles R, Lämmle B. Blood. 1996;87:4223–4234. [PubMed] [Google Scholar]
- 12.Tsai H-M. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 13.Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lammle B. Blood. 1997;89:3097–3103. [PubMed] [Google Scholar]
- 14.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle P A, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, et al. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 15.Furlan M, Robles R, Solenthaler M, Lammle B. Blood. 1998;91:2839–2846. [PubMed] [Google Scholar]
- 16.Tsai H M, Lian E C. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerritsen H E, Robles R, Lammle B, Furlan M. Blood. 2001;98:1654–1661. doi: 10.1182/blood.v98.6.1654. [DOI] [PubMed] [Google Scholar]
- 18.Hurskainen T L, Hirohata S, Seldin M F, Apte S S. J Biol Chem. 1999;274:25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 19.Upshaw J D., Jr N Engl J Med. 1978;298:1350–1352. doi: 10.1056/NEJM197806152982407. [DOI] [PubMed] [Google Scholar]
- 20.Schulman I, Pierce M, Lukens A, Currimbhoy Z. Blood. 1960;16:943–957. [PubMed] [Google Scholar]
- 21.Tsai H M, Rice L, Sarode R, Chow T W, Moake J L. Ann Int Med. 2000;132:794–799. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett C L, Connors J M, Carwile J M, Moake J L, Bell W R, Tarantolo S R, McCarthy L J, Sarode R, Hatfield A J, Feldman M D, et al. N Engl J Med. 2000;342:1773–1777. doi: 10.1056/NEJM200006153422402. [DOI] [PubMed] [Google Scholar]
- 23.Dong J F, Moake J L, Nolasco L, Bernardo A, Arceneaux W, Shrimpton C N, Schade A J, McIntire L V, Fujikawa K, López J A. Blood. 2002. www.bloodjournal.org , e-Print archive, www.bloodjournal.org, 10.1182/blood-2002-05-1401. , 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 24.Tsai H M, Sussman I I, Nagel R L. Blood. 1994;83:2171–2179. [PubMed] [Google Scholar]
- 25.Yagi H, Konno M, Kinoshita S, Matsumoto M, Ishizashi H, Matsui T, Titani K, Fujimura Y. Br J Haematol. 2001;115:991–997. doi: 10.1046/j.1365-2141.2001.03222.x. [DOI] [PubMed] [Google Scholar]
- 26.Ajzenberg N, Denis C V, Veyradier A, Girma J P, Meyer D, Baruch D. Thromb Haemostasis. 2002;87:808–811. [PubMed] [Google Scholar]
- 27.Arya M, Anvari B, Romo G M, Cruz M A, Dong J F, McIntire L V, Moake J L, Lopez J A. Blood. 2002;99:3971–3917. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- 28.Savage B, Almus-Jacobs F, Ruggeri Z M. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 29.André P, Hainaud P, Bal dit Sollier C, Garfinkel L I, Caen J P, Drouet L O. Arterioscler Thromb Vasc Biol. 1997;17:919–924. doi: 10.1161/01.atv.17.5.919. [DOI] [PubMed] [Google Scholar]
- 30.Hara T, Kitano A, Kajiwara T, Kondo T, Sakai K, Hamasaki Y. Am J Pediatr Hematol Oncol. 1986;8:324–328. doi: 10.1097/00043426-198624000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Barbot J, Costa E, Guerra M, Barreirinho M S, Isvarlal P, Robles R, Gerritsen H E, Lämmle B, Furlan M. Br J Haematol. 2001;113:649–651. doi: 10.1046/j.1365-2141.2001.02808.x. [DOI] [PubMed] [Google Scholar]